Abstract
Lobular capillary hemangioma (LCH) is a specific entity among vascular lesions of the head and neck that may be diagnosed loosely as “pyogenic granuloma”. In contrast to granulation polyps and other reactive conditions, LCH is now regarded as likely a benign vascular neoplasm. Recent series assert lack of postoperative recurrence of LCH. We have observed otherwise, and to clarify this issue performed a systematic review of our institutional experience with LCH, tabulating clinicopathologic, histologic, and follow-up parameters of nasal or sinus lesions diagnosed as LCH or PG between 1989 and 2009. Lesions meeting strict criteria for LCH were included, and statistical analyses were performed using t tests, χ2 tests, and Kaplan–Meier analysis. Of cases identified, 38 of 46 (86 %) met criteria for LCH. Presenting symptoms included epistaxis (75 %), obstruction (36 %), and pain (3 %), with no sex predilection (17/17; M/F), and a median age of 39 years. Pregnancy was associated with 5/34 (15 %) cases, while antecedent trauma was reported in 4/34 (12 %). Histologically, ulceration was frequent (68 %) and mitotic activity highly variable (0–38 mitoses/10 HPF). Of cases with follow-up (31/34), we observed 13 local recurrences (42 %), including unbiopsied clinical recurrence (6/31, 19.4 %) and biopsy-documented recurrence (7/31, 22.6 %). Subjects with recurrence were significantly older (P = 0.04). Demographic, clinical, and histopathologic features were similar to prior studies; in contrast to recent series, recurrence in this cohort was frequent and comparable to that originally reported. Awareness of this may aid in avoiding misdiagnosis of these lesions as more aggressive entities such as angiofibroma and angiosarcoma.
Keywords: Lobular capillary hemangioma, Pyogenic granuloma, Sinonasal tract
Introduction
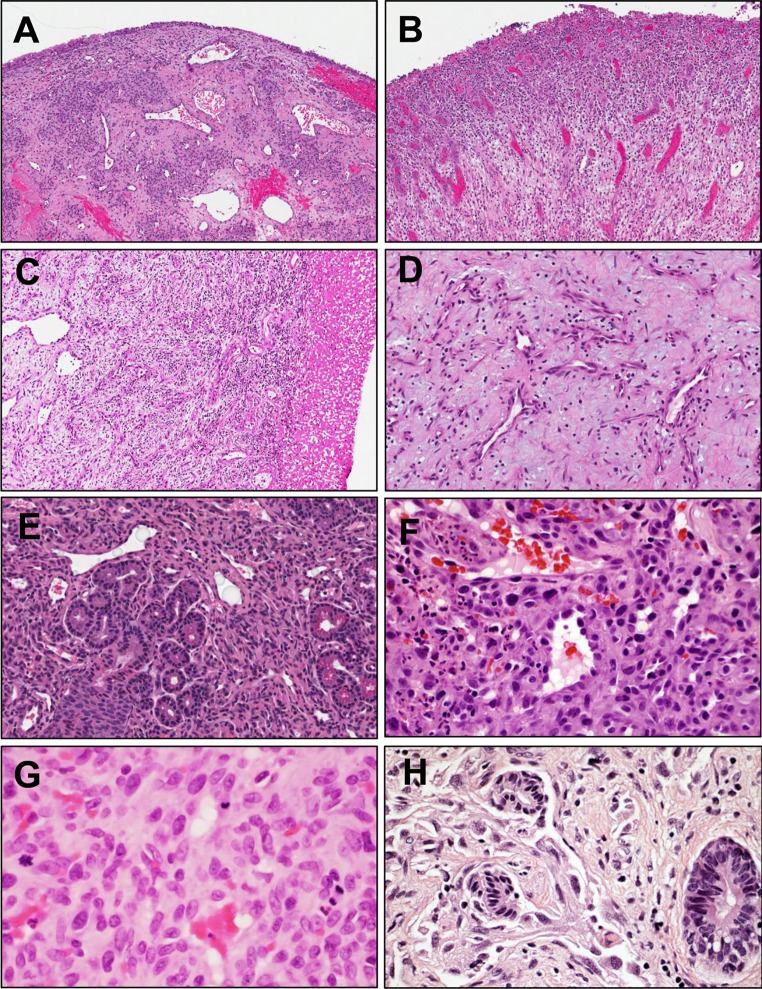
In 1980, Mills et al. [1] described lobular capillary hemangioma (LCH) as a distinctive subset of vascular lesions of the skin and mucosal membranes of the head and neck commonly diagnosed as “pyogenic granuloma” (PG). This descriptive term for the entity was intended to describe the singular morphology of these lesions, reflecting the lobular arrangement of bland capillaries encircling angulated feeder vessels without endothelial atypia (Fig. 1a). This was in contradistinction to polypoid granulation tissue or reactive processes (Fig. 1b) that may mimic LCH on clinical exam or be very loosely diagnosed as PG.
Fig. 1.
a Lobular capillary hemangioma (LCH) demonstrates distinctive architecture of lobules of capillaries surrounding angular vessels lined by bland endothelial cells (H&E, ×10). b Polypoid granulation tissue shows stroma with edema and prominent inflammation admixed with radially proliferating vessels with reactive atypia (H&E, ×10). c LCH (left third of figure panel) may be traumatized and acquire secondary surface granulation tissue (right third of panel) (H&E, ×20). d One case studied showed prominent myxoid stroma with low cellularity, sinonasal angiofibroma was considered in the differential but areas of typical LCH were present focally (H&E, ×20). e Rapidly proliferating LCH vessels may entrap sinus epithelial elements, imparting an infiltrative appearance (H&E, ×20), while subacutely traumatized LCH may acquire striking reactive atypia and brisk mitotic activity (panels f and g, respectively, both H&E, ×40), raising concern for a more aggressive lesion. However, LCHs lack the true invasive growth and endothelial atypia characteristic of angiosarcoma (panel h, H&E, ×60)
From the diagnostic standpoint, distinction of LCH from reactive mimics may be rendered difficult due to secondary superimposition of granulation tissue onto LCH (Fig. 1c), presumably due to local trauma, a phenomenon identified before by Mills et al. Moreover, authors have documented the sometimes difficult distinction of the full range of LCH lesions (Fig. 1c–g) from more aggressive vascular neoplasms, such as rare sinonasal angiosarcomas (Fig. 1h), with which they share overlapping histologic and immunophenotypic features [2]. Consistent with the clinicopathologic impression that LCH constitutes a vascular neoplasm, clonality has been recently demonstrated cytogenetically in a case from the nasal cavity, showing deletion within the long arm of chromosome 12 [3].
From a clinical standpoint, a key concern regarding LCH is its propensity to recur (locally) after excision. The Mills et al. series observed a low rate recurrence with 2 of 7 (28.6 %) oral lesions and 2 of 18 (11.1 %) of nasal lesions demonstrating this phenomenon. Three additional nasal lesions previously treated by cauterization recurred, the excisions of which were included among the cohort (total recurring 5/18, 27.8 %). Older series of LCH from the oral cavity or in the dermatology literature have observed similar recurrence rates [4–6], as well as one recurrence of in a cohort of 12 LCH in the sinonasal tract [7]. However, the only two recent, larger series of sinonasal LCH have observed lack of recurrence among cohorts of 40 [8] and 18 cases [9], respectively. These latter observations are contrary to our experience with recurrence in sinonasal LCH. To clarify this issue, we performed a systematic clinicopathologic review of our institution’s experience with sinonasal LCH in order to better understand this lesion and its biologic potential.
Methods
After Institutional Review Board approval, we performed a retrospective review of departmental cases and institutional medical records from 1989 through 2009 to identify cases. Inclusion criteria included a diagnosis as LCH or PG, lesion site in the nasal cavities or paranasal sinuses, and availability of institutional medical records. For these cases, original slides were pulled to confirm the diagnoses, and deidentified clincopathologic data were reviewed and tabulated. Diagnostic criteria of LCH included vascular lesions composed of circumscribed “lobules” of capillaries arrayed around angular, more dilated feeder vessels, each with cytologically bland endothelium, as detailed by Mills et al. [1]. Where available, clinical and demographic data were tabulated, including age, gender, pregnancy (at time of lesion appearance), antecedent trauma or procedure, symptomatology (pain, bleeding, obstruction), and anatomic location, including laterality and configuration (whether polypoid/pendunculated). For each case, the type of first procedure performed was tabulated including whether a biopsy (including “biopsy”, “punch”, or “shave”), excision (including “excision” or “resection”), or other primary nonsurgical treatment (e.g., silver nitrate without removal of tissue) was performed. Data were also tabulated for whether such a procedure was performed with or without ancillary treatments (e.g., cautery or silver nitrate).
Recurrence was tabulated as clinically documented, using the last head and neck examination documented in the University of Michigan medical record to right censor cases without evidence of disease. Pathologic data collected included largest dimension on gross exam, ulceration (presence or absence), cellularity (density of lobules; low, medium, or high), and mitoses (figures/10 high power fields). Statistical analysis included Student’s t test (homoscedastic, two tailed, and paired as appropriate), Chi squared test, and Kaplan–Meier estimation of recurrence free survival, performed in Prism (GraphPad Software, La Jolla, CA, USA). Archival slide material was reviewed to confirm diagnosis and score histologic parameters (SCS, JBM), and cases of interest were recut for digital whole slide imaging (ScanScope XT and ImageScope, both from Aperio, Vista, CA, USA).
Results
Of 46 candidate cases identified, diagnosis, using strict criteria for LCH, was confirmed 38 cases (82.6 %), representing a total of 34 subjects (i.e., including four recurrences). Slides were available for review in all cases. Of the eight cases that were misdiagnosed, six were purely granulation tissue, while the remaining two cases were a cavernous hemangioma and a tangentially oriented, richly vascular section of nasal turbinate tissue without histopathologic abnormality. Examples of LCH and its morphologic mimics are summarized in Fig. 1. Digital whole slide images (WSIs) for several representative cases are available for review at our institution’s resource: www.wsirepository.org.
Table 1 summarizes the clinical and demographic parameters of the cohort. Of the subjects, 17/34 were male and 17/34 were female; the mean ages were 39.2 and 38.7 years, respectively (P = 0.96, t test). Of female subjects, 5/17 (29.4 %) developed LCH during current or recent pregnancy. Of all subjects, antecedent trauma was referenced in 4/34 (11.8 %). Where presenting symptoms were recorded in clinic notes, the most common presenting symptom was epistaxis (24/32; 75.0 %), followed by obstruction (11/31; 35.5 %), then pain (1/30; 3.2 %). Of 33 cases where records for type of first surgical procedure was available, excision was most commonly employed (20/33; 54.5 %), less commonly biopsy (13/33; 39.4 %) or other procedures (“piecemeal polyp removal” and silver nitrate only; 2/31; 6.1 %). Ancillary treatments used at time of biopsy or excision, whether for hemostasis or adjuvant effect, included electrocautery/fulguration (N = 5), silver nitrate (N = 14), gel foam (N = 1), and cellulose absorbable hemostatic product (N = 1). At least one of such treatments was used in the majority of cases (20/31; 64.5 %).
Table 1.
Clinical and Demographic Cohort Data
| Clinical characteristics | Subject data | # Missing data | ||
|---|---|---|---|---|
| Gender, # (%) | 0 | |||
| Female | 17 (50) | |||
| Male | 17 (50) | |||
| Age (years) | 0 | |||
| Range | <1–81 | |||
| Mean | 38.9 | |||
| Female, mean | 38.7 | |||
| Male, mean | 39.2 | |||
| Presentation | As below | |||
| Pregnancy, # (%) | 5 (14.7) | 0 | ||
| Antecedent trauma # (%) | 4 (11.8) | 0 | ||
| Range symptom duration (mo) | Congenital-60 | 9 | ||
| Mean duration (months) | 10.5 | 9 | ||
| Epistaxis, # (%) | 24 (75) | 2 | ||
| Obstructive symptoms | 11 (35.5) | 3 | ||
| Pain | 1 (3.3) | 4 | ||
| Anatomic site, laterality | Left | Right | All | 1 |
| Septum, # (%) | 15 (68.2) | 7 (31.8) | 22 (66.7) | |
| Turbinate, # (%) | 1 (25) | 3 (75) | 4 (12.1) | |
| Vestibule, # (%) | 1 (16.7) | 5 (83.3) | 6 (18.2) | |
| Ethmoid sinus, # (%) | 1 (100) | 0 (0) | 1 (3) | |
| All sites, # (%) | 18 (54.5) | 15 (45.5) | 33 (100) | |
Anatomically we observed essentially equal distributions of laterality (right, 18/33, 54.5 %; left, 15/33, 45.5 %), excepting one older case where site/laterality was not recorded. Lesions occurred on the septum most frequently (22/33; 66.7 %), followed by vestibule (6/33; 18.2 %), nasal turbinate (4/33; 12.1 %), and ethmoid sinus (1/33; 3.0 %). The mean largest dimension of all cases (N = 32, using data from gross exam) was 0.90 cm ± 0.55 cm (SD), median 0.80 cm, range [0.20–2.5 cm]. In terms of configuration, clinical notes described a slight minority as pedunculated or polypoid (14/34; 41.2 %).
Histologic parameters scored included presence or absence of ulceration, degree of cellularity (on a subjective semiquantitative three-tiered scale), and mitotic activity among cases with follow-up data (31/34, 91.2 %). A significant majority of cases were ulcerated (21/31; 67.7 %). Cellularity of lesions was most frequently moderate (20/31; 64.5 %), less frequently low (7/31; 22.6 %), and least frequently high (4/31; 12.9 %). Overall mitotic activity was low to moderate [mean 3.6 mitoses/HPF ± 3.6 SD, range (0–15)]. Table 2 summarizes data for the cases with follow-up.
Table 2.
Comparison of non-recurrent and recurrent cases
| All subjects | Non-recurrent | Recurrent | Significance* | |
|---|---|---|---|---|
| Follow-up data (months) | ||||
| Available data, # (mean) | 31 (58.6) | 18 (59.0) | 13 (58.1) | |
| Follow-up range | 0–186.5 | 0.9–186.5 | 3.2–124.1 | P = 0.97 |
| RFS (mean) | 36.7 | 58.9 | 5.74 | |
| Gender | P = 1.0 | |||
| Males, # | 14 | 8 | 6 | |
| Females, # (# pregnant) | 17 (5) | 10 (2) | 7 (3) | P = 0.62 |
| Age, mean | 39.3 | 31.5 | 50.1 | P = 0.04 |
| Pathologic feature | ||||
| Size, mean | 0.84 | 0.89 | 0.77 | P = 0.53 |
| Mitoses, mean | 3.59 | 4.2 | 2.8 | P = 0.38 |
| Ulceration | 21 | 8 | 5 | P = 0.70 |
| Cellularity | ||||
| Low | 7 | 3 | 4 | P = 0.56 |
| Intermediate | 20 | 12 | 8 | |
| High | 4 | 3 | 1 | |
RFS recurrence free survival
* Student’s two-tailed homoscedastic t test, Fisher’s exact test, or χ2 test, as appropriate, with P < 0.05 in bold type
Over a mean follow-up of 58.6 months, we observed local recurrence in 13 of 31 cases (41.9 %), including cases of clinically documented, unbiopsied recurrence (6/31, 19.4 %) and biopsy-documented recurrence (7/31, 22.6 %). No case showed more than one recurrence, and no case evinced malignant degeneration or metastasis. Among recurrent cases where gross measurements and archival slides were available for both the primary and recurrent lesion (N = 6), a nonsignificant trend toward increased size was noted in recurrent lesions (mean largest dimension of primary lesion 0.8 cm, mean of recurrent 1.25 cm; P = 0.20, paired t test), as was a trend toward increased mitotic activity in the recurrence [mean 4.2 mitoses/HPF primary, mean 15.8 in recurrent lesions, recurrent mitosis range (3–38), P = 0.13, paired t test]. Pedunculated/polypoid configuration was equally distributed across recurrent and nonrecurrent cases (5/13; 38.5 % versus 9/21; 42.9 %).
In terms of types of procedures performed, a nonsignificant trend was observed toward a higher proportion of primary treatment described as “biopsy” as opposed to “excision” in cases that subsequently recurred (7/13; 53.8 %) than in cases that did not recur (6/20; 30.0 %), P = 0.14, χ2 test. However, in one recurrent case the surgeon noted concern for incomplete resection of tissue at time of biopsy, and in a second case where shave biopsy was performed, the surgeon documented planned “re-excision if neoplasm is demonstrated by pathology” at the time of biospy. An additional trend, approaching significance, was observed toward less frequent use of adjuvant/hemostatic agents in recurrent cases than nonrecurrent cases (6/13; 46.2 % versus 14/18; 77.8 %, respectively, P = 0.069, χ2 test).
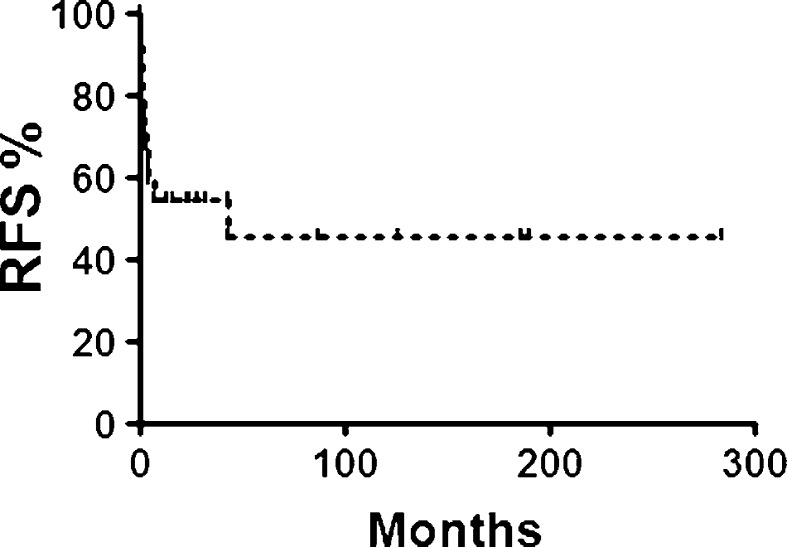
The mean time to recurrence was 5.7 months; see Fig. 2 for Kaplan–Meier estimation of recurrence free survival. Gender was equally distributed across recurrence (recurrent M/F 6/7; non-recurrent M/F 8/10; P = 1.0; χ2 test). A significant trend toward older age was identified among recurrent cases (recurrent mean 50.1 years, nonrecurrent mean 31.5; P = 0.04; t test).
Fig. 2.
Kaplan–Meier estimation of recurrence free survival (RFS) by LCH subjects for whom follow-up exam data were available (31/34; 91.2 %). The pattern of recurrence was predominantly early
Discussion
Review of our institution’s experience with sinonasal lobular capillary hemangiomas suggests that recurrence is relatively frequent. In contrast to recent reports that have only documented a single recurrence in series of N = 40, 18 and 12 subjects [7–9], we observed a significant rate of recurrence of 42 % (23 % confirmed histologically, 19 % treated without additional pathologic evaluation), comparable to the report first characterizing this lesion (observed 11 and 17 %, respectively [1]). Clinicopathologic parameters were in our cohort were similar to a recent cohort of similar size (N = 40) reported by Puxeddu et al. As in the prior study, we observed essentially equal proportions of male and female subjects, similar age (mean age 39 years herein versus 40 years), similar prevalence of pregnancy (both ~15 %), and similar symptomatology (epistaxis 75 % herein versus 95 %; obstruction in both ~35 %; pain 3 % herein versus 7.5 %). Anatomic distribution was also similar (septum 67 % herein versus 55 %; vestibule, both ~18 %) [8]. The significantly older age among recurrent cases in our study has not been observed before. Importantly, no malignant behavior was noted; all recurrent cases were cured by the second treatment.
In the Puxeddu [8] or El-Sayed [7] series, histologic features were not studied; however, we observed similar rates of ulceration (61 % herein versus 71 %) to that described among the subgroup of 18 nasal LCH described by Mills et al. [1]. We also observed a similar range of mitotic activity (0–38 herein versus 0–53 per HPF). To our knowledge, comparison of primary and recurrent LCH lesions has not been undertaken before, and though slight trends in favor of increased mitotic activity and size upon recurrence were noted, the number of subjects in this study with recurrences evaluable histologically (N = 6) was not sufficient to exclude this may have occurred by chance alone.
Consistent with our identification of cases by searching surgical pathology records, all cases were managed by at least one operative biopsy or excision resulting in a specimen, and thus cases treated by other nonsurgical modalities, including cryotherapy, were not identified. Our use of surgeons’ own descriptions of their procedure as “biopsy” or “excision” to classify what type of procedure was performed comes with the caveat that such distinctions may be arbitrary and difficult to ascertain in terms of intent (whether diagnostic versus therapeutic, e.g., a significant number of procedures called “biopsy” were actually curative). However, we did find trends toward higher proportion of biopsy procedures and lower frequency of ancillary procedures (cautery, silver nitrate etc.) among cases that recurred. Though systematic review of types of procedures, treatments, and outcomes has not been undertaken for sinonasal LCHs, large cohorts have been analyzed for LCH at more accessible sites, including the oral mucous membranes [10, 11] and skin [12]. Consistent with our findings regarding excisions, these reports overall identify lowest rates of recurrence among lesions treated with surgical excision (as low as 15 of 510 skin lesions, or ~3 % [12]). We speculate that the constrained operating space and increased vascularity of the nasal passages may be related to the higher recurrence rate for excisions that we have observed (~25 %).
The primary limitation of this study, though similar in size to the largest nasal LCH to date [8], remains its statistical power, and we acknowledge the possibility of sampling bias. Another important caveat concerns the status of our institution as referral center, which might have resulted in a selection bias in favor of more aggressive lesions with greater propensity for recurrence than those described in previous series, though these were similarly based in academic medical centers [7–9]. As is true with any recurrence study of a disease with clinical symptomatology, this study may be biased in favor of ascertainment of recurrence, though follow-up data were available for >90 % of cases. Variation in surgical and clinical follow-up practice between institutions might also have played a role in favor of detecting recurrence. We cannot exclude diagnostic variability as well, especially given that the distinction of LCH, especially when traumatized, from granulation tissue may be difficult (as below).
From the standpoint of histology, we observed a significant range of morphology in LCH, including pseudomalignant features concerning for more worrisome lesions. Ulceration and mitotic activity were remarkable in a number of cases (Fig. 1c, g), leading understandably to the consideration of angiosarcoma (Fig. 1h) [2]. For example, cases showing traumatization may show prominent reactive atypia among endothelial cells and stroma (Fig. 1f). One case (44 year old female) within the cohort showed prominently hyalinized stroma with areas of myxoid change (Fig. 1d), where the diagnosis of sinonasal angiofibroma would have been more strongly considered in a more appropriate context. Other cases showed areas of dilation of the angular “feeder vessels,” leading to consideration of cavernous hemangioma. In the case of well-formed and cellular LCH, large, ectatic, thin-walled vessels might be construed as so-called “hemangiopericytoma”-like vessels, causing consideration of sinonasal-type hemangiopericytoma/glomangiopericytoma [13] or solitary fibrous tumor, though such lesions lack the well-defined lobular capillary architecture of LCH.
In the end, we hope these findings are of use in the recognition, diagnosis, and management of LCH. Future studies should examine the issue of recurrence in detail among larger cohorts, while emerging molecular technologies may be of use in testing definitively whether these lesions are clonal and clarifying their nosologic relationship to other vascular neoplasms.
References
- 1.Mills SE, Cooper PH, Fechner RE. Lobular capillary hemangioma: the underlying lesion of pyogenic granuloma. A study of 73 cases from the oral and nasal mucous membranes. Am J Surg Pathol. 1980;4(5):470–479. doi: 10.1097/00000478-198010000-00007. [DOI] [PubMed] [Google Scholar]
- 2.Nelson BL, Thompson LD. Sinonasal tract angiosarcoma: a clinicopathologic and immunophenotypic study of 10 cases with a review of the literature. Head Neck Pathol. 2007;1(1):1–12. doi: 10.1007/s12105-007-0017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Truss L, Dobin SM, Donner LR. Deletion (21) (q21.2q22.12) as a sole clonal cytogenetic abnormality in a lobular capillary hemangioma of the nasal cavity. Cancer Genet Cytogenet. 2006;170(1):69–70. doi: 10.1016/j.cancergencyto.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 4.Bhaskar SN, Jacoway JR. Pyogenic granuloma—clinical features, incidence, histology, and result of treatment: report of 242 cases. J Oral Surg. 1966;24(5):391–398. [PubMed] [Google Scholar]
- 5.Patrice SJ, Wiss K, Mulliken JB. Pyogenic granuloma (lobular capillary hemangioma): a clinicopathologic study of 178 cases. Pediatr Dermatol. 1991;8(4):267–276. doi: 10.1111/j.1525-1470.1991.tb00931.x. [DOI] [PubMed] [Google Scholar]
- 6.Tay YK, Weston WL, Morelli JG. Treatment of pyogenic granuloma in children with the flashlamp-pumped pulsed dye laser. Pediatrics. 1997;99(3):368–370. doi: 10.1542/peds.99.3.368. [DOI] [PubMed] [Google Scholar]
- 7.El-Sayed Y, Al-Serhani A. Lobular capillary haemangioma (pyogenic granuloma) of the nose. J Laryngol Otol. 1997;111(10):941–945. doi: 10.1017/S0022215100139027. [DOI] [PubMed] [Google Scholar]
- 8.Puxeddu R, Berlucchi M, Ledda GP, Parodo G, Farina D, Nicolai P. Lobular capillary hemangioma of the nasal cavity: a retrospective study on 40 patients. Am J Rhinol. 2006;20(4):480–484. doi: 10.2500/ajr.2006.20.2878. [DOI] [PubMed] [Google Scholar]
- 9.Baradaranfar MH, Dabirmoghaddam P. Endoscopic endonasal surgery for resection of benign sinonasal tumors: experience with 105 patients. Arch Iran Med. 2006;9(3):244–249. [PubMed] [Google Scholar]
- 10.Jafarzadeh H, Sanatkhani M, Mohtasham N. Oral pyogenic granuloma: a review. J Oral Sci. 2006;48(4):167–175. doi: 10.2334/josnusd.48.167. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar B. Minimally invasive approach to eliminate pyogenic granuloma: a case report. Case Rep Dent. 2012;2012:909780. doi: 10.1155/2012/909780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Sinno H, Tahiri Y, Gilardino MS. Treatment options for cutaneous pyogenic granulomas: a review. J Plast Reconstr Aesthet Surg. 2011;64(9):1216–1220. doi: 10.1016/j.bjps.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 13.Thompson LD, Miettinen M, Wenig BM. Sinonasal-type hemangiopericytoma: a clinicopathologic and immunophenotypic analysis of 104 cases showing perivascular myoid differentiation. Am J Surg Pathol. 2003;27(6):737–749. doi: 10.1097/00000478-200306000-00004. [DOI] [PubMed] [Google Scholar]