Abstract
Epstein–Barr virus (EBV) is known to be associated with the development of lymphomas in immunocompromised patients. Recently, age-related immune impairment has been recognized as a predisposing factor in the development of EBV-driven lymphoproliferative disorders (LPDs) in elderly patients without any known immunodeficiency or prior lymphoma. In approximately 70 % of reported cases, the affected sites have been extranodal, such as the skin, lung, tonsil and stomach. However, age-related EBV-associated B cell (EBV + B cell) LPD is extremely rare in the oral cavity. Here we report a 71-year-old Japanese man who developed an EBV + B cell LPD resembling classical Hodgkin lymphoma (CHL)—so-called polymorphous subtype—of the mandible. Histopathologically, infiltration of large atypical lymphoid cells including Hodgkin or Reed-Sternberg-like cells into granulation tissue with marked necrosis was found in the mandibular bone. Immunohistochemical analysis revealed that the large atypical Hodgkin or Reed-Sternberg-like cells were CD3–, CD15–, CD20+, CD30+ and Epstein–Barr virus (EBV)-latent infection membrane protein-1 (LMP-1)+. In situ hybridization (ISH) demonstrated EBV-encoded small RNA (EBER) + in numerous Hodgkin or Reed-Sternberg-like cells. EBNA-2 was detected by polymerase chain reaction (PCR) using an extract from the formalin-fixed, paraffin-embedded specimen. To our knowledge, this is the first reported case of the polymorphous subtype of age-related EBV + B cell LPD affecting the mandible.
Keywords: Age-related Epstein–Barr virus (EBV)-associated B cell lymphoproliferative disorder (age-related EBV + B cell LPD), Epstein–Barr virus (EBV), Polymorphous subtype, Mandible
Introduction
Age-related Epstein–Barr virus-associated B cell lymphoproliferative disorder (EBV + B cell -LPD) is a group of clinicopathologic entities, originally described by Oyama et al. [1], that differs from immunodeficiency-associated LPDs in the World Health Organization (WHO) classification [2], occurring predominantly in elderly patients and sharing features of EBV-positive (EBV+) B cell neoplasms seen in patients with immunologic impairment despite absence of any predisposing immunodeficiency [1, 3]. Age-related EBV + B cell LPD may be associated with immune senescence in the elderly, and is now incorporated into the 2008 WHO lymphoma classification as EBV-positive diffuse large B cell lymphoma (DLBCL) of the elderly [4]. This newly described disease is characterized by proliferation of atypical large B cells including Hodgkin and Reed-Sternberg-like cells with reactive inflammatory components, which may be diagnostically problematic for pathologists. Recently, Shimoyama et al. [5] have reported that age-related EBV + B cell LPDs are divisible into three morphological types: polymorphous, large cell lymphoma, and reactive lymphoid hyperplasia subtypes, among which the polymorphous subtype is the most difficult to distinguish from EBV + classic Hodgkin lymphoma (CHL) [5, 6]. Age-related EBV + B cell LPD occurs in patients over the age of 50 years (median age 71 years), and approximately 70 % of reported cases have involved extranodal sites, such as the skin, lung, tonsil and stomach [4]. However, to our knowledge, only four articles in English (two by the present authors’ group) have reported age-related EBV + B cell LPDs of the oral cavity in elderly patients [1, 7, 8, 10]. Previous studies of the relationship between EBV + LPD in the oral cavity and age-associated immunosuppression in patients over 50 years old are listed in Table 1. In fact, little is known about the clinicopathological features of extranodal EBV + B cell LPD in oral sites in elderly patients. We report a rare case of age-related EBV + B cell LPD of the polymorphous subtype, resembling classical Hodgkin lymphoma (CHL), arising in the mandible of an elderly Japanese patient.
Table 1.
Summary of clinicopathological findings in oral cavity age-related EBV + B cell LPDs
| Site | Number of cases | Age [average] | Sex (M/F) | PT | EBV | Histology | R-S like cells | Extensive necrosis | Treatment | Outcome | Author [ref.] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Tongue | (6) | 64–89 [78] | (2/4) | Ulcer | + | B cell type | + | NA (±, ?) | N(5)/Rad(1) | CR(1)/SR(2)/SR-RR(1)/SD(1)/NA(1) | Dojcinov et al. [7, 8] |
| (1) | 83 [83] | (1/0) | ? | + | Polymorphos subtype | + | + | Cem(1) | CR(1) | Oyama et al. [1, 10] | |
| Lip | (2) | 82–82 [82] | (1/1) | Ulcer | + | B cell type | + | NA (±, ?) | N(2) | SR(1)/SR-RR(1) | Dojcinov et al. [7, 8] |
| Gingiva | (1) | 70 [70] | (1/0) | ? | + | Large-cell lymphoma subtype | + | + | Cem(1) | CR(1) | Oyama et al. [1, 10] |
| Palate | (1) | 80 [80] | (0/1) | Ulcer | + | B cell type | + | NA (±, ?) | Rad(1) | CR(1) | Dojcinov et al. [7, 8] |
| Mandibular bone | (1) | 71 [71] | (1/0) | Poor healing of dental extraction wound | + | Polymorphos subtype | + | + | Sur(1) | Follow up stage, and unremarkable change(1) | Our case |
M mail, F female, PT presentation, EBV Epstein–Barr virus, N none-treatment, Cem chemotherapy, Rad radiotherapy, Sur surgical resection, CR complete remission, SR spontaneous remission, SD stable disease, RR relapsing/remitting, + positive, – negative, NA not available, ? unknown (), (total number)
Materials and Methods
Case Report
A 71-year-old Japanese man was referred to Hanyu General Hospital because of poor healing of a dental extraction wound in the left lower canine fossa. Intra-oral examination revealed a lesion that was not covered with mucosal epithelium. Although the surrounding mucosa had a normal color without ulceration or exposure of the alveolar bone, marked alveolar bone loss was found in the upper and lower jaws due to severe periodontitis (Fig. 1). Extra-oral examination revealed no remarkable features, or any regional or generalized lymphadenopathy. Radiographic examination revealed a radiolucency with an ill-defined border (Fig. 2a), and CT and 3D-CT demonstrated apparent “moth-eaten” osteolysis (Fig. 2b, c). The results of blood tests were all within the normal ranges, except for HbA1C (6.2 %), and human immunodeficiency virus (HIV) was negative. Review of clinical data and patient history did not disclose a known immunodeficiency or underlying tumor of the hematopoietic or lymphoid tissue. The clinical diagnosis was a suspected intra-osseous tumor, and biopsy of the gingival mucosa surrounding the post-extraction socket was performed. The pathologic diagnosis was inflammation, with no evidence of any atypical tumor cells in the biopsy specimen (data not shown). Despite treatment with antibiotics, no improvement of the intra-osseous lesion was observed. In view of the possibility of an intra-osseous tumor, at the patient’s request the lesion was subsequently resected and curetted by intraoral surgery under general anesthesia. The patient recovered and had a good post-operative course although long-term follow up is lacking.
Fig. 1.
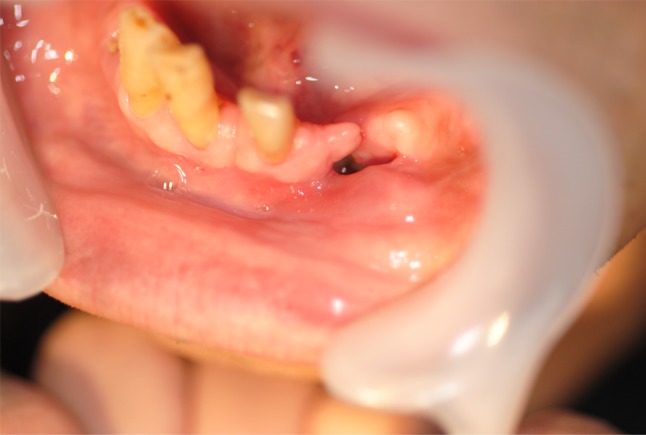
Intra-oral appearance of the mandible, showing swollen non-ulcerative mucosa in the buccal gingiva around a poorly healed canine extraction wound
Fig. 2.
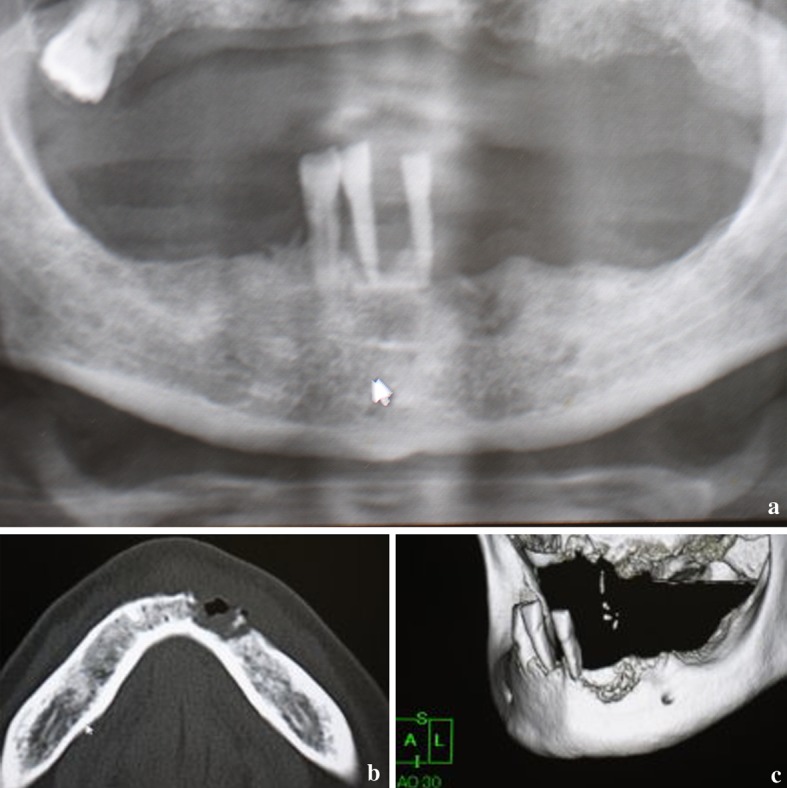
Radiologic findings before therapy. a Panoramic radiograph showing irregular radiolucent lesion in the mandible located around the site of canine extraction. b CT showing marked cortical bone osteolysis with sequestrated loose bony tissue. c 3D-CT view showing a so-called “moth-eaten” irregular bone resorptive surface, and remaining teeth with severe periodontitis
The case study protocol was reviewed and approved by the Research Ethics Committee of Meikai University School of Dentistry (A0832).
Light Microscopy
The resected and curetted specimens were fixed in 10 % neutral buffered formalin, and embedded in paraffin wax after decalcification of the bone. Sections (about 5 μm thick) were stained routinely with hematoxylin-eosin (HE).
Immunohistochemistry
Deparaffinized sections were immersed in absolute methanol containing 0.3 % H2O2 for 15 min at room temperature to block endogenous peroxidase activity. After washing, the sections were immersed in 0.01 M citrate buffer, pH 6.0, and heated in a microwave oven for 5 min at high voltage and then for 15 min at low voltage. They were then incubated with appropriately diluted mouse monoclonal antibodies against human CD3, CD4, CD8, CD15, CD20, CD79a, CD30, CD45RB (LCA), CD56, TdT, c-kit (CD117) and bcl-6 (Nichirei Co., Tokyo, Japan), CD45RO (UCHL-1), CD68, CD138, EBV-latent infection membrane protein-1 (EBV-LMP-1) and Ki-67 (Dako, A/S, Denmark). After washing, the sections were incubated with a pre-diluted anti-mouse or rabbit IgG antibody conjugated with peroxidase (Nichirei, Tokyo, Japan) for 30 min at room temperature. They were then immersed for 8 min in 0.05 % 3,3′-diaminobenzidine tetrahydrochloride (DAB) in 0.05 M Tris–HCl buffer (pH 8.5) containing 0.01 % H2O2, and counterstained with Mayer’s haematoxylin for 90 s.
Immunoreactivities for CD15, CD20, CD30, CD45RB (LCA) and EBV-LMP-1 were used for differential diagnosis between classical Hodgkin lymphoma: CHL [CD15(+), CD20(±), CD30(+), CD45RB(−), LMP-1(±)] and nodular lymphocyte-predominant Hodgkin lymphoma: NLPHL [CD15(−), CD20(+), CD30(−), CD45RB(+), LMP-1(−)]. Immunoreactivities indicative of age-related EBV + B cell LPDs [CD15 (−), CD20 (+), CD30 (+), LMP-1 (+)] were also examined, in addition to EBNA-2(+) [1, 5, 7, 9]. However, anti-human EBNA-2 antibody for paraffin-embedded sections is currently not available commercially. Additionally, immunoreactivities for CD3, CD4, CD8, CD56, CD20, CD79a, CD138, CD68 and CD117 (c-kit) were also examined in infiltrating cells of the inflammatory backgrounds.
In situ Hybridization
In situ hybridization (ISH) with EBV-encoded small RNA(EBER) oligonucleotides was performed to test for the presence of EBV small RNA in formalin-fixed paraffin-embedded sections using a hybridization kit (Dako, A/S, Denmark) in accordance with the manufacturer’s instructions.
Polymerase Chain Reaction (PCR)
To demonstrate the presence of the EBV gene (EBNA-2) in surgical specimens, formalin-fixed, paraffin-embedded materials were subjected to polymerase chain reaction (PCR) analysis using a TaKaRa DEXPAT™ kit (TaKaRa, Japan) in accordance with the manufacturer’s instructions. The primer designs and amplification programs are listed in Table 2 [10]. The Raji (EBV-positive Burkitt’s lymphoma) cell line was used as a positive control, having been subcultured from material obtained from the Japan Health Sciences Foundation Health Science Research Resources Bank (HSRRB).
Table 2.
Primer design of EBV, sequences and amplification programs for polymerase chain reaction
| Target | Sequence (5′ to 3′) | Size | 1 | 2 | 3 | 4 | 5 | 6 | Cycle | |
|---|---|---|---|---|---|---|---|---|---|---|
| GAPDH | (F) | GCACCGTCAAGGCTGAGAAC | 138 bp | 45 | ||||||
| (R) | TGGTGAAGACGCCAGTGGA | 94 °C | 94 °C | 58 °C | 72 °C | 72 °C | 4 °C | |||
| EBNA-2 | (F) | AGGCTGCCCACCCTGAGGAT | 186 bp | 5 m | 30 s | 30 s | 1 m | 7 m | 45 | |
| (R) | GCCACCTGGCAGCCCTAAAG |
Results
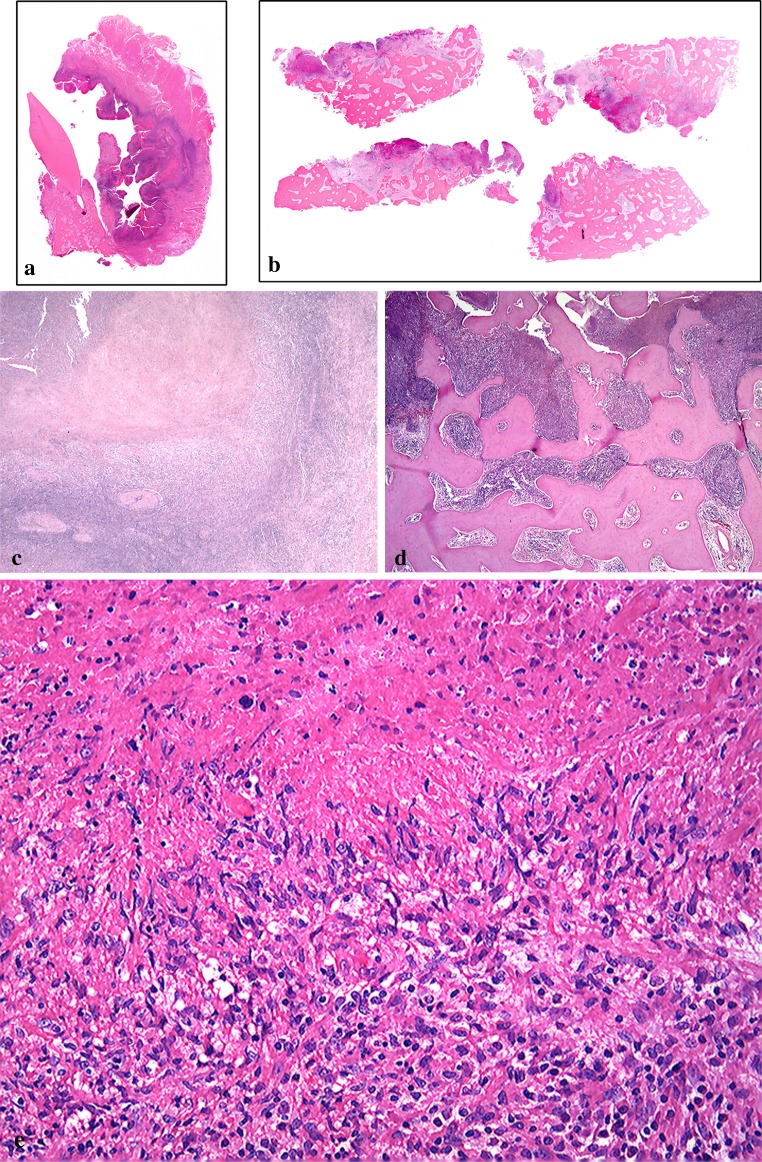
Macroscopically, the surgical specimen showed granulomatous tissues with fragments of bone and tooth (data not shown). Microscopically, both extra- (Fig. 3a) and intra-osseous (Fig. 3b) surgical specimens had associated necrosis, and a band-like rim of marked inflammatory cell infiltration was evident on the outer side (Fig. 3a). The base of the necrotic lesion was rimmed by band-like infiltration of small lymphocytes (Fig. 3c). There was marked lymphoid cell infiltration into the destroyed bone-marrow space with necrosis (Fig. 3d). Granulomatous lesions with caseous-like necrosis were composed of palisade-like arrangements of epithelioid cells, and lymphocyte-based severe inflammatory cell infiltration with many atypical lymphoid cells in the extra- (data not shown) and intra-osseous areas (Fig. 3e). The atypical lymphoid cells were localized in the granulomatous lesion in the extra-osseous area (data not shown). In the intra-osseous area, on the other hand, large Hodgkin and Reed-Sternberg-like giant cells were found (Fig. 4a). Small lymphocytes without atypia were seen in the area around the granulomatous lesion (data not shown). In the deepest portion of the involved bone marrow space, large atypical lymphoid cells were observed in a chronic inflammatory background (Fig. 4b).
Fig. 3.
Microscopic features of the resected specimen (extra- and intra-osseous areas). (a, c show the extra-osseous lesion, and b, d, e show the intra-osseous lesion). a A survey view of the extra-osseous area shows alveolar bone loss with an ulcerative post-extraction fossa. b A survey view of the intra-osseous area shows an osteomyelitis-like appearance. c Marked necrosis with granulomatous proliferation in the extra-osseous area; the base of the lesion is mainly rimmed off by a band of small lymphocytes (HE, original magnification ×12.5). d Intra-osseous area, showing marked lymphoid or inflammatory cell infiltration into the bone-marrow space, with geographical necrosis and bone destruction (HE, original magnification ×20). e Many degenerated large atypical lymphoid cells are present in the necrotic focus, and a palisade-like arrangement of epithelioid cells is evident in the intra-osseous area (HE, original magnification ×100)
Fig. 4.
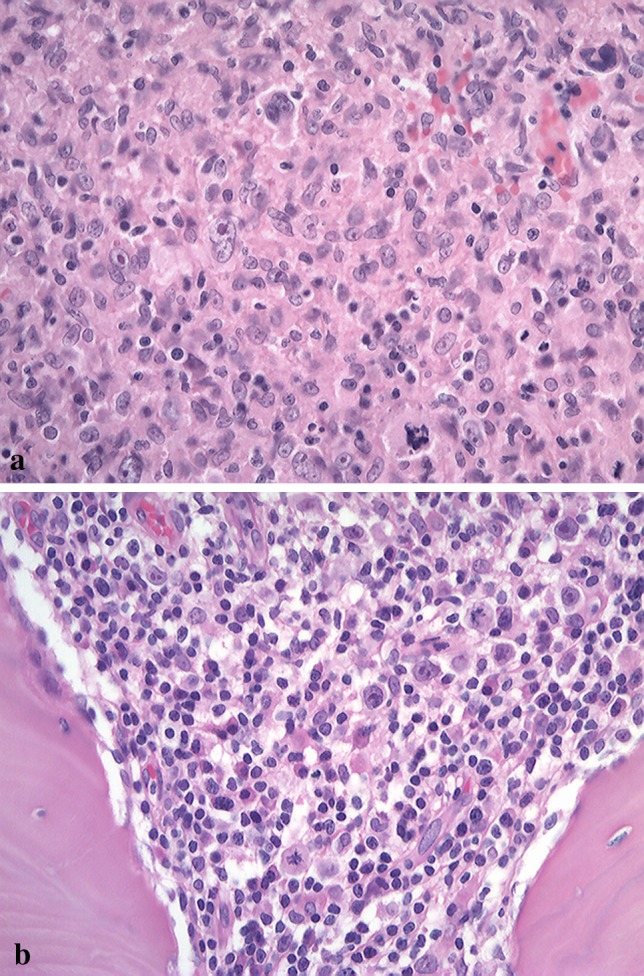
a Bizarre giant cells with a Hodgkin and Reed-Sternberg-like appearance are evident in an area of granulomatous proliferation in the intra-osseous area (HE, original magnification ×200). b In the deepest portion of the involved bone marrow, atypical large lymphoblastic cells are observed in a chronic inflammatory background, including small lymphocytes, plasma cells, histiocytes and endothelial cells (HE, original magnification ×200)
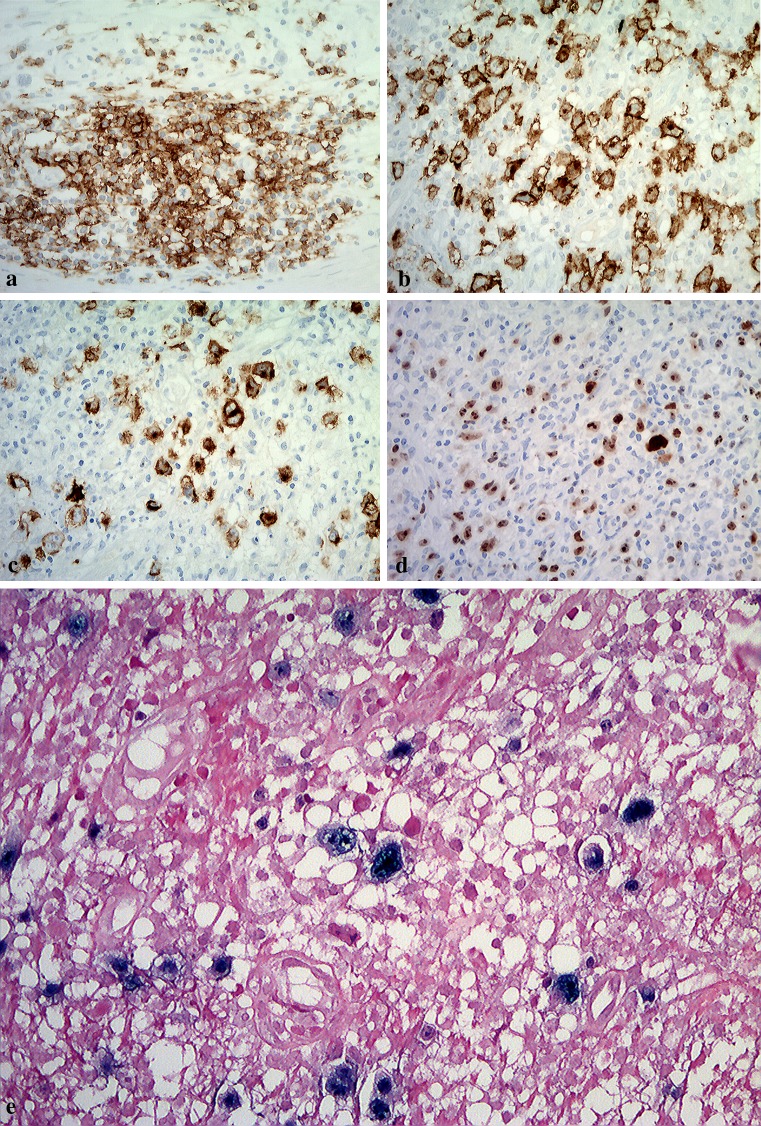
Immunohistochemical analysis revealed that many large atypical cells in the intra-osseous area were positive for CD20 (Fig. 5a), CD30 (Fig. 5b), EBV-LMP-1 (Fig. 5c) and Ki-67 (Fig. 5d), and negative for CD3, CD15, CD8 and CD68 (data not shown). EBV-encoded small RNA (EBER) was detected in these cells mainly located at between areas of necrotic to necrotic foci by ISH (Fig. 5e). Additionally, the large atypical lymphoid cells were negative for CD4, CD56, TdT, CD45RO (UCHL1), CD117 (c-kit) and bcl-6 (data not shown). The patient was diagnosed as having age-related EBV + B cell LPD.
Fig. 5.
Immunohistochemical features of the surgical specimen. a Atypical large lymphoid cell in the intra-osseous area is positive for CD20 (original magnification ×200). b Atypical large lymphoid cell in the intra-osseous area is positive for CD30 (original magnification ×200). c EBV-LMP-1 positive cells, including Hodgkin and Reed-Sternberg-like cells, were present in multiple foci of granulomatous tissue surrounding severely necrotic foci (original magnification ×200). d The nuclei of large atypical cells are markedly positive for Ki-67 (original magnification ×200). e In situ hybridization for EBV shows large lymphoblastic cells are strong staining in the nuclei of Hodgkin or Reed-Sternberg-like giant cells (original magnification ×400)
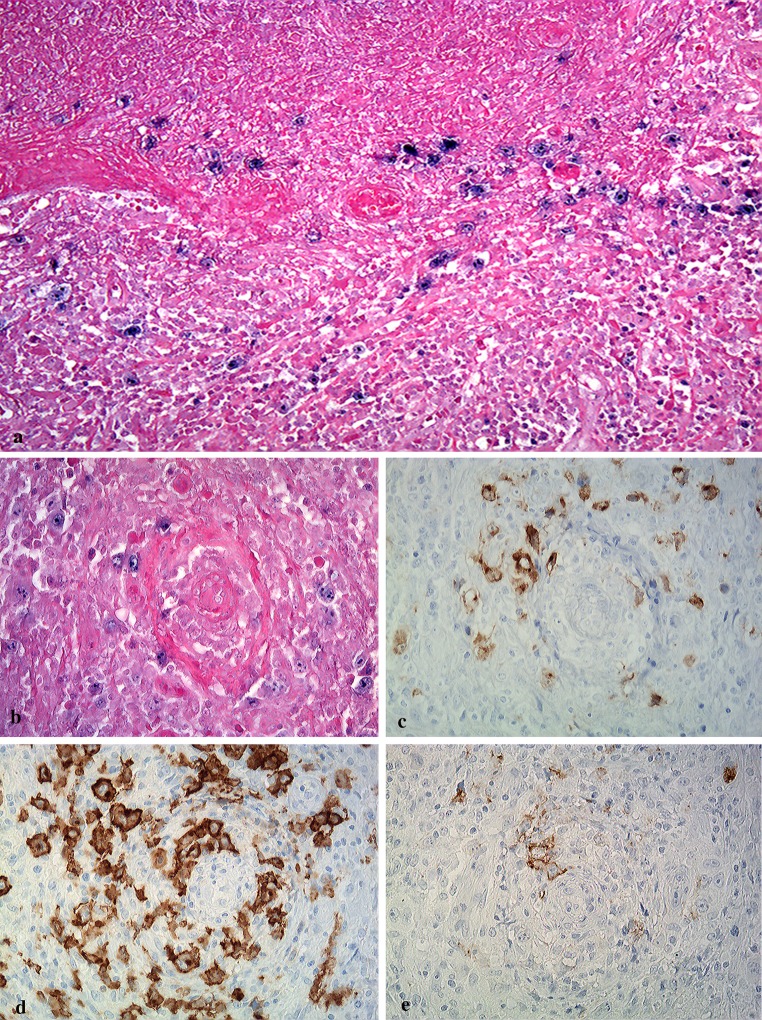
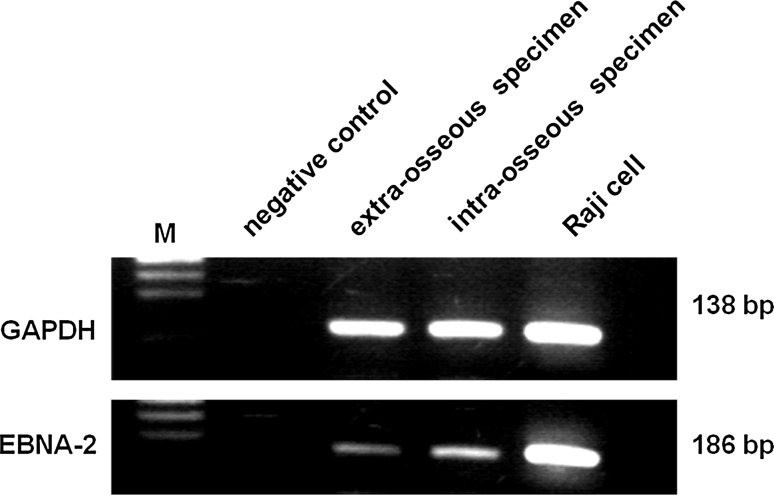
Large EBV + atypical lymphoid cells within hemorrhagic necrosis and granulomatous background were distributed in areas of perivascular- (Fig. 6a) or vascular- invasion (Fig. 6b). These large atypical cells were positive for LMP-1 (Fig. 6c), strongly positive for CD30 (Fig. 6d), and positive for CD20 (Fig. 6e). CD15-positive cells were distributed mainly in necrotic foci, and morphologically appeared small or intermediate in size (data not shown). Using PCR, EBNA-2 was detected in the extract from formalin-fixed paraffin-embedded sections of the surgical samples (extra-osseous and intra-osseous specimens). Raji cells were used as a positive control (Fig. 7).
Fig. 6.
Distribution of EBV + large atypical lymphoid cells in hemorrhagic necrosis and granulomatous areas. EBER-positive cells showing angiocentric/angiodestructive pattern are present in areas of hemorrhagic necrosis (a) and granulomatous formation (b) (original magnification ×100, and ×200). Large atypical lymphoid cells in perivascular or vascular areas are positive for LMP-1 (c), CD30 (d), and CD20 (e) (original magnification ×200)
Fig. 7.
PCR analysis of the Epstein–Barr virus (EBV) gene from formalin-fixed and paraffin-embedded tissue. EBNA-2 (186 bp) was detected in each specimens (extra-osseous and intra-osseous area), and GAPDH and EBNA-2 bands showed the same levels as those in Raji cells used as a positive control
These histological as well as clinical findings indicated that the lesion was compatible with age-related EBV + B cell LPD.
Discussion
EBV is associated with a variety of lymphoproliferative disorders and malignant lymphomas [2, 12–14]. EBV-driven B cell LPDs occur in immunosuppressed patients with primary immune deficiency, HIV infection, or post-transplantation, or who have received other treatments including methotrexate and tumor necrosis factor-α antagonists [1, 15]. Recently, a new group of EBV-associated B cell LPDs has been described in the elderly population (aged more than 50 years; median age 71 years) without any known immunodeficiency or prior lymphoma [1, 3–6, 8, 10, 16], and has been given several different names in the literature, including “senile EBV-associated B cell LPD”, “age-related EBV + B cell LPD”, “EBV-associated B cell LPD of the elderly”, and “EBV-positive diffuse large B cell lymphoma of the elderly” [4]. Clinically, the disease is often extranodal, frequently involving the skin, lung, tonsil and lung (with or without simultaneous lymph node involvement) [4]. However, it is very rare in the oral cavity, and to our knowledge, only four articles in English (two from the authors’ group) on age-related EBV + B cell LPDs of the oral cavity have been published [1, 7, 8, 10]. However, the reported cases of primary oral age-related EBV + B cell LPDs have usually involved intractable mucous ulcer [7], and none have involved intra-osseous lesions of the mandible. Some of the reported age-related EBV + B cell LPDs have arisen from, or involved bone at initial diagnosis, while the site of involvement for others was unknown [1, 5, 6].
Pathologically, age-related EBV + B cell LPDs are characterized by proliferation of atypical large B cells including Hodgkin- or Reed-Sternberg-like giant cells, and there is some morphologic and metachronous overlap with classical Hodgkin lymphoma (CHL) and diffuse large B cell lymphoma (DLBCL) [4, 5, 17]. Furthermore, Shimoyama et al. [5] have reported that age-related EBV + B cell LPDs are divisible into three morphological types: polymorphous, large cell lymphoma, and reactive lymphoid hyperplasia. Additionally, the polymorphous and large cell lymphoma subtypes may show large areas of geographic necrosis [1, 4, 10] and the histology is often variable from area to area, indicating a continuous spectrum between the two subtypes [4, 5]. The polymorphous subtype shows a broad range of B cell maturation and a variable component of reactive elements such as small lymphocytes, plasma cells, histiocytes and epithelioid cells [4, 5]; their histological features are similar to those of EBV + mucocutaneous ulcer, including age-related or methotrexate-related EBV + B cell LPDs of the oral cavity [7, 11]. The most important pathological characteristics of age-related EBV + B cell LPDs are, 1) a clinical predilection for older patients (aged >50 years) without any known immunodeficiency or prior lymphoma [4], 2) morphological similarity to both Hodgkin-like lesion (polymorphous subtype) and DLBCL-like lesion (large-cell lymphoma subtype) with or without marked necrosis (typically, the presence of geographic necrosis), and 3) immunohistochemical positivity for CD20, CD79a, CD30 (variably), EBER (100 %), LMP-1(94 %) and EBNA-2 (28 %) in atypical large lymphoid cells, and negativity for CD15, CD10 and bcl-6 [4, 5]. Clinically, the present case involved an elderly patient (aged 71 years) without any known immunodeficiency or prior lymphoma, and showed morphological similarity to a Hodgkin-like lesion (polymorphous subtype) with marked necrosis. Immunohistochemically, the large atypical lymphoid cells were CD20(+), CD79a(±), CD30(+), EBER(+) and LMP-1(+), the non-necrotic foci were bcl-6 (–) and mostly CD15 (–) and additionally PCR using formalin-fixed paraffin-embedded tissue revealed EBNA-2 (+). Therefore, the pathological diagnosis in the present case seemed most compatible with age-related EBV + B cell LPD.
The clinical course of age-related EBV + B cell LPD is aggressive, with a median patient survival of about two years [3, 4, 17]. Patients with age-related EBV + B cell LPD have a worse prognosis than those with EBV-negative DLBCL or EBV-positive classical Hodgkin lymphoma (CHL) of the elderly [3, 6]. Many patients with age-related EBV + B cell LPD/EBV + B cell lymphoma show a poor outcome in spite of chemo-radiotherapy [3, 4, 16, 18], and patients with non-germinal center B cell-like DLBCL with EBER positivity have a particularly poor survival rate [18, 19]. In the oral cavity (Table 1), age-related EBV + B cell LPD show only a low rate of complete remission (33 %), and most cases show a course of relapse and spontaneous remission even with combined chemo-radiotherapy [7]. The presence of B symptoms and a patient age of >70 years appear to be reliable prognostic factors [3, 4]. In all cases of age-related EBV + B cell LPD affecting the oral cavity, the age of the patients (Table 1), including the present one has been more than 70 years (median age 77 years). On the other hand, in Western population, Dojcinov et al. [8] have recently reported that the 5-year survival rate of patients with reactive lymphoid hyperplasia (100 %) and polymorphic extranodal LPD (93 %) was higher than that of patients with polymorphic nodal LPD (57 %) and DLBCL (25 %). Moreover patients with EBV + mucocutaneous ulcer showed extremely good 5-year survival (100 %) [8]. Although age-related EBV + B cell LPDs associated with oral mucosal ulcer apparently have a good prognosis [8], no previous clinical report has documented the prognosis of patients with lesions of the mandible.
In the present case, it was unclear why the large atypical lymphoid cells that had infiltrated into the oral cavity became located in the alveolar part of the mandible after tooth extraction. The only limited information about the primary site in the present case being similar to another we have reported previously [11, 20], was that the site of lymphoma/LPDs infiltration coincided with severe periodontitis in the oral cavity. In elderly patients, the onset of primary oral lymphoma/LPD may be related to chronic inflammation that has been regarded as “periodontitis”. In fact, EBV has been reportedly detected in apical and marginal periodontitis [21–27]. Most EBV-related oral tumors involve the tongue and the parotid gland, but some are located in the periodontium [1, 7, 8, 10, 11, 20, 27], as was the case in the present patient. Moreover, poor glycemic control (HbA1C level ≥10 %) in patients with type 2 diabetes mellitus (DM) can increase the occurrence of EBV in shallow periodontal pockets [28]. The present patient with age-related EBV + B cell LPD also had type 2 DM and severe chronic periodontitis, but the DM had been well controlled (HbA1C level 6.2 %).
Primary EBV infection is usually asymptomatic, and the EBV then establishes latent infection in memory B cells, which do not permit viral replication [29]. Although newly infected naive B cells have the phenotypes of transformed cells, they are controlled by both EBV-specific cytotoxic T lymphocytes (CTL) and natural killer (NK) cells unless immunity is suppressed [29, 30]. In immunocompromised hosts, transformed cells become proliferating blasts that can result in symptomatic disease, such as immunodeficiency-associated LPD [2, 15, 29, 30]. The factor responsible for oral mucosal or intra-osseous involvement of primary lymphoma/LPDs is unclear, but there may be some association with bacteria in and around the teeth together with chronic inflammation such as apical and marginal periodontitis. The copy number of EBV-DNA in subgingival plaque is associated with the presence of some periodontal bacteria [31]. A recent study has shown that periodontal disease could act as a risk factor for HIV reactivation [32], and similarly induce EBV re-activation [33]. Thus there may be a relationship between EBV-infected B cells and the presence of periodontal bacteria. More studies, including reports of cases in the oral cavity, will be needed to confirm the causal link between periodontitis and EBV + LPDs in the oral cavity.
We have reported a case of extranodal age-related EBV + B cell LPD showing a polymorphous subtype (Hodgkin-like features) and affecting the oral cavity. Very few such cases have been reported in the English literature [1, 7, 8, 10]. Although part of the aging process is thought to cause EBV + B cell LPDs in elderly patients, little is known about the histological pattern and EBV reactivation mechanisms of the lesions in extranodal sites, including the oral cavity. It will be important to accumulate and evaluate further cases of extranodal age-related EBV + B cell LPDs in oral cavity and examine their clinical correlates, as well as their immunohistochemical and genotypic characteristics.
References
- 1.Oyama T, Ichimura K, Suzuki R, et al. Senile EBV + B-cell lymphoproliferative disorders: a clinicopathological study of 22 patients. Am J Surg Pathol. 2003;27:16–26. doi: 10.1097/00000478-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Borisch B, Raphael M, Swerdlow SH, Jaffe ES, et al. Immunodeficiency associated lymphoproliferative disorders. In: Harris NL, Stein H, Vardiman JW, Jaffe ES, et al., editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 255–271. [Google Scholar]
- 3.Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinico pathologic group: a study of 96 patients. Clin Cancer Res. 2007;13:5124–5132. doi: 10.1158/1078-0432.CCR-06-2823. [DOI] [PubMed] [Google Scholar]
- 4.Nakamura S, Jaffe ES, Swerdlow SH. EBV-positive diffuse large B-cell lymphoma of the elderly. In: Swerdlow SJ, Campo E, Harris NL, Jaff ES, Pileri SA, Stein H, Thiele J, Vardiman JW, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. pp. 243–244. [Google Scholar]
- 5.Shimoyama Y, Asano N, Kojima M, et al. Age-related EBV-associated B-cell lymphoproliferative disorders: diagnostic approach to a newly recognized clinicopathological entity. Pathol Int. 2009;59:835–843. doi: 10.1111/j.1440-1827.2009.02466.x. [DOI] [PubMed] [Google Scholar]
- 6.Asano N, Yamamoto K, Tamaru J, et al. Age-related Epstein–Barr virus (EBV)-associated B-cell lymphoproliferative disorders: comparison with EBV-positive classic Hodgkin lymphoma in elderly. Blood. 2009;113:2629–2636. doi: 10.1182/blood-2008-06-164806. [DOI] [PubMed] [Google Scholar]
- 7.Dojcinov SD, Venkataraman G, Raffeld M, et al. EBV-positive mucocutaneous ulcer: a study of 26 cases associated with various sources of immunosuppression. Am J Surg Pathol. 2010;34:405–417. doi: 10.1097/PAS.0b013e3181cf8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dojcinov SD, Venkataraman G, Pittaluga S, et al. Age-related EBV-associated lymphoproliferative disorders in the Western population: a spectrum of reactive lymphoid hyperplasia and lymphoma. Blood. 2011;117:4726–4735. doi: 10.1182/blood-2010-12-323238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kamel OW, Weiss LM, van de Rijn M, et al. Hodgkin’s disease and lymphoproliferations resembling Hodgkin’s disease in patients receiving long-term low-dose methotrexate therapy. Am J Surg Pathol. 1996;20:1279–1287. doi: 10.1097/00000478-199610000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Shimoyama Y, Oyama T, Asano N, et al. Senile Epstein–Barr virus-associated B-cell lymphoproliferative disorders: a mini review. J Clin Exp Hematopathol. 2006;46:1–4. doi: 10.3960/jslrt.46.1. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi K, Miyazaki Y, Tanaka A, et al. Methotrexate-related Epstein–Barr virus (EBV)-associated lymphoproliferative disorder—so-called “Hodgkin-like lesion”—of the oral cavity in a patient with rheumatoid arthritis. Head Neck Pathol. 2010;4:305–311. doi: 10.1007/s12105-010-0202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambinder RF. Epstein–Barr virus-associated lymphoproliferative disorders. Rev Clin Exp Hematol. 2003;7:362–374. [PubMed] [Google Scholar]
- 13.Diebold J, Jaffe ES, Raphael M, Warnke RA. Burkitt lymphoma. In: Harris NL, Stein H, Vardiman JW, Jaffe ES, editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 181–185. [Google Scholar]
- 14.Chan JKC, Jaffe ES, Ralfkiaer E. Extranodal NK/T-cell lymphoma, nasal type. In: Harris NL, Stein H, Vardiman JW, Jaffe ES, editors. World Health Organization Classification of Tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2001. pp. 204–207. [Google Scholar]
- 15.Jaffe ES, Harris NL, Swerdlow SJ, et al. Immunodeficiency-associated lymphoproliferative disorder. In: Swerdlow SJ, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW, et al., editors. WHO classification of tumors of haematopoietic and lymphoid tissues. Lyon: IARC Press; 2008. pp. 335–351. [Google Scholar]
- 16.Shimoyama Y, Yamamoto K, Asano N, et al. Age-related Epstein–Barr virus-associated B-cell lymphoproliferative disorders: special references to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci. 2008;99:1085–1091. doi: 10.1111/j.1349-7006.2008.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murase T, Fujita A, Ueno H, et al. A case of age-related EBV-associated B-cell lymphoproliferative disorder metachronously showing two distinct morphologic appearance, one of a polymorphic disease resembling classical Hodgkin lymphoma, and the othere of a large-cell lymphoma. Int Hematol. 2009;89:80–85. doi: 10.1007/s12185-008-0214-0. [DOI] [PubMed] [Google Scholar]
- 18.Park S, Lee J, Ko YH, et al. The impact of Epstein–Barr virus on clinical outcome in diffuse lage B-cell lymphoma. Blood. 2007;110:972–978. doi: 10.1182/blood-2007-01-067769. [DOI] [PubMed] [Google Scholar]
- 19.Sarah E, Gibson MD, Eric D, et al. Epstein–Barr virus-positive B-cell lymphoma of the elderly at a United States tertiary medical center: an uncommon aggressive lymphoma with a nongerminal center B-cell phenotype. Hum Pathol. 2009;40:653–661. doi: 10.1016/j.humpath.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka A, Shigematsu H, Kojima M, et al. Methotrexate-associated lymphoproliferative disorder arising in a patient with adult Still’s disease. J Oral Maxillofac Surg. 2008;66:1492–1495. doi: 10.1016/j.joms.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Hernádi K, Szalmás A, Mogyorósi R, et al. Prevalence and activity of Epstein–Barr virus and human cytomegalovirus in symptomatic and asymptomatic apical periodontitis lesions. JOE. 2010;36:1485–1489. doi: 10.1016/j.joen.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Sabeti M, Kermani V, Sabeti S, et al. Significance of human cytomegalovirus and Epstein–Barr virus in inducing cytokine expression in periapical lesions. JOE. 2012;38:47–50. doi: 10.1016/j.joen.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 23.Li H, Chen V, Chen Y, et al. Herpesviruses in endodontic pathoses: association of Epstein–Barr virus with irreversible pulpitis and apical periodontitis. JOE. 2009;35:23–29. doi: 10.1016/j.joen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 24.Contreras A, Nowzari H, Slots J. Herpesviruses in periodontal pocket and gingival tissue specimens. Oral Microbiol Immunol. 2000;15:15–18. doi: 10.1034/j.1399-302x.2000.150103.x. [DOI] [PubMed] [Google Scholar]
- 25.Bilichodmath S, Mangalekar SB, Sharma DCG, et al. Herpesviruses in chronic and aggressive periodontitis patients in an Indian population. J Oral Sci. 2009;51:79–86. doi: 10.2334/josnusd.51.79. [DOI] [PubMed] [Google Scholar]
- 26.Nibali L, Atkinson C, Griffiths P, et al. Low prevalence of subgingival viruses in periodontitis patients. J Clin Periodontol. 2009;36:928–932. doi: 10.1111/j.1600-051X.2009.01476.x. [DOI] [PubMed] [Google Scholar]
- 27.Slot J, Saygun I, Sabeti M, et al. Epstein–Barr virus in oral diseases. J Periodont Res. 2006;41:235–244. doi: 10.1111/j.1600-0765.2006.00865.x. [DOI] [PubMed] [Google Scholar]
- 28.Casarin RC, Duarte PM, Santos VR, et al. Influence of glycemic control on Epstein–Barr and cytomegalovirus infection in periodontal pocket of type 2 diabetic subjects. Arch Oral Biol. 2010;55:902–906. doi: 10.1016/j.archoralbio.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H, Ito Y, Suzuki R, et al. Measuring Epstein–Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305–319. doi: 10.1002/rmv.582. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JI. Epstein–Barr virus infection. N Eng J Med. 2000;343:481–492. doi: 10.1056/NEJM200008173430707. [DOI] [PubMed] [Google Scholar]
- 31.Dawson DR, III, Wang C, Danaher RJ, et al. Real-time polymerase chain reaction to determine the prevalence and copy number of Epstein–Barr virus and cytomegalovirus DNA in subgingival plaque at individual healthy and periodontal disease sites. J Periodontol. 2009;80:1133–1140. doi: 10.1902/jop.2009.080644. [DOI] [PubMed] [Google Scholar]
- 32.Imai K, Ochiai K, Okamoto T. Reactivation of latent HIV-1 infection by the periodontopathic bacterium Porphyromonas gingivalis involves histone modification. J Immunol. 2009;182:3688–3695. doi: 10.4049/jimmunol.0802906. [DOI] [PubMed] [Google Scholar]
- 33.Imai K, Inoue H, Tamura M, Cueno ME, Inoue H, Takeichi O, Kusama K, Saito I, Ochiai K. The periodontal pathogen Porphyromonas gingivalis induces the Epstein–Barr virus lytic switch transactivator ZEBRA by histone modification. Biochimie. 2012;94:839–846. doi: 10.1016/j.biochi.2011.12.001. [DOI] [PubMed] [Google Scholar]