Abstract
Damage to one vestibular labyrinth or nerve causes a central tone imbalance, reflected by prominent spontaneous nystagmus. Central adaptive mechanisms eliminate the nystagmus over several days, and the mechanisms underlying this process have received extensive study. The characteristics of vestibular compensation when the tone imbalance is presented gradually or repeatedly have never been studied. We used high-frequency electrical stimulation of semicircular canal afferents to generate a vestibular tone imbalance and recorded the nystagmus produced when the stimulation was started abruptly or gradually and when it was repeatedly cycled on and off. In the acute-onset protocol, brisk nystagmus occurred when stimulation started, gradually resolved within 1 day, and reversed direction when the stimulation was stopped after 1 week. Repeated stimulation cycles resulted in progressively smaller nystagmus responses. In the slow-onset protocol, minimal nystagmus occurred while the stimulation ramped-up to its maximum rate over 12 h, but a reversal still occurred when the stimulation was stopped after 1 week, and repeated stimulation cycles did not affect this pattern. The absence of nystagmus during the 12 h ramp of stimulation demonstrates that central vestibular tone can rebalance relatively quickly, and the reduction in the stimulation-off nystagmus with repeated cycles of the acute-onset but not the slow-onset stimulation suggests that dual-state adaptation may have occurred with the former paradigm but not the latter.
Keywords: vestibular, nystagmus, compensation, adaptation, electrical stimulation
Introduction
The central vestibular system is highly plastic and can adapt to changes in peripheral labyrinthine inputs or changes in visually mediated demands. The former is typically studied with lesions of the labyrinth or vestibular nerve (e.g., Fetter and Zee 1988) that eliminate all input from the affected ear and produce substantial static and dynamic abnormalities in eye movements, posture, and perception (Dieringer 1995). Many studies have focused on vestibular-mediated eye movements as a way to quantify adaptation. Ablative lesions such as labyrinthectomy produce strong spontaneous nystagmus, the static oculomotor abnormality that results from a central imbalance in tonic activity between the vestibular nuclei on the ablated and normal sides (Vidal et al. 1998). The process of vestibular compensation eliminates the spontaneous nystagmus over a period of days, but this form of adaptation has been studied exclusively as a monophasic process that occurs when an acute lesion of the vestibular periphery produces a large, abrupt imbalance in central vestibular tone (Curthoys 2000). This approach mimics the changes that occur clinically after acute peripheral damage (e.g., vestibular neuritis, Halmagyi et al. 2010).
While vestibular deficits can also develop gradually (e.g., with a vestibular schwannoma, Day et al. 2008), the compensation that occurs when a central tone imbalance is introduced gradually has never been explored. Furthermore, it has become clear that the vestibulo-ocular reflex (VOR) can adapt to more than one condition simultaneously and that the appropriate motor responses can be accessed using sensory cues linked to the condition. By tailoring retinal image motion to different contexts, for example, the VOR gain can be differentially adapted for different orbital eye positions (Shelhamer et al. 1992). This context-specific adaptation allows rapid changes in motor output to occur when the demands on the system change abruptly. Since the training that elicits this multi-state adaptation requires repeated switching between two or more sensori-motor conditions, an experimental approach to evaluate the possibility of multi-state adaptation of static vestibular tone has not been available.
It has been observed that high-frequency electrical stimulation of vestibular afferents from one ear closely mimics the static behavioral syndrome (e.g., spontaneous nystagmus, postural instability) that occurs after unilateral vestibular ablation (Vidal et al. 1998; Merfeld et al. 2006). Electrical stimulation, however, allows one to alter central vestibular tone in a controlled and reversible manner that cannot be replicated with standard ablative procedures. Using this approach, an imbalance in central vestibular tone can be introduced gradually or rapidly. Furthermore, since electrical stimulation can be switched on and off, this method allows one to probe the capacity for dual-state adaptation of the vestibular tone balance between the stimulated and unstimulated sides. In the current study, we compared the eye movements elicited by gradual and rapid changes in the central vestibular tone balance produced by electrical stimulation of canal afferents to investigate if the rate at which the tone imbalance develops affects static compensation. We also examined the effects of repeated cycles of electrical stimulation to investigate the capacity for dual-state adaptation of vestibular tone.
Methods
-
General methods and surgical procedures: Most of the methods used in this study have been described in a prior publication (Merfeld et al. 2006) so they will be summarized briefly. The elements of this study that are new will be described in detail. Four mature guinea pigs were used in this study. All experimental protocols were approved by the institutional animal care and use committee and were in accordance with USDA guidelines.
The animals had three separate surgical procedures, each of which was performed under general anesthesia using inhaled isoflurane. For the eye coil surgery, an 11-mm diameter three-turn frontal coil was inserted under the conjunctiva. For the headbolt procedure, the guinea pig’s head was restrained in a stereotaxic frame and four stainless-steel screws heads were surgically inserted beneath the skull roughly equidistant from the bregma. A small fiberglass head bolt was placed between the screws and attached to the skull and screws using dental acrylic. A fiberglass headcap was attached to the headbolt and served to hold the stimulation circuitry and batteries. For the ear electrode surgery, a stimulating electrode was inserted near afferent neurons innervating the left lateral canal. The electrodes were Teflon coated platinum wire, 150 μm in diameter, with about 500 μm of Teflon stripped from the electrode’s tip. The electrodes were positioned with a micromanipulator while pulsatile electrical stimulation (600 pulses-per-second) was provided and when large eye responses were observed without evidence of facial nerve activation, they were fixed in place with dental acrylic (Merfeld et al. 2006). The return electrode was inserted in the ipsilateral temporalis muscle.
-
Stimulation protocols: The ear electrodes were connected to the prosthesis stimulation circuit we have previously described in detail (Gong and Merfeld 2000, 2002). All electrical stimulation consisted of a series of charge-balanced biphasic current pulses that consisted of a 200 μs cathodic pulse, followed by a 200-μs “rest” phase, followed by a 200-μs anodic pulse. The amplitude of the current pulses was determined empirically to maximize eye movement responses without activating facial musculature, and ranged from 50 to 200 μA across animals. Once determined during the initial electrode characterization, the current amplitude was maintained constant for each animal during the course of all experiments. The efficacy of stimulation was assessed by monitoring the amplitude of eye movements evoked by single biphasic current pulses during intervals when the tonic stimulation was turned off. Like our prior studies which used chronic electrical stimulation (e.g., Lewis et al. 2010), the eye movements elicited in this manner remained stable during the experiment. We also monitored the back voltage from the stimulating electrode (Lewis et al. 2010) and this remained stable as well in all the guinea pigs we tested, indicating that the electrodes remained functional throughout the experiment.
Two stimulation protocols were used in this study:- Acute-onset stimulation protocol: One guinea pig had stimulation abruptly turned-on at a rate of 250 pulses-per-second (pps), was maintained in this state for 1 week, and then stimulation was abruptly turned-off and remained off for 1 week. This pattern was repeated such that stimulation was turned-on at the start of weeks 1, 3, and 5, and was turned-off at the start of weeks 2, 4, and 6. Since this protocol is identical to that used in our prior study (Merfeld et al. 2006), the results from this guinea pig were analyzed in combination with data from two of the three guinea pigs in our previous study. Guinea pig G in that study was excluded because it was an extreme outlier, with nystagmus responses two orders of magnitude greater than those recorded in the other two animals (Merfeld et al. 2006, see Fig. 5C). This marked difference is almost certainly due to the experimental preparation in this one guinea pig (e.g., the position of the electrode tip relative to the canal afferents) which rendered its electrical stimulation one thousand times more effective than the other animals, rather than a variation in vestibular physiology.
- Slow-onset stimulation protocol: Three guinea pigs (GP1, GP2, and GP3) were tested with a similar stimulation protocol but when the stimulation was started it increased slowly in a linear manner from 0 to 250 pps over a period of 12 h (e.g., increased at a rate of about 0.35 pps/min for 720 min) and then was maintained at 250 pps for the remainder of the week. At the end of the week the stimulation was abruptly turned-off. Like the abrupt-onset protocol, for these three guinea pigs the electrical stimulation was provided during weeks 1, 3, and 5, and stimulation was off during weeks 2, 4, and 6. During each week of stimulation, the slow-onset protocol provided 96.4 % as many biphasic current pulses as the acute-onset protocol.
-
Data collection and analysis: Eye movements were measured with the animals alert, stationary, and in complete darkness, using a standard Robinson-style search coil system with the head tilted forward to bring the horizontal canals roughly parallel to the earth-horizontal (Curthoys 1975). Horizontal eye position signals were sampled at 5,000 Hz and were digitally differentiated twice to yield eye acceleration. Nystagmus quick phases were identified using an interactive Matlab program using standard acceleration criteria, and all data were reviewed and corrected, if necessary, by an experienced investigator.
In both protocols, at the onset of stimulation, eye movements were recorded in the dark for 30 min. For the acute-onset protocol the stimulation rate was 250 pps throughout this period but for the slow-onset protocol the stimulation rate was 0 at the start and reached only 10.4 pps over this 30 min period. After 30 min the room was illuminated for 10 min (allowing access to retinal slip signals associated with nystagmus) with the guinea pig’s head restrained, and then eye movements were recorded in the dark for another 5 min. In between subsequent periods of data collection, the guinea pigs were returned to their cages and were free to move normally in a lit environment. Eye movements were recorded for 5 min three additional times during the first day of stimulation, and for 5 min after 24 and 32 h, and 5 days of stimulation. The exact same recording schedule was followed after the stimulation was turned-off.
Like prior studies in guinea pigs (Escudero et al. 1993), we choose to quantify the nystagmus response by calculating the rate and cumulative number of fast phases because vestibular slow phases in guinea pigs are scallop-shaped (Merfeld et al. 2006) and hence accurate measures of slow phase velocity are not possible. The nystagmus rate was calculated as the inverse of the time interval between fast phases, and the high-frequency variability of the nystagmus rate was reduced with a low-pass filter (Merfeld et al. 2006). The cumulative number of fast phases was also calculated since it captures both the rate and temporal course of the fast phase response with less variability than the nystagmus rate. While the nystagmus rate was determined for all periods of data collection, the cumulative fast phase count was only calculated for the first 30 min that followed each cycle of abrupt termination of stimulation for both the acute and slow-onset protocol. The cumulative count was not calculated during the first 30 min of stimulation because no quick phases were evoked by the very low rate of stimulation reached during this time frame with the slow-onset protocol, so no quantitative comparison of fast phase counts between the acute- and slow-onset protocols was possible during stimulation-onset.
The standard right-handed sign convention was used for the eye movements, so the positive direction was leftward. Since the left ear was stimulated in each guinea pig, the slow phases during stimulation were directed to the right (negative) and the fast phases were directed to the left (positive). When the stimulation was abruptly terminated, a nystagmus beating in the opposite direction (leftward slow phases, rightward fast phases) was always observed. Fast phases were therefore assigned a value of +1, if they were directed towards the left (the fast phase direction when stimulation was initiated), and −1, if they were towards the right (the fast phase direction when the stimulation was abruptly terminated). Since nystagmus can reverse direction during or after prolonged vestibular stimulation (Hain et al. 1987), we determined the peak value of the cumulative fast phase count that was in the appropriate physiologic (negative) direction after each termination of stimulation. The peak value was often reached prior to the end of the 30 min of data collection.
Data are reported below as mean ± one standard deviation, and all statistical tests were performed using the SigmaStat 3.5 software package.
Results
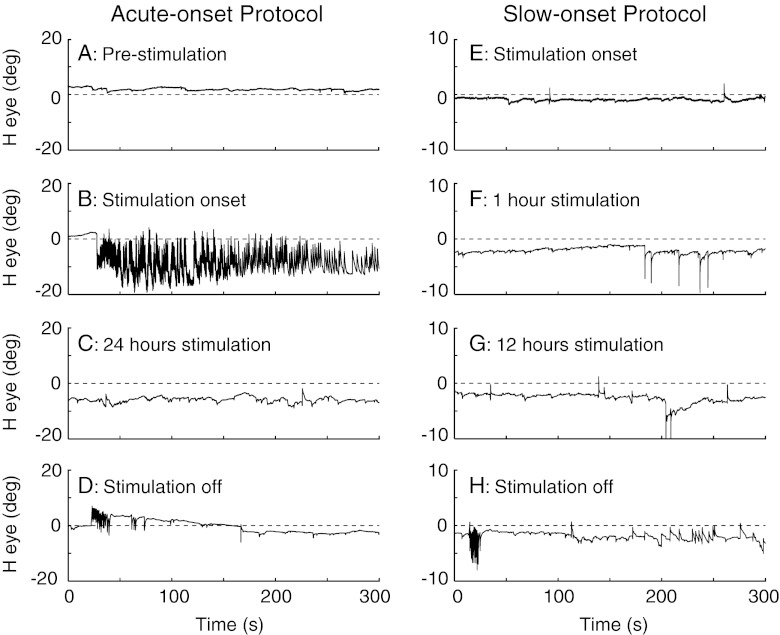
Overview: Figure 1 illustrates eye movement traces from the first cycle of stimulation for the guinea pig tested with the acute-onset protocol and for one guinea pig tested with the slow-onset protocol. For the acute-onset protocol, no nystagmus was recorded prior to stimulation (Fig. 1A) but when the stimulation was acutely started at 250 pps a prominent nystagmus was observed with slow phases contralateral and fast phases ipsilateral to the stimulated left ear (Fig. 1B). Within 1 day of tonic high-frequency stimulation the nystagmus had completely resolved (Fig. 1C), but when the stimulation was acutely turned-off after 1 week, nystagmus beating in the opposite direction (e.g., fast phases contralateral to the stimulated ear) occurred (Fig. 1D). For the slow-onset protocol, almost no fast phases were recorded at any point during the gradual ramp up to the stimulation rate of 250 pps (Fig. 1E–G) or during the subsequent 6.5 days of tonic stimulation at this rate. When the tonic stimulation was abruptly stopped (Fig. 1H), however, a clear burst of nystagmus was observed with fast phases contralateral to the stimulated ear (e.g., in the same direction as the off-response for guinea pig tested with the acute-onset protocol). The eye movement response characteristics for the acute- and slow-onset protocols and the effects of repeated cycles of stimulation are analyzed in detail below.
Stimulation-onset: The nystagmus rates for all three stimulation cycles are shown in Figure 2 for the one guinea pig tested with the acute-onset protocol and for the three guinea pigs tested with the slow-onset protocol. For the acute-onset protocol, the stimulation-on fast phases were positive (towards the stimulated ear) and the nystagmus rate attenuated fairly rapidly and became negligible after about 100 min of tonic stimulation. Compared to the first week of stimulation (week 1 in Fig. 2), when the stimulation was repeatedly cycled between the on and off states, the nystagmus produced by the subsequent on-transitions (weeks 3 and 5) became weaker. This pattern is identical to the responses we previously described (Merfeld et al. 2006) using the same stimulation protocol. For the slow-onset protocol, during both the 12 h ramp-onset and the following 6.5 days of tonic stimulation at 250 pps, there was an almost complete absence of fast phases during the first week of stimulation (week 1) or during each of the two subsequent weeks of stimulation (weeks 3 and 5) in all three guinea pigs (Fig. 2).
-
Stimulation-offset: The nystagmus rates and cumulative fast phase counts for all three stimulation cycles are shown for the four guinea pigs in Figure 3. For the guinea pig tested with the acute-onset protocol, when the stimulation was abruptly terminated the nystagmus beat in the opposite (negative) direction compared to when the stimulation was turned on. For all three off-transitions (weeks 2, 4, and 6), the nystagmus was weaker and resolved faster than it did for the corresponding on-transitions (Fig. 2). The direction of the fast phases also clearly reversed after each of the three off-transitions, as the cumulative counts, which always began as negative, shifted towards the positive after a variable duration (Fig. 3). Similar to our prior study using the same protocol (Merfeld et al. 2006), when the stimulation was repeatedly cycled between the on and off states, the nystagmus produced by the off-transition became progressively weaker. This is most readily observed in the cumulative count plot, which demonstrates that the stimulation-off fast phases that beat contralateral to the stimulated ear (e.g., in the negative direction) were markedly reduced after the first off-transition. The peak (negative) cumulative fast phase count in this guinea pig dropped from 26 (week 2) to 2 (week 6).
For the three guinea pigs tested with the slow-onset protocol, when the stimulation was abruptly turned-off after 1 week of stimulation (Fig. 3, week 2), the nystagmus response was qualitatively similar to the stimulation-off response that followed the first week of acute-onset stimulation. The nystagmus rate was generally smaller and resolved more rapidly with the slow-onset protocol, as reflected in the smaller peak (negative) cumulative counts for the slow-onset guinea pigs following the first off-transition (Fig. 3, week 2). Like the acute-onset guinea pig, two of the slow-onset animals had a delayed reversal of the nystagmus direction after the first off-transition (GP1 reversed after about 800 s and GP3 after about 200 s). During the subsequent off-transitions (weeks 4 and 6), GP1 showed a modest reduction in the nystagmus rate and the peak (negative) cumulative fast phase count (which declined from 22 to 18 for week 6 compared to week 2). The other two guinea pigs, conversely, showed an enhancement of the stimulation-off nystagmus with repeated stimulation cycles, with the peak (negative) cumulative fast phase count increasing from 6 to 17 in GP2 and 15 to 27 in GP3 for week 6 compared to week 2.
-
Comparison of the stimulation-off transitions in the two protocols: Since essentially no nystagmus was generated during stimulation with the slow-onset protocol, quantitative comparison of the two protocols must focus on the nystagmus responses that occurred when the stimulation was abruptly terminated. A benefit of this approach is that the stimulation-off response, commonly referred to as an after-effect, is considered to be a specific feature of central adaptation (see further discussion below). Figure 4 plots the average (and standard deviation) of the peak cumulative fast phase count for the two protocols for all three stimulation-off transitions. The three acute-onset guinea pigs (black icons) consist of the one animal in the current study and the two prior guinea pigs described in Merfeld et al. 2006, while the three slow-onset guinea pigs (grey icons) are the three animals in the current study.
There are two notable differences between the two sets of animals: (a) for the first stimulation-off transition, significantly more fast phases occurred with the acute-onset protocol than with the slow-onset protocol (t test, T4 = 2.91, P = 0.04); and (b) the acute-onset guinea pigs showed a substantial reduction in the peak cumulative fast phase count for sequential stimulation-off transitions. Despite the small number of animals and the variation in responses between guinea pigs, the fast phase count was negatively correlated with the off-transition number for the acute-onset protocol (Pearson correlation, r = −0.68, P = 0.046). In contrast, for the guinea pigs tested with the slow-onset protocol, the fast phase count was statistically independent of the number of off-transitions (Pearson correlation: r = 0.41, P = 0.28).
FIG. 1.
Horizontal eye position plotted against time for the first cycle of the acute-onset and slow-onset protocols. For the acute-onset protocol, the panels show 300 s segments before the stimulus was started (A), when it was first turned-on (B), after 1 day of tonic stimulation (C), and when the stimulation was first turned-off after 1 week of tonic stimulation (D). For the slow-onset protocol, the panels show 300 s segments during the first 5 min of stimulation (E, stimulation ramping from 0 → 1.7 pulses-per-second [pps]), after 1 h of stimulation (F, 20.8 → 22.5 pps), after 12 h of stimulation (G, tonic stimulation at 250 pps), and when the stimulation was abruptly turned-off after 1 week of stimulation (H). The right-handed sign convention was used in this and all subsequent figures; positive values are leftward eye movements.
FIG. 2.
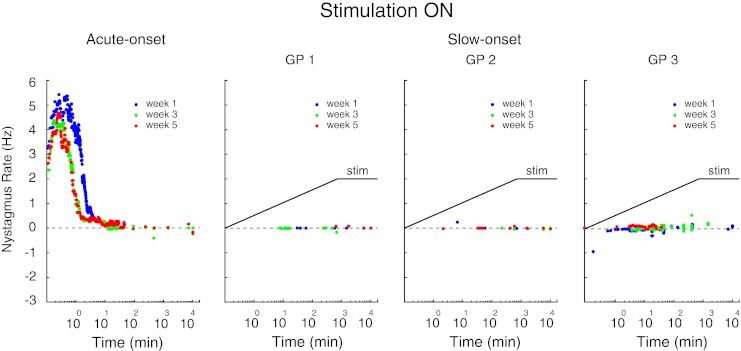
The nystagmus rate for the acute-onset guinea pig and for the three slow-onset guinea pigs (GP1, GP2, GP3) for each of the three stimulation-on transitions (weeks 1, 3, 5). The nystagmus rate is shown for the entire week of data collection in the stimulation-on state. For the slow-onset protocol, the rate of stimulation (stim) is shown schematically by the solid line: the stimulation rate increased linearly for the first 12 h of each stimulation cycle and then remained constant at 250 pps for the remainder of the week (note that for simplicity the stimulation rate is shown as a linear ramp even though time on the x axis is plotted on a log scale).
FIG. 3.
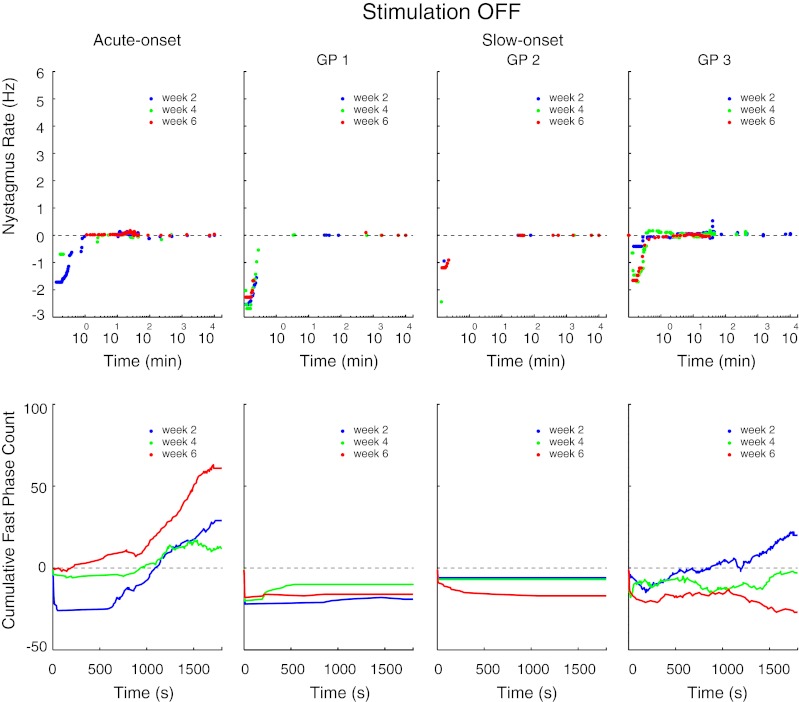
The nystagmus rate and cumulative fast phase count for each of the three stimulation-off transitions (weeks 2, 4, 6), in the acute-onset guinea pig and the three slow-onset guinea pigs. The nystagmus rate is shown for the entire week of data collection that followed each acute termination of stimulation but the cumulative count is only shown for the first 30 min following each off-transition.
FIG. 4.
The peak cumulative fast phase count that followed each off-transition. Black icons are the means for the three acute-onset guinea pigs (including the new guinea pig and the two prior guinea pigs studied with this protocol) and gray icons are the means for the three slow-onset guinea pigs. Error bars are one standard deviation.
Discussion
Vestibular adaptation and habituation: Before we discuss our results in detail, it is helpful to consider the characteristics of vestibular adaptation and habituation, since both of these processes could potentially contribute to our findings. Adaptation is defined as a form of associative learning that minimizes an error signal, such as the tone imbalance between the two vestibular nuclei complexes or the nystagmus produced by this tone imbalance (Welch 1978; Cohen et al. 1992; Quinn 1998). If adaptation reduced the error signal while the stimulus was present, the error signal reverses when the stimulus is removed, resulting in a reversal of the behavioral error. This reversal, termed an “after-effect,” is characteristic of adaptation and does not occur with non-adaptive mechanisms such as habituation (Welch 1978; Kandel et al. 2000). Habituation, in contrast, is defined as a non-associative reduction in sensitivity to a sensory stimulus that occurs after exposure to the stimulus (Welch 1978; Cohen et al. 1992; Kandel et al. 2000). As discussed below, in our experiments both adaptation and habituation most likely contributed to the nystagmus response during stimulation, while adaptation was responsible for the nystagmus reversal when stimulation was stopped.
First stimulation cycle—evidence for adaptation and habituation: When electrical stimulation was first introduced abruptly with the acute-onset protocol, a brisk nystagmus was generated but attenuated quickly and reversed direction when the stimulation was stopped 1 week later. The reversal is clear evidence that adaptation contributed to the resolution of nystagmus during stimulation. When the stimulation was introduced gradually with the slow-onset protocol, essentially no nystagmus occurred as the stimulation rate increased to 250 pps over 12 h or while it was maintained at that rate for the first week. This indicates that the brain was able to maintain a central balance in vestibular tone between the stimulated and un-stimulated sides when the high-frequency stimulation was introduced more gradually. The finding that a high rate of tonic afferent stimulation could be achieved over 12 h without generating a meaningful nystagmus response is surprising, as it implies that central vestibular tone could be continually rebalanced as stimulation from one labyrinth increased at a rate of 20.8 pps/h. The presence of an after-effect when the stimulation was abruptly stopped indicates that adaptation did occur with the slow-onset protocol, but it is notable that the after-effect was significantly smaller with this protocol compared to the acute-onset protocol (see Fig. 4, first off-transition). One possible explanation for this observation is that habituation was more prominent with the slow-onset protocol than with the acute-onset protocol during the first week of stimulation.
-
Repeated stimulation cycles—evidence for dual-state adaptation: As we previously observed (Merfeld et al. 2006), the magnitude of the nystagmus produced by the abrupt on- and off-transitions with the acute-onset protocol became progressively smaller for subsequent cycles of stimulation. In contrast, the nystagmus produced by the abrupt off-transitions with the slow-onset protocol did not change with subsequent stimulation cycles. Although the first stimulation-off response was smaller for the slow-onset protocol, the third stimulation-off response was not larger (Fig. 4). These results can be interpreted in two alternate ways. First, it seems probable that some feature of the large tone imbalance that was recurrently introduced with acute-onset protocol resulted in a form of dual-state adaptation (Shelhamer et al. 1992). This implies that the brain learned to use a cue related to the abrupt change in central vestibular tone to switch between two adapted states (stimulation-on and stimulation-off) to compensate in part for these changes. This would explain the reduction in nystagmus produced by the on- and off-transitions with the acute-onset protocol since the brain was repeatedly exposed to a large central tone imbalance at stimulation-onset and offset. In contrast, the slow-onset protocol did not introduce a large asymmetry in central vestibular tone during the periods of stimulation (evidenced by an absence of nystagmus during the ramp up to the tonic stimulation rate) but only at stimulation-offset, and a similar reduction in the off-responses did not occur with this protocol.
A second potential explanation, which appears less likely, is that these results are due to habituation or other non-adaptive mechanisms (Courjon et al. 1987; Balter et al. 2004; Tykocinski et al. 1995). By approximately matching the total amount of stimulation provided by the two protocols while changing the dynamics of stimulus-onset, we sought to control for the possibility that the decreases in stimulation-on and stimulation-off nystagmus observed with the acute-onset protocol were due to a progressive non-adaptive reduction in sensitivity to the electrical stimulation. Our results, however, suggest that more habituation was engendered during the first stimulation cycle with the slow-onset than the acute-onset protocol (given the smaller after-effect with the former, Fig. 4). We therefore cannot exclude the possibility that the first slow-onset stimulation cycle produced the maximal habituation possible. In this scenario, the amount of habituation (which is inversely correlated with the size of the after-effect) would not change with subsequent stimulation cycles. The acute-onset protocol, conversely, produced less habituation with the first stimulation cycle and it remains possible that the reduction in the after-effect with subsequent stimulation cycles reflects a progressive increase in habituation rather than a form of dual-state adaptation. This explanation seems improbable for two reasons. First, it is unlikely that maximal habituation would occur with the first cycle of the slow-onset stimulation such that no further habituation was possible with subsequent stimulation cycles. Second, this explanation predicts that the size of the after-effect produced by the acute- and slow-onset protocols should converge at the same value, after maximal habituation has been achieved by repetitions of the acute-onset protocol. Our results suggest, however, that the after-effect produced by the acute-onset protocol may have become smaller than the after-effect produced by the slow-onset protocol by the third off-transition (Fig. 4).
-
Possible neuronal mechanisms: The mechanisms responsible for compensation of the static abnormalities that occur after peripheral ablation have received extensive attention and appear to be multi-factorial and complex (reviewed in Darlington and Smith 2000). In particular, changes in synaptic efficacy (e.g., Ris and Godaux 1998a, b; Berquest et al. 2008; Lim et al. 2010) and intrinsic neuronal excitability (reviewed in Beraneck and Idoux 2012) appear to contribute to rebalancing central tone after peripheral ablation. Although the electrical stimulation model of central tone imbalance differs substantially from the ablative model (Lewis et al. 2010), it is interesting that similar changes in synaptic efficacy and intrinsic neuronal excitability can be observed after electrical stimulation of vestibular afferents (Pettorossi et al. 2011; Gittis and du Lac 2006). These observations suggest that, in a general sense, the types of processes that contribute to the central rebalancing of vestibular tone in both the stimulation and ablation models may be qualitatively similar, although certainly substantial differences in the pattern and distribution of these processes would be expected.
The mechanisms underlying dual-state adaptation remain unknown but it has been suggested that the cerebellum may play an important role in this process (Thach 1996) and there is some experimental evidence supporting this contention (Lewis and Tamargo 2001; Norris et al. 2011). Since the cerebellum clearly contributes to the vestibular compensation that minimizes the static behavioral deficits after peripheral labyrinthine ablation (Beraneck et al. 2008; Johnston et al. 2002), a reasonable hypothesis is that the dual-state component of the vestibular tone adaptation that probably occurred in this study was mediated in some manner by the cerebellum. More generally, it may be that small differences in vestibular tone (as provided by the slow-onset protocol) can be corrected in the brainstem without cerebellar involvement, but that cerebellar mechanisms are recruited when a large tone imbalance is acutely introduced (as occurs with peripheral ablation and with the acute-onset protocol).
Future directions: Recording activity in peripheral afferents and central vestibular neurons would provide very useful information in future studies, as it could help determine the extent and location of the adaptation and habituation that occurred with these protocols. In particular, it would help define the extent that reduced sensitivity of primary afferents contributes to the nystagmus responses with both stimulation protocols. Based on prior work, irregular afferents may be preferentially activated with the low stimulation rates provided early in the slow-onset protocol (Goldberg et al. 1984). Since irregular fibers have more phasic responses than regular fibers (Goldberg and Fernandez 1971), this could potentially explain why the slow-onset protocol produced nystagmus patterns that appear to reflect a greater reduction in the sensitivity of peripheral afferents (and therefore smaller after-effects) than the acute-onset protocol. Measuring the VOR elicited by head rotations at different times during the stimulation paradigms could also be useful. Since the canal stimulation was tonic and not modulated by head motion, however, it is likely that reduction in spontaneous nystagmus is the principal adaptive change engendered in this situation, and this would be predicted to reduce, rather than augment the VOR gain during tonic stimulation (Lewis et al. 2010).
Acknowledgments
We thank S. Fukuda, M. Saginaw, and J-P Guyot. This work was supported by the Geneva Charity Foundation “Valeria Rossi di Montelera” and the Swiss Foundation for Fellowships in Medicine and Biology (PASMP3-123225) in collaboration with the Swiss National Science Foundation (K. Nicoucar); by the National Institute of Deafness and Other Communication Disorders Grants DC-6909 and DC-8362 to R.F. Lewis and DC-8167 to D.M. Merfeld; and by the European Commission contract 225929 to D.M. Merfeld.
Contributor Information
Richard F. Lewis, Phone: +1-617-5733501, FAX: +1-617-5734154, Email: Richard_lewis@meei.harvard.edu
Keyvan Nicoucar, Email: Keyvan_nicourcar@meei.harvard.edu.
Wangsong Gong, Email: Wangsong_gong@meei.harvard.edu.
Csilla Haburcakova, Email: Csilla_haburckova@meei.harvard.edu.
Daniel M. Merfeld, Email: Dan_merfeld@meei.harvard.edu
References
- Balter SG, Stokroos RJ, Eterman RM, Paredis SA, Orbons J, Kingma H. Habituation to galvanic vestibular stimulation. Acta Otolaryngol. 2004;124:941–945. doi: 10.1080/00016480410017350. [DOI] [PubMed] [Google Scholar]
- Beraneck M, Idoux E. Reconsidering the role of neuronal intrinsic properties and neuromodulation in vestibular homeostasis. Front Neurol. 2012;3:1–13. doi: 10.3389/fneur.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beraneck M, McKee JL, Aleisa M, Cullen KE. Asymmetric recovery in cerebellar-deficient mice following unilateral labyrinthectomy. J Neurophysiol. 2008;100:945–958. doi: 10.1152/jn.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berquest F, Ludwig M, Dutia MB. Role of the commissural inhibitory system in vestibular compensation in the rat. J Physiol. 2008;586:4441–4452. doi: 10.1113/jphysiol.2008.155291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen H, Cohen B, Raphan T, Waespe W. Habituation and adaptation of the vestibuloocular reflex: a model of differential control by the vestibulocerebellum. Exp Brain Res. 1992;90:526–538. doi: 10.1007/BF00230935. [DOI] [PubMed] [Google Scholar]
- Courjon JH, Precht W, Sirkin DW. Vestibular nerve and nuclei unit responses and eye movement responses to repetitive galvanic stimulation of the labyrinth in the rat. Exp Brain Res. 1987;66:41–48. doi: 10.1007/BF00236200. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. The orientation of the semicircular canals in guinea pigs. Acta Otolaryngol. 1975;80:197–205. doi: 10.3109/00016487509121319. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. Vestibular compensation and substitution. Curr Opin Neurol. 2000;13:27–30. doi: 10.1097/00019052-200002000-00006. [DOI] [PubMed] [Google Scholar]
- Darlington CL, Smith PF. Molecular mechanisms of recovery from vestibular damage in mammals: recent advances. Prog Neurobiol. 2000;62:313–325. doi: 10.1016/S0301-0082(00)00002-2. [DOI] [PubMed] [Google Scholar]
- Day AS, Wang CT, Chen CN, Young YH. Correlating the cochlearvestibular deficits with tumor size of acoustic neuroma. Acta Otolaryngol. 2008;128:756–760. doi: 10.1080/00016480701749240. [DOI] [PubMed] [Google Scholar]
- Dieringer N. ‘Vestibular compensation’: neural plasticity and its relations to functional recovery after labyrinthine lesions in frogs and other vertebrates. Prog Neurobiol. 1995;46:97–129. [PubMed] [Google Scholar]
- Escudero M, de Waele C, Vibert N, Berthoz A, Vidal PP. Saccadic eye movements and the horizontal vestibulo-ocular and vestibule-collic reflexes in the intact guinea pig. Exp Brain Res. 1993;97:254–262. doi: 10.1007/BF00228694. [DOI] [PubMed] [Google Scholar]
- Fetter M, Zee DS. Recovery from unilateral labyrinthectomy in rhesus monkey. J Neurophysiol. 1988;59:370–393. doi: 10.1152/jn.1988.59.2.370. [DOI] [PubMed] [Google Scholar]
- Gittis AH, du Lac S. Intrinsic and synaptic plasticity in the vestibular system. Curr Opin Neurobiol. 2006;16:385–390. doi: 10.1016/j.conb.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Fernandez C. Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. III. Variations among units in their discharge properties. J Neurophysiol. 1971;34:676–684. doi: 10.1152/jn.1971.34.4.676. [DOI] [PubMed] [Google Scholar]
- Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- Gong W, Merfeld DM. Prototype neural semicircular canal prosthesis using patterned electrical stimulation. Ann Biomed Eng. 2000;28:572–581. doi: 10.1114/1.293. [DOI] [PubMed] [Google Scholar]
- Gong W, Merfeld DM. System design and performance of a unilateral horizontal semicircular prosthesis. IEEE Trans Biomed Eng. 2002;49:175–181. doi: 10.1109/10.979358. [DOI] [PubMed] [Google Scholar]
- Hain TC, Fetter M, Zee DS. Head-shaking nystagmus in patients with unilateral peripheral vestibular lesions. Am J Otolaryngol. 1987;8:36–47. doi: 10.1016/S0196-0709(87)80017-0. [DOI] [PubMed] [Google Scholar]
- Halmagyi GM, Weber KP, Curthoys IS. Vestibular function after acute vestibular neuritis. Restor Neurol Neurosci. 2010;28:37–46. doi: 10.3233/RNN-2010-0533. [DOI] [PubMed] [Google Scholar]
- Johnston AR, Seckl JR, Dutia MB. Role of the flocculus in mediating vestibular nucleus neuron plasticity during vestibular compensation in the rat. J Physiol. 2002;545:903–911. doi: 10.1113/jphysiol.2002.024281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E, Kupferman I, Iverson S. Learning and memory. In: Kandel E, Schwartz J, Jessel T, editors. Principals of neural science. New York: McGraw-Hill; 2000. pp. 1227–1246. [Google Scholar]
- Lewis RF, Tamargo RJ. Cerebellar lesions impair context-dependent adaptation of reaching movements in primates. Exp Brain Res. 2001;138:263–267. doi: 10.1007/s002210100719. [DOI] [PubMed] [Google Scholar]
- Lewis RF, Haburcakova C, Gong W, Makary C, Merfeld DM. Vestibuloocular reflex adaptation investigated with chronic motion-modulated electrical stimulation of semicircular canal afferents. J Neurophysiol. 2010;103:1066–1079. doi: 10.1152/jn.00241.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Callister RJ, Brichta AM. An increase glycinergic quantal amplitude and frequency during early vestibular compensation in the mouse. J Neurophysiol. 2010;103:16–24. doi: 10.1152/jn.91223.2008. [DOI] [PubMed] [Google Scholar]
- Merfeld DM, Gong W, Morrissey J, Saginaw M, Haburcakova C, Lewis RF. Acclimation to chronic constant-rate peripheral stimulation provided by a vestibular prosthesis. IEEE Trans Biomed Eng. 2006;53:2362–2372. doi: 10.1109/TBME.2006.883645. [DOI] [PubMed] [Google Scholar]
- Norris SA, Hathaway EN, Taylor JA, Thach WT. Cerebellar inactivation impairs memory of learned prism gaze-reach calibrations. J Neurophysiol. 2011;105:2248–2259. doi: 10.1152/jn.01009.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettorossi VE, Dieni CV, Scarduzio M, Grassi S. Long-term potentiation of synaptic response and intrinsic excitability in neurons of the rat medial vestibular nuclei. Neuroscience. 2011;187:1–14. doi: 10.1016/j.neuroscience.2011.04.040. [DOI] [PubMed] [Google Scholar]
- Quinn KJ. Classical conditioning using using vestibular reflexes. J Vestib Res. 1998;8:117–132. doi: 10.1016/S0957-4271(96)00179-6. [DOI] [PubMed] [Google Scholar]
- Ris L, Godaux E. Spike discharge regularity of vestibular neurons in labyrinthectomized guinea pigs. Neurosci Lett. 1998;253:131–134. doi: 10.1016/S0304-3940(98)00631-4. [DOI] [PubMed] [Google Scholar]
- Ris L, Godaux E. Neuronal activity in the vestibular nuclei after contralateral or bilateral labyrinthectomy in the alert guinea pig. J Neurophysiol. 1998;80:2352–2367. doi: 10.1152/jn.1998.80.5.2352. [DOI] [PubMed] [Google Scholar]
- Shelhamer M, Robinson DA, Tan HS. Contex-specific adaptation of the gain of the vestibulo-ocular reflex in humans. J Vestib Res. 1992;2:89–96. [PubMed] [Google Scholar]
- Thach WT. Context-response linkage. Int Rev Neurobiol. 1996;41:599–611. doi: 10.1016/S0074-7742(08)60372-4. [DOI] [PubMed] [Google Scholar]
- Tykocinski M, Shepard RK, Clark G. Reduction in excitability of the auditory nerve following electrical stimulation at high stimulus rates. Hear Res. 1995;88:124–142. doi: 10.1016/0378-5955(95)00108-G. [DOI] [PubMed] [Google Scholar]
- Vidal P-P, de Waele C, Vibert N, Muhlethaler M. Vestibular compensation revisited. Otolaryngol Head Neck Surg. 1998;119:34–42. doi: 10.1016/S0194-5998(98)70171-8. [DOI] [PubMed] [Google Scholar]
- Welch R. Perceptual modification: adapting to altered sensory environments. New York: Academic; 1978. [Google Scholar]