Background: Tie2 is essential for angiogenesis and vascular stabilization.
Results: Tie2, but not Tie1, forms ligand-independent dimers on the cell surface.
Conclusion: The inactive monomer mutant Tie2YIA/LAS decreases Ang1/Tie2 signaling.
Significance: The Tie2 ligand-independent dimer induces strong phosphorylation upon high dose Ang1 binding.
Keywords: Angiogenesis, Endothelial Cell, Imaging, Tyrosine Protein Kinase (Tyrosine Kinase), Vascular Biology, Angiopoietin
Abstract
Tie2 is a receptor tyrosine kinase expressed on vascular endothelial cells (ECs). It has dual roles in promoting angiogenesis and stabilizing blood vessels, and it has been suggested that Tie2 forms dimers and/or oligomers in the absence of angiopoietin-1 (Ang1); however, the mechanism of ligand-independent dimerization of Tie2 and its biological significance have not been clarified. Using a bimolecular fluorescence complementation assay and a kinase-inactive Tie2 mutant, we show here that ligand-independent Tie2 dimerization is induced without Tie2 phosphorylation. Moreover, based on the fact that Tie1 never forms heterodimers with Tie2 in the absence of Ang1 despite having high amino acid sequence homology with Tie2, we searched for ligand-independent dimerization domains of Tie2 by reference to the difference with Tie1. We found that the YIA sequence of the intracellular domain of Tie2 corresponding to the LAS sequence in Tie1 is essential for this dimerization. When the YIA sequence was replaced by LAS in Tie2 (Tie2YIA/LAS), ligand-independent dimer was not formed in the absence of Ang1. When activation of Tie2YIA/LAS was induced by a high dose of Ang1, phosphorylation of Tie2 was limited compared with wild-type Tie2, resulting in retardation of activation of Erk downstream of Tie2. Therefore, these data suggest that ligand-independent dimerization of Tie2 is essential for a strong response upon stimulation with high dose Ang1.
Introduction
The functions of angiopoietin-1 (Ang1),2 a ligand for receptor tyrosine kinase Tie2 expressed on endothelial cells (ECs), in both EC-to-EC and EC-to-mural cell adhesion are well established (1–4). Although Ang1-Tie2 signaling is involved in promoting maturation and quiescence of blood vessels mainly regulated by Akt signal transduction via the p85 subunit of PI3K, Tie2 also has proangiogenic activity mediated by MAPK signaling (5–7). Because Tie2 possesses both anti-angiogenic as well as proangiogenic properties, it is important to investigate how Tie2 activation is altered during angiogenesis. The Tie receptor family consists of Tie2 and Tie1 (8–10). Recent studies show that Ang1 activates Tie1 indirectly by interactions with Tie2 (11). When Tie1 expression is silenced, Tie2 signaling especially via the MAPK pathway is enhanced; accordingly, Tie1-deficient mice show hyperproliferative vascular formation and vascular abnormalities (12, 13). This suggests that Tie1 may negatively regulate angiogenic signaling by Tie2.
Tie2 is composed of an extracellular domain, one transmembrane domain, and an intracellular tyrosine kinase domain split into two by a non-kinase sequence. Tie1 has high amino acid sequence homology with Tie2 (76 and 33% identity in intracellular and extracellular domains, respectively). Based on the isolation of Tie2 ligands and analysis of signal transduction through Tie2, it is widely accepted that Ang1 activates Tie2, but Ang2 binding inhibits its signaling. Thus, with certain exceptions Ang2 acts as an Ang1 antagonist (4, 13–18).
It is known that Tie2 is present on the EC surface in the form of dimers and higher order oligomers, as established by electron microscopy (19). It has been reported that EGF receptor, erythropoietin receptor, and TNF receptor also dimerize in a ligand-independent manner (20–25). Ligand-independent dimerization of EGF receptor does not lead to tyrosine phosphorylation of EGF receptor. It has been suggested that a conformational change of the EGF receptor induces kinase activity on ligand binding. Therefore, ligand-independent dimers may mediate rapid signal transduction responses. Although Tie2 also forms ligand-independent dimers, the importance of this has not yet been determined.
In the present study, we established a system for visualization of Tie2 dimers using bimolecular fluorescence complementation (BiFC) assays in living cells (26). Using this system, we sought Ang1-independent Tie2-Tie2 dimerization domains. We generated a Tie2 mutant that does not form dimers in the absence of Ang1. We investigated the biological significance of ligand-independent Tie2 dimerization using this mutant.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Recombinant human Ang1 was purchased from R&D Systems. In Western blot analysis, mouse anti-phosphotyrosine (4G10) and anti-Tie2 (Ab33) antibodies (Abs) (Upstate), anti-HA.11 mAb (COVANCE), anti-c-Myc (9E10) and Tie1 (C-18) mAbs (Santa Cruz Biotechnology, Inc.), anti-HA-tag rabbit serum (Medical & Biological Laboratories Co., Ltd.), p44/42, phospho-p44/42 (Thr202/Tyr204), phospho-Tie2 (Tyr992) Abs (Cell Signaling Technology, Inc.) and mouse anti-HA (12CA5) mAb (Roche Diagnostics) were used as the first Abs. Anti-phospho-Tie2 (Tyr992) and anti-phosphotyrosine Abs were diluted 1:500, and others were diluted 1:1000. HRP-conjugated anti-rabbit and anti-mouse IgG (Jackson ImmunoResearch Laboratories) was used as the secondary antibody (dilution: 1:1000). For the immunofluorescence analysis, HA and Myc were used as the first Abs (dilution, 1:100). Alexa Fluor 546-conjugated goat anti-rabbit Igs and Alexa Fluor 647-conjugated goat anti-mouse Igs were used as the secondary Abs (Invitrogen) (dilution, 1:200).
Plasmid Construction
Mouse Tie2 and Tie1 were fused to sequences encoding full-length Venus and Venus residues 1–173 amino acids (VN) or 155–238 amino acids (VC). The coding regions were connected with linker sequences encoding RSAIT (Arg-Ser-Ala-Ile-Thr). RSAIT is a non-adhesion sequence (26). HA or Myc epitopes were inserted as tags between linker and fluorescent genes. Genes were inserted at the multicloning site in pEGFPN1 vector or pMRX virus vector. Basing the work on the pE-Tie2-linker-Myc-Venus, pE-Tie2-linker-HA-VN and pE-Tie2-linker-Myc-VC, we cut between the BamHI and MluI sites and the Tie2 mutant (Tie2K854R, Tie2R848W) was created. Tie2 kinase-dead (Tie2K854R) or Tie2 constitutive-active (Tie2R848W) mutants were amplified from wild-type Tie2 using Tie2K854R-N, -C primers or Tie2R848W-N, -C primers, respectively (supplemental Table S1).
For the generation of Tie1*, the signal sequence of Tie2 was amplified from the Tie2 plasmid using oligonucleotide primers (forward primer, 5′-GTA GGC GTG TAC GGT GGG AGG TCT-3′ and reverse primer, 5′-GTT AAG TCA ACA GAG CCT TCT ACT ACT CC-3′) and 5′-Tie1 core sequence excluding signal sequence was amplified from Tie1 using oligonucleotide primers (forward primer, 5′-GGA GTA GTA GAA GGC TCT GTT GAC TTA AC-3′ and reverse primer, 5′-CCA CTT CTG AGC TTC ACA GCC TCG CAC GAT-3′). These two products were amplified with the forward primer for Tie2 and the reverse primer for Tie1. This PCR product was placed into EcoRI and AgeI sites of the Tie1 plasmid. For the generation of Tie2/Tie1 chimeric plasmids, mutagenesis was performed on Tie2 and Tie1 plasmids as templates by using specific primer sets (supplemental Table S1). For generation of 1–3 amino acid mutants of each Tie2 plasmid, mutagenesis was performed using specific primers (supplemental Table S1).
Retroviral Infection
Plat-E cells were transfected with pMRX-Tie2-linker-Myc-Venus, pMRX-Tie2YIA/LAS-linker-Myc-Venus, pMRX-Tie2-linker-HA-VN173 and pMRX-Tie2-linker-Myc-VC155, pMRX-Tie2YIA/LAS-linker-HA-VN173 and pMRX-Tie2YIA/LAS-linker-Myc-VC155 vectors as indicated in each experiment (1.0 μg each) using Lipofectamine 2000 (Invitrogen) and then incubated for 24 h at 37 °C after which the medium was changed. After 12 (36 h from transfection) and 24 h (48 h from transfection), conditioned medium was harvested, sterilized by filtration, and used to infect NIH3T3 cells. 8 μg/ml polybrene was added for enhancement of infection. Stable cell lines were selected by culture in medium containing puromycin (5 μg/ml) or blasticidin (10 μg/ml) (27, 28).
Cell Culture
HEK293T and NIH3T3 lines were grown in DMEM supplemented with 10% FBS. Platinum-E cells (Plat-E; packaging cells) and stable cell lines transfected by pMRX virus vector were cultured in 10% FBS containing DMEM.
Transfection and Bimolecular Fluorescence Complementation Analysis
To carry out BiFC in living cells, cells were co-transfected with the expression vectors indicated in each experiment (1.0 μg each) using Lipofectamine 2000. The fluorescence emissions were acquired in living cells 22–48 h after transfection using a fluorescence microscope with a cooled CCD camera, or by flow cytometry. Protein expression levels were assessed by Western blotting.
Cell Lysis, Immunoprecipitation, SDS-PAGE, and Western Blotting
Cells were washed with ice-cold PBS and lysed with radioimmune precipitation assay lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS). The cells were incubated on ice for 10 min followed by centrifugation at 15,000 rpm for 5 min at 4 °C. Cells were immunoprecipitated from the supernatant using 1–2 μg of anti-Myc Ab that had been precoupled to 20 μl of protein A-Sepharose 4 Fast Flow (GE Healthcare).
Proteins electrophoretically separated using 7.5% SDS gels were transferred to nylon membranes (Amersham Biosciences) by a wet blotting procedure (140 V, 200 mA, 120 min). The membrane was blocked with 5% skim milk/TBST for 60 min, subsequently incubated with the Abs as indicated in the figures and processed for chemiluminescence detection with ECL solution. Densitometry was performed with NIH ImageJ software (version 1.43u).
FACS Analysis
BiFC was analyzed by flow cytometry. After fluorescence complementation, cells were washed with PBS and resuspended in PBS. FACS analysis was performed with a FACSCalibur (BD Biosciences) using the 488 nm laser for excitation and a 515–545 nm band pass filter for detection. For quantitative evaluation of BiFC fluorescence, we used % Gated (fluorescent cells) × X Geo Mean (average of fluorescent intensity) as arbitrary fluorescence units.
Confocal Laser Scanning Microscopy
Transfected cells on 0.1% gelatin-coated glass dishes (Sigma Aldrich) were rinsed, fixed for 10 min in 4% paraformaldehyde-PBS (pH 7.5), and washed with PBS. Subsequently, the cells were permeabilized with 0.1% Triton X-100 for 10 min. After washing with PBS, cells were blocked with PBS containing 5% normal goat serum and 1% BSA for 30 min and immunostained with first Ab (1:100) for 1 h. Protein reacting with Ab was visualized with secondary Abs (1:200). HA or Myc epitopes were inserted as tags between Tie2 or Tie2YIA/LAS and the BiFC tag (VN or VC). HA-VN fused with Tie2 or Tie2YIA/LAS was stained by rabbit anti-HA Ab (Medical & Biological Laboratories Co., Ltd.) and Alexa Fluor 546 (red)-conjugated anti-rabbit Igs. Myc-VC fused with Tie2 or Tie2YIA/LAS was stained with mouse anti-Myc Ab and Alexa Fluor 647 (blue)-conjugated anti-mouse Igs. BiFC fluorescence was detected using a filter for Alexa Fluor 488 (green). The slides were observed under a Leica TCS SP5 Ver1.6 (Leica Microsystems) using HCX PL APO lambda blue 63 × 1.4 oil. Images were processed using Adobe Photoshop CS5 Extended software (Adobe Systems).
Statistical Analysis
All data are displayed as the mean ± S.D. and were analyzed by two-tailed Student t test. A probability value of < 0.05 was considered statistically significant.
RESULTS
Establishment of Imaging Methods for Investigating the Dimerization of Tie2 Receptors
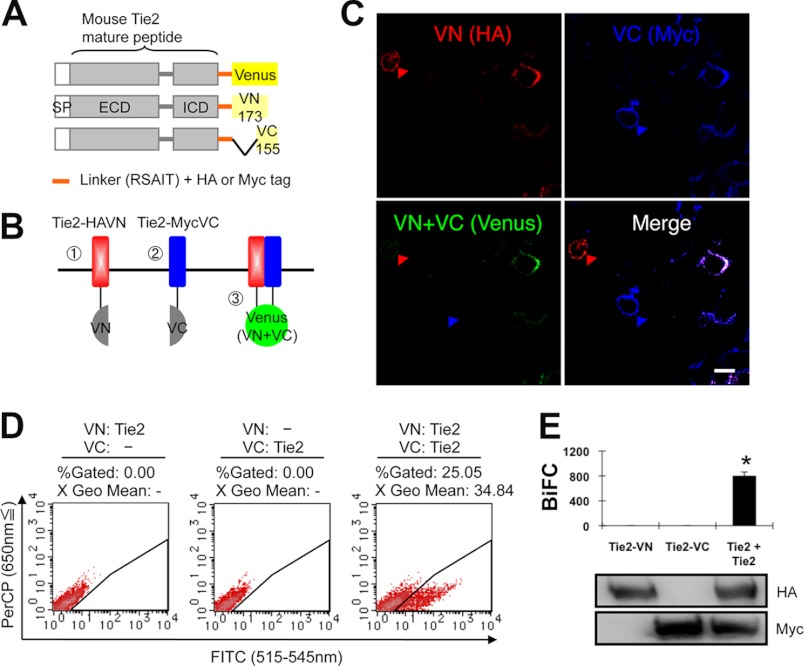
It has been reported that Tie2 is present in the form of dimers and/or oligomers on the cell surface (19). We also detected ligand-independent dimers of endogenous Tie2 in human umbilical vein endothelial cells (supplemental Fig. S1A). To assess Tie2 dimerization in the absence of Ang1, we utilized the BiFC assay (26). First, we prepared amino (N)-terminal (1–173: VN173) and carboxyl (C)-terminal (155–238: VC155) components of Venus fluorescent protein, a modifier of yellow fluorescent protein, fused with the C-terminal domain of wild-type (WT) mouse Tie2 with an HA or Myc tag linked to the molecule (Fig. 1A). When Tie2 (Tie2-HAVN, Tie2-MycVC) dimerizes, the fluorescent complex should be reconstituted (Fig. 1B). As expected, when Tie2-HAVN and Tie2-MycVC were cotransfected into HEK293T cells, cells expressing both HA and Myc developed fluorescence (Fig. 1C). Flow cytometry showed that transfection with both Tie2-HAVN and Tie2-MycVC vectors, but not with either alone, resulted in cells having high FITC intensity (Fig. 1, D and E). We confirmed that these co-transfectants developed BiFC fluorescence in cells expressing physiological levels of Tie2 as observed in ECs (supplemental Fig. S1, B and C).
FIGURE 1.
BiFC analysis of Tie2 receptor homodimerization in living cells. A and B, schematic representation of Tie2 tagged with either the N- or C-terminal of the Venus fragment (VN or VC). SP, signal peptide; ECD, extracellular domain; ICD, intracellular domain. When Tie2 dimerizes, fluorescence should reconstitute. C, HEK293T cells expressing Tie2-HAVN (red) and Tie2-MycVC (blue) observed by confocal microscopy. Cells were co-transefected with Tie2-HAVN and Tie2-MycVC expression vectors. Note that cells expressing Tie2-HAVN alone (red arrowhead) or Tie2-MycVC alone (blue arrowhead) develop no Venus fluorescence. Bar indicates 20 μm. D, flow cytometric analysis for evaluation of receptor dimerization as indicated. E, quantitative evaluation of Tie2 homodimerization in BiFC as observed in D (*, p < 0.05; n = 3). Protein expression level of each receptor was assessed by immunoblotting with anti-HA or anti-Myc Ab.
Analysis of the Dimerization of Tie2-Tie1 using BiFC Assays
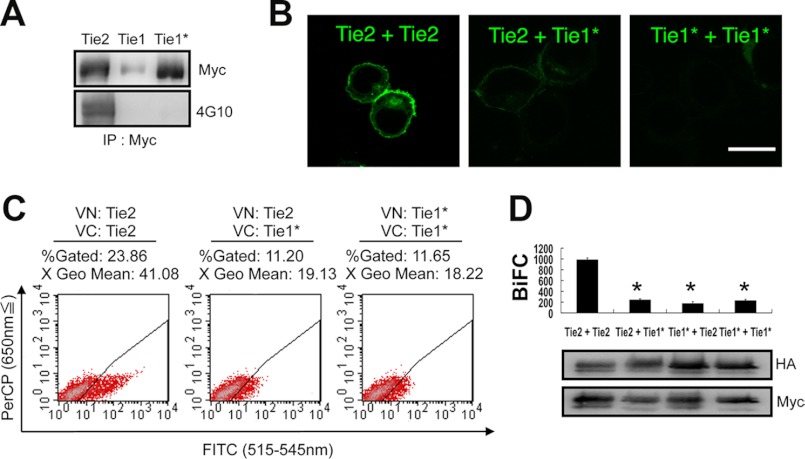
Ang1 activates Tie1 indirectly, mediated by its interaction with Tie2 (11, 12). It has been suggested that co-localization of Tie2 and Tie1 is induced upon activation of Tie2 by Ang1 (6). We investigated whether Tie2 and Tie1 also form heterodimers in a ligand-independent manner. Relative to Tie2, we found that the Tie1 protein was difficult to express in HEK293T cells following transfection of full-length Tie1 cDNA. However, when the original native signal sequence of Tie1 was excised and replaced with the Tie2 signal sequence (designated Tie1*), Tie1 expression was easily induced (Fig. 2A). Using this Tie1* construct, we evaluated Tie2-Tie1 and Tie1-Tie1 associations by BiFC. Although it has recently been reported that Tie2 and Tie1 associate following Ang1 stimulation and on cell-cell contact, we failed to detect any Tie2-Tie1 or Tie1-Tie1 associations (Fig. 2, B–D). This suggests that a Tie2 and Tie1 interaction is required for Ang1 binding to Tie2 and Tie1 and that Tie1 never gives rise to inactive dimers and/or oligomers in the absence of Ang1.
FIGURE 2.
BiFC analysis comparing Tie2 and Tie1*. A, signal peptide of Tie1 was replaced with that of Tie2 (Tie1*). All receptors were C-terminally tagged with Myc. The levels of Tie2, Tie1, and Tie1* protein were analyzed with Myc or 4G10 Ab. B–D, HEK293T cells were transiently transfected in combination with Tie2-HAVN and Tie2-MycVC, Tie2-HAVN and Tie1*-MycVC, or Tie1*-HAVN and Tie1*-MycVC. B, cells were analyzed by confocal microscopy. Bar indicates 20 μm. C, flow cytometric analysis for evaluation of receptor dimerization as indicated. D, quantitative evaluation of receptor dimerization in BiFC as shown in C (*, p < 0.05; n = 3). Protein expression level of each receptor was assessed by immunoblotting with anti-HA or anti-Myc Ab.
Analysis of the Dimerization of Tie2 Mutants Using BiFC Assays
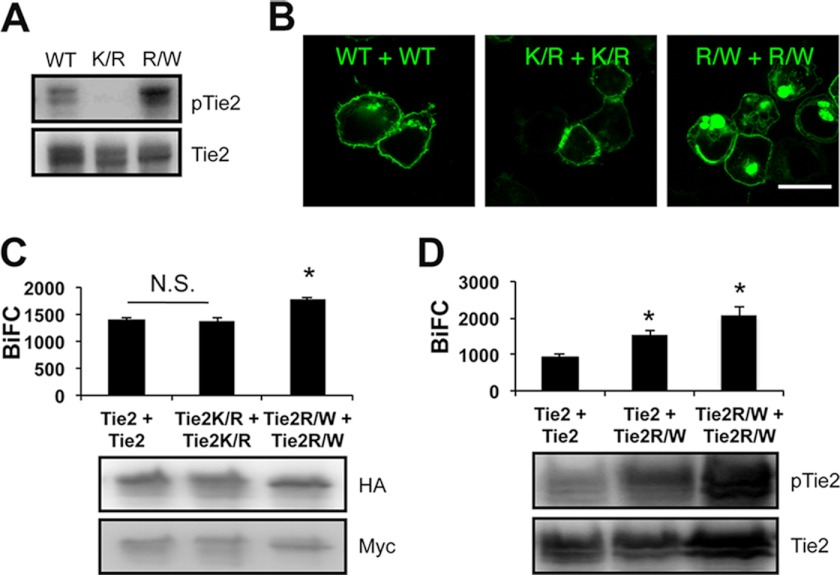
Phosphorylation of overexpressed Tie1 and Tie2 was observed, but only Tie2 and not Tie1 was autophosphorylated in the absence of Ang1 stimulation (Fig. 2A). To test whether phosphorylation of Tie2 affects Tie2-Tie2 dimerization, we generated a kinase-inactive Tie2 mutant (Tie2K854R) (Fig. 3A). However, loss of phosphorylation did not affect Tie2 dimerization (Fig. 3, B and C, and supplemental Fig. S2A). We further confirmed that it was not until Ang1 bound Tie2 that dimerized Tie2 was internalized (supplemental Fig. S2B). Although dimerized WT Tie2 was observed in the cytoplasm, dimerized kinase-inactivated Tie2 did not internalize from the cell surface into the cytoplasm (Fig. 3B). Next, we constructed a constitutively active mutant of Tie2 (Tie2R848W) (Fig. 3A) (29). In HEK293T cells overexpressing Tie2R848W-HAVN and Tie2R848W-MycVC, more abundant Venus fluorescence was observed in the cytoplasm than in wt Tie2 or Tie2K854R (Fig. 3, B and C). Interestingly, Tie2R848W can dimerize with WT Tie2, resulting in BiFC intensity enhanced compared with Tie2-Tie2 dimers (Fig. 3D). These results suggest that our BiFC system mimics canonical receptor down-modulation only after activation of the receptor.
FIGURE 3.
BiFC analysis comparing Tie2 and Tie2 mutant. A, detection of Tie2, kinase-dead mutant Tie2K854R (K/R) and constitutively active mutant Tie2R848W (R/W) phosphorylation. B and C, HEK293T cells were transiently transfected in combination with Tie2-HAVN and Tie2-MycVC, Tie2K854R-HAVN and Tie2K854R-MycVC, or Tie2R848W-HAVN and Tie2R848W-MycVC. B, cells were analyzed by confocal microscopy. Bar indicates 20 μm. C, quantitative evaluation of receptor dimerization in BiFC as shown in B (*, p < 0.05; n = 3). Protein expression level of each receptor was assessed by immunoblotting with anti-HA or anti-Myc Ab. D, quantitative evaluation of receptor dimerization in BiFC of Tie2 and Tie2R848W (*, p < 0.05; n = 3). Protein expression level of each receptor was assessed by immunoblotting with anti-Tie2. N.S., not significant.
Identification of the Domain That Induces Ligand-independent Tie2 Homodimerization
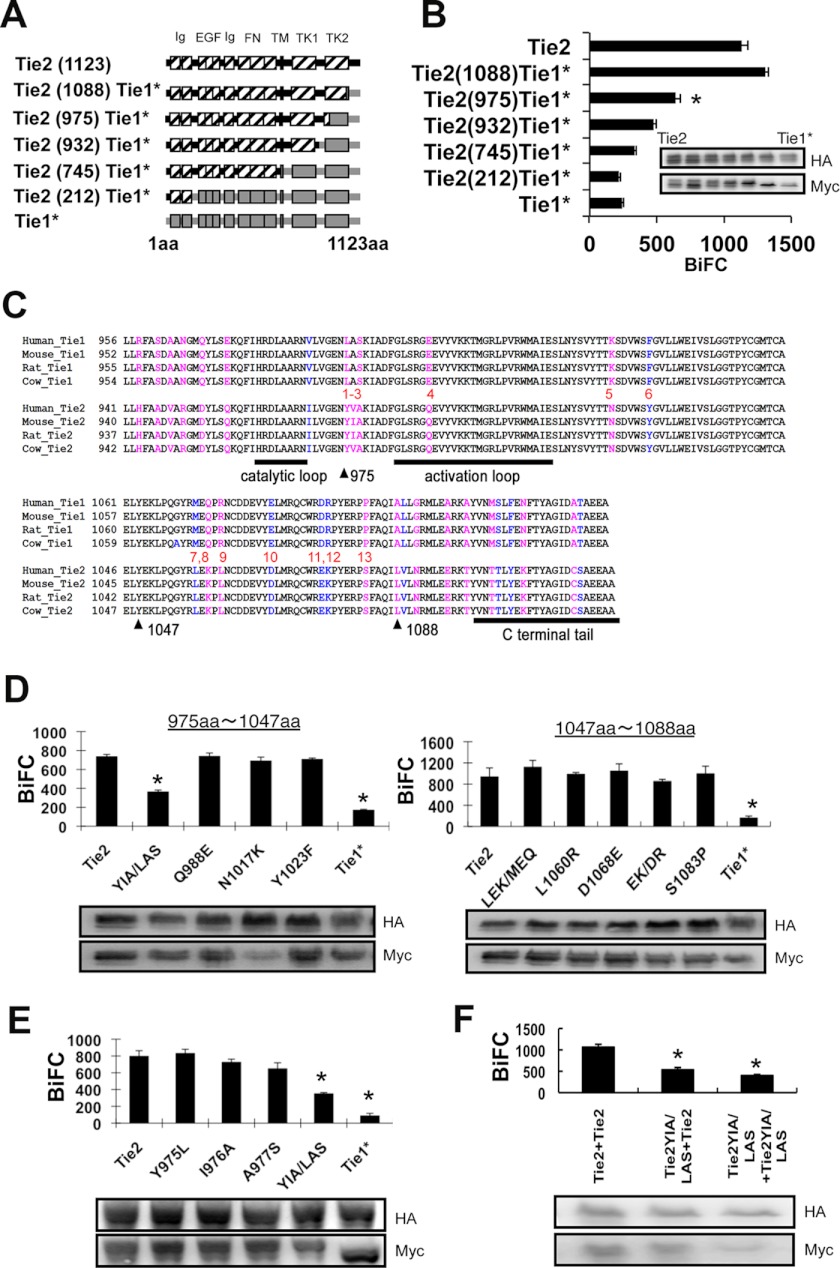
We found that Tie2, but not Tie1, forms homodimers in a ligand-independent manner. Hence, we attempted to isolate the Tie2 ligand-independent dimerizing region. First, we sought domains responsible for Tie2-Tie2 association by replacing part of Tie2 with the Tie1 homologous domain (Fig. 4A). We found that lack of the extracellular domain of Tie2 did not affect BiFC (supplemental Fig. S3), suggesting that BiFC caused by Tie2-Tie2 interaction is mainly induced by the intracellular domain of Tie2 in our model. Therefore, we focused on the intracellular domain of Tie2 for dimerization in our next experiments.
FIGURE 4.
YIA sequence of Tie2 induces ligand-independent dimerization. A, schematic of Tie2/Tie1* chimeras. B, in HEK293T cells, Tie2-HAVN was transiently transfected in combination with Tie2, Tie2(1088)Tie1*, Tie2(975)Tie1*, Tie2(932)Tie1*, Tie2(745)Tie1*, Tie2(212)Tie1*, or Tie1* C-terminally fused with MycVC, and close associations of receptors assessed by BiFC and flow cytometry (*, p < 0.05; n = 3). The protein expression level of each receptor was confirmed by immunoblotting with anti-HA or anti-Myc Ab (inset). C, comparison of amino acid sequences of Tie1 and Tie2 C terminus from different species. Pink, different amino acids; blue, same amino acids (aa). D and E, in HEK293T cells, Tie2-HAVN was transiently transfected in combination with Tie2, Tie2YIA/LAS, Tie2Q988E, Tie2N1017K, Tie2Y1023F, Tie2LEK/MEQ, Tie2L1060R, Tie2D1068E, Tie2EK/DR, Tie2S1083P, or Tie1* C-terminally fused with MycVC (D) or Tie2, Tie2Y975L, Tie2I976A, Tie2A977S, Tie2YIA/LAS, or Tie1* C-terminally fused with MycVC (E), and close associations of receptors assessed by BiFC and flow cytometry. Protein expression level of each receptor was confirmed by immunoblotting with anti-HA or anti-Myc Ab (inset) (*, p < 0.05; n = 3). F, YIA domain of Tie2 was replaced by LAS sequence (Tie2YIA/LAS). Association of Tie2-Tie2, Tie2-Tie2YIA/LAS, and Tie2YIA/LAS-Tie2YIA/LAS was observed by BiFC as described above. Protein expression level of each receptor was confirmed by immunoblotting with anti-HA or anti-Myc Ab (inset) (*, p < 0.05; n = 3).
We transfected Tie2-HAVN and Tie2/Tie1* chimeric genes fused with Myc-tagged VC155 into HEK293T cells. When the C-terminal of Tie2 (from 975 to 1088 amino acids) was replaced by the Tie1 sequence, BiFC was significantly attenuated (Fig. 4B). There are differences in 13 amino acids between Tie2 and Tie1 (Fig. 4C). Therefore, we mutated Tie2 where its sequence is different from Tie1 domain by domain and observed Tie2-Tie2/mutant dimerization. We found that a YIA sequence within Tie2 (975–977) is critical for dimerization (Fig. 4D). Next, we introduced point mutations into this YIA domain. We found that no single mutation was responsible for reducing Tie2 dimerization, but rather the whole YIA tandem sequence was involved (Fig. 4E). We generated mutant Tie2 (Tie2YIA/LAS) in which the YIA domain of Tie2 was replaced by LAS. Tie2-Tie2YIA/LAS and Tie2YIA/LAS-Tie2YIA/LAS dimerization was not significantly different, suggesting that both Tie2 YIA domains in the cytoplasmic region are required for dimerization (Fig. 4F). When phosphorylation of Tie2YIA/LAS was assessed, it was found that mere overexpression did not induce it (supplemental Fig. S4).
Tie2YIA/LAS Monomer Mutants Can Be Dimerized and Phosphorylated by Ligand Binding
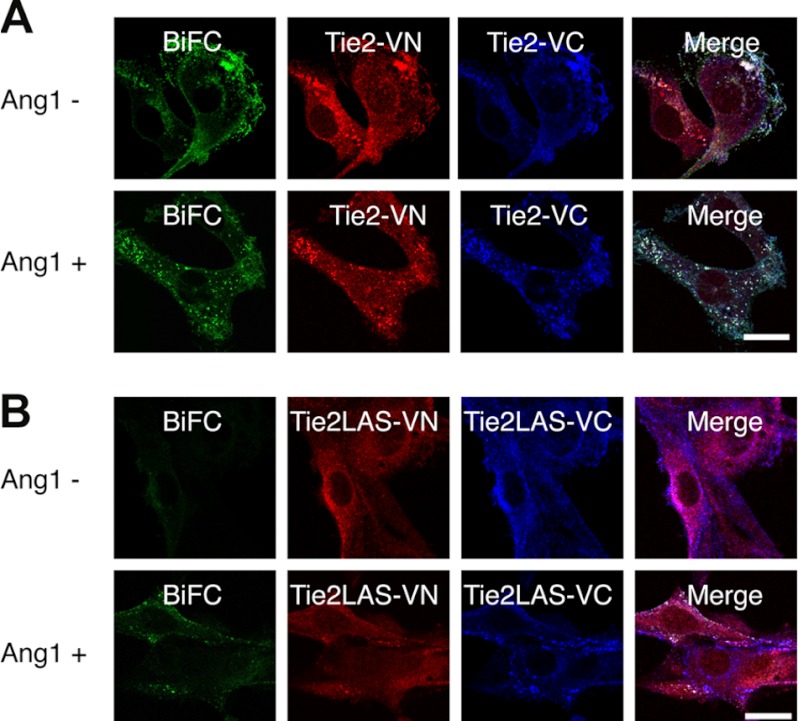
Tie2 can form ligand-independent inactive dimers; it has therefore been suggested that receptor dimerization and activation are mechanistically distinct and separable events (19, 30). Next, we analyzed whether Ang1 binding to the inactive monomer mutant Tie2YIA/LAS induced dimerization and activation of Tie2. Phosphorylation of WT Tie2 by exogenous Ang1 did not increase the intensity of BiFC developed by either Tie2-Tie2 (Fig. 5A). On the contrary, Ang1 stimulation decreased BiFC intensity after 30 min. This suggests that internalization and degradation of Tie2 was induced after Tie2 phosphorylation (30). Interestingly, we found that Tie2YIA/LAS prominently enhanced BiFC intensity under Ang1 stimulation for 1 h (Fig. 5B). Microscopy showed that Tie2 formed ligand-independent dimers and was internalized upon Ang1 stimulation (Fig. 6A). In contrast, Tie2YIA/LAS dimerization was not detected in the absence of Ang1. However, BiFC signals due to dimerization did occur upon stimulation with Ang1, although to a lesser extent than in WT Tie2. This suggests that YIA mutations in Tie2 did not completely prevent Tie2 dimerization (Fig. 6B).
FIGURE 5.
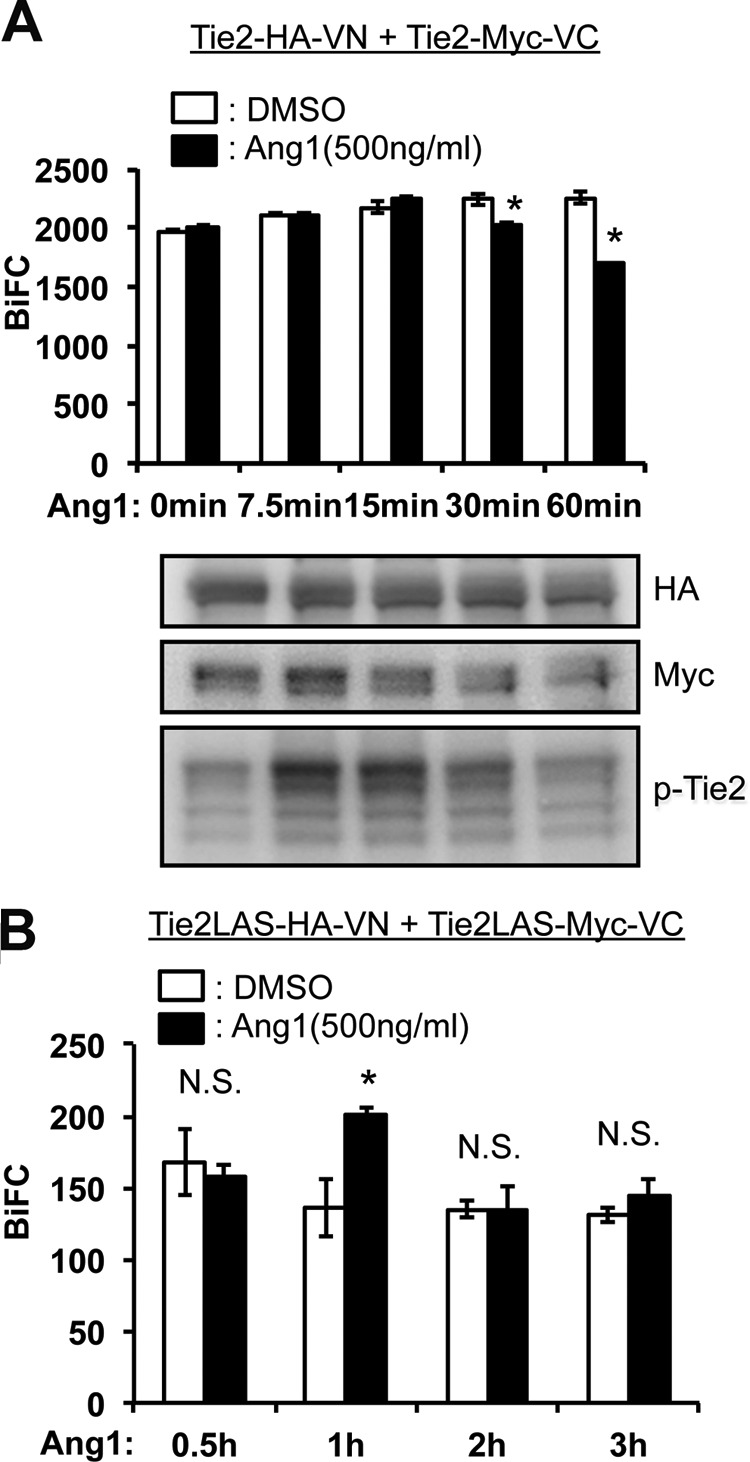
BiFC analysis of ligand-dependent dimerization of Tie2. A, dimerization of Tie2 was observed in Tie2-VN- and Tie2-VC-coexpressing NIH3T3 cells in the presence or absence of Ang1 stimulation. At each time point, cell lysates were analyzed for Tie2-HAVN and Tie2-MycVC as well as the degree of Tie2 phosphorylation (lower panel). Note that Ang1 stimulation did not enhance BiFC level but rather attenuated it 30 min after stimulation with Ang1. B, time course of dimerization of Tie2YIA/LAS (Tie2LAS) was observed in Tie2 YIA/LAS-VN- and Tie2 YIA/LAS -VC-coexpressing HEK293T cells in the presence or absence of Ang1 stimulation (*, p < 0.05; n = 3). DMSO, dimethyl sulfoxide; N.S., not significant.
FIGURE 6.
Tie2YIA/LAS cannot form ligand-independent dimers but is dimerized and phosphorylated upon stimulation with Ang1. A, dimerization and localization of Tie2 were observed in Tie2-VN- and Tie2-VC-coexpressing NIH3T3 cells in the presence or absence of Ang1. Wild-type Tie2 can form dimers irrespective of Ang1 stimulation, as confirmed by BiFC. However, this dimerized Tie2 forms cluster-like aggregations and is internalized upon stimulation with Ang1. B, similar to A, dimerization and localization of Tie2YIA/LAS (Tie2LAS) is observed in Tie2YIA/LAS-VN- and Tie2YIA/LAS-VC-coexpressing NIH3T3 cells. In the absence of Ang1, Tie2LAS did not dimerize but formed cluster-like aggregations upon stimulation with Ang1. Bar indicates 20 μm.
Finally, we investigated how the lack of Tie2 ligand-independent dimerization affected its phosphorylation and downstream Erk signaling. When the time course of Tie2 phosphorylation was recorded in the presence of a fixed dose of Ang1 (200 ng/ml), no significant differences between wild-type Tie2 and Tie2YIA/LAS were observed (Fig. 7A). However, when phosphorylation was measured after stimulation for 10 min with different doses of Ang1, Tie2 and Erk phosphorylation by Tie2YIA/LAS decreased at a high dose (350∼500 ng/ml) of Ang1 compared with wild-type Tie2 (Fig. 7, B and C). These findings suggest that the YIA domain of Tie2 is not indispensable for dimerization of Tie2 but is used for forming non-ligand-mediated dimerization of Tie2 to effectively react to a higher dose of Ang1.
FIGURE 7.
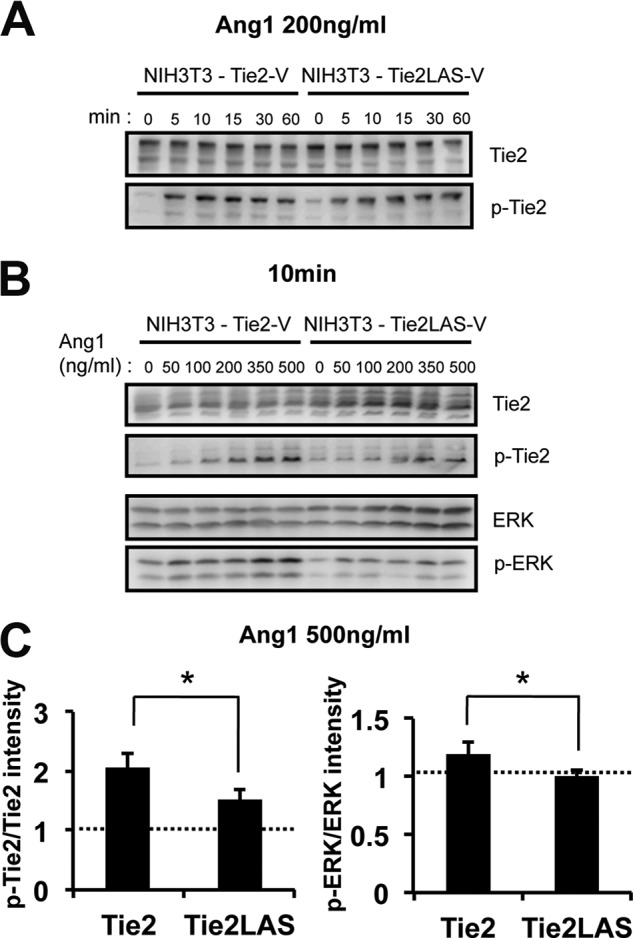
Phosphorylation of Tie2 and Tie2 downstream molecule Erk. A, Ang1 reactivity of Tie2 and Tie2YIA/LAS. After exposure to 200 ng/ml of Ang1, Tie2 and Tie2YIA/LAS phosphorylation was detected in a time-dependent fashion between 0 and 60 min. B, Ang1 reactivity of Tie2 and Tie2YIA/LAS. Ang1-mediated Tie2, Tie2YIA/LAS, and Erk phosphorylation was detected in a dose-dependent fashion between 0 and 500 ng/ml for 10 min. C, Tie2 and Erk phosphorylation on stimulation with 500 ng/ml Ang1 was quantified. The ratio of pTie2/Tie2 or pErk/Erk in cells on stimulation with Ang1 was compared with Ang1-untreated cells. (*, p < 0.05; n = 3).
DISCUSSION
In the present study, we visualized Tie2 dimerization by the BiFC method and sought ligand-independent dimerization domains of Tie2. A previous report showed that Tie2 clusters are expressed on the apical and basolateral plasma membranes (19). However, it was not clear whether Tie2 phosphorylation results in dimer formation. Here, we showed that kinase-inactive Tie2 mutants also form dimers in the absence of Ang1. Thus, Tie2 can indeed form dimers without Ang1. To analyze the role of ligand-independent dimerization of Tie2, a mutant that cannot form dimers in the absence of Ang1 is required. In the present study, we utilized a mutant with no evidence of Tie1-Tie1 dimerization even when overexpressed. Based on the amino acid sequence difference between Tie2 and Tie1, we found that YIA in the Tie2 cytoplasmic domain is important for ligand-independent Tie2 dimerization.
We show that the YIA domain required to form ligand-independent Tie2 dimers is situated between the catalytic and activation loops in the intracellular region of the molecule. Previous reports show that the Tie2 C-terminal tail has a negative regulatory role in Tie2 signaling and function (31, 32). To activate Tie2, conformational changes in the intracellular loop structure and C-terminal tail are required for ATP and substrate binding. Therefore, it is possible that YIA domains control the movement of these loop and C-terminal tails. Further structural analysis of Tie2 will be necessary to assess how the YIA domain controls ligand-independent dimerization of Tie2 for folding and Tie2-Tie2 associations.
Unlike Tie2 homodimer formation, the BiFC method reveals that Tie2 and Tie1 scarcely interact. Recently, it has been reported that Tie2-Tie1 heterodimer formation is induced in the extracellular domain of Tie2 and Tie1, respectively, and that this occurs in the absence of angiopoietin ligation (33). Heterodimerization was observed using Tie receptors lacking intracellular domains. At present, it is difficult to explain this discrepancy, but it may simply be due to the absence of receptor cytoplasmic regions in the previous report. Indeed, when endogenous Tie2 and Tie1 localization in human umbilical vein endothelial cells was observed in the absence of Ang1, we found that Tie2 and Tie1 did not co-localize on the cell surface (supplemental Fig. S5). However, as previously reported, upon Ang1 stimulation, co-localization of these receptors does occur. In contrast, when NIH3T3 cells expressing Tie2-VN and Tie1*-VC were stimulated with Ang1, BiFC intensity was not enhanced (supplemental Fig. S6A). In addition, Ang1 activates both Tie2 and Tie1, but we did not observe a strong physical association between Tie2 and Tie1 in the immunoprecipitation analysis (supplemental Fig. S6, B and C). It has been reported that shedding of Tie1 extracellular domain itself induces Tie2 activation and that Ang2 acts as a Tie2 agonist upon Tie1 shedding (34–36). This suggests that Tie1 ectodomain shedding plays important roles in promoting Tie2 conformation changes and activation. Therefore, we cannot completely exclude the possibility that full-length Tie2 and Tie1 may heterodimerize under certain specific conditions in ECs.
It has been reported that Tie2 forms oligomers on the cell membrane (19); however, the function of such forms of Tie2 has not been elucidated. We found that a lack of ligand-independent dimerization of Tie2 led to attenuation of high dose Ang1-mediated activation of Tie2. This suggests that ligand-independent Tie2 dimerization plays a role in the rapid clustering of Tie2 upon activation with higher doses of Ang1 or in the preformation of Tie2 oligomers to respond to higher doses of Ang1. Further precise analysis of how ligand-independent dimerization of Tie2 relates to the extent of Tie2 phosphorylation at higher Ang1 doses is still required, including elucidation of the biological significance of Tie2 oligomers.
In humans, an amino acid substitution of tryptophan for arginine at residue (Tie2R849W) leads to ligand-independent constitutive activation; it is associated with familial venous malformations and causes thickness or lack of smooth muscle cells in the veins systemically (14, 29, 37). In the present study, we showed that the intensity of BiFC signals from Tie2R848W-Tie2R848W was enhanced. Interestingly, Tie2R848W interactions with WT Tie2 were stronger than Tie2-Tie2 interactions. This suggests that Tie2R848W may heterodimerize with WT Tie2 and induce constitutive phosphorylation of WT Tie2. Therefore, analysis of regulatory mechanisms in ligand-independent dimerization domains may be useful for developing therapeutic strategies to inhibit Tie2 activation in patients suffering from venous malformation.
Acknowledgments
We thank T. Kamimoto, N. Fujimoto, and K. Fukuhara for technical assistance.
This work was supported in part by a grant from the Ministry of Education, Science, Sports, and Culture of Japan.

This article contains supplemental Table S1 and Figs. S1–S6.
- Ang1
- angiopoietin-1
- EC
- endothelial cell
- BiFC
- bimolecular fluorescence complementation
- Ab
- antibody.
REFERENCES
- 1. Dejana E., Tournier-Lasserve E., Weinstein B. M. (2009) The control of vascular integrity by endothelial cell junctions: molecular basis and pathological implications. Dev. Cell 16, 209–221 [DOI] [PubMed] [Google Scholar]
- 2. Augustin H. G., Koh G. Y., Thurston G., Alitalo K. (2009) Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell Biol. 10, 165–177 [DOI] [PubMed] [Google Scholar]
- 3. Sato T. N., Tozawa Y., Deutsch U., Wolburg-Buchholz K., Fujiwara Y., Gendron-Maguire M., Gridley T., Wolburg H., Risau W., Qin Y. (1995) Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature 376, 70–74 [DOI] [PubMed] [Google Scholar]
- 4. Davis S., Aldrich T. H., Jones P. F., Acheson A., Compton D. L., Jain V., Ryan T. E., Bruno J., Radziejewski C., Maisonpierre P. C., Yancopoulos G. D. (1996) Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell 87, 1161–1169 [DOI] [PubMed] [Google Scholar]
- 5. Fukuhara S., Sako K., Minami T., Noda K., Kim H. Z., Kodama T., Shibuya M., Takakura N., Koh G. Y., Mochizuki N. (2008) Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat. Cell Biol. 10, 513–526 [DOI] [PubMed] [Google Scholar]
- 6. Saharinen P., Eklund L., Miettinen J., Wirkkala R., Anisimov A., Winderlich M., Nottebaum A., Vestweber D., Deutsch U., Koh G. Y., Olsen B. R., Alitalo K. (2008) Angiopoietins assemble distinct Tie2 signalling complexes in endothelial cell-cell and cell-matrix contacts. Nat. Cell Biol. 10, 527–537 [DOI] [PubMed] [Google Scholar]
- 7. Takakura N., Watanabe T., Suenobu S., Yamada Y., Noda T., Ito Y., Satake M., Suda T. (2000) A role for hematopoietic stem cells in promoting angiogenesis. Cell 102, 199–209 [DOI] [PubMed] [Google Scholar]
- 8. Dumont D. J., Gradwohl G., Fong G. H., Puri M. C., Gertsenstein M., Auerbach A., Breitman M. L. (1994) Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 8, 1897–1909 [DOI] [PubMed] [Google Scholar]
- 9. Puri M. C., Rossant J., Alitalo K., Bernstein A., Partanen J. (1995) The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J. 14, 5884–5891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Puri M. C., Partanen J., Rossant J., Bernstein A. (1999) Interaction of the TEK and TIE receptor tyrosine kinases during cardiovascular development. Development 126, 4569–4580 [DOI] [PubMed] [Google Scholar]
- 11. Saharinen P., Kerkelä K., Ekman N., Marron M., Brindle N., Lee G. M., Augustin H., Koh G. Y., Alitalo K. (2005) Multiple angiopoietin recombinant proteins activate the Tie1 receptor tyrosine kinase and promote its interaction with Tie2. J. Cell Biol. 169, 239–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yuan H. T., Venkatesha S., Chan B., Deutsch U., Mammoto T., Sukhatme V. P., Woolf A. S., Karumanchi S. A. (2007) Activation of the orphan endothelial receptor Tie1 modifies Tie2-mediated intracellular signaling and cell survival. FASEB J. 21, 3171–3183 [DOI] [PubMed] [Google Scholar]
- 13. Patan S. (1998) TIE1 and TIE2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc. Res. 56, 1–21 [DOI] [PubMed] [Google Scholar]
- 14. Morris P. N., Dunmore B. J., Tadros A., Marchuk D. A., Darland D. C., D'Amore P. A., Brindle N. P. (2005) Functional analysis of a mutant form of the receptor tyrosine kinase Tie2 causing venous malformations. J. Mol. Med. 83, 58–63 [DOI] [PubMed] [Google Scholar]
- 15. Maisonpierre P. C., Suri C., Jones P. F., Bartunkova S., Wiegand S. J., Radziejewski C., Compton D., McClain J., Aldrich T. H., Papadopoulos N., Daly T. J., Davis S., Sato T. N., Yancopoulos G. D. (1997) Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 277, 55–60 [DOI] [PubMed] [Google Scholar]
- 16. Thomas M., Augustin H. G. (2009) The role of the Angiopoietins in vascular morphogenesis. Angiogenesis 12, 125–137 [DOI] [PubMed] [Google Scholar]
- 17. Eklund L., Olsen B. R. (2006) Tie receptors and their angiopoietin ligands are context-dependent regulators of vascular remodeling. Exp. Cell Res. 312, 630–641 [DOI] [PubMed] [Google Scholar]
- 18. Kim I., Kim J. H., Moon S. O., Kwak H. J., Kim N. G., Koh G. Y. (2000) Angiopoietin-2 at high concentration can enhance endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Oncogene 19, 4549–4552 [DOI] [PubMed] [Google Scholar]
- 19. Bogdanovic E., Coombs N., Dumont D. J. (2009) Oligomerized Tie2 localizes to clathrin-coated pits in response to angiopoietin-1. Histochem. Cell Biol. 132, 225–237 [DOI] [PubMed] [Google Scholar]
- 20. Jura N., Endres N. F., Engel K., Deindl S., Das R., Lamers M. H., Wemmer D. E., Zhang X., Kuriyan J. (2009) Mechanism for activation of the EGF receptor catalytic domain by the juxtamembrane segment. Cell 137, 1293–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Red Brewer M., Choi S. H., Alvarado D., Moravcevic K., Pozzi A., Lemmon M. A., Carpenter G. (2009) The juxtamembrane region of the EGF receptor functions as an activation domain. Mol. Cell 34, 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yu X., Sharma K. D., Takahashi T., Iwamoto R., Mekada E. (2002) Ligand-independent dimer formation of epidermal growth factor receptor (EGFR) is a step separable from ligand-induced EGFR signaling. Mol. Biol. Cell 13, 2547–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tao R. H., Maruyama I. N. (2008) All EGF (ErbB) receptors have preformed homo- and heterodimeric structures in living cells. J. Cell Sci. 121, 3207–3217 [DOI] [PubMed] [Google Scholar]
- 24. Livnah O., Stura E. A., Middleton S. A., Johnson D. L., Jolliffe L. K., Wilson I. A. (1999) Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283, 987–990 [DOI] [PubMed] [Google Scholar]
- 25. Chan F. K., Chun H. J., Zheng L., Siegel R. M., Bui K. L., Lenardo M. J. (2000) A domain in TNF receptors that mediates ligand-independent receptor assembly and signaling. Science 288, 2351–2354 [DOI] [PubMed] [Google Scholar]
- 26. Kerppola T. K. (2006) Design and implementation of bimolecular fluorescence complementation (BiFC) assays for the visualization of protein interactions in living cells. Nat. Protocols 1, 1278–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Morita S., Kojima T., Kitamura T. (2000) Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 7, 1063–1066 [DOI] [PubMed] [Google Scholar]
- 28. Saitoh T., Nakano H., Yamamoto N., Yamaoka S. (2002) Lymphotoxin-β receptor mediates NEMO-independent NF-κB activation. FEBS Lett. 532, 45–51 [DOI] [PubMed] [Google Scholar]
- 29. Vikkula M., Boon L. M., Carraway K. L., 3rd, Calvert J. T., Diamonti A. J., Goumnerov B., Pasyk K. A., Marchuk D. A., Warman M. L., Cantley L. C., Mulliken J. B., Olsen B. R. (1996) Vascular dysmorphogenesis caused by an activating mutation in the receptor tyrosine kinase TIE2. Cell 87, 1181–1190 [DOI] [PubMed] [Google Scholar]
- 30. Bogdanovic E., Nguyen V. P., Dumont D. J. (2006) Activation of Tie2 by angiopoietin-1 and angiopoietin-2 results in their release and receptor internalization. J. Cell Sci. 119, 3551–3560 [DOI] [PubMed] [Google Scholar]
- 31. Shewchuk L. M., Hassell A. M., Ellis B., Holmes W. D., Davis R., Horne E. L., Kadwell S. H., McKee D. D., Moore J. T. (2000) Structure of the Tie2 RTK domain: self-inhibition by the nucleotide binding loop, activation loop, and C-terminal tail. Structure 8, 1105–1113 [DOI] [PubMed] [Google Scholar]
- 32. Niu X. L., Peters K. G., Kontos C. D. (2002) Deletion of the carboxyl terminus of Tie2 enhances kinase activity, signaling, and function. Evidence for an autoinhibitory mechanism. J. Biol. Chem. 277, 31768–31773 [DOI] [PubMed] [Google Scholar]
- 33. Seegar T. C., Eller B., Tzvetkova-Robev D., Kolev M. V., Henderson S. C., Nikolov D. B., Barton W. A. (2010) Tie1-Tie2 interactions mediate functional differences between angiopoietin ligands. Mol. Cell 37, 643–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yabkowitz R., Meyer S., Black T., Elliott G., Merewether L. A., Yamane H. K. (1999) Inflammatory cytokines and vascular endothelial growth factor stimulate the release of soluble tie receptor from human endothelial cells via metalloprotease activation. Blood 93, 1969–1979 [PubMed] [Google Scholar]
- 35. Marron M. B., Singh H., Tahir T. A., Kavumkal J., Kim H. Z., Koh G. Y., Brindle N. P. (2007) Regulated proteolytic processing of Tie1 modulates ligand responsiveness of the receptor-tyrosine kinase Tie2. J. Biol. Chem. 282, 30509–30517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Singh H., Milner C. S., Aguilar Hernandez M. M., Patel N., Brindle N. P. (2009) Vascular endothelial growth factor activates the Tie family of receptor tyrosine kinases. Cell Signal. 21, 1346–1350 [DOI] [PubMed] [Google Scholar]
- 37. Limaye N., Wouters V., Uebelhoer M., Tuominen M., Wirkkala R., Mulliken J. B., Eklund L., Boon L. M., Vikkula M. (2009) Somatic mutations in angiopoietin receptor gene TEK cause solitary and multiple sporadic venous malformations. Nat. Genet. 41, 118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]