Abstract
Regeneration of synaptic connections between hair cells and spiral ganglion neurons would be required to restore hearing after neural loss. Here we demonstrate by immunohistochemistry the appearance of afferent-like cochlear synapses in vitro after co-culture of de-afferented organ of Corti with spiral ganglion neurons from newborn mice. The glutamatergic synaptic complexes at the ribbon synapse of the inner hair cell contain markers for presynaptic ribbons and postsynaptic densities. We found postsynaptic density protein PSD-95 at the contacts between hair cells and spiral ganglion neurons in newly formed synapses in vitro. The postsynaptic proteins were directly facing the CtBP2-positive presynaptic ribbons of the hair cells. BDNF and NT-3 promoted afferent synaptogenesis in vitro. Direct juxtaposition of the postsynaptic densities with the components of the preexisting ribbon synapse indicated that growing fibers recognized components of the presynaptic sites. Initiation of cochlear synaptogenesis appeared to be influenced by glutamate release from the hair cell ribbons at the presynaptic site since the synaptic regeneration was impaired in glutamate vesicular transporter 3 mutant mice. These insights into cochlear synaptogenesis could be relevant to regenerative approaches for neural loss in the cochlea.
Keywords: synaptic regeneration, auditory neurons, hair cells, VGLUT3, neurotrophins
Introduction
Sound inputs are converted into electrical signals by cochlear hair cells (HCs), the sensory cells of the organ of Corti in the inner ear, and conveyed to the central auditory system by spiral ganglion neurons (SGNs), representing the first step of the ascending auditory pathway from the cochlea (Fuchs et al. 2003; Weisz et al. 2009). After excessive exposure to noise, both HCs and SGNs can be damaged (Liberman and Kiang 1978; Harding et al. 2002; Kujawa and Liberman 2009). Despite the capacity of peripheral neurons to regenerate, SGNs in mammals do not spontaneously recover from injury (Starr et al. 1996; White et al. 2000; McFadden et al. 2004; Kujawa and Liberman 2009). Thus, regenerating SGNs, either alone or in combination with HCs, could be important for treating hearing loss.
The afferent synapse between SGNs and HCs is suited for fast and synchronized neurotransmitter release. The presynaptic ribbons are located at the basolateral membrane of the HCs and are directly faced by the postsynaptic glutamate receptors on the afferent fibers. Upon sound stimulation, the synaptic vesicles tethered with HC ribbons quickly release the neurotransmitter glutamate into the synaptic zone. Primary or secondary damage to SGNs results in loss of the afferent synapses (Wang et al. 2002; Kujawa and Liberman 2006, 2009), which must be replaced as a part of any strategy to restore transmission of information from the cochlea to the brain.
At the inner HC (IHC) afferent synapse, vesicular glutamate transporter 3 (VGLUT3), one of three transporter types in mammals (VGLUT1-3) (Fremeau et al. 2004), is required for packaging glutamate into synaptic vesicles, and mice lacking VGLUT3 are profoundly deaf due to the lack of glutamate release into the synaptic zone (Ruel et al. 2008; Seal et al. 2008). Glutamate release from axonal or presynaptic neurons plays important roles in establishing synaptic contacts in various neuronal systems (Wong and Wong 2001; Tashiro et al. 2003; Sabo et al. 2006; McAllister 2007) and is essential for cochlear development and maturation (Housley et al. 2006; Tritsch et al. 2010).
In previous studies, neurons extended processes to HCs in vitro after prior removal of SGNs (Martinez-Monedero et al. 2006; Flores-Otero et al. 2007; Martinez-Monedero et al. 2008). In the present study we evaluated the contacts generated between cultured SGNs and denervated HCs and found PSD-95 immunopositive puncta directly facing the HC ribbons. We also found that neurotrophins, BDNF and NT-3, known to promote SGN survival and neurite outgrowth (Pirvola et al. 1992; Ernfors et al. 1995; Fritzsch et al. 1997b), significantly increased the number of new synapses, whereas innervation of HCs was decreased in organ of Corti from VGLUT3 knockout mice.
Methods
Animals
C57BL/6J mice (Jackson Laboratory, Bar Harbor, Maine) between postnatal days 4 and 6 (P4–P6) were used for the organ of Corti explants. VGLUT3 mutant mice, a kind gift from Rebecca Seal and Robert Edwards (Seal et al. 2008), were crossed with C57BL/6J mice and used as homozygous (VGLUT3−/−) or wild type (VGLUT3+/+) littermates. All procedures were approved by the Massachusetts Eye and Ear Infirmary Institutional Animal Care and Use Committee.
Explants of de-afferented organ of Corti
The afferent innervation of HCs was removed by physical ablation as compared to pharmacological targeting with β-bungarotoxin used in previous studies (Martinez-Monedero et al. 2006, 2008). At postnatal days 4–6 (P4–P6), the cochlea was dissected and transferred to Petri dishes. The inner HC (IHC), outer HC (OHC) and surrounding supporting cells of the organ of Corti, were separated from the SGN region at the greater epithelial ridge with a surgical micro-blade, to obtain an intact sensory epithelium devoid of neurons. The de-afferented organ of Corti was then transferred to a cover glass coated with laminin (25 μg/ml, BD Biosciences) and poly-L-ornithine (0.01 %, Sigma) in a 4-well Petri dish (Greiner) and maintained overnight at 37 °C in a humidified incubator with 5 % CO2 in DMEM/F12 (Gibco) supplemented with N2 and B27 (Gibco). During the first day of culture, 10 % FBS was added for attachment to the glass. FBS was removed after 2 days, and growth medium was changed every 2–3 days.
Isolation of SGNs and co-culture with de-afferented organ of Corti
SGNs were dissected from P4–P6 mouse cochlea by trypsinization as described previously (Martinez-Monedero et al. 2006, 2008). Briefly, after removing the sensory epithelium from the organ of Corti, the SGNs were isolated from the modiolus and treated with 0.25 % trypsin for 15 min at 37 °C. After neutralization of the trypsin with 10 % FBS in DMEM, neurons were collected by centrifugation, triturated to a single-cell suspension and co-cultured with the de-afferented organ of Corti in DMEM/F12 supplemented with N2 and B27. In some explants the medium was supplemented with NT-3 (10 ng/ml; Chemicon) and BDNF (10 ng/ml; Chemicon).
Immunofluorescence
Cultures were fixed with 4 % paraformaldehyde at room temperature for 20 min, followed by permeabilization and blocking with 0.1 % Triton-X-100 and 15 % normal goat serum for one h. Primary antibodies – anti-CtBP2 (mouse monoclonal IgG1; BD Biosciences), anti-PSD-95 (mouse monoclonal IgG2a; NeuroMab), anti-neurofilament (NF) heavy chain (chicken polyclonal; Chemicon) and anti-myosin VIIa (rabbit polyclonal; Proteus) — were added to the tissue overnight at 4 °C. After rinsing three times for ten min with 0.01 M PBS, pH 7.4, explants were incubated with secondary antibodies — cyanine-5-conjugated goat anti-mouse IgG1 (Caltad Laboratories), biotin-conjugated Fluor goat anti-mouse IgG2a (Caltad Laboratories), Alexa Fluor 568-Streptavidin (Molecular Probes), Alexa Fluor 488 goat anti-chicken (Molecular Probes) or Alexa Fluor 647 goat anti-rabbit (Molecular Probes) — for 2 h at RT. Tissues were placed onto a glass microscope slide with a drop of fluorescent mounting medium (Dako Cytomation) after three PBS rinses and viewed using a Leica confocal microscope. Images were analyzed with Metamorph software and processed with Adobe Photoshop. Triple labeling of CtBP2, PSD-95 and NF was quantified. We excluded PSD-95 puncta that were not associated with CtBP2 puncta on the HC surface from the analysis. Significance was determined by the Student’s t-test.
Statistical analysis
All results were presented as mean ± standard error. Comparisons were made using unpaired two-tailed Student’s t-test (MATLAB, MathWorks). A P value <0.05 was considered statistically significant.
Results
Postsynaptic density protein PSD-95 is expressed in mouse cochlea
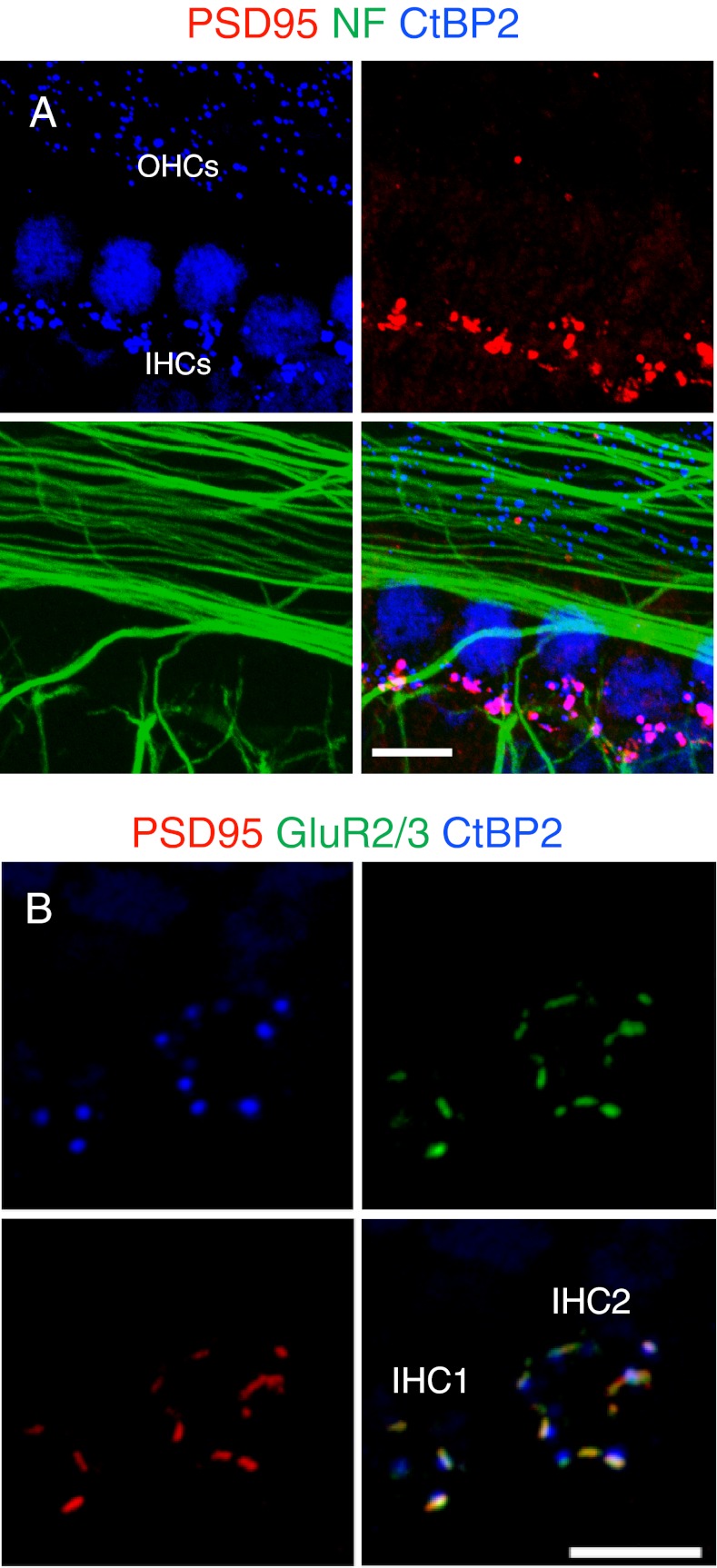
The cochlear synapse in mouse organ of Corti undergoes a dynamic transition during the first several weeks after birth, and the exact developmental expression profiles of synaptic proteins are largely unknown (Sobkowicz et al. 1982; Eatock and Hurley 2003; Housley et al. 2006). We performed immunohistochemistry to identify the pre- and postsynaptic specializations of neonatal and adult mouse organ of Corti. In the neonatal mouse cochlea, SGN fibers could be stained with an antibody against NF and the HC ribbons could be stained with an antibody against C-terminal-binding protein 2 (CtBP2), a component of ribbon protein, RIBEYE. The presynaptic HC ribbons were seen in the basal–lateral aspects of the IHCs and OHCs of the organ of Corti. The postsynaptic densities, stained for an antibody against PSD-95, a membrane-associated guanylate kinase (MAGUK) scaffolding protein, were mostly seen at the endings of the SGN fibers innervating the IHCs. Pre- and postsynaptic puncta of CtBP2 and PSD-95 were closely associated at the synaptic zone of the IHCs (Fig. 1A). In the mature mouse cochlea, refined PSD-95 puncta were found to co-localize with glutamate AMPA receptors, GluR2/3, on the SGN afferents and were juxtaposed to the IHC ribbons (Fig. 1B). Thus, PSD-95 faithfully marked the afferent ribbon synapses between the SGNs and the IHCs in newborn and mature cochleae.
FIG. 1.
Components of afferent synapses in mouse cochlea. A HC ribbon synaspes from a P5 mouse were detected using antibodies against PSD-95 (red), NF (green) and CtBP2 (blue). Ribbons of both IHCs and OHCs were positive for CtBP2 and appeared as puncta. IHC nuclei were also positive for CtBP2. Postsynaptic densities were observed at the endings of SGNs in the IHC region with the PSD-95 antibody. B Representative confocal projections of the basal membranes of two IHCs are shown from a P30 mouse. The ribbon synapses were immunostained with antibodies to PSD-95 (red), GluR2/3, against AMPA receptors (green) and CtBP2 (blue). GluR2/3 and PSD-95 were co-localized, and apposed to the HC ribbons. Scale bars: 10 μm.
Afferent-like re-innervation of HCs by newborn SGNs
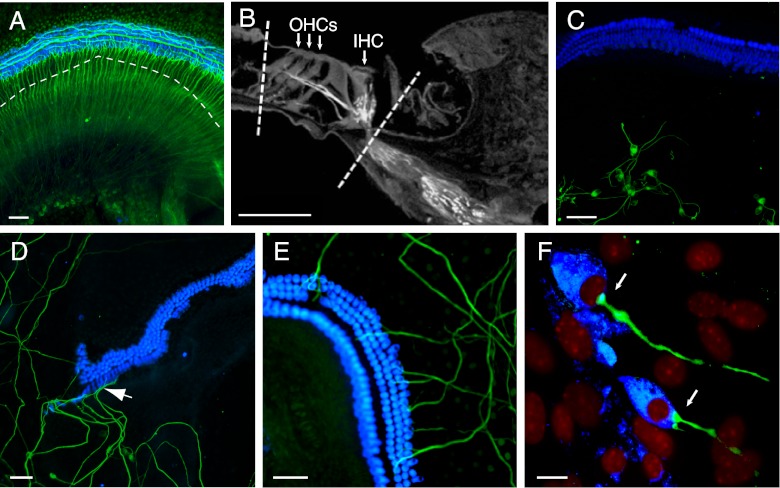
We co-cultured the isolated SGNs with the denervated HCs to examine whether nerve fibers would form afferent endings. The de-afferentation of newborn mouse organ of Corti was accomplished by physically dissecting the HCs from the SGNs (see Methods section, Fig. 2A and B), and newly dissociated SGNs from an age-matched mouse were randomly placed in the culture around the de-afferented organ of Corti. This protocol was less damaging to the remaining cells than the previously published β-bungarotoxin method (Martinez-Monedero et al. 2006). Isolated SGNs, identified by a NF antibody at day 1, did not contact HCs in the explant, identified by a myosin VIIa antibody (Fig. 2C). However, after 6 days, the SGNs grew into the organ of Corti with fibers extending toward IHCs (Fig. 2D) and OHCs (Fig. 2E), as previously observed in the toxin-treated cultures (Martinez-Monedero et al. 2006). Many fibers contacted the basal membrane of HCs, suggesting normal innervation of the synaptic zone (Fig. 2F).
FIG. 2.
Co-culture of de-afferented organ of Corti with SGNs. A An organ of Corti from a P5 mouse was immunostained with antibodies against NF (green) and myosin VIIa (blue) to label the SGNs and HCs. B A cross-sectional view shows the lines of separation followed with a micro-blade to mechanically axotomize the SGN fibers at the GER region close to IHCs and at the outer sulcus (dashed lines). C A de-afferented organ of Corti (P5) was co-cultured with isolated SGNs for 1 day. D, E De-afferented organs of Corti (P6) were co-cultured with isolated SGNs for 6 days. The fibers of SGNs extended to both IHCs (arrow, D) and OHCs (E). F SGN fibers innervated HCs at their basal surface after 6 days. Nuclei were stained with DAPI (red). Scale bars in A–E: 50 μm, F: 10 μm.
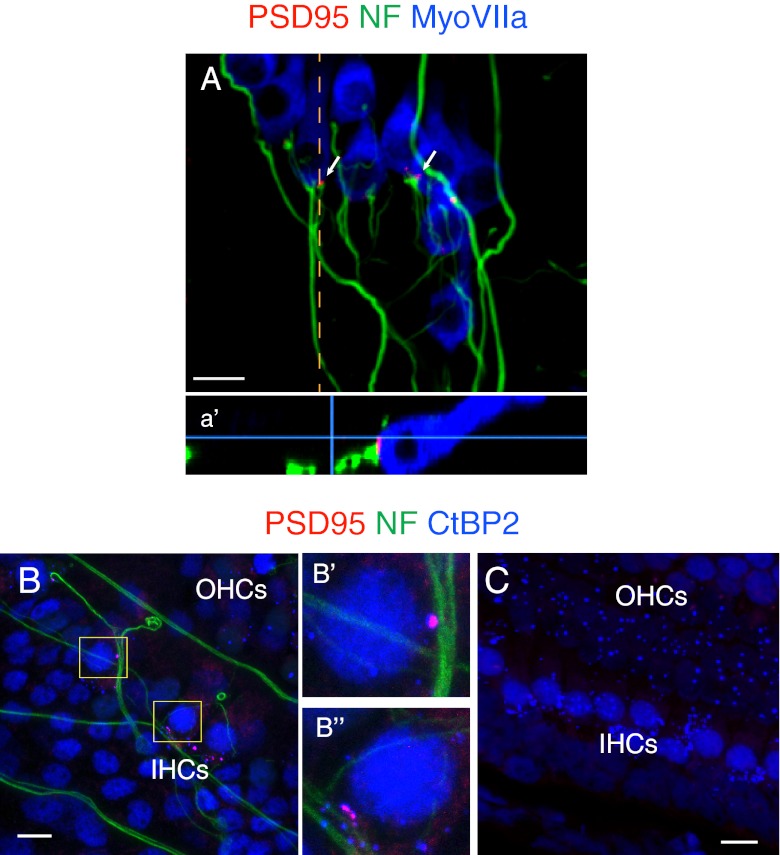
We then examined the innervation sites between the SGNs and the basolateral membranes of the HCs for afferent synapses. SGN fibers extended to IHCs of the de-afferented organ of Corti forming postsynaptic densities that expressed PSD-95 at the termini of the fibers apposed to the HC synaptic zones (Fig. 3A). Immunostaining with CtBP2 antibody revealed that PSD-95 puncta on the SGN fibers were in direct apposition to the presynaptic ribbons of IHCs (Fig. 3B), as is observed in afferent ribbon synapses in the organ of Corti in vivo. Thus, afferent-like cochlear synapses could be regenerated in the in vitro explants by co-culturing the isolated SGNs and the de-afferented HCs from newborn mice. In cases where the de-afferented organ of Corti was not reached by SGN fibers, postsynaptic densities were not seen but both IHCs and OHCs retained presynaptic ribbons after 6 days (Fig. 3C).
FIG. 3.
Newly generated afferent-like cochlear synapses in vitro. A A de-afferented organ of Corti (P5) was co-cultured with isolated SGNs for 6 days. PSD-95-positive puncta were detected at the sites of innervation of the IHCs (arrows). An orthogonal view shows PSD-95 apposed to the basal surface of the IHC, presumably the synaptic zone (a′, dashed line in A). B After 6 days, immunostaining with an antibody against CtBP2 showed afferent-like synapses at the sites of IHC innervation. Enlarged views demonstrate that PSD-95 puncta were directly apposed to the CtBP2-positive presynaptic ribbons of the HCs (B′ and B″). C After 6 days, HC ribbons in the section of the de-afferented organ of Corti that was not innervated by SGNs were preserved, whereas postsynaptic densities were no longer present at synaptic zones.
Neurotrophins promote synaptogenesis in vitro
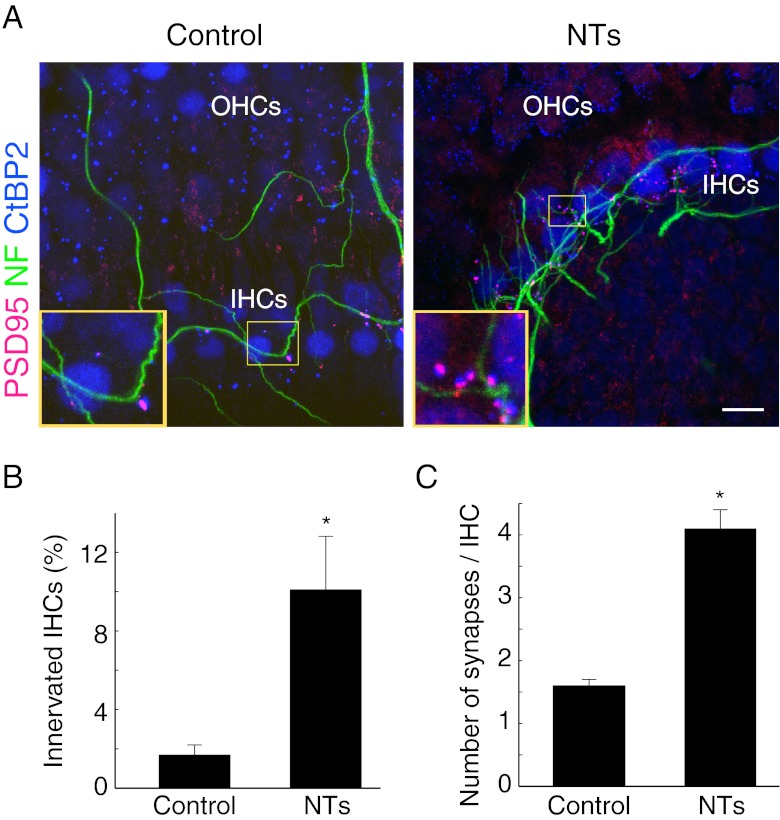
As the overall percentage of HCs innervated by SGNs, based on the appearance of PSD-95 and CtBP2 immunopositive puncta in the cochlear synaptic region, was low, we sought methods to facilitate synaptogenesis. BDNF (10 ng/ml) and NT-3 10 ng/ml), required for inner ear development and SGN survival and neurite growth (Fritzsch et al. 2004, 2005), added to the culture, promoted afferent-like innervation of HCs by SGNs in the explant, identified by co-immunostaining of CtBP2-positive ribbons apposed by PSD-95-positive endings. The number of PSD-95 puncta and percentage of innervated IHCs were significantly increased in the explants with addition of NTs (Fig. 4A and B; T(16) = −3.89, P = 0.012). The average number of new synapses per IHC, measured by calculating the number of paired puncta of CtBP2 and PSD-95 of individual innervated IHCs in de-afferented organs of Corti, was also dramatically increased by the addition of neurotrophins to the explant (Fig. 4C; T(198) = 3.64, P = 0.003).
FIG. 4.
Neurotrophins promoted cochlear synaptogenesis. A De-afferented organs of Corti were co-cultured with SGNs. In one group of explants, NT-3 (10 ng/ml) and BDNF (10 ng/ml) were added to the culture. After 6 days, the explants were immunostained with antibodies to PSD-95, NF and CtBP2. Insets show enlarged views of synaptic zones in the yellow boxes. B After 6 days, addition of NTs significantly increased the percentage of IHCs innervated by SGNs in the co-cultured explant (Control, n = 8 explants; NTs, n = 11 explants). C After 6 days, addition of NTs significantly increased the average numbers of new synapses per IHC in the co-cultured explant (Control, n = 59 IHCs; NTs, n = 209 IHCs). *P < 0.05, Student’s t-test. Scale bar: 10 μm.
Role of glutamate in cochlear synaptogenesis in vitro
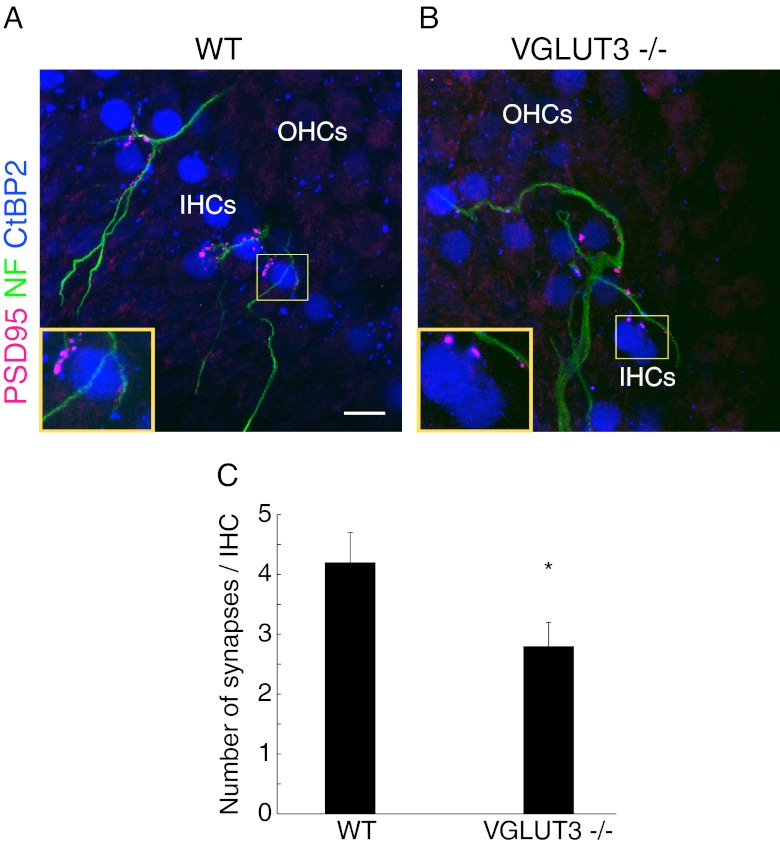
Glutamate is released from presynaptic vesicles of ribbons at the cochlear afferent synapse. We speculated that glutamate could act as a chemoattractant for the isolated SGNs guiding the dendrites to de-afferented HCs to form new synapses at the sites adjacent to presynaptic ribbons on the HC membrane. We tested this hypothesis by taking advantage of mice that have no glutamate release from HCs due to a lack of VGLUT3 (Ruel et al. 2008; Seal et al. 2008). The SGNs from age-matched wild type mice were co-cultured with de-afferented organs of Corti from homozygous VGLUT3 knockout mice and wild type littermates for 6 days, followed by immunohistochemistry with antibodies against NF, CtBP2 and PSD-95. Cochlear afferent synapses were generated in de-afferented organs of Corti from both groups (Fig. 5A and B), but the number of new synapses per IHC was significantly reduced in VGLUT3 knockout cochlear explants (Fig. 5D; T(44) = −2.07, P = 0.043). Heterozygous littermates were excluded from the study because the degree of synaptic release is unknown.
FIG. 5.
Glutamate was not required for cochlear synaptogenesis. A–B De-afferented organs of Corti dissected from wild type (WT) and VGLUT3 knockout (VGLUT3−/−) littermates were co-cultured with isolated SGNs from C57BL/6J mice for 6 days. PSD-95-positive puncta were detected in both groups. Enlarged views of the synaptic zones demonstrated that postsynaptic densities were formed along the SGN fibers and juxtaposed to the IHC ribbons. C The average number of new synapses generated per IHC was decreased in the explants of VGLUT3−/− organs of Corti (WT, n = 23; VGLUT3−/−, n = 23). *P < 0.05, Student’s t-test. Scale bars: 10 μm.
Discussion
Here, we demonstrate afferent cochlear synapses in vitro after co-culture of de-afferented organ of Corti with SGNs from newborn mice. In previous studies, neurons extended processes to HCs in vitro and expressed synaptic markers (Martinez-Monedero et al. 2006; Flores-Otero et al. 2007; Martinez-Monedero et al. 2008). However, postsynaptic structures of the auditory HC synapse were not assessed. We have exploited the synaptic complexes at the ribbon of the IHC and postsynaptic densities in the SGN, characteristic features of the glutamatergic synapse, for the detection and quantification of new synapses. We found postsynaptic density protein, PSD-95, as well as glutamate receptors, in the fibers that extended from SGNs, and CtBP2 in the presynaptic ribbons.
The loss of PSD-95 and its appearance when new synapses were formed provided a useful measure of synaptogenesis. PSD-95 was closely associated with glutamate receptors in the postsynaptic terminals, and was also closely associated with CtBP2 staining, thus marking the synapses from both sides. In a recent study using organotypic rat culture, nerve endings of type 1 SGNs injured by the application of glutamate agonists regrew fibers to HCs and also showed synaptic regeneration (Wang and Green 2011). In contrast to those explants, in which the cochlear explant included the organ of Corti and the attached neurons, our model involved the complete removal of SGNs from the organ of Corti, and could be used to study reinnervation by growth of exogenously added cells.
Contacts between SGNs and HCs could be quantified in the cochlear explants, providing a unique opportunity to identify regulatory mechanisms for synaptogenesis. BDNF and NT-3, which are known to promote SGN survival and neurite outgrowth (Pirvola et al. 1992; Lefebvre et al. 1994; Ernfors et al. 1995; Malgrange et al. 1996; Fritzsch et al. 1997a; Hegarty et al. 1997; Mou et al. 1997), promoted the formation of pre- and postsynaptic markers of the afferent synapse, in agreement with a previous study (Wang and Green 2011).
In addition to showing that the postsynaptic densities were present and could be quantified, confocal microscopy revealed that PSD-95 was directly apposed to presynaptic CtBP2. Thus, the new fibers seemed to recognize the sites of the ribbon synapses and the direct alignment of the components in the newly formed ribbon synapse indicated that signaling from HCs directed growing fibers to the preexisting site of the ribbon synapse (Wang and Green 2011), suggesting that cochlear specific synaptic connections similar to the ribbon synapse in vivo were regenerated in vitro.
The coincidence and alignment of the pre- and postsynaptic processes in regenerated synapses has been observed in vertebrate neuromuscular junctions, modulated by presynaptic secretion of agrin (Gautam et al. 1996; Sanes and Lichtman 1999). Several models have been proposed to guide the initial formation of glutamatergic synapses in the CNS. In one, a mobile or stochastic model, synaptic proteins are randomly transported prior to synapse formation. The encounter of axon and dendrite initiates the recruitment of scaffolding complexes on both sides of the synapse (Friedman et al. 2000; Washbourne et al. 2002; Ziv and Garner 2004; Dean and Dresbach 2006). In a second, a predetermined model, synaptic proteins are assembled at specific sites on one side of the axodendritic synapse before the formation of the synapse, followed by attraction of the synaptic components on the opposite side (Gerrow et al. 2006; Sabo et al. 2006). In the case of cochlear synaptogenesis, our study strongly favored the predetermined model, in which presynaptic signals dictate the initiation of postsynaptic components, because ribbons, the dense structure anchoring presynaptic components, were not lost from the de-afferented HCs and were therefore present before the formation of the new synapse.
Glutamate released from presynaptic neurons is known to play an important role in establishing synaptic contact (Wong and Wong 2001; Tashiro et al. 2003; Sabo et al. 2006; McAllister 2007). In the auditory system, glutamate is loaded into synaptic vesicles of IHCs by VGLUT3, and mice lacking VGLUT3 are profoundly deaf due to the lack of release of glutamate into synaptic zones (Ruel et al. 2008; Seal et al. 2008). Since glutamate release from HCs precedes formation of synapses during development, we tested whether glutamate from presynaptic terminals of HCs induced cochlear synaptogenesis during regeneration. Synaptic regeneration in VGLUT3 mutant mice was still seen but the number of new synapses was reduced, suggesting that glutamate release facilitated synaptogenesis.
Abnormal morphology of ribbon synapses due to the dysfunction of synchronized synaptic release (Roux et al. 2006) has been demonstrated in otoferlin null mice, a model for progressive hearing loss in humans (Yasunaga et al. 1999). Partial deletion of presynaptic scaffolding protein, bassoon, resulted in ribbons detaching from the membrane (Dick et al. 2003; Khimich et al. 2005). Deletion of synaptotagmin IV disrupted the linear dependence of synaptic release on Ca2+ (Johnson et al. 2010). Interestingly, although synaptic functions were disrupted in these mice the process of initial ribbon synapse formation was unaffected. This is consistent with the results of our study and suggests that recognition of the HC target by regenerating cochlear neurons could be mediated by other proteins, possibly guidance molecules or adhesion proteins (Ziv and Garner 2004; Sabo et al. 2006; McAllister 2007).
Our results provide fundamental insights into cochlear synaptogenesis that will be relevant to regenerative approaches for neural loss in the cochlea. In the damaged mammalian nervous system, spontaneous regeneration of injured neurons is always insufficient or incomplete (Lie et al. 2004; Chen et al. 2007; Okano et al. 2007; Kawabuchi et al. 2011), and this insufficiency is pronounced in the auditory system. A better understanding of mechanisms that increase regeneration of synaptic connections would be valuable in achieving reversal of hearing loss.
Acknowledgements
We thank Robert Edwards and Rebecca Seal (University of California, San Francisco) for the VGLUT3 knockout mouse. This work was supported by grants from the National Institute on Deafness and other Communicative Disorders (RO1 DC007174 and P30 DC05209).
References
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–29. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Dick O, tom Dieck S, Altrock WD, Ammermuller J, Weiler R, Garner CC, Gundelfinger ED, Brandstatter JH. The presynaptic active zone protein bassoon is essential for photoreceptor ribbon synapse formation in the retina. Neuron. 2003;37:775–786. doi: 10.1016/S0896-6273(03)00086-2. [DOI] [PubMed] [Google Scholar]
- Eatock RA, Hurley KM. Functional development of hair cells. Curr Top Dev Biol. 2003;57:389–448. doi: 10.1016/S0070-2153(03)57013-2. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–1164. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- Flores-Otero J, Xue HZ, Davis RL. Reciprocal regulation of presynaptic and postsynaptic proteins in bipolar spiral ganglion neurons by neurotrophins. J Neurosci. 2007;27:14023–14034. doi: 10.1523/JNEUROSCI.3219-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Voglmaier S, Seal RP, Edwards RH. VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 2004;27:98–103. doi: 10.1016/j.tins.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/S0896-6273(00)00009-X. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Farinas I, Reichardt LF. Lack of neurotrophin 3 causes losses of both classes of spiral ganglion neurons in the cochlea in a region-specific fashion. J Neurosci. 1997;17:6213–6225. doi: 10.1523/JNEUROSCI.17-16-06213.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–164. doi: 10.1016/S0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Pauley S, Matei V, Katz DM, Xiang M, Tessarollo L. Mutant mice reveal the molecular and cellular basis for specific sensory connections to inner ear epithelia and primary nuclei of the brain. Hear Res. 2005;206:52–63. doi: 10.1016/j.heares.2004.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs PA, Glowatzki E, Moser T. The afferent synapse of cochlear hair cells. Curr Opin Neurobiol. 2003;13:452–458. doi: 10.1016/S0959-4388(03)00098-9. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Moscoso L, Rupp F, Scheller RH, Merlie JP, Sanes JR. Defective neuromuscular synaptogenesis in agrin-deficient mutant mice. Cell. 1996;85:525–535. doi: 10.1016/S0092-8674(00)81253-2. [DOI] [PubMed] [Google Scholar]
- Gerrow K, Romorini S, Nabi SM, Colicos MA, Sala C, El-Husseini A. A preformed complex of postsynaptic proteins is involved in excitatory synapse development. Neuron. 2006;49:547–562. doi: 10.1016/j.neuron.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Harding GW, Bohne BA, Ahmad M. DPOAE level shifts and ABR threshold shifts compared to detailed analysis of histopathological damage from noise. Hear Res. 2002;174:158–171. doi: 10.1016/S0378-5955(02)00653-6. [DOI] [PubMed] [Google Scholar]
- Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–1970. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Marcotti W, Navaratnam D, Yamoah EN. Hair cells—beyond the transducer. J Membr Biol. 2006;209:89–118. doi: 10.1007/s00232-005-0835-7. [DOI] [PubMed] [Google Scholar]
- Johnson SL, Franz C, Kuhn S, Furness DN, Ruttiger L, Munkner S, Rivolta MN, Seward EP, Herschman HR, Engel J, Knipper M, Marcotti W. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nat Neurosci. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabuchi M, Tan H, Wang S. Age affects reciprocal cellular interactions in neuromuscular synapses following peripheral nerve injury. Ageing Res Rev. 2011;10:43–53. doi: 10.1016/j.arr.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci. 2006;26:2115–2123. doi: 10.1523/JNEUROSCI.4985-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre PP, Malgrange B, Staecker H, Moghadass M, Van de Water TR, Moonen G. Neurotrophins affect survival and neuritogenesis by adult injured auditory neurons in vitro. Neuroreport. 1994;5:865–868. doi: 10.1097/00001756-199404000-00003. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Kiang NY. Acoustic trauma in cats. Cochlear pathology and auditory-nerve activity. Acta Otolaryngol Suppl. 1978;358:1–63. [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Malgrange B, Lefebvre PP, Martin D, Staecker H, Van de Water TR, Moonen G. NT-3 has a tropic effect on process outgrowth by postnatal auditory neurones in vitro. Neuroreport. 1996;7:2495–2499. doi: 10.1097/00001756-199611040-00019. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Corrales CE, Cuajungco MP, Heller S, Edge AS. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66:319–331. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Monedero R, Yi E, Oshima K, Glowatzki E, Edge AS. Differentiation of inner ear stem cells to functional sensory neurons. Dev Neurobiol. 2008;68:669–684. doi: 10.1002/dneu.20616. [DOI] [PubMed] [Google Scholar]
- McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–539. doi: 10.1002/(SICI)1096-9861(19971006)386:4<529::AID-CNE1>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Okano H, Sakaguchi M, Ohki K, Suzuki N, Sawamoto K. Regeneration of the central nervous system using endogenous repair mechanisms. J Neurochem. 2007;102:1459–1465. doi: 10.1111/j.1471-4159.2007.04674.x. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Ylikoski J, Palgi J, Lehtonen E, Arumae U, Saarma M. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89:9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Ruel J, Emery S, Nouvian R, Bersot T, Amilhon B, Van Rybroek JM, Rebillard G, Lenoir M, Eybalin M, Delprat B, Sivakumaran TA, Giros B, El Mestikawy S, Moser T, Smith RJ, Lesperance MM, Puel JL. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 2008;83:278–292. doi: 10.1016/j.ajhg.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, Lustig LR, Edwards RH. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 2008;57:263–275. doi: 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz HM, Rose JE, Scott GE, Slapnick SM. Ribbon synapses in the developing intact and cultured organ of Corti in the mouse. J Neurosci. 1982;2:942–957. doi: 10.1523/JNEUROSCI.02-07-00942.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Tashiro A, Dunaevsky A, Blazeski R, Mason CA, Yuste R. Bidirectional regulation of hippocampal mossy fiber filopodial motility by kainate receptors: a two-step model of synaptogenesis. Neuron. 2003;38:773–784. doi: 10.1016/S0896-6273(03)00299-X. [DOI] [PubMed] [Google Scholar]
- Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nat Neurosci. 2010;13:1050–1052. doi: 10.1038/nn.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Green SH. Functional role of neurotrophin-3 in synapse regeneration by spiral ganglion neurons on inner hair cells after excitotoxic trauma in vitro. J Neurosci. 2011;31:7938–7949. doi: 10.1523/JNEUROSCI.1434-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol. 2002;3:248–268. doi: 10.1007/s101620020028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P. The postsynaptic function of type II cochlear afferents. Nature. 2009;461:1126–1129. doi: 10.1038/nature08487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Burgess BJ, Hall RD, Nadol JB. Pattern of degeneration of the spiral ganglion cell and its processes in the C57BL/6J mouse. Hear Res. 2000;141:12–18. doi: 10.1016/S0378-5955(99)00204-X. [DOI] [PubMed] [Google Scholar]
- Wong WT, Wong RO. Changing specificity of neurotransmitter regulation of rapid dendritic remodeling during synaptogenesis. Nat Neurosci. 2001;4:351–352. doi: 10.1038/85987. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Cohen-Salmon M, El-Amraoui A, Mustapha M, Salem N, El-Zir E, Loiselet J, Petit C. A mutation in OTOF, encoding otoferlin, a FER-1-like protein, causes DFNB9, a nonsyndromic form of deafness. Nat Genet. 1999;21:363–369. doi: 10.1038/7693. [DOI] [PubMed] [Google Scholar]
- Ziv NE, Garner CC. Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci. 2004;5:385–399. doi: 10.1038/nrn1370. [DOI] [PubMed] [Google Scholar]