Abstract
The adsorption of colipase is essential for pancreatic triglyceride lipase activity and efficient dietary fat digestion. Yet, little is known about which specific amino acids in the hydrophobic surface of colipase influence adsorption. In this study, we systematically substituted alanine or tryptophan at residues implicated in adsorption of colipase to an interface. We expressed, purified recombinant colipase mutants and characterized the ability of each alanine mutant to restore activity to lipase in the presence of bile salts. The functions of L16A, Y55A, I79A and F84A colipase were most impaired with activities ranging from 20 to 60% of wild-type colipase. We next characterized the fluorescence properties of the tryptophan mutants in the absence and presence of bile-salt-oleic acid mixed micelles. We performed steady-state emission spectra to determine peak shift and I330/I350 ratio and acrylamide quenching curves to characterize the environment of the residues. The analysis supports a model of adsorption that includes residues Leu 34 and Leu 36 on the 2nd loop, Tyr 55 and Tyr 59 on the 3rd loop and Ile 75 and Ile 79 on the 4th loop. The analysis confirms that Phe 84 is not part of the adsorption surface and likely stabilizes the conformation of colipase. Contrary to the predictions of computer modeling, the results provide strong support for an essential role of Tyr 55 in colipase adsorption to mixed micelles. The results indicate that the adsorption of colipase to mixed micelles is mediated by specific residues residing in a defined surface of colipase.
Keywords: Colipase, Dietary lipid, Mixed Micelle, Pancreatic Lipase, Site-directed mutagenesis
1. Introduction
Fatty acids must be released from dietary triglycerides before they are absorbed by intestinal enterocytes and distributed throughout the body. In the duodenum, lipases catalyze the efficient release of fatty acids from dietary lipid emulsions. Prior to hydrolysis, lipases must first bind to the surface of the lipid particles and hydrolysis occurs at that interface. In vivo, dietary lipids form multilamellar emulsion particles with phospholipids, cholesterol and cholesterol esters located in the surface layers and a bulk phase of triglyceride inside the outer layers [1]. Dietary proteins and carbohydrates and bile salts cover the surface of the emulsion particles. By itself, the predominant duodenal lipase, pancreatic triglyceride lipase (PTL)1 cannot bind to the surface of the particles and is effectively inactive [2]. Another pancreatic protein, colipase, acts in a complex with PTL to restore activity.
Despite years of study, the molecular details of the interactions of colipase or the colipase-PTL complex with substrate and bile salt micelles remain obscure. X-ray crystallography of the porcine colipase-human PTL complex revealed the orientation of colipase to PTL in the complex [3-4]. Colipase, a flat and rectangular molecule with four loops or fingers, interacts with the C-terminal domain of PTL. The region of colipase opposite the PTL binding site forms a large hydrophobic plateau with residues in the open lid domain of PTL. It was proposed that residues in the hydrophobic plateau interact with the lipid substrate or bile salt micelles or both [3-5]. Hydrophobic residues reside in the first finger (Leu 16 and Met 18 (Leu 18 in the pig)), the second finger (Ala 33 (Ile 33 in the pig), Leu 34 and Leu 36), the third finger (Leu 54, Tyr 55, Ile 57 (Val 57 in the pig) and Tyr 59) and the fourth finger (Leu 75, Val 76, Ile 79 and Phe 84) [5].
Of these residues, only the tyrosines have experimental evidence for a role in binding lipid emulsions or bile salt micelles. NMR studies implicated the tyrosine-rich region in the binding of porcine colipase to bile salt micelles [6-8]. Fluorescence studies suggested Tyr 55 can insert into the interior of bile salt micelles [9-11]. Neutron crystallography of the porcine colipase-PTL complex also provided evidence that Tyr 55 interacts with bile salt micelles [12]. Site directed mutagenesis of Tyr 55 and Tyr 59 in human colipase to aspartic acid decreased the ability of colipase to restore lipase activity against long-chain triglycerides emulsified in bile salts [13]. No direct evidence for the interaction of Tyr 55 and Tyr 59 with the substrate interface was presented in this study.
In silico modeling of potential interactions between porcine colipase and bile salt micelles or lipid droplets argued against a role for Tyr 55and Tyr 59 in the interaction with either interface [14]. The predicted orientations of colipase on the surface of bile salt micelles or lipid droplets placed these tyrosines near but not in the interface. The authors suggested this proximity to the interface could explain earlier experimental results implicating Tyr 55 and Tyr 59 in the absorption of colipase to bile salt micelles or lipid droplets. The modeling also did not identify Phe 84 as a residue that might interact with an interface. The modeling suggested that the tyrosine cluster and Phe 84 influence the function of colipase by stabilizing the conformation of colipase rather than by inserting into a hydrophobic interface.
To directly identify residues that mediate colipase absorption to interfaces, we substituted alanine for each of the putative residues in the hydrophobic plateau, expressed and purified the recombinant human colipase variants and characterized the ability of the variants to restore lipase activity and to mediate binding of lipase to various substrates. We then took advantage of the absence of tryptophan residues in human colipase and made tryptophan substitution mutants in selected residues. We measured interactions of the tryptophan mutants with bile salt micelles and mixed micelles of bile salt and oleic acid by tryptophan fluorescence.
2. Materials and methods
2.1. Construction of colipase mutants
All manipulations of DNA were done by standard methods unless otherwise noted[15]. Substitution of alanine or tryptophan was accomplished by site-directed mutagenesis using the QuickChange II XL Site-directed Mutagenesis Kit according to the manufacturer’s instructions. Synthetic oligonucleotide primers were designed with the desired mutation and were generally 27-bp long. The template was human colipase cDNA cloned into pPIC9 as previously described[16]. Amplification was with 10 cycles with 15 m extension times in an Applied Biosystems 9800 Fast Thermal Cycler. The amplified product was transformed into E. coli as described by the manufacturer. Plasmid DNA was prepared from single colonies with the Qiagen Spin MiniPrep Kit according to the directions. The presence of the desired base changes and the absence of unwanted mutations were confirmed by dideoxynucleotide sequencing of both strands of the entire cDNA insert.
2.2. Production and purification of colipase
The plasmids containing the desired colipase construct were transformed into Pichia pastoris strain GS115 and expressed as previously described [16]. The cells were removed by centrifugation at 3000 g for 5 min and the medium was concentrated using a Labscale TFF System over a Pellicon XL Biomax 5 membrane (Millipore, Bedford, MA). The sample was diluted 1:1 with 0.1 M NaPO4 containing 2 M ammonium sulfate and the pH adjusted to 6.0. The sample was applied to two 5-ml HiTrap Phenyl HP columns (Amersham Biosciences) connected in tandem and equilibrated in 50 mM NaPO4 containing 1 M ammonium sulfate pH 6.0. Chromatography was controlled with an AktaExplorer system (Amersham Biosciences). The column was washed with the equilibration buffer until the OD280 returned to baseline and the bound colipase was eluted with a 10 column volume gradient from 1 M to 0 M ammonium sulfate in the phosphate buffer. Fractions containing colipase were identified by monitoring the OD280 and by activity assay. The fractions were pooled and concentrated over an Amicon Ultra-15 5,000 MWCO centrifuge filter according to the parameters described by the manufacturer (Millipore). The buffer was exchanged by gel filtration over a Superdex 75 HR 10/30 column equilibrated in 50 mM TrisCl, pH 8.0 and 150 mM NaCl. Fractions containing colipase were pooled and concentrated over the centrifugation filter as above.
The concentration of wild-type colipase was determined by ultraviolet spectrophotometry at OD280 and an extinction coefficient of E = 3.0 (g/100 ml)-1cm-1. The concentration of the mutants was determined by ELISA with wild-type colipase as the standard. 96-well plates were coated with a mouse monoclonal antibody against human colipase by incubating each well with 50 μl of a 1:6400 dilution of the antibody in PBS overnight at 4°C. The wells were rinsed 3 times with 400 μl distilled water and 3 times with 400 μl SuperBlock buffer (Pierce). Next, diluted colipase in 100 mM Tris-Cl, pH 8.0, 150 mM NaCl, 1mM CaCl2 and 0.1% BSA. The colipase standard contained 10 to 200 pg/50 μl. The amount of unknown was estimated by spectrophotometry and two different dilutions were prepared. All assays were done in triplicate. 50 μl was placed in each well and the plate incubated at room temperature for 2 h. The wells were rinsed 3 times with 400 μl of PBS with 0.5% Tween-20. Then, 50 μl of a rabbit polyclonal antibody against human colipase diluted 1:6400 in SuperBlock buffer was added to each well and incubated 2 h at room temperature. After rinsing the wells 3 times with 400 μl of PBS with 0.5% Tween-20, 50 μl of a goat anti-rabbit IgG antibody conjugated with horseradish peroxidase (Pierce) diluted 1:12,500 in SuperBlock buffer was added to each well. After 1 hour incubation at room temperature, the wells were washed 3 times with 400 μl distilled water. 50 μl of substrate (1-Step Ultra TMB-ELISA, Pierce) was added to each well. The reaction was allowed to proceed for 10-30 minutes and stopped by adding 50 μl 2MH2SO4 to each well. The plate was read at 450 nm. The homogeneity of the purified colipase was confirmed by SDS-PAGE and staining with GELCODE Blue according to the manufacturer’s instructions (Pierce) [17].
2.3. Activity and adsorption assays
Activity against tributyrin, tricaprylin and triolein was done in the pH stat as described previously[13]. For each assay, 0.5 ml of the substrate was added to 14.5 ml of 2 mM Tris-HCl, pH 8.0, 1 mM CaCl2, 150 mM NaCl and 4 mM NaTDC. The final concentration of the substrate was 310 mM for tributyrin and 100 mM for tricaprylin or triolein. Adsorption to tributyrin was measured by a centrifugation assay as previously described[13, 16]. For these assays, 0.5 ml of substrate was added to 14.5 ml of 50 mM Tris-Cl, pH 8.0, 1 mM CaCl2, 150 mM NaCl, and 4 mM sodium taurodeoxycholate. Recombinant human pancreatic triglyceride lipase (PTL) was prepared as previously described and used in all assays [18]. Colipase and lipase were added in a 1:1 molar ratio. Under these assay conditions, colipase concentration was the limiting factor for lipase activity. Curves were fitted to a rectangular hyperbola function using SigmaPlot 11. To determine the apparent Kd values we fit the curves assuming the maximum binding or activity was the same for wild-type and the mutants. Since the PTL concentration is not varied, it will determine the maximum values.
2.4. Steady-state fluorescence spectroscopy
Fluorescence measurements were performed at room temperature on a Jasco FP-6300 spectrofluorimeter using a 1 cm quartz cell. The excitation and emission monochromators were set at 5 and 10 nm slit widths respectively. Excitation was at 295 nm to selectively excite tryptophan residues. Emission was monitored between 300 and 450 nm. The average of five separate scans was analyzed. The buffer was 20 mM Tris-Cl at pH 8.0. When included, the concentration of NaTDC or oleic acid was 4 mM. For quenching experiments, acrylamide was added in 2 μl aliquots of an 8.4 M acrylamide stock to 1 ml of protein solution up to a final concentration of 0.4 M acrylamide. The fluorescence intensity changes were recorded at the maximum emission wavelength for the mutant PTL and corrected for dilution. The Stern-Volmer plots were created with the simplified equation [19-20].
F0 and F are the fluorescence intensities in the absence and presence of the quencher, respectively. Q is the molar concentration of acrylamide and Kq is the Stern-Volmer constant for bimolecular collisional quenching. The data was fitted by linear regression using SigmaPlot 11.
2.5. Statistics
Comparisons were done with the software package, SigmaStat (SPSS, Chicago, IL). Pairwise comparisons were done by t-test. Multiple comparisons were done by one-way ANOVA followed by pair-wise multiple comparisons with the Holm-Sidak method. P < 0.05 was considered significant.
Results
2.6. Functional properties of the alanine mutations
We expressed wild-type and alanine substitution mutants in P. pastoris. All of the mutants were secreted into the medium and were purified in mg quantities. Each mutant was assayed for function in the standard pH-stat assay with 4 mM NaTDC using tributyrin, tricaprylin or triolein. The mutants divided into three groups where activity with all three substrates was significantly different from the other groups (P = <0.001) (Fig. 1). L34A, L36A, L54A, I57A, Y59A, I75A and V76A had function close to that of wild-type colipase regardless of substrate. L16A and I79A had intermediate function, 50-60%, with all three substrates. Y55A and F84A had less than 30% function with all three substrates. Since triolein, Y55A had a lag time of 9 min and F84A had a lag time of 30 min with triolein, longer assays were done and the activity was determined from the linear portion of the burst phase.
Fig 1.
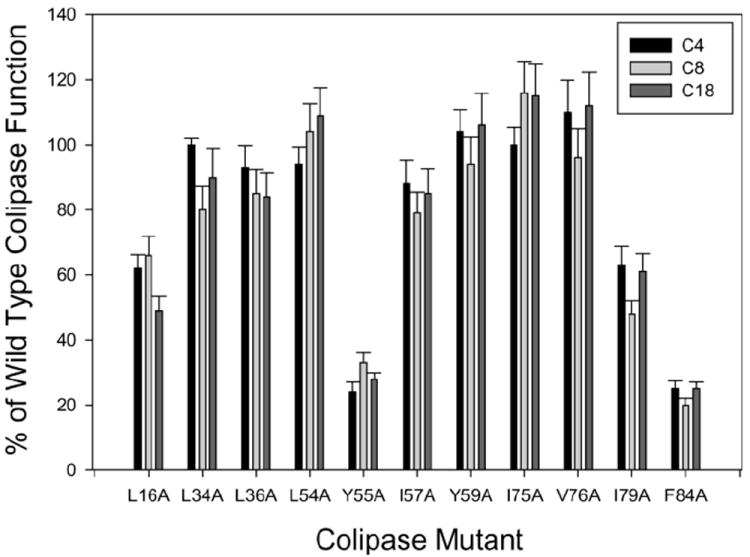
Relative function of the alanine substitution mutations of colipase. Assays were done by the pH-stat method as described in Experimental Procedures. All assays were done in 4 mM NaTDC with a 1:1 molar ratio of colipase to recombinant human PTL. The concentrations of substrate were 310 mM tributyrin, 100 mM trioctanoin and 100 mM triolein. The results are expressed as a percentage of wild-type colipase function. Each bar is the mean ± S.D. of at least 5 determinations.
To further characterize the properties of the L16A, Y55A, I79A and F84A mutants, we took two different approaches. First, we determined the ability of the alanine mutants to mediate PTL absorption on tributyrin and NaTDC emulsion particles by measuring adsorption over a range of colipase concentrations (Fig 2.). We calculated the half-maximal absorption (Kd) by nonlinear regression. I16A and I79A anchored PTL as effectively as wild-type colipase and the Kd values were similar (Fig 2 and Table 1). Both Y55A and F84A were less effective at anchoring PTL. For these mutants, the Kd values were about 5-fold higher than for wild-type colipase.
Fig 2.
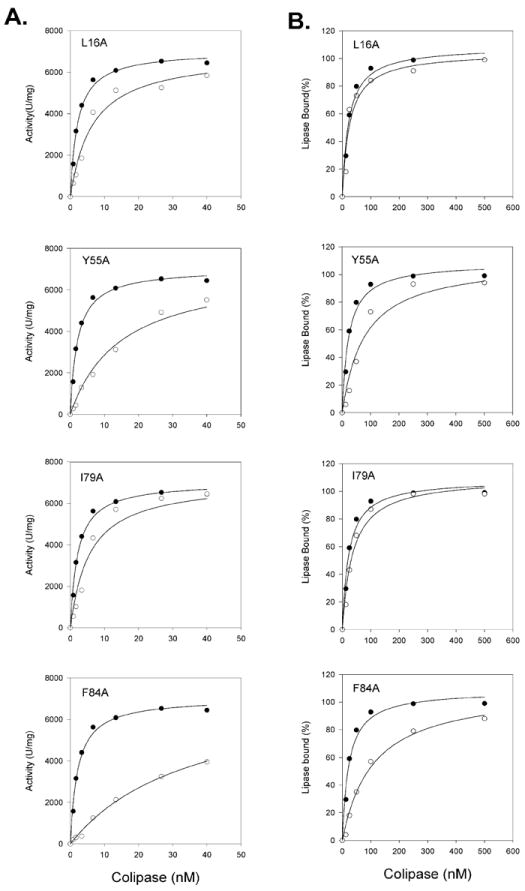
Characterization of L16A, Y55A, I79A and F84A colipase mutants. A. Colipase dependence of PTL with wild-type and selected colipase alanine substitution mutants. The assays were done with the standard 5-min pH-stat assay with tributyrin emulsified in 4 mM NaTDC. 2.7 nM recombinant human PTL was included in each assay. The concentration of colipase was varied. The points are the mean of 4 assays for wild-type and 3 assays for each mutant. Dark circles, wild-type colipase; Open circles, mutant colipase. The lines were best fits of a rectangular hyperbola function determined by SigmaStat 11 software. B. Absorption of human PTL to tributyrin mediated by wild-type colipase and the alanine substitution mutants. Absorption to tributyrin was done in a centrifugation assay as described in Experimental Procedures. 20 nM recombinant human PTL was added to the incubation which included 310 mM tributyrin in 4 mM NaTDC. Colipase was added at various concentrations. Dark circles, wild-type colipase; Open circles, mutant colipase. The lines were best fits of a rectangular hyperbola function determined by SigmaStat 11 software.
Table 1.
Apparent Kd of colipase mutants for absorption of PTL to tributyrin in 4 mM NaTDCa
| Colipase | Kd | Colipase:PTL |
|---|---|---|
|
| ||
| nM | Molar Ratio at Kd | |
| Wild type | 22.8 ± 4.1 | 1.1 |
| L16A | 25.7 ± 7.8 | 1.3 |
| Y55A | 78.6 ± 20.0 | 3.9 |
| I79A | 36.0 ± 7.2 | 1.8 |
| F84A | 107.7 ± 19.0 | 5.4 |
Absorption assays were done with 310 mM tributyrin in 4 mM NaTDC as described in Experimental Procedures. Each assay contained 20 nM recombinant human PTL. ± 1 S.D. is given.
Second, we determined the ability of the 4 colipase mutants to restore activity to PTL (Fig. 3.). From these data, we determined the concentration of colipase to restore half-maximal activity (Kd) by nonlinear regression with a rectangular hyperbola function. The activity curves shifted right for each of the alanine mutants compared to wild-type colipase although each of the mutants restored PTL activity to essentially the same SAmax as wild-type colipase (Fig. 3. and Table 2). L16A and I79A had apparent Kd values about 3-fold greater than wild-type whereas Y55A and F84A were 11- and 18-fold higher, respectively (Table 2). The molar ratio for wild-type colipase:PTL at the Kd is about 1.0 in both assays. In contrast, the molar ratio is lower at the Kd in the absorption assay than in the activity assay for each of the colipase mutants suggesting the mutants are more efficient at mediating PTL absorption than restoring activity (Table 1 and Table 2).
Fig 3.
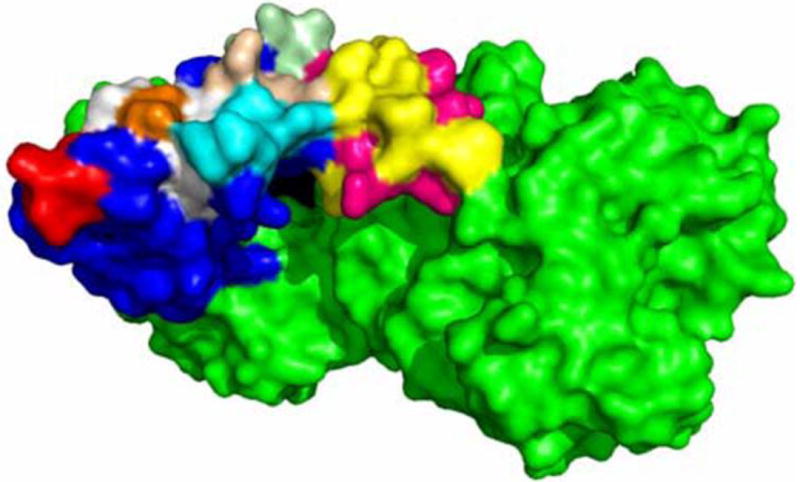
Crystal structure of the colipase-PTL complex in the active, open-lid conformation. The surface of the colipase-PTL complex (PDB: 1LPB) is represented. The crystal structure was accomplished with porcine colipase and human PTL. We used PyMOL software to convert the targeted residues that differ between porcine and human colipase to the corresponding residues in human colipase. PTL is colored green. The lid domain is magenta for polar residues and yellow for apolar residues. Colipase residues 7-9 are in light cyan; residues 16 and 17 in wheat; residues 33-37 in cyan; residues 54-59 in white with Tyr55 in orange; residues 75-79 in red. The remaining colipase residues are blue. The figure was constructed with PyMOL software (Schrodinger, LLC).
Table 2.
Apparent Kd of wild-type and mutant colipases to restore PTL activity with tributyrin in 4 mM NaTDCa
| Colipase | Kd | Colipase:PTL |
|---|---|---|
|
| ||
| nM | Molar Ratio at Kd | |
| Wild type | 2.1 ± 0.23 | 0.8 |
| L16A | 6.5 ± 1.55 | 2.4 |
| Y55A | 14.8 ± 3.4 | 5.5 |
| I79A | 5.6 ± 1.8 | 2.1 |
| F84A | 32.3 ± 6.0 | 12.0 |
All assays were done over 5 min in the pH-stat with 310 mM tributyrin in 4 mM NaTDC as described in Experimental Procedures. Each assay included 2.7 nM recombinant human PTL. ± 1 S.D. is given.
2.2. Characterization of tryptophan mutants
Our analysis of the alanine mutants supports a role for Leu 16, Tyr 55, Ile 79 and Phe 84 in the function of colipase. The analysis does not prove that the function of the targeted residues is to mediate the interaction of colipase with the lipid interface. To provide direct evidence for the interaction of the specific residues with an interface, we took advantage of the absence of native tryptophan residues in human colipase and substituted tryptophan for colipase residues Leu 16, Leu 34, Leu 36, Leu 54, Tyr 55, Ile 57, Tyr 59, Ile 75, Val 76, Ile 79 and Phe 84. This permitted analysis of the tryptophan’s environment by steady state fluorescence under various conditions [21-22]. A potential disadvantage to this approach is that tryptophan may disrupt structure because it is a bulky residue and its Van der Waals volume is larger than the residues it is replacing. On the other hand, the targeted residues, except for the single valine, have large volumes and most are on the surface of the molecule. We judged the effect of the mutations on the structure of colipase by two criteria, secretion levels during expression and the specific activity of the purified colipase. We successfully expressed and purified all but one of the mutants, L16W. Although the medium of yeast transformed with the L16W plasmid had low, detectable levels of colipase, we were unable to purify the mutant protein in sufficient quantities for analysis. The expression level of the other mutants was similar to that of wild-type colipase for all but L36W, I57W and Y58W (Table 3). Analysis by one-way ANOVA showed a statistically significant difference in function among the proteins with only I57W function being significantly lower than that of wild-type colipase and the other mutants (P = <0.001).
Table 3.
Secretion and activity of tryptophan mutants against tributyrina
| Colipase | Expression | Activity ± SD | Colipase | Expression | Activity ± SD |
|---|---|---|---|---|---|
|
| |||||
| μg/ml | U/mg | μg/ml | U/mg | ||
| Wild-type | 15.6 ± 2.1 | 12675 ± 227 | I57W | 1.9 ± 0.5 | 8760 ± 251 |
| L16W | ND | ND | Y59W | 17.7 ± 2.0 | 12435 ± 210 |
| L34W | 13.3 ± 1.5 | 13955 ± 159 | I75W | 10.8 ± 1.3 | 12915 ± 291 |
| L36W | 5.9 ± 1.1 | 12515 ± 193 | V76W | 14.0 ± 1.7 | 12775 ± 210 |
| L54W | 1.9 ± 0.7 | 12655 ± 277 | I79W | 12.7 ± 1.6 | 13550 ± 246 |
| Y55W | 18.4 ± 1.9 | 13110 ± 290 | F84W | 15.4 ± 1.8 | 12810 ± 118 |
Recombinan colipase was expressed in P. pastoris. The colipase function in the medium was measured in the standard pH-stat assay using tributyrin in 4 mM NaTDC. The amount of colipase in the medium was calculated from the specific activity of the purified colipase. Mean ± S.D. is given.
2.3. Interaction of tryptophan mutants with bile salt micelles and mixed micelles
We determined the steady-state fluorescence parameters for each of the tryptophan mutants in the presence of NaTDC micelles and mixed micelles of NaTDC and oleic acid as described in Methods (Table 4 and Supplemental Fig 1.). In buffer alone, L34W, L36W, Y55W, I75W and I79W had λMAX around 350 indicating these residues are on the surface of colipase and freely accessible to water. The λMAX of the other residues was blue shifted indicating they are located in a more apolar environment although none of them appears to be completely buried within the protein and are relatively exposed to water. The location is supported by the I330/I350 ratio. Tryptophans have a λMAX around 330 when buried in proteins and a λMAX around 350 when fully exposed to water. Lower I330/I350 ratios indicate a more polar environment. Thus, L34W, Y55W and I79W have the lowest I330/I350 ratio whereas Y59W, V76W and F84W have the highest values consistent with the less accessible position identified in the NMR and crystal structures.
Table 4.
Fluorescence characterization of tryptophan mutantsa
| Colipase | Buffer | NaTDC | NaTDC & Oleic Acid | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| λMAX | Peak | I330/I350 | Peak Shift | Peak | I330/I350 | Peak Shift | Peak | I330/I350 | |
|
| |||||||||
| nm | Intensity | nm | Intensity | nm | Intensity | ||||
| L34W | 350 | 10.1 | 0.67 | -14 | 19.8 | 1.17 | -10 | 10.7 | 1.03 |
| L36W | 349 | 4.4 | 0.70 | -6 | 8.6 | 0.93 | -6 | 9.2 | 0.90 |
| L54W | 347 | 3.9 | 0.77 | -3 | 5.0 | 0.90 | -3 | 4.0 | 0.85 |
| Y55W | 350 | 4.3 | 0.63 | -6 | 6.6 | 0.90 | -19 | 5.3 | 1.23 |
| I57W | 347 | 4.7 | 0.75 | -4 | 6.9 | 0.94 | -4 | 5.3 | 0.93 |
| Y59W | 345 | 9.7 | 0.81 | -2 | 9.8 | 0.96 | -2 | 6.0 | 0.89 |
| I75W | 349 | 5.8 | 0.70 | -3 | 6.8 | 0.80 | -5 | 5.5 | 0.90 |
| V76W | 346 | 10.2 | 0.81 | -3 | 10.3 | 0.91 | -5 | 6.9 | 0.98 |
| I79W | 350 | 3.7 | 0.64 | -4 | 5.2 | 0.83 | -7 | 3.9 | 0.91 |
| F84W | 345 | 5.7 | 0.84 | 0 | 5.4 | 0.87 | 0 | 3.8 | 0.86 |
Fluorescence measurements were done in 0.02 M Tris-Cl, pH 8.0 as described in Experimental Procedures. When added, NaTDC was 4 mM and oleic acid was 4 mM. Excitation was at 295 nm. Emission was scanned from 300 to 450 nm. The excitation slit was set at 4 nm and the emission slit at 10 nm. Each scan was done 5 times and the results determined from the average fluorescence curve of those scans.
In the presence of NaTDC micelles, the peak intensity increases for all of the targeted residues except Y59W, V76W, and F84W. The largest increase occurs for L34W and L36W, which nearly double their peak intensity. The fluorescence peak is blue-shifted and the I330/I350 ratio increased for all of the tryptophan mutants except for F84W where these parameters do not change. The largest change is for L34W with a λMAX of 335 and an increase in the I330/I350 ratio to 1.17 indicating the residue is in a more apolar environment and has limited exposure to water. L36W and Y55W have the next largest blue-shift and increase in the I330/I350 ratio. When oleic acid is added to the micelles, the peak fluorescence intensity decreases for all mutants except for L36W where the peak intensity increases slightly. L34W retains a large blue-shift and increased I330/I350 ratio. Y55W exhibits a much larger blue-shift than with NaTDC alone. There is also a corresponding increase in the I330/I350 ratio. The changes in the other targeted residues are minimal and F84W does not change.
2.4. Fluorescence quenching by acrylamide
To confirm the location of the tryptophan residues when incubated with NaTDC micelles or mixed micelles of NaTDC and oleic acid, we performed quenching experiments with acrylamide. Acrylamide is a water-soluble fluorescence quencher. Blue-shifted tryptophans are essentially inaccessible to acrylamide and not quenched whereas red-shifted tryptophans are almost as accessible as tryptophan in water. In all conditions, the curves for each tryptophan mutant were linear when fit to the Stern-Volmer equation (Supplemental Fig 2). In these plots, the steeper slopes indicate more quenching and the bimolecular quenching constant, Kq, can be calculated from the slope (Table 5). The most accessible residue in buffer is L34W. It is almost completely quenched at 0.4 M acrylamide. The other residues are protected from quenching to various degrees. Of these, L36W and Y55W are the most accessible and I57W and F84W are the least accessible although they are still quenched by acrylamide. When incubated with either NaTDC micelles or mixed micelles, at least three distinct patterns emerge. L36W, Y55W, Y59W, and I75W are the least accessible in mixed micelles. Y55W and Y59W are strongly protected from quenching with Kq approaching 1 or complete protection from quenching. Y34W, L54W, I57W, V76W and I79W are quenched to about the same extent in NaTDC micelles and mixed micelles. Finally, F84W showed little to no change in the Kq regardless of the conditions.
Table 5.
Stern-Volmer bimolecular quenching constants for acrylamide and the tryptophan mutantsa
| Conditions | L34W | L36W | L54W | Y55W | I57W | Y59W | I75W | V76W | I79W | F84W |
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | 23.6 | 8.3 | 7.0 | 8.0 | 4.0 | 6.5 | 6.7 | 6.6 | 4.8 | 4.0 |
| NaTDC | 4.4 | 6.5 | 4.5 | 4.8 | 3.3 | 4.8 | 4.1 | 4.5 | 3.4 | 3.7 |
| NaTDC & Oleic | 3.6 | 3.3 | 3.6 | 1.4 | 2.4 | 1.7 | 2.7 | 3.0 | 3.0 | 3.3 |
The conditions for fluorescence are given in the legend for Table IV and Fig. 5. Excitation was at 295 nm and emission measured at the peak maximum for the incubation conditions (Table 4). The slopes of the best fit lines were determined by linear regression. Two to three replicates were done for each assay.
3. Discussion
Alanine mutagenesis decreased the function of colipase when substituted for Leu 16, Tyr 55, Ile 79 and Phe 84. Of these, Phe 84 is the least likely to contribute to colipase adsorption to a lipid interface [14]. Fluorescence analysis of the tryptophan substitution mutation, F84W, supported this contention. There was no shift in peak intensity, no change in the I330/I350 ratio and no change in acrylamide quenching compared to buffer indicating that F84W does not change interact with micelles. More likely, the decreased function of F84A results from effects on colipase structure. Five disulfide bridges and apolar interactions of Tyr 58, Tyr 59 and Phe 84 largely determine colipase structure [5, 14]. The removal of Phe 84 by substitution with alanine may disrupt the cluster of aromatic residues and the core structure of colipase. Eliminating interactions of Phe 84 with other aromatic residues has the potential to disrupt the orientation of the conserved tyrosine loop (residues 52-59) and of the conserved region containing the PTL binding site (residues Glu 45, Glu 64, Arg 65 and Asp 89) [5, 23]. Either effect could alter the ability of F84A colipase to anchor PTL on the surface of a substrate emulsion particle.
The other alanine substitution mutants that significantly impaired function of colipase were L16A, Y55A and I79A. Interestingly, L16A and I79A colipase had relatively preserved ability to anchor PTL to substrate emulsions. That observation coupled with their decreased ability to restore PTL activity suggests that L16A and I79A colipase anchor PTL on the surface of the substrate in an orientation or conformation that is suboptimal for activity. The analysis of the I79W mutant provides more insight into the role of this residue. In buffer, the tryptophan is completely exposed to water. In micelles, there is a blue-shift of the peak intensity and a widening of the I330/I350 ratio. The results suggest that I79W partially inserts into the micelles. The interaction of Ile79 with micelles was predicted by computational modeling and identified in the neutron diffraction structure of a ternary complex of colipase, PTL and taurodeoxycholate micelles[12, 14]. In the ternary complex, the detergent micelle contacted residues in colipase and the carboxyl-terminus of PTL. Mutagenesis of residues in the β5’ loop of the PTL C2-like domain provided additional evidence that the function of PTL required colipase and micelles, likely mixed micelles, or a lipid interface or both [24-25]. It is possible that the decreased function of I79A relates to decreased ability of the colipase-PTL complex to absorb efficiently to micelles or the surface of the lipid emulsion particle.
Ile 75 and Val 76 also reside in the loop containing Ile 79. Alanine mutagenesis of these residues had no effect on colipase function. Still, steady state fluorescence measurements suggest that the environments of I75W and V76W become more apolar in the presence of micelles. Both residues have a blue-shift and increase in the I330/I350 ratio in the presence of micelles. The changes are most pronounced in mixed micelles. Acrylamide quenching confirms that the residues are in a more hydrophobic environment when colipase is incubated with micelles. Taken together these findings implicate a role for the Ile79 loop, the 4th finger, in colipase adsorption to a lipid interface. These results agree with an earlier study implicating this region in colipase adsorption. The investigators studied colipase prepared from porcine pancreas (27). During purification the bond between Ile 79 and Thr 80 was cleaved by a protease. The isolated colipase fully restored activity to bile-salt inhibited PTL but the kinetics showed much longer lag times than were seen with intact colipase and the cleaved form had weaker binding to hydrophobic surfaces. These results are consistent with a role of the 4th finger in colipase-lipid interactions.
The other loop that has been implicated in the adsorption of colipase to interfaces is the one containing Leu-34 and Leu-36. The alanine substitution mutants in these positions had a small, statistically significant decrease in function with trioctanoin and triolein but not with tributyrin. L34W had the largest blue-shift and increase in the I330/I350 ratio of all the tryptophan mutants and its Stern-Volmer constant for acrylamide quenching decreases 5-fold in the presence of bile salt micelles. The large change is likely a result of the fully exposed location of L34W in buffer where its peak fluorescence, I330/I350 ratio and Stern-Volmer bimolecular quenching constant in buffer were similar to the values for tryptophan in water. L36W also had a blue-shift, widening of the I330/I350 ratio and decrease in the Stern-Volmer constant, particularly with mixed micelles. The findings suggest that Leu 34 and Leu 36 insert into micelles as proposed by NMR structures and computer modeling that implicated residues 31-36 as a major site of interaction between colipase and interfaces. The structure of Leu 34 is unstable and it is the residue most frequently predicted to insert into lipid droplets [14]. The residue at position 36 is most often Leu but Val and Ile are also found in colipase from some species whereas Leu 34 is absolutely conserved in colipase from a variety of species further supporting a critical role for these residues in colipase function.
The last region we fully interrogated was the loop containing the neighboring tyrosines. Because our earlier study showed no role for Tyr 58, we focused on other residues in the loop, Leu 54, Tyr 55, Ile 57 and Tyr 59 [13]. Alanine substitutions at positions 54, 57 and 59 had little to no effect on the function of the mutant colipase. Even so, our steady state fluorescence suggested all three residues influence adsorption of colipase onto micelles. Each had a blue-shift and broadening of the I330/I350 ratio in the presence of micelles and each had decrease in the Stern-Volmer constant when quenched by acrylamide in the presence of micelles. Intriguingly, Y59W had a Stern-Volmer constant approaching 1 in the presence of mixed micelles indicating it is buried in an apolar environment under these conditions.
Of the residues in the tyrosine loop, Tyr 55 appears to have a dominant role in the function of colipase. Y55A had greatly decreased function with all three substrates. It was almost 5-fold less efficient than wild-type colipase at mediating absorption of PTL onto NaTDC-tributyrin emulsion particles. Interestingly, the function of Y55A appears more impaired than the function of the previously reported Y55D colipase [13]. The concentration of Y55A to restore PTL activity to half-maximal activity is over 10-fold greater than for wild-type colipase and 5-fold greater than found for Y55D. Fluorescence analysis of Y55W suggested that the residue is located on the surface of colipase and mostly accessible to water. Y55W had a peak intensity of 350 nm, I330/I350 ratio close to 0.6 and a Stern-Volmer constant of 8.0, all consistent with a polar environment. An accessible location on the surface is in agreement with the previously reported crystal structures of colipase. In those structures, Tyr 55 had no observable electron density indicating it is highly mobile [3, 26]. The residue is also accessible in most of the NMR models of colipase [14]. Additionally, the reactivity of Tyr 55 to dansylation was greater than for the other two tyrosine groups and fluorescence anisotropy decay measurements were consistent with a surface location of Tyr55 and with a highly mobile side-chain [11, 27]. The environment changes dramatically when Y55W is incubated with bile salt micelles or mixed micelles. There is a blue-shift and widening of the I330/I350 ratio in micelles. The changes are significantly larger for mixed micelles and the values show that Trp 55 is completely buried in an apolar environment. An earlier study on the interaction of Tyr 55 with mixed micelles also concluded that Tyr 55 inserts into mixed micelles. In that study, dansylated Tyr 55 colipase had a marked fluorescence blue-shift in the presence of mixed micelles indicating it resides in a hydrophobic environment and mediates colipase absorption to mixed micelles.
The conclusion that Tyr 55 participates in absorption of colipase to mixed micelles is in contradiction to the conclusion from computational modeling that Tyr 55 cannot participate because it is on the hydrophilic side of colipase opposite the hydrophobic fingers [14]. In contrast, Egloff et al concluded that Tyr 55 is on the tip of the tyrosine loop (finger 3) and contributes to the hydrophobic plateau based on the crystal structure complexed with PTL [5]. The explanation may lie in the differences of colipase structure between the NMR and crystallography studies[12]. The NMR file of porcine colipase (PDB:1PCO) contains 25 different structures with great diversity. In the crystal structure of porcine colipase and human PTL, Tyr 55 contributes to the hydrophobic plateau formed by the lid domain of PTL and colipase (Fig 6). Furthermore, it is on a plane with residues 31-35. It may be that the structure of colipase absorbed to a micelle more closely resembles that observed in the crystal structures.
One caveat with our fluorescence studies is they were done in the absence of PTL. It is possible that the formation of a colipase-PTL complex would change the results. Still, our studies likely have physiological relevance since multiple studies suggest that colipase binds to the interface prior to complex formation. Two models have been proposed. In the first, colipase binds to the emulsified substrate and anchors PTL to the surface [28-29]. In the second, colipase binds to mixed micelles and the colipase-PTL complex forms on the mixed micelle [30-32]. Others and we have argued for the second mechanism based on the relative dissociation constants of colipase, PTL, emulsion particles and mixed micelles [12, 24]. Studies with monolayers of phospholipids also support a model whereby colipase absorbs before PTL [33-34]. Consequently, results with micelles and colipase alone contribute to our understanding of the molecular details underlying the absorption of colipase to an interface.
Still, the studies do not provide direct evidence for the involvement of the tested residues in the absorption to a substrate emulsion. L16A, Y55A and I79A all had decreased ability to restore activity to PTL in the presence of bile salts. The result suggests these residues contribute to the absorption of colipase onto lipid particles. The other residues implicated by the fluorescence studies as participants in the absorption of colipase to mixed micelles may not contribute to absorption to lipid particles since they had minimal to no effect on colipase function. Even so, it seems likely that the same hydrophobic surface that interacts with mixed micelles interacts with lipid particles given the orientation of the hydrophobic plateau with the lid domain of PTL.
In conclusion, we have confirmed the importance of Tyr55 in colipase interactions with mixed micelles and in its function. Additional support for an interaction of the tyrosine loop with mixed micelles was provided by the analysis of the tryptophan substitutions at position 54, 57, and 59. In the presence of micelles, the environment of these residues becomes more hydrophobic. Although alanine mutagenesis of residues in the second loop (33-36) did not affect the function, fluorescence analysis of the single tryptophan mutants clearly demonstrated the interaction of this loop with micelles. The orientation of the second and third loops with the lid domain of PTL suggests they have a major role in colipase absorption alone and in complex with PTL. In this orientation, the fourth loop is less likely to contribute to absorption onto the surface of a mixed micelle or substrate emulsion. The loop may contribute to absorption of a micelle located between colipase and the carboxyl-terminus of PTL as observed by neutron crystallography and supported by mutagenesis studies of the β5’ loop of PTL [12, 24-25]. Finally, we provide evidence that Phe 84 does not insert into micelles and that its effect on activity is likely secondary to disruption of colipase structure.
Supplementary Material
Highlights.
Tyr 55 of colipase inserts into mixed micelles
Phe 84 is critical for colipase structure
Phe 84 does not interact with lipid or mixed micelle interfaces
Amino acids 31-36 influence colipase adsorption to lipid interfaces
The second and third loops have a major role colipase adsorption to interfaces
Acknowledgments
We thank Dr. David Perlmutter for ongoing support and Dr. Al-Walid Mohsen for advice on fluorescence spectrophotometry. This research was supported by NIH grant DK080820 to MEL.
Footnotes
Abbreviations used: nuclear magnetic resonance, NMR; pancreatic triglyceride lipase, PTL; sodium taurodeoxycholate, NaTDC.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Carey MC, Hernell O. Digestion and absorption of fat. Sem Gastrointest Dis. 1992;3:189–208. [Google Scholar]
- 2.Lowe ME. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 3.van Tilbeurgh H, Egloff MP, Martinez C, Rugani N, Verger R, Cambillau C. Interfacial activation of the lipase-procolipase complex by mixed micelles revealed by x-ray crystallography. Nature. 1993;362:814–820. doi: 10.1038/362814a0. [DOI] [PubMed] [Google Scholar]
- 4.van Tilbeurgh H, Gargouri Y, Dezan C, Egloff MP, Nesa MP, Ruganie N, Sarda L, Verger R, Cambillau C. Crystallization of pancreatic procolipase and of its complex with pancreatic lipase. J Mol Biol. 1993;229:552–554. doi: 10.1006/jmbi.1993.1054. [DOI] [PubMed] [Google Scholar]
- 5.Egloff MP, Sarda L, Verger R, Cambillau C, van Tilbeurgh H. Crystallographic study of the structure of colipase and of the interaction with pancreatic lipase. Prot Sci. 1995;4:44–57. doi: 10.1002/pro.5560040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wieloch T, Borgstrom B, Karl-Erik F, Forsen S. High-resolution proton magnetic resonance study of porcine colipase and its interaction with taurodeoxycholate. Biochemistry. 1979;18:1622–1628. doi: 10.1021/bi00575a038. [DOI] [PubMed] [Google Scholar]
- 7.Cozzone PJ, Canioni P, Sarda L, Kaptein R. 360-MHz nuclear magnetic resonance and laser photochemically induced dynamic nuclear polarization studies of bile salt interaction with porcine colipase A. Eur J Biochem. 1981;114:119–126. doi: 10.1111/j.1432-1033.1981.tb06181.x. [DOI] [PubMed] [Google Scholar]
- 8.Canioni P, Cozzone PJ, Sarda L. Conformation of colipase. Prediction of the secondary structure, circular dichroism and 360 MHz proton NMR studies of porcine colipase A. Biochim Biophys Acta. 1980;621:29–42. doi: 10.1016/0005-2795(80)90059-8. [DOI] [PubMed] [Google Scholar]
- 9.Ernst EG, Behnke WD. Construction and expression of synthetic wild-type and mutant genes encoding porcine pancreatic colipase: tryptophan fluorescence studies. Biochemica et Biphysica Acta. 1991;1089:331–338. doi: 10.1016/0167-4781(91)90173-j. [DOI] [PubMed] [Google Scholar]
- 10.McIntyre J, Hundley P, Behnke W. The role of aromatic side chain residues in micelle binding by pancreatic colipase. Biochemical Journal. 1987;245:821–829. doi: 10.1042/bj2450821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntyre J, Schroeder F, Behnke W. The interaction of bile salt micelles with the dansyltyrosine derivatives of porcine colipase. Biophysical Chemistry. 1990;38:143–154. doi: 10.1016/0301-4622(90)80049-d. [DOI] [PubMed] [Google Scholar]
- 12.Hermoso J, Pignol D, Penel S, Roth M, Chapus C, Fontecilla-Camps JC. Neutron crystallographic evidence of lipase-colipase complex activation by a micelle. The EMBO Journal. 1997;16:5531–5536. doi: 10.1093/emboj/16.18.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordle R, Lowe ME. The hydrophobic surface of colipase influences lipase activity at an oil-water interface. J Lip Res. 1998;39:1759–1767. [PubMed] [Google Scholar]
- 14.Kerfelec B, Allouche M, Colin D, Van Eyck MH, Brasseur R, Thomas A. Computational study of colipase interaction with lipid droplets and bile salt micelles. Proteins. 2008;73:828–838. doi: 10.1002/prot.22109. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook J, Russell DW. A Laboratory Manual. Third. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. Molecular Cloning. [Google Scholar]
- 16.Cordle RA, Lowe ME. Purification and characterization of human procolipase expressed in yeast cells. Prot Exp Purif. 1998;13:30–35. doi: 10.1006/prep.1998.0873. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli U. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Yang Y, Lowe ME. Human pancreatic triglyceride lipase expressed in yeast cells: purification and characterization. Protein Expr Purif. 1998;13:36–40. doi: 10.1006/prep.1998.0874. [DOI] [PubMed] [Google Scholar]
- 19.Eftink MR, Ghiron CA. Exposure of tryptophanyl residues in proteins. Quantitative determination by fluorescence quenching studies. Biochemistry. 1976;15:672–680. doi: 10.1021/bi00648a035. [DOI] [PubMed] [Google Scholar]
- 20.Eftink MR, Ghiron CA. Fluorescence quenching studies with proteins. Anal Biochem. 1981;114:199–227. doi: 10.1016/0003-2697(81)90474-7. [DOI] [PubMed] [Google Scholar]
- 21.Yau WM, Wimley WC, Gawrisch K, White SH. The preference of tryptophan for membrane interfaces. Biochemistry. 1998;37:14713–14718. doi: 10.1021/bi980809c. [DOI] [PubMed] [Google Scholar]
- 22.Killian JA, von Heijne G. How proteins adapt to a membrane-water interface. Trends Biochem Sci. 2000;25:429–434. doi: 10.1016/s0968-0004(00)01626-1. [DOI] [PubMed] [Google Scholar]
- 23.Crandall WV, Lowe ME. Colipase residues Glu64 and Arg65 are essential for normal lipase-mediated fat digestion in the presence of bile salt micelles. J Biol Chem. 2001;276:12505–12512. doi: 10.1074/jbc.M009986200. [DOI] [PubMed] [Google Scholar]
- 24.Bourbon Freie A, Ferrato F, Carriere F, Lowe ME. Val407 and Ile408 in the beta 5’ loop of pancreatic lipase mediate lipase-colipase interactions in the presence of bile salt micelles. J Biol Chem. 2006;281:7793–7800. doi: 10.1074/jbc.M512984200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahinian H, Bezzine S, Ferrato F, Ivanova MG, Perez B, Lowe ME, Carriere F. The beta 5’ loop of the pancreatic lipase C2-like domain plays a critical role in the lipase-lipid interactions. Biochemistry. 2002;41:13725–13735. doi: 10.1021/bi0257944. [DOI] [PubMed] [Google Scholar]
- 26.van Tilbeurgh H, Sarda L, Verger R, Cambillau C. Structure of the pancreatic lipase-procolipase complex. Nature. 1992;359:159–162. doi: 10.1038/359159a0. [DOI] [PubMed] [Google Scholar]
- 27.McIntyre J, Schroeder F, Behnke W. Synthesis and characterization of the dansyltyrosine derivatives of porcine pancreatic colipase. Biochemistry. 1990;29:2092–2101. doi: 10.1021/bi00460a019. [DOI] [PubMed] [Google Scholar]
- 28.Patton JS, Albertsson PA, Erlanson C, Borgstrom B. Binding of porcine pancreatic lipase and colipase in the absence of substrate studied by two-phase partition and affinity chromatography. J Biol Chem. 1978;253:4195–4202. [PubMed] [Google Scholar]
- 29.Patton JS, Donner J, Borgstrom B. Lipase-colipase interactions during gel filtration. Biochim Biophys Acta. 1978;529:67–78. doi: 10.1016/0005-2760(78)90104-2. [DOI] [PubMed] [Google Scholar]
- 30.Bernback S, Blackberg L, Hernell O. Fatty acids generated by gastric lipase promote human milk triacylglycerol digestion by pancreatic colipase-dependent lipase. Biochim Biophys Acta. 1989;1001:286–293. doi: 10.1016/0005-2760(89)90113-6. [DOI] [PubMed] [Google Scholar]
- 31.Lairon D, Nalbone G, Lafont H, Leonardi J, Domingo N, Hauton JC, Verger R. Possible roles of bile lipids and colipase in lipase adsorption. Biochemistry. 1978;17:5263–5269. doi: 10.1021/bi00617a028. [DOI] [PubMed] [Google Scholar]
- 32.Sari H, Nurit S, Entressangles B. On the formation of a ternary complex between lipase, colipase and micelles of amphipathic compounds. Biochimie. 1975;57:1045–1050. doi: 10.1016/s0300-9084(75)80360-9. [DOI] [PubMed] [Google Scholar]
- 33.Dahim M, Brockman H. How colipase-fatty acid interactions mediate adsorption of pancreatic lipase to interfaces. Biochemistry. 1998;37:8369–8377. doi: 10.1021/bi973015r. [DOI] [PubMed] [Google Scholar]
- 34.Chu BS, Gunning AP, Rich GT, Ridout MJ, Faulks RM, Wickham MS, Morris VJ, Wilde PJ. Adsorption of bile salts and pancreatic colipase and lipase onto digalactosyldiacylglycerol and dipalmitoylphosphatidylcholine monolayers. Langmuir. 2010;26:9782–9793. doi: 10.1021/la1000446. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


