Background: IMP3 is an mRNA-binding protein associated with aggressive cancers but whose function is unknown in these cancers.
Results: IMP3 promotes chemoresistance in breast cancer cells by interacting directly with the mRNA that encodes a specific drug transporter.
Conclusion: IMP3 has a causal role in the chemoresistance of breast cancer cells.
Significance: These data provide a mechanism for how IMP3 contributes to breast cancer.
Keywords: Breast Cancer, Chemoresistance, Drug Transport, mRNA, RNA-binding Protein
Abstract
IMP3, a member of a family of insulin-like growth factor II (IGF-II) mRNA-binding proteins (IMPs), is expressed preferentially in triple-negative breast cancers, which are resistant to many chemotherapeutics. However, the mechanisms by which it impacts breast cancer have not been elucidated. We hypothesized a role for IMP3 in chemoresistance based on these observations. Depletion of IMP3 expression in triple-negative breast cancer cells increased their sensitivity to doxorubicin and mitoxantrone significantly but not to taxol. Given that doxorubicin and mitoxantrone are effluxed by breast cancer resistance protein (BCRP), we assessed whether IMP3 regulates BCRP. The data obtained demonstrate that IMP3 binds to BCRP mRNA and regulates BCRP expression. These findings are significant because they provide insight into the mechanism by which IMP3 contributes to aggressive cancers, and they highlight the potential for targeting this mRNA-binding protein for the clinical management of cancer.
Introduction
The foundation for this study is the compelling evidence that IMP3,2 a member of a family of insulin-like growth factor II (IGF-II) mRNA-binding proteins (IMPs) that function in RNA trafficking, stabilization, and localization (1), is expressed preferentially in triple-negative breast cancer (2). Clinically, triple-negative breast cancers are usually of high histological grade, poorly differentiated, and more aggressive as compared with other subtypes of breast cancer (3). Most, if not all, breast tumors that contain inactivating mutations in the BRCA1 gene, which is a major determinant of hereditary breast cancer, exhibit a triple-negative phenotype (4). Importantly, treatment of triple-negative breast cancer remains a challenge because of the lack of targeted therapeutic options and resistance to standard chemotherapy (3). What is not known is whether there is a causal link between IMP3 and the aggressive behavior of triple-negative breast cancer and, if so, the mechanism by which this RNA-binding protein contributes to such behavior.
In pursuit of a causal role for IMP3 in triple-negative breast cancer, we explored the hypothesis that this mRNA-binding protein contributes to chemoresistance. The results obtained validate this hypothesis and establish a mechanism that involves IMP3-mediated regulation of breast cancer resistance protein (BCRP), also known as ABCG2, a member of the ATP-binding cassette (ABC) transporters and a major effector of drug resistance in breast cancer (5).
EXPERIMENTAL PROCEDURES
Cells and Reagents
The human breast cancer cell line SUM-1315 was obtained from Dr. Stephen Ethier (Medical College of South Carolina, Charleston, SC). HEK293T and MDA-468 cell lines were obtained from the American Type Culture Collection (ATCC). SUM-1315 cells were maintained in F-12 medium supplemented with 5% fetal bovine serum, insulin (5 μg/ml), epidermal growth factor (10 ng/ml), and 1% penicillin-streptomycin. MDA-468 cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. HEK293T cells were cultured in DMEM (high glucose) supplemented with 10% fetal bovine serum, nonessential amino acids (1×, Gibco), HEPES (pH 7.4, 1 mm, Gibco), sodium pyruvate (1 mm, Gibco), and 1% penicillin-streptomycin. All cell lines were grown at 37 °C and 5% CO2.
IMP3-specific shRNAs (TRCN0000074673 and TRCN0000074675) were obtained from Open Biosystems. Doxorubicin, mitoxantrone, and taxol were procured from Sigma-Aldrich. Doxorubicin was solubilized in water, whereas mitoxantrone and taxol were solubilized in dimethyl sulfoxide (DMSO). A BCRP expression vector (Plasmid ID: 25983) was obtained from Addgene. The control vector was generated by excising BCRP cDNA from the expression vector. IMP3 and BCRP antibodies were purchased from DAKO and Abcam, respectively. Lipofectamine 2000 and FuGENE 6 were procured from Invitrogen and Promega, respectively.
IMP3-depleted cell lines (SUM-1315 and MDA-468) were generated by infecting them with PLKO.1-based lentiviruses (produced in HEK293T cells by transfecting plasmid DNA using Lipofectamine) expressing shRNAs targeting IMP3 mRNA and subsequent selection under puromycin (2 μg/ml). Stable cell lines were maintained regularly under puromycin (1 μg/ml). BCRP expression was rescued by infecting IMP3-depleted SUM-1315 cells with a lentivirus expressing full-length BCRP cDNA and subsequent selection under G418 (600 μg/ml). The lentivirus was generated from HEK293T cells.
MTT Cytotoxicity Assay
The chemosensitivity of breast cancer cells was determined using a standard 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cytotoxicity assay (6). The assay was performed 48 h after drug treatment.
Immunoblotting
Cell extracts were prepared using radioimmune precipitation buffer containing EDTA and EGTA (Boston BioProducts). A protease and phosphatase inhibitor mixture was added separately (Roche Applied Biosciences). Extracts (40–50 μg of protein) were blotted with the appropriate primary antibodies and then incubated with either mouse or rabbit IgG horseradish peroxidase-conjugated secondary antibody. An ECL kit (Thermo Scientific) was used to develop the blots.
RNA Isolation and Real-time PCR Analysis
Total RNA was isolated from cultured cells using TRIzol reagent (Invitrogen) following the manufacturer's protocol. cDNA was synthesized using SuperScript-II reverse transcriptase (Invitrogen). mRNAs were quantified by real-time PCR analysis (ABI Prism, Applied Biosystems) using Power SYBR Green PCR master mix (Applied Biosystems). Quantification was performed using ΔΔCt method, and GAPDH was used as reference gene. The following primer pairs were used for real-time PCR analysis: IMP3 forward, 5′-CCGCAGTTTGAGCAATCAGAA-3′, and IMP3 reverse, 5′-CGAGAAAGCTGCTTGATGTGC-3′; IGF-II forward, 5′-CCGAAACAGGCTACTCTCCT-3′, and IGF-II reverse, 5′-AGGGTGTTTAAAGCCAATCG-3′; BCRP forward, 5′-GTTGTGATGGGCACTCTGAC-3′, and BCRP reverse, 5′-CCCTGTTAATCCGTTCGTTT-3′; and ESR2 forward, 5′-AAGGTTAGTGGGAACCGTTG-3′, and ESR2 reverse, 5′-ACATCCTTCACACGACCAGA-3′.
Ribo-immunoprecipitation Assay
The interaction between IMP3 protein and BCRP mRNA was determined using a ribo-immunoprecipitation quantitative PCR assay as described previously (7). Briefly SUM-1315 cells (∼2 × 107) were harvested and extracted for 15 min on ice in 250 μl of ice-cold lysis buffer (100 mm KCl, 5 mm MgCl2, 10 mm HEPES (pH 7.0), 0.5% Nonidet P-40, 10 μm dithiothreitol) supplemented with RNase and protease inhibitors. Extracts were cleared by centrifugation for 15 min at 13,000 rpm, and supernatant was transferred to a fresh 1.5-ml tube. To preclear the cytoplasmic extracts, 25 μg of nonimmune rabbit IgG (Sigma) was added to the supernatant and kept on ice for 45 min and then incubated with 50 μl of a 50% (v/v) suspension of protein G-Sepharose beads (BioVision) for 3 h at 4 °C with rotation. This was centrifuged at 13,000 rpm, and the supernatant was recovered (precleared lysate). For immunoprecipitation, the precleared extract was incubated with 100 μl of a 50% suspension of protein G-Sepharose beads (Sigma) precoated with the same amount of either nonimmune mouse IgG (Sigma) or anti-human IMP3 antibody (25 μg) in 800 μl of NT-2 buffer (150 mm NaCl, 1 mm MgCl2, 50 mm Tris-HCl (pH 7.4), 0.05% Nonidet P-40) containing RNase inhibitor and protease inhibitors overnight at 4 °C with rotation. Beads were washed 10 times using ice-cold NT-2 buffer, digested with 20 units of RNase-free DNase I (Promega) in 100 of μl of NT-2 buffer for 20 min at 30 °C, washed with NT-2 buffer, and further digested with 0.5 mg/ml protease K (Ambion) in 100 μl of NT-2 buffer containing 0.1% SDS at 55 °C for 30 min. RNA was extracted with TRIzol (Invitrogen). Glycogen (Roche Applied Science) was added to facilitate precipitation of RNA. Real-time PCR was performed on equivalent amounts of sample to quantify protein-bound mRNAs.
Generation of IMP3 Expression Construct Resistant to shIMP3-2
The IMP3 expression construct resistant to shIMP3-2 was generated by mutating two nucleotides within the target sequence (located in the coding region of wild-type IMP3) of shIMP3-2. The wild-type IMP3 construct was generated by cloning full-length cDNA of IMP3 in pCDH-CMV-MCS-EF1-GFP lentiviral vector (System Biosciences, Mountain View, CA) at EcoRI/NotI sites. The desired mutation (underlined) was carried out by site-directed mutagenesis (QuikChange XL site-directed mutagenesis kit, Agilent Technologies). The target sequence of shIMP3-2 is 5′-CGGTGAATGAACTTCAGAATT and located 1782 bp downstream of transcription start site. The sequence was mutated to CGGTGAATGAATTGCAGAATT (The underlined sequences indicate the desired mutation). Primers used for mutagenesis are: forward, 5′-GGAGGCAAAACGGTGAATGAATTGCAGAATTTGTCAAGTGCAGAAG; reverse, 5′-CTTCTGCACTTGACAAATTCTGCAATTCATTCACCGTTTTGCCTCC.
RESULTS AND DISCUSSION
Depletion of IMP3 Expression Increases Chemosensitivity of Triple-negative Breast Cancer Cells
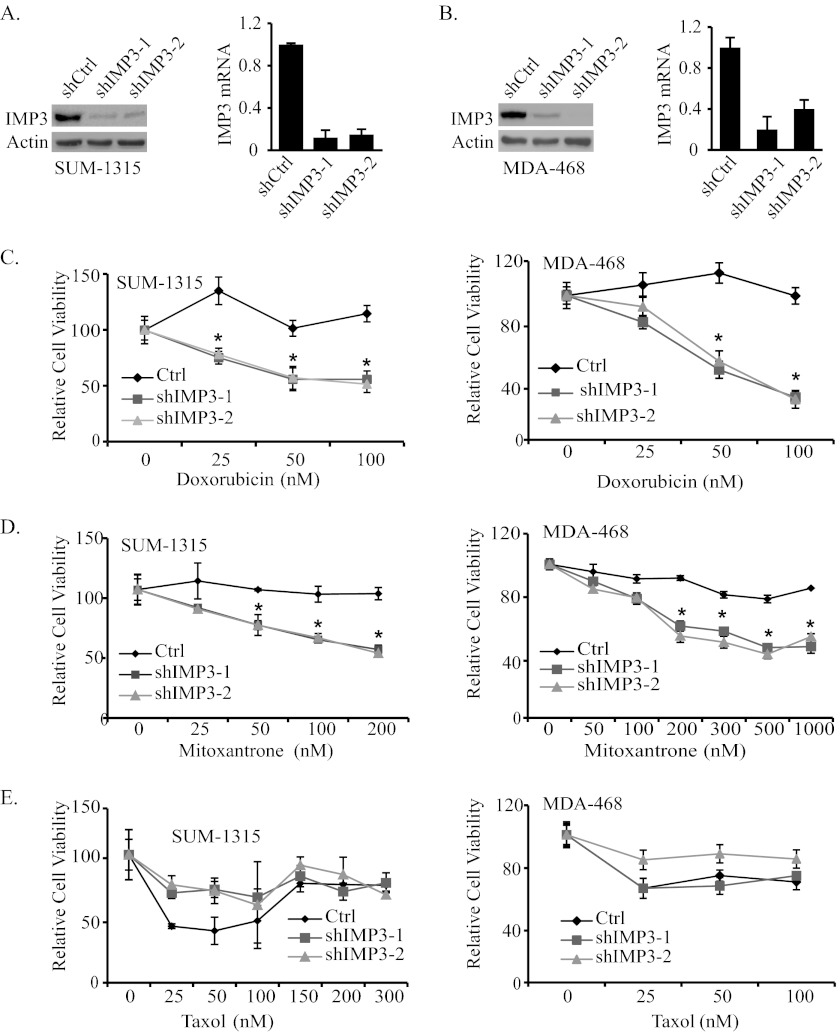
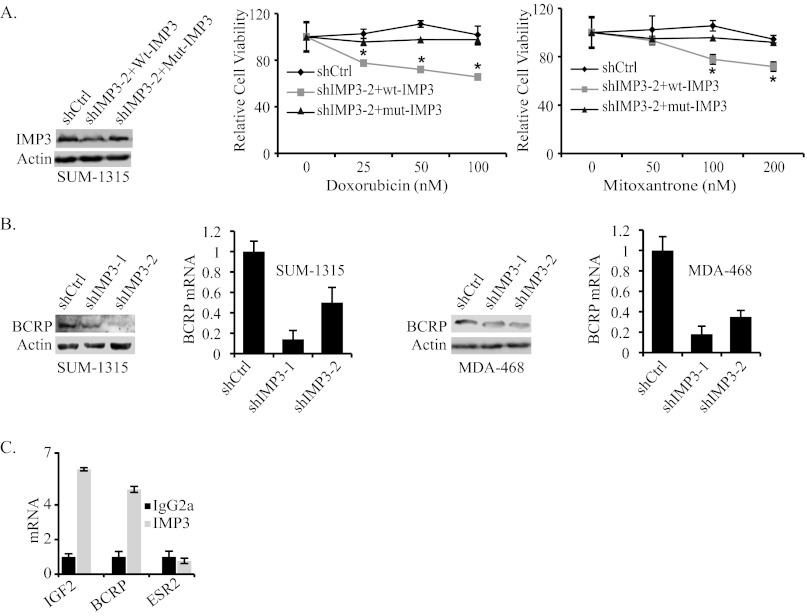
To test the possible role of IMP3 in promoting chemoresistance, we depleted IMP3 expression in the triple-negative breast cancer cell lines SUM-1315 and MDA-468 using two different short hairpin RNAs (shRNAs) (Fig. 1, A and B). Control and IMP3-depleted cells were assessed for their sensitivity to doxorubicin, mitoxantrone, and taxol, chemotherapeutic agents used in breast cancer therapy (8–10). As shown in Fig. 1, C and D, depletion of IMP3 expression increased the sensitivity of both cell lines to doxorubicin and mitoxantrone significantly as measured by the MTT assay. In contrast, IMP3-depleted SUM-1315 cells were more resistant to taxol than control cells (Fig. 1E). Similar results were also obtained with MDA-468 cells. To demonstrate the specificity of shRNA used to deplete IMP3 expression, we rescued IMP3 expression in IMP3-depleted SUM-1315 cells using a lentiviral construct that is resistant to shIMP3-2 (Fig. 2A). As shown in Fig. 2A, restoration of IMP3 expression decreases sensitivity to both doxorubicin and mitoxantrone as compared with cells expressing wild-type IMP3, which is targeted by shIMP3.
FIGURE 1.
IMP3 promotes chemoresistance in triple-negative breast cancer cells. A and B, IMP3 was depleted using two shRNAs (shIMP3-1 and shIMP3-2) in SUM-1315 (A) and MDA-468 cells (B), and its expression was analyzed by immunoblotting and real-time PCR. shCtrl, short hairpin control. C–E, control (Ctrl, shRNA targeting GFP) and IMP3-depleted SUM-1315 (C–E, left panel) and MDA-468 cells (C–E, right panel) were treated with either vehicle or varying concentrations of doxorubicin, mitoxantrone, or taxol for 48 h, and cytotoxicity was measured by the MTT assay. The relative cell viability of the cells treated with vehicle was normalized to 100. Data presented are the mean of three independent experiments. Error bars in all panels indicate S.D. *, p ≤ 0.05.
FIGURE 2.
IMP3 promotes chemoresistance by regulating BCRP. A, the immunoblot shows the restoration of IMP3 expression in IMP3-depleted cells (shIMP3-2) using a lentiviral expression construct that is resistant to shIMP3-2 (mut-IMP3). The chemoresistance of these cells was compared with either control cells (cells expressing shRNA targeting GFP) or IMP3-depleted cells infected with wild-type IMP3 expression construct, which can be targeted by shIMP3-2. shCtrl, short hairpin control. B, BCRP expression (protein and mRNA) was assessed by immunoblotting and real-time PCR in IMP3-depleted SUM-1315 and MDA-468 cells. C, IMP3-associated RNAs were isolated from the cytoplasmic extracts of SUM-1315 cells by immunoprecipitation using an IMP3 antibody (25 μg). Nonimmune mouse IgG was used as negative control. Expression of IGF2, ESR2, and BCRP was analyzed by real-time PCR. Error bars in all panels indicate S.D. *, p ≤ 0.05.
IMP3 Promotes Drug Resistance by Binding to BCRP mRNA and Regulating Its Expression
The finding that IMP3-depleted cells are sensitive to doxorubicin and mitoxantrone is noteworthy because these drugs are effluxed by BCRP (11, 12). Interestingly, taxol is not effluxed by BCRP (13). These observations prompted us to examine the possible role of IMP3 in regulating BCRP expression. To test this possibility, we assessed BCRP mRNA and protein expression in IMP3-depleted SUM-1315 and MDA-468 cells. As shown in Fig. 2B, depletion of IMP3 reduced the level of BCRP mRNA and protein significantly in both cell lines.
An important consideration based on the above findings is whether IMP3 interacts directly with BCRP or regulates its expression indirectly. To address this issue, we performed ribo-immunoprecipitation quantitative PCR, which detects specific protein-RNA interactions. IGF2 was used as a positive control for this experiment because IMP3 was defined initially as an IGF2 mRNA-binding protein (14). Indeed, IMP3 binds to BCRP mRNA at a level comparable with its binding to IGF2 mRNA (Fig. 2C). We used ESR2 (estrogen receptor β) as a negative control for this experiment because IMP3 has not been reported to regulate its expression or function. These data demonstrate that IMP3 binds to BCRP mRNA and, as a consequence, regulates its expression.
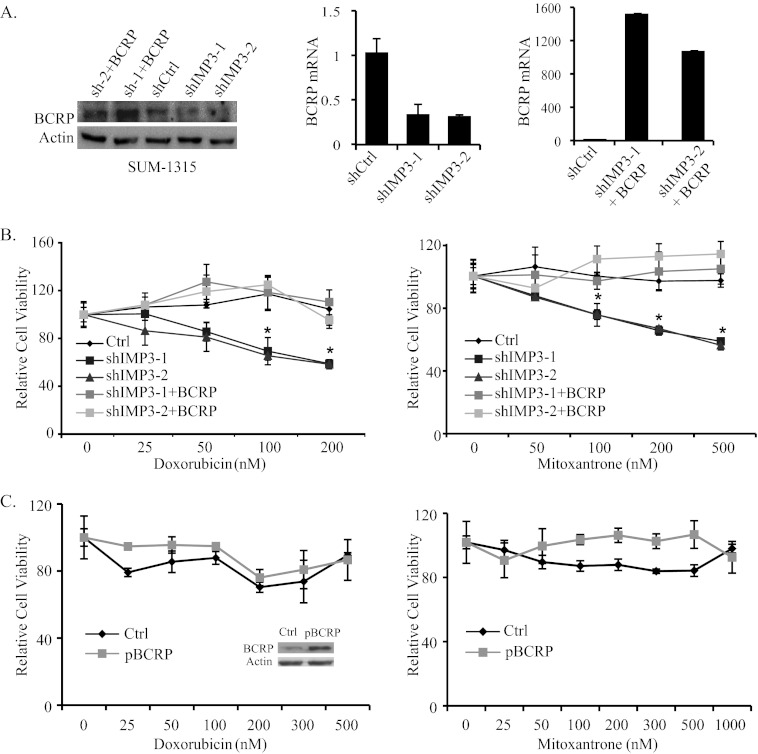
To demonstrate that IMP3 promotes chemoresistance by regulating BCRP, we rescued BCRP expression in IMP3-depleted SUM-1315 cells by transfecting them with a lentivirus-based BCRP expression vector (Fig. 3A) and assayed for chemoresistance. As shown in Fig. 3B, restoration of BCRP expression in IMP3-depleted cells decreased their sensitivity to doxorubicin and mitoxantrone significantly. To control for the possibility that this result could be attributed solely to BCRP overexpression and not linked to IMP3, we overexpressed BCRP in parental SUM-1315 cells and assayed chemoresistance. As shown in Fig. 3C, BCRP overexpression in parental cells caused a slight, if any, increase in resistance to doxorubicin and mitoxantrone in comparison with the increase observed for IMP3-depleted cells (Fig. 3B). We conclude from these data that IMP3 promotes chemoresistance in triple-negative breast cancer cells by regulating BCRP.
FIGURE 3.
Restoration of BCRP expression in IMP3-depleted cells increases chemoresistance. A, IMP3-depleted SUM-1315 cells were infected with a lentivirus expressing BCRP, and its expression was determined by immunoblotting and real-time PCR. Sh-1+BCRP and sh-2+BCRP designate BCRP restoration in IMP3-depleted cells (shIMP3-1 and shIMP3-2). shCtrl, short hairpin control. B, control (Ctrl, shGFP), IMP3-depleted, and IMP3-depleted cells with restored BCRP expression were treated with either vehicle or varying concentrations of doxorubicin (left) and mitoxantrone (right), and cytotoxicity was measured using the MTT assay. C, SUM-1315 cells were infected with the lentivirus expressing BCRP and treated with vehicle or varying concentrations of doxorubicin and mitoxantrone. Cytotoxicity was measured by MTT assay. The immunoblot inside the graph (left) shows BCRP expression. Data are representative of three independent experiments. Error bars in all panels indicate S.D. *, p ≤ 0.05.
The findings presented in this study are significant for several reasons. Although IMP3 expression correlates with the aggressive behavior of many cancers and is used clinically for the prognostic assessment of specific cancers (15, 16), the mechanism by which it functions in this context had been elusive. Our demonstration that IMP3 promotes the chemoresistance of triple-negative breast cancers by regulating a specific drug transporter provides the first insight into this mechanism. These findings are consistent with the recent study reporting that other IMPs contribute to the initiation of glioblastomas (17) and highlight the potential for targeting IMPs as a therapeutic approach to cancer. Targeting IMP3 is a potentially feasible and effective approach to the clinical management of triple-negative breast cancer for several reasons. IMP3 is not expressed in normal breast (2), its mechanism of action is known (binding to specific RNA sequences), and its inhibition should increase susceptibility to standard chemotherapy.
Acknowledgment
We thank Dr. Stephen Ethier for providing the SUM-1315 cell line.
This work was supported, in whole or in part, by National Institutes of Health Grant CA168464.
- IMP3
- insulin-like growth factor-II mRNA-binding protein
- BCRP
- breast cancer resistance protein
- IGF-II
- insulin-like growth factor
- ESR2
- estrogen receptor 2
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Mueller-Pillasch F., Pohl B., Wilda M., Lacher U., Beil M., Wallrapp C., Hameister H., Knöchel W., Adler G., Gress T. M. (1999) Expression of the highly conserved RNA binding protein KOC in embryogenesis. Mech. Dev. 88, 95–99 [DOI] [PubMed] [Google Scholar]
- 2. Walter O., Prasad M., Lu S., Quinlan R. M., Edmiston K. L., Khan A. (2009) IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Hum. Pathol. 40, 1528–1533 [DOI] [PubMed] [Google Scholar]
- 3. Griffiths C. L., Olin J. L. (2012) Triple negative breast cancer: a brief review of its characteristics and treatment options. J. Pharm. Pract. 25, 319–323 [DOI] [PubMed] [Google Scholar]
- 4. Turner N., Tutt A., Ashworth A. (2004) Hallmarks of 'BRCAness' in sporadic cancers. Nat. Rev. Cancer 4, 814–819 [DOI] [PubMed] [Google Scholar]
- 5. Doyle L. A., Ross D. D. (2003) Multidrug resistance mediated by the breast cancer resistance protein BCRP (ABCG2). Oncogene 22, 7340–7358 [DOI] [PubMed] [Google Scholar]
- 6. Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65, 55–63 [DOI] [PubMed] [Google Scholar]
- 7. Liao B., Hu Y., Herrick D. J., Brewer G. (2005) The RNA-binding protein IMP-3 is a translational activator of insulin-like growth factor II leader-3 mRNA during proliferation of human K562 leukemia cells. J. Biol. Chem. 280, 18517–18524 [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim N. K., Buzdar A. U., Asmar L., Theriault R. L., Hortobagyi G. N. (2000) Doxorubicin-based adjuvant chemotherapy in elderly breast cancer patients: the M.D. Anderson experience, with long-term follow-up. Ann. Oncol. 11, 1597–1601 [DOI] [PubMed] [Google Scholar]
- 9. Petit T., Borel C., Theobald S., Serin D., Rodier J. F., Prevot G., Brettes J. P., Klein T. (2003) Randomized multicentric study of perioperative chemotherapy with mitoxantrone in early breast cancer. Ann. Surg. Oncol. 10, 369–375 [DOI] [PubMed] [Google Scholar]
- 10. Rowinsky E. K., Onetto N., Canetta R. M., Arbuck S. G. (1992) Taxol: the first of the taxanes, an important new class of antitumor agents. Semin. Oncol. 19, 646–662 [PubMed] [Google Scholar]
- 11. Hazlehurst L. A., Foley N. E., Gleason-Guzman M. C., Hacker M. P., Cress A. E., Greenberger L. W., De Jong M. C., Dalton W. S. (1999) Multiple mechanisms confer drug resistance to mitoxantrone in the human 8226 myeloma cell line. Cancer Res. 59, 1021–1028 [PubMed] [Google Scholar]
- 12. Robey R. W., Honjo Y., Morisaki K., Nadjem T. A., Runge S., Risbood M., Poruchynsky M. S., Bates S. E. (2003) Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br. J. Cancer 89, 1971–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sharom F. J. (2008) ABC multidrug transporters: structure, function, and role in chemoresistance. Pharmacogenomics 9, 105–127 [DOI] [PubMed] [Google Scholar]
- 14. Nielsen J., Christiansen J., Lykke-Andersen J., Johnsen A. H., Wewer U. M., Nielsen F. C. (1999) A family of insulin-like growth factor II mRNA-binding proteins represses translation in late development. Mol. Cell. Biol. 19, 1262–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jiang Z., Chu P. G., Woda B. A., Rock K. L., Liu Q., Hsieh C. C., Li C., Chen W., Duan H. O., McDougal S., Wu C. L. (2006) Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol. 7, 556–564 [DOI] [PubMed] [Google Scholar]
- 16. Ikeda K., Tate G., Suzuki T., Kitamura T., Mitsuya T. (2011) Diagnostic usefulness of EMA, IMP3, and GLUT-1 for the immunocytochemical distinction of malignant cells from reactive mesothelial cells in effusion cytology using cytospin preparations. Diagn. Cytopathol. 39, 395–401 [DOI] [PubMed] [Google Scholar]
- 17. Janiszewska M., Suvà M. L., Riggi N., Houtkooper R. H., Auwerx J., Clément-Schatlo V., Radovanovic I., Rheinbay E., Provero P., Stamenkovic I. (2012) Imp2 controls oxidative phosphorylation and is crucial for preserving glioblastoma cancer stem cells. Genes Dev. 26, 1926–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]