Abstract
Acute otitis media (AOM) is a rapid infection of middle ear due to bacterial or viral invasion. The infection commonly leads to negative pressure and purulent effusion in the middle ear. To identify how these changes affect tympanic membrane (TM) mobility or sound transmission through the middle ear, we hypothesize that pressure, effusion, and structural changes of the middle ear are the main mechanisms of conductive hearing loss in AOM. To test the hypothesis, a guinea pig AOM model was created by injection of Streptococcus pneumoniae. Three days post inoculation, vibration of the TM at umbo in response to input sound in the ear canal was measured at three experimental stages: intact, pressure-released, and effusion-drained AOM ears. The vibration of the incus tip was also measured after the effusion was removed. Results demonstrate that displacement of the TM increased mainly at low frequencies when pressure was released. As the effusion was removed, the TM mobility increased further but did not reach the level of the normal ear at low frequencies. This was caused by middle ear structural changes or adhesions on ossicles in AOM. The structural changes also affected movement of the incus at low and high frequencies. The results provide new evidence for understanding the mechanism of conductive hearing loss in AOM.
Keywords: acute otitis media, conductive hearing loss, middle ear pressure, middle ear effusion, laser vibrometer
Introduction
Acute otitis media (AOM) is the most frequently diagnosed illness in children in the United States and the leading indication for antibiotics therapy in children (Hoberman et al. 2011). AOM is characterized by a rapid onset of middle ear infection, and the signs and symptoms of AOM usually include a hyperemic tympanic membrane (TM), purulent middle ear effusion, and purulent otorrhea (Bluestone and Klein 1983A; Shaikh et al. 2011). AOM is commonly associated with reduction of TM mobility as a result of middle ear pressure and middle ear effusion (Bluestone and Klein 1983B).
Middle ear fluid and abnormal pressure are also commonly observed in another type of otitis media—otitis media with effusion, which causes serous effusion without infection in the middle ear. Effects of otitis media with effusion on middle ear function have been well studied in human temporal bones (Dai et al. 2008, 2007; Gan et al. 2006; Ravicz et al. 2004) and animal models (Dai and Gan 2008; Guan and Gan 2011; Qin et al. 2010; Turcanu et al. 2009). It is generally accepted that the middle ear fluid decreases the mobility of TM mainly at high frequencies due to increased mass on the TM, and middle ear fluid also causes TM mobility loss at low frequencies due to reduction of middle ear air space. Several studies have investigated the effect of positive and negative pressure on middle ear function (Dai et al. 2008; Gan et al. 2006; Lee and Rosowski 2001; Murakami et al. 1997; Petrova et al. 2006; Rosowski and Lee 2002). In human temporal bone studies, Gan et al. (2006) and Dai et al. (2008) showed that the positive or negative middle ear pressure mainly reduced the TM movement at low frequencies, which agrees with the results in human temporal bones reported by Murakami et al. In a guinea pig otitis media with effusion model, Dai and Gan (2008) reported that the middle ear under-pressure caused reduction of TM displacement mainly at frequencies less than 2 kHz.
Previous studies have revealed mechanisms of TM mobility loss associated with fluid or pressure in the middle ear cavity. However, a quantitative evaluation of the effects of the pressure and effusion on TM mobility in AOM ear has not been reported yet. In addition to pressure and effusion, infection-related middle ear changes may also affect TM mobility. For example, purulent effusion induced by the infection in AOM can be more viscous than serous middle ear effusion (Carrie et al. 1992; Yagi et al. 1986). Yagi et al. measured viscosity of middle ear effusion from 110 patients and reported that there was a close relation between the viscosity values and gross appearance of the effusion (serous less than seropurulent less than purulent). Although it has been reported that the viscosity of middle ear fluid was not correlated with the TM mobility loss (Jeselsohn et al. 2005; Marsh et al. 1985; Ravicz et al. 2004; Wiederhold et al. 1980), the effects of the purulent effusion on TM movement have not been examined in living AOM ears. Additionally, bacteria can form surface-attached communities on the mucosal layer of the middle ear cavity during the infectious process of AOM (Ehrlich et al. 2002; Hoa et al. 2009; Reid et al. 2009), which promotes formation of purulent adhesions in narrow spaces of the cavity such as the round window niche (Caye-Thomasen et al. 1996). The adhesions can form between ossicles and surrounding bony wall and cause fibrous fixation of the TM or ossicular chain (Caye-Thomasen et al. 1996; Caye-Thomasen and Tos 2000; von Unge et al. 1997). Furthermore, the infection of AOM can induce pathological changes of the TM, and thus, the mechanical properties of the TM in AOM are different from that in normal ears (Larsson et al. 2003; von Unge et al. 1993, 1997). TM property changes may also impact its mechanical response to input sound. How these different mechanisms contribute to sound transmission in AOM ears needs further studies. We hypothesize that the middle ear pressure, effusion, and infection-induced structural changes are the main mechanisms associated with TM mobility loss in AOM ears.
As a continuation of our previous study of otitis media in guinea pigs, in this paper, we report the recent study on AOM ears by measuring TM vibration at the umbo at three experimental stages in a guinea pig AOM model: (1) the intact or original AOM ear with middle ear pressure and effusion, (2) pressure-released, and (3) effusion-drained ear. After the middle ear effusion was removed, the vibration of the incus tip was also measured. The vibrations of the TM and incus tip in response to sound stimuli were measured by a laser Doppler vibrometer (LDV). The results quantify the effects of the middle ear pressure, effusion, and TM or ossicular changes on sound transmission through the AOM ear.
Methods
Animal Preparation
Twelve Hartley guinea pigs weighing between 300 and 400 g were included in this study. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma and met the guideline of the National Institutes of Health. The animals were free from middle ear disease as evaluated by otoscopy examination.
The animals were divided into AOM and control groups. The AOM group includes eight animals and the control group includes four animals. The AOM model was created by injection of Streptococcus pneumoniae type 3 (Sp3) (ATTC 6303, Manassas, VA, USA) suspension into the left middle ear (N = 8) following the procedure reported by Dai et al. (2009). S. pneumoniae is one of the most common bacteria found in AOM effusion (Gould and Matz 2010). It has been reported that the intrabullar injection of Sp3 successfully induced AOM symptoms in small rodents such as gerbils (Larsson et al. 2003; von Unge et al. 1997) and rats (Spratley et al. 2002). Briefly, under general anesthesia, skin of the mastoid area was disinfected with povidone-iodine and a transbullar injection of 0.15–0.2 ml Sp3 suspension at a concentration of 2.6–4.5 × 107 CFU/ml was performed using a 1-cc syringe with 26-gauge needle under a surgical microscope. The right ear was untreated but was not used as control since cross-infection may occur. After inoculation, the animal was examined by an otoscope and body temperature was monitored twice a day.
Three days postinoculation, the animal was anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg). Additional anesthesia was administered, as required, to maintain areflexia. The TM of the inoculated ear (left ear) was examined under a microscope and then surgery was performed before tympanometry and TM vibration measurement (see section below). In control animals, the experiment was conducted bilaterally (N = 8). For both control and AOM groups, body temperature of the animal was maintained throughout the experiment at approximately 38 °C by placing the animal on heating blanket. The animal was placed in prone position during the measurement.
Experimental Protocol
In order to place the sound source close to the TM, a surgery was performed to remove the pinna and the most cartilaginous part of the ear canal. A laser reflective tape (0.2 × 0.2 mm2, <0.01 mg, 3 M, St. Paul, MN, USA) was placed on the center of the lateral surface of the TM over the umbo as the laser target for measuring TM vibration (see “Laser Vibrometry Measurement”).
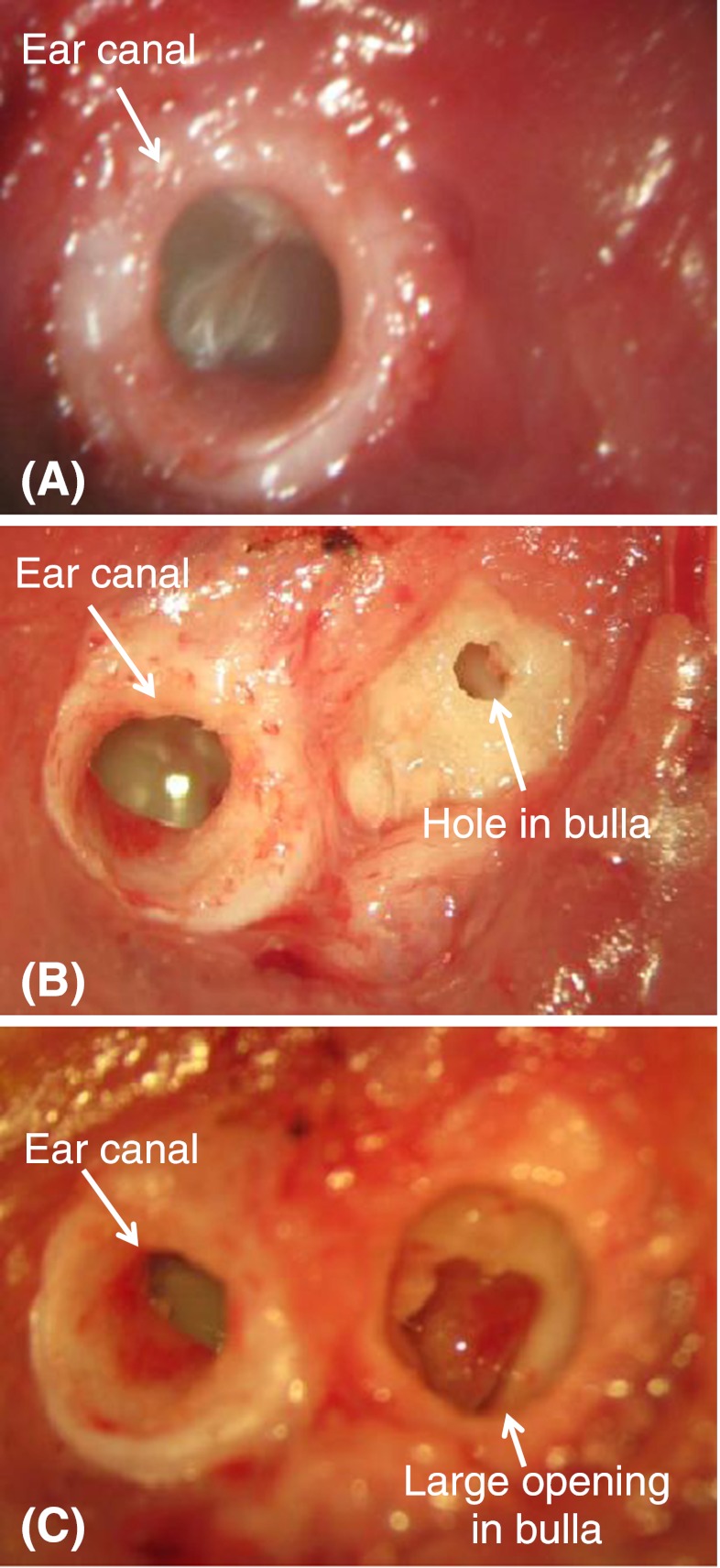
In the AOM ears, middle ear pressure in the intact bulla was measured by using a multiple-frequency tympanometer at frequency of 1 kHz (Maico MI 24, Maico Diagnostics, Eden Prairie, MN, USA) before vibration measurement. Then, measurement of TM vibration was performed in three stages of the experiment: AOM-1, intact bulla containing middle ear pressure and effusion; AOM-2, pressure released from the middle ear; and AOM-3, effusion drained from the middle ear. Figure 1 shows the surgical preparation in these three stages from one AOM ear of guinea pig. Figure 1A displays the lateral view of intact bulla for AOM-1 experiment. The sound delivery tube was placed on the bony canal wall and the movement of the TM at the umbo was measured.
FIG. 1.
Surgical approaches of the three experimental stages in AOM ear: A AOM-1, intact bulla; B AOM-2, middle ear pressure released from the hole in the bulla; C AOM-3, middle ear effusion drained from the opening in the bulla.
Upon completion of the vibration measurement in AOM-1, the posterior bony wall of the bulla was exposed and a hole of 1 mm diameter was drilled at about 3–4 mm from the ear canal on the posterior–superior area of the bulla to release the middle ear pressure as shown in Figure 1B. The AOM-2 experimental stage of measuring the TM movement at the umbo was then conducted, and the small hole was sealed using dental cement (PD-135, Pac-Dent, CA, USA) before the measurement. The possibility of middle ear pressure buildup due to anesthesia is addressed at the end of this section. After the completion of the vibration measurement in AOM-2, the hole was opened and enlarged to about 3 mm in diameter as shown in Figure 1C. A tube made of silicone (1 mm of outer diameter) was inserted deeply into the middle ear cavity through the hole. The other end of the tube was connected to a 1-cc syringe. The middle ear effusion was then aspirated manually from the cavity into the syringe. The aspiration usually started at the anterior–inferior portion of the cavity and ended at the posterior–inferior. During the draining process, the middle ear cavity was inspected frequently under a microscope through the opening of the bulla until almost no fluid remained in the tympanic cavity. The amount of the effusion in each AOM ear was recorded.
Note that some dense purulence was commonly found on the ossicles or between the ossicles and cavity bony walls in AOM ears. Those ossicular adhesions remained in the middle ears after completing aspiration of the effusion. In order to examine the effect of the adhesions on ossicular movement, the pus around the incus-stapedial joint was removed. A reflective tape was placed on the tip of the incus that was the lateral surface of the most ventral point on the incus long process for measuring the vibration of incus tip. It was difficult to access the stapes in guinea pig, thus the ossicular movement was measured at the incus tip (Guan and Gan 2011). Note that the incus was not able to be accessed in AOM-1 and AOM-2 experiments. The opening in the bulla was then covered by a thin glass sheet, and the gap between the glass sheet and bony wall was sealed by dental cement. Then, the AOM-3 experiment of measuring the vibration at the TM and incus tip was performed. After all experimental measurements were completed, the bulla was harvested and widely opened to allow a microscopy examination of the middle ear ossicles and cavity.
In control ears, the ear canal was prepared the same as that in AOM ears. To exclude the effect of possible nonzero middle ear pressure in healthy ear due to anesthesia (Guinan and Peake 1967) and to expose the tip of the incus long process, a hole of approximately 3 mm diameter was drilled at about 3–4 mm from the ear canal on the posterior–superior side of the bulla. The location of the bulla hole in control ears was comparable to its location in inoculated ears. The reflective tape was placed on the umbo and the incus tip, respectively. The opening in the bulla was covered by a thin glass sheet and the gap between the glass sheet and the bulla was sealed by dental cement. Vibrations of the TM and incus tip were measured by laser vibrometry as described in the next section. Middle ear pressure measurement was not performed in the control ears, since any unbalanced pressure had been released in the preparation.
Laser Vibrometry Measurement
The experimental setup for measuring movements of the TM and tip of incus was similar to that reported by Guan and Gan (2011). Briefly, 80-dB sound pressure level (SPL) pure tones over the frequency range of 0.2–40 kHz, including 20 points per octave from a function generator (Signal Analyzer, HP 35670A, CA, USA), power amplifier (Type 2718 B&K, Denmark), and speaker (CF1 Tucker-Davis Technologies, Alachua, FL, USA) were presented into the ear canal at about 4 mm away from the umbo through a sound delivery tube. The tones were played sequentially from 200 to 40 kHz for 50 cycles at each tone. The probe microphone (Model 4938 B&K, Denmark, frequency up to 100 kHz) was used to monitor the input SPL. The probe tubes of the microphone and speaker were integrated into the sound delivery tube that was placed in the ear canal and sealed to the canal wall with the dental cement. The tip of the probe microphone tube was positioned approximately 2 mm from the umbo. One end of the sound delivery tube was covered by a transparent glass sheet.
Movements of the TM and incus tip induced by sound stimuli in the ear canal were measured by a laser vibrometer (Polytec CLV-2534, Tustin, CA, USA). The output scaling factor of the laser vibrometer was set at 2 mm/s/V. The velocity amplitude and phase measurements from the laser vibrometer were sent to the signal analyzer and recorded on a personal computer. The peak-to-peak displacements (dp-p in micrometers) of the TM and incus were calculated from the voltage output of the laser vibrometer velocity decoder by 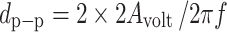 , where Avolt is the amplitude of vibrometer output (velocity) in volts, f is the frequency of pure tone in kilohertz, a factor of 2 is from the LDV sensitivity, and another factor of 2 converts amplitude to peak-to-peak displacement. The reference of the laser targets' velocity phase (umbo or incus tip) was the pure tone stimuli in the ear canal. To derive the displacement phase, the velocity phase was reduced by 90 °. Note that the phase data reported in Guan and Gan (2011) were velocity phase.
, where Avolt is the amplitude of vibrometer output (velocity) in volts, f is the frequency of pure tone in kilohertz, a factor of 2 is from the LDV sensitivity, and another factor of 2 converts amplitude to peak-to-peak displacement. The reference of the laser targets' velocity phase (umbo or incus tip) was the pure tone stimuli in the ear canal. To derive the displacement phase, the velocity phase was reduced by 90 °. Note that the phase data reported in Guan and Gan (2011) were velocity phase.
In the measurement on the TM, the direction of the laser beam was adjusted so that it was approximately normal to the lateral surface over the umbo. In the measurement on the incus tip, the angle between the laser beam and the axis of stapes piston motion was approximately 40–50 °. The detailed methods to evaluate the angles were described in Guan and Gan (2011). Since the angle correction for the ossicular chain may only be valid at low frequencies (Chien et al. 2006), no correction factor was applied in the displacement data of the incus tip, which was the same as the incus tip measurement by Guan and Gan (2011).
The surgery preparation for each ear usually took 15–20 min. The tympanometry took about 2 min and the laser measurement at each experimental stage usually took about 5 min. The TM vibration data for the intact bulla (AOM-1) were collected within 30 min after anesthesia. For control and other experimental stages of AOM ear, the TM and incus vibration data were obtained within 5 min after resealing the opening on the bulla. Therefore, no significant amount of middle ear pressure would build before the laser measurement.
Statistical Analysis
In this study, the laser measurements were conducted in two animal populations: AOM and control. In AOM group, the TM vibration at umbo was measured at three experimental stages from the same population. Therefore, at each tested frequency, one-way repeated-measures analysis of variance (ANOVA) test was used to analyze the changes of TM displacement at all three AOM stages, followed by Turkey test (post hoc test) between the different AOM stages.
To statistically compare the TM or incus displacement between control and AOM-3, an unpaired Student's t test (equal sample size, two sided) was used because the control group and AOM group were independent. The statistical analysis was performed using Prism software (GraphPad, La Jolla, CA, USA). The p values of post hoc test were reported as inequalities in the software.
Results
Evaluation of AOM Model
The TM was first observed under a microscope to identify the AOM symptoms. Figure 2A shows a typical control guinea pig TM, which was usually translucent, and Figure 2B shows the TM in an AOM ear, which was commonly hyperemic and opaque. In this study, all eight AOM ears had purulent effusion and the color of the effusion was white or yellow. The volume of the middle ear effusion in the AOM ears ranged from 0.06 to 0.14 ml with a mean value of 0.09 ± 0.03 ml (SD, standard deviation). Note that the middle ear air space of a normal guinea pig is about 0.2–0.25 ml (Guan and Gan 2011). It was observed that the purulent effusion was more viscous compared with saline or serous effusion in guinea pig otitis media with effusion model reported by Dai and Gan (2008). However, in this study, we did not measure the viscosity of the effusion in the AOM ears.
FIG. 2.
Microscopic photographs of A control eardrum, B AOM eardrum, C middle ear cavity and ossicles in control ear (right ear), and D middle ear cavity in ear of AOM-3 (left ear). IS-joint incudo-stapedial joint, RW round window.
The middle ear pressure in the intact AOM ear was assessed by the tympanogram and determined as the pressure when middle ear compliance reached a peak value. Two AOM ears had a flat tympanogram, in which the compliance over the negative pressure range was slightly greater than that over the positive pressure range. The pressure in those two ears seemed to be negative but the exact value could not be determined. In other AOM ears, the middle ear pressures were all negative with a mean value of −162 dPa (±40 dPa, SD).
The middle ear cavity was examined at the end of the experiment. In all control ears, there were no signs of middle ear inflammation, and the middle ear structure was clearly identified as shown in Figure 2C. In AOM ears, it is noted that the middle ear effusion had been removed before the microscopic examination. In all eight AOM ears, purulent adhesions were formed between the ossicles and the middle ear bony wall. The adhesions were commonly observed between the manubrium and promontory, around the oval and round window niches, and in the small cavity that contained the malleus–incus complex as shown in Figure 2D. Those adhesions were formed during the infectious process and remained on the ossicles after aspiration of the middle ear fluid.
TM Mobility Change in AOM Ear
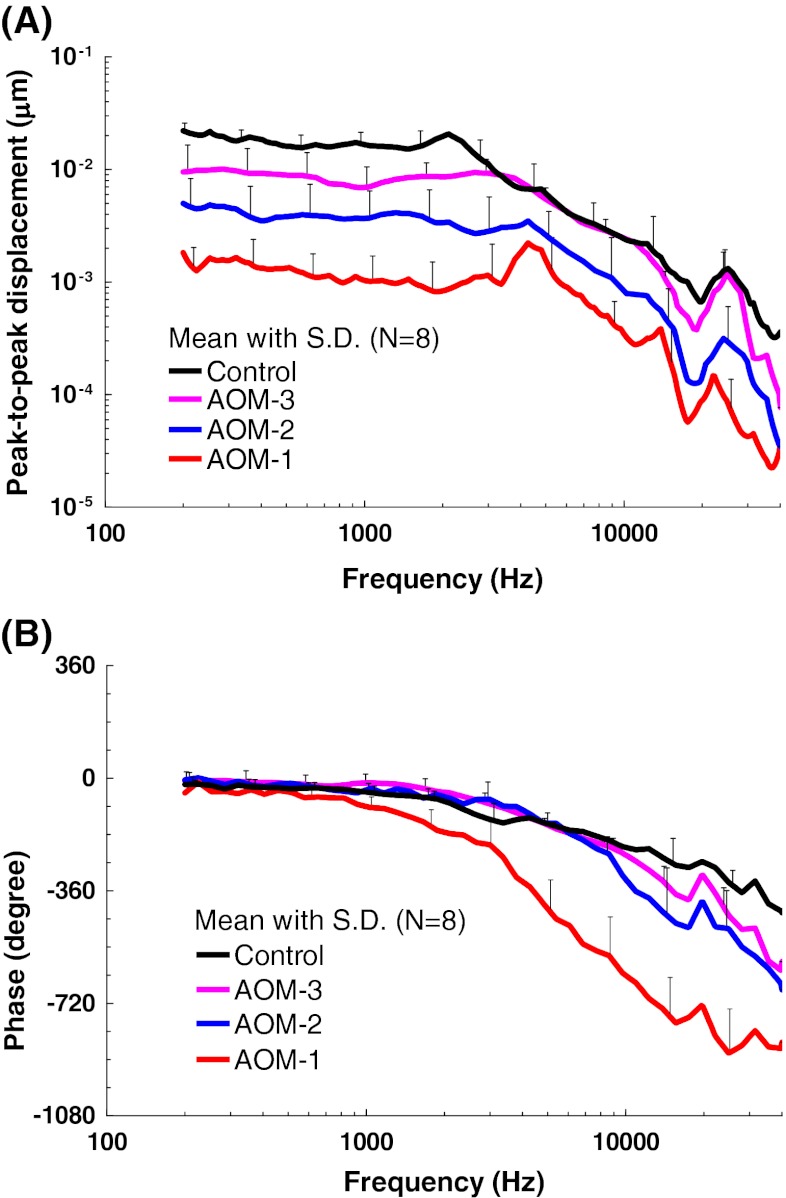
Figure 3 shows the individual and mean curves of the TM displacement at the umbo in response to 80-dB SPL pure tones over frequencies of 0.2–40 kHz measured from eight AOM and eight control ears. Note that in each AOM ear, the TM movement was recorded at three experimental stages. The upper panel displays the frequency–response curves of the magnitude of the peak-to-peak TM displacement. The displacement phase curves are shown in the lower panel.
FIG. 3 .
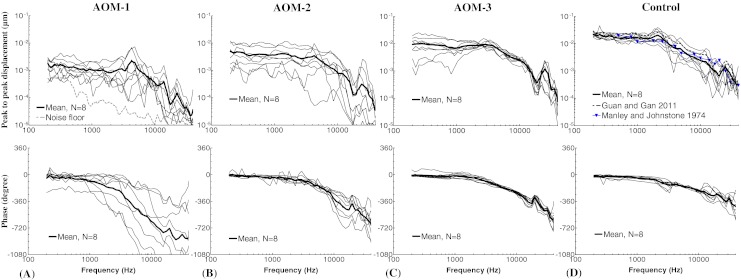
Peak-to-peak displacement magnitude (upper panel) and phase angle (lower panel) of the TM at umbo in response to 80 dB SPL sound input at the ear canal in A AOM-1, B AOM-2, C AOM-3, and D control ears. The solid lines represent the mean curves (N = 8); the dotted lines represent the individual curves; the gray dashed line represents the noise floor of the laser measurement; the black dashed line represents the TM displacement magnitude of normal guinea pig ears; the blue line with triangles represents the TM displacement magnitude of normal guinea pig ears. (Guan and Gan 2011; Manley and Johnstone 1974).
TM Displacement in AOM-1
The bulla was intact at the experimental stage of AOM-1, and the TM movement was affected by a combination of the middle ear pressure, effusion, and structural changes of the ossicles. Figure 3A shows the TM displacement at umbo measured in AOM-1. The mean TM magnitude plateaued from 0.2 to 3 kHz with a value of 0.001–0.0015 μm, peaked at 4.3 kHz, and decreased at higher frequencies. A second peak was observed between 22 and 25 kHz. The noise level of LDV measurement was plotted in Figure 3A as well. As shown in Figure 3A, the TM displacement magnitude in AOM-1 was at least 10 dB greater than the noise level for most tested frequencies (except two individual measurements at 0.2–0.4 kHz and 15–40 kHz). Considering that the TM mobility in AOM-1 was the lowest compared to the other ear conditions, the LDV measurement in this study should be reliable.
As shown in the lower panel of Figure 3A, the mean phase gradually decreased from 0.2 to 1 kHz and dropped fast after 1 kHz. It is noted that there was a large variance in both TM magnitude and phase among individuals in AOM-1. The variations in AOM-1 may be caused by different middle ear conditions among individuals because the middle ear condition in AOM-1 was more variable than those in AOM-2 and AOM-3. The middle ear pressure, effusion, and ossicular adhesions all varied from ear to ear.
TM Displacement in AOM-2
Figure 3B shows the magnitude and phase of TM displacement at umbo measured in AOM-2 after the middle ear pressure had been released. The mean displacement plateaued at 0.2–4 kHz with a value of 0.004–0.003 μm and decreased after 4 kHz as frequency increased. A local peak was observed in the mean displacement curve at 25 kHz. There were variations in the TM displacement in AOM-2 due to the different effusion levels and degree of purulent adhesions among individuals. It is noted that there was a correlation between the effusion volume and the reduction of TM displacement. This agreed with the results in studies of human temporal bones (Ravicz et al. 2004; Gan et al. 2006) and guinea pigs (Guan and Gan 2011) in which the middle ear effusion was simulated by saline.
Less individual difference was observed in the phase curves compared to that in AOM-1. The mean phase decreased slowly at 0.2–3 kHz and at a higher rate at frequencies over 3 kHz.
TM Displacement in AOM-3
Figure 3C displays the TM displacement curves measured in AOM-3, where the effusion was removed from the middle ear cavity but purulent adhesions were still present. The variation of TM displacement between animals was mainly at frequencies below 2 kHz. The mean value had a plateau from 0.2 to 3 kHz with a value of about 0.01 μm and decreased at higher frequencies with a local peak at 25 kHz. The mean phase curve of AOM-3 changed with frequency in a similar way to that in AOM-2.
TM Displacement in Control
The TM displacement magnitude and phase measured from control ears are shown in Figure 3D. The mean magnitude plateaued from 0.2 to about 1.6 kHz with a value of 0.015–0.022 μm, reached a small peak at 2 kHz and then decreased as frequency increased. A second peak of the mean curve was at 25 kHz. As shown in Figure 3D, the control TM displacement at umbo in this study was generally consistent with the published data from the normal guinea pig ears (Manley and Johnstone 1974; Guan and Gan 2011). The phase curves of the control ears gradually decreased across the whole frequency range.
Comparison of TM Displacement Between AOM-1, AOM-2, and AOM-3, and Control
The mean TM displacement curves in Figure 3 are displayed in Figure 4 with SD bars for control and three AOM experiments. The statistical analysis results (p values) of TM displacement magnitude data in Figure 4A are listed in Table 1. Note that repeated-measures ANOVA and Turkey post hoc tests were used between the three AOM stages, since the TM displacements were measured from the same population. Unpaired t test was used between the AOM-3 and control ears because the two middle ear conditions were from different populations.
FIG. 4.
Mean (N = 8) peak-to-peak displacement magnitude (A) and phase angle (B) of the TM at umbo with SD in response to 80 dB SPL sound input at the ear canal in AOM-1 (red line), AOM-2 (blue line), AOM-3 (purple line), and control ears (black line).
TABLE 1.
List of P values derived from: (1) one-way repeated-measures ANOVA test and Turkey post hoc test on the TM displacement magnitude data between three AOM stages; (2) unpaired Student's t test on the TM magnitude data between AOM-3 and control

The P values with gray background represent significant difference between groups (P < 0.05). The P values of Turkey post hoc test are shown as inequalities since no exact value is reported by the software
The ANOVA test indicates that there were significant changes of TM displacement among the three AOM stages (see column 2 of Table 1). As shown in Figure 4A and Table 1, the TM displacement of AOM-3 was significantly greater than that of AOM-1 across frequencies of 0.2–40 kHz (see column 3 of Table 1). When pressure in the middle ear was released (AOM-2), the TM displacement at umbo increased significantly at low frequencies (0.2–3 kHz except 0.5 kHz, see column 4 of Table 1). As the effusion was drained from the cavity (AOM-3), the TM displacement showed a significant increase compared to AOM-2 ears over all tested frequencies, except 32 kHz (see column 5 of Table 1). Comparing AOM-3 with control, there was a significant difference at f < 3 kHz but no significant difference at 3–40 kHz, except 32 kHz (see column 6 of Table 1).
Figure 4B shows the mean ± SD of the TM displacement phase measured in control and three AOM experimental stages. There was no significant difference between control and AOMs at frequencies below 500 Hz. The mean TM phase in intact AOM ears (AOM-1) was significantly lower than that in control by up to 400 ° at 3–40 kHz (unpaired Student's t test, p < 0.05). After releasing the pressure (AOM-2), the TM phase generally overlapped with the control at frequencies below 8 kHz and was lower than the control by up to 200 ° at higher frequencies. After effusion was drained (AOM-3), the TM phase was almost the same as that of control at 0.2–8 kHz and was less than the control by up to 150 ° at higher frequencies.
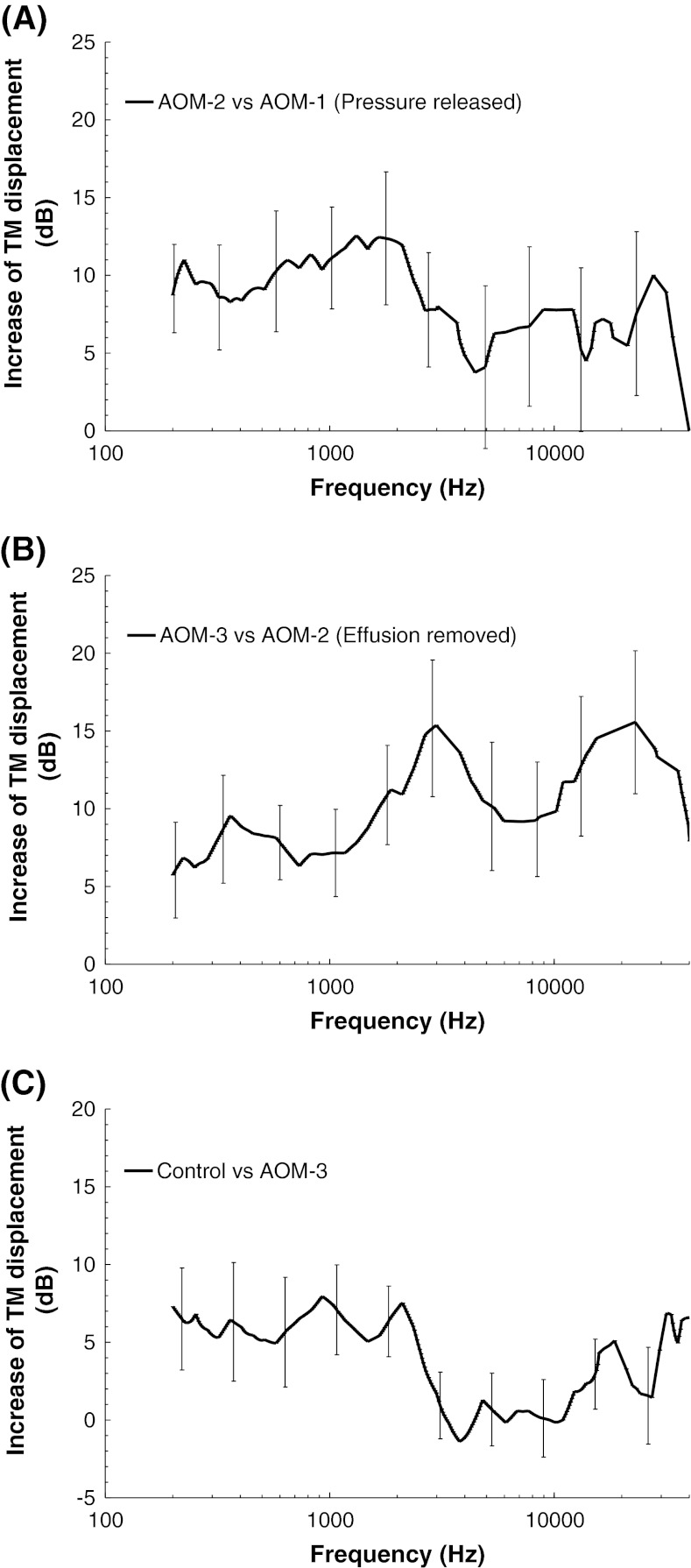
To quantitatively investigate the frequency responses of TM mobility changes caused by the different middle ear factors such as the pressure, effusion, and structural changes, the results in Figure 4A were conveyed as the mean increase of TM displacement (in decibel, with SD) at an experimental stage with respect to the previous experimental stage as shown in Figure 5. Figure 5A displays the increase of TM displacement in pressure-released AOM ears (AOM-2) with respect to the intact ears (AOM-1). An average of 8–12 dB increase of the TM displacement at frequencies below 3 kHz was caused by the middle ear pressure release. An increase of 3–8 dB at higher frequencies was observed after the release of pressure; however, those changes, generally, were not significant due to high variations. Figure 5B shows the TM displacement increase after the middle ear effusion was drained. Removing of fluid resulted in an average of 5–9 dB increase of TM movement at low frequencies (<1 kHz) and 10–15 dB increase at 2–40 kHz. When the TM displacement measured in AOM-3 ears was compared to that in control ears, the displacement of control was higher than that of AOM-3 as shown in Figure 5C. This demonstrates that the middle ear structural changes such as the adhesions of ossicles to the middle ear wall or the mechanical property changes of soft tissues reduced the TM movement. An average of 5–8 dB difference between AOM-3 and control was observed at f < 3 kHz. At high frequencies, except 32 kHz, no significant difference was observed between the two groups.
FIG. 5.
A Mean increase (with SD) of TM displacement at umbo after release of middle ear pressure (AOM-2 vs. AOM-1). B Mean increase of TM displacement after removal of middle ear effusion (AOM-3 vs. AOM-2). C Mean difference of TM displacement between control and AOM-3 (control vs. AOM-3).
It is noted that at low frequencies (f < 2 kHz), the middle ear pressure, generally, had a greater effect on the TM mobility than the effusion and structural changes in the AOM ears. At high frequencies (f > 2 kHz), the effusion demonstrated a greater effect than the middle ear pressure and structural changes.
Incus Tip Mobility in AOM-3 and Control Ears
In addition to displacement measurement at the TM, we did measure the movement of the incus tip in control and AOM-3 ears in response to 80 dB SPL pure tones over 0.2–40 kHz. The analysis of AOM-3 and control incus data was the same as for the TM data. Figure 6 shows the mean curves with SD obtained from the eight control and eight AOM-3 ears. Note that there was still some dense purulence around the middle ear ossicles in AOM-3. An unpaired t test was used for the analysis of the incus displacement data between the control and AOM-3. The magnitude of incus displacement in AOM-3 ears was significantly lower than that in control ears approximately by 8 dB at 0.2–3 kHz and up to 12 dB at 18–40 kHz (Student's t test, p < 0.05). However, there was no significant difference between the control and AOM-3 at frequencies of 3–16 kHz. Figure 6B shows that the mean phase curve of the incus in AOM-3 overlapped with that of the control at 0.2–8 kHz and was less than the control by up to 150 ° at frequencies greater than 8 kHz. The phase of AOM-3 was significantly different from that of control at f > 24 kHz (Student's t test, p < 0.05).
FIG. 6.
Mean (N = 8) peak-to-peak displacement magnitude (A) and phase angle (B) of the incus tip with SD in response to 80 dB SPL sound input at the ear canal in AOM-3 (purple line) and control ears (black line).
Discussion
In this study, the biomechanical changes of the middle ear in AOM were investigated at three stages: intact bulla, middle ear pressure released, and middle ear effusion drained. We measured the TM displacement at umbo in control and three AOM stages. The incus tip movement was measured in control and AOM-3 only. We hypothesize that the TM mobility loss was caused by three factors: middle ear pressure, fluid, and structural changes of the middle ear ossicles and TM. This study has provided evidence to identify the effects from these three factors.
Effect of Middle Ear Pressure on TM Movement
Negative middle ear pressure is frequently found in AOM ears probably due to the oxygen consumption of bacteria and inflammatory cells in the middle ear (Kitaoka et al. 2009). The positive pressure has also been found in other studies (Shaikh et al. 2011). Both negative and positive pressures affected the TM motion as reported by Murakami et al. (1997); Gan et al. (2006), and Dai et al. (2008) in human temporal bones. The middle ear pressure decreases the TM displacement at low frequencies primarily due to an increased stiffness of the TM. As the middle ear pressure exists, the TM is stretched and its stiffness is increased regardless of whether the pressure is negative or positive (Lee and Rosowski 2001).
In this study, the middle ear pressure in the AOM ears was negative, which was one of the main factors that reduced the TM movement in AOM ear. After the pressure was released by drilling a hole in the bulla, the TM displacement at umbo generally increased over 0.2–40 kHz (Figs. 4A and 5A), but significant changes occurred only at low frequencies (f < 4 kHz, see column 4 in Table 1, AOM-1 vs. AOM-2). The stiffness of the TM affects the TM movement at low frequencies (Dai et al. 2008). When the negative pressure was removed from the middle ear, the TM became less tense. As a consequence, the TM mobility at umbo was significantly improved at low frequencies from AOM-1 to AOM-2.
Dai and Gan (2008) created the otitis media with effusion model in guinea pigs and they found that when the middle ear pressure was released, the TM displacement increased at frequencies below 2 kHz, while not much change was observed at higher frequencies (Fig. 6 in their paper). Findings in this study of AOM model agrees with the published results. Though an increase of TM displacement from AOM-1 to AOM-2 at higher frequencies was observed in Figure 4A, and those changes were not statistically significant. The middle ear pressure contributed to the TM mobility loss mainly at low frequencies in AOM ears.
Effect of Middle Ear Effusion on TM Movement
The effect of middle ear fluid on middle ear mechanics has been investigated in human temporal bones and animal models (Ravicz et al. 2004; Gan et al. 2006; Guan and Gan 2011). These studies show that fluid in the middle ear decreases the TM movement mainly at high frequencies. The TM vibration at high frequency is dominated by the mass of the TM; therefore, the fluid behind the TM increases TM mass and reduces its mobility at high frequencies (Ravicz et al. 2004). In this study, the TM displacement at umbo significantly increased at all tested frequencies in AOM ears after the middle ear effusion was removed (Table 1, column 4, AOM-2 vs. AOM-3). The TM displacement changes displayed in Figure 5B reveal that the effusion affected the TM mobility mostly at high frequencies (f > 2 kHz), which is generally consistent with the mechanism of the middle ear fluid reported in the previous studies (Ravicz et al. 2004; Gan et al. 2006; Guan and Gan 2011). We also observed from Figure 5 that the middle ear effusion dominated the reduction of TM movement at high frequencies (10–15 dB) compared to other factors, which suggests that the purulent effusion was the leading cause of TM mobility loss at high frequencies in the AOM ear.
The middle ear fluid can also reduce the TM mobility at low frequencies due to the decrease of the air space in the middle ear cavity (Ravicz et al. 2004). As can be seen in Figure 5B and Table 1 (column 4, AOM-2 vs. AOM-3), a smaller but significant increase of TM mobility did occur at low frequencies after the effusion was drained from the cavity. This was probably due to the restoration of the middle ear air space. Ravicz et al. (2004) reported that the umbo mobility loss at low frequencies could be associated with the reduction of the air space in the middle ear. As a consequence of the aspiration of fluid, the middle ear air space increased and led to the increase of TM mobility at low frequencies. Thus, in general, the middle ear effusion in AOM was the leading factor that contributed to the TM mobility loss at high frequencies, but the effusion also caused reduction of TM mobility at low frequencies.
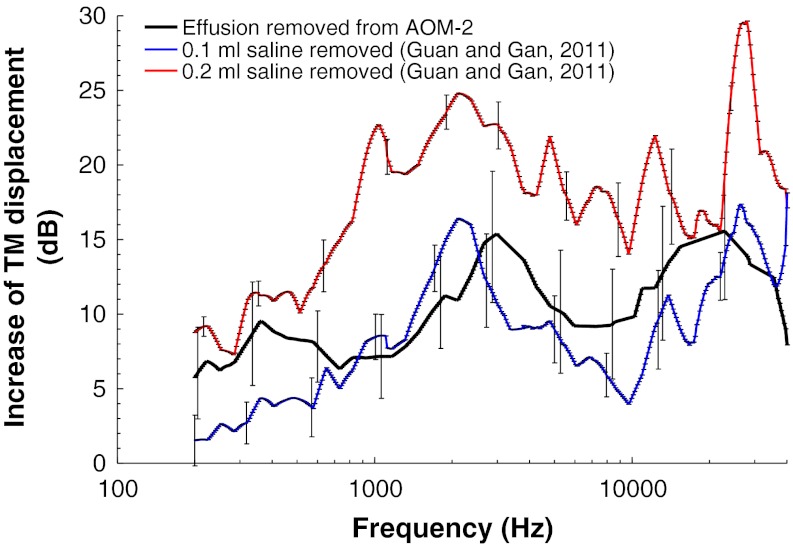
In our previous study on guinea pigs (Guan and Gan 2011), the TM vibration was measured at umbo when the middle ear was injected with saline from 0 to 0.2 ml (filled middle ear cavity). In this study, the middle ear effusion in all AOM ears was purulent type. To investigate whether the purulent effusion in the AOM ears had a distinct effect on TM mobility compared to the saline, the TM displacement increase from AOM-2 to AOM-3 (removing the middle ear effusion) was compared with the previous saline results as illustrated in Figure 7. Saline (0.1 and 0.2 ml) was introduced into the middle ear and the reduction of TM movement was recorded in the previous study, which is equivalent to the increase of TM displacement when the same amount of saline was removed (red and blue lines in Fig. 7). The black line in Figure 7 represents the increase of TM mobility as the effusion was removed from the AOM ears. It is noticed that the mean volume of the effusion in AOM ears was 0.09 ml, not much different from 0.1 ml of saline reported by Guan and Gan (2011). However, the effect of the effusion in AOM ears was greater than that of 0.1 ml saline on TM movement at most tested frequencies. The significant difference was observed at 0.2–0.6, 3–4, and 8–20 kHz (except 12 kHz, unpaired t test, p < 0.05), which suggests that the purulent effusion reduced TM mobility more than saline over certain frequencies in guinea pigs. This might be related to the viscosity difference between the purulent effusion and saline. The purulent effusion seemed more viscous with higher density than saline.
FIG. 7.
Comparison of the mean TM (at umbo) displacement magnitude changes (with SD) between guinea pig ears with purulent effusion, 0.1 ml saline, and 0.2 ml saline. The black line represents the TM displacement increase as the effusion was removed from the AOM-2 ears, a comparison between AOM-2 and AOM-3. The blue line represents the TM displacement increase as 0.1 ml saline was removed from the middle ear and the red line represents the TM displacement increase as 0.2 ml saline was removed from the middle ear. (Guan and Gan 2011).
Although several studies showed that the viscosity of the middle ear effusion had no significant effect on sound transmission in the middle ear (Wiederhold et al. 1980; Ravicz et al. 2004), some contradictory results were found in the literature (Hartley and Moore 2003; Majima et al. 1988). The comparison in Figure 7 suggests that the purulent effusion may result in more TM mobility loss than the same amount of saline. However, it should be noted that the bases of the two compared situations are different. The effect of saline shown in Figure 7 was observed from the normal ears, which is not the same as the structural damaged (AOM-3) or structural damaged plus the effusion (AOM-2) ears. The combination of the structural changes and the effusion may have an additional effect on the reduction of TM mobility. We also noticed that 0.2 ml saline in the middle ear cavity had a greater effect on TM movement than that of the purulent effusion (up to 16 dB). This indicates that there is a significant effect on reduction of TM mobility when the middle ear cavity was filled with fluid.
Effect of Middle Ear Structural Changes on TM Movement
Adhesions on the middle ear ossicular chain have been observed in AOM models of gerbil (von Unge et al. 1997) and rat (Caye-Thomasen et al. 1996; Caye-Thomasen and Tos 2000). Caye-Thomasen and Tos proposed that the middle ear adhesion is a pathological phenomenon associated with AOM. However, the effect of ossicular adhesion on TM vibration has not been reported in the literature. In this study, eight AOM ears, more or less, all had purulent adhesions formed in the narrow spaces between the ossicles and surrounding bony wall, which was a striking structural change of the middle ear in AOM. The comparison between control and AOM-3 ears (Fig. 5C) indicates that a significant difference of TM mobility occurred at frequencies less than 3 kHz, where the TM movement is controlled by the middle ear stiffness. The reduction of TM movement at low frequencies suggested an increase of middle ear stiffness. It is possible that the adhesions in AOM ears could fix the handle of the malleus and other parts of the ossicles to a certain degree and, therefore, increase the overall stiffness of the middle ear. In a clinical study, Rosowski et al. (2008) measured the umbo vibration in patients with malleus and stapes fixation. Their results indicated that significant loss of the umbo mobility occurred at f < 3 kHz in both stapes-fixed and malleus-fixed groups (Fig. 9 in their paper). Therefore, the effect of the ossicular adhesions on TM vibration is similar to that of ossicular fixation, and they both can increase middle ear stiffness.
Another factor that needs to be considered is the TM's own structural changes due to infection. Several histological studies in animal AOM models have shown that the TM became edematous and the thickness was increased at a few days postinoculation (MacArthur et al. 2006; von Unge et al. 1997). Those structural changes can affect stiffness of the TM. In a gerbil AOM model, von Unge et al. (1997) reported that the mechanical stiffness of the TM without middle ear adhesions was reduced at 1–4 days post-Sp3 inoculation compared to control; however, the stiffness of the TM with the adhesions was greater than the control (Fig. 10 in their paper), which agrees with our TM measurements at AOM-3. In our AOM model, it is highly possible that the TM movement in AOM-3 was affected by a combination of the ossicular adhesions and TM structural changes. A reasonable conclusion is that the middle ear adhesion increased the overall middle ear stiffness and contributed to the TM mobility loss mainly at low frequencies in AOM ears. It is noticed that the TM movement at umbo in AOM-3 was significantly different from control at frequencies of 32 kHz (Table 1), and the mechanism causing this difference is not clear. Future studies are needed to differentiate the effect on TM movement between the ossicular adhesion and TM's own structural change.
It is noted that the low frequency response of the TM can be decreased also by reduction of the air space of the middle ear. Based on our observation, the middle ear air space was reduced by 10–20 % of its original volume by the adhesions in AOM-3 ears. Ravicz et al. (2004) reported that a 30 % reduction in the middle ear air space of the human temporal bone resulted in only a 2-dB reduction in umbo mobility at low frequencies. In this study, the TM displacement was reduced by 5–8 dB at 0.2–2 kHz in AOM-3. Therefore, the reduction of the middle ear air space by the adhesions was not likely to be the primary mechanism of the TM mobility loss in AOM-3 ears.
We noticed that the reduction of incus displacement was similar to that of the TM in AOM-3 at most tested frequencies. The TM and incus tip in AOM-3 both had a loss of displacement up to 8 dB at 0.2–3 kHz (Fig. 4A and Fig. 6A). The incus displacement of AOM-3 overlapped with control at 3–10 kHz, which was similar to that observed in the TM displacement. This suggested that the displacement transfer ratio from TM to incus tip in AOM-3 ears was not much different from that in control ears. At frequencies over 10 kHz, the reduction of incus movement in AOM-3 showed a different pattern compared with the TM, which may be related to the different motion of ossicular chain at high frequencies. Several studies in human temporal bones have shown that the motion of stapes at high frequencies became more complex than the simple pistonlike motion at low frequencies (Chien et al. 2006; Hato et al. 2003; Heiland et al. 1999). The complex motion of stapes at high frequencies was also reported in guinea pig (Huber et al. 2008), cat, and gerbil ears (Decraemer et al. 1999). Note that there was a measurement angle of 40–50 ° between the laser beam and the axis of stapes piston motion, and the measurement of incus from one direction may not be able to reveal the ossicular chain movement at high frequencies. More studies are needed to investigate the effects of ossicular adhesions and TM mechanical changes on middle ear transfer function in AOM ears.
Conclusion
The AOM model was successfully created in guinea pigs. The mechanisms of the TM mobility loss in the AOM ears were investigated at three experimental stages: the intact middle ear with pressure and effusion, pressure-released, and effusion-drained ears. The effects of middle ear pressure, effusion, and structural changes were quantified by the changes of TM mobility between the different AOM stages and control ears. The middle ear pressure reduced the TM mobility mainly at low frequencies. The middle ear effusion decreased TM movement at all tested frequencies and was the leading cause of TM mobility loss at high frequencies compared to other mechanisms. The infection-induced adhesions on ossicles provided a plausible explanation for the difference of TM mobility at low frequencies between the control and the AOM ears without pressure and effusion. The effects of the middle ear pressure, effusion, and ossicular adhesions on TM mobility changes were quantified. The results provide new evidence for understanding the mechanism of conductive hearing loss in AOM.
Acknowledgments
The authors thank Don Nakmali at Hough Ear Institute for his expert technical assistance. This work was supported by OCAST (HR09-033) and NIH (R01DC006632 and R01DC011585).
References
- Bluestone CD, Klein JO. Otitis media with effusion, atelectasis, and eustachian tube dysfuncion. In: Bluestone CD, Stool SE, editors. Pediatric otolaryngology. PA: W.B. Saunders; 1983. pp. 419–432. [Google Scholar]
- Bluestone CD, Klein JO. Intratemporal complications and sequelae of otitis media. In: Bluestone CD, Stool SE, editors. Pediatric otolaryngology. Philadelphia: W. B. Saunders Company; 1983. pp. 513–515. [Google Scholar]
- Carrie S, Hutton DA, Birchall JP, Green GG, Pearson JP. Otitis media with effusion: components which contribute to the viscous properties. Acta Otolaryngol. 1992;112(3):504–511. doi: 10.3109/00016489209137432. [DOI] [PubMed] [Google Scholar]
- Caye-Thomasen P, Tos M. Polyp and fibrous adhesion formation in acute otitis media caused by non-typeable or type b Haemophilus influenzae or Moraxella catarrhalis. Acta Otolaryngol. 2000;120(7):810–814. doi: 10.1080/000164800750061651. [DOI] [PubMed] [Google Scholar]
- Caye-Thomasen P, Hermansson A, Tos M, Prellner K. Pathogenesis of middle ear adhesions. Laryngoscope. 1996;106(4):463–469. doi: 10.1097/00005537-199604000-00013. [DOI] [PubMed] [Google Scholar]
- Chien W, Ravicz ME, Merchant SN, Rosowski JJ. The effect of methodological differences in the measurement of stapes motion in live and cadaver ears. Audiol Neurootol. 2006;11(3):183–197. doi: 10.1159/000091815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Gan RZ. Change of middle ear transfer function in otitis media with effusion model of guinea pigs. Hear Res. 2008;243(1–2):78–86. doi: 10.1016/j.heares.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Wood MW, Gan RZ. Tympanometry and laser Doppler interferometry measurements on otitis media with effusion model in human temporal bones. Otol Neurotol. 2007;28(4):551–558. doi: 10.1097/mao.0b013e318033f008. [DOI] [PubMed] [Google Scholar]
- Dai C, Wood MW, Gan RZ. Combined effect of fluid and pressure on middle ear function. Hear Res. 2008;236(1–2):22–32. doi: 10.1016/j.heares.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Li W, Gan RZ (2009) Change of cochlear mechanics in acute otitis media and otitis media with effusion. Association for Research in Otolaryngology (ARO) - Midwinter Meeting, Vol. 32: 984, Baltimore, MD, February 14-19, 2009
- Decraemer W, Khanna S, Funnell W (1999) Measurement and modeling of the three-dimensional vibration of the stapes in cat. Symposium on recent developments in auditory mechanics, Sendai, Japan
- Ehrlich GD, Veeh R, Wang X, Costerton JW, Hayes JD, Hu FZ, Daigle BJ, Ehrlich MD, Post JC. Mucosal biofilm formation on middle-ear mucosa in the chinchilla model of otitis media. JAMA. 2002;287(13):1710–1715. doi: 10.1001/jama.287.13.1710. [DOI] [PubMed] [Google Scholar]
- Gan RZ, Dai C, Wood MW. Laser interferometry measurements of middle ear fluid and pressure effects on sound transmission. J Acoust Soc Am. 2006;120(6):3799–3810. doi: 10.1121/1.2372454. [DOI] [PubMed] [Google Scholar]
- Gould JM, Matz PS (2010) Otitis media. Pediatr Rev 31:102–110 [DOI] [PubMed]
- Guan X, Gan RZ. Effect of middle ear fluid on sound transmission and auditory brainstem response in guinea pigs. Hear Res. 2011;277(1–2):96–106. doi: 10.1016/j.heares.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Jr, Peake WT. Middle-ear characteristics of anesthetized cats. J Acoust Soc Am. 1967;41(5):1237–1261. doi: 10.1121/1.1910465. [DOI] [PubMed] [Google Scholar]
- Hartley DE, Moore DR. Effects of conductive hearing loss on temporal aspects of sound transmission through the ear. Hear Res. 2003;177(1–2):53–60. doi: 10.1016/S0378-5955(02)00797-9. [DOI] [PubMed] [Google Scholar]
- Hato N, Stenfelt S, Goode RL. Three-dimensional stapes footplate motion in human temporal bones. Audiol Neurootol. 2003;8(3):140–152. doi: 10.1159/000069475. [DOI] [PubMed] [Google Scholar]
- Heiland KE, Goode RL, Asai M, Huber AM. A human temporal bone study of stapes footplate movement. Am J Otol. 1999;20(1):81–86. [PubMed] [Google Scholar]
- Hoa M, Syamal M, Sachdeva L, Berk R, Coticchia J. Demonstration of nasopharyngeal and middle ear mucosal biofilms in an animal model of acute otitis media. Ann Otol Rhinol Laryngol. 2009;118(4):292–298. doi: 10.1177/000348940911800410. [DOI] [PubMed] [Google Scholar]
- Hoberman A, Paradise JL, Rockette HE, Shaikh N, Wald ER, Kearney DH, Colborn DK, Kurs-Lasky M, Bhatnagar S, Haralam MA, Zoffel LM, Jenkins C, Pope MA, Balentine TL, Barbadora KA. Treatment of acute otitis media in children under 2 years of age. N Engl J Med. 2011;364(2):105–115. doi: 10.1056/NEJMoa0912254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AM, Sequeira D, Breuninger C, Eiber A. The effects of complex stapes motion on the response of the cochlea. Otol Neurotol. 2008;29(8):1187–1192. doi: 10.1097/MAO.0b013e31817ef49b. [DOI] [PubMed] [Google Scholar]
- Jeselsohn Y, Freeman S, Segal N, Sohmer H. Quantitative experimental assessment of the factors contributing to hearing loss in serous otitis media. Otol Neurotol. 2005;26(5):1011–1015. doi: 10.1097/01.mao.0000185051.69394.01. [DOI] [PubMed] [Google Scholar]
- Kitaoka K, Kaieda S, Takahashi H, Yoshida H, Takasaki K, Kumagami H. Oxygen consumption by bacteria: a possible cause of negative middle ear pressure in ears with otitis media. Acta Otolaryngol Suppl. 2009;562:63–66. doi: 10.1080/00016480902933064. [DOI] [PubMed] [Google Scholar]
- Larsson C, Dirckx JJ, Decraemer WF, Bagger-Sjoback D, von Unge M. Pars flaccida displacement pattern in purulent otitis media in the gerbil. Otol Neurotol. 2003;24(3):358–364. doi: 10.1097/00129492-200305000-00002. [DOI] [PubMed] [Google Scholar]
- Lee CY, Rosowski JJ. Effects of middle-ear static pressure on pars tensa and pars flaccida of gerbil ears. Hear Res. 2001;153(1–2):146–163. doi: 10.1016/S0378-5955(00)00269-0. [DOI] [PubMed] [Google Scholar]
- MacArthur CJ, Hefeneider SH, Kempton JB, Parrish SK, McCoy SL, Trune DR. Evaluation of the mouse model for acute otitis media. Hear Res. 2006;219(1–2):12–23. doi: 10.1016/j.heares.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Majima Y, Hamaguchi Y, Hirata K, Takeuchi K, Morishita A, Sakakura Y. Hearing impairment in relation to viscoelasticity of middle ear effusions in children. Ann Otol Rhinol Laryngol. 1988;97(3 Pt 1):272–274. doi: 10.1177/000348948809700311. [DOI] [PubMed] [Google Scholar]
- Manley GA, Johnstone BM (1974) Middle-ear function in the guinea pig. J Acoust Soc Am 56(2):571–576 [DOI] [PubMed]
- Marsh RR, Baranak CC, Potsic WP. Hearing loss and visco-elasticity of middle ear fluid. Int J Pediatr Otorhinolaryngol. 1985;9(2):115–120. doi: 10.1016/S0165-5876(85)80011-2. [DOI] [PubMed] [Google Scholar]
- Murakami S, Gyo K, Goode RL. Effect of middle ear pressure change on middle ear mechanics. Acta Otolaryngol. 1997;117(3):390–395. doi: 10.3109/00016489709113411. [DOI] [PubMed] [Google Scholar]
- Petrova P, Freeman S, Sohmer H. The effects of positive and negative middle ear pressures on auditory threshold. Otol Neurotol. 2006;27(5):734–738. doi: 10.1097/01.mao.0000226296.28704.de. [DOI] [PubMed] [Google Scholar]
- Qin Z, Wood M, Rosowski JJ. Measurement of conductive hearing loss in mice. Hear Res. 2010;263(1–2):93–103. doi: 10.1016/j.heares.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravicz ME, Rosowski JJ, Merchant SN. Mechanisms of hearing loss resulting from middle-ear fluid. Hear Res. 2004;195(1–2):103–130. doi: 10.1016/j.heares.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Reid SD, Hong W, Dew KE, Winn DR, Pang B, Watt J, Glover DT, Hollingshead SK, Swords WE. Streptococcus pneumoniae forms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis. 2009;199(6):786–794. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Lee CY. The effect of immobilizing the gerbil's pars flaccida on the middle-ear's response to static pressure. Hear Res. 2002;174(1–2):183–195. doi: 10.1016/S0378-5955(02)00655-X. [DOI] [PubMed] [Google Scholar]
- Rosowski JJ, Nakajima HH, Merchant SN. Clinical utility of laser-Doppler vibrometer measurements in live normal and pathologic human ears. Ear Hear. 2008;29(1):3–19. doi: 10.1097/AUD.0b013e31815d63a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaikh N, Hoberman A, Kaleida PH, Rockette HE, Kurs-Lasky M, Hoover H, Pichichero ME, Roddey OF, Harrison C, Hadley JA, Schwartz RH. Otoscopic signs of otitis media. Pediatr Infect Dis J. 2011;30(10):822–826. doi: 10.1097/INF.0b013e31822e6637. [DOI] [PubMed] [Google Scholar]
- Spratley J, Hellstrom S, Eriksson PO, Pais-Clemente M. Early structural tympanic membrane reactions to myringotomy: a study in an acute otitis media model. Acta Otolaryngol. 2002;122(5):479–487. doi: 10.1080/00016480260092264. [DOI] [PubMed] [Google Scholar]
- Turcanu D, Dalhoff E, Muller M, Zenner HP, Gummer AW. Accuracy of velocity distortion product otoacoustic emissions for estimating mechanically based hearing loss. Hear Res. 2009;251(1–2):17–28. doi: 10.1016/j.heares.2009.02.005. [DOI] [PubMed] [Google Scholar]
- von Unge M, Decraemer WF, Bagger-Sjoback D, Dirckx JJ. Displacement of the gerbil tympanic membrane under static pressure variations measured with a real-time differential moire interferometer. Hear Res. 1993;70(2):229–242. doi: 10.1016/0378-5955(93)90161-S. [DOI] [PubMed] [Google Scholar]
- von Unge M, Decraemer WF, Bagger-Sjoback D, Van den Berghe D. Tympanic membrane changes in experimental purulent otitis media. Hear Res. 1997;106(1–2):123–136. doi: 10.1016/S0378-5955(97)00008-7. [DOI] [PubMed] [Google Scholar]
- Wiederhold ML, Zajtchuk JT, Vap JG, Paggi RE. Hearing loss in relation to physical properties of middle ear effusions. Ann Otol Rhinol Laryngol Suppl. 1980;89(3 Pt 2):185–189. doi: 10.1177/00034894800890s343. [DOI] [PubMed] [Google Scholar]
- Yagi N, Fukazawa T, Kurata K, Honjo I. A new microviscometer for determining the viscosity of middle ear effusion. Am J Otolaryngol. 1986;7(6):407–409. doi: 10.1016/S0196-0709(86)80016-3. [DOI] [PubMed] [Google Scholar]