Abstract
Human papillomavirus (HPV) is associated with oropharyngeal squamous cell carcinomas. Persistent viral infection is postulated to lead to carcinogenesis, although infection of benign adjacent epithelium is not typically observed. It is known that immune evasive tumor cells can provide an ideal niche for a virus. The B7-H1/PD-1 cosignaling pathway plays an important role in viral immune evasion by rendering CD8+ cytotoxic T cells anergic. We hypothesized that HPV-related oropharyngeal squamous cell carcinomas express B7-H1 as a mechanism for immune evasion. A tissue microarray was utilized, for which HPV E6/E7 mRNA by in situ hybridization was previously performed. Immunohistochemistry was performed to detect B7-H1 and staining was characterized by pattern, distribution, and intensity. B7-H1 was expressed by 84 of the 181 (46.4 %) cases. Both tumor cell membranous and cytoplasmic expression were present and cytoplasmic expression was identified in some peritumoral lymphocytes. Expression was analyzed in several different ways and then considered binarily as positive versus negative. Tumors expressing B7-H1 were more likely to be HPV positive (49.2 vs. 34.1 %, p = 0.08). B7-H1 expression showed no correlation with disease recurrence in the entire cohort (OR = 1.09, p = 0.66), HPV positive cohort (OR = 0.80, p = 0.69) or HPV negative cohort (OR = 2.02, p = 0.22). However, B7-H1 expression intensity did correlate with the development of distant metastasis (p = 0.03), and B7-H1 intensity of 3+ (versus all other staining) showed a strong trend towards distant metastasis in the HPV positive (OR = 6.67, p = 0.13) and HPV negative (OR = 9.0, p = 0.13) cohorts. There was no correlation between B7-H1 expression and patient survival for any of the different ways in which staining was characterized, whether binarily, by distribution, intensity, or combined scores. B7-H1 is expressed in the majority of oropharyngeal squamous cell carcinomas with transcriptionally-active HPV. This suggests that B7-H1 expression by tumor cells may play a role in harboring persistent HPV infection.
Keywords: Human papillomavirus, Oropharyngeal squamous cell carcinoma, p16, B7-H1, Immune
Introduction
Human papillomavirus (HPV)-related oropharyngeal squamous cell carcinoma (OSCC) is recognized as a distinct clinicopathologic entity. The younger age of patients with HPV-related tumors, the lower smoke and alcohol exposure rates, and increased rates of sexual exposure suggest that HPV has a central role in carcinogenesis [1–4]. The prevalence of HPV in OSCC is between 60 % and 80 %, and rates are steadily increasing, with the mechanism believed to be chronic persistent infection leading to carcinogenesis [5–8]. Studies have demonstrated that oral infection by HPV is not uncommon [3, 9]. However, evidence suggests that virtually all immune competent patients clear the viral infections [10–13], supporting the hypothesis that most individuals experience only transient infection.
Patients with HIV have a high prevalence of oral HPV infections due to their systemic immune suppression [14, 15], and smokers, both of tobacco and marijuana, have high prevalences as well, also thought to be related to some form of immune suppression, either local or systemic. In HPV-related OSCC, viral DNA is seen in tumor cells but typically is absent in the surrounding mucosa, suggesting localized immune suppression within tumor cells [16]. We hypothesized that HPV creates a niche for itself in host tumor cells which allows for persistent infection and immune evasion.
B7-H1 is an immune modulatory molecule that can be aberrantly expressed on the surface of epithelial cells [17]. Cytotoxic T-lymphocytes become anergic when their PD-1 receptor is bound to B7-H1, hence silencing immune surveillance. Expression of B7-H1 in the tumor microenvironment enables the tumor cell to evade the immune system by causing either cytotoxic T-lymphocyte apoptosis or tumor cell resistance to lysis [17–21]. Increased cell surface expression of B7-H1 has been demonstrated in chronic infection and carcinomas [19, 20, 22–26]. Studies have demonstrated immune evasion via B7-H1 expression to be associated with metastasis and poor prognosis in many different carcinomas, such as those arising in the kidney, skin, lung, and pancreas [21–29]. Effective blockade of PD-1 has shown to reverse T-cell anergy and improve outcomes [19, 20]. While the main theory regarding HPV-related carcinogenesis is that there is chronic persistent infection leading to carcinogenesis, we hypothesize that dysplastic or carcinomatous tumor cells, with a microenvironment of B7-H1 expression, can provide the ideal niche for HPV to develop persistent infection. We sought to investigate the expression of B7-H1 in HPV-related OSCC as a potential model for viral and tumor cell immune evasion and to see if its expression correlated with patient outcomes.
Materials and Methods
With approval from the university Human Research Protection Office, newly diagnosed cases of oropharyngeal SCC from the databases of Radiation Oncology (WLT) and Otolaryngology Head and Neck Surgery were identified. Clinical follow up information was obtained, including survival data, smoking, and other clinical variables. All cases were reviewed by one study pathologist and typed histologically according to our established system as keratinizing SCC, nonkeratinizing SCC with maturation, and nonkeratinizing SCC. Other pathologic features were obtained by report review from the working Copath database. All patients were treated either with primary surgery with or without postoperative radiation and chemotherapy or were treated definitively with radiation therapy with or without chemotherapy. All radiation therapy was intensity modulated and was administered by a single radiation oncologist (WLT). The paraffin blocks were obtained from department files, and a tissue microarray constructed. According to the amount of available biopsied or resected tumor tissue, duplicate two millimeter punches (or if inadequate tumor tissue present, then 0.6 mm punches) were taken from each case. Since most of the cases (75 %) were treated with primary surgery, the majority of cases on the array had the larger (2 mm) punches.
B7-H1 Immunohistochemistry
Paraffin-embedded tissue blocks were cut into 5 μm sections and deparaffinized in xylene and rehydrated in a graded series of ethanols. Antigen retrieval was performed by heating tissue sections in Target Retrieval Solution pH 6.0 (Dako #S1699, Carpenteria, CA, USA) to 121 °C using a Digital Decloaking Chamber (Biocare Medical, Walnut Creek, CA, USA), cooled to 90 °C, and incubated for an additional 5 min before opening the Decloaking Chamber. Sections were washed in running DH20 for 5 min and then incubated for 5 min in Wash Buffer (Dako #S3006) before being placed on the Autostainer Plus (Dako) for the following protocol utilizing a CSA II kit (Dako #K1497). Sections were blocked for endogenous peroxidase for 5 min using Endogenous Blocking Solution, washed in wash buffer followed by incubation for 60 min with a a B7-H1 antibody (clone A3; monoclonal; dilution 1:300). Antibody was diluted with antibody diluent containing background reducing components. Sections were then washed in wash buffer and incubated 15 min in horseradish peroxidase-labeled anti-mouse immunoglobulins, washed with wash buffer, and incubated for 15 min in amplification reagent. Sections were then washed with wash buffer and incubated for 15 min in anti-FITC HRP, washed and visualized in DAB for 8 min. Sections were washed with DH20, counterstained with hematoxylin, dehydrated in ethanol, cleared in xylene, and coverslipped with permanent mounting media. Staining was read by two study pathologists (JSL and OCU), and all positive cases demonstrated both membranous and cytoplasmic staining but to variable degrees. Staining intensity was graded as: 0 = no staining, 1+ = weak staining, 2+ = moderate staining and 3+ = strong staining. Distribution was graded by percentage of tumor cells that were positive and then divided into quartiles as: 0–5 % = no staining, 1+ = 6–25 % staining; 2+ = 26–50 %; 3+ = 51–75 % and 4+ = 76–100 %. Cases were analyzed in several ways, including by intensity alone, by a composite score of distribution plus intensity (resulting in scores of 0 or 2 through 7), and finally, simply binarily as positive when there was any staining (regardless of intensity) in greater than 5 % of the tumor cells versus negative or those with less than 5 % staining.
p16 Immunohistochemistry
Immunohistochemistry was performed for p16 on representative 5 μm whole tumor sections cut from formalin-fixed, paraffin-embedded tissue blocks from each case using a monoclonal antibody to p16 (MTM Laboratories; clone E6H4; monoclonal; 1:1 dilution) on a Ventana Benchmark automated immunostainer (Ventana Medical Systems, Inc., Tucson AZ) according to standard protocols with appropriate positive control including normal tonsil and a known positive OSCC case. Antigen retrieval, standard on the machine, utilized the Ventana CC1, EDTA-Tris, pH 8.0 solution. Staining was read by one study pathologist (JSL), and all positive cases demonstrated both nuclear and cytoplasmic staining. Staining was graded in a quartile manner as follows: 0 = no staining, 1+ = 1–25 % staining; 2+ = 26–50 %; 3+ = 51–75 % and 4+ = 76–100 %. Cases were then classified binarily as strong positive when there was extensive staining (3+) and negative when there was no or only partial staining (0, 1+, or 2+). More than 90 % of cases were either strongly and diffusely positive or completely negative.
RNA In situ Hybridization for High Risk HPV
In situ hybridization for high risk HPV E6/E7 mRNA was performed by hand using the RNAscope™ HPV kit (Advanced Cell Diagnostics, Inc., Hayward, CA, USA) [30] as previously described. The array (and corresponding control) slides had been read by the two study pathologists (JSL and OSU) and classified in a binary manner as either positive or negative. Positive cases had to have granular cytoplasmic and/or nuclear brown staining that was above the signal on the DapB negative control slide. Discrepant cases were resolved by consensus review. Cases where there was less than 10 % surface area consisting of tumor were excluded.
Statistics
Binary variables were analyzed using Chi square or Fisher’s exact test when appropriate. Univariate Cox proportional analysis was used in overall survival, disease specific survival, and recurrence. Overall survival was considered as the time from start of treatment to death from any cause, and disease specific survival was considered as the time from start of treatment to death with known persistent or recurrent cancer. Kaplan–Meier curves were constructed for overall and disease specific survival utilizing the log-rank test for statistical significance. A p value less than 0.05 was considered significant. Kappa statistics were utilized for interobserver agreement for staining results between the reviewing pathologists.
Results
The tissue microarray had adequate tumor present for B7-H1 immunohistochemistry evaluation for 181 out of the total of 198 cases on it. The mean age was 55.8 years (±9.4) with a male:female ratio of 8:1. HPV mRNA and p16 expression were detected in 138 (77.1 %) and 148 (82.0 %) cases, respectively. A total of 84 (46.4 %) cases showed expression of B7-H1 (Table 1; Fig. 1). Considering staining binarily as positive or negative, there was substantial interobserver agreement for the two pathologists (OCU and JSL) (kappa statistic = 0.89). The distribution and extent of staining are presented in Table 2. Staining intensity for the entire cohort was as follows: negative = 97 (53.5 %), weak (1+) = 21 (11.6 %), moderate (2+) = 41 (22.7 %), and strong (3+) = 22 (12.2 %). Distribution of staining for the entire cohort for just the positive cases was as follows: 5–25 % = 34 (40.4 %), 26–50 % = 27 (32.1 %), 51–75 % = 12 (6.6 %), and 76–100 % = 11 (13.1 %). Dispersed lymphoid staining (with no discernible pattern) was observed in 150 (82.8 %) cases.
Table 1.
Patient characteristics
| Group (%) | All (181) | B7-H1 positivea N = 84 |
B7-H1 negative N = 97 |
p value |
|---|---|---|---|---|
| Age (mean ± SD) | 55.8 ± 9.4 n/a |
56.6 ± 10.0 | 55.1 ± 8.8 | 0.28 |
| Gender (%) | ||||
| Male | 186 (88.2) | 79 (94.0) | 83 (85.6) | 0.06 |
| Female | 23 (11.8) n/a |
5 (6.0) | 14 (14.4) | |
| Smoking (%) | ||||
| Yes (current/former) | 124 (68.5) | 54 (64.3) | 70 (72.2) | 0.26 |
| No (never) | 51 (28.2) | 27 (32.1) | 24 (24.7) | |
| Missing | 6 (3.3) | 3 (3.6) | 3 (3.1) | |
| T stage (%) | ||||
| T1/T2 | 105 (58.0) | 48 (57.1) | 57 (58.8) | 0.42 |
| T3/T4 | 66 (36.5) | 34 (40.5) | 32 (33.0) | |
| Missing | 10 (5.5) | 2 (2.4) | 8 (8.2) | |
| N stage (%) | ||||
| N0 | 16 (8.8) | 7 (8.3) | 9 (9.3) | 0.82 |
| N1–3 | 165 (91.2) | 77 (91.7) | 88 (90.7) | |
| AJCC stage | ||||
| I/II | 9 (5.0) | 2 (2.4) | 7 (7.2) | 0.12 |
| III/IV | 170 (94.0) | 82 (97.6) | 88 (90.7) | |
| Missing | 2 (1) | 2 (2.1) | ||
| Distant metastasis (%) | ||||
| Yes | 10 (5.5) | 7 (8.3) | 3 (3.1) | 0.10 |
| No | 171 (94.5) | 77 (91.7) | 94 (96.9) | |
| IMRT (%) | ||||
| Definitive | 38 (21.0) | 15 (17.9) | 23 (23.7) | 0.34 |
| Post-operative | 143 (79.0) | 69 (82.1) | 74 (76.3) | |
| Surgery only | 0 (0) | 0 (0) | 0 (0) | |
| Chemotherapy (%) | ||||
| Yes | 80 (44.2) | 29 (34.5) | 38 (39.2) | 0.10 |
| No | 74 (40.9) | 42 (50) | 45 (46.4) | |
| Unknown | 27 (14.9) | 13 (15.5) | 14 (14.4) | |
| Recurrence (%) | ||||
| Yes | 28 (15.5) | 12 (14.2) | 16 (16.5) | 0.66 |
| No | 152 (84.0) | 72 (85.8) | 80 (82.4) | |
| Missing | 1 (0.5) | 1 (1.9) | ||
| Histologic type (%) | ||||
| K SCC (type 1) | 37 (20.4) | 17 (20.2) | 22 (22.7) | 0.74 |
| NK SCC with | 45 (24.9) | 19 (22.6) | 24 (24.7) | |
| Maturation (type 2) | ||||
| NK SCC (type 3) | 98 (54.1) | 47 (56.0) | 51 (52.6) | |
| Missing | 1 (0.6) | 1 (1.2) | 0 | |
| p16 Status (%)b | ||||
| Positive | 134 (79.3) | 61 (72.6) | 73 (75.2) | 0.78 |
| Negative | 35 (19.3) | 15 (17.8) | 20 (20.6) | |
| Missing | 12 (1.4) | 8 (9.6) | 4 (4.2) | |
IMRT intensity modulated radiation therapy, K SCC keratinizing type squamous cell carcinoma, NK SCC nonkeratinizing squamous cell carcinoma
aB7-H1 expression considered binarily as positive (>5 % staining) versus negative (no staining or less than 5 % staining)
bp16 immunohistochemistry status considered binarily as positive ≥50 % tumor cell staining and negative = no staining or <50 % tumor cell staining
Fig. 1.
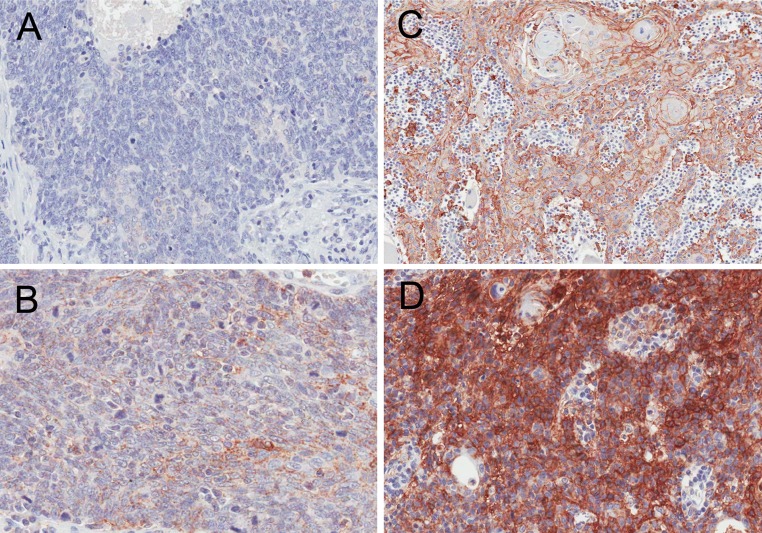
B7-H1 immunohistochemistry by intensity. a B7-H1 negative, b B7-H1 positive with 1+ intensity, c 2+ intensity, and d 3+ intensity. (images all at 200X magnification)
Table 2.
Clinical and pathologic characteristics by B7-H1 intensity score and distribution
| B7-H1 intensity score (%) | B7-H1 staining distribution (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1+ | 2+ | 3+ | 0 % (0) | 5–25 % (1+) | 26–50 % (2+) | 51–75 %(3+) | 76–100 % (4+) | |
| Entire cohort | 97 (46.0) | 21 (10.0) | 41 (19.4) | 22 (10.4) | 97 (46.0) | 34 (16.0) | 27 (12.8) | 12 (5.7) | 11 (5.2) |
| HPV RNA ISH positive | 70 (50.7) | 14 (10.2) | 37 (26.8) | 17 (12.3) | 70 (50.7) | 28 (20.3) | 21 (15.2) | 10 (7.24) | 9 (6.55) |
| HPV RNA ISH negative | 27 (65.6) | 7 (17.2) | 3 (7.41) | 4 (9.79) | 27 (65.6) | 6 (14.7) | 6 (14.7) | 0 (0) | 2 (4.98) |
| T1/T2 | 57 (54.2) | 12 (11.4) | 25 (24.0) | 11 (10.4) | 57 (54.2) | 21 (20.0) | 15 (14.2) | 5 (4.8) | 7 (6.6) |
| T3/T4 | 32 (48.4) | 9 (13.6) | 15 (22.7) | 10 (15.2) | 32 (48.4) | 13 (19.6) | 12 (18.2) | 5 (7.6) | 4 (6.1) |
| N0 | 9 (56.2) | 2 (12.5) | 3 (18.8) | 2 (12.5) | 9 (56.2) | 3 (18.8) | 2 (12.5) | 1 (6.25) | 1 (6.25) |
| N1–N3 | 88 (53.3) | 19 (11.5) | 38 (23.0) | 20 (12.1) | 88 (53.3) | 31 (18.8) | 25 (15.2) | 11 (6.67) | 10 (6.03) |
| M0 | 94 (54.9) | 19 (11.2) | 40 (23.4) | 18 (10.5) | 94 (54.9) | 33 (19.4) | 25 (14.6) | 9 (5.26) | 10 (5.84) |
| M1 | 3 (30.0) | 2 (20.0) | 1 (10.0) | 4 (40.0) | 3 (30.0) | 1 (10.0) | 2 (20.0) | 3 (30.0) | 1 (10.0) |
ISH in situ hybridization, HPV human papillomavirus, N0 no nodal metastases, N1–N3 nodal metastasis present, M0 no distant metastasis, M1 distant metastasis present
B7-H1 expression was present in 68 of the 138 HPV mRNA positive cases (49.2 %) versus 14 of the 41 (34.1 %) that were HPV negative [OR = 3.4, p = 0.08 (Table 3)]. Nodal metastasis was present in 77 (91.7 %) of the B7-H1 positive cases compared to 88 (90.7 %) of the B7-H1 negative cases (p = 0.82). Distant metastasis was observed in only 10 cases, 7 (70.0 %) of which were positive for B7-H1 (OR = 2.85 p = 0.10). Incremental B7-H1 staining intensity score (as ordinal results 0, 1+, 2+, or 3+) showed a significant association with distant metastasis in the entire cohort (OR = 1.68; p = 0.03). Binary analysis of B7-H1 intensity of 3+ versus all others (0, 1+, 2+) also showed a positive association between intense B7-H1 expression and the development of distant metastasis in the entire cohort, but this was not quite statistically significant (OR = 4.37, p = 0.06). Considering B7-H1 intensity of 3+ versus all others in just the HPV-positive or just the HPV-negative cohorts, there again were strong trends towards the development of distant metastasis with intense B7-H1 expression (OR = 6.67, p = 0.13 for HPV-positive and OR = 9.0, p = 0.13 for HPV-negative), but these again were not statistically significant. B7-H1 distribution and intensity scores did not show any associations with tumor stage, p16 expression, or other clinical parameters. Patients with tumors that were p16 and HPV mRNA negative were more likely to be either current or former smokers (Table 4).
Table 3.
B7-H1 and E6/E7 mRNA correlation (p = 0.08)
| B7-H1a | Total | ||
|---|---|---|---|
| Negative | Positive | ||
| HPV E6/E7 mRNA | |||
| Negative | 27 | 14 | 41 |
| 15.1 % | 7.8 % | 22.9 % | |
| Positive | 70 | 68 | 138 |
| 39.1 % | 38.0 % | 77.1 % | |
| Total | 97 | 82 | 179 |
| 54.2 % | 45.8 % | 100.0 % | |
HPV human papillomavirus
aB7-H1 staining considered binarily as positive (>5 % staining) versus negative (no staining or <5 % staining)
Table 4.
Smoking status comparison to HPV and p16 results
| HPV mRNA positive | HPV mRNA negative | p value | p16 positive | p16 negative | p value | |
|---|---|---|---|---|---|---|
| Smokinga | ||||||
| Yes | 92 (63.8) | 46 (97.8) | <0.001 | 102 (68.9) | 41 (93.2) | 0.001 |
| No | 52 (36.2) | 1 (2.2) | 46 (31.2) | 3 (6.8) | ||
HPV human papillomavirus
aas positive = current or former smoker and negative = lifetime non-smoker
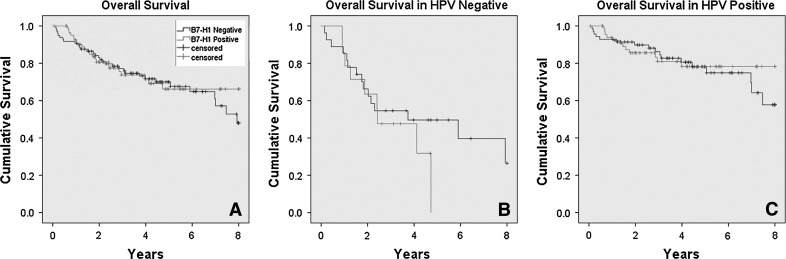
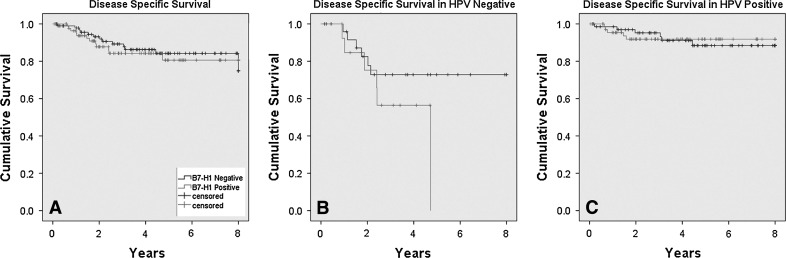
B7-H1 expression (binarily as positive versus negative), when analyzed for disease recurrence of any kind (local, regional, or distant), was not associated with these in the entire cohort (OR = 1.09, p = 0.66), HPV positive cohort (OR = 0.80, p = 0.69) or HPV negative cohort (OR = 2.02, p = 0.22). It was also not associated with overall survival for the entire cohort (HR = 0.91, p = 0.73), the HPV positive cohort (HR = 0.84, p = 0.64), or HPV negative cohort (HR = 1.65, p = 0.38), although there was a slight trend towards worse overall survival for the patients with HPV negative tumors that were B7-H1 positive (Fig. 2). B7-H1 expression was not associated with disease specific survival in the entire cohort, (HR = 0.95, p = 0.93) or HPV positive cohort (HR = 1.26, p = 0.56), but did show a trend toward correlation in the HPV negative cohort (HR = 2.15, p = 0.19) (Fig. 3). Univariate survival analysis of incremental B7-H1 staining intensity and also separately for the presence of lymphoid stromal staining versus none showed no statistically significant correlations with disease recurrence or survival (Table 5). Finally, we analyzed a composite score by adding intensity and distribution scores to provide results of 0 or 2 through 7 for each case and compared these results (as ordinal variables and also binarily as high scores—6 or 7—versus others) in univariate survival analysis. We did not find any significant correlations between B7-H1 expression and patient survival by this analysis, either (data not shown).
Fig. 2.
Kaplan-Meier overall survival curves for B7-H1 expression (binarily as positive versus negative) for the a entire cohort (p = 0.73), b HPV negative cohort (p = 0.38), and c HPV positive cohorts (p = 0.64)
Fig. 3.
Kaplan-Meier disease specific survival curves for B7-H1 expression (binarily as positive versus negative) for the a entire cohort (p = 0.56), b HPV negative cohort (p = 0.13), and c HPV positive cohorts (p = 0.93)
Table 5.
Outcomes in entire cohort by incremental B7-H1 staining intensity (0, 1+, 2+, or 3+) and lymphoid staining (present or absent)
| Overall survival HRa (p value) | Disease specific survival HR (p value) | Disease free survival HR (p value) | Nodal metastasis HR (p value) | Distant metastasis HR (p value) | |
|---|---|---|---|---|---|
| B7-H1 tumor cell staining intensity | 0.95 (0.69) | 1.09 (0.61) | 1.00 (0.96) | 1.03 (0.82) | 1.68 (0.03) |
| B7-H1 lymphoid stromal staining | 0.65 (0.20) | 0.47 (0.11) | 0.5 (0.15) | 1.00 (0.23) | 0.05 (0.85) |
HR hazard ratio
Discussion
In this cross-sectional study we assessed for an association between B7-H1 OSCC tumor expression and transcriptionally-active HPV. Oral HPV infection is not uncommon [10, 11, 31]. However, persistent viral infection is postulated to be the preceding event to carcinogenesis. Several studies show that HPV infection is effectively cleared in patients with an intact immune system, and infected cells are restricted just to those within tumors [9–11]. An alternative hypothesis to HPV-mediated carcinogenesis is that immune-evasive dysplastic or frankly invasive cells become persistently infected by HPV. We found transcriptionally-active high risk HPV E6/E7 in 49.2 % of cases with B7-H1 expression versus 34.1 % of B7-H1 negative cases (p = 0.08). Although these findings were not statistically significant, the effect size suggests that tumor mediated immune evasion could play a role in the subset of HPV-related OSCCs.
The highest prevalence of HPV oral infection is seen in HIV patients [14], which further suggests the necessity of a compromised immune system for persistent infection. We propose that the compromise of the immune system could be partially a localized phenomenon, mediated at the individual tumor cell level. Although overall, we observed B7-H1 expression in 46 % of cases, we saw a higher proportion of B7-H1 positive cases among the HPV positive tumors. It is possible that tumors could lose their expression of B7-H1 later and therefore cannot be captured in this study. In vivo studies addressing the likelihood of persistent B7-H1 expression amongst HPV positive tumor cells would be informative.
We did not find an association between B7-H1 expression and nodal metastases. However our study population was rich with patients with nodal metastasis (91.2 %), as would be expected for a contemporary OSCC cohort. In this cohort, only 16 patients showed no evidence of nodal metastasis. This suggests our study may be underpowered to make any specific associations here. Carcinomas in other anatomic regions with B7-H1 expression are associated with distant metastasis and with poorer survival. B7-H1 staining intensity as an integral variable significantly correlated with the development of distant metastasis in the entire cohort, and comparing binary strong intensity staining (3+) versus others (0, 1+, 2+) showed strong trends with the development of distant metastases. The findings suggest that there may be a biologically significant role for B7-H1 expression in the development of distant metastasis, perhaps akin to the findings in numerous studies on other carcinomas [23, 24, 27, 32–34] and on melanomas [25]. In our study, this association did not carry through to a benefit in overall, disease free, or disease specific survival. This is likely due to the small numbers of patients that had distant metastasis in the whole cohort of patients. So, although an interesting finding, one must be careful not to over interpret our finding of an association between B7H1 expression and metastasis. To more definitively address if B7-H1 expression truly increases the risk of death in OSCC, quite sizeable numbers of patients would appear to be needed.
As an additional unrelated finding, we observed a large proportion of current or former smoking amongst the HPV negative and also the HPV positive patients. This is in contrary to the common misconception that HPV and smoking-related carcinogenesis pathways are either partially or totally exclusive of each other. The larger literature also shows most HPV-related OSCC patients to be current or former smokers [2, 8, 35–39], although it is clear that there are less overall smokers amongst the HPV-related cases and their amounts of exposure are less. Dysplastic changes secondary to tobacco smoke could be accompanied by aberrant expression of B7-H1. If true, a dysplastic cell would provide an ideal niche for persistent infection by HPV setting up an environment for malignant transformation.
Our study has a few other limitations. The inherently “snap shot” nature of cross sectional studies make it difficult to determine any temporal relationship between infection and carcinogenesis. Also, the distribution of patients with recurrent versus nonrecurrent disease in this cohort is fairly skewed due to the favorable prognosis of HPV-related OSCC. This perhaps limits the power to identify factors associated with recurrence. Lastly, some have observed B7-H1 expression in the lymphocytes and tumor cells at the leading edge of invasive tumors, notably in melanomas. Because we utilized a tissue microarray, we could not assess the leading edge of invasive tumor to examine for this phenomenon.
In summary, a significant subset of HPV-related OSCC express B7-H1, and B7-H1 expression is somewhat higher in patients with distant metastases. This suggests a mechanism of tumor mediated immune evasion. Future in vitro studies could delineate the role of B7-H1 expression in tumor cell infection by HPV. No strong association with disease recurrence or prognosis was observed, which may reflect the overall positive effect of HPV on these variables or may be due to limited sample sizes. Future studies will be needed to better define the role of B7-H1 in OSCC.
Acknowledgments
We would like to acknowledge Xiao-Jun Ma PhD and Yuling Luo PhD from Advanced Cell Diagnostics, Inc. for the prior RNAscope™ in situ hybridization studies that were performed on the tissue microarray cohort for a previous study. We would also like to acknowledge Haidong Dong MD, PhD for providing the B7-H1 immunostaining on this study.
References
- 1.Adelstein DJ, Ridge JA, Gillison ML, et al. Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting, November 9–10, 2008, Washington, DC. Head Neck. 2009;31(11):1393–1422. doi: 10.1002/hed.21269. [DOI] [PubMed] [Google Scholar]
- 2.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, Broutian T, Pickard RK, et al. Prevalence of oral HPV infection in the United States, 2009–2010. JAMA. 2012;307(7):693–703. doi: 10.1001/jama.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92(9):709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 5.Cerovac Z, Sarcevic B, Kralj Z, Ban J. Detection of human papillomavirus (HPV) type 6, 16 and 18 in head and neck squamous cell carcinomas by in situ hybridization. Neoplasma. 1996;43(3):185–194. [PubMed] [Google Scholar]
- 6.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104(3):336–344. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 7.Snow AN, Laudadio J. Human papillomavirus detection in head and neck squamous cell carcinomas. Adv Anat Pathol. 2010;17(6):394–403. doi: 10.1097/PAP.0b013e3181f895c1. [DOI] [PubMed] [Google Scholar]
- 8.Ukpo OC, Pritchett CV, Lewis JE, Weaver AL, Smith DI, Moore EJ. Human papillomavirus-associated oropharyngeal squamous cell carcinomas: primary tumor burden and survival in surgical patients. Ann Otol Rhinol Laryngol. 2009;118(5):368–373. doi: 10.1177/000348940911800509. [DOI] [PubMed] [Google Scholar]
- 9.Duray A, Descamps G, Bettonville M, et al. High prevalence of high-risk human papillomavirus in palatine tonsils from healthy children and adults. Otolaryngol Head Neck Surg. 2011;145(2):230–235. doi: 10.1177/0194599811402944. [DOI] [PubMed] [Google Scholar]
- 10.Rice PS, Mant C, Cason J, et al. High prevalence of human papillomavirus type 16 infection among children. J Med Virol. 2000;61(1):70–75. doi: 10.1002/(SICI)1096-9071(200005)61:1<70::AID-JMV11>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 11.Rintala M, Grenman S, Puranen M, Syrjanen S. Natural history of oral papillomavirus infections in spouses: a prospective Finnish HPV Family Study. J Clin Virol. 2006;35(1):89–94. doi: 10.1016/j.jcv.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Rintala MA, Grenman SE, Jarvenkyla ME, Syrjanen KJ, Syrjanen SM. High-risk types of human papillomavirus (HPV) DNA in oral and genital mucosa of infants during their first 3 years of life: experience from the Finnish HPV Family Study. Clin Infect Dis. 2005;41(12):1728–1733. doi: 10.1086/498114. [DOI] [PubMed] [Google Scholar]
- 13.Rintala MA, Grenman SE, Puranen MH, et al. Transmission of high-risk human papillomavirus (HPV) between parents and infant: a prospective study of HPV in families in Finland. J Clin Microbiol. 2005;43(1):376–381. doi: 10.1128/JCM.43.1.376-381.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beachler DC, Weber KM, Margolick JB, et al. Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21(1):122–133. doi: 10.1158/1055-9965.EPI-11-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreuter A, Wieland U. Human papillomavirus-associated diseases in HIV-infected men who have sex with men. Curr Opin Infect Dis. 2009;22(2):109–114. doi: 10.1097/QCO.0b013e3283229fc8. [DOI] [PubMed] [Google Scholar]
- 16.Begum S, Gillison ML, Nicol TL, Westra WH. Detection of human papillomavirus-16 in fine-needle aspirates to determine tumor origin in patients with metastatic squamous cell carcinoma of the head and neck. Clin Cancer Res. 2007;13(4):1186–1191. doi: 10.1158/1078-0432.CCR-06-1690. [DOI] [PubMed] [Google Scholar]
- 17.Tamura H, Ogata K, Dong H, Chen L. Immunology of B7-H1 and its roles in human diseases. Int J Hematol. 2003;78(4):321–328. doi: 10.1007/BF02983556. [DOI] [PubMed] [Google Scholar]
- 18.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 19.Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7–H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119(2):317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 20.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56(5):739–745. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao Y, Zhang L, Kamimura Y, et al. B7–H1 overexpression regulates epithelial-mesenchymal transition and accelerates carcinogenesis in skin. Cancer Res. 2011;71(4):1235–1243. doi: 10.1158/0008-5472.CAN-10-2217. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Zhang L, Ritprajak P, et al. Immunoregulatory molecule B7-H1 (CD274) contributes to skin carcinogenesis. Cancer Res. 2011;71(14):4737–4741. doi: 10.1158/0008-5472.CAN-11-0527. [DOI] [PubMed] [Google Scholar]
- 23.Chen J, Li G, Meng H, et al. Upregulation of B7-H1 expression is associated with macrophage infiltration in hepatocellular carcinomas. Cancer Immunol Immunother. 2012;61(1):101–108. doi: 10.1007/s00262-011-1094-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 25.Gadiot J, Hooijkaas AI, Kaiser AD, van Tinteren H, van Boven H, Blank C. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer. 2011;117(10):2192–2201. doi: 10.1002/cncr.25747. [DOI] [PubMed] [Google Scholar]
- 26.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 27.Lu B, Chen L, Liu L, et al. T-cell-mediated tumor immune surveillance and expression of B7 co-inhibitory molecules in cancers of the upper gastrointestinal tract. Immunol Res. 2011;50(2–3):269–275. doi: 10.1007/s12026-011-8227-9. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci USA. 2004;101(49):17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang L, Ma Q, Chen X, Guo K, Li J, Zhang M. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg. 2010;34(5):1059–1065. doi: 10.1007/s00268-010-0448-x. [DOI] [PubMed] [Google Scholar]
- 30.Ukpo OC, Flanagan JJ, Ma XJ, Luo Y, Thorstad WL, Lewis JS., Jr High-risk human papillomavirus E6/E7 mRNA detection by a novel in situ hybridization assay strongly correlates with p16 expression and patient outcomes in oropharyngeal squamous cell carcinoma. Am J Surg Pathol. 2011;35(9):1343–1350. doi: 10.1097/PAS.0b013e318220e59d. [DOI] [PubMed] [Google Scholar]
- 31.Turner DO, Williams-Cocks SJ, Bullen R, et al. High-risk human papillomavirus (HPV) screening and detection in healthy patient saliva samples: a pilot study. BMC Oral Health. 2011;11:28. doi: 10.1186/1472-6831-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malaspina TS, Gasparoto TH, Costa MR, et al. Enhanced programmed death 1 (PD-1) and PD-1 ligand (PD-L1) expression in patients with actinic cheilitis and oral squamous cell carcinoma. Cancer Immunol Immunother. 2011;60(7):965–974. doi: 10.1007/s00262-011-1007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD. Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol. 2011;47(12):1148–1153. doi: 10.1016/j.oraloncology.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 34.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7–H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15(20):6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]
- 35.Chernock RD, El-Mofty SK, Thorstad WL, Parvin CA, Lewis JS., Jr HPV-related nonkeratinizing squamous cell carcinoma of the oropharynx: utility of microscopic features in predicting patient outcome. Head Neck Pathol. 2009;3(3):186–194. doi: 10.1007/s12105-009-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis JS, Jr, Thorstad WL, Chernock RD, et al. p16 positive oropharyngeal squamous cell carcinoma:an entity with a favorable prognosis regardless of tumor HPV status. Am J Surg Pathol. 2011;34(8):1088–1096. doi: 10.1097/PAS.0b013e3181e84652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus–associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24(5):736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 38.Hannisdal K, Schjolberg A, De Angelis PM, Boysen M, Clausen OP. Human papillomavirus (HPV)-positive tonsillar carcinomas are frequent and have a favourable prognosis in males in Norway. Acta Otolaryngol. 2010;130(2):293–299. doi: 10.3109/00016480903071377. [DOI] [PubMed] [Google Scholar]
- 39.D’Souza G, Zhang HH, D’Souza WD, Meyer RR, Gillison ML. Moderate predictive value of demographic and behavioral characteristics for a diagnosis of HPV16-positive and HPV16-negative head and neck cancer. Oral Oncol. 2010;46(2):100–104. doi: 10.1016/j.oraloncology.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]