Abstract
Medial olivocochlear efferent neurons can control cochlear frequency selectivity and may be activated in a reflexive manner by contralateral sounds. The present study investigated the significance of the contralateral medial olivocochlear reflex (MOCR) on human psychoacoustical tuning curves (PTCs), a behavioral correlate of cochlear tuning curves. PTCs were measured using forward masking in the presence and in the absence of a contralateral white noise, assumed to elicit the MOCR. To assess MOCR effects on apical and basal cochlear regions over a wide range of sound levels, PTCs were measured for probe frequencies of 500 Hz and 4 kHz and for near- and suprathreshold conditions. Results show that the contralateral noise affected the PTCs predominantly at 500 Hz. At near-threshold levels, its effect was obvious only for frequencies in the tails of the PTCs; at suprathreshold levels, its effects were obvious for all frequencies. It was verified that the effects were not due to the contralateral noise activating the middle-ear muscle reflex or changing the postmechanical rate of recovery from forward masking. A phenomenological computer model of forward masking with efferent control was used to explain the data. The model supports the hypothesis that the behavioral results were due to the contralateral noise reducing apical cochlear gain in a frequency- and level-dependent manner consistent with physiological evidence. Altogether, this shows that the contralateral MOCR may be changing apical cochlear responses in natural, binaural listening situations.
Keywords: medial olivocochlear reflex, efferent control, frequency selectivity, forward masking, cochlear model, cochlear nonlinearity
Introduction
Humans have the (limited) ability to separately perceive the multiple frequency components of a complex sound. This property, termed auditory frequency selectivity, has its physiological origin in the frequency selective nature of basilar membrane (BM) responses (Evans 2001; Shera et al. 2002). BM frequency selectivity depends upon the status of cochlear outer hair cells (Robles and Ruggero 2001). Medial olivocochlear (MOC) efferent neurons innervate these cells and convey signals that are capable of controlling BM sensitivity and tuning (Murugasu and Russell 1996; Russell and Murugasu 1997; Cooper and Guinan 2006). MOC efferents may be activated in a reflexive manner by contralateral sounds (Guinan 2006, 2010). Therefore, cochlear responses may change dynamically in binaural listening situations. The present study aimed at investigating the effects of the contralateral medial olivocochlear reflex (MOCR) on human cochlear frequency selectivity.
MOC neurons can be activated by acoustic stimuli (Robertson and Gummer 1985), and there is a wealth of evidence that contralateral stimulation reduces the levels of human otoacoustic emissions, a physiological indicator of cochlear function (Collet et al. 1990; Souter 1995; Guinan et al. 2003; Atcherson et al. 2008; Sun 2008; Lilaonitkul and Guinan 2009a, b; Francis and Guinan 2010). Psychoacoustical tuning curves (PTCs) (Small 1959) are regarded as the behavioral correlate of physiological cochlear tuning curves (Evans 2001; Shera et al. 2002). Therefore, the role and significance of the contralateral MOCR on human cochlear frequency selectivity may be assessed by measuring PTCs in the presence and in the absence of contralateral stimulation. Few studies have used this approach, and they report greater effects in the tails than in the tips of the PTCs (Kawase et al. 2000; Quaranta et al. 2005; Vinay and Moore 2008). At first sight, this appears inconsistent with the well-established physiological evidence that efferent activation affects mostly the tip of basal BM tuning curves, that is, the stimulus frequencies subject to cochlear compression (Guinan 2006, 2010). This inconsistency, however, is likely more apparent than real as, in contrast to BM tuning curves, two stimuli are required to measure a PTC (a probe tone and a masker sound) and so efferents may affect either or both of them. Indeed, most reported behavioral effects are broadly consistent with a reduction in cochlear gain (e.g., Kawase et al. 2000; Strickland 2001; Jennings et al. 2009; Jennings and Strickland 2012b). The size of the behavioral effects, however, is typically small and their direction is not always consistent across studies (see below).
We hypothesize that the conditions used thus far in previous behavioral studies were insufficient to properly reveal contralateral MOCR effects. Indeed, previous experimental studies have focused on PTCs at levels near threshold. Since the effects of efferent activation vary with level (Cooper and Guinan 2006), the effect of contralateral stimulation on PTCs might be more obvious for suprathreshold conditions. A study published during the revision of the present manuscript suggests that this is possibly the case (Jennings and Strickland 2012b). Furthermore, with one exception (Vinay and Moore 2008), previous studies have focused on cochlear regions above 1 kHz, where contralateral MOCR effects may be smaller than at lower frequencies (Lilaonitkul and Guinan 2009a, b, 2012). Indeed, the latency of click-evoked otoacoustic emissions, a presumed indirect measure of cochlear frequency selectivity, is reduced by contralateral broadband noise more at low than at high test frequencies (Francis and Guinan 2010). In the present study, contralateral MOCR effects were investigated for near-threshold and suprathreshold PTCs and for probe frequencies of 500 Hz and 4 kHz. It will be shown that the effects of contralateral stimulation are more obvious at 500 Hz and for supra threshold PTCs.
We further hypothesize that it is not possible to assess the direction and size of contralateral MOCR effects on physiological cochlear tuning curves directly from behavioral PTCs. This is particularly true at low frequencies, where compression and hence active cochlear mechanisms occur for a comparatively broader range of stimulus frequencies than at high frequencies (Rhode and Cooper 1996; Lopez-Poveda et al. 2003; Plack and Drga 2003). Here, a physiologically inspired, phenomenological computer model of forward masking with efferent control is used to explain the effects of contralateral stimulation on PTCs. Complementary control tests were performed to verify that the effects were not the result of the contralateral noise activating the middle-ear muscle reflex or changing the postmechanical rate of recovery from forward masking. A preliminary version of this work is to be published as a conference proceeding (Lopez-Poveda et al. 2013).
Materials and methods
Rationale and assumptions
The main experiment consisted of presenting subjects with a brief pure tone probe preceded by a longer pure tone masker and measuring the level of the masker at the detection threshold of the probe. The probe level was fixed only a few decibels above the subject’s detection threshold for the probe in the absence of the masker. In these conditions, the masker level at threshold is assumed to depend on the relative excitation of the masker and the probe at the cochlear region activated by the probe. The graphical representation of the threshold masker levels as a function of masker frequency is called a PTC (Small 1959). Because the probe is fixed in frequency and level, all maskers in any given PTC are assumed to produce the same excitation at the probe cochlear region. Therefore, PTCs are thought of as isoresponse curves and indeed are regarded as behavioral correlates of cochlear tuning curves (Evans 2001; Shera et al. 2002).
To assess the strength of the MOCR on apical and basal cochlear responses, PTCs were measured here for probe frequencies of 500 Hz and 4 kHz, respectively. Contralateral white noise (CWN) is effective in activating the MOCR (Lilaonitkul and Guinan 2009b). Therefore, the effect of the MOCR was assessed here by comparing PTCs measured in the presence and in the absence of a CWN. The sound pressure level (SPL) of the CWN was fixed at 60 dB because this level is capable of activating the MOCR without activating the MEM reflex (Lilaonitkul and Guinan 2009a, b). Nevertheless, a test was carried out to ensure that our CWN in combination with the maskers involved in the PTC measurements did not activate the MEM reflex.
The threshold masker level also depends on the time gap between the masker and the probe. As this gap increases, recovery from forward masking increases and so it becomes necessary to increase the masker level to achieve a masking threshold. Therefore, it is possible to assess cochlear tuning across levels by measuring PTCs for different masker–probe time gaps. Here, the effect of the CWN on near-threshold cochlear frequency selectivity was assessed by measuring PTCs for a short (2 ms) masker–probe time gap; the effect of the CWN on suprathreshold tuning was assessed by measuring PTCs for longer masker–probe time gaps. The measured rate of recovery from forward masking is assumed to simultaneously depend on the cochlear (mechanical) response to the masker and on the postmechanical rate of recovery from cochlear excitation (Oxenham and Moore 1994; Nelson et al. 2001). We will refer to the latter contribution as the postmechanical rate of recovery from forward masking. Insofar as the CWN is expected to change cochlear responses (e.g., to reduce cochlear gain, compression and/or tuning), it is also expected to change the measured rate of recovery from forward masking (e.g. Krull and Strickland 2008). An implicit assumption of our approach, however, is that the postmechanical rate of recovery from forward masking is not affected by the CWN. A control experiment (described below) was carried out to confirm this assumption. One last implicit assumption of our approach is that the postmechanical rate of recovery from forward masking is frequency and level independent. This is a common assumption (e.g. Oxenham and Moore 1994; Nelson et al. 2001; Lopez-Poveda et al. 2003, 2007; Yasin and Plack 2003) but has been recently disputed for masker levels above around 83 dB SPL (Wojtczak and Oxenham 2009). The accuracy of this assumption and its implication for the interpretation of the present data will be discussed in “Discussion.”
All experimental procedures were approved by the Ethical Review Board of the University of Salamanca.
Subjects
Two female (S1 and S4) and three male (S2, S3, and S5) subjects with normal audiometry and tympanometry participated in the experiments. Subjects’ ages (in years) were 31 (S1), 31 (S2), 43 (S3), 27 (S4), and 25 (S5). All subjects were tested in their right ears, having the CWN delivered in their left ears. Subjects S1, S2, and S3 participated in all experiments; subjects S4 and S5 participated only in measures of near-threshold PTCs. Subjects were volunteers and were not paid for their services.
Probe thresholds and PTCs
Unless otherwise stated, probe thresholds and PTCs were measured using equipment and procedures identical to those of Lopez-Poveda et al. (2007). Probe frequencies were 500 Hz and 4 kHz. Masker frequencies ranged from 0.5 to 1.3 times the probe frequency. The durations of the probes and the maskers were 10 and 200 ms, respectively, including 5-ms cosine-squared onset and offset ramps. PTCs were measured for masker–probe time gaps (defined from masker offset to probe onset) of 2, 10, 30, and 50 ms. All PTCs were measured with and without a CWN. The CWN had a duration of 1,210 ms. It started 500 ms before and ended well after the stimuli used to measure the PTCs. For example, the total stimulus duration for the longest 50 ms masker–probe gap was 260 ms (200-ms masker + 50-ms gap + 10-ms probe); hence, the noise ended 450 ms after the probe offset (1,210-ms total noise duration–500-ms prenoise segment–260-ms stimulus duration). The level of the CWN was fixed at 60 dB SPL. The level of the probe was held constant at 10 dB above individual absolute threshold for the probe measured without the CWN. It is important to note that the probe SPL remained identical with and without the CWN.
A two-interval, two-alternative, and forced-choice adaptive procedure was employed to estimate the masker level at threshold. The time period between the two intervals was 500 ms. Feedback was provided to the listener. Masker level was changed according to a two-up, one-down adaptive procedure to estimate the 71 % point on the psychometric function (Levitt 1971). The masker level step was 6 dB for the first two reversals and 2 dB thereafter. Threshold was calculated as the mean of the masker levels for the last 12 reversals. The measured value was discarded if the standard deviation exceeded 6 dB. Each masker threshold was measured at least three times and reported values are the mean of the three measurements. If the SD of these three measurements exceeded 6 dB, a fourth measure was obtained and included in the mean. A threshold measurement was aborted when the adaptive procedure called for masker levels higher than the maximum system output (100 dB SPL) on three successive trials. Stimuli were digitally generated (sampling frequency of 44100 Hz), output via an RME Fireface 400 sound card, and played through Etymotic ER2 insert earphones with nominal interaural attenuation of 70+ dB. Subjects performed the task while sitting in a double-wall sound attenuating chamber.
Probe absolute thresholds were measured using identical procedures and equipment except that a two-down, one-up adaptive rule was used. Absolute thresholds for the 500 Hz probe were 23 (S1), 19 (S2), 21 (S3), 21 (S4), and 18 (S5) dB SPL, respectively. Absolute thresholds for the 4 kHz probe were 18 (S1), 23 (S2), 20 (S3), 14 (S4), and 13 (S5) dB SPL, respectively.
Postmechanical recovery from forward masking test
A test was carried out to ensure that the CWN did not affect the postmechanical rate of recovery from forward masking. Recovery from forward masking is commonly characterized by measuring the level of a forward pure tone masker at the detection threshold of a brief fixed-level, fixed-frequency pure tone probe as a function of the masker–probe time gap. The resulting curve is referred to as a temporal masking curve (TMC). The slope of a TMC is thought to simultaneously depend on the postmechanical rate of recovery from forward masking and on BM compression on the masker (Nelson et al. 2001). Therefore, the postmechanical rate of recovery from forward masking may be inferred from the slope of a TMC for a masker frequency that is subject to linear (rather than compressive) cochlear responses. Basal BM responses to low frequency stimuli are linear (Robles and Ruggero 2001). In human, sinusoidal maskers with a frequency of 1,600 Hz evoke linear responses when masking a 4-kHz sinusoidal probe (Lopez-Poveda and Alves-Pinto 2008; Plack and Arifianto 2010). Therefore, the test consisted of comparing the slope of a TMC for a probe frequency of 4 kHz and a masker frequency of 1,600 Hz, referred to as a linear reference TMC, as measured in the presence and in the absence of the CWN. Stimuli and methods were identical to those used to measure the PTCs (described in the preceding section). Masker–probe time gaps ranged from 10 to 50 ms in 10-m steps, with an additional step of 2 ms. The test was performed in the three subjects for whom suprathreshold PTCs were measured (S1, S2, and S3).
Middle-ear muscle contraction test
The middle-ear muscle (MEM) contraction test of Lilaonitkul and Guinan (2009b) was performed to ensure that the CWN did not activate the MEM reflex over the range of frequencies involved in the PTC measurements. When a test tone is presented in the ear canal of a subject, the ear-canal level for the tone depends on the middle-ear impedance and so on the activation state of the MEM reflex. Therefore, the test consisted of measuring the ear-canal level of test tones of various frequencies in the presence and in the absence of a CWN. A potential confounding factor is that the ear-canal level of the test tone also depends upon the level of the stimulus frequency otoacoustic emission (SFOAE) generated by the test tone in the inner ear. To account for this confounding factor, a suppressor tone 110 Hz above the test tone frequency was employed to suppress the SFOAE contribution (for further details of this test, see Lilaonitkul and Guinan 2009b). Test tone frequencies ranged from 396 Hz to 4 kHz, covering most of frequencies involved in PTC measurements. The threshold level of activation of the MEM reflex by pure tones is around 65 dB SPL (Neumann et al. 1996). Hence, to confirm that the CWN by itself was unable to activate the MEM reflex, the test was performed using low level (40 dB SPL) test tones. In this case, the level of the suppressor was 65 dB SPL. Obviously, the maskers involved in the PTC measurements with levels above around 65 dB SPL can, in principle, activate the MEM reflex both in the presence and in absence of the CWN. This was not a problem because we were interested in the differential effect of the CWN. The question is whether the CWN in combination with the (ipsilateral) masker elicited a stronger MEM reflex than the masker alone. To assess this possibility, the test was repeated using test tones of 80 dB SPL, the highest possible level that our system could deliver without significant distortion. In this case, the suppressor level was 80 dB SPL.
Measurements were made using an IHS Smart system (with SMARTOAE software version 4.52) equipped with an Etymotic ER-10D probe. Three measurements were made for each subject and condition, and the results were averaged. All measurements were obtained in a single recording session. Measurements with and without CWN were alternated with an intermeasurement time interval of approximately 10 s. During the measurements, subjects sat comfortably in a double-wall sound attenuating chamber and were asked to remain as steady as possible.
Computer model
A phenomenological computer model of forward masking with efferent control was used to simulate and explain the effects of the CWN on the PTCs. The model is a crude approximation to what the cochlea and auditory system actually do, but it nevertheless served to test the hypothesis that the only effect of the CWN was to activate the MOCR and hence to reduce cochlear gain in a level- and frequency-dependent manner. The model was inspired by the BM-temporal window model (Plack et al. 2002). It consisted of a stage that mimics nonlinear frequency selectivity at a single cochlear site, followed by a squaring device, followed by a temporal integrator (or temporal window) that integrates masker and probe energy over time, followed by a decision device. In this model, forward masking is assumed to occur from a persistence of neural activity produced by the masker (Oxenham 2001). The temporal window accounts for the postmechanical rate of recovery from forward masking. It was centered at the time when the probe occurred in the stimulus. The model output was calculated for the masker + probe and the masker alone stimuli. Probe detection was assumed to occur when the ratio of the two outputs exceeded a certain criterion value, k (see Plack et al. 2002 for details).
In the original version of the model (Plack et al. 2002), cochlear mechanical responses were simulated using a dual resonance nonlinear (DRNL) filter (Lopez-Poveda and Meddis 2001; Meddis et al. 2001). In the current model, however, the DRNL filter was replaced by a more recent version with efferent control (Ferry and Meddis 2007), termed here EDRNL. The latter accounts for physiological efferent effects on BM and auditory nerve by means of a single parameter that attenuates the input signal to the nonlinear path of the standard DRNL filter (Ferry and Meddis 2007).
Another novelty of the present forward masking model is that it accounted for off-frequency listening at 500 Hz. When measuring a PTC, probe detection is assumed to occur via the cochlear site (or filter) giving the largest masker–probe response ratio. This site need not be the site giving the strongest response to the probe. If cochlear tuning is asymmetric in frequency and narrower than the probe bandwidth, then probe detection may occur via a cochlear site different from the probe site. This phenomenon is known as off-frequency listening (Patterson and Nimmo-Smith 1980). The present PTCs were obtained using a brief (10 ms) probe, and this may have caused energy splatter, hence off-frequency listening at 500 Hz. To account for off-frequency listening, a bank of 11 EDRNL filters was used to model mechanical responses at cochlear sites with characteristic frequencies (CFs) from 250 to 850 Hz. The local filterbank was constructed assuming a linear dependence of filter parameters with CF on a log–log scale, as follows:
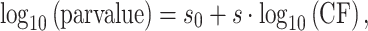 |
1 |
where s0 and s are regression coefficients, and parvalue denotes the value of the corresponding EDRNL filter parameter. This approach seemed reasonable considering that any continuous function may be approximated by a linear segment in a local domain.
The model was implemented digitally in the time domain (sampling frequency = 44,100 Hz) and evaluated for identical stimuli and conditions as used in the experiments. Its parameters were optimized automatically as follows. First, the parameters of the standard DRNL filter (without efferent attenuation) and the temporal window were optimized simultaneously to mimic the 4-kHz PTCs and the linear reference TMCs measured without the CWN. The resulting temporal window parameters were held constant for the other test frequencies and conditions. In other words, it was assumed that the postmechanical rate of recovery from forward masking is constant across conditions. Second, the parameters of the standard DRNL filter (without efferent attenuation) were optimized at 500 Hz using the experimental PTCs measured without the CWN. Lastly, the parameter controlling the amount of efferent attenuation (ATT) was optimized to maximize the fit between simulated and experimental PTCs measured with CWN. In other words, it was assumed that the only effect of the CWN was to attenuate cochlear gain (via MOCR activation) in a physiologically inspired way (Ferry and Meddis 2007). The value of this parameter was allowed to differ across probe frequencies.
Importantly, rather than fitting the model to the mean PTCs, a different set of model parameters was obtained for each individual data set, and the individual model responses were averaged and compared to the mean experimental PTCs. The resulting EDRNL filter parameters at 4 kHz and 500 Hz are given in Tables 1 and 2, respectively. The resulting parameters of the temporal window and the detection efficiency are given in Table 3.
TABLE 1.
Individual values of the EDRNL filter parameters at 4 kHz
| Parameter | S1 | S2 | S3 |
|---|---|---|---|
| EDRNL linear path | |||
| Number of GT filters | 2 | 2 | 2 |
| CFlin (Hz) | 3,971.90 | 3,590.70 | 3,306.80 |
| BWlin (Hz) | 358.85 | 418.20 | 318.13 |
| g | 1,000.00 | 299.02 | 999.17 |
| LPlin (Hz) | 5,999.70 | 5,947.50 | 5,993.30 |
| Number of LP filters | 2 | 2 | 2 |
| EDRNL nonlinear path | |||
| Number of GT filters | 3 | 3 | 3 |
| CFnl (Hz) | 4,142.60 | 3,946.40 | 3,861.90 |
| BWnl (Hz) | 421.75 | 474.64 | 523.10 |
| a | 54,574.97 | 17,420.05 | 54,574.97 |
| b | 0.384 | 0.087 | 0.347 |
| c | 0.24 | 0.25 | 0.25 |
| LPnl (Hz) | 3,900.60 | 4,640.00 | 3,753.00 |
| Number LP filters | 3 | 3 | 3 |
| ATT (dB) | 4.36 | 0.5 | −1.5 |
TABLE 2.
Individual regression parameters of the EDRNL local filberbanks at 500 Hz (see “Materials and methods”)
| Subject | S1 | S2 | S3 | |||
|---|---|---|---|---|---|---|
| Parameter | s0 | s | s0 | s | s0 | s |
| EDRNL linear path | ||||||
| CFlin (Hz) | 1.218 | 0.675 | 0.850 | 0.756 | 1.673 | 0.513 |
| BWlin (Hz) | 2.918 | −0.120 | 3.535 | −0.183 | 3.526 | −0.248 |
| g | 0.472 | 0.693 | 2.521 | −0.0062 | 0.858 | 0.622 |
| LPlin (Hz) | −0.749 | 1.346 | −1.074 | 1.372 | −0.923 | 1.347 |
| EDRNL nonlinear path | ||||||
| CFnl (Hz) | 0.023 | 0.994 | 0.073 | 0.977 | 0.159 | 0.953 |
| BWnl (Hz) | 1.907 | 0.207 | 1.818 | 0.197 | 1.313 | 0.376 |
| a | 3.382 | 0 | 3.900 | 0 | 3.803 | 0 |
| b | −4.654 | 1.087 | −2.669 | 0.568 | −4.008 | 0.975 |
| c | −1.176 | 0.204 | −0.677 | 0.024 | −1.212 | 0.199 |
| LPnl (Hz) | 1.119 | 0.742 | 0.478 | 0.885 | 1.127 | 0.761 |
| ATT (dB)a | −5.06 | n/a | −6.89 | n/a | −8.32 | n/a |
Notation is as for Table 1. The number of cascaded gammatone (GT) and Butterworth lowpass (LP) filters in the linear and nonlinear paths were identical at 500 and 4 kHz and so are shown in Table 1
aNote that Eq. (1) does not apply to the ATT parameter; its value was constant across the filterbank and equal to the value shown in the table
TABLE 3.
Temporal window and detection efficiency parameters
| Subject | S1 | S2 | S3 |
|---|---|---|---|
| w | 0.93 | 0.92 | 0.93 |
| τb1 (ms) | 0.00168 | 9.47 | 6.88 |
| τb2 (ms) | 31.32 | 19.22 | 31.97 |
| τa (ms) | 3.50 | 3.50 | 3.50 |
| k at 500 Hz (dB) | 8.01 | 3.08 | 5.63 |
| k at 4 kHz (dB) | 1.66 | 0.90 | 0.83 |
For detailed descriptions on the meaning of these parameters, see Oxenham (2001)
Results
MEM reflex test
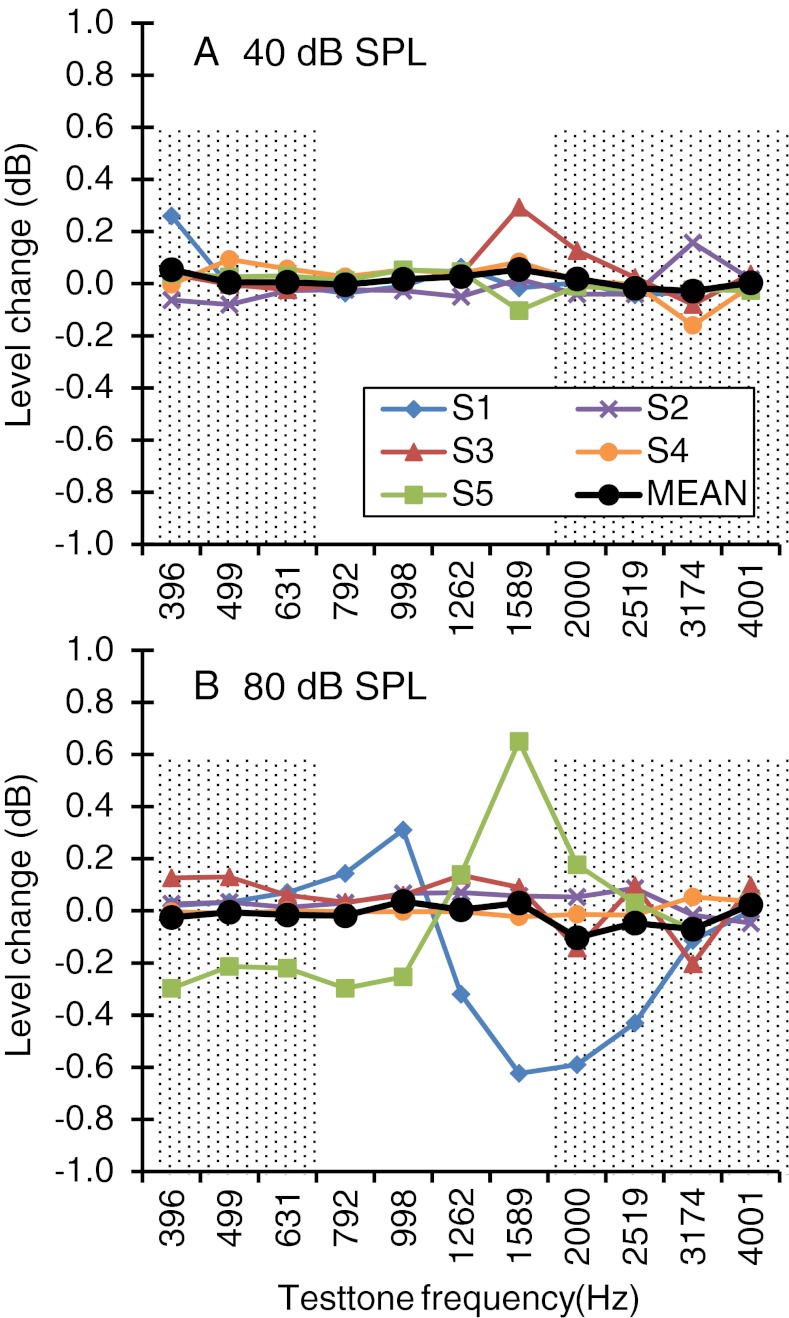
If the CWN had activated the MEM reflex in addition to (or instead of) the MOCR, this could confound the interpretation of the results. The aim of this test was to ensure that this did not happen. Figure 1A shows that the CWN used in the main experiment (60 dB SPL) hardly changed the ear-canal level of 40 dB SPL test tones within the frequency range involved in the PTC measurements (illustrated by the shaded areas). Therefore, the CWN did not evoke the MEM reflex. Figure 1B shows the results for 80 dB SPL test tones, i.e., for tones that can activate the MEM by themselves. In this case, the CWN possibly evoked a MEM reflex for subjects S1 and S5. The latter is irrelevant for the present discussion because he did not take part in suprathreshold PTC measurements (his MEM reflex results are shown only for completeness). As for S1, MEM reflex effects were small (<0.65 dB) and occurred over a frequency range (between 1 and 2 kHz) outside the present range of interest.
FIG. 1.
Mean change in test tone ear-canal level induced by the CWN. Each curve is for a different subject or the mean. The change is expressed as the ear-canal level measured without the CWN minus the level measured with the CWN. Negative values indicate that the CWN increased the middle-ear impedance, presumably by activation of the MEM reflex. Shaded areas highlight the approximate range of masker frequencies used in the PTC measurements. A For test tones of 40 dB SPL. B For test tones of 80 dB SPL.
Altogether, this shows that the MEM reflex played no significant role on the present measurements (at least for masker levels below 80 dB SPL) and supports our assumption that any significant effect of the CWN on the PTCs will be likely due to the MOCR.
Effect of CWN on postmechanical recovery from forward masking
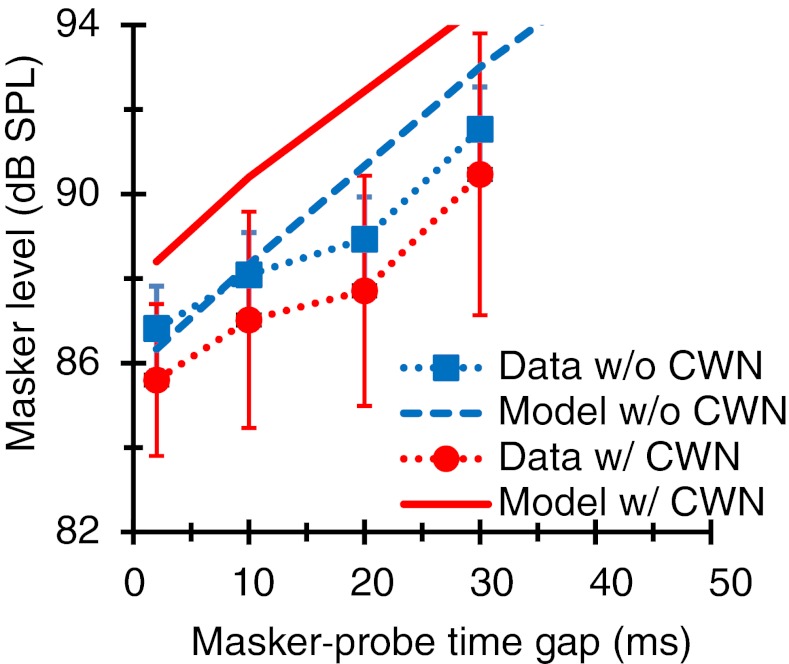
A test was carried out to ensure that the CWN did not alter the postmechanical rate of recovery from forward masking. Figure 2 shows mean recovery functions measured with and without the CWN. When fitted with straight lines, the two functions had virtually identical slopes (0.16 dB/ms). Assuming that the effect of the CWN was to elicit the MOCR, this suggests that the MOCR had no effect on the postmechanical rate of recovery from forward masking. In other words, it suggests that any effect of the CWN on the PTCs will be almost certainly the result of a change in cochlear responses.
FIG. 2.
Postmechanical recovery from forward masking with and without the CWN. The probe and masker frequencies were 4 and 1.6 kHz, respectively. Symbols and dotted lines depict mean experimental data; error bars illustrate ± one standard deviation. Continuous and dashed lines depict computer model simulations.
Figure 2 also suggests that the CWN shifted the mean linear reference TMC downward by ∼1.14 dB. This would be consistent with the expected effect of the CWN activating the MOCR; it reduces cochlear gain and thereby the response to the fixed-SPL probe. The observed shift, however, was neither statistically significant nor consistent across subjects. Indeed, it was smaller than the masker-level step size (2 dB) used in the adaptive procedure that we employed to measure masker levels at threshold. This suggests that either the CWN did not activate the MOCR at 4 kHz or that the effect of MOCR activation on the fixed-SPL 4-kHz probe was so small that it became undetectable by our measuring method. Physiological studies demonstrate that a 60 dB SPL CWN is sufficient to evoke the MOCR at 4 kHz (Lilaonitkul and Guinan 2009b). Therefore, the latter explanation seems more likely (see “Discussion”).
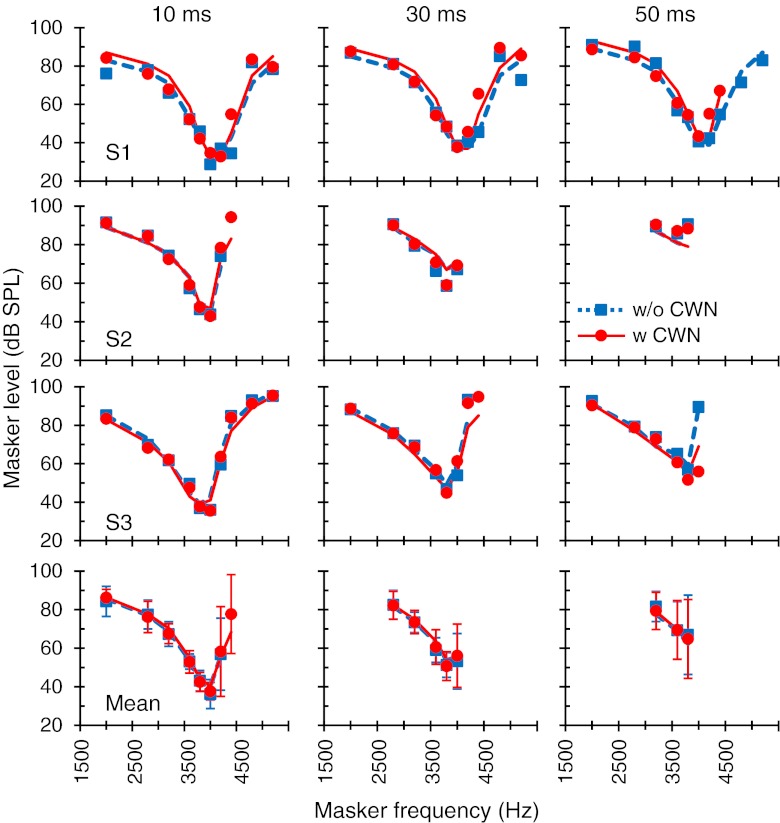
Effect of contralateral noise on near-threshold PTCs
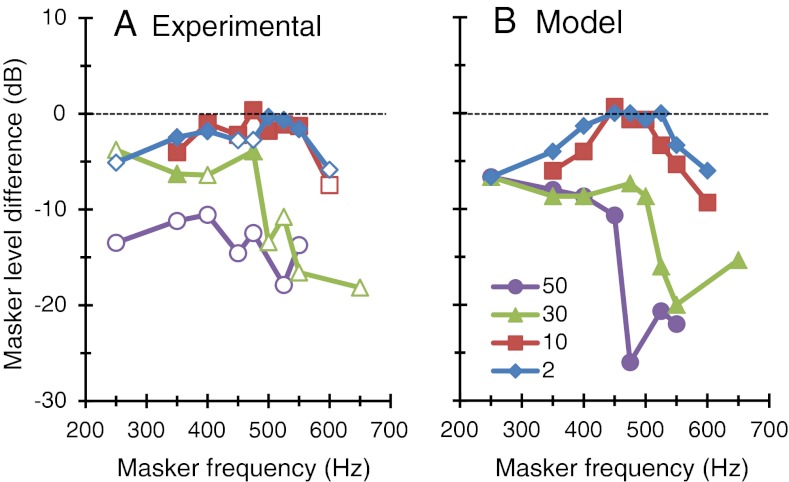
Figure 3 shows the effect of the CWN on the PTCs for a 2-ms masker–probe time gap; the near-threshold condition. The CWN had no significant effect of the PTCs 4 kHz. At 500 Hz, the CWN did not change masker levels near the PTC tip but reduced masker levels in the low- and high-frequency tails of the PTCs of all subjects. The mean reduction, shown in Figure 4, was 5.1 and 5.8 dB for the 250- and 600-Hz maskers, respectively, and was statistically significant (single-tailed, paired, Student’s t test, N = 5; p = 0.0014 and p = 0.024 at 250 and 600 Hz, respectively). Thus, the net effect of the CWN was a slight broadening of the near-threshold PTC at 500 Hz.
FIG. 3.
The effect of the CWN on near-threshold PTCs for probe frequencies of 500 Hz (left) and 4 kHz (right). PTCs were measured using a masker–probe time gap of 2 ms with (circles) and without (squares) CWN. Symbols and lines illustrate measured and modeled PTCs, respectively. Each row shows results for a single subject or the mean, as indicated in the bottom-left corner of each panel. In the bottom panels, error bars are associated to the symbols and illustrate ± one standard deviation. Missing symbols correspond to conditions for which it was not possible to obtain three or more valid measurements using the criteria explained in “Materials and methods.” Model simulations were not obtained for subjects S4 and S5 and so mean model results are for S1, S2, and S3, while mean experimental data are for all five subjects.
FIG. 4.
The effect of the CWN on the 500-Hz PTCs as a function of masker frequency. The effect is expressed as the difference in mean masker level measured with CWN minus levels measured without CWN. Negative values indicate that the CWN reduced the masker level. Each series is for a different masker–probe gap, as indicated by the legend, in units of ms. A Experimental data. Open symbols illustrate statistically significant differences (p < 0.05, single-tailed, paired, t test, N = 5 for the 2 ms series, N = 3 for the other series). B Model predictions.
The present results appear inconsistent with those of Vinay and Moore (2008). They showed that a contralateral narrowband noise of 60 dB SPL had a different effect on PTCs depending on the probe frequency. At 4 kHz, it reduced masker levels in the tails of the PTCs but left masker levels near the PTC tip unchanged, a result qualitatively consistent with the present pattern of results at 500 Hz. They further reported that the narrowband contralateral noise typically increased masker level in the low-frequency tail of PTCs at 500 Hz, an effect opposite to the present findings. The present results at 500 Hz are qualitatively and quantitatively consistent with those of Kawase et al. (2000) at 2 kHz.
The discrepancies across studies may reflect methodological differences. Kawase et al. (2000) used broadband contralateral noises as MOCR elicitors and measured PTCs using very narrowband (1/6 octave) noise maskers. Vinay and Moore (2008) used narrowband contralateral noises and used comparatively broader (1/3 and 1/2 octave) noise maskers. Furthermore, Vinay and Moore (2008) measured PTCs using simultaneous masking, while Kawase et al. (2000) measured PTCs using simultaneous and forward masking. Masker thresholds in simultaneous masking can be lower than in forward masking partly due to suppression of the probe’s cochlear excitation by the maskers (Moore et al. 1984; Rodriguez et al. 2010). The amount of suppression is inversely proportional to cochlear sensitivity or gain [e.g., Fig. 11 in Ruggero et al. (1992)]. Therefore, insofar as the contralateral MOCR elicitor reduces cochlear gain, it can concurrently reduce cochlear suppression to an uncertain extent and this may increase, rather than decrease, masker levels. In other words, it is almost certain that in simultaneous masking the contralateral MOCR elicitor concomitantly reduces cochlear gain and suppression, and this can confound the interpretation of the results. Suppression affects a comparatively broader range of frequencies in the apex than in the base (Cooper and Rhode 1996; Robles and Ruggero 2001). Therefore, the confounding effects of suppression likely affected the simultaneous masking 500-Hz PTCs of Vinay and Moore (2008) more than the 2-kHz PTCs of Kawase et al. (2000). The present PTCs were measured using forward masking and so were not affected by suppression.
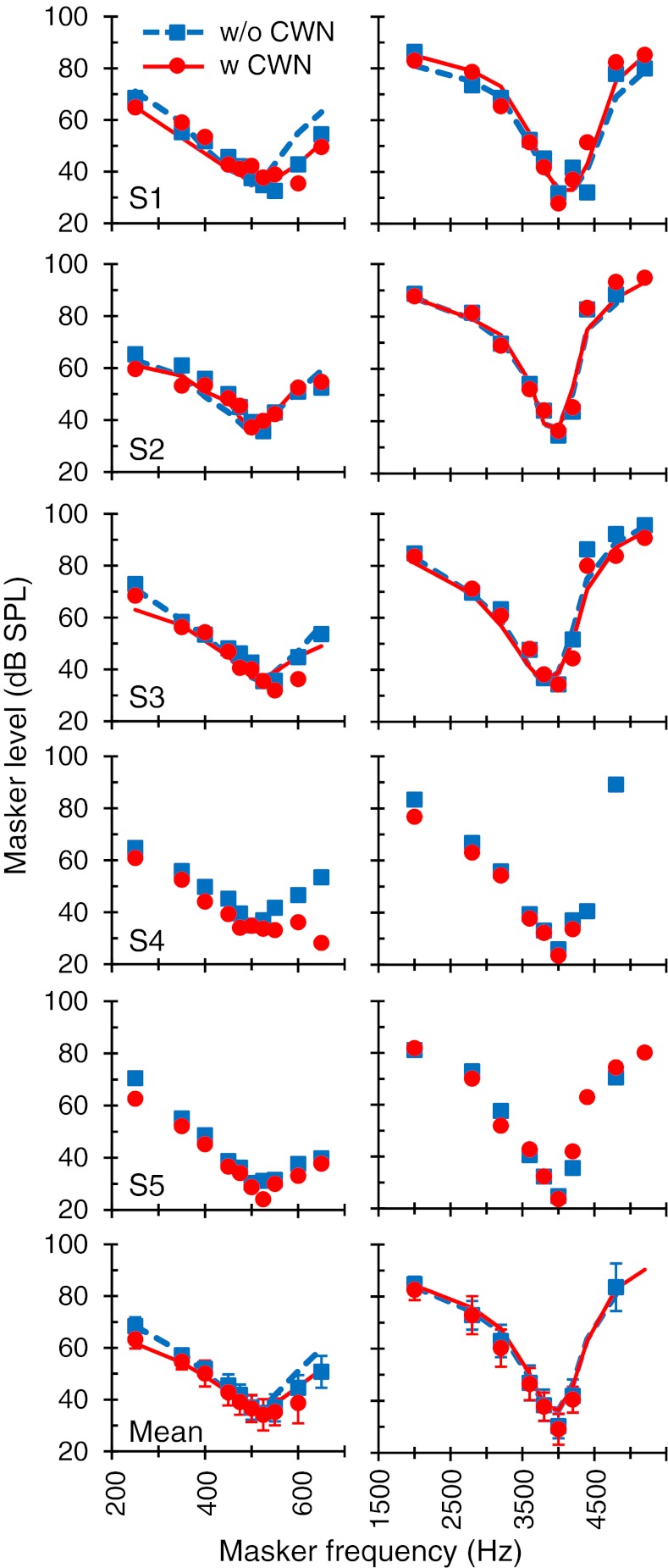
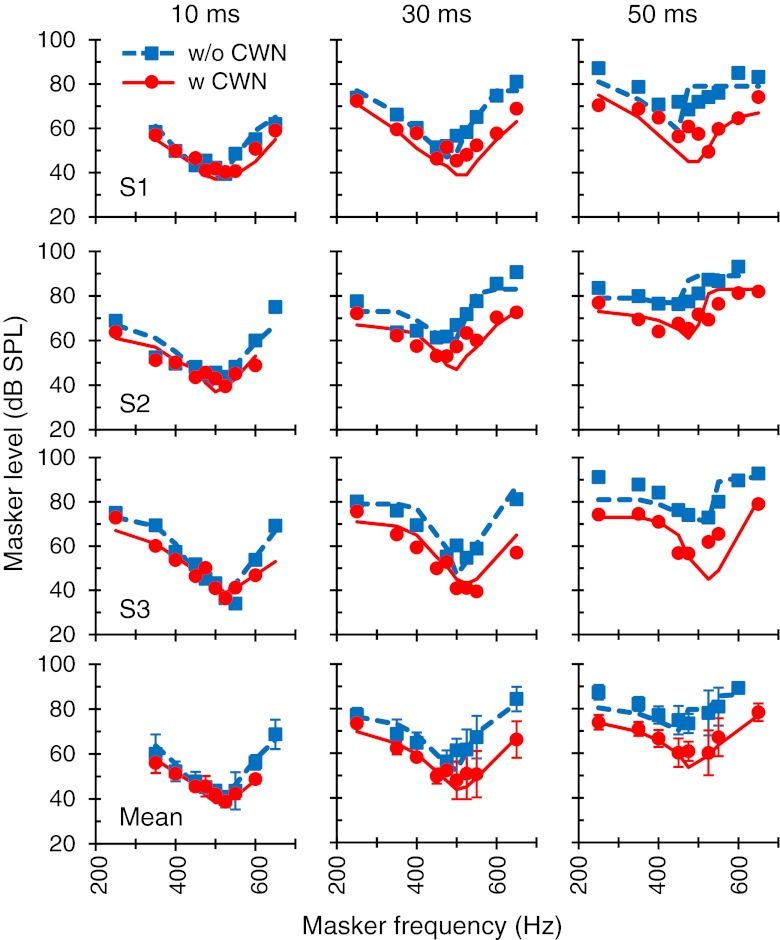
Effects of the CWN on suprathreshold PTCs
Figure 5 shows the effects of the CWN on 4 kHz PTCs for masker–probe time gaps of 10, 30, and 50 ms, regarded here as suprathreshold conditions. Figure 6 shows corresponding results for a 500-Hz probe. Clearly, the CWN had no significant effect on the 4 kHz PTCs. At 500 Hz, by contrast, the CWN reduced masker levels. The magnitude of the reduction is shown in Figure 4A. For the 10 ms gap, the reduction was greater for masker frequencies remote from the probe frequency, consistent with the pattern of results for near-threshold PTCs (2-ms series in Fig. 4A). For 30- and 50-ms gaps, however, the CWN reduced the level of all maskers across the frequency range. On average, the reduction was greater the longer the masker–probe time gap: Mean reduction was 2.3, 8.3, and 14.7 dB for gaps of 10, 30, and 50 ms, respectively. The pattern of results was virtually identical for all subjects.
FIG. 5.
The effect of the CWN on suprathreshold PTCs for a probe frequency of 4 kHz. Each column illustrates PTCs measured using a different masker–probe time gap, as indicated at the top. Symbols and lines illustrate measured and modeled data, respectively. Each row shows results for a single subject or the mean, as indicated in the bottom-left corner of the left panels. Missing symbols correspond to conditions for which it was not possible to obtain three or more valid measurements using the criteria explained in “Materials and methods.” In the bottom panels, error bars are associated to the symbols and illustrate ± one standard deviation.
FIG. 6.
As for Figure 5, but for a probe frequency of 500 Hz.
In summary, the CWN did not have a measurable effect on PTCs at 4 kHz. At 500 Hz, by contrast, the CWN reduced the level of maskers remote from the probe frequency near threshold and of all maskers in supra threshold conditions. Given that (1) that the CWN did not activate the MEM reflex (Fig. 1), (2) the postmechanical rate of recovery from forward masking was unaffected by the CWN (Fig. 2), and (iii) that the used CWN is able to activate the MOCR (Lilaonitkul and Guinan 2009b), altogether the present results suggest that contralateral activation of the MOCR has a stronger effect on apical than on basal cochlear tuning curves. Interestingly, at first sight, the pattern of the present results appears inconsistent with the effects of electrical activation of the MOCR on high-frequency BM responses, which shifts only the tip of the tuning curves upward (Cooper and Guinan 2006). The computer model simulations described below will show that this inconsistency is more apparent than real.
Model simulations
The dashed line in Figure 2 illustrates the mean linear reference TMC resulting from optimizing the model to simultaneously fit the experimental linear reference TMCs and the 4-kHz PTCs. Its slope was slightly steeper than that of the corresponding experimental curve (0.22 vs. 0.16 dB/ms). Although the difference in slope was not statistically significant, the goodness-of-fit to the experimental PTCs became poorer when the temporal window parameters were independently optimized to match the slope of the linear reference TMCs. This suggests that the actual postmechanical recovery from forward masking could be slightly steeper than suggested by the measured linear reference TMC. This is not unreasonable considering that the experimental curve is based on measured values and it disregards runs that called for values higher than the maximum system output (100 dB SPL). In other words, the experimental linear reference TMC might have been steeper if the masker level had been allowed to go higher.
The model predicts a slight upward rather than downward shift of the linear reference TMC with CWN. This is because at 4 kHz, the effect of the CWN in the model was to slightly increase rather than decrease cochlear sensitivity to the probe, i.e., the mean ATT parameter was positive (+1.12 dB) (individual ATT values are shown in Table 1). Nonetheless, the difference between model masker levels with and without CWN was not statistically significant.
PTCs simulated with the model are shown as lines in Figures 3, 5, and 6. The model reproduced individual and mean PTCs measured with and without CWN reasonably well, both qualitatively and quantitatively (the root mean square error of the fits are given in Table 4). Particularly, the model simulated the observation that the CWN reduced the levels of the maskers in the low- and high-frequency tails of near-threshold PTCs at 500 Hz while leaving masker levels near the PTC tip unchanged (2-ms series in Fig. 4B). It also simulated the observation that the CWN shifted suprathreshold PTCs downwards and that the shift was larger for longer masker–probe time gaps, or equivalently for higher masker levels (Figs. 4 and 6). The model performance was comparatively poorer for the 50-ms masker–probe time interval (i.e., compare the 50-ms series in Figure 4A and B). In this case, the model underestimated the size of the effect for masker frequencies below the probe frequency and overestimated it for frequencies at and above the probe frequency.
TABLE 4.
Root mean square error (in dB) of the model fits for different conditions and subjects (or the mean)
| Frequency (Hz) | Condition | S1 | S2 | S3 | Mean |
|---|---|---|---|---|---|
| 500 | w/o CWN | 5.37 | 4.77 | 4.79 | 3.60 |
| w/CWN | 5.69 | 4.79 | 5.65 | 2.70 | |
| 4,000 | w/o CWN | 4.54 | 4.55 | 3.77 | 2.99 |
| w/CWN | 5.65 | 5.15 | 5.14 | 3.27 |
At 4 kHz, the error includes the error of fits to PTCs and recovery functions
The overall good model performance is rather remarkable considering that the effect of the CWN was accounted for by a single model parameter, namely, the efferent attenuator (ATT). The individual values for this parameter are given in the last row of Tables 1 and 2. Mean values were −6.76 and +1.12 dB at 500 Hz and 4 kHz, respectively. The difference across test frequencies was significant (p = 0.0098, N = 3, two-tailed paired Student’s t test). The efferent attenuation is attributed here to MOCR activation by the CWN. Hence, the model suggests that contralateral MOCR efferent attenuation is significantly greater at 500 Hz than at 4 kHz.
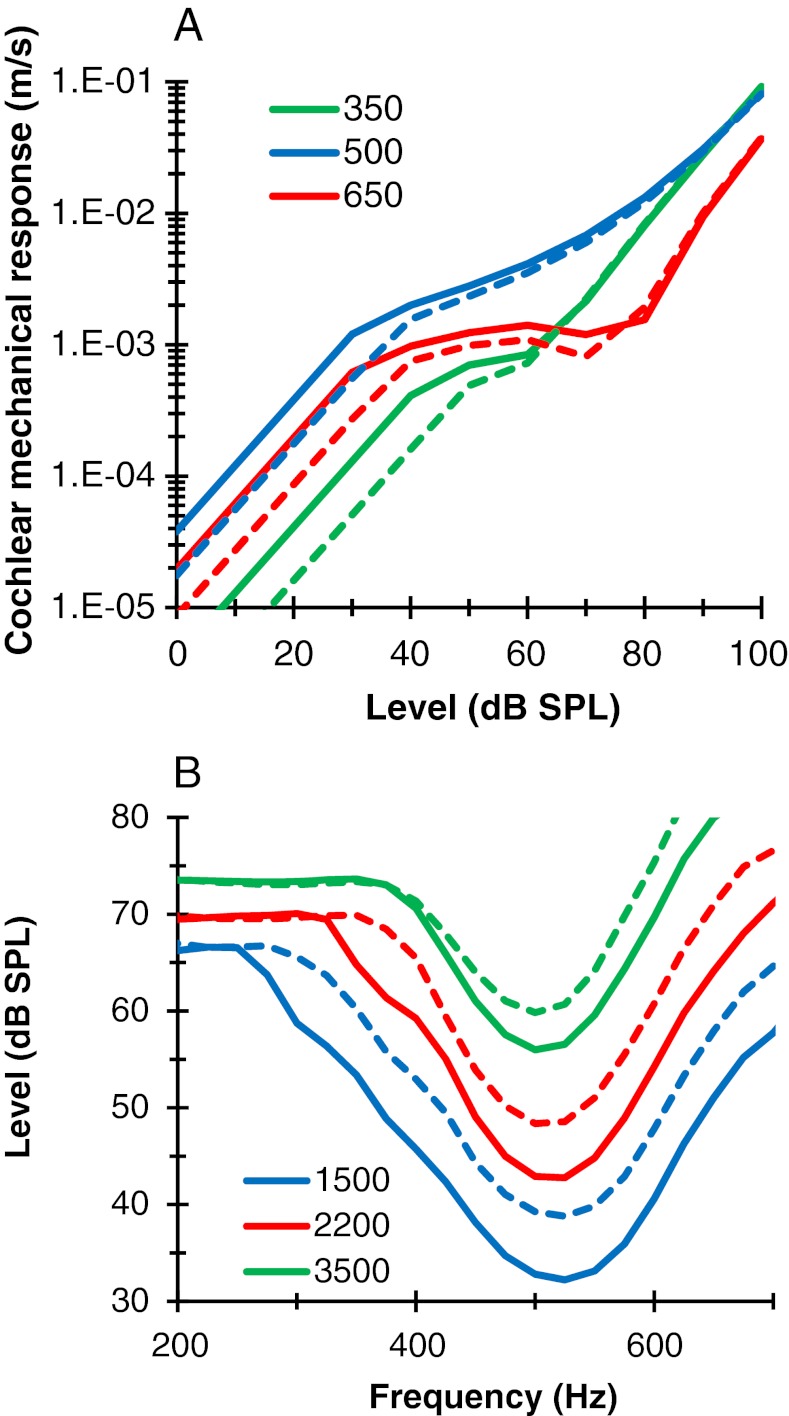
Figure 7 illustrates the effects of efferent attenuation on the model mechanical response for an example subject (S2). The top and bottom panels illustrate input/output (I/O) and tuning curves, respectively. As explained above, in the model, it was assumed that the CWN activated the MOCR, which in turn reduced cochlear gain by an amount determined by the efferent attenuation parameter (ATT = −6.89 dB for this subject). This shifted the low- and moderate-level portions of the I/O curves to the right (Fig. 7A). It also shifted a great portion of the PTCs upwards (Fig. 7B). The present model predicts that efferent attenuation in the apex (CF∼500 Hz) extends over a wider range of stimulus frequencies than suggested by the physiological data for a basal BM region (Cooper and Guinan 2006). This is because, in order to account for the behavioral data, compressive responses in the model affected a comparatively broader range of frequencies (relative to CF) at 500 Hz than at 4 kHz. This model prediction is not a new result; it has been suggested by earlier physiological studies (Rhode and Cooper 1996) and by earlier behavioral studies based on experimental methods and assumptions identical (Lopez-Poveda et al. 2003; Plack and Drga 2003) as well as different to those employed here (Plack and Drga 2003; Lopez-Poveda and Alves-Pinto 2008). Hence, the present model and data are consistent with earlier reports. To our knowledge, however, this is the first model to account for this phenomenon, which is important for simulating the present behavioral results.
FIG. 7.
Model cochlear mechanical responses in the 500-Hz model of subject S2 without (continuous lines) and with (dashed lines) efferent attenuation (ATT = −6.9 dB). A Input/output curves for three sinusoidal maskers with frequencies as indicated in the legend (in Hz). B Tuning curves for three different response criteria, as indicated by the legend (in μm/s).
The example model mechanical responses shown in Figure 7 emerged from fitting the forward masking model to the experimental PTCs. In addition to the previously discussed broad bandwidth of compression, the mechanical stage of the model also predicts greater compression for some tones above CF than for tones at CF and sometimes even a notch in the I/O curve (in the example responses of Fig. 7, these two characteristics occur for a tone of 650 Hz). It is technically difficult to record mechanical cochlear responses in the apex, but these model predictions are nevertheless supported by the limited available data. For example, tectorial membrane responses in the 600 Hz region show greater compression for a tone of 800 Hz than for an on-CF tone [see Fig. 6A in Rhode and Cooper (1996)]. Greater compression for tones above than at CF as well as notches is also seen in recordings from Reissner’s membrane [e.g. Figs. 3 and 4 of Cooper and Rhode (1995)]. Auditory nerve responses from low-CF units also support there being notches in the output of the cochlea (Gifford and Guinan 1983). Further support also comes from basal BM responses. For example, greater compression for tones above CF than at CF as well as notches are regular features of BM responses from mid and basal regions in good preparations [see Figs. 3 and 11 and their related discussion in Rhode and Recio (2000)]. In the present mechanical model, notches occur as a result of cancellation between the output signals from the two resonances of the DRNL filter, as explained elsewhere (Lopez-Poveda and Meddis 2001).
The present forward masking model offers an explanation for the effects of the CWN on the 500 Hz PTCs (shown in Fig. 4). An accurate explanation involves a complex interaction of frequency- and level-dependent tuning and compression with off-frequency listening (discussed below). An approximate but clearer explanation may be obtained from the analysis of the response of a single model filter with a CF near the probe frequency. In the model, it was assumed that masking threshold occurred at a fixed probe-to-masker cochlear response ratio (p/m) and that this ratio was identical in the presence and in the absence of a CWN; that is, p′/m′ = p/m, where prime denotes mechanical cochlear response in the presence of CWN. It follows that: m/m′ = p/p′. In other words, in the model, masking threshold occurred when the ratio of mechanical excitation produced by any masker in the absence and in the presence of the CWN was equal to the ratio of probe excitation in the two corresponding conditions. Here, the SPL of the probe used to measure the PTCs was fixed and identical with and without the CWN. Therefore, p/p′ was greater than unity as a result of efferent attenuation. The key issue is that the masker levels required to achieve a fixed masker excitation ratio (m/m′) depend on the amount of compression affecting the masker in question over the masker excitation range under study. Figure 8 illustrates various possibilities using a cartoon based on the model mechanical responses for subject S2 (adapted from Fig. 7A). The probe excitation ratio (p/p′) is illustrated by a vertical double-headed arrow near the ordinate of Figure 8A and D. The top and bottom panels illustrate near- and suprathreshold conditions, respectively; that is, masker excitation in the top panels is lower than in the bottom panels. Note that the masker excitation ratio (m/m′) is identical in the two cases and equal to the probe excitation ratio.
FIG. 8.
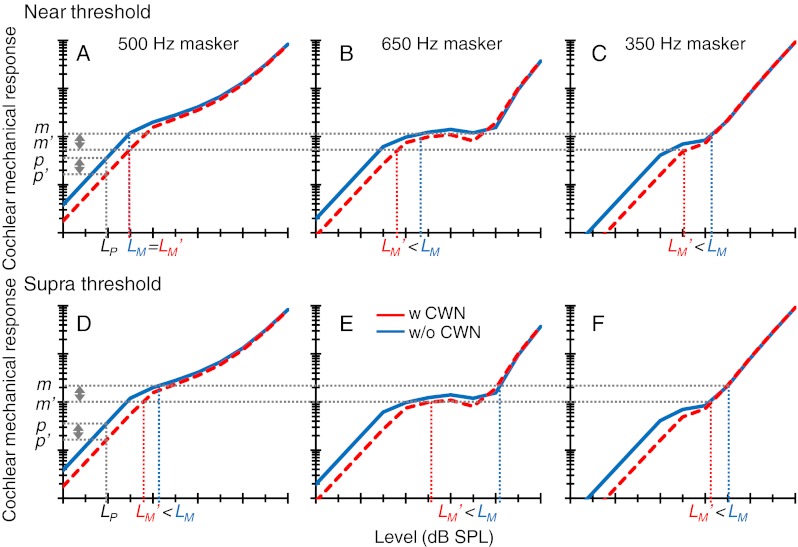
A supporting diagram to explain the effects of the CWN on the 500-Hz PTCs based on an individual’s (S2) BM model. For a full explanation, see “Model simulations.” Top row near-threshold condition. Each panel shows BM input/output curves (adapted from Fig. 7A) with (dashed line) and without (continuous line) efferent attenuation. Each panel is for a different stimulus (masker) frequency, as indicated at the top of the panel. LP denotes probe sound pressure level, and p′ and p denote BM responses evoked by the probe with and without efferent attenuation, respectively. L'M and LM denote levels of a given masker at masked threshold with and without efferent attenuation, respectively, and m and m′ denote corresponding BM responses. Bottom row As the top row but for a suprathreshold condition. The input/output curves are identical in the top and bottom rows.
Let us analyze the near-threshold case first (Fig. 8 top). For the on-frequency masker (Fig. 8A), efferent attenuation equally affects the probe and the masker. Hence, identical masker levels are required to just mask the probe with and without the CWN (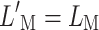 ). Low and high off-frequency maskers are compressed over the masker excitation range (Fig. 8C and B, respectively). Hence, masker levels are lower with than without the CWN (
). Low and high off-frequency maskers are compressed over the masker excitation range (Fig. 8C and B, respectively). Hence, masker levels are lower with than without the CWN (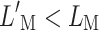 ). This explains why (in our model) the CWN did not change masker levels around the near-threshold PTC tip but reduced the levels of remote maskers (Fig. 4).
). This explains why (in our model) the CWN did not change masker levels around the near-threshold PTC tip but reduced the levels of remote maskers (Fig. 4).
Let us now analyze the suprathreshold case (Fig. 8 bottom). In this case, a higher masker excitation is required to just mask the probe as a result of using a longer masker–probe time gap. The response of the on-frequency masker (Fig. 8D) is compressed over the range of masker excitation. Hence, the level of the on-frequency masker is lower with than without the CWN (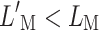 ). The response to the low off-frequency masker (Fig. 8F) is approximately linear over the excitation range under study, but over this excitation range, this masker is unaffected by efferent attenuation. Therefore, its level is lower with than without the CWN. By contrast, the response to the high off-frequency masker (Fig. 8E) is linear without the CWN over the excitation range under study but falls in the compressive region of the I/O curve with the CWN. Therefore, the level of the high off-frequency masker is significantly lower with than without the CWN, consistent with the data (cf. 30 ms series in Fig. 4). This explains why the CWN noise reduced the levels of all maskers in suprathreshold conditions, but the magnitude of the reduction was greater for high than for low off-frequency maskers.
). The response to the low off-frequency masker (Fig. 8F) is approximately linear over the excitation range under study, but over this excitation range, this masker is unaffected by efferent attenuation. Therefore, its level is lower with than without the CWN. By contrast, the response to the high off-frequency masker (Fig. 8E) is linear without the CWN over the excitation range under study but falls in the compressive region of the I/O curve with the CWN. Therefore, the level of the high off-frequency masker is significantly lower with than without the CWN, consistent with the data (cf. 30 ms series in Fig. 4). This explains why the CWN noise reduced the levels of all maskers in suprathreshold conditions, but the magnitude of the reduction was greater for high than for low off-frequency maskers.
In summary, the effects of the CWN and so of efferent attenuation on the PTCs depend both on the reduction in the cochlear response to the probe and on the amount of compression affecting the maskers over the range of levels required for masking. Crucially, the latter may be different with and without CWN and for maskers below and above the CF. The match between observed and model effects was poorest for the longest masker–probe time gap (or, correspondingly, for the largest masker levels tested), specifically for low frequency maskers (cf. 50 ms series in Fig. 4), probably because compression on these maskers was larger experimentally than in the model.
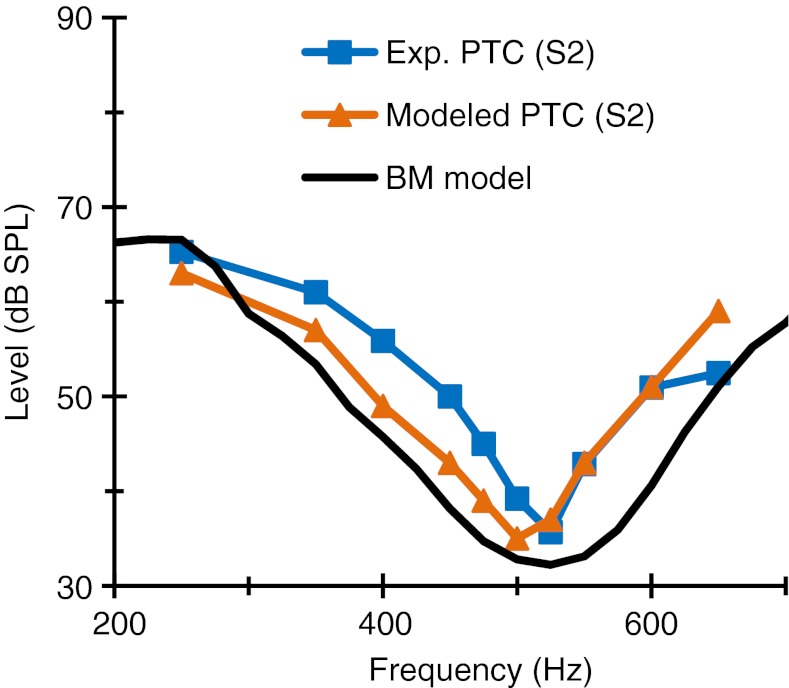
This explanation for the effects of the CWN at 500 Hz, however, is only approximate. Indeed, it would not be possible to work out the actual masker levels of the modeled PTCs (Figs. 3 and 6) directly from Figure 8. An accurate explanation involves a complex interaction of the previously described effects with off-frequency listening. Off-frequency listening facilitates probe detection, which leads to higher masker levels and sharper PTCs (O’Loughlin and Moore 1981). One way to assess the significance of off-frequency listening is by comparing a modeled PTC with the tuning curve for a single cochlear filter with a characteristic frequency equal to the probe frequency. The comparison in question is shown in Figure 9. Clearly, the modeled PTC is sharper than the tuning curve of the BM filter in question. This suggests that the model PTC is sharper as result of off-frequency listening. Insofar as the modeled PTC resembles the experimental PTC, this further suggests that experimental PTCs were also affected by the off-frequency listening. Indeed, it was not possible to accurately reproduce the experimental PTCs using a model version without off-frequency listening. In summary, the present results suggest that apical cochlear tuning curves are actually broader than suggested by the present PTCs.
FIG. 9.
The significance of off-frequency listening on modeled and experimental PTCs. Note that the modeled PTC (and its corresponding experimental counterpart) is sharper than the tuning curve of a single BM filter as a result of off-frequency listening.
Discussion
It has been shown that CWN has a stronger effect on PTCs at 500 Hz than at 4 kHz. At 500 Hz, it has been shown that (1) for near-threshold PTCs, the CWN reduces the level of low- and high-frequency maskers remote from the PTC tip while leaving masker levels around the tip unchanged, and (2) for suprathreshold PTCs, the CWN reduces the levels of all maskers (Fig. 4A). A phenomenological computer model of forward masking with efferent BM control has been provided that accounts for the main experimental findings based on the assumption that the only effect of the CWN is to apply efferent attenuation. It has been shown that these effects are not due to CWN activating the MEM reflex, at least for the conditions involving masker levels below 80 dB SPL, which were the great majority (Fig. 6). Indeed, the model suggests that the observed effects are the result of using an identical probe SPL with and without the CWN combined with level- and frequency-dependent compression and off-frequency listening. In other words, altogether, the present evidence suggests that the observed effects of the CWN on the 500 Hz PTCs are due to MOCR activation and that the magnitude of efferent attenuation is significantly greater at 500 Hz than at 4 kHz.
The present forward masking model is convenient to explain the complex interactions between the stimuli involved to measure a PTC but represents only a crude description of what the cochlea and the auditory system actually do. For example, in the model, BM motion is regarded as the mechanical output of the cochlea. This is probably inaccurate. A more accurate output would be the mechanical drive to inner hair cell stereocilia, and there can be important differences between that and BM motion (Chen et al. 2011; Guinan 2012). Indeed, Chen et al. (2011) directly show that compression in the reticular lamina, which is closer to what drives inner hair cell stereocilia than BM motion, is different from BM compression. Furthermore, it is possible that some of the compression involved in the PTCs is not mechanical but rather originates within the inner hair cell (Plack et al. 2004; Lopez-Poveda et al. 2005; Lopez-Poveda and Eustaquio-Martin 2006).
The present model and interpretation rest on three assumptions about the postmechanical rate of recovery from forward masking: (1) that it is identical across cochlear regions, (2) that it is identical across masker frequencies over the range of masker levels tested, and (3) that it is unaffected by the CWN. One study has disputed the first assumption (Stainsby and Moore 2006), but another study has supported it (Lopez-Poveda and Alves-Pinto 2008). If the recovery rate were faster in the apex than in the base, as suggested elsewhere (Stainsby and Moore 2006), then compression in the apex would be less than suggested by the present model. As for the second assumption, it holds true for a probe frequency of 4 kHz and for off-frequency masker levels of 83 dB SPL but not for off-frequency masker levels of 92 dB SPL (Wojtczak and Oxenham 2009). It remains to be shown that it also holds for a probe frequency of 500 Hz. Nonetheless, virtually, all masker levels reported here were below 83 dB SPL, particularly at 500 Hz (Figs. 5 and 6). As for the third assumption, we have experimentally shown here that the CWN has no effect on the recovery rate in the 4 kHz region (Fig. 2), but it remains to be shown that the same holds for the apex. If the CWN reduced the postmechanical recovery rate in the apex, then this could account, at least in part, for the present findings. Compression in the apex likely extends to a broad range of frequencies (Rhode and Cooper 1996; Lopez-Poveda et al. 2003) and so our control experiment may not be easily extended to the apex. In any case, the present model of forward masking incorporates the three assumptions in question (i.e., the temporal window was identical across all conditions) and still accounts for the observations very well. This suggests that the three assumptions are not unreasonable.
The present evidence also suggests that human apical cochlear tuning curves are affected by contralateral stimulation via MOCR efferent activation. It is important to stress, however, that the form and magnitude of these effects may not be inferred directly from the present behavioral data (PTCs). Instead, a nonlinear computer model is necessary to properly infer MOCR effects from the PTCs. MOCR effects on cochlear tuning curves, however, might be inferred directly from PTCs obtained using probes with different SPLs but identical sensation levels with and without the CWN and by taking precautions to minimize off-frequency listening.
The present model offers an explanation for CWN effects on PTCs based on the relative amount of MOCR attenuation on the mechanical response to the probe and the maskers (Fig. 8). A mechanism qualitatively similar has been previously proposed to explain the effects of contralateral (Kawase et al. 2000) and ipsilateral (Strickland 2001; Jennings et al. 2009) MOCR elicitors on near-threshold PTCs. In a very recent study, Jennings and Strickland (2012b) explained the effect of ipsilateral MOCR elicitors on near- and supratreshold PTCs at 4 kHz using a model that is conceptually similar to the present one. The input to the squaring device in their model, however, was the response of a cat inner hair cell with a CF of 11 kHz, and hence, their explanation of their human data was only qualitative. The present model has been carefully tuned to quantitatively reproduce human PTCs at 500 Hz and 4 kHz and so it provides an approximate estimate of the magnitude of cochlear gain attenuation by the contralateral MOCR at these frequencies (Fig. 7). An emergent property of the present model is that it accounts for the broad bandwidth of compression at 500 Hz reported elsewhere (Rhode and Cooper 1996; Lopez-Poveda et al. 2003; Plack and Drga 2003).
The present results suggest that the effects of MOCR activation by CWN are stronger in apical than in basal cochlear regions. This is consistent with recent human OAE studies that have reported that CWNs have stronger effects on the compression threshold (Bhagat and Carter 2010), cochlear response latency (Francis and Guinan 2010), and SFOAE levels (Lilaonitkul and Guinan 2009a, 2012) at lower than at higher test frequencies. Although the present results showed no statistically significant effects at 4 kHz, we cannot rule them out. Indeed, there is evidence in the present data as well as in earlier studies that such effects do occur at 4 kHz. Here, the CWN reduced the mean masker levels of the linear reference TMC (Fig. 2). This is qualitatively consistent with previously reported effects of the CWN reducing the level of forward maskers with frequencies between one and two octaves below a probe frequency of 2 kHz (Kawase et al. 2000). The reduction, however, was smaller here (1.14 dB) than in the study of Kawase et al. (∼5 dB), but this may be due to differences in the probe frequency (4 kHz here vs. 2 kHz in Kawase et al.), masking threshold definition (threshold was defined here as the masker level yielding 71 % correct probe detection compared to 50 % in Kawase et al.), or measurement precision (the masker-level step size was 2 dB here vs. 1 dB in Kawase et al.). Another piece of evidence in support of MOCR effects at 4 kHz is that electrical activation of the midline olivo-cochlear bundle induced bigger effects on single-unit auditory nerve tuning curves at 4 kHz than at 500 Hz (Guinan and Gifford 1988). It is, however, difficult to compare the latter results with the present ones because they were for cat rather than human and for electrical rather than acoustical activation of the olivo-cochlear bundle. Electrical shocks on the midline olivo-cochlear bundle may have activated both the ipsi- and contralateral MOCR, hence exaggerating the effects. A further piece of evidence in support of contralateral MOCR effects at 4 kHz is that Lilaonitkul and Guinan (2009a) showed that contralateral broadband noise reduced the level of human SFOAE both at 500 Hz and 4 kHz. The magnitude of the reduction was about 2.4 times larger at 500 Hz than at 4 kHz (e.g., their Fig. 3C). Assuming that the magnitude of SFOAE reduction reflects an identical amount of reduction in cochlear gain, and since the present results suggest a mean gain reduction of 6.76 dB at 500 Hz, this would suggest a gain reduction of 2.81 dB at 4 kHz. This figure is within the variability of our measurements. Altogether this suggests that it is possible that the CWN did have a (small) effect at 4 kHz but the precision of our experimental methods was insufficient to reveal it.
The present results differ from those in other behavioral studies that report that ipsilateral precursors designed to activate the MOCR significantly change cochlear responses at 4 kHz (Jennings et al. 2009). The reason for this difference is uncertain, but there is evidence that the ratio of ipsilateral to contralateral MOCR may vary with test frequency (Guinan 2006). To our knowledge, the magnitude of the effect of ipsilateral precursors at 500 Hz is uncertain.
The ipsilateral forward maskers used in our experiments were long enough to also activate the MOCR. It is unlikely, however, that the ipsilateral MOCR affected the PTCs (Jennings and Strickland 2012a). Even if it did, its effects would be identical for PTCs obtained with and without CWN and would cancel out in the comparison [this is unless the effects of ipsi- and contralateral MOCR elicitors add up nonlinearly, which is unlikely the case as suggested by Fig. 3C of Lilaonitkul and Guinan (2009a)]. Therefore, the present approach may still be reasonably used to characterize the effect of contralateral sounds on cochlear responses. The proposed forward masking model could be expanded to include the time course of MOCR activation by ipsi- and contralateral sounds to differentiate between MOCR effects due ipsilateral and/or contralateral elicitors.
Many theories of auditory perception and related technologies (including hearing aids, cochlear implants, loudness models, or bit-rate compression algorithms) are based on behavioral estimates of nonlinear cochlear frequency selectivity. In developing these theories and applications, it has been commonly assumed that the response characteristics of the human cochlea are fixed. That is, that the characteristics inferred in laboratory conditions using simple monaural sounds are representative of cochlear responses in natural, binaural listening to time-varying complex sounds, such as speech or music. The present evidence shows that this assumption could be wrong and that apical cochlear responses may be changing depending on the presence of contralateral sounds. Further research is necessary to fully characterize the size of these changes, their dependence on the type of contralateral sound, and their significance to auditory perception in natural environments.
Acknowledgments
We are most grateful to two anonymous reviewers for their helpful comments and to Peter T. Johannesen for technical assistance with the middle-ear muscle reflex measurements. Work supported by a grant of the Spanish MICINN (BFU2009-07909) to ELP and a predoctoral studentship of the Chilean CONICYT to EA.
References
- Atcherson SR, Martin MJ, Lintvedt R. Contralateral noise has possible asymmetric frequency-sensitive effect on the 2F1–F2 otoacoustic emission in humans. Neurosci Lett. 2008;438:107–110. doi: 10.1016/j.neulet.2008.04.050. [DOI] [PubMed] [Google Scholar]
- Bhagat SP, Carter PH. Efferent-induced change in human cochlear compression and its influence on masking of tones. Neurosci Lett. 2010;485:94–97. doi: 10.1016/j.neulet.2010.08.069. [DOI] [PubMed] [Google Scholar]
- Chen F, Zha D, Fridberger A, Zheng J, Choudhury N, Jacques SL, Wang RK, Shi X, Nuttall AL. A differentially amplified motion in the ear for near-threshold sound detection. Nat Neurosci. 2011;14:770–774. doi: 10.1038/nn.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collet L, Kemp DT, Veuillet E, Duclaux R, Moulin A, Morgon A. Effect of contralateral auditory stimuli on active cochlear micro-mechanical properties in human subjects. Hear Res. 1990;43:251–261. doi: 10.1016/0378-5955(90)90232-E. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Guinan JJ. Efferent-mediated control of basilar membrane motion. J Physiol. 2006;576:49–54. doi: 10.1113/jphysiol.2006.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper NP, Rhode WS. Nonlinear mechanics at the apex of the guinea-pig cochlea. Hear Res. 1995;82:225–243. doi: 10.1016/0378-5955(94)00180-X. [DOI] [PubMed] [Google Scholar]
- Cooper NP, Rhode WS. Two-tone suppression in apical cochlear mechanics. Audit Neurosci. 1996;3:123–134. [Google Scholar]
- Evans EF. Latest comparison between physiological and behavioral frequency selectivity. In: Breebaart J, Houtsma AJM, Kohlraush A, Prijs VF, Schoonhoven R, editors. Physiological and psychophysical bases of auditory function. Maastricht: Shaker; 2001. pp. 382–387. [Google Scholar]
- Ferry RT, Meddis R. A computer model of medial efferent suppression in the mammalian auditory system. J Acoust Soc Am. 2007;122:3519–3526. doi: 10.1121/1.2799914. [DOI] [PubMed] [Google Scholar]
- Francis NA, Guinan JJ. Acoustic stimulation of human medial olivocochlear efferents reduces stimulus-frequency and click-evoked otoacoustic emission delays: implications for cochlear filter bandwidths. Hear Res. 2010;267:36–45. doi: 10.1016/j.heares.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford ML, Guinan JJ. Effects of crossed-olivocochlear-bundle stimulation on cat auditory nerve fiber responses to tones. J Acoust Soc Am. 1983;74:115–123. doi: 10.1121/1.389728. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Olivocochlear efferents: anatomy, physiology, function, and the measurement of efferent effects in humans. Ear Hear. 2006;27:589–607. doi: 10.1097/01.aud.0000240507.83072.e7. [DOI] [PubMed] [Google Scholar]
- Guinan JJ. Cochlear efferent innervation and function. Curr Opin Otolaryngol Head Neck Surg. 2010;18:447–453. doi: 10.1097/MOO.0b013e32833e05d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ. How are inner hair cells stimulated? Evidence for multiple mechanical drives. Hear Res. 2012;292:35–50. doi: 10.1016/j.heares.2012.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Gifford ML. Effects of electrical stimulation of efferent olivocochlear neurons on cat auditory-nerve fibers. III. Tuning curves and thresholds at CF. Hear Res. 1988;37:29–45. doi: 10.1016/0378-5955(88)90075-5. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Backus BC, Lilaonitkul W, Aharonson V. Medial olivocochlear efferent reflex in humans: otoacoustic emission (OAE) measurement issues and the advantages of stimulus frequency OAEs. J Assoc Res Otolaryngol. 2003;4:521–540. doi: 10.1007/s10162-002-3037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA. Auditory filter tuning inferred with short sinusoidal and notched-noise maskers. J Acoust Soc Am. 2012;132:2497–2513. doi: 10.1121/1.4746029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA. Evaluating the effects of olivocochlear feedback on psychophysical measures of frequency selectivity. J Acoust Soc Am. 2012;132:2483–2496. doi: 10.1121/1.4742723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings SG, Strickland EA, Heinz MG. Precursor effects on behavioral estimates of frequency selectivity and gain in forward masking. J Acoust Soc Am. 2009;125:2172–2181. doi: 10.1121/1.3081383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase T, Ogura M, Hidaka H, Sasaki N, Suzuki Y, Takasaka T. Effects of contralateral noise on measurement of the psychophysical tuning curve. Hear Res. 2000;142:63–70. doi: 10.1016/S0378-5955(00)00010-1. [DOI] [PubMed] [Google Scholar]
- Krull V, Strickland EA. The effect of a precursor on growth of forward masking. J Acoust Soc Am. 2008;123:4352–4357. doi: 10.1121/1.2912440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt H (1971). Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49:467–477 [PubMed]
- Lilaonitkul W, Guinan JJ. Human medial olivocochlear reflex: effects as functions of contralateral, ipsilateral, and bilateral elicitor bandwidths. J Assoc Res Otolaryngol. 2009;10:459–470. doi: 10.1007/s10162-009-0163-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ. Reflex control of the human inner ear: a half-octave offset in medial efferent feedback that is consistent with an efferent role in the control of masking. J Neurophysiol. 2009;101:1394–1406. doi: 10.1152/jn.90925.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilaonitkul W, Guinan JJ. Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J Neurophysiol. 2012;107:1598–1611. doi: 10.1152/jn.00549.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Alves-Pinto A. A variant temporal-masking-curve method for inferring peripheral auditory compression. J Acoust Soc Am. 2008;123:1544–1554. doi: 10.1121/1.2835418. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Eustaquio-Martin A. A biophysical model of the inner hair cell: the contribution of potassium currents to peripheral auditory compression. J Assoc Res Otolaryngol. 2006;7:218–235. doi: 10.1007/s10162-006-0037-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Meddis R. A human nonlinear cochlear filterbank. J Acoust Soc Am. 2001;110:3107–3118. doi: 10.1121/1.1416197. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Plack CJ, Meddis R. Cochlear nonlinearity between 500 and 8000 Hz in listeners with normal hearing. J Acoust Soc Am. 2003;113:951–960. doi: 10.1121/1.1534838. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Plack CJ, Meddis R, Blanco JL. Cochlear compression in listeners with moderate sensorineural hearing loss. Hear Res. 2005;205:172–183. doi: 10.1016/j.heares.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Barrios LF, Alves-Pinto A. Psychophysical estimates of level-dependent best-frequency shifts in the apical region of the human basilar membrane. J Acoust Soc Am. 2007;121:3646–3654. doi: 10.1121/1.2722046. [DOI] [PubMed] [Google Scholar]
- Lopez-Poveda EA, Aguilar E, Johannesen PT, Eustaquio-Martín A. Contralateral efferent regulation of human cochlear tuning: behavioral observations and computer model simulations. In: Moore BCJ, Gockel H, Carlyon RP, Winter IM, Patterson RD, editors. Basic aspects of hearing: a compilation of papers from the 16th International Symposium on Hearing. New York: Springer; 2013. [DOI] [PubMed] [Google Scholar]
- Meddis R, O’Mard L, Lopez-Poveda EA. A computational algorithm for computing nonlinear auditory frequency selectivity. J Acoust Soc Am. 2001;109:2852–2861. doi: 10.1121/1.1370357. [DOI] [PubMed] [Google Scholar]
- Moore BCJ, Glasberg BR, Roberts B. Refining the measurement of psychophysical tuning curves. J Acoust Soc Am. 1984;76:1057–1066. doi: 10.1121/1.391425. [DOI] [PubMed] [Google Scholar]
- Murugasu E, Russell IJ. The effect of efferent stimulation on basilar membrane displacement in the basal turn of the guinea pig cochlea. J Neurosci. 1996;16:325–332. doi: 10.1523/JNEUROSCI.16-01-00325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DA, Schroder AC, Wojtczak M. A new procedure for measuring peripheral compression in normal-hearing and hearing-impaired listeners. J Acoust Soc Am. 2001;110:2045–2064. doi: 10.1121/1.1404439. [DOI] [PubMed] [Google Scholar]
- Neumann J, Uppenkamp S, Kollmeier B. Detection of the acoustic reflex below 80 dB HL. Audiol Neuro Otol. 1996;1:359–369. doi: 10.1159/000259219. [DOI] [PubMed] [Google Scholar]
- O’Loughlin BJ, Moore BCJ. Off-frequency listening: effects on psychoacoustical tuning curves obtained in simultaneous and forward masking. J Acoust Soc Am. 1981;69:1119–1125. doi: 10.1121/1.385691. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ. Forward masking: adaptation or integration? J Acoust Soc Am. 2001;109:732–741. doi: 10.1121/1.1336501. [DOI] [PubMed] [Google Scholar]
- Oxenham AJ, Moore BC. Modeling the additivity of nonsimultaneous masking. Hear Res. 1994;80:105–118. doi: 10.1016/0378-5955(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Patterson RD, Nimmo-Smith I. Off-frequency listening and auditory-filter asymmetry. J Acoust Soc Am. 1980;67:229–245. doi: 10.1121/1.383732. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Arifianto D. On- and off-frequency compression estimated using a new version of the additivity of forward masking technique. J Acoust Soc Am. 2010;128:771–786. doi: 10.1121/1.3455844. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Drga V. Psychophysical evidence for auditory compression at low characteristic frequencies. J Acoust Soc Am. 2003;113:1574–1586. doi: 10.1121/1.1538247. [DOI] [PubMed] [Google Scholar]
- Plack CJ, Oxenham AJ, Drga V. Linear and nonlinear processes in temporal masking. Acta Acustica United with Acustica. 2002;88:348–358. [Google Scholar]
- Plack CJ, Drga V, Lopez-Poveda EA. Inferred basilar-membrane response functions for listeners with mild to moderate sensorineural hearing loss. J Acoust Soc Am. 2004;115:1684–1695. doi: 10.1121/1.1675812. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Scaringi A, Nahum S, Quaranta A. Effects of efferent acoustic reflex activation on psychoacoustical tuning curves in humans. Acta Otolaryngol. 2005;125:520–523. doi: 10.1080/00016480510026214. [DOI] [PubMed] [Google Scholar]
- Rhode WS, Cooper NP. Nonlinear mechanics in the apical turn of the chinchilla cochlea in vivo. Audit Neurosci. 1996;3:101–121. [Google Scholar]
- Rhode WS, Recio A. Study of mechanical motions in the basal region of the chinchilla cochlea. J Acoust Soc Am. 2000;107:3317–3332. doi: 10.1121/1.429404. [DOI] [PubMed] [Google Scholar]
- Robertson D, Gummer M. Physiological and morphological characterization of efferent neurones in the guinea pig cochlea. Hear Res. 1985;20:63–77. doi: 10.1016/0378-5955(85)90059-0. [DOI] [PubMed] [Google Scholar]
- Robles L, Ruggero MA. Mechanics of the mammalian cochlea. Physiol Rev. 2001;81:1305–1352. doi: 10.1152/physrev.2001.81.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J, Neely ST, Patra H, Kopun J, Jesteadt W, Tan H, Gorga MP. The role of suppression in psychophysical tone-on-tone masking. J Acoust Soc Am. 2010;127:361–369. doi: 10.1121/1.3257224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggero MA, Robles L, Rich NC. Two-tone suppression in the basilar membrane of the cochlea: mechanical basis of auditory-nerve rate suppression. J Neurophysiol. 1992;68:1087–1099. doi: 10.1152/jn.1992.68.4.1087. [DOI] [PubMed] [Google Scholar]
- Russell IJ, Murugasu E. Medial efferent inhibition suppresses basilar membrane responses to near characteristic frequency tones of moderate to high intensities. J Acoust Soc Am. 1997;102:1734–1738. doi: 10.1121/1.420083. [DOI] [PubMed] [Google Scholar]
- Shera CA, Guinan JJ, Oxenham AJ. Revised estimates of human cochlear tuning from otoacoustic and behavioral measurements. Proc Natl Acad Sci U S A. 2002;99:3318–3323. doi: 10.1073/pnas.032675099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small AM. Pure-tone masking. J Acoust Soc Am. 1959;31:1619–1625. doi: 10.1121/1.1907670. [DOI] [Google Scholar]
- Souter M. Suppression of stimulus frequency otoacoustic emissions by contralateral noise. Hear Res. 1995;91:167–177. doi: 10.1016/0378-5955(95)00187-5. [DOI] [PubMed] [Google Scholar]
- Stainsby TH, Moore BC. Temporal masking curves for hearing-impaired listeners. Hear Res. 2006;218:98–111. doi: 10.1016/j.heares.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Strickland EA. The relationship between frequency selectivity and overshoot. J Acoust Soc Am. 2001;109:2062–2073. doi: 10.1121/1.1357811. [DOI] [PubMed] [Google Scholar]
- Sun XM. Contralateral suppression of distortion product otoacoustic emissions and the middle-ear muscle reflex in human ears. Hear Res. 2008;237:66–75. doi: 10.1016/j.heares.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Vinay, Moore BCJ. Effects of activation of the efferent system on psychophysical tuning curves as a function of signal frequency. Hear Res. 2008;240:93–101. doi: 10.1016/j.heares.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Wojtczak M, Oxenham AJ. Pitfalls in behavioral estimates of basilar-membrane compression in humans. J Acoust Soc Am. 2009;125:270–281. doi: 10.1121/1.3023063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasin I, Plack CJ. The effects of a high-frequency suppressor on tuning curves and derived basilar-membrane response functions. J Acoust Soc Am. 2003;114:322–332. doi: 10.1121/1.1579003. [DOI] [PubMed] [Google Scholar]