Abstract
Many human subjects suffering from chronic tinnitus also suffer from hyperacusis, a heightened perception of loudness at moderate to intense sound levels. While numerous studies suggest that animals develop chronic tinnitus following intense noise exposure, it is not yet clear whether sound exposure also induces chronic hyperacusis-like responses in animals. We addressed this question by examining the chronic effects of intense sound exposure on the acoustic startle response (ASR) and its suppression by background noise containing brief gaps. We compared startle amplitudes in intense tone-exposed (10 kHz, 115 dB SPL, 4 h) and age-matched controls at 2–28 weeks post-exposure. While both groups showed similar startle thresholds, exposed animals showed a hyperacusis-like augmentation of ASR at high stimulus levels. Addition of background noise had little effect on ASR in controls but had a strong suppressive effect on startle in exposed animals, indicating a sensitization to background noise. When the background noise contained a gap preceding the startle stimulus, ASR was suppressed in control animals, but exposed animals showed a marked weakening of gap-induced suppression of ASR. This weakening of gap-induced startle suppression is consistent with the interpretation that the gap may have been masked by tinnitus. The associated hyper-responsiveness to startle stimuli presented alone and the sensitization to background noise suggest that hyperacusis may have also been induced. The results indicate that noise exposure leads to increases in the gain of auditory responsiveness and may offer a model of the association of hyperacusis with tinnitus.
Keywords: tinnitus, hyperacusis, acoustic startle, gap-induced suppression of startle, hearing loss
Introduction
In recent years, there has been a surge of interest in understanding the neural basis of tinnitus and how the spontaneous auditory percepts that define it arise in the absence of a driving acoustic stimulus. While a great deal has been learned about the mechanisms underlying tinnitus, relatively little attention has been given to its stimulus-elicited counterpart, hyperacusis. Hyperacusis is a condition characterized by heightened perception of the loudness or annoyance level of sounds, particularly those in the moderate to intense range (Gu et al. 2010; Fournier and Hébert 2013; Dauman and Bouscau-Faure 2005).
It is well known that tinnitus and hyperacusis often occur together. Earlier studies estimated the prevalence of hyperacusis in tinnitus patients at 40 % (Bartnik et al. 1999; Jastreboff and Jastreboff 2000), but a study by Dauman and Bouscau-Faure (2005), which employed more rigorous methods to assess both tinnitus and hyperacusis, found that a vast majority (79 %) of patients in treatment for tinnitus (n = 259) also had hyperacusis (Dauman and Bouscau-Faure 2005). The prevalence of tinnitus in subjects being treated for hyperacusis was also found to be high (86 %) (Anari et al. 1999). These findings raise the possibility that the two disorders are at some level linked, such that disruptions in the auditory system leading to tinnitus may also lead to heightened responses to moderate and high levels of sound.
An important step toward understanding why tinnitus and hyperacusis are so often associated is the establishment of animal models in which the presence of both disorders can be demonstrated simultaneously. Some important progress in this direction has recently been made. Manipulations that cause tinnitus in human subjects, such as sodium salicylate treatment or exposure to intense sound, have been shown to cause deficits in gap detection in animals (Rybalko and Syka 2005; Turner et al. 2006; Turner and Parrish 2008; Longenecker and Galazyuk 2011; Dehmel et al. 2012). These deficits have been interpreted as evidence of tinnitus based on the concept that the tinnitus effectively fills in the gap, rendering it more difficult to resolve. Further support for this view comes from a study reporting similar gap detection deficits in human subjects with tinnitus but no hearing loss (Fournier and Hébert 2013).
Evidence has also been reported for hyperacusis-like behavioral responses in animals following similar manipulations. Turner and Parrish (2008) found that prepulse inhibition of the acoustic startle was increased in rats treated with salicylate (Turner and Parrish 2008). Sun and colleagues described acute (transient) enhancements of the acoustic startle reflex following salicylate treatment (Sun et al. 2009) and intense noise exposure (Sun et al. 2012). Tinnitus with hyperacusis in the same animals is suggested by the results of Turner et al. (2012), showing improvement in behavioral responses to prepulse stimuli in the first 3 weeks after noise exposure, although these disappeared by the third week and gave way to more normal prepulse inhibition followed by gap detection deficits beginning at 7 weeks post-exposure (Turner et al. 2012). A more chronic form of hyperacusis-like response is suggested by the exaggeration in the amplitude of the acoustic startle reflex observed in aging mice (Ison et al. 2007) and by increased strength of prepulse inhibition in noise-exposed guinea pigs (Dehmel et al. 2012). However, no previous studies, to our knowledge, have presented evidence of chronic hyperacusis-like enhancements of acoustic startle amplitudes following noise exposure and its possible association with tinnitus in the same animals.
Here we conducted a systematic study of acoustic startle responses in hamsters weeks to months, following their exposure to intense sound. Our primary goal was to determine whether the same exposure conditions that have been shown to cause chronic tinnitus also induce chronic hyperacusis-like responses to startle stimuli. We first characterized the startle as a function of startle stimulus level and how it is affected after extended periods following intense sound exposure. The hypothesis was that animals with hyperacusis would show evidence of hyper-responsiveness to acoustic startle stimuli. We then examined the effects of adding background noise with and without gaps on the acoustic startle in control and sound-exposed animals. We used various combinations of startle stimulus and background noise levels with and without gaps to determine whether hamsters display gap-induced suppression of startle and, if so, whether that suppression is weakened following intense sound exposure, consistent with tinnitus induction.
Methods
Animal subjects
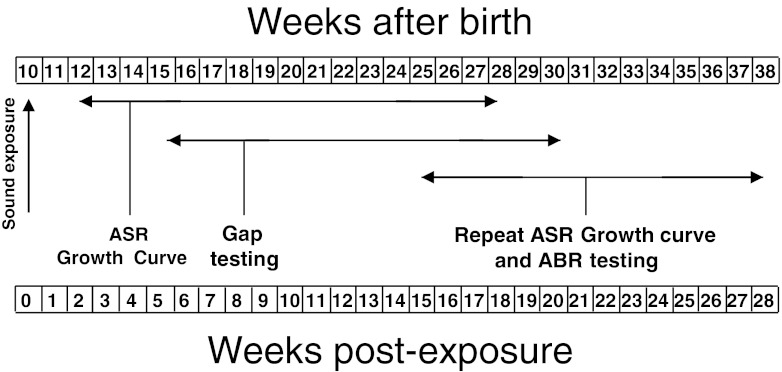
Hamsters (LVG strain) were obtained from Charles River and were between 60 and 70 days of age on the day of arrival. The animals were housed in a vivarium and put on a 12:12-h day/night cycle. After a quarantine period of 4 days, they were divided into two groups of nine animals. One group consisted of experimentals, which were to be exposed to intense sound, while the second group included unexposed animals serving as controls. The exposures were conducted when the animals were between 67 and 68 days of age. The various tests of the startle responses were performed beginning 2 weeks post-exposure and continuing through auditory brainstem responses (ABR) measurements, which were completed at 28 weeks after exposure (see time line of Fig. 1). The animals were housed and cared for in accordance with NIH guidelines for the care and use of animals in research.
FIG. 1.
Time line showing the animals’ ages (top row of numbers) and post-exposure recovery times (bottom row of numbers) at the times when the various experimental tests were performed.
Sound exposure
Sound exposures were conducted by placing the animals in an acrylic cylindrical chamber located inside a sound attenuation booth (Acoustic Systems). The chamber was subdivided into four compartments, one for each animal. The lid of the chamber contained a 6-in.-diameter speaker (Beyma CP-25) which was open toward the bottom of the chamber. Sound calibrations were performed before exposures using a microphone (Etymotic) placed in the center of the chamber at 2 cm above the chamber’s floor to approximate the level where the animals’ ears would be during exposure. The lid was then placed over the chamber, and a 10-kHz tone was presented. The intensity of the tone was adjusted until it reached 115 dB SPL. Sound levels were measured at multiple locations throughout the chamber and varied by ± 6 dB within each compartment. The sound was then turned off, and an animal was placed inside each of the four compartments. The animals were given a period of a few minutes to adjust to the enclosure. Then, the sound was turned back on, but initially only at a level of 80 dB SPL. The sound level was gradually increased in 5-dB steps at one step every few minutes until the desired exposure level (115 dB SPL) was reached. The tone was left on continuously for a period of 4 h. Animal reactions to the tone were found to be minimal as long as the sound level was gradually increased in steps. Control animals were placed inside an Acoustic Systems booth for 4 h but were not exposed to intense noise. Following the exposure period, the animals were returned to the vivarium where they were allowed a period of 2 weeks before beginning measures of the acoustic startle reflex.
Acoustic startle apparatus
Measures of acoustic startle were obtained using a Kinder Scientific Acoustic Startle system (Model SM100). The apparatus consisted of a chamber measuring 28 × 36 × 50 cm. The interior of the chamber was insulated with a 1-in.-thick layer of dense foam material to reduce sound reflections (Longenecker and Galazyuk 2012). Startle responses were detected with a plate containing a pressure-sensitive piezoelectric transducer on the floor of a small plexiglass housing into which the animal was placed. Sound stimuli were introduced to the chamber from two speakers, one to elicit the acoustic startle, which was directly above the housing, and the other to introduce background noise with and without gaps, which was placed a few inches away from the startle speaker. The sound input and measurement of startle responses were controlled remotely using Kinder software. The sensitivity of the sensor plate and amplification of its signal were adjusted so that a 1-N calibrated pressure pulse produced a readout value of 1 ± 0.05 N on the first positive peak of the pressure pulse waveform. All startle response measures were based on the amplitude of the peak of the startle waveform observed during a collection window that spanned a period of 100 ms from startle stimulus onset. The animals were weighed before each startle session to determine whether group differences in weight existed between exposed and control animals, which could bias measures of startle amplitude. This might be expected since larger animals could exert greater force on the pressure sensor, which could lead to larger startle amplitudes.
Acoustic stimuli
Calibration of the sound level was performed using a 0.25-in. microphone and sound measurement system (B&K). The first experiment was designed to quantify the amplitude of the acoustic startle as a function of startle stimulus level. Each test session consisted of five sets of 15 trials of startle stimuli consisting of 12 20-ms bursts of broadband noise varied in level from 57 to 120 dB SPL in steps of 3–6 dB plus three no-stimulus trials. In each session, the different level startle stimuli were presented in double random order so that a different random sequence was used in successive test sessions to minimize habituation related to repeating identical sequences of stimuli. The times between successive stimuli were varied randomly between 15 and 20 s so that the animals would have no way of predicting the timing of each stimulus. The same test session, which evaluated the responses to the full range of 15 trials, was repeated on four successive days, with all control and exposed animals being tested on the same days and at the same average times of day.
The second experiment was designed to assess the effect of background noise with and without gaps on the startle response. For this test, the same startle stimulus was presented in the presence of a continuous narrowband background noise (12–14 kHz). This frequency range was selected to approximate that, which in earlier studies, has been found to be maximally affected by the 10-kHz exposure tone (Kaltenbach et al. 1998). In each session, startle responses were tested in four sets of eight trials consisting of one of the following startle stimulus levels: 100, 105, 110, and 115 dB SPL plus two no-stimulus trials and two startle stimulus trials with no background noise. On each day, the startle stimulus level was held constant, while the level of the background noise was randomly varied to include the following: no background noise, 70, 75, and 80 dB SPL. Responses to the no-stimulation condition were tested to quantify the background level of pressure associated with random movements of the animal. To avoid giving the animal temporal cues on the timing of the startle stimulus, the times between successive startle stimuli were varied between 15 and 20 s. For the determination of gap suppression effects, the background noise contained a brief gap (50 ms) that began 100 ms before the onset of the startle stimulus. All the different sound conditions were intermixed and their order was double randomized within and across trials for each test session as in the first experiment. Since only one startle stimulus level was tested on a given day, the full complement of sessions for all four startle stimulus levels required 4 days. The 4-day series of test sessions was repeated four times, with control and exposed animals being tested on the same days. The level of sound was randomized over the 4-day test period.
Auditory brainstem responses
Each animal was anesthetized by intramuscular injection of ketamine/xylazine (58 mg/kg–9 mg/kg). Temperature was monitored and maintained at 37 °C using a heating pad with rectal probe. ABRs were recorded using an Intelligent Hearing System. Needle electrodes were placed subcutaneously in the following configuration: non-inverting in the vertex, linked inverting electrodes behind the left and right ears, and ground in the right hind limb. Electrode signals were amplified 100,000× and bandpass filtered (clicks, 150–3,000 Hz; tone pips, 30–3,000 Hz). Stimuli were presented at a rate of 17.7 stimuli per second. Responses were averaged over at least 250 stimulus repetitions over a time epoch of 12 ms for clicks and 26 ms for tone pips, including a 1-ms prestimulus period. Clicks (100 μs) and tone pips (4, 8, 12. and 16 kHz, all with 2.5-ms duration) were used to elicit responses and were delivered binaurally through ER2 ear inserts. For the click stimuli, recordings were obtained using rarefaction polarity. For the tone stimuli, recordings were obtained using alternating polarity. Stimuli were presented initially at 80 dB SPL then lowered in 20-dB steps until no responses were recorded. Stimuli were presented at levels greater than 80 dB SPL if no response or a weak response was present at 80 dB SPL. Intensity was bracketed in 5-dB steps to determine threshold. At each level of stimulation, at least two responses were obtained to confirm response or lack of a response. The ABR waveforms were generally consistent with those described previously in displaying five to six biphasic responses (Church and Kaltenbach 1993), with P4 being the response of most interest.
Data analysis
For each stimulus condition, measures were averaged first across trials for each session then across the days of identical trials for each animal. The final mean response amplitude for each group (exposed and controls) was obtained by averaging across animals. The results were used to generate plots comparing startle growth curves (startle amplitude vs. startle stimulus levels) for exposed and control animals in the absence of background noise. A second comparison was made between the startle response amplitudes with and without different levels of background noise in exposed and control animals. We then compared the effects of background noise containing gaps on the acoustic startle response with different levels of background noise. Next, we measured the degree of gap-induced changes in startle in exposed animals with those in controls. For the latter comparison, we examined both the absolute startle response amplitudes in each group as well as the startle ratio between the startle amplitude with gap to the startle amplitude with no gap. In all comparisons, ages in control animals were matched to those in exposed animals. Animal weights were first averaged across sessions to obtain a mean weight for each animal. The mean weight (± S.E.M.) for each group was then determined by averaging the weights across animals for each experiment. Group differences were tested using ANOVAs or two-tailed paired t-tests, performed using Excel and Prism GraphPad software. For each of the respective comparisons, differences were considered significant if P ≤ 0.05.
Results
In all 18 animals (nine exposed and nine controls), we obtained complete sets of behavioral data that included measures of acoustic startle responses to startle stimuli alone (no background noise) and acoustic startle stimuli with background noise with and without gaps. In seven of the control animals and eight of the exposed animals, successful recordings of ABR were also obtained. The results are summarized in the following paragraphs.
Effects of intense noise exposure on acoustic startle growth curves
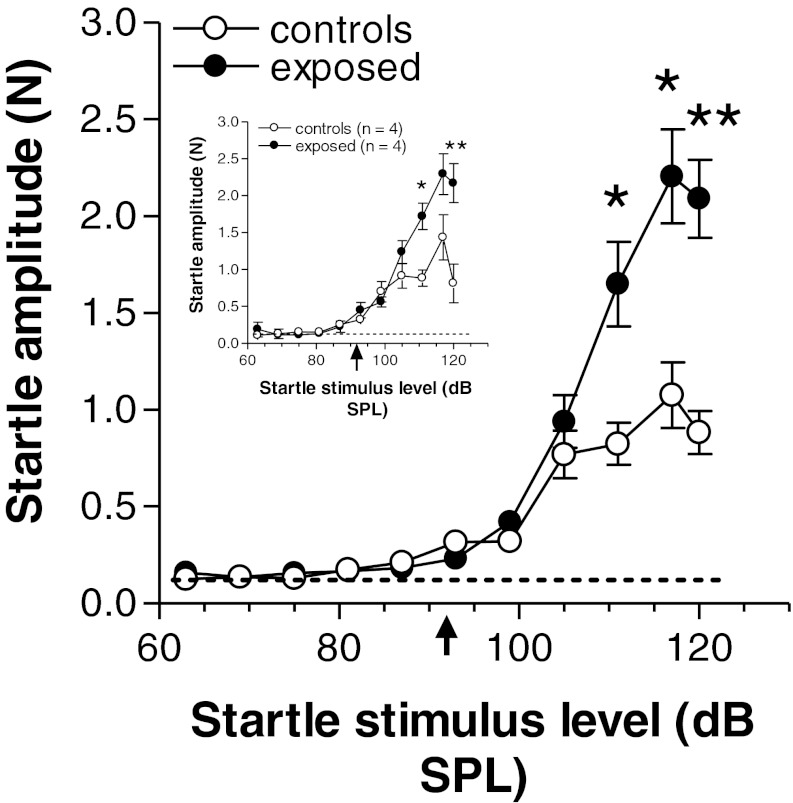
In this first experiment, we were interested in knowing how the acoustic startle response amplitude varied with changes in startle stimulus intensity and whether previous sound exposure might induce a change in the startle growth curve that could be related to hearing loss and hyperacusis. We studied the startle response as a function of startle stimulus level over a range of 57 to 120 dB SPL. The results of this analysis are presented in Fig. 2. The data show that the thresholds of the startle response in exposed animals were essentially the same as those in controls, occurring at approximately 92 dB SPL (arrow below abscissa). The curves for the two groups were similar up to a stimulus level of 105 dB SPL, but above this level, exposed animals showed a clear elevation of their startle responses. This hyper-responsiveness was statistically significant at all startle stimulus levels from 110 to 120 dB SPL (F(1,16) = 11.46, P = 0.004 for 110 dB SPL; F(1,16) = 14.53, P = 0.002 for 115 dB SPL; and F(1,16) = 27.6, P = 0.0002 for 120 dB SPL). The hyper-responsive state was found to be persistent over time since the same animals, when retested many weeks later (i.e., after completion of the gap suppression tests which will be described later), continued to display stronger startle responses in the exposed group than in controls (Fig. 2, inset). The larger responses in exposed hamsters are all the more impressive given that the mean weight of this group (155 ± 4.08 g, mean ± S.E.M.) was slightly lower than that of the control group (158 ± 4.00 g). This difference, however, was not statistically significant (T8 = 1.307, P = 0.228).
FIG. 2.
Comparisons of the growth of acoustic startle response amplitude in exposed and control animals with increasing startle stimulus level with no background noise present. Each point represents the mean ± S.E.M. of measures from nine animals. Note in each graph that the thresholds of the startle responses are 92 dB SPL in exposed and control animals (arrow below abscissa), and the much stronger responses (hyper-responsiveness) in exposed animals at higher stimulus levels. The inset shows a similar graph for the same animals retested after the completion of the studies that included background noise in the startle paradigm (summarized in Figs. 3, 4, 5 and 6). Asterisks indicate significance levels as follows: *P ≤ 0.024; **P = 0.0026. The dashed curve at the bottom of each graph represents the baseline level of pressure changes caused by random movement of the animal in the absence of stimulation.
Effect of background noise on startle responses in control and exposed animals
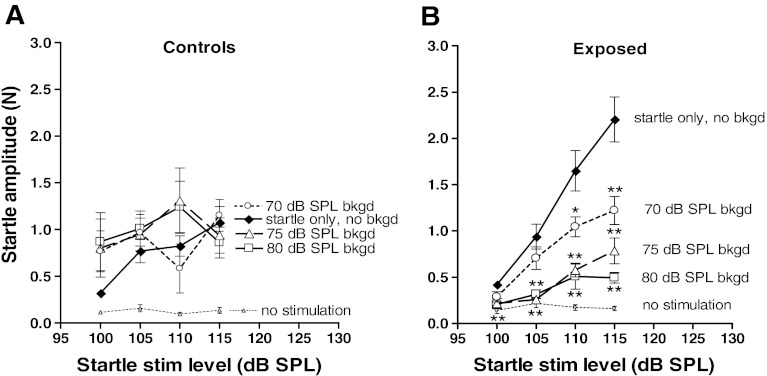
Addition of background noise affected startle responses very differently in control and exposed animals (Fig. 3). In control animals (Fig. 3A), there were no clear differences between startle amplitudes with and without background noise (i.e., startle responses were generally not suppressed by the addition of background noise). Instead startle responses in controls were generally slightly stronger in the presence of background noise, regardless of level (an exception to this was the response to the 110-dB SPL startle stimulus using a 70-dB SPL background noise, which was slightly lower than the response to the startle stimulus-only condition); none of these increases, however, was found to be statistically significant.
FIG. 3.
Effect of fixed levels of background noise on the acoustic startle growth curves. A In control animals, addition of background noise caused a slight but not significant increase in acoustic startle amplitudes. B In exposed animals, addition of background noise reduced the gain of the startle growth curve, indicating sensitization to background noise. Note the greater decrements in the growth curves with increases in background noise level. The dashed curve represents random pressure changes in the absence of stimulation (see legend to Fig. 2). Asterisks above the symbols in B represent measures showing significant differences from those in the uppermost curve (asterisks for the 80 dB SPL background noise level curve are shown below the symbols for that curve). *P < 0.05; **P < 0.002.
In contrast, the exposed animals showed an enhanced sensitivity to the background noise compared to controls. This is shown in Fig. 3B. In this group, startle amplitudes were reduced by background noise at all startle stimulus levels, and the degree of this reduction increased with the level of the background noise. The differences between the growth curves for the no-background condition (top curve in Fig. 3B) and that for each of the three background noise conditions in exposed animals (curves below top curve in Fig. 3B) are apparent. The stronger effect of background noise in exposed animals was not related to the larger size of these animals as their mean weight (186.6 ± 3.78) was very close to that of controls (187.3 ± 3.58 g).
The different effects of background noise on the startle responses in control and exposed animals were further dissected by comparing the startle amplitudes as a function of background noise level for each of the startle stimulus levels tested. As can be seen in Fig. 4A, there was no consistent trend towards higher startle amplitudes in controls as the background noise level was increased. However, a clear trend toward decreasing startle amplitudes with increases in background noise was observed in exposed animals at all startle stimulus levels (Fig. 4B). Thus, despite the fact that increasing the level of startle-eliciting stimuli caused increases in startle amplitude in both exposed and control animals (Fig. 2), increasing the level of the background noise had the opposite effect in exposed animals but not in controls.
FIG. 4.
Effect of increasing the level of background noise on startle amplitudes while holding startle stimulus level constant in control (A) and exposed (B) animals. Each graph compares startle responses with and without background noise. Note that, in contrast to control animals, exposed animals showed a sensitization to background noise manifested as a suppression of startle with the addition of background noise, and the degree of suppression increased with the level of background noise. Startle stimulus-only condition (no background noise) is based on the same data as that used for Fig. 2.
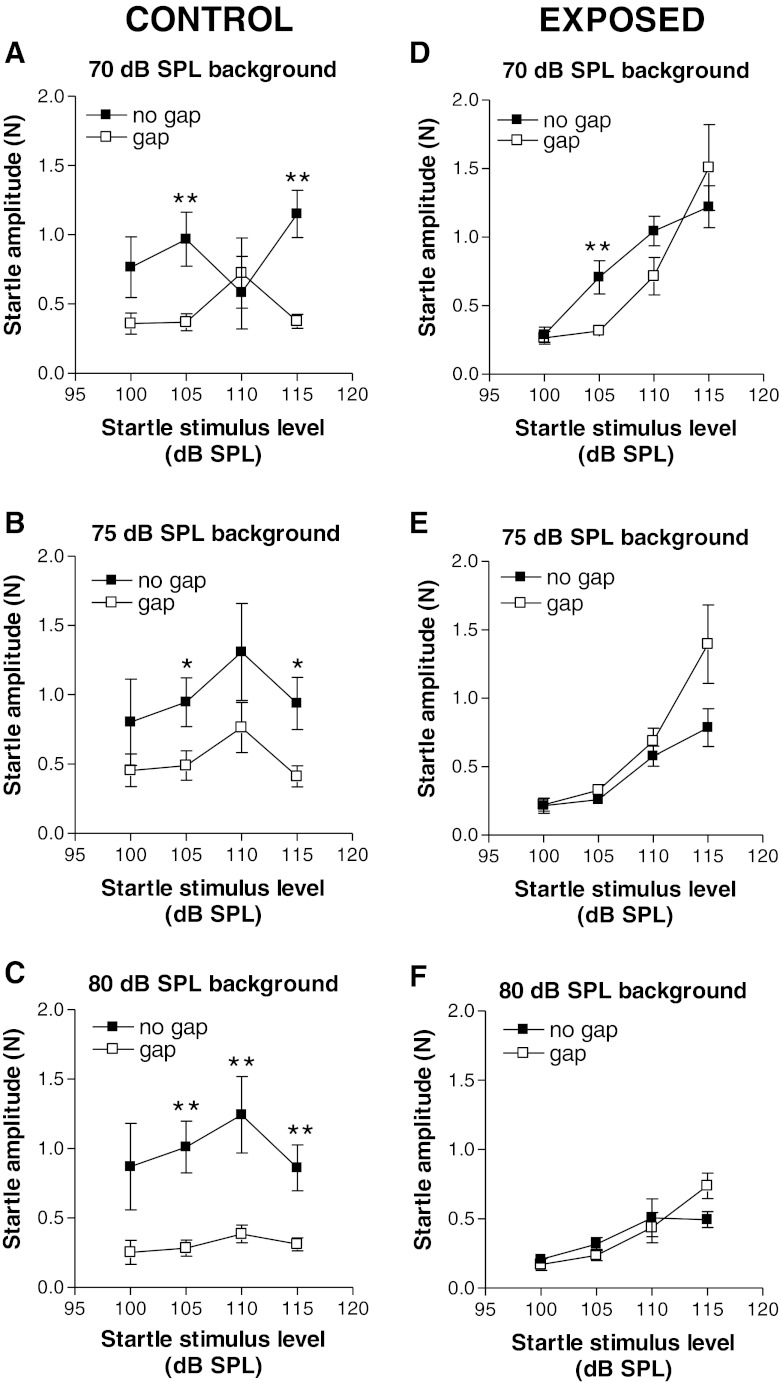
Effect of gaps in noise on startle response in control and exposed animals
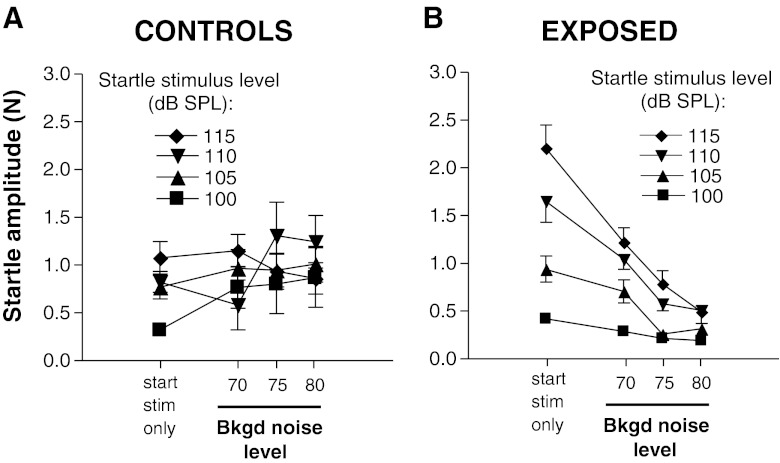
In control animals, startle responses were generally suppressed by insertion of a gap in the background of noise. This is shown in Fig. 5A–C, plotting the startle response amplitudes as a function of startle stimulus level (startle growth curves) for each of the three background noise levels tested. For just more than half of the stimulus combinations tested (7/12), the suppression of startle by gaps was significant (asterisks in each panel). For the 70-dB SPL background noise conditions (Fig. 5A), gap-induced suppression of the startle response was most robust and was statistically significant at startle stimulus levels of 105 and 115 dB SPL (T8 = 4.10, P = 0.003 and T8 = 5.339, P = 0.0007 at 105 and 115 dB SPL startle stimulus levels, respectively). A suppressive effect of gaps for the 80-dB background noise condition (Fig. 5C) was significant at the three highest startle stimulus levels (T8 = 5.195, P = 0.0008 at 105 dB SPL; T8 = 3.737, P = 0.0057 at 110 dB SPL, and T8 = 3.764, P = 0.006 at 115 dB SPL). Note that the stronger gap suppression at 80-dB SPL background noise level occurred despite the fact that the startle amplitudes in the no-gap conditions were in approximately the same range at all background noise levels (0.75 to 1.3 N) (compare filled squares in Fig. 5A–C). Thus, the higher gap-induced suppression observed at the highest level of background noise was not due to differences in the degree of masking of the startle stimulus or of the gap by the background noise.
FIG. 5.
Comparisons of responses to startle stimuli with background noise with and without gaps in control (A–C) and exposed (D–F) animals. Each pair of graphs plots a segment of the startle growth curve (100–115 dB SPL) with background noise at one of the following levels: 70 dB SPL (A and D), 75 dB SPL (B and E), and 80 dB SPL (C and F). Note that the generally wide separations between curves for the gap and no-gap conditions in control animals (A–C) were greatly diminished in exposed animals (D–F), indicating a weakening of gap suppression of startle. Each point represents the mean ± S.E.M. of measures from nine animals. *P < 0.05; **P ≤ 0.007.
Exposed animals generally showed greatly weakened gap-induced suppression of the startle. These results are shown in Fig. 5D–F. Some evidence for a persistence of strong and statistically significant gap-induced suppression of startle was seen when the background noise level was 70 dB SPL and the startle stimulus level was 105 dB SPL (Fig. 5D). However, for all other combinations of startle stimulus and background noise levels, no statistically significant differences were seen between the gap and no-gap test conditions. In fact, for the 75- and 80-dB SPL background noise conditions (Fig. 5E, F), the startle responses with gaps were almost the same as those without gaps over the range of 100 to 110 dB SPL startle stimulus levels; at the highest startle stimulus level (115 dB SPL), the startle responses with gap were actually stronger than those without gap for the 75- and 80-dB background noise levels, the opposite of what was observed in controls (Fig. 5B, C). These results are therefore consistent with the interpretation that the weakening of gap-induced suppression was not due to a floor effect resulting from suppression by the background noise, beyond which further suppression would not be possible.
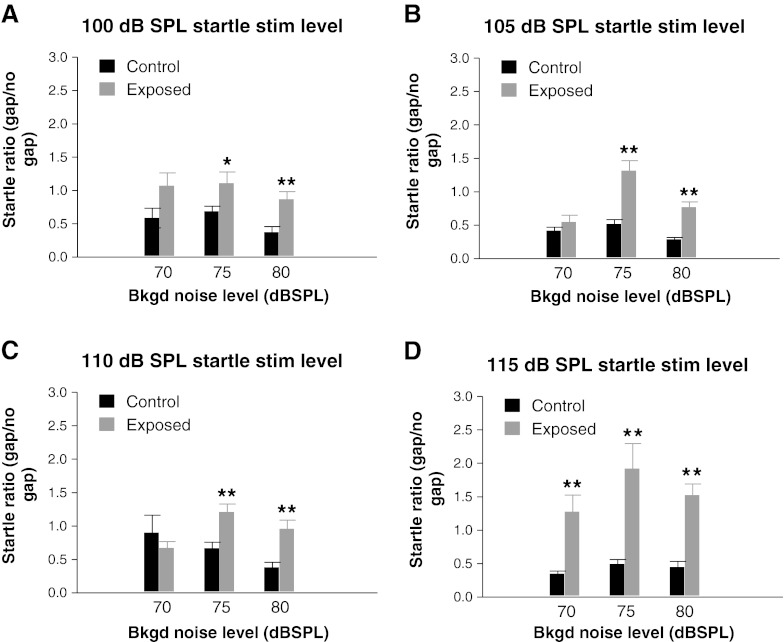
To further describe the losses of gap suppression that were induced by intense sound exposure, we next compared the relative startle amplitudes in exposed animals with those in controls (Fig. 6). By relative startle response, we are referring to the ratio of the startle amplitude with gap to the startle amplitude without gap (i.e., the percent of the original startle amplitude obtained without gap that remains when a gap is present). As can be seen, for almost every stimulus condition tested, there was an increase in the startle ratio in exposed animals compared to controls, indicating a weakening of the suppressive effect of gaps. For all four startle stimulus levels, the increases in startle ratio, representing the greatest loss of the gap-induced suppression effect, were significant when the background noise levels were either 75 or 80 dB SPL (P values between 0.038 and 0.0002). At a startle stimulus level of 115 dB SPL, all levels of background noise tested yielded highly significant increases in the startle ratio (Fig. 6D).
Fig. 6.
Effect of intense sound exposure on startle ratios as a function of background noise level for four different startle stimulus levels: a 100 dB SPL, b 105 dB SPL, c 110 dB SPL, and d 115 dB SPL. Note that higher startle ratios, indicative of a weakening of gap-induced suppression, were found in exposed animals at almost all startle stimulus levels, although there was considerable variability in the magnitude of this increase relative to those in controls, as a function both of startle stimulus level and background noise level. Each histogram bar represents the mean (± SEM) of measures from nine animals. *P < 0.05; **P < 0.005
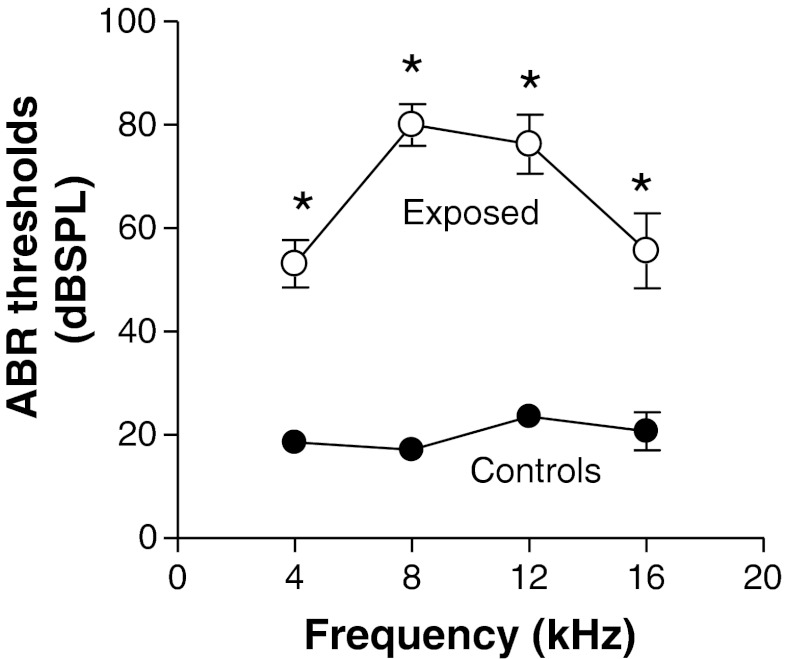
ABR thresholds
ABR were measured at the end of the startle tests to determine whether the animals experienced significant binaural hearing loss. The measures of the auditory brainstem responses revealed that the tone exposure resulted in marked increases in pure tone thresholds (Fig. 7). Thresholds of tone-evoked ABR in exposed animals were considerably higher than those in control animals at all frequencies tested. Mean thresholds were between 17 and 22 dB SPL in control animals, while those in exposed animals were between 53 and 80 dB SPL. Thresholds in exposed animals were shifted the most at 8 and 12 kHz where they were 63 and 54 dB higher than controls levels, respectively. Thus, ABR thresholds in both groups were well below the threshold of the startle response in both animal groups.
FIG. 7.
Comparison of mean ABR thresholds in exposed and control animals. The compromising effect of tone exposure on auditory sensitivity is demonstrated by the higher thresholds of the ABR at each of the eliciting tone frequencies tested. Each point represented the mean threshold ± S.E.M. of measures from seven control animals or eight exposed animals.
Discussion
In this study, we systematically measured startle responses over a wide range of startle intensities and background noise conditions with and without gaps. The wide range of stimulus conditions was tested because it became evident in the preliminary phases of our study that considerable variations in the startle responses were observed depending on the relationships among the startle stimulus level, the background noise level, and the presence or absence of gaps. Without a clear perspective of how these parameters of stimulation affected startle and how they interact, a meaningful interpretation of the effects of intense sound exposure on startle would have been difficult. We now summarize and discuss the major trends in our data and their relationships with the hearing disorders which were the focus of this study.
Intense sound exposure induced a chronic hyperacusis-like state of hyper-responsiveness to acoustic startle stimuli
Our comparison of startle growth curves from exposed and control animals revealed a clear hyper-responsiveness to startle-eliciting stimulus levels of 110 dB SPL and above (Fig. 2). Below this level, startle responses in the two groups were virtually indistinguishable, indicating that, despite shifts in ABR thresholds (see Fig. 7), intense sound exposure had no measurable effect on the threshold of the startle response (Fig. 2). Enhancements of startle have been observed in animals and proposed as correlates of hyperacusis following intense noise exposure (Sun et al. 2012), following treatment with sodium salicylate (Sun et al. 2009; Lu et al. 2011), and in aging animals (Ison et al. 2007). However, such enhancements of startle amplitude after noise exposure and salicylate treatment have not previously been shown to be chronic. Moreover, Longenecker and Galazyuk (2012) showed a decrease in acoustic startle amplitudes in mice following noise exposure. A similar result was obtained in rats by Lobarinas et al. (2013). It is possible that these differences may be species-related, but some potentially important methodological differences could also play a role. An important difference was the method of sound exposure. In our study, sound exposures were conducted binaurally with neither ear plugged. In the studies of Lobarinas et al. and Longenecker and Galazyuk, the exposures were conducted monaurally with the opposite ear plugged. If the induction of hyper-responsiveness is a result of a compensatory increase in response gain, the pathways involved in compensation or the degree of compensation itself may differ, depending on the relative differences in hearing loss in the two ears. Our ABR results, which were based on measures of binaural thresholds, indicate that the hearing losses in our sample of exposed animals were bilateral, whereas the studies employing the unilateral exposure would seem likely to have resulted in more asymmetric hearing losses.
Intense sound exposure caused a chronic hyperacusis-like sensitization to background noise
Our results revealed a sensitization of the startle response to background noise in exposed animals that was not seen in controls. In control animals, background noise tested at levels of 70, 75, and 80 dB SPL had no statistically significant effect on startle amplitude, although there was a suggestion of a slight enhancement of startle responses at certain startle stimulus levels (100, 105, and 110 dB SPL; see Fig. 3A). This result is similar to that observed over a similar range of background noise levels in normal-hearing rats (see Fig. 9 of Ison and Hammond 1971). In contrast, startle responses in our exposed animals were clearly suppressed by the addition of background noise. Thus, the exaggerated startle that was observed in exposed animals in response to the startle stimulus-only condition (Fig. 2) was abolished in the presence of background noise. Further weakening of the startle response was seen with increases in the level of background noise, such that at the two highest levels of background noise tested the growth of startle with increases in startle stimulus intensity was weaker in exposed animals than in controls (compare startle-only curve in Fig. 3A with curves for 75- and 80-dB SPL background noise in Fig. 3B). Also in exposed animals, increasing the level of background noise increased the degree of startle suppression relative to that evoked by startle stimulation alone. This was true at all intensities of startle stimulation tested above 100 dB SPL and for all levels of background noise tested (Fig. 4B). This sensitization to background noise is another demonstration of a hyperacusis-like response and may be related to the greater sensitivity to noise that has been found to be a common feature of hyperacusis in humans (Dauman and Bouscau-Faure 2005; Coelho et al. 2007).
Intense sound exposure induced a weakening of gap-induced suppression of startle
In control hamsters, gap-induced suppression of startle was observed for almost all stimulus conditions tested (Fig. 5, left column), although there was considerable variability in the strength and significance of this suppression across the different stimulus conditions. In contrast, significant suppression of startle by gaps was observed in only one case in exposed hamsters (Fig. 5D), indicating a general loss of gap suppression for the great majority (11/12) of startle conditions tested. Loss of gap-induced suppression of startle was most consistent at the middle and highest levels of background noise tested (75 and 80 dB SPL) (Fig. 5D–F). These results are in most respects consistent with those of other investigators using different species (Turner et al. 2012; Nowotny et al. 2011; Longenecker and Galazyuk (2011); Dehmel et al. 2012; Gaese et al. 2009; Lobarinas et al. 2013).
Weakening of gap suppression is often taken to mean that there is a decrease of gap detectability. While this may be true, it needs to be reconciled with the finding that exposed animals sometimes displayed startle ratios greater than 1 (Fig. 6). Startle ratios greater than 1 in exposed animals can also be found in Fig. 4 of Longenecker and Galazyuk (2011). Startle ratios greater than 1 could be a measurement artifact resulting from a few trials yielding very high ratios due to measurement error. However, this did not appear to be the case. For most of the examples in which startle ratios were above 1, several animals in the exposed group showed repeated occurrences of much stronger startle responses to the gap condition than to the no-gap conditions, and for each animal there were no outlier measurements that could explain the results. Startle ratios >1 could mean that the gaps somehow become more detectable after noise exposure, perhaps because the tinnitus interacts with the offset of the background sound to create a hybrid sound that is not present in the control group. Another possibility is that some feature about the tinnitus might result in a facilitation of startle response. We suggest that the facilitation may result from a slight mismatch between the spectrum of the induced tinnitus and that of the background noise. A slight mismatch would increase the probability that the tinnitus filling in the gap would be perceived differently from the background noise or from the tinnitus that occurs during the background noise period and would be detected as a separate pulse of sound of its own (i.e., a “tinnitus pulse”) spectrally distinct from the background noise. If this occurs, the resulting percept could compete with the background noise on the startle response, weakening its suppressive effect and making startle responses stronger. Alternatively, the tinnitus pulse could facilitate the startle response, independent of the background noise. This would be consistent with a previous study in mice reporting that startle responses can sometimes be facilitated when a pulse of sound preceding the startle stimulus consists of a high-pitch tone (Plappert et al. 2004).
How confident can we be that the weakening of gap suppression represents evidence for tinnitus?
Numerous studies have interpreted the weakening of gap suppression of the acoustic startle after noise exposure or salicylate treatment as evidence for tinnitus (Turner et al. 2006, 2012; Wang et al. 2009, 2011; Middleton et al. 2011; Middleton and Tzounopoulos 2012; Longenecker and Galazyuk 2011; Dehmel et al. 2012; Nowotny et al. 2011; Lobarinas et al. 2013). The reasoning is that tinnitus whose pitch is approximately matched to the spectrum of the background noise would fill in the gap more effectively, making gap detection more difficult. While hearing loss could also cause a similar weakening of gap suppression, others have found that gap-induced suppression of startle is either not weakened by conductive hearing loss (Turner et al. 2006) or is weakened in animals even after they have recovered from hearing loss (Dehmel et al. 2012; Turner et al. 2012). Moreover, weakened gap suppression has recently been observed in human subjects with tinnitus but no hearing loss (Fournier and Hébert 2013).
In the present study, we used exposure conditions which caused severe binaural threshold shifts from which the animals did not recover. ABR measures conducted after acoustic startle response (ASR) testing demonstrated major chronic shifts in response thresholds at all frequencies tested. Previously, we have shown that exposure conditions similar to those used here resulted in tinnitus-related changes in activity in auditory brainstem nuclei of hamsters (Finlayson and Kaltenbach 2009; Manzoor et al. 2012, 2013). Increases in spontaneous activity were found to occur broadly across the tonotopic range but generally peaked in the high-frequency region between 12 and 14 kHz. Since the background noise used in our experiments had a frequency band of 12–14 kHz, the animals’ tinnitus would be expected to be in the bandwidth encompassed by our background noise conditions. The presence of tinnitus within this range would be expected to weaken gap suppression due to its masking effect on the gap.
An issue that needs to be addressed is the possibility that the weakening of gap suppression in our exposed animals was more related to hearing loss than to tinnitus. Hearing loss could, theoretically, also cause a weakening of gap suppression and an increase in startle ratio if the hearing loss leads to a decrease in the sensitivity to the background noise containing the gap. However, this was clearly not the case since the responses to background noise were not weakened in exposed animals but were instead strengthened. This was evidenced by the fact that background noise had a suppressive effect on the startle in exposed animals but no significant effect in controls (Figs. 3 and 4). Moreover, despite the induction of hearing loss, the thresholds of the startle responses in exposed and control animals were essentially the same. Thus, hearing loss apparently did not elevate the thresholds of responses to either the startle stimulus or the background noise. This may seem paradoxical, but it is often the case that hearing loss is associated with enhanced responses to suprathreshold stimuli (e.g., background noise), which is why hearing loss and hyperacusis are often associated.
Another possibility is that weakened gap suppression could be more related to hyperacusis than to tinnitus. The two types of hyperacusis-like effects observed in exposed animals, including the enhancement of the startle response (Fig. 2) and the sensitization to background noise on startle (Figs. 3 and 4), could theoretically have weakened gap-induced suppression of startle. If the startle amplitude is enhanced in exposed animals, the stronger startle response might be more difficult to suppress, leading to a relative weakening of startle suppression by gaps. This explanation can be readily dismissed because there was no enhancement of the startle response in exposed animals when the startle stimulus was presented with background noise. On the other hand, an increase in the suppressive effect of background noise on startle could be associated with an increase in suppression of the response to the gap (i.e., suppression of the gap’s suppressive effects). This explanation is also unlikely since increasing the background noise level in control animals while holding the startle stimulus level constant did not result in a weakening of gap suppression (Fig. 6); indeed one set of results showed a trend toward an increase in gap suppression as the background noise level was increased (see black bars in Fig. 6C). Taken together, these findings indicate that the induced hyperacusis-like behaviors exhibited by exposed animals are unlikely to account for the weakening of gap suppression that our exposed animals displayed.
Another possible explanation of the weakened gap suppression that occurred in noise-exposed animals is that it reflects a slowing of temporal processing, independent of tinnitus or hyperacusis. For example, gaps might be less likely to have a suppressive effect if the gap is filled in by activity that carries over into the gap interval from previous activation by the preceding background noise. However, had spillover of activity from the response to the background noise been the reason for reduced gap suppression of startle, we would have expected to see an increase in this weakening with background noise level. Our results showed no consistent trend toward greater loss of gap suppression as background noise level was increased. We believe that the view, that weakening of gap-induced suppression of startle represents evidence for tinnitus, continues to be a reasonable interpretation of such results, although additional confirmatory evidence based on studies of the effects of noise exposure on temporal processing of the gaps themselves will be necessary before this explanation can be completely ruled out.
Different gain controls of acoustic startle are affected differently by intense sound exposure
The augmented startle response seen in the absence of background noise, the emergence of suppressive effects of background noise on startle, and the weakening of suppressive effect of gaps on startle imply the existence of multiple gain controls of acoustic startle. The basic circuitry underlying the startle response has previously been shown to involve input from the auditory nerve to the cochlear root nucleus and its connections with the pontine reticular nucleus (Davis et al. 1982; Lee et al. 1996; Koch and Friauf 1995; Lingenhöhl and Friauf 1992, 1994) which mediates startle responses via the reticulo-spinal pathway. This circuit likely represents the essential core on which ascending and descending pathways that modulate the startle gain exert their influence. The gain that is background noise-dependent may involve a circuit more concerned with spectral context, while the gain that is gap-dependent may involve a pathway specialized for processing of temporal cues. Although little direct information about the substrates underlying these different gain controls is available, it has been shown that gap detection can be modulated by blocking acetylcholine receptors (Ison and Bowen 2000). Detection of short-duration gaps (<30 ms) but not gaps longer than 100 ms is dependent on the integrity of the auditory cortex (Bowen et al. 2003). Whether the cortex is also required for detection of gaps of 50 ms, such as those used here, has not yet been determined.
Acknowledgments
The authors would like to express their gratitude to Dr. Alexander Galazyuk and Ryan Longenecker, at Northeastern Ohio Medical University, for comments on the manuscript and for offering their technical expertise in the use of the Kinder system during the early stages of our study. This work was supported by NIH grant R01 DC009097.
References
- Anari M, Axelsson A, Eliasson A, Magnusson L. Hypersensitivity to sound—questionnaire data, audiometry and classification. Scand Audiol. 1999;28:219–230. doi: 10.1080/010503999424653. [DOI] [PubMed] [Google Scholar]
- Bartnik G, Fabijanska A, Rogowski M. Our experience in treatment of patients with tinnitus and/or hyperacusis using the habituation method. In: Hazell JWP, editor. Proc. of the VIth Internat. Tinnitus Sem. London: The Tinnitus and Hyperacusis Centre, 6; 1999. pp. 416–417. [Google Scholar]
- Bowen GP, Lin D, Taylor MK, Ison JR. Auditory cortex lesions in the rat impair both temporal acuity and noise increment thresholds, revealing a common neural substrate. Cereb Cortex. 2003;13:815–822. doi: 10.1093/cercor/13.8.815. [DOI] [PubMed] [Google Scholar]
- Church MW, Kaltenbach JA (1993) The hamster’s auditory brain stem response as a function of stimulus intensity, tone burst frequency, and hearing loss. Ear Hear 14:249–257 [DOI] [PubMed]
- Coelho CB, Sanchez TG, Tyler RS. Hyperacusis, sound annoyance, and loudness hypersensitivity in children. Prog Brain Res. 2007;166:169–178. doi: 10.1016/S0079-6123(07)66015-4. [DOI] [PubMed] [Google Scholar]
- Dauman R, Bouscau-Faure F. Assessment and amelioration of hyperacusis in tinnitus patients. Acta Otolaryngol. 2005;125:503–509. doi: 10.1080/00016480510027565. [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM. A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci. 1982;2:791–805. doi: 10.1523/JNEUROSCI.02-06-00791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Syst Neurosci. 2012;6:42. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson PG, Kaltenbach JA. Alterations in the spontaneous discharge patterns of single units in the dorsal cochlear nucleus following intense sound exposure. Hear Res. 2009;256:104–117. doi: 10.1016/j.heares.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, Hébert S (2013) Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: does tinnitus fill in the gap? Hear Res 295:16–23 [DOI] [PubMed]
- Gaese BH, Nowotny M, Pilz PK. Acoustic startle and prepulse inhibition in the Mongolian gerbil. Physiol Behav. 2009;98:460–466. doi: 10.1016/j.physbeh.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Gu JW, Halpin CF, Nam EC, Levine RA, Melcher JR. Tinnitus, diminished sound-level tolerance, and elevated auditory activity in humans with clinically normal hearing sensitivity. J Neurophysiol. 2010;104:3361–3370. doi: 10.1152/jn.00226.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ison JR, Bowen GP. Scopolamine reduces sensitivity to auditory gaps in the rat, suggesting a cholinergic contribution to temporal acuity. Hear Res. 2000;145:169–176. doi: 10.1016/S0378-5955(00)00088-5. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of the startle reflex in the rat by changes in the auditory and visual environments. J Comp Physiol Psychol. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE. Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J Assoc Res Otolaryngol. 2007;8:539–550. doi: 10.1007/s10162-007-0098-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jastreboff PJ, Jastreboff MM. Tinnitus retraining therapy (TRT) as a method for treatment of tinnitus and hyperacusis patients. J Am Acad Audiol. 2000;11:162–177. [PubMed] [Google Scholar]
- Kaltenbach JA, Godfrey DA, Neumann JB, McCaslin DL, Afman CE, Zhang J. Changes in spontaneous neural activity in the dorsal cochlear nucleus following exposure to intense sound: relation to threshold shift. Hear Res. 1998;124:78–84. doi: 10.1016/S0378-5955(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Koch M, Friauf E. Glycine receptors in the caudal pontine reticular formation: are they important for the inhibition of the acoustic startle response? Brain Res. 1995;671:63–72. doi: 10.1016/0006-8993(94)01309-6. [DOI] [PubMed] [Google Scholar]
- Lee Y, López DE, Meloni EG, Davis M. A primary acoustic startle pathway: obligatory role of cochlear root neurons and the nucleus reticularis pontis caudalis. J Neurosci. 1996;16:3775–3789. doi: 10.1523/JNEUROSCI.16-11-03775.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the caudal pontine reticular formation receive short latency acoustic input: an intracellular recording and HRP-study in the rat. J Comp Neurol. 1992;325:473–492. doi: 10.1002/cne.903250403. [DOI] [PubMed] [Google Scholar]
- Lingenhöhl K, Friauf E. Giant neurons in the rat reticular formation: a sensorimotor interface in the elementary acoustic startle circuit? J Neurosci. 1994;14:1176–1194. doi: 10.1523/JNEUROSCI.14-03-01176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobarinas E, Hayes SH, Allman BL (2013) The gap-startle paradigm for tinnitus screening in animal models: Limitations and optimization. Hear Res 295:150–160 [DOI] [PMC free article] [PubMed]
- Longenecker RJ, Galazyuk AV. Development of tinnitus in CBA/CaJ mice following sound exposure. J Assoc Res Otolaryngol. 2011;12:647–658. doi: 10.1007/s10162-011-0276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker RJ, Galazyuk AV (2012) Methodological optimization of tinnitus assessment using prepulse inhibition of the acoustic startle reflex. Brain Res 1485:54–62 [DOI] [PubMed]
- Lu J, Lobarinas E, Deng A, Goodey R, Stolzberg D, Salvi RJ, Sun W. GABAergic neural activity involved in salicylate-induced auditory cortex gain enhancement. Neuroscience. 2011;189:187–198. doi: 10.1016/j.neuroscience.2011.04.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor NF, Licari FG, Klapchar M, Elkin RL, Gao Y, Chen G, Kaltenbach JA. Noise-induced hyperactivity in the inferior colliculus: its relationship with hyperactivity in the dorsal cochlear nucleus. J Neurophysiol. 2012;108:976–988. doi: 10.1152/jn.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoor NF, Gao Y, Licari F, Kaltenbach JA (2013) Comparison and contrast of noise-induced hyperactivity in the dorsal cochlear nucleus and inferior colliculus. Hear Res 295:114–123 [DOI] [PMC free article] [PubMed]
- Middleton JW, Tzounopoulos T. Imaging the neural correlates of tinnitus: a comparison between animal models and human studies. Front Syst Neurosci. 2012;6:35. doi: 10.3389/fnsys.2012.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton JW, Kiritani T, Pedersen C, Turner JG, Shepherd GM, Tzounopoulos T. Mice with behavioral evidence of tinnitus exhibit dorsal cochlear nucleus hyperactivity because of decreased GABAergic inhibition. Proc Natl Acad Sci USA. 2011;108:7601–7606. doi: 10.1073/pnas.1100223108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowotny M, Remus M, Kössl M, Gaese BH. Characterization of the perceived sound of trauma-induced tinnitus in gerbils. J Acoust Soc Am. 2011;130:2827–2834. doi: 10.1121/1.3646902. [DOI] [PubMed] [Google Scholar]
- Plappert CF, Pilz PK, Schnitzler HU. Factors governing prepulse inhibition and prepulse facilitation of the acoustic startle response in mice. Behav Brain Res. 2004;152:403–412. doi: 10.1016/j.bbr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Rybalko N, Syka J. Effect of noise exposure on gap detection in rats. Hear Res. 2005;200:63–72. doi: 10.1016/j.heares.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ. Salicylate increases the gain of the central auditory system. Neuroscience. 2009;159:325–334. doi: 10.1016/j.neuroscience.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Deng A, Jayaram A, Gibson B. Noise exposure enhances auditory cortex responses related to hyperacusis behavior. Brain Res. 2012;1485:108–116. doi: 10.1016/j.brainres.2012.02.008. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish J. Gap detection methods for assessing salicylate-induced tinnitus and hyperacusis in rats. Am J Audiol. 2008;17:S185–S192. doi: 10.1044/1059-0889(2008/08-0006). [DOI] [PubMed] [Google Scholar]
- Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM. Gap detection deficits in rats with tinnitus: a potential novel screening tool. Behav Neurosci. 2006;120:188–195. doi: 10.1037/0735-7044.120.1.188. [DOI] [PubMed] [Google Scholar]
- Turner J, Larsen D, Hughes L, Moechars D, Shore S. Time course of tinnitus development following noise exposure in mice. J Neurosci Res. 2012;90:1480–1488. doi: 10.1002/jnr.22827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Turner JG, Ling L, Parrish JL, Hughes LF, Caspary DM. Plasticity at glycinergic synapses in dorsal cochlear nucleus of rats with behavioral evidence of tinnitus. Neuroscience. 2009;164:747–759. doi: 10.1016/j.neuroscience.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Brozoski TJ, Caspary DM. Inhibitory neurotransmission in animal models of tinnitus: maladaptive plasticity. Hear Res. 2011;279:111–117. doi: 10.1016/j.heares.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]