Abstract
Progressive supranuclear palsy (PSP) is the most common atypical parkinsonian disorder. Abnormal tau inclusions, in selected regions of the brain, are a hallmark of the disease and the H1 haplotype of MAPT, the gene encoding tau, is the major risk factor in PSP. A 3-repeat and 4-repeat tau isoform ratio imbalance has been strongly implicated as a cause of disease. Thus, understanding tau isoform regional expression in disease and pathology-free states is crucial to elucidating mechanisms involved in PSP and other tauopathies. We used a tau-isoform specific fluorescent assay to investigate relative 4R-tau expression in 6 different brain regions in PSP cases and healthy controls. We identified marked difference in 4R-tau relative expression, both across brain regions and between MAPT haplotypes. Highest 4R-tau expression levels were identified in the globus pallidus as compared to pons, cerebellum and frontal cortex. 4R-tau expression levels were related to both the MAPT H1 and H1c haplotypes. Similar regional variation was seen in both PSP cases and controls.
1. Introduction
Progressive supranuclear palsy (PSP) is an atypical parkinsonian disorder characterised by the presence of abnormal hyperphosphorylated tau inclusions in selected regions of the brain (Dickson et al., 2007). These inclusions, or neurofibrillary tangles (NFTs), are the hallmark of a group of neurodegenerative disorders referred to as tauopathies. These include cortical basal degeneration (CBD), Pick’s Disease (PiD), Fronto-temporal dementia (FTD) and Alzheimer’s disease (AD), each featuring a disease-specific composition of tau tangles.
The human microtubule-associated protein tau gene (MAPT) is located on chromosome 17q21; its protein tau is involved in microtubule stability and interaction with the cytoskeleton. In the adult human brain, MAPT gives rise to 6 major isoforms through alternative splicing of exons 2, 3 and 10 (Andreadis, 2005). Inclusion or exclusion of exon 10, which encodes for an additional microtubule-binding repeat in the C-terminal domain, leads to a protein containing either three (3R, 10-) or four (4R, 10+) imperfect repeats. In the normal adult brain, the 3R and 4R isoforms are found in equal amounts (Goedert et al., 1989). In PSP, the NFTs contain an excess of 4R-tau and isoform ratio imbalance has been implicated in disease (Chambers et al., 1999; Takanashi et al., 2002; Ezquerra et al., 2007; Dickson et al., 2007).
Two major MAPT haplotypes, H1 and H2, have been described: H1 is significantly over-represented in PSP cases whereas H2 acts as a protective haplotype in Europeans (Baker et al., 1999; Hoglinger et al., 2011). Additionally, due to the presence of numerous common variants on the H1 background, the H1 clade has been refined to a number of subhaplotypes using 6 tagging SNPs, and association with PSP was further linked to the H1c subhaplotype (Pittman et al., 2005; Rademakers et al., 2005). In AD, association with the H1c subhaplotype and tagSNP rs7521 was reported by some (Myers et al., 2005) but not all (Abraham et al., 2009) studies. Recently, genome-wide association studies have provided striking evidence for an effect of MAPT and its H1 haplotype in Parkinson’s disease (Simon-Sanchez et al., 2009; Nalls et al., 2011).
The mechanisms leading from risk alleles and haplotypes to disease are largely unknown; for example, specific variants may affect total gene expression or alternative splicing. It has been suggested that some or all of the genetic risk for disease associated with MAPT variants is driven by differences in expression levels of the gene or its isoforms (Kwok et al., 2004; Rademakers et al., 2005; Caffrey et al., 2006; Ezquerra et al., 2007; Myers et al., 2007). Furthermore, the basis for regional susceptibility in neurodegeneration is unknown but may relate to variation in gene expression and the effect of risk alleles in different brain areas.
In this study we have addressed these issues by investigating a series of brain regions in PSP and healthy individuals. We designed a fluorescent assay to target haplotype-specific 4R-tau expression in these cohorts and identified marked regional variation, particularly in relative 4R-tau expression, in both cases and controls.
2. Methods
2.1 Cohort
We studied tau expression in post-mortem brain tissue from PSP cases and controls unaffected by neurological disease (Table 1). Samples were obtained from the Sara Koe PSP Brain Research Centre, UCL, London, UK and the Newcastle Brain Tissue Resource, University of Newcastle, UK. PSP was defined using standard pathological criteria. Additional controls (frontal cortex, temporal cortex, cerebellum and pons) were obtained from the Neuropathology department, John Hopkins Hospital and the University of Maryland Brain Bank, as previously described (Gibbs et al., 2010). The samples had previously been genotyped using the Infinium HumanHap550 beadchip (Illumina, Inc., San Diego, CA; Gibbs et al., 2010) and detailed haplotypes were determined using the PLINK software tool (Purcell et al., 2007). Phenotypic data for the samples are summarized in Tables 1 and 2.
Table 1.
Multiple brain regions assayed in case and control samples.
| Cohort | Haplotype | Brain regions | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Frontal/Motor cortex | Globus pallidus | Occipital lobe | Temporal | Pons | Cerebellum | ||
| PSP cases | H1/H1 | 58 | 14 | 9 | – | – | – |
|
| |||||||
| Controls | H1/H1 | 88 | 3 | 3 | 38 | 32 | 38 |
| H1/H2 | 59 | 4 | 4 | 24 | 21 | 21 | |
Table 2.
Phenotypic data in cases and controls.
| Cohort | Phenotype | |||
|---|---|---|---|---|
| Females | Males | Mean age at death | Range | |
| PSP cases | 26 | 41 | 73 years old | 61–86 years old |
| Controls | 53 | 99 | 77 years old | 15–101 years old |
RNA was extracted using TriReagent (Ambion/Applied Biosystems Inc, Foster City, CA) and treated with DNase before conversion to cDNA with random decamers using the RETROscript kit (Ambion/Applied Biosystems Inc, Foster City, CA). Each sample was subjected to two parallel reverse transcriptase reactions. DNase-treated RNA was quantified and checked for integrity using an Agilent bioanalyser (Agilent Technologies, Santa Clara, CA). DNA was extracted using standard phenol chloroform method.
2.2 Haplotype-specific mRNA isoform quantification
We used two forward primers, each specific to an allele of the H1/H2-defining SNP rs1052553 located in exon 9 of MAPT. The reverse primer was placed in exon 11 to capture both the 4R- and 3R-tau isoforms, expressed by the MAPT H1 and H2 haplotypes. The forward primers were designed with a locked nucleic acid (LNA) base at the 3′ end, for stringency of binding to rs1052553, and a fluorescent label at the 5′ end (Hex/Fam). The primer sequences were checked for common variants using the 1000 Genomes Project (www.1000genomes.org/) and the Exome Variant Server, NHLBI Exome Sequencing Project (http://evs.gs.washington.edu/EVS/, accessed July 2012). The rare variants identified (rs114900761, rs63749855 and rs63751231) are unlikely to contribute to assay variability. The accuracy of the assay for relative quantitation was confirmed by a control curve analysis performed with each PCR, using a sequential dilution of pure clonal 4R-tau and a constant dilution of pure clonal 3R-tau. The PCR products were analysed on an ABI 3100 genotyper (Applied Biosystems Inc, Foster City, CA) and quantified using the peak height ratios of 4R/(3R+4R), where a 1:1 4R:3R ratio is 0.5. Variations in expression were confirmed using a two-sample t-test for pairwise comparison.
3. Results
3.1 Relative 4R-tau expression patterns in multiple brain regions
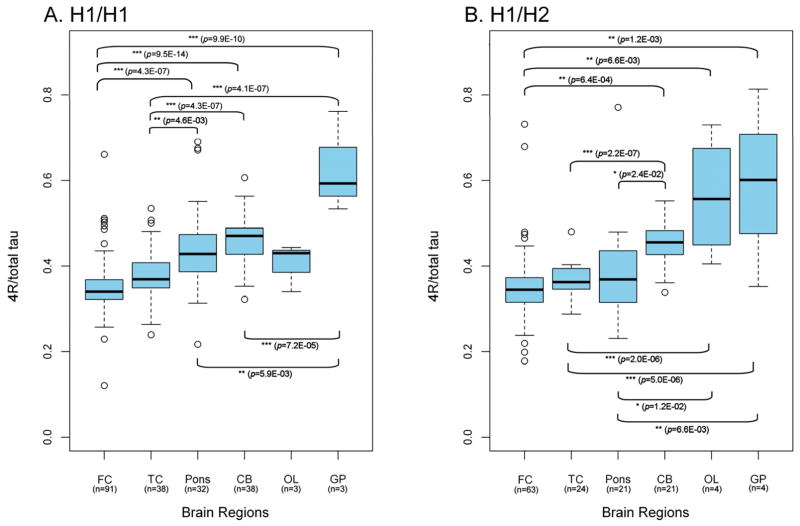
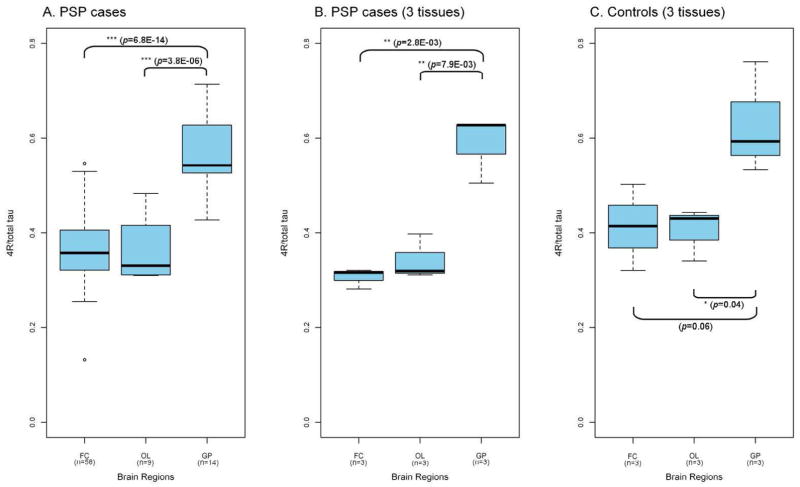
We observed marked difference in tau splicing between brain regions. The highest relative 4R-tau expression was seen in globus pallidus, with intermediate expression in pons and cerebellum and lowest expression in the motor/frontal cortex. These patterns of expression were consistent, irrespective of haplotype or disease state (Figure 1 and 2). In both the H1/H1 and H1/H2 controls, the regional variations showed robust statistical significance (Figure 1). In PSP cases, the difference in relative 4R-tau expression was particularly striking between frontal cortex and globus pallidus (p=6.8E-14, equal variance two sample t-test; Figure 2). Pair-wise comparison of the H1/H1 frontal cortex control group (n=91) and PSP frontal cortex group (n=58) did not show differences in 4R-tau expression between PSP cases and controls. Consequently, general patterns of expression support regional, rather than disease-related, differences in expression in post-mortem tissue.
Figure 1. Regional variation in relative 4R-tau expression in controls.
Regional patterns in H1/H1 homozygotes (A) and H1/H2 heterozygotes (B) show the lowest expression in frontal cortex and the highest expression in globus pallidus. Significant differences in relative 4R-tau expression are indicated as p values (equal variance two-sample t-test). FC, frontal cortex; TC, temporal cortex; CB, cerebellum; OL, occipital lobe; GP, globus pallidus, n, number of samples available for each group.
Figure 2. Regional variation in relative 4R-tau expression in PSP cases.
Relative 4R-tau expression shows significant regional variation in the PSP cohort, with the highest expression in globus pallidus (A., equal variance two-sample t-test). These observations held true in a small subset of PSP patients (B.) and controls (C.) where all 3 brain regions were available from a single donor. This suggest that the regional variations identified in the larger cohorts are not due to sample heterogeneity. FC, frontal cortex; OL, occipital lobe; GP, globus pallidus, n, number of samples available for each group.
3.2 Effect of H1-tau and H1c-tau haplotype on 4R-tau expression
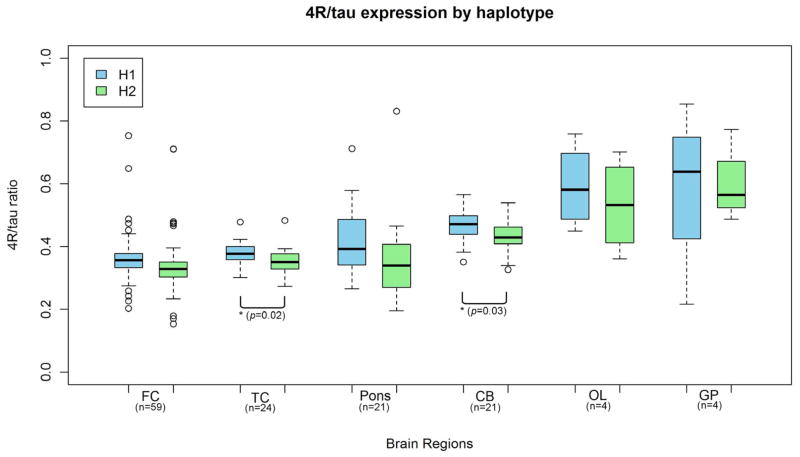
We observed a trend for higher expression of 4R-tau from the neurodegeneration risk haplotype H1 as compared to H2 haplotype, in all control brain regions (Figure 3). The difference in 4R-tau expression between H1 and H2 reached statistical significance in the temporal cortex (p=0.02; equal variance two sample t-test) and cerebellum (p=0.03; equal variance two sample t-test; Figure 4). Overall, relative expression of 4R-tau was significantly increased on the H1 background (p=0.016, one-way ANOVA) and across brain regions (p=0.004, two-way ANOVA).
Figure 3. Variation in relative 4R-tau expression by haplotype in controls.
There was a trend for higher expression of 4R-tau from the H1 haplotype compared to the H2 haplotype. The variation reached statistical significance in the temporal cortex and cerebellum (p <0.05; equal variance two-sample t-test). FC, frontal cortex; TC, temporal cortex; CB, cerebellum; OL, occipital lobe; GP, globus pallidus, n, number of samples available for each group.
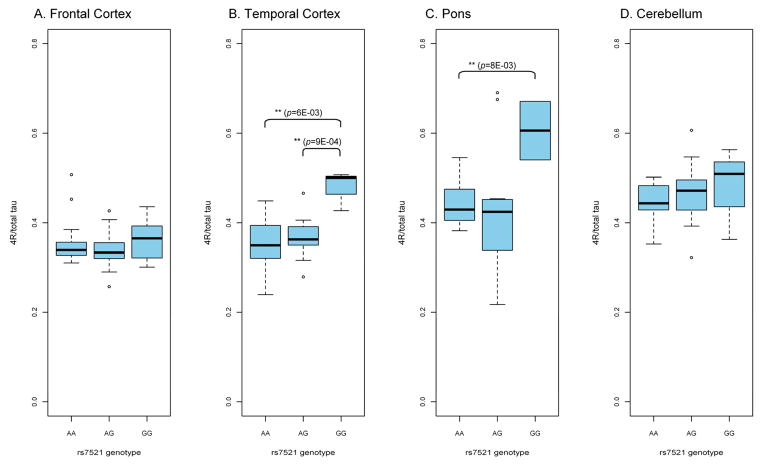
Figure 4. Effect of SNP rs7521 on 4R-tau expression in H1/H1 controls.
The G allele, on the risk haplotype H1c background, is particularly associated with higher relative 4R-tau expression in the temporal cortex and pons.
To further analyse H1 subhaplotypes and SNPs previously implicated in neurodegeneration, we stratified the H1/H1 control and PSP data by SNPs rs242557 and rs7521, both of which distinguish the PSP risk haplotype H1c from the other common H1 haplotypes. Data stratification by SNP rs242557 only showed inconsistent effects on relative 4R-tau expression (data not shown). This suggests that additional SNPs on the same background may affect tau splicing and a larger sample set will be required to accurately dissect these effects. Stratification of the data by H1c-tagging SNP rs7521 showed a moderate (frontal cortex, cerebellum) to strong (temporal cortex, pons) genotypic effect on 4R-tau expression (Figure 4). Although the cohort numbers were small, there was more relative 4R-tau expression from the G/G genotype. The G allele of rs7521 is found primarily on the H1c subhaplotype, on the H1 background (Pittman et al., 2005). Of note, 5/7 control brains that carried the G/G genotype were heterozygotes for H1c and a rare H1 subhaplotype. A similar trend for 4R-tau overexpression from the G/G haplotype was observed in the PSP frontal cortex cases (data not shown).
4. Discussion
Regional disease gene expression is likely to be important in the pathogenesis of regionally selective neurodegenerative diseases. It is important that disease gene expression studies are considered in the light of normal regional variations that occur within a pathology-free brain. We investigated multiple brain regions for their relevance to PSP and further studied the expression of 4R-tau from the tau H1 and H2 backgrounds. The fluorescent PCR method described here allows simultaneous analysis of the 3R- and 4R-tau isoform populations across MAPT haplotypes. By design, the assay is thus not susceptible to quantification artefacts that have been seen in probe arrays (Trabzuni et al., 2012).
Both the common MAPT H1 haplotype and the H1c defining SNP rs7521 are associated with higher 4R-tau expression. In agreement with previous reports (Caffrey et al., 2006), there was a trend for higher expression of 4R-tau from the H1 haplotype compared to H2 in all our samples. In PSP, the presence of one or multiple trans-acting variants acting in concert may promote enough additional expression of 4R-tau to tip the balance of the 4R/3R tau ratio into becoming pathogenic, in critical brain regions already exhibiting a high level of 4R-tau (compared to 3R-tau). The effect of H1c-SNP rs7521 genotype on 4R-tau expression appeared to vary between brain regions. These differences may also be compounded, in each brain region, by the effect of additional risk variants which were not analyzed here. Incidently, a recent genome-wide association study in PSP identified two distincts signals at the MAPT locus, rs242557 and H1-tagging SNPs (Hoglinger et al., 2012). While H1-tagging SNPs have been implicated as MAPT expression modifiers in the Hoglinger study, the mechanism underlying rs242557 association as a risk factor for PSP is currently unknown. It has been hypothesized that rs242557 could exert an effect on MAPT splicing (Caffrey et al., 2006; Hoglinger et al., 2012), but we could not confirm such a role in the present study.
Importantly, we also observed strong regional variations in 4R-tau expression in our series. Genome-wide expression studies have previously uncovered striking regional variations in gene expression in the same control samples (Gibbs et al., 2010) and this study provides further evidence that mRNA isoform expression is region-specific. This is of particular interest in neurodegeneration, as discrete brain regions are targeted in disease. For example, we observed a strong relative expression of 4R-tau in the globus pallidus compared to all other brain regions. This is in line with some (Caffrey et al., 2006), but not all (Ezquerra et al., 2007) observations of 4R-tau over-expression in the globus pallidus compared to frontal cortex in pathology-free brains. The globus pallidus is particularly affected by PSP pathology and conceivably this may relate to the background variation in normal tau expression. Secondly, in control cases, we observed a trend for higher 4R-tau expression from the temporal compared to the frontal cortex. The temporal cortex is known to develop neurofibrillary tangles in the early stages of AD and others have described similar expression patterns (Glatz et al., 2006).
We did not identify differences between PSP cases and controls. Generally, inter-regional variations in the control series showed a robust expression pattern that was mirrored in cases. This study was likely underpowered to detect significant differences between cases and controls. It should also be noted that lack of difference between cases and controls might be in part attributed to cell death in PSP cases. Nevertheless, it is tempting to speculate that brain regions most affected in disease may in fact be more susceptible to damage because the expression level of key genes (or, in the case of MAPT, the shift from an equimolar 4R/3R-tau ratio) is closer to a “threshold” required for disease. Furthermore, RNA extraction in this study is based on frozen tissue chips which will contain neurons, glia and inflammatory cells. Potentially, methods which assess gene expression in defined cell types using laser capture microdissection may provide more more evidence of case-control differences. It has been suggested that an imbalance in the 4R/3R-tau isoforms, rather than overexpression of total tau is pivotal to PSP pathology (Takanashi et al., 2002) and recent in vitro studies suggested that even subtle variations in 4R/3R-tau ratio can affect tau aggregation (Adams et al., 2010).
Recent work by Trabzuni and colleagues has identified variation in MAPT exon 3 expression realted to the MAPT H2 haploptype. Due to the differences in tissues, approaches and techniques employed, direct comparisons of our study with this work is difficult. Nevertheless, in their only overlapping dataset (222 H1/H1 individuals interrogated for H1c eQTL using the Illumina HumanHT-12 v3 Expression BeadChips in the Trabzuni study), both studies failed to detect an effect for SNP rs242557 on tau expression. Certainly, our results provide an additional piece to the MAPT puzzle and complement recent studies of overall MAPT expression and N-terminal isoform splicing (Trabzuni et al., 2012).
In conclusion, our data touched on different facets of an extremely complex mechanism where cis-acting elements and region-specific (and very likely cell-specific) events influence subtle tau isoform expression. Understanding how this mechanism is regulated, both in control and disease states, might open the way to new therapeutic approaches that correct the abnormal tau isoform imbalance seen in PSP.
Acknowledgments
This work was supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health, Department of Health and Human Services; project number Z01 AG000932-03. This study was funded by the PSP (Europe) Association.
Tissue for this study was provided by the Newcastle Brain Tissue Resource which is funded in part by a grant from the UK Medical Research Council (G0400074), the Alzheimer’s Society and Alzheimer’s Research Trust as part of the Brains for Dementia Research Project, and by the National Institute for Health Research (NIHR) Newcastle Biomedical Research Centre based at Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Disclosure statement
All authors declare that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham R, Sims R, Carroll L, et al. An association study of common variation at the MAPT locus with late-ons zheimer’s disease. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:1152–1155. doi: 10.1002/ajmg.b.30951. [DOI] [PubMed] [Google Scholar]
- Adams SJ, DeTure MA, McBride M, et al. Three repeat isoforms of tau inhibit assembly of four repeat tau filaments. PLoS One. 2010;5(5):e10810. doi: 10.1371/journal.pone.0010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreadis A. Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Biophys Acta. 2005;1739:91–103. doi: 10.1016/j.bbadis.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Baker M, Litvan I, Houlden H, et al. Association of an extended haplotype in the tau gene with progressive supranuclear palsy. Hum Mol Genet. 1999;8:711–715. doi: 10.1093/hmg/8.4.711. [DOI] [PubMed] [Google Scholar]
- Caffrey TM, Joachim C, Paracchini S, et al. Haplotype-specific expression of exon 10 at the human MAPT locus. Hum Mol Genet. 2006;15:3529–3537. doi: 10.1093/hmg/ddl429. [DOI] [PubMed] [Google Scholar]
- Chambers CB, Lee JM, Troncoso JC, et al. Overexpression of four-repeat tau mRNA isoforms in progressive supranuclear palsy but not in Alzheimer’s disease. Ann Neurol. 1999;46:325–332. doi: 10.1002/1531-8249(199909)46:3<325::aid-ana8>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Dickson DW, Rademakers R, Hutton ML. Progressive supranuclear palsy: pathology and genetics. Brain Pathol. 2007;17:74–82. doi: 10.1111/j.1750-3639.2007.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezquerra M, Gaig C, Ascaso C, et al. Tau and saitohin gene expression pattern in progressive supranuclear palsy. Brain Res. 2007;1145:168–176. doi: 10.1016/j.brainres.2007.01.098. [DOI] [PubMed] [Google Scholar]
- Gibbs JR, van der Brug MP, Hernandez DG, et al. Abundant quantitative trait loci exist for DNA methylation and gene expression in human brain. PLoS Genet. 2010;6(5):e1000952. doi: 10.1371/journal.pgen.1000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz DC, Rujescu D, Tang Y, et al. The alternative splicing of tau exon 10 and its regulatory proteins CLK2 and TRA2-BETA1 changes in sporadic Alzheimer’s disease. J Neurochem. 2006;96:635–644. doi: 10.1111/j.1471-4159.2005.03552.x. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG, Jakes R, et al. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer’s disease. Neuron. 1989;3:519–526. doi: 10.1016/0896-6273(89)90210-9. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Melhem NM, Dickson DW, et al. Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nature Genetics. 2011;43:699–705. doi: 10.1038/ng.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok JB, Hallupp M, Loy CT, et al. GSK3B polymorphisms alter transcription and splicing in Parkinson’s disease. Ann Neurol. 2005;58:829–839. doi: 10.1002/ana.20691. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Kaleem M, Marlowe L, et al. The H1c haplotype at the MAPT locus is associated with Alzheimer’s disease. Hum Mol Genet. 2005;14:2399–2404. doi: 10.1093/hmg/ddi241. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Pittman AM, Zhao AS, et al. The MAPT H1c risk haplotype is associated with increased expression of tau and especially of 4 repeat containing transcripts. Neurobiol Dis. 2007;25:561–570. doi: 10.1016/j.nbd.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Nalls MA, Plagnol DG, Hernandez DG, et al. Imputation of sequence variants for identification of genetic risks for Parkinson’s disease: a meta-analysis of genome-wide association studies. Lancet. 2011;377:641–649. doi: 10.1016/S0140-6736(10)62345-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman AM, Myers AJ, Abou-Sleiman P, et al. Linkage disequilibrium fine mapping and haplotype association analysis of the tau gene in progressive supranuclear palsy and corticobasal degeneration. J Med Genet. 2005;42:837–846. doi: 10.1136/jmg.2005.031377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademakers R, Melquist S, Cruts M, et al. High-density SNP haplotyping suggests altered regulation of tau gene expression in progressive supranuclear palsy. Hum Mol Genet. 2005;14:3281–3292. doi: 10.1093/hmg/ddi361. [DOI] [PubMed] [Google Scholar]
- Simon-Sanchez J, Schulte C, Bras JM, et al. Genome-wide association study reveals genetic risk underlying Parkinson’s disease. Nat Genet. 2009;41(12):1308–12. doi: 10.1038/ng.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takanashi M, Mori H, Arima K, et al. Expression patterns of tau mRNA isoforms correlate with susceptible lesions in progressive supranuclear palsy and corticobasal degeneration. Brain Res Mol Brain Res. 2002;104:210–219. doi: 10.1016/s0169-328x(02)00382-0. [DOI] [PubMed] [Google Scholar]
- Trabzuni D, Wray S, Vandrovcova J, et al. MAPT expression and splicing is differentially regulated by brain region: relation to genotype and implication for tauopathies. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds238. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]