Abstract
CD44 is a transmembrane adhesion molecule which has been previously shown to be useful in the differentiation of benign papillary lesions from invasive carcinoma in several different areas including sinonasal mucosa and breast tissue. CD44 expression has previously been shown to be lost in invasive carcinoma and retained in benign papillary lesions in both of the above locations. In addition, studies have evaluated oral mucosal lesions for CD44 expression and found a loss with invasive squamous cell carcinoma when compared to normal epithelium, hyperplasia, and squamous papillomas, which stained particularly strongly. To the best of our knowledge, no study has evaluated CD44 expression when comparing salivary ductal papillomas in comparison to oral papillary SCCA. In this study 18 cases of intraductal papilloma were compared to 19 cases of oral papillary SCCA. Within the ductal papilloma group, all cases stained either absent (6 %), weakly (33 %), or moderately (61 %) with 76 % expressing the stain diffusely and 24 % focally. In comparison, the papillary squamous cell carcinoma cases expressed the CD44 moderately (26 %) or strongly (74 %) with 100 % showing diffuse staining. Thus, the CD44 expression was contrary to expectation based on previous studies, which we hypothesize is due to the extremely well differentiated nature of papillary SCCA which expressed CD44 staining compatible with levels previously reported with oral squamous papillomas than invasive carcinoma.
Keywords: CD44, Salivary ductal papillomas, Papillary squamous cell carcinoma, Immunohistochemistry
Introduction
CD44, a transmembrane cell adhesion molecule, has been studied for the potential to differentiate benign papillary lesions from invasive carcinoma in several different types of tissue. It has been shown to be universally present in sinonasal ductal papillomas but lose expression when invasive squamous cell carcinoma (SCCA) arises in these lesions [1]. Similar results have been shown comparing intraductal breast papillomas to invasive papillary carcinoma [2–4].It has also been demonstrated to be downregulated in oral dysplasia and SCCA when compared to normal oral epithelium, epithelial hyperplasia, and oral squamous papillomas [5]. Oral squamous papillomas in particular have been shown to stain strongly to CD44, often more strongly than the normal oral epithelium [5].
Salivary intraductal papillomas are a family of benign lesions that occur mainly in the minor salivary glands and include sialadenoma papilliferum, inverted ductal papilloma, and intraductal papilloma. Sialadenoma papilliferum are a papillary growth which project out of a minor salivary gland duct onto the surface epithelium. Inverted and intraductal salivary papillomas occur within the duct, though inverted ductal papillomas maintain a connection to the surface epithelium of the oral cavity. Both generally present as submucosal masses and are histologically similar to papillary proliferation of ductal-type epithelium inside a salivary duct [6]. Prior reports of immunohistochemistry (IHC) of oral ductal papillomas have found positivity to cytokeratin (CK) markers such as AE1/3, Cam5.2, CK7, 8, 13, 14, 18, and 19 along with epithelial markers such as epithelial membrane antigen (EMA) and carcinoembryonic antigen (CEA) [7–12]. The lesions have very little to no potential for malignant transformation, and in most cases no recurrence is reported after simple excision [6]. Salivary ducts, however, may be secondarily affected by oral SCCA which may sometimes result in difficulty in diagnostic distinction from the more cellular forms of ductal papillomas.
Papillary SCCA is a variant of oral SCCA which may display a rather benign appearance due to the extremely well differentiated form it often takes, resulting in a diagnostic dilemma and confusion with benign papillary conditions, particularly larger lesions in high risk locations such as floor of the mouth or ventral tongue. Papillary SCCA is most often found in the oropharynx, oral cavity, hypopharynx, larynx, and sinonasal tract, all areas where benign squamous papilloma occurrence is also high [13, 14]. It has been linked to human papillomavirus (HPV), though this association has not been conclusively proven [14]. It has been associated with a better prognosis than other forms of SCCA in the head and neck, but exhibits a high rate of local recurrence [14, 15].
To the best of our knowledge, no study has compared the expression of CD44 in oral salivary intraductal papillomas and papillary SCCA. This study compared 18 cases of salivary intraductal papilloma to 19 cases of oral SCCA with immunohistochemical staining for CD44 to determine whether this stain may be useful in supporting a histologic diagnosis and discriminating between these two entities.
Materials and Methods
After obtaining institutional review board approval, blocks were identified from the archives of the University of Florida College of Dentistry oral biopsy service over a time frame from 1980 to 2011 for salivary ductal papillomas and oral papillary SCCA. 19 initial cases of each lesion were chosen, although one case of salivary ductal papilloma was later dropped from the study due to insufficient tissue available. Of the final 18 cases of salivary ductal papilloma, 12 cases were diagnosed as sialadenoma papilliferum, and 6 cases as inverted or intraductal papillomas. The 19 cases of papillary SCCA were from multiple locations within the oral cavity including floor of the mouth, tongue, gingiva, and buccal mucosa.
Four micron sections of the tissue samples were obtained and dried for 2 h in a 60 °C oven. A Ventana Benchmark automated immunostainer was used and they were first dewaxed then heat inducted epitope retrieval was performed via the Ventana CC1 retrieval solution for 30 min at 95–100 °C. Anti-Human CD44s, Clone DF1485 (Dako North America Inc, Carpinteria CA), a mouse monoclonal antibody, was diluted to 1:40 and applied to the sections at 37 °C for 16 min. The antigen was visualized by use of the Ultra View DAB detection kit (Ventana Medical Systems Inc, Tucson AZ). Counterstaining was performed with Ventana brand hematoxylin followed by dehydration, clearing, and mounting on glass slides.
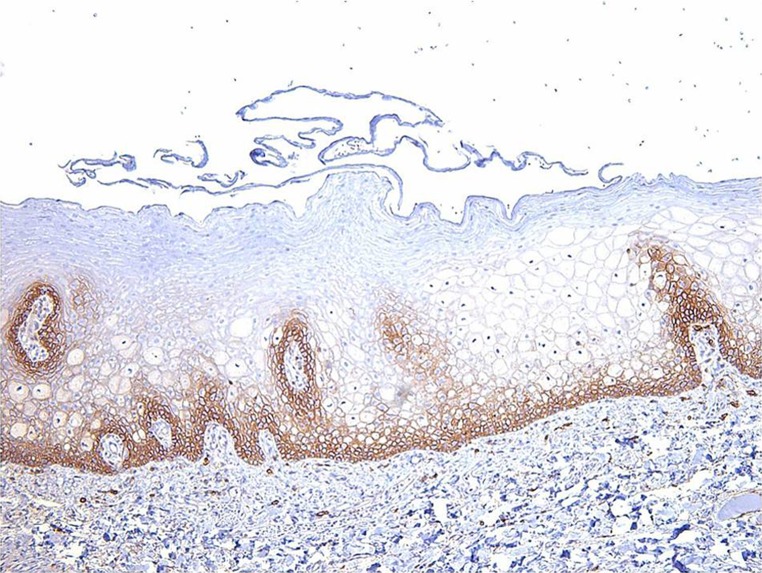
A grading system was devised for evaluating the staining patterns based on a control of the staining level in normal oral epithelium, of which the basal and spinous layers stain with CD44 in a membraneous staining pattern (Fig. 1). The following grades were assigned to each CD44 preparation in both the salivary ductal papilloma group and the papillary SCCA group: 0 = absent or no staining, 1 = weaker staining than normal oral epithelial control, 2 = comparable staining to normal oral epithelial control, 3 = stronger staining than normal oral epithelial control. In addition, slides were graded as to presence of focal versus diffuse staining of CD44 (diffuse staining being equal to greater than 70 % of cell membranes of the appropriate areas of the basal third of the epithelium showing expression).
Fig. 1.
Normal oral epithelium shows moderate membraneous staining to CD44 in the basal third. This level of intensity was used as a control for this study (CD44 immunostain magnification ×10)
Results
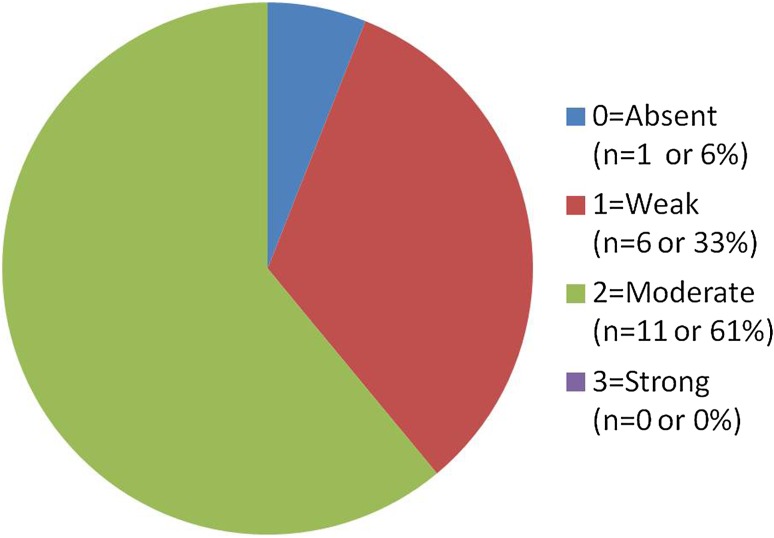
Results of CD44 staining in the salivary ductal papilloma group (Fig. 2) showed the following (of 18 total cases): 1 case (6 %) showed “0” (absent staining), 6 cases (33 %) showed “1” (weak staining), 11 cases (61 %) showed “2” (comparable to control), and 0 cases (0 %) showed “3” staining (strong staining). Of the cases that stained positively in this group 76 % (13 cases) stained diffusely and 24 % (4 cases) showed focal staining only. Figure 3 demonstrates a typical staining pattern of weak to moderate staining in the basal third of the epithelium in an intraductal papilloma.
Fig. 2.
Ductal papilloma group CD44 results. CD44 staining on the ductal papilloma group revealed the majority of cases (94 %) exhibiting weak or moderate staining
Fig. 3.
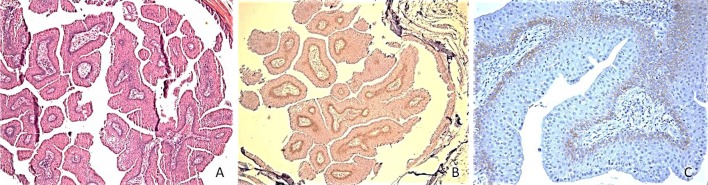
Typical staining pattern of ductal papilloma group with weak staining mainly confined to the basal third of the epithelium demonstrated in this case of intraductal papilloma (a hematoxylin and eosin magnification ×5, b CD44 immunostain magnification ×5, c CD44 immunostain magnification ×20)
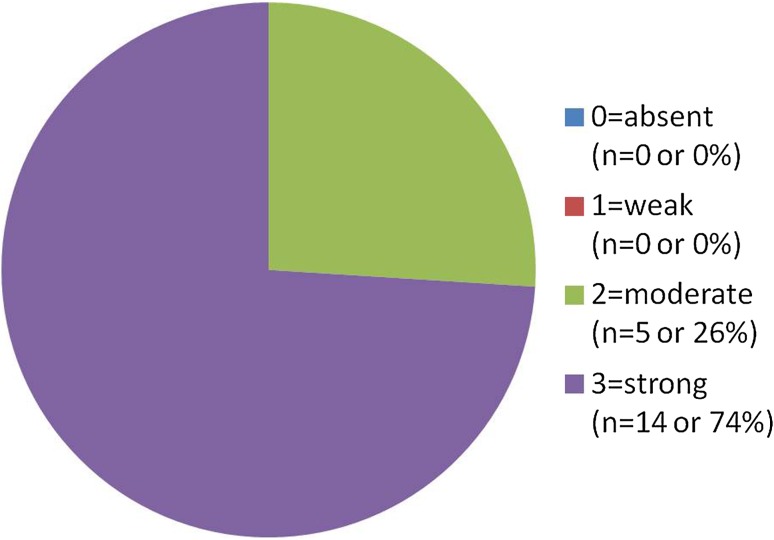
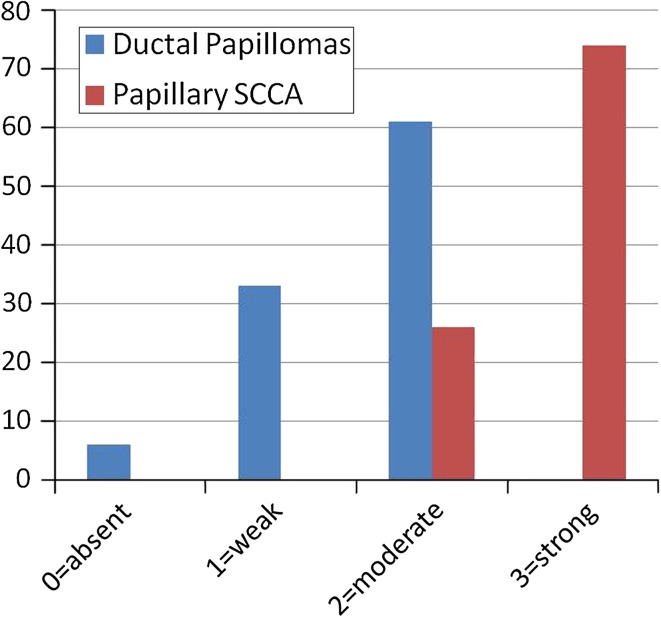
In the papillary SCCA cases the following results (Fig. 4) were obtained (of 19 cases): 0 cases (0 %) “0” (absent staining), 0 cases (0 %) “1” (weak staining), 5 cases (26 %) of “2” (comparable to control), and 14 cases (74 %) of “3” (strong staining). All cases (100 %) stained diffusely. Figure 5 demonstrates a typical staining response for this group with strong and diffuse staining extending above the basal third in a papillary SCCA. A table comparing the two groups may be seen in Fig. 6.
Fig. 4.
Papillary SCCA group CD44 results. CD44 staining on the papillary SCCA group revealed 100 % of cases exhibiting moderate or strong staining
Fig. 5.
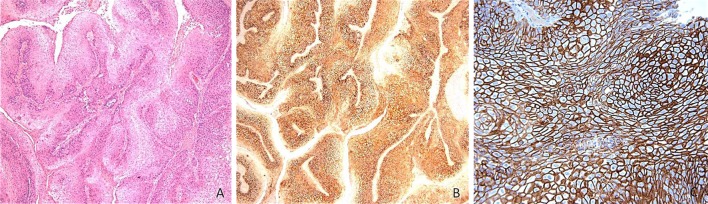
Typical staining pattern of the papillary SCCA group with strong and diffuse staining demonstrated (a hematoxylin and eosin magnification ×5, b CD44 immunostain magnification ×5, c CD44 immunostain magnification ×20)
Fig. 6.
Comparison of Ductal Papilloma group to papillary SCCA group for CD44 expression. A distinct staining profile emerged with with lower expression of CD44 in the ductal papilloma group. In addition, positivity through the entire thickness of the epithelium was a feature noted uniformly within the papillary SCCA group while the ductal papilloma group only exhibited positivity in the basal third of the epithelium
Discussion
CD44 is a receptor for hyaluronate, which is a component of the extracellular matrix, thus allowing it to act as a transmembrane adhesion molecule [16]. It also binds fibronectin and collagen [17]. It functions as a lymphocyte homing receptor as well [17]. CD44 has many uses in histopathology, including as an epithelial marker, in the differentiation of some forms of lymphoma, and in evaluating early urothelial carcinomas [18, 19]. Expression of CD44 has been studied in many different types of cancer, and the majority show loss of CD44 expression to be linked to a worse prognosis [20]. This loss of CD44 expression has been linked to a worse prognosis in laryngeal carcinoma, tongue carcinoma, breast carcinoma, ovarian carcinoma, prostate carcinoma, urothelial carcinoma, melanoma, gastro-intestinal stromal tumor (GIST), and carcinoid tumors, although in some of these tumors an association has failed to have been replicated in other studies [20–28]. Conversely, other tumors have shown the opposite effect with high expression signaling worse prognosis, particularly hematologic malignancies such as multiple myeloma and non-Hodgkin lymphoma [29, 30]. CD44s is the standard subtype, but splice variants (CD44v) have been identified and evaluated as well in which up to 10 variant exons can be inserted into the molecule [31]. It has been hypothesized that it is the loss of adhesion properties with loss of CD44 expression that allows for increased levels of invasion and worse prognosis in some types of malignancy [20].
The use of CD44 in distinguishing benign from malignant papillary breast lesions has been evaluated in several studies. Saddik and Lai [2] studied 11 intraductal papillomas of the breast and 10 papillary carcinoma cases. Their results showed that 100 % of intraductal papillomas of the breast showed staining to CD44s of greater than 70 % while 80 % of papillary carcinomas showed staining of less than 10 %, and they concluded that the stain was “useful to distinguish” between the two pathologic entities [2]. Another study looked at 101 intraductal papillomas and 59 papillary carcinomas of the breast in 2005[4]. Their results demonstrated that 45 % of ductal papillomas were positive for CD44s, as opposed to only 8 % of the papillary carcinomas, a significant difference, and concluded that CD44 may be useful as an adjunct stain to differentiate [4]. Finally, Troxell et al. [3] studied 39 intraductal papillomas, 3 atypical papillomas, 25 carcinomas arising from ductal papillomas, and 28 papillary carcinomas of the breast. Their results showed that 79 % of intraductal papillomas were positive for CD44s as compared to 42 % of the carcinomas, but due to the high rate of positivity in the carcinoma group they concluded that CD44 was not helpful in differentiating between the two [3].
A separate study evaluated CD44s staining in sinonasal papillomas when compared to invasive squamous cell carcinoma. Ingle et al. evaluated 76 cases of sinonasal inverted papillomas (SIP), 2 cases of SIP with carcinoma-in situ, and 10 cases of SIP with frank invasive SCCA [1]. They found that 100 % of the SIP and the SIP with carcinoma-in situ were positive for the stain, while a loss of CD44 expression was found in the SIP with SCCA group (60 % negative, 40 % weakly positive) [1]. They concluded that for SCCA arising in SIP, loss of CD44 was associated with carcinoma development [1].
Bahar et al. [5] evaluated a different variant of CD44, CD44v6, with intraoral lesions. They studied 10 cases of oral epithelial hyperplasia, 8 cases of oral squamous papilloma, 19 cases of oral epithelium with dysplasia, and 38 cases of oral SCCA using the staining of normal oral epithelium as a benchmark for staining level. While epithelial hyperplasia stained compatible to normal epithelium and squamous papillomas stained comparable or stronger to oral epithelium, 80 % of dysplastic lesions and 100 % of SCCA showed loss of expression to CD44v6 [5]. They concluded that CD44v6 might be useful as a progression marker of oral dysplastic and invasive lesions [5]. An earlier study by Herold-Mende et al. evaluated multiple splice variants of CD44 in normal oral epithelium, dysplasia, and oral squamous cell carcinoma and found downregulation of certain variants (CD44v7,v8, and v10) with the maintenance of others (CD44v5 and v6) in the cases of squamous cell carcinoma [31]. They found no alteration of CD44v expression in dysplastic oral epithelium [31].
Based on the previous studies, the expected outcome of this study was that the salivary ductal papillomas would be positive for CD44, and a loss of expression would be seen in the papillary SCCA cases. The results were contrary to these expectations, but distinctive staining patterns with CD44 did emerge between the two groups. The salivary ductal papillomas showed weak or moderately positive staining, but the papillary SCCA cases all stained moderately to strongly positive with the antibody. In addition, in most cases, staining was limited to the basal and lower levels of the ductal papillomas similar to normal control oral epithelium but in the majority of papillary SCCA, staining of CD44 extended throughout the entire thickness of the lesion. The level of staining intensity of the papillary SCCA in this study was more compatible with that reported by Bahar et al. for oral squamous papillomas than that reported for most types of invasive carcinoma in the sinonasal and breast locations previously studied as well [1–5]. No prior studies evaluating the expression of CD44 in papillary SCCA as compared to other variants of oral SCCA could be found in a search of the literature.
This study was initiated after encountering in our biopsy service a case of papillary SCCA invading the submandibular salivary duct which appeared so well-differentiated that it mimicked a very cellular intraductal or inverted ductal papilloma with extremely minimal mucous cell differentiation. This case was also stained for CD44 and showed diffuse and strong (“3”) membraneous staining throughout the lesion. This case is illustrated in Fig. 7. In this case, staining with CD44 proved a useful adjunct to confirm the diagnosis of papillary SCCA.
Fig. 7.
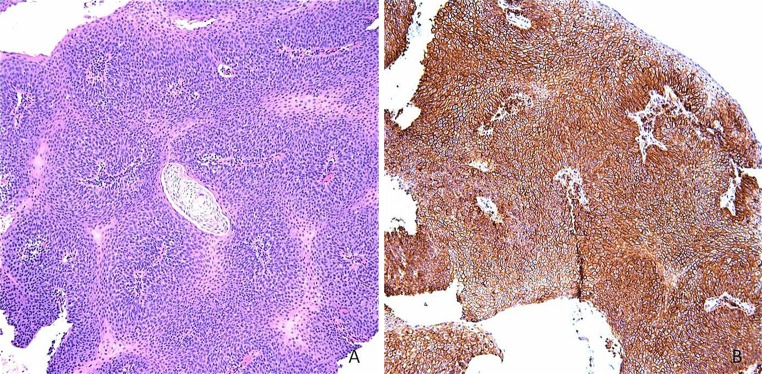
a Special case of papillary SCCA invading the submandibular salivary duct showing a very cellular papillary proliferation with little atypia (hematoxylin and eosin magnification ×10). b Staining for CD44 demonstrates strong and diffuse membraneous expression (CD44 immunostain magnification ×10)
Conclusion
We hypothesize that it is the well differentiated nature of papillary SCCA that causes the strongly positive staining pattern for CD44. Given the somewhat better prognosis that has been generally reported for the papillary form of SCCA, the staining pattern may be a reflection of this. The results of this study do not corroborate the findings of lower CD44 expression in carcinomas and thereby do not aid in differentiating salivary ductal papillomas and papillary SCCA. However, the findings may help to illustrate better the extremely well differentiated nature of papillary SCCA in comparison to other forms of invasive oral SCCA, especially the potential retention of cell-adhesion properties in this tumor as demonstrated by the strong expression of CD44. This may support to the view that papillary SCCA is a distinct very well differentiated variant of SCCA with a much better prognosis as observed in many cases [32, 33].
Acknowledgments
Special thanks to Ms. Elaine Dooley, University of Florida Pathology Laboratory, for immunohistochemical staining, slide preparation, and explanation of the technical processes.
References
- 1.Ingle R, Jennings TA, Goodman ML, Pilch BZ, Bergman S, Ross JS. CD44 expression in sinonasal inverted papillomas and associated squamous cell carcinoma. Am J Clin Pathol. 1998;109:309–314. doi: 10.1093/ajcp/109.3.309. [DOI] [PubMed] [Google Scholar]
- 2.Saddik M, Lai R. CD44s as a surrogate marker for distinguishing intraductal papilloma from papillary carcinoma of the breast. J Clin Pathol. 1999;52:862–864. doi: 10.1136/jcp.52.11.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Troxell ML, Masek M, Sibley RK. Immunohistochemical staining of papillary breast lesions. Appl Immunohistochem Mol Morphol. 2007;15:145–153. doi: 10.1097/01.pai.0000210420.45869.f4. [DOI] [PubMed] [Google Scholar]
- 4.Tse GMK, Tan P-H, Ma TKF, Kilks CB, Poon CSP, Law BKB. CD44s is useful in the differentiation of benign and malignant papillary lesions of the breast. J Clin Pathol. 2005;58:1185–1188. doi: 10.1136/jcp.2005.026906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahar R, Kunishi M, Kayada Y, Yoshiga K. CD44 variant 6 (CD44v6) expression as a progression marker in benign, premalignant, and malignant oral epithelial tissues. Int J Oral Maxillofac Surg. 1997;26:443–446. doi: 10.1016/S0901-5027(97)80010-0. [DOI] [PubMed] [Google Scholar]
- 6.Ellis GL, Auclair PL. Benign epithelial neoplasms. In: Silverberg SG, Sobin LH, editors. AFIP atlas of tumor pathology: tumors of the salivary glands. Washington, DC: American Registry of Pathology; 2008. pp. 49–171. [Google Scholar]
- 7.Gomes APN, Sobral APV, Loducca SVL, de Araujo VC. Sialadenoma papilliferum: immunohistochemical study. Int J Oral Maxillofac Surg. 2004;33:621–624. doi: 10.1016/j.ijom.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 8.Ubaidat MA, Robinson RA, Belding PJ, Merryman DJ. Sialadenoma papilliferum of the hard palate: report of 2 cases and immunohistochemical evaluation. Arch Pathol Lab Med. 2001;125:1595–1597. doi: 10.5858/2001-125-1595-SPOTHP. [DOI] [PubMed] [Google Scholar]
- 9.de Sousa SO, Sesso A, de Araujo NS, de Araujo VC. Inverted ductal papilloma of minor salivary gland origin: a report of 19 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2001;92:68–77. doi: 10.1067/moe.2001.115978. [DOI] [PubMed] [Google Scholar]
- 10.Koutlas IG, Jessurun J, Iamaroon A. Immunohistochemical evaluation and in situ hybridization in a case of oral inverted ductal papilloma. J Oral Maxillofac Surg. 1994;52:503–506. doi: 10.1016/0278-2391(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa T, Imada S, Ijuhin N. Intraductal papilloma of the anterior lingual salivary gland: case report and immunohistochemical study. Int J Oral Maxillofac Surg. 1993;22(2):116–117. doi: 10.1016/S0901-5027(05)80816-1. [DOI] [PubMed] [Google Scholar]
- 12.Tomonao A, Kishino M, Masuda T, Isomura ET, Tanaka S, Namikawa M, Iida S. Intraductal papilloma arising from sublingual minor salivary gland: case report and immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:e34–e37. doi: 10.1016/j.tripleo.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Suarez PA, Adler-Storthz K, Luna MA, El-Naggar AK, Abdul-Karim FW, Batsakis JG. Papillary squamous cell carcinomas of the upper aerodigestive tract: a clinicopathologic and molecular study. Head Neck. 2000;22(4):360–368. doi: 10.1002/1097-0347(200007)22:4<360::AID-HED8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 14.Russell JO, Hoschar AP, Scharpf J. Papillary squamous cell carcinoma of the head and neck: a clinicopathologic series. Am J Otolaryngol. 2011;32(6):557–563. doi: 10.1016/j.amjoto.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Colby C, Klein AM. Papillary squamous cell carcinoma of the larynx. Ear Nose Throat J. 2011;90(8):E13–E15. doi: 10.1177/014556131109000817. [DOI] [PubMed] [Google Scholar]
- 16.Zoller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nature. 2011;11:254–267. doi: 10.1038/nrc3023. [DOI] [PubMed] [Google Scholar]
- 17.Ioachim HL, Medeiros LT, editors. Flow cytometry. In: Ioachim’s lymph node pathology. Philadelphia, PA: Lippincott Williams and Wilkins; 2009. p. 48–58.
- 18.Schniederjan SD, Li S, Saxe DF, Lechowicz MJ, Lee KL, Terry PD, Mann KP. A novel flow cytometric antibody panel for distinguishing Burkitt lymphoma from CD10+ diffuse large B-cell lymphoma. Am J Clin Pathol. 2010;133:718–726. doi: 10.1309/AJCP0XQDGKFR0HTW. [DOI] [PubMed] [Google Scholar]
- 19.McKenney JK, Desai S, Cohen C, Amin MB. Discriminatory immunohistochemical staining of urothelial carcinoma in situ and non-neoplastic urothelium: an analysis of cytokeratin 20, p53, and CD44 antigens. Am J Surg Pathol. 2001;25(8):1074–1078. doi: 10.1097/00000478-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Esteban F, Bravo JJ, Gonzales-Moles MA, Bravo M, Ruiz-Avila I, Gil-Montoya JA. Adhesion molecule CD44 as a prognostic factor in laryngeal cancer. Anticancer Res. 2005;25:1115–1122. [PubMed] [Google Scholar]
- 21.Gonzales-Moles MA, Bravo M, Ruiz-Avila I, Esteban F, Rodriguez-Archilla A, Gonzales-Moles S, Arias B. Adhesion molecule CD44 as a prognostic factor in tongue cancer. Anticancer Res. 2003;23(6D):5197–5202. [PubMed] [Google Scholar]
- 22.Ahmed MA, Aleskandarany MA, Rakha EA, Moustafa RZ, Benhasouna A, Nolan C, Green AR, Ilyas M, Ellis IO. A CD44(−)/CD24(+) phenotype is poor prognostic marker in early invasive breast cancer. Breast Cancer Res Treat. 2011 Nov 27. Epub ahead of print. [DOI] [PubMed]
- 23.Sillanpaa S, Anttila MA, Voutilainen K, Tammi RH, Tammi MI, Saarikoski SV, Kosma VM. CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clin Cancer Res. 2003;9(14):5318–5324. [PubMed] [Google Scholar]
- 24.Aaltomaa S, Lipponen P, Ala-Opas M, Kosma V-M. Expression and prognostic value of CD44 standard and variant v3 and v6 isoforms in prostate cancer. Eur Urol. 2001;39:138–144. doi: 10.1159/000052428. [DOI] [PubMed] [Google Scholar]
- 25.Stavropoulos NE, Filliadis I, Ioachim E, Michael M, Mermiga E, Hastazeris K, Nseyo UO. CD44 standard form expression as a predictor of progression in high risk superficial bladder tumors. Int Urol Nephrol. 2001;33(3):479–483. doi: 10.1023/A:1019589923706. [DOI] [PubMed] [Google Scholar]
- 26.Sviatoha V, Tani E, Kleina R, Sperga M, Skoog L. Immunohistochemical analysis of the S100A1, S100B, CD44 and Bcl-2 antigens and the rate of cell proliferation assessed by Ki-67 antibody in benign and malignant melanocytic tumors. Melanoma Res. 2010;20(2):118–125. doi: 10.1097/CMR.0b013e3283350554. [DOI] [PubMed] [Google Scholar]
- 27.Montgomery E, Abraham SC, Fisher C, Robinette Deasel M, Amr SS, Sheikh SS, House M, Lilliemoe K, Choti M, Brock M, Ephron DT, Zahuruk M, Chadburn A. CD44 loss in gastric stromal tumors as a prognostic marker. Am J Surg Pathol. 2004;28:168–177. doi: 10.1097/00000478-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 28.Coppola D, Clarke M, Landreneau R, Weyant RJ, Cooper D, Yousem SA. Bcl-2, p53, CD44, and CD44v6 isoform expression in neuroendocrine tumors of the lung. Mod Pathol. 1996;9(5):484–490. [PubMed] [Google Scholar]
- 29.Jalkanen S, Joensuu H, Soderstrom K-O, Klemi P. Lymphocyte homing and clinical behavior of non-Hodgkin’s lymphoma. J Clin Invest. 1991;87:1835–1840. doi: 10.1172/JCI115205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eisterer W, Bechter O, Hilbe W, van Driel M, Lokhorst HM, Thaler J, Bloem AC, Gunthert U, Stauder R. CD44 isoforms are differentially regulated in plasma cell dyscrasias and CD44v9 represents a new independent prognostic parameter in multiple myeloma. Leuk Res. 2001;25(12):1051–1057. doi: 10.1016/S0145-2126(01)00075-3. [DOI] [PubMed] [Google Scholar]
- 31.Herold-Mende C, Seiter S, Born AI, Patzelt E, Schupp M, Zoller J, Bosch FX, Zoller M. Expression of CD44 splice variants in squamous epithelia and squamous cell carcinomas of the head and neck. J Pathol. 1996;179:66–73. doi: 10.1002/(SICI)1096-9896(199605)179:1<66::AID-PATH544>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Chrissman JD, Kessis T, Shah KV, Fu YS, Stoler MH, Zarbo RJ, Weiss MA. Squamous papillary neoplasia of the adult upper aerodigestive tract. Hum Pathol. 1988;19:1387–1396. doi: 10.1016/S0046-8177(88)80231-4. [DOI] [PubMed] [Google Scholar]
- 33.Thompson LDR, Wenig BM, Heffner DK, Gnepp DR. Exophytic and papillary squamous cell carcinomas of the larynx: a clinicopathologic series of 104 cases. Otolaryngol Head Neck Surg. 1999;120:718–724. doi: 10.1053/hn.1999.v120.a92773. [DOI] [PubMed] [Google Scholar]