Background: How the activity of Smo on the plasma membrane is regulated is not fully understood.
Results: Smo forms oligomers/higher order clusters in lipid rafts of cell plasma membrane to transduce high level Hh signaling.
Conclusion: Both lipid rafts and the oligomerization/higher order clustering of Smo are critical for high Hh activity transduction.
Signification: Lipid rafts are essential for Smo activation and Hh gradient signal transduction.
Keywords: Cancer, Drosophila, Hedgehog, Lipid Raft, Signal Transduction, Oligomer, Smo
Abstract
The Hedgehog (Hh) signaling pathway plays evolutionarily conserved roles in controlling embryonic development and tissue homeostasis, and its dysregulation has been implicated in many human diseases including congenital disorder and cancer. The Hh pathway has a unique signal reception system that includes two membrane proteins, the receptor Patched (Ptc) and the transducer Smoothened (Smo). In the Hh signaling cascade, Smo plays a critical role in controlling transduction of Hh gradient signal from the outside into the inside of cells. Although the Smo downstream signal transduction has been intensively studied, the mechanism by which Smo on the plasma membrane is regulated has not been fully understood. As a specific membrane structure of metazoan cells, lipid rafts act as a platform to regulate signal transduction by forming a nanoscale cluster through protein-protein or protein-lipid interactions. However, it remains largely unknown whether lipid rafts are also involved in the regulation of Hh signal transduction. Here, we show that Smo extracellular domain (N terminus) and transmembrane domains form oligomers/higher order clusters in response to Hh signal. Furthermore, we identify that lipid rafts on the plasma membrane are essential for high level activity of Smo during the Hh signal transduction. Finally, our observation suggests that oligomerization/higher order clustering of Smo C-terminal cytoplasmic tail (C-tail) is essential for the transduction of high level Hh signal. Collectively, our data support that in response to Hh gradient signals, Smo transduces high level Hh signal by forming oligomers/higher order clusters in the lipid rafts of cell plasma membrane.
Introduction
Hedgehog (Hh)6 signaling is essential for organ patterning of both invertebrates and vertebrates (1, 2). Malfunction of the Hh signaling pathway leads to numerous human congenital disorders and cancers (3). The core components and regulatory mechanisms of the Hh signaling are conserved among invertebrates and vertebrates with few exceptions (1, 4). In Drosophila wing discs, Hh proteins secreted by posterior (P) compartment cells move into the anterior (A) compartment to form a local concentration gradient. Genetic and biochemical studies revealed that two multipass transmembrane proteins, Patched (Ptc, 12-transmembrane domain protein) and Smoothened (Smo, 7-transmembrane domain protein), function as a reception system for signal transduction in Hh-receiving cells (5).
Correlative studies in Drosophila have revealed several important steps in the regulation of Smo activity and the Hh signaling (3, 6). In the absence of Hh, Ptc inhibits Smo activity, thereby blocking the Hh signal transduction. Under this condition, Smo plasma membrane accumulation is prevented and its C-terminal cytoplasmic tail (C-tail) assumes a “closed” inactive conformation, whereas its extracellular N terminus forms a constitutive dimer. At the same time, full-length Cubitus interruptus (Ci), the transcription factor of Hh pathway, is sequentially phosphorylated by protein kinase A, glycogen synthase kinase 3, and casein kinase I, and processed to generate Ci75, to block downstream gene expression as a transcriptional repressor (7, 8). In the presence of Hh, the Hh ligand physically interacts with Ptc and relieves its inhibition on Smo, resulting in Smo accumulation on plasma membrane and its C-tail conformational switch to an “open” active dimer form, which regulates distinct downstream target gene expression in a Hh concentration-dependent manner through controlling Ci nuclei translocation (3, 9–13). Low levels of Hh are sufficient to induce the expression of decapentaplegic (dpp), whereas middle and high levels of Hh are required to induce patched (ptc) and engrailed (en) expression, respectively (10, 14).
In the Hh signaling pathway, the mechanism by which Ci leads to downstream target gene expression is understood in general (2, 3). However, the regulation mechanism of Smo on the plasma membrane is poorly defined. Lipid rafts are known as a spatiotemporally regulated dynamic lipid-dependent segregation of specific membrane components. They range from unstable nanoscale clusters of protein to relatively stable microdomains formed from preexisting small clusters through protein-protein or protein-lipid interactions, mediating signal transduction as platforms on the plasma membrane. For example, lipid rafts have been found to aid protein dimerization during certain signal transductions (15–18). Given that Smo dimerization is essential for the Hh signal transduction across the plasma membrane (6, 9), it is interesting to study whether Smo accumulates in lipid rafts to form dimers and even oligomers/higher order clusters in the presence of Hh.
In the present study, we demonstrated that the extracellular and transmembrane domains of Smo form oligomers/higher order clusters in the presence of Hh. Our results also suggested that the closer proximity of Smo intracellular subdomain 3 (third internal loop) is triggered by Hh. Importantly, we identified that lipid rafts on the plasma membrane are essential for Smo activity induced by high level of Hh. Furthermore, we showed that oligomerization of Smo C-tail is essential for high level Hh signaling transduction.
EXPERIMENTAL PROCEDURES
DNA Constructs
All Drosophila genes used in this study were constructed into pUAST vectors. Myc-SmoΔC-EphB2CT was generated by replacing the Smo C-tail (amino acids 556-end) with mouse EphB2 C-tail (amino acids 610–1029). Constructs of Myc (or FLAG)-SmoΔN (amino acids 32–255 were deleted), Myc (or FLAG)-SmoΔC (amino acids 556-end were deleted), Myc (or FLAG)-SmoΔNΔC (amino acids 256–555 were kept) and Myc (or FLAG)-SmoCT (amino acids 1–555 were deleted) were generated from full-length Myc (or FLAG)-Smo by site-directed mutagenesis. For SmoCFPL3/SmoYFPL3, CFP or YFP was inserted between amino acids 451 and 452 of Smo. SmoCFPN/SmoYFPN and SmoCFPC/SmoYFPC were generated as described previously (9). For constructs of Myr-FLAG-CCm/-CCd/-CCt-SmoCT, CCm, CCd, or CCt (19–21) were inserted downstream of myristoylation signal (Myr-) (amino acid sequence: MGNKCCSKRQ) and FLAG tag, upstream of the Smo C-tail.
Fly Stocks
Drosophila strains used in this study were maintained under standard conditions. The yw strain was used as host for all of the P-element mediated transformations. MS1096, Ci-Gal4, Hh-Gal4, dpp-LacZ, ptc-LacZ, FLAG-SmoWT, FLAG-SmoSD and HA-Ci were described before (9, 11). Myr-FLAG-CCm/-CCd/-CCt-SmoCT transgenic flies were generated following standard protocols.
Immunostaining for Wing Imaginal Discs
Standard protocol for immunostaining of imaginal discs was used (9). Primary antibodies used in this study were mouse anti-Smo (DSHB), rabbit anti-FLAG (Sigma), rat anti-Ci (2A1) (DSHB), mouse anti-Ptc (DSHB), mouse anti-En 4D9 (DSHB), rabbit anti-LacZ (MP Biomedicals), mouse anti-phosphotyrosine antibody 4G10 (Millipore), and CTB-FITC (Sigma). Secondary antibodies used in this study were bought from Millipore.
Cell Culture, Transfection, Immunoprecipitation, and Western Blotting
S2 cells were cultured in Schneider's Drosophila Medium (Invitrogen) and transfected with Lipofectamine 2000 (Invitrogen) under standard conditions and protocol (13). To detect the Myc-SmoΔC-EphB2CT phosphorylation, S2 cells were transfected with the indicated constructs. After 48-h transfection, S2 cells were harvested, and the cell lysate was treated with the phosphatase, LAR (Sigma), a protein-tyrosine phosphatase, for 30 min at 30 °C. The sample was immunoprecipitated with anti-Myc antibody and subjected to standard SDS-PAGE and Western blotting with anti-Myc and 4G10 antibodies. To detect the oligomerization of Smo, S2 cells were transfected with the indicated constructs. The cell lysates were treated as described (22), and then Smo signals were detected by Western blotting with anti-Myc antibody. Immunoprecipitation, Western blotting, and the native gel electrophoresis were performed following standard protocols (22, 23).
Fluorescence Resonance Energy Transfer (FRET) Analysis
FRET was performed as described previously (9, 24). Briefly, S2 cells were transfected with the indicated constructs. Cells were then treated and mounted as described. Fluorescence signals were acquired with Leica TCS SP5 Confocal microscope. The CFP signal was obtained once before (BP) and once after (AP) photobleaching YFP at the top half of each cell, leaving the bottom half of each cell as the internal control. Each data set was based on >30 individual cells. Three to five interest areas in the bleached or unbleached half of cell were selected for analysis. The intensity change of CFP was analyzed using Leica FRET AB Wizard software. FRET efficiency was calculated using the formula: FRET% = ((CFPAP − CFPBP)/CFPAP) × 100. Statistical significance was determined using Student's t test.
Gradient Centrifugation
The centrifugation was conducted as reported previously (17, 25) and modified for Drosophila cells. Specifically, S2 cells were transfected with Myc-Smo and HA-dGq constructs and treated with or without Hh. The cell lysates were centrifuged at 10,000 × g for 45min at 4 °C. The membrane pellet was resolved in the lysis buffer, loaded onto 1.12 m sucrose cushion in the same lysis buffer, centrifuged at 100,000 × g for 2 h at 4 °C. The fraction of 1 ml at the interface, which contained the plasma membrane, was collected and mixed with 1 ml of 1 m Na2CO3 (pH 11.0) and 2 ml of 90% sucrose in 50 mm MES, 0.3 m NaCl (pH 6.5). The mixture was placed at the bottom of the 12.5-ml tube, overlaid with 3 ml of 35%, 4 ml of 21%, 1 ml of 5% sucrose solution in 0.25 m Na2CO3 (final pH 11.0), 50 mm MES, 0.3 m NaCl. Percentage sucrose here is standard for weight/volume. The samples were then centrifuged at 200,000 × g for 20 h at 4 °C. Twenty-four fractions were collected for detection.
Luciferase Assay
S2 cells cultured in a 24-well plate were co-transfected with 250 ng of Gal4, 250 ng of Ci, 250 ng of ptc-luciferase reporter, 25 ng of Renilla, 250 ng of the indicated constructs, and 250 ng HhN or pUAST in each well. After transfection for 48 h, dual-luciferase measurements were performed in triplicate using the Dual-GloTM luciferase assay system (Promega). Statistical significance was determined using Student's t test.
Lipid Raft Disruption
Lipid raft stability was disrupted by perturbation with exogenous polyunsaturated fatty acid (eicosapentaenoic acid (20:5(n-3), PUFA)) (Sigma). Stearic acid (18:0) (SA) (Sigma) was used as the negative control as recommended (26). For S2 cells, after transfection for 12 h, cell culture medium was replaced with serum-free one supplemented with 0.4% BSA and 50 μm PUFA or SA. Cells were cultured for another 48 h following detection. For in vivo assay, the dissected discs from third instar larvae were cultured in serum-free M3 Medium with 5% BSA and 50 μm PUFA or SA for 6 h at 25 °C. After treatment, the immunostaining was followed.
Cell Surface Immunostaining and Z Section Image Processing
Cell surface staining was described before (9). The Z sections were scanned under Leica Confocal microscope, x-y images were scanned in the z axis at 0.25-μm intervals for three-dimensional reconstruction. The Z plane sections, Z plane stacks, and videos were analyzed and exported using Leica software.
RESULTS
Smo Extracellular Domains Form Oligomers/Higher Order Clusters in the Presence of Hh
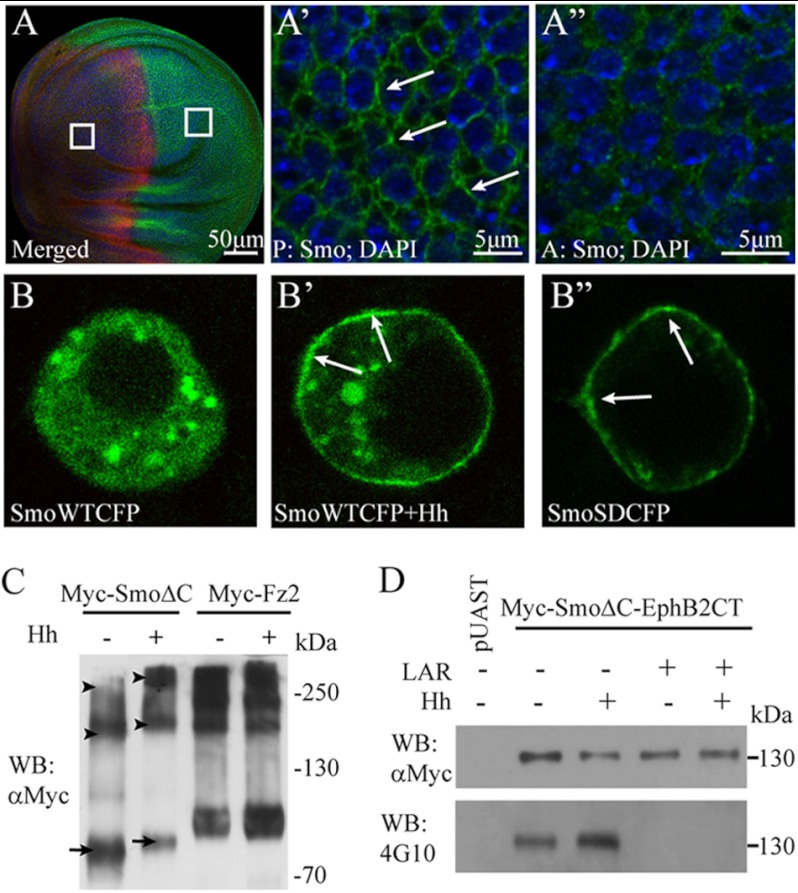
Regardless of Hh, the Smo N termini form a constitutive dimer, and its C-tail has a conformational switch between closed and open states (6, 9). To understand the molecular mechanism by which Smo activity is regulated on the plasma membrane, we sought to investigate whether the extracellular domains of Smo form oligomers/higher order clusters in the presence of Hh. First, we used antibody to label the endogenous Smo in Drosophila wing imaginal disc. As shown in Fig. 1, A–A″, in the A compartment of wing disc where Hh signal was absent, Smo is located in the cytoplasm. However, in the P compartment of wing disc where high level Hh was present, Smo accumulated on the plasma membrane and formed clusters.
FIGURE 1.
Smo extracellular domains form oligomers/higher order clusters in the presence of Hh. A–A″, endogenous Smo forms clusters in the presence of Hh. The wild-type Drosophila wing disc was stained with anti-Smo and anti-Ci antibodies to label Smo (green) and Ci (red), and DAPI was used to mark nucleus (blue). A, Ci staining positive part (red) or negative part (red signal negative) delimits the A compartment, in which there is no Hh, and the P compartment, in which there is high level Hh. The squares delimit the magnified areas in A′ and A″. The image magnified from the P compartment (A′) or from the A compartment (A″) shows Smo and DAPI staining. Scale bars, 50 μm (A) or 5 μm (A′ and A″). B–B″, S2 cells were transfected with the indicated constructs and observed under a Leica Confocal microscope. B, SmoWTCFP locates in the cytoplasm in the absence of Hh. B′ and B″, SmoWTCFP in the presence of Hh or SmoSDCFP accumulates on the plasma membrane and forms clusters. Arrows point the clustered Smo in A′, B′, and B″. C, to detect the oligomers of SmoΔC, a native gel electrophoresis was employed. To compare the ratio between oligomer and monomer of SmoΔC with or without Hh treatment, the loading amount of the first two samples was adjusted to make the total SmoΔC to a comparable level. Western blotting shows that, upon Hh treatment, SmoΔC forms oligomers; meanwhile, the level of SmoΔC monomer decreases. As the control, the level of Fz2 monomer, dimer, or oligomer does not change with or without Hh treatment. Arrows point to the monomer, and arrowheads point to the dimer and oligomer, respectively. D, in Western blots, the autophosphorylated tyrosine level of Myc-SmoΔC-EphB2CT was detected by 4G10 antibody. In the presence of Hh, the tyrosine phosphorylation level of Myc-SmoΔC-EphB2CT is increased. Tyrosine phosphorylation could be erased after lysates were treated with LAR, a protein-tyrosine phosphatase.
Phosphorylation of Smo C-tail is very important for its activity and localization and could be mimicked by SmoSD mutations, in which three protein kinase A sites (Ser667, Ser687, and Ser740) and adjacent casein kinase I sites are mutated to Asp (9, 11). We found that overexpressed SmoSD also aggregated and formed clusters on the cell membrane, as wild-type Smo upon Hh treatment (Fig. 1, B–B″).
Smo belongs to G protein-coupled receptor (GPCR) superfamily (27). Certain studies have suggested that GPCRs might form oligomeric structures for signal transduction (28, 29). Together with our observations, we presume that Smo may form oligomers/higher order clusters in response to Hh. To test this hypothesis, we performed a series of Western blots. The C-tail of activated Smo recruits Hh pathway components in the presence of Hh (3, 13, 30) and may disturb the molecular mass of Smo in Western blots. To avoid this possibility, we generated a truncated form of Smo (SmoΔC), in which the Smo C-tail was deleted. As shown in Fig. 1C, upon Hh treatment, SmoΔC formed oligomers/higher order clusters with high molecular mass, whereas the level of Smo monomer decreased dramatically. In this experiment, we used Myc-tagged frizzled2 (Myc-Fz2) as control because Fz2 forms constitutive dimers/oligomers in Wnt signaling pathway (31).
EphB2 is a receptor tyrosine kinase. As part of cell signaling machinery, the ligand binding-induced N-terminal dimerization/higher order clustering of EphB2 has been correlated with its C-terminal autophosphorylation level (32). To further verify our notion, we generated a fusion protein, Smo-EphB2CT, in which the Smo C-tail was replaced with the kinase tail of EphB2. The tyrosine phosphorylation levels of the fusion protein were then detected by 4G10, a specific tyrosine phosphorylation antibody. As shown in Fig. 1D, Myc-SmoΔC-EphB2CT was autophosphorylated, which indicated a constitutive dimer of the Smo N terminus, consistent with a previous finding (9). After treated with Hh, the detected tyrosine phosphorylation level was dramatically increased (Fig. 1D). Moreover, this effect could be blocked if the lysate was treated with a specific protein-tyrosine phosphatase, LAR, before immunoprecipitation and the gel electrophoresis. Taken together, these observations suggest that in response to Hh, the Smo N terminus/transmembrane domains (TMs)-mediated constitutive dimer may further aggregate to form oligomers/higher order clusters.
Hh Induces Close Proximity of the Third Internal Loop of Smo
Although it is generally accepted that Smo activity correlates with its accumulation on cell surface, Hh-induced Smo activation may be regulated through additional mechanisms, such as oligomerization. To further address how Smo forms oligomers/higher order clusters in response to Hh, we employed FRET analysis, which is a powerful tool to detect protein-protein interaction in living cells (33). We initially made several pairs of tagged Smo constructs by fusing CFP/YFP to the end of C-tail (SmoCFPC/SmoYFPC), N terminus (SmoCFPN/SmoYFPN), the first, second, or third internal loop (SmoCFPL1/SmoYFPL1, SmoCFPL2/SmoYFPL2 or SmoCFPL3/SmoYFPL3) of Smo, respectively. Meanwhile, we used the CFP/YFP pair as a negative control and the SmoCFPC/SmoYFPC pair as a positive control.
Consistent with the previous finding that Smo C-tails form a dimer upon Hh treatment (9), we observed FRET between SmoCFPC and SmoYFPC changed from ∼1.4% to ∼12.3% in S2 cells upon Hh treatment (Fig. 2, A and B). Under similar conditions, FRET between SmoCFPL1/SmoYFPL1 and SmoCFPL2/SmoYFPL2 kept unchanged regardless of Hh (data not shown). However, FRET between SmoCFPL3 and SmoYFPL3 significantly increased from ∼11.8% to ∼16.8% upon Hh stimulation (Fig. 2, A and B), and FRET between SmoCFPN and SmoYFPN increased from ∼11.5% to ∼16.3% (Fig. 2B). FRET between negative control pairs (CFP/YFP) was <0.5% regardless of Hh (Fig. 2B). These observations strongly indicate that Hh may induce close proximity of Smo N terminus and TMs during Smo oligomerization.
FIGURE 2.
The proximity of Smo intracellular subdomains is induced by Hh. A, S2 cells were transfected with indicated constructs and treated with or without Hh, respectively. CFP and YFP signals were acquired for FRET before (BP) and after (AP) photobleaching YFP at the top half of each cell (white rectangle frame). B, FRET efficiency is shown from the indicated CFP/YFP-tagged constructs, which were transfected into S2 cells and treated with or without Hh (mean ± S.D. (error bars), n ≥ 30; *, p < 0.05). C, diagram shows the Smo truncations which were used in D and E. D, S2 cells were co-transfected with Myc-tagged or FLAG-tagged full-length Smo (SmoFL) or Smo truncations, respectively. The immunoprecipitation was followed with mouse anti-FLAG antibody, and Western blotting (WB) was followed with rabbit anti-FLAG or anti-Myc antibodies for immunoprecipitation product and cell lysates. Full-length and truncated Smo, beside SmoCT, interact with each other. E, S2 cells were transfected with the indicated constructs, and then native gel electrophoresis was employed. Western blotting shows that full-length and truncated Smo, beside SmoCT, form dimer or oligomer. Arrows point to monomer, and arrowheads point to dimer and oligomer.
We next performed FRET analysis using phosphorylation-mimicking mutants SmoSDCFPL3 and SmoSDYFPL3. As shown in Fig. 2,A and B, upon Hh stimulation, FRET between SmoSDCFPL3 and SmoSDYFPL3 increased significantly from ∼12.9% to ∼17.6%, which was comparable with that of wild-type Smo. These results suggested that the increased proximity of Smo third internal loop is triggered by Hh signal and is not fully dependent on the Smo C-tail phosphorylation.
To further map the Smo subdomain(s) essential for oligomerization/higher order clustering, we generated a series of Smo mutants, including SmoΔN, SmoΔC, SmoΔNΔC, and SmoCT (see details under “Experimental Procedures” and Fig. 2C). The wild-type and mutant Smo were subject to native gel and immunoprecipitation analysis (Fig. 2, D and E). Without the Smo N terminus and TMs, SmoCT could not form dimer/oligomers (Fig. 2E). The tetramer of SmoΔNΔC or SmoΔC was obviously observed in the lysates (Fig. 2E). To further confirm this finding, different tagged wild-type or truncated Smo were co-expressed in S2 cells. After being immunoprecipitated with FLAG, the samples were detected by anti-FLAG or anti-Myc antibodies (Fig. 2D). The result suggested the indicated Smo, beside SmoCT, could form dimer/oligomer. Taking these results and those from FRET experiments (Fig. 2B), it suggested that both the N terminus and the TMs play an important role in Smo oligomer/higher order cluster formation.
Lipid Rafts Are Essential for Smo Activation Induced by High Level Hh
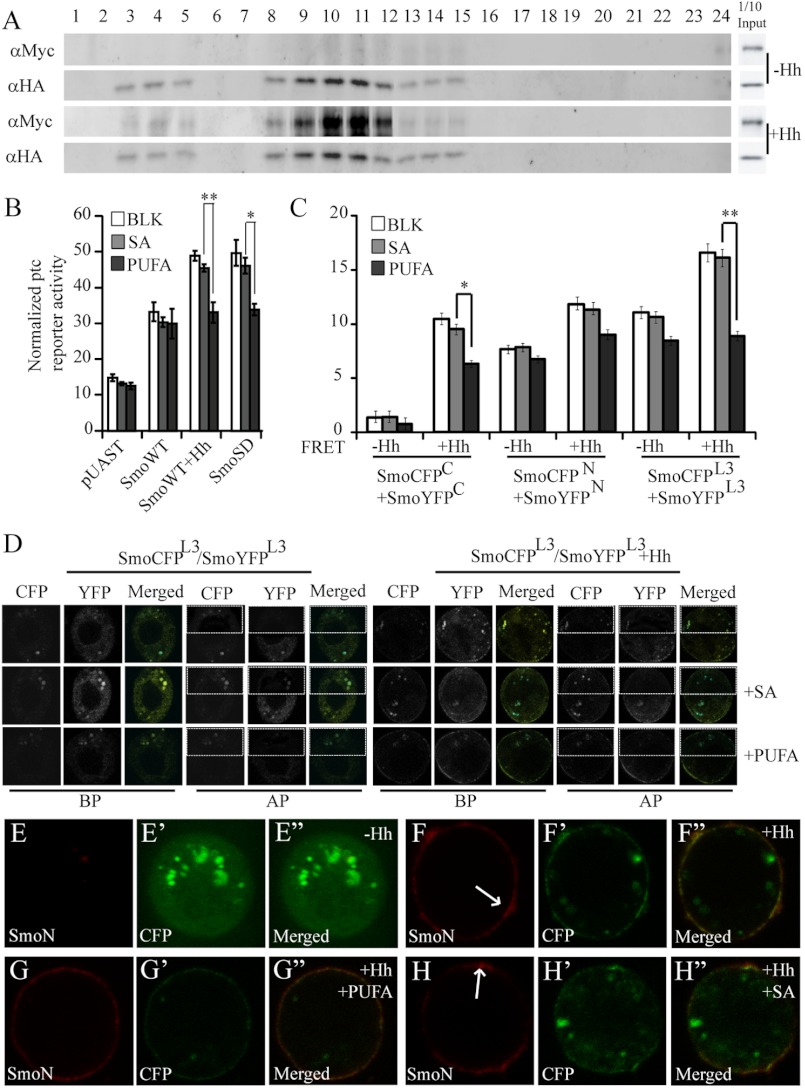
As aforementioned, lipid rafts may function as a signaling platform to mediate signal transduction, especially those involving membrane protein dimerization/oligomerization (15, 18). To determine whether lipid rafts play a role in the regulation of Hh signal transduction, specifically their effect on Smo activation, we performed gradient centrifugation assay. As shown in Fig. 3A, not like lipid raft marker, dGq, Smo was isolated in low and middle density insoluble membrane fractions only in the presence of Hh. This finding suggested that Smo locates in lipid rafts on the plasma membrane in the presence of Hh.
FIGURE 3.
Lipid rafts are important for Smo function. A, S2 cells were co-transfected with Myc-tagged Smo and HA-tagged dGq and then treated with or without Hh, respectively. Membrane lipid microdomains were fractionated with a three-step sucrose gradient centrifugation. Myc-tagged Smo and HA-tagged dGq were detected by Western blotting. Myc-Smo or HA-dGq in cell lysates shows the indicated protein expression level. B, ptc-Luc reporter assay of S2 cells transfected with the indicated constructs and treated with or without Hh, PUFA, or SA is shown (mean ± S.D. (error bars), triplicate wells; *, p < 0.05; **, p < 0.01). C and D, to detect FRET efficiency, S2 cells were transfected with the indicated CFP/YFP-tagged constructs and treated with or without Hh, SA, or PUFA, respectively. C, graph shows that PUFA treatment reduces the FRET of SmoCFPC/SmoYFPC and SmoCFPL3/SmoYFPL3 (mean ± S.D., n ≥ 30; *, p < 0.05' **, p < 0.01). D, CFP and YFP signals from SmoCFPL3/SmoYFPL3 in the absence/presence of Hh were acquired for FRET before (BP) and after (AP) photobleaching YFP (for details of FRET, see “Experimental Procedures” and legend of Fig. 2A). E–H″, cell surface accumulation of CFP-tagged Smo treated with (F–H″) or without (E–E″) Hh, PUFA (G–G″), or SA (H–H″). S2 cells were transfected with CFP-tagged Smo followed by immunostaining with anti-SmoN antibody before membrane permeabilization. Cell surface accumulated Smo (red) and total Smo (green, CFP) are shown. Arrows in F and H point to Smo clusters on the plasma membrane. The images were taken from videos (please refer to supplemental Videos 1–4).
PUFA can disrupt lipid rafts on the membrane and inhibit related signal transduction, whereas the noninhibitory fatty acid SA (18:0) does not affect the lipid raft structure (26, 34). Indeed, we found that PUFA but not SA treatment could dramatically decrease ptc reporter luciferase activity induced by Hh as well as SmoSD (Fig. 3B). However, PUFA treatment could not fully inhibit the activity of SmoSD or Smo stimulated by Hh (see more below). Together with the results shown in Fig. 3A, these observations indicated that lipid rafts are important in the Hh signal transduction from the outside into the inside of cell.
Given that Smo C-tail forms dimer (9), we further investigated whether lipid rafts are important for the oligomerization and activation of Smo. We found that PUFA decreased the FRET induced by Hh between Smo C termini (SmoCFPC/SmoYFPC), yet SA did not (Fig. 3C and data not shown). Meanwhile, the FRET induced by Hh between SmoCFPL3 and SmoYFPL3 was blocked by PUFA (Fig. 3, C and D, and data not shown). Smo clustering on the plasma membrane induced by Hh was also reduced by PUFA although Smo membrane accumulation was not affected (Fig. 3, E–H″ and supplemental Videos 1–4). Taken together, these results indicated that lipid rafts are required for both Smo oligomerization/higher order clustering and Smo activation.
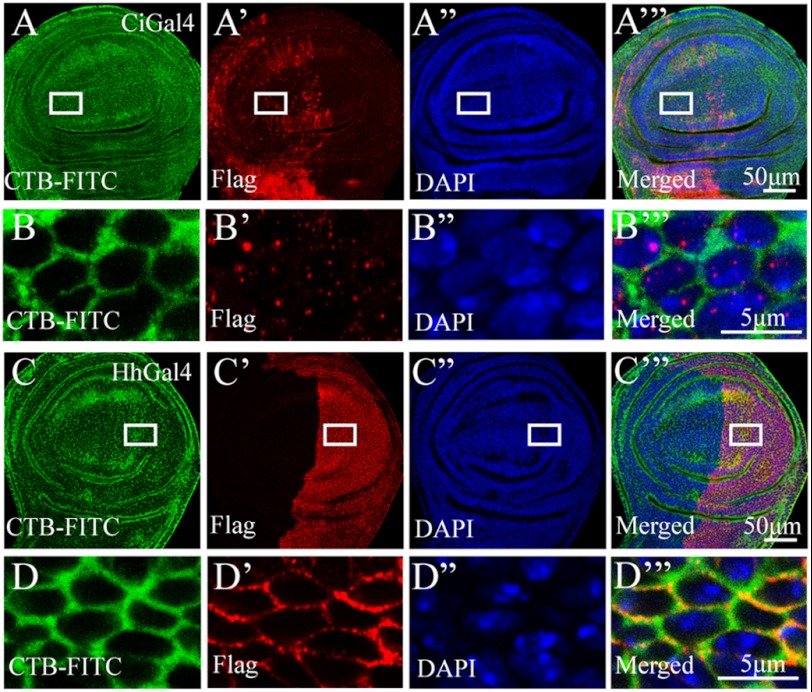
To further investigate whether Smo locates in lipid rafts in vivo, we overexpressed Smo with Ci-Gal4 or Hh-Gal4 in the A or P compartment, respectively. We then examined whether Smo could co-localize with cholera toxin B subunit (CTB), which has been widely used as a marker of membrane lipid rafts (35). As shown in Fig. 4, A–B‴, we found that Smo locates in the cytosol and does not co-localize with CTB in the absence of Hh (A compartment). However, Smo co-localizes with CTB in the presence of Hh (P compartment) (Fig. 4, C–D‴). This finding suggested that Smo relocates to lipid rafts only in the presence of Hh.
FIGURE 4.
Smo locates in lipid rafts in vivo in the presence of Hh. FLAG-tagged SmoWT was expressed in the A compartment with Ci-Gal4 (A–A‴ and B–B‴), or in the P compartment with Hh-Gal4 (C–C‴ and D–D‴). FITC-CTB (green) was employed to mark lipid rafts on the plasma membrane. Anti-FLAG antibody was used to immunostain the overexpressed Smo (red), and DAPI was used to mark nucleus (blue). The squares in A–A‴ and C–C‴ delimit the magnified areas in B–B‴ and D–D‴, respectively. The magnified images showed the details of Smo, CTB, and DAPI staining (B–B‴ and D–D‴). In the absence of Hh, Smo located in cytoplasm and could not co-localize with CTB (B and B′). In the presence of Hh, however, Smo co-localized with CTB on the plasma membrane (D and D′). Scale bars, 50 μm (A‴ and C‴) or 5 μm (B‴ and D‴).
Because PUFA treatment decreased ptc reporter activity induced by Hh (Fig. 3B), we asked whether it could down-regulate Hh pathway activity in vivo. To address this question, we overexpressed FLAG-tagged SmoWT or SmoSD in fly wing imaginal disc with MS1096. When SmoWT was overexpressed, it could turn on dpp expression. However, the dpp expression level was not affected by PUFA (data not shown). We further investigated whether lipid rafts play an important role in high Hh activity transduction. SmoSD has been used to mimic highly activated Smo in response to high level of Hh signal. When overexpressed in wing disc and treated with SA, SmoSD cannot only turn on ptc expression, which responds to the middle level of Hh, but also en expression, which responds to high Hh level (Fig. 5, A–A‴ and C–C‴). After being treated by PUFA, the ptc expression level had no significant alteration (Fig. 5, A and B). However, the en expression level decreased dramatically by PUFA treatment (Fig. 5, C and D). The magnified image demonstrated that PUFA treatment decreased clustering level of SmoSD in vivo (Fig. 5, E, E′, F, and F′). However, PUFA did not reduce the en expression level induced by overexpressing downstream component of Hh pathway, HA-tagged wild-type full-length Ci (Fig. 5, G, G′, H, and H′). In addition, Western blotting results suggested that PUFA treatment reduced the oligomerization level of Smo in the presence of Hh (Fig. 5, I and J). These results suggested that PUFA treatment mainly affects the high level Hh activity by reducing Smo oligomerization/higher order clustering on the cell membrane. Combining these result with the observation in Figs. 3, B and C, and 4, D–D‴, our results indicated that lipid raft localization and oligomerization/higher order clustering of Smo on the plasma membrane are essential for transduction of high level Hh activity.
FIGURE 5.
Lipids rafts are essential for high level Hh signal transduction. Wing imaginal discs of third instar larvae expressing FLAG-tagged SmoSD (A–F′) or HA-tagged full-length Ci (G–H′) with MS1096;ptc-LacZ are shown. Discs were dissected and treated with SA (served as control, A–A‴, C–C‴, E–E′, and G–G′) or PUFA (B–B‴, D–D‴, F–F′, and H–H′) in serum-free M3 medium for 6 h and then immunostained with different antibodies to show the expression of ptc-LacZ (blue), En (blue), Ci (red), and SmoSD (green). E–E′ and F–F′ are the magnified views of FLAG staining to show the clusters on the plasma membrane of SmoSD. DAPI staining labeled the nucleus. Arrows in E point to the oligomers/clusters of SmoSD on the cell surface. Scale bars, 5 μm (E′ and F′). G–H′, wild-type Ci was overexpressed with MS1096 to induce En expression in the A compartment. PUFA treatment did not reduce En expression level (G and H). I, S2 cells were transfected with Myc-SmoΔC and treated with SA or PUFA in the absence/presence of Hh. Cell lysates were harvested for native gel electrophoresis and then detected by anti-Myc antibody in Western blotting (WB). J, graph shows the ratio change of oligomer/monomer in the experiment.
Oligomerization/Higher Order Clustering of Smo Is Required for High Level Activity of Hh
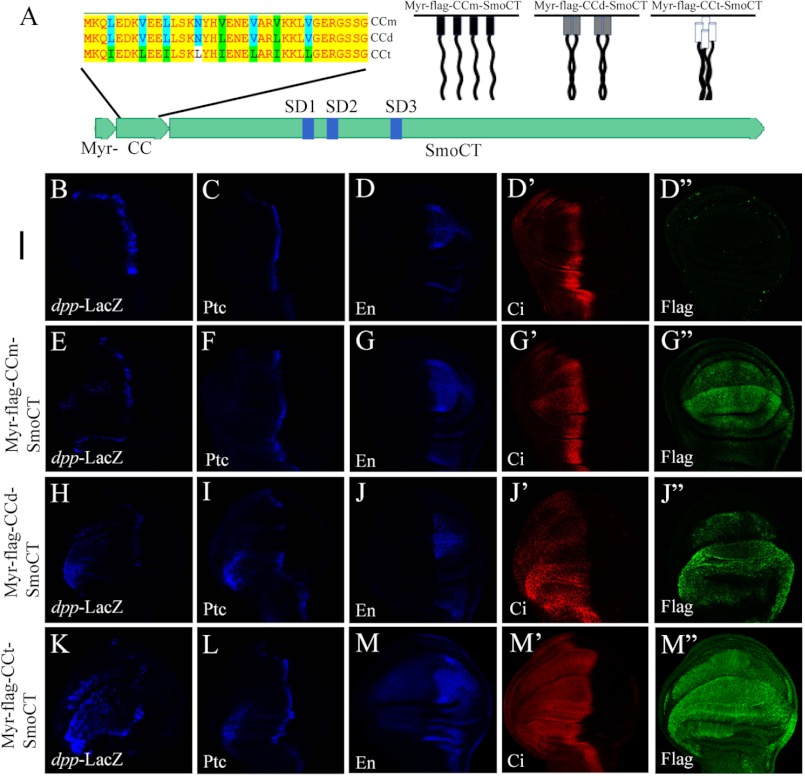
Given the C-tail conformational switch as a key determinant for Smo activation (9), it is conceivable that, in the response to Hh, Smo N terminus and TMs mediated oligomerization may greatly promote the clustering of the Smo C-tails, and therefore transduce high level Hh signal. To further verify this hypothesis, we generated several FLAG-tagged Smo C-tail mutants: Myr-FLAG-CCd-SmoCT, Myr-FLAG-CCm-SmoCT, and Myr-FLAG-CCt-SmoCT, all of which contained a myristoylation signal at the N terminus of Smo C-tail (see Fig. 6A, right). A coiled-coil dimerization motif from yeast GCN4 (21) (referred to as CCd), and its dimerization-deficient version (referred to as CCm) or its tetramerization version (referred to as CCt) (19, 20) were inserted into the N terminus of the Smo C-tail, respectively. To determine whether higher order clustering of Smo C-tail activates the Hh pathway in vivo, UAS transgenes expressing Myr-FLAG-CCm-SmoCT, Myr-FLAG-CCd-SmoCT, or Myr-FLAG-CCt-SmoCT were generated, respectively. Expression of Myr-FLAG-CCm-SmoCT, Myr-FLAG-CCd-SmoCT, or Myr-FLAG-CCt-SmoCT with MS1096;dpp-LacZ resulted in ectopic expression of dpp in a gradient manner (compare dpp level in Fig. 6, B, E, H, and K). We also noticed that Myr-FLAG-CCd-SmoCT or Myr-FLAG-CCt-SmoCT, but not Myr-FLAG-CCm-SmoCT, induced Ptc protein level increase in the A compartment far away from A/P boundary (compare Ptc level in Fig. 6, C, F, I, and L) in a gradient manner, and only the expression of Myr-FLAG-CCt-SmoCT could turn on en expression (compare En level in Fig. 6, D, G, J, and M). These results suggested that, along with Smo C-tail oligomerization ranging from monomer, dimer to high order cluster, Smo activates the Hh pathway in a gradient manner in vivo. It appears that Smo C-tail oligomerization/higher order clustering is essential for transduction of high level Hh signaling.
FIGURE 6.
Oligomerization/higher order clustering of Smo C-tail is essential for high activity of Hh pathway. A, upper left, amino acid sequences of CCm, CCd, and CCt, with green and blue letters indicating different amino acids among them. Upper right, diagrams of Myr-FLAG-CCm-SmoCT, Myr-FLAG-CCd-SmoCT, and Myr-FLAG-CCt-SmoCT. The lower diagram shows Myr-FLAG-CC-SmoCT variants. B–M″, wing discs dissected from third instar larvae, which were expressing the indicated Smo C-tail mutants (E–M″) or wild-type fly (B–D″) with MS1096;dpp-LacZ, were immunostained to show the expression level of dpp-LacZ (blue), Ptc (blue), En (blue), Ci (red) and FLAG (green), respectively.
DISCUSSION
It is generally accepted that Smo transduces the Hh signaling via intracellular complexes consisting of many proteins such as Fu/Cos2 and Ci and that Smo activation is regulated via a cytoplasmic conformational switch as well as its extracellular loops (9, 13, 36–38). However, whether Smo forms oligomers/higher order clusters in the presence of Hh remains largely unknown. If so, how do Smo oligomers/higher order clusters regulate its activity? Here, we provide substantial evidence that the Smo extracellular N terminus and its TMs are important for oligomerization/higher order clustering in the presence of Hh. Moreover, we found that Hh signal triggers closer proximity of the Smo intracellular subdomain 3 (third internal loop). We also demonstrated that lipid rafts are essential for high level Smo activation, and oligomerization/higher order clustering of the Smo C-tail is required for the transduction of high level Hh signaling. Thus, our studies propose a novel role of lipid rafts and Smo oligomerization/higher order clustering in regulation of the Hh signal transduction.
A graded signal mediated by morphogen, including the Hh graded signal, has been proposed to regulate differential gene expressions in a concentration-dependent manner (3, 39). However, as a fundamental aspect of the Hh signaling pathway, how Smo activity on the plasma membrane is regulated by Hh gradient has not been fully addressed. Drosophila wing discs provide an excellent model system to study the regulatory mechanism of the Hh signal transduction. In wing discs, Hh signal molecules are secreted from the P compartment cells and form a signal concentration gradient in the A compartment cells adjacent to the A/P boundary, which has a high level of Hh, to induce the expression of downstream target genes, including dpp, ptc, and en. Because Smo has a critical positive role in the Hh signaling pathway, its activity must be tightly controlled by the concentration of Hh to ensure proper strength and duration of the Hh signaling. Previous studies have proposed a model by which the Smo N terminus forms a constitutive dimer and its C-tails have a conformational change to transduce Hh gradient information (6, 9). However, it is still unknown whether Smo forms oligomers/higher order clusters in responding to Hh gradient. In this study, we demonstrated that the N terminus and the TMs contribute to the oligomerization/higher order clustering of Smo on the cell plasma membrane. Meanwhile, our data suggested that the N terminus and the TMs of Smo may induce its C-tails to form oligomers/higher order clusters for transducing the information of differential Hh concentration. To test this hypothesis, we mimicked Smo C-tail monomer, dimer, and tetramer (oligomer) on the plasma membrane by expressing Smo mutants (Myr-FLAG-CCm-SmoCT, Myr-FLAG-CCd-SmoCT, or Myr-FLAG-CCt-SmoCT), respectively. We found that, by mimicking N terminus oligomers/higher order clusters, oligomerized Smo C-tails exhibit higher level of Hh signaling activity than dimerized or monomer one. We concluded that the oligomerization/higher order clustering of the Smo C-tail is essential for high Hh activity transduction.
Lipid rafts play a critical role in mediating certain signal transductions by acting as signaling and oligomerization platforms (15–18, 35). However, the functional relevance of lipid rafts regarding Hh signaling has not been studied. In this work, we identified an important role of lipid rafts in Hh pathway. We found that Smo locates in lipid rafts on the plasma membrane in the presence of Hh, implying that lipid rafts are involved in regulating Hh signal transduction. Given that the disruption of lipid rafts down-regulates high level Hh pathway activity and decreases the oligomers/higher order clusters of Smo on the plasma membrane, lipid rafts appear to play a critical role in transducing high level Hh activity by acting as a platform for Smo oligomers/higher order clusters. In fact, lipid rafts function not only in cell signaling and but also in protein trafficking (35). As a future direction, it would be intriguing to investigate their potential function in regulating Smo trafficking in the presence of Hh. In addition, the functional conservation of Smo in development of vertebrate and invertebrate warrants further studies into the importance of lipid rafts in mammalian Hh signaling.
Acknowledgments
We apologize to colleagues whose works were not cited because of space limitations. We thank Drs. Kan Liao and Jinqiu Zhou for sharing the reagents and useful discussion.
This work was supported by National Basic Research Program 973 of China Grants 2010CB912101, 2011CB943902, and 2012CB945001, the National Natural Science Foundation of China Grants 31171414 and 31171394, and the Strategic Priority Research Program of the Chinese Academy of Sciences Grants XDA01010405 and XDA01010406.

This article contains supplemental Videos 1–4.
- Hh
- Hedgehog
- A compartment
- anterior compartment
- C-tail
- C-terminal cytoplasmic tail
- CCd
- peptides corresponding to the wild-type leucine zipper of the yeast GCN4
- CCm
- dimerization-deficient version of CCd
- CCt
- tetramerization version of CCd
- Ci
- Cubitus interruptus
- CFP
- cyan fluorescent protein
- CTB
- cholera toxin B subunit
- dGq
- Drosophila Gαq
- dpp
- decapentaplegic
- En
- Engrailed
- LAR
- leukocyte antigen-related
- Myr
- myristoylation
- P compartment
- posterior compartment
- Ptc
- Patched
- PUFA
- polyunsaturated fatty acid
- SA
- stearic acid
- Smo
- Smoothened
- SmoCT
- Smo C-terminal cytoplasmic tail
- SmoΔC
- Smo deleted C terminus
- SmoΔN
- Smo deleted N terminus
- SmoΔNΔC
- Smo deleted both N and C termini
- TM
- transmembrane domain.
REFERENCES
- 1. Ingham P. W., McMahon A. P. (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 2. Ingham P. W., Nakano Y., Seger C. (2011) Mechanisms and functions of Hedgehog signalling across the metazoa. Nat. Rev. 12, 393–406 [DOI] [PubMed] [Google Scholar]
- 3. Jiang J., Hui C. C. (2008) Hedgehog signaling in development and cancer. Dev. Cell 15, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilson C. W., Chuang P. T. (2010) Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137, 2079–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hooper J. E., Scott M. P. (1989) The Drosophila patched gene encodes a putative membrane protein required for segmental patterning. Cell 59, 751–765 [DOI] [PubMed] [Google Scholar]
- 6. Zhao Y., Tong C., Jiang J. (2007) Transducing the Hedgehog signal across the plasma membrane. Fly 1, 333–336 [DOI] [PubMed] [Google Scholar]
- 7. Price M. A., Kalderon D. (2002) Proteolysis of the Hedgehog signaling effector Cubitus interruptus requires phosphorylation by glycogen synthase kinase 3 and casein kinase 1. Cell 108, 823–835 [DOI] [PubMed] [Google Scholar]
- 8. Smelkinson M. G., Kalderon D. (2006) Processing of the Drosophila Hedgehog signaling effector Ci-155 to the repressor Ci-75 is mediated by direct binding to the SCF component Slimb. Curr. Biol. 16, 110–116 [DOI] [PubMed] [Google Scholar]
- 9. Zhao Y., Tong C., Jiang J. (2007) Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450, 252–258 [DOI] [PubMed] [Google Scholar]
- 10. Hooper J. E., Scott M. P. (2005) Communicating with Hedgehogs. Nat. Rev. Mol. Cell Biol. 6, 306–317 [DOI] [PubMed] [Google Scholar]
- 11. Jia J., Tong C., Wang B., Luo L., Jiang J. (2004) Hedgehog signalling activity of Smoothened requires phosphorylation by protein kinase A and casein kinase I. Nature 432, 1045–1050 [DOI] [PubMed] [Google Scholar]
- 12. Camp D., Currie K., Labbé A., van Meyel D. J., Charron F. (2010) Ihog and Boi are essential for Hedgehog signaling in Drosophila. Neural Dev. 5, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang Y., Mao F., Lu Y., Wu W., Zhang L., Zhao Y. (2011) Transduction of the Hedgehog signal through the dimerization of Fused and the nuclear translocation of Cubitus interruptus. Cell Res. 21, 1436–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabata T., Kornberg T. B. (1994) Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell 76, 89–102 [DOI] [PubMed] [Google Scholar]
- 15. Jacobson K., Mouritsen O. G., Anderson R. G. (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat. Cell Biol. 9, 7–14 [DOI] [PubMed] [Google Scholar]
- 16. Simons K., Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 17. Yao Y., Hong S., Zhou H., Yuan T., Zeng R., Liao K. (2009) The differential protein and lipid compositions of noncaveolar lipid microdomains and caveolae. Cell Res. 19, 497–506 [DOI] [PubMed] [Google Scholar]
- 18. Simons K., Ikonen E. (1997) Functional rafts in cell membranes. Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 19. Harbury P. B., Zhang T., Kim P. S., Alber T. (1993) A switch between two-, three-, and four-stranded coiled coils in GCN4 leucine zipper mutants. Science 262, 1401–1407 [DOI] [PubMed] [Google Scholar]
- 20. O'Shea E. K., Klemm J. D., Kim P. S., Alber T. (1991) X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science 254, 539–544 [DOI] [PubMed] [Google Scholar]
- 21. Shi Q., Li S., Jia J., Jiang J. (2011) The Hedgehog-induced Smoothened conformational switch assembles a signaling complex that activates Fused by promoting its dimerization and phosphorylation. Development 138, 4219–4231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wittig I., Karas M., Schägger H. (2007) High resolution clear native electrophoresis for in-gel functional assays and fluorescence studies of membrane protein complexes. Mol. Cell. Proteomics 6, 1215–1225 [DOI] [PubMed] [Google Scholar]
- 23. Robbins D. J., Nybakken K. E., Kobayashi R., Sisson J. C., Bishop J. M., Thérond P. P. (1997) Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90, 225–234 [DOI] [PubMed] [Google Scholar]
- 24. Vogel S. S., Thaler C., Koushik S. V. (2006) Fanciful FRET. Sci. STKE 2006, re2. [DOI] [PubMed] [Google Scholar]
- 25. Song K. S., Li S., Okamoto T., Quilliam L. A., Sargiacomo M., Lisanti M. P. (1996) Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains: detergent-free purification of caveolae microdomains. J. Biol. Chem. 271, 9690–9697 [DOI] [PubMed] [Google Scholar]
- 26. Stulnig T. M., Huber J., Leitinger N., Imre E. M., Angelisova P., Nowotny P., Waldhausl W. (2001) Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. J. Biol. Chem. 276, 37335–37340 [DOI] [PubMed] [Google Scholar]
- 27. Polizio A. H., Chinchilla P., Chen X., Manning D. R., Riobo N. A. (2011) Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci. Signal. 4, pt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bouvier M. (2001) Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2, 274–286 [DOI] [PubMed] [Google Scholar]
- 29. Avissar S., Amitai G., Sokolovsky M. (1983) Oligomeric structure of muscarinic receptors is shown by photoaffinity labeling: subunit assembly may explain high- and low-affinity agonist states. Proc. Natl. Acad. Sci. U.S.A. 80, 156–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang W., Zhao Y., Tong C., Wang G., Wang B., Jia J., Jiang J. (2005) Hedgehog-regulated Costal2-kinase complexes control phosphorylation and proteolytic processing of Cubitus interruptus. Dev. Cell 8, 267–278 [DOI] [PubMed] [Google Scholar]
- 31. Carron C., Pascal A., Djiane A., Boucaut J. C., Shi D. L., Umbhauer M. (2003) Frizzled receptor dimerization is sufficient to activate the Wnt/β-catenin pathway. J. Cell Sci. 116, 2541–2550 [DOI] [PubMed] [Google Scholar]
- 32. Brückner K., Pasquale E. B., Klein R. (1997) Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275, 1640–1643 [DOI] [PubMed] [Google Scholar]
- 33. Centonze V. E., Sun M., Masuda A., Gerritsen H., Herman B. (2003) Fluorescence resonance energy transfer imaging microscopy. Methods Enzymol. 360, 542–560 [DOI] [PubMed] [Google Scholar]
- 34. Stulnig T. M., Berger M., Sigmund T., Raederstorff D., Stockinger H., Waldhäusl W. (1998) Polyunsaturated fatty acids inhibit T cell signal transduction by modification of detergent-insoluble membrane domains. J. Cell Biol. 143, 637–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Janes P. W., Ley S. C., Magee A. I. (1999) Aggregation of lipid rafts accompanies signaling via the T cell antigen receptor. J. Cell Biol. 147, 447–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Denef N., Neubüser D., Perez L., Cohen S. M. (2000) Hedgehog induces opposite changes in turnover and subcellular localization of Patched and Smoothened. Cell 102, 521–531 [DOI] [PubMed] [Google Scholar]
- 37. Carroll C. E., Marada S., Stewart D. P., Ouyang J. X., Ogden S. K. (2012) The extracellular loops of Smoothened play a regulatory role in control of Hedgehog pathway activation. Development 139, 612–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rohatgi R., Milenkovic L., Scott M. P. (2007) Patched1 regulates Hedgehog signaling at the primary cilium. Science 317, 372–376 [DOI] [PubMed] [Google Scholar]
- 39. Ashe H. L., Briscoe J. (2006) The interpretation of morphogen gradients. Development 133, 385–394 [DOI] [PubMed] [Google Scholar]