Background: The Tim-Tipin complex plays a critical role in the S phase checkpoint and replication fork stability by a molecular mechanism not yet elucidated.
Results: The human Tim-Tipin complex specifically enhances the synthetic activity of DNA polymerase ϵ.
Conclusion: The Tim-Tipin complex could modulate the DNA polymerase ϵ function at the replication fork.
Significance: These findings further our understanding of the replication fork dynamics in metazoans.
Keywords: DNA Damage, DNA Enzymes, DNA Polymerase, DNA Repair, DNA Replication, DNA-Protein Interaction, Fork Protection Complex, Tim-Tipin Complex
Abstract
The Tim-Tipin complex plays an important role in the S phase checkpoint and replication fork stability in metazoans, but the molecular mechanism underlying its biological function is poorly understood. Here, we present evidence that the recombinant human Tim-Tipin complex (and Tim alone) markedly enhances the synthetic activity of DNA polymerase ϵ. In contrast, no significant effect on the synthetic ability of human DNA polymerase α and δ by Tim-Tipin was observed. Surface plasmon resonance measurements and co-immunoprecipitation experiments revealed that recombinant DNA polymerase ϵ directly interacts with either Tim or Tipin. In addition, the results of DNA band shift assays suggest that the Tim-Tipin complex (or Tim alone) is able to associate with DNA polymerase ϵ bound to a 40-/80-mer DNA ligand. Our results are discussed in view of the molecular dynamics at the human DNA replication fork.
Introduction
Accurate transfer of the genetic information is critical for survival of living organisms. Checkpoint control pathways prevent the transmission of incompletely replicated or damaged DNA to daughter cells. Genotoxic insults, spontaneous errors arising during replication as well as endogenous blocks (the so-called replication fork barriers), impede replisome progression and may trigger chromosomal rearrangements and genomic instability. To cope with these problems, cells have evolved S phase checkpoints, which sense stalled replication forks and DNA damage and delay cell cycle progression until the problems are solved (1–3).
Stabilization of stalled replication forks in the presence of DNA damage or at difficult-to-replicate templates is necessary to prevent their collapse and inability to restart synthesis after recovery (4). It was proposed that in yeast stabilization of paused forks is carried out by three proteins: Mrc1, Tof1, and Csm3 in Saccharomyces cerevisiae (5) and Mrc1, Swi1, and Swi3 in Schizosaccharomyces pombe (6). Swi1 and Swi3 or Tof1 and Csm3 form a stable complex (fork protection complex). These proteins are all mediators of the replication checkpoint (7–13) and were found to move with the replication forks in normal S phase (5, 14). Therefore, they are thought to play a role even during unperturbed DNA replication that is independent of their checkpoint function (15). Similar mechanisms of stabilization of the stalled forks are believed to operate also in metazoans, where Claspin, Tim, and Tipin are the orthologs of Mrc1, Tof1 (Swi1), and Csm3 (Swi3), respectively (16–19). Like the yeast counterparts, these proteins participate in S phase checkpoint acting as mediators and are associated with the replisome, as demonstrated either in human cells (19) or in Xenopus egg extracts (20, 21). Tim and Tipin, whose level of expression peaks at the G1/S border during the cell cycle, form a heterodimeric stable complex located in the nucleus (18, 19). Several studies revealed that in budding yeast Tof1 is required for the regulation of the normal progression of DNA replication (8, 12, 15), and a reduction in the expression levels of mammalian Tim (and Tipin) resulted in a decreased rate of DNA synthesis (16–19). Furthermore, experiments performed in the Xenopus egg extracts system showed that Tim and Tipin interact and collaborate with the replication factor And1 to ensure a stable association of DNA polymerase α to the replisome and that the Tim-Tipin complex is required for fork restart after aphidicolin treatment (20, 22). This is likely due to the ability of these factors to stabilize paused replication forks and to prevent disassembly of the replisome. Consequently, forks are ready to resume DNA replication once the blockage and/or damage has been removed. Claspin, an additional important S phase checkpoint mediator, is also believed to play a critical role during unperturbed DNA replication. As a matter of fact, DNA replication in Xenopus egg extracts occurred somewhat more slowly than normal after immunodepletion of Claspin (23). Interestingly, in human cells overexpression of Claspin enhanced the rate of cell proliferation (24), and Claspin was shown to promote normal replication fork rate either in HeLa cells or in primary fibroblasts by means of DNA-combing experiments (25). In S. cerevisiae Mrc1 was demonstrated to interact directly with Pol2, the catalytic subunit of DNA polymerase ϵ, either during normal S phase or after checkpoint activation (26). This interaction is believed to be important to avoid dissociation of DNA polymerase ϵ from the leading strand during checkpoint. In fact, Pol2 dissociated from the replisome after hydroxyurea treatment in yeast cells lacking Mrc1 and the same behavior was also observed in Tof1-depleted cells, in line with the proposal that Mrc1 associates with Tof1 at the replication fork. Co-immunoprecipitation experiments revealed that Mrc1 interacted even with the Mcm2 subunit of the Mcm2–7 complex (26).
Overall, genetic and biochemical analyses carried out in yeasts and metazoans indicate the existence of an evolutionarily conserved molecular mechanism that functionally couples the replicative DNA helicase (the Cdc45-Mcm2–7-GINS (CMG)2 complex (27)) with DNA polymerase α on the lagging strand by the action of And1 and Tipin (Ctf4 in yeast) and with DNA polymerase ϵ on the leading strand by means of Claspin and Tim-Tipin (Mrc1 and Tof1/Csm3 or Swi1/Swi3 in budding yeast) (3). In line with this proposal, we report here evidence that the human Tim-Tipin complex (and Tim alone) directly interacts with DNA polymerase ϵ and specifically enhances its synthetic activity.
EXPERIMENTAL PROCEDURES
Plasmids and Baculoviruses
Human Tipin open reading frame (ORF) was PCR-amplified from an OriGene clone (OriGene accession number NM_017858) and cloned into the pGEX-4T-1 vector (GE Healthcare) using the following oligonucleotides: Tipin-BamHI forward (5′-TTTGGGGGATCCATGCTAGAACCACAGGAGAATGGCGTGATT-3′) and Tipin-XhoI-His6 reverse (5′-GGGTTTCATATGCTAGAACCACAGGAGAATGGCGTG-3′). The ORF sequence was confirmed by sequencing analysis (PRIMM; Milan, Italy). The plasmid expressing the truncated form of human Tim (FLAG-Tim5) was already described (28, 29). The baculoviruses expressing the human DNA polymerase ϵ subunits (p261, FLAG-p59, p17, and p12) were a generous gift from J. Hurwitz (Memorial Sloan Kettering Cancer Center, New York, NY).
Purification of Human Tipin
Escherichia coli Rosetta cells (Novagen) were transformed with the plasmid pGEX-4T-1-Tipin-His6 and grown at 37 °C in 2 liters of LB (Luria-Bertani) medium containing 100 μg/ml ampicillin and 30 μg/ml cloramphenicol. When cells reached an A600 of 0.8, protein expression was induced by adding isopropyl 1-thio-β-d-galactopyranoside (at 0.2 mm) to the medium, and the culture was incubated for additional 4 h at 22 °C. Cells were harvested by centrifugation (10,800 × g for 10 min at 4 °C), and the resulting pellet was resuspended in 40 ml of Tipin-lysis buffer (20 mm Hepes-NaOH, pH 7.5, 300 mm NaCl, 1% Triton X-100; 5 mm β-mercaptoethanol, 800 nm aprotinin, 4 mm benzamidine, 2 mm phenylmethylsulfonyl fluoride (PMSF) and subjected to three consecutive passages through a French pressure cell apparatus (Aminco Co.) at 1500 p.s.i. After centrifugation at 4 °C for 30 min at 65,000 × g the cell extract was incubated with 4 ml of glutathione-Sepharose beads (GE Healthcare) for 1 h at 4 °C. Then the resin was washed extensively with glutathione-washing buffer (20 mm Hepes-NaOH, pH 7.5, 500 mm NaCl, 1% Triton X-100, 5 mm β-mercaptoethanol) at room temperature, and the protein was eluted with glutathione-elution buffer (20 mm Hepes-NaOH, pH 7.5, 150 mm NaCl, 5 mm β-mercaptoethanol, 20 mm l-glutathione). The fractions were pooled and incubated with thrombin protease (GE Healthcare) in a dialysis tube to remove the GST fused at the N terminus of Tipin. The dialysis step was performed at room temperature for 16 h against 3 liters of a buffer having the following composition: 20 mm Hepes-NaOH, pH 7.5, 150 mm NaCl, 5 mm β-mercaptoethanol. The dialyzed protein was mixed with 4 ml of glutathione-Sepharose resin and incubated for 1 h at 4 °C to remove the cleaved GST. The resulting flow-through fraction (which contained Tipin-His6 but not GST) was then mixed with 4 ml of nickel-nitrilotriacetic acid-agarose resin (Qiagen) and incubated for 1 h at 4 °C. The resin was washed extensively with nickel-washing buffer (20 mm Hepes-NaOH, pH 7.5, 300 mm NaCl, 5 mm β-mercaptoethanol), and finally the protein was eluted with an imidazole gradient (25–500 mm) in nickel-washing buffer. The fractions containing Tipin-His6 were pooled, and the pool was dialyzed against QA buffer (20 mm Hepes-NaOH, pH 7.5, 100 mm NaCl, 5 mm β-mercaptoethanol, 10% glycerol) and loaded onto a Mono Q (10/100) column at 0.5 ml/min using an ÄKTA apparatus (GE Healthcare). The column was washed extensively with the same buffer, and the bound proteins were eluted with a linear gradient of NaCl (0.1–1 m). Elution fractions were collected and concentrated. 150 μg of Tipin-His6 was loaded on a Superdex 200 (10/300) column using an ÄKTA system in buffer containing 20 mm Hepes-NaOH, pH 7.5, 200 mm NaCl, 5 mm β-mercaptoethanol, 10% glycerol. The column was calibrated by running a set of gel filtration markers that included ferritin (440 kDa), yeast alcohol dehydrogenase (150 kDa), bovine serum albumin (67 kDa), and chymotrypsinogen (25 kDa). Finally, Tipin-His6 was concentrated and stored in aliquots at −80 °C.
Purification of Human Tim
Sf9 insect cells (20 × 106 cells/15-cm dish × 6 dishes) were grown at 27 °C in Sf900 medium (GIBCO) supplemented with 10% fetal bovine serum and gentamycin (10 μg/ml). The cells were infected with a fresh amplified recombinant baculovirus stock expressing FLAG-Tim. 48 h after infection cells were harvested by centrifugation at 800 × g for 10 min, washed with ice-cold PBS, and resuspended in 5 ml of Tim-lysis buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% glycerol, 0.1% Nonidet P-40, 1 mm dithiothreitol (DTT), 50 mm NaF, 1 mm Na3VO4, 2 mm PMSF) containing a protease inhibitors mixture (Roche Applied Science). Cells were disrupted with five cycles of sonication and centrifuged at 65,000 × g for 30 min at 4 °C. α-FLAG M2 beads (1 ml; Sigma) were incubated with cell extract for 2 h at 4 °C and then washed with Tim-washing buffer (50 mm Tris-HCl, pH 7.5, 300 mm NaCl, 0.1% Nonidet P-40, 10% glycerol, 1 mm DTT). Bound proteins were eluted with 5 ml of FLAG-elution buffer (50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 10% glycerol, 1 mm DTT, 200 μg/ml FLAG peptide (Sigma)). The pool of fractions containing FLAG-Tim was dialyzed against 1 liter of FLAG-elution buffer without FLAG peptide and then concentrated up to 0.5 mg/ml. FLAG-Tim (100 μg) was layered onto a 8-ml preformed glycerol gradient (from 15 to 40% glycerol in 50 mm sodium phosphate buffer, pH 7.5, 30 mm NaCl, 1 mm DTT, 0.005% Triton X-100) and subjected to centrifugation at 250,000 × g for 12 h at 4 °C. A parallel gradient was used to fractionate a mixture of the following standard proteins: ferritin (440 kDa), catalase (232 kDa), yeast alcohol dehydrogenase (150 kDa), and bovine serum albumin (67 kDa). Fractions containing FLAG-Tim were pooled and dialyzed against the glycerol gradient buffer containing 10% glycerol, concentrated, and stored in aliquots at −80 °C.
Purification of the Human Tim-Tipin Complex and the Truncated Form Tim 5
The human Tim-Tipin complex was purified from HEK 293T cells transiently co-transfected with plasmids expressing His6-Tim-HA and His6-Tipin-3×FLAG, as recently described (28, 29). FLAG-Tim 5 was produced and purified using the same procedure utilized for the Tim-Tipin complex.
The human Tim-Tipin complex was also produced using the baculovirus/insect cells system. Sf9 insect cells (20 × 106 cells/15-cm dish × 25 dishes) were grown at 27 °C in Sf900 medium supplemented with 10% fetal bovine serum and gentamycin (10 μg/ml). The cells were infected with fresh amplified recombinant baculovirus stocks expressing FLAG-Tim and Tipin. 48 h after infection cells were harvested by centrifugation at 800 × g for 10 min, washed with ice-cold PBS, and resuspended in 20 ml of Tim-lysis buffer containing a protease inhibitors mixture. Cells were disrupted with 5 cycles of sonication and centrifuged at 65,000 × g for 30 min at 4 °C. α-FLAG M2 beads (1 ml) were incubated with cell extract for 2 h at 4 °C and then washed with Tim-washing buffer containing 2 mm PMSF. Bound proteins were eluted with 4 ml of FLAG-elution buffer. The eluted sample containing the Tim-Tipin complex was dialyzed overnight against buffer A (Tris-HCl, pH 7.5, 50 mm NaCl, 0.5 mm EDTA, 1 mm DTT, 10% glycerol, 0.01% Triton-X-100) and loaded onto a Mini Q 4.6/50 PE column at 0.5 ml/min using an ÄKTA apparatus. The column was washed extensively with the same buffer, and the bound proteins were eluted with 8-ml linear gradient of NaCl (50 mm–1 m). Fractions (0.25 ml each) were analyzed by SDS-PAGE, and the ones that contained either Tim or Tipin were dialyzed separately overnight against buffer B (50 mm sodium phosphate, pH 7.5, 30 mm NaCl, 10% glycerol, 1 mm DTT, 0.005% Triton X-100). Aliquots of each fraction were tested for the presence of DNA polymerase ϵ stimulatory activity. Then, the dialyzed fractions were pooled and dialyzed overnight against buffer C (50 mm sodium phosphate, pH 6.0, 50 mm NaCl, 0.5 mm EDTA, 10% glycerol, 1 mm DTT, 0.01% Triton X-100). The dialyzed pool was loaded onto a Mini S 4.6/50 PE column at 0.5 ml/min using an ÄKTA apparatus. The column was washed extensively with the same buffer, and the bound proteins were eluted with a 5-ml linear gradient of NaCl (50 mm–1 m). Fractions (0.25 ml each) were analyzed by SDS-PAGE, and the ones that contained either Tim or Tipin were dialyzed overnight against buffer B. Aliquots of each fraction were tested for the presence of DNA polymerase ϵ stimulatory activity in enzymatic assays.
Purification of the Human Replicative DNA Polymerases
The human DNA polymerases α and ϵ used for the enzymatic assays were purified from exponentially growing HeLa cells, as described (30). DNA polymerase δ was produced in recombinant form in Sf9 insect cells and purified as described (31). The human DNA polymerase ϵ used for the protein/protein interaction studies was purified from Sf9 insect cells (20 × 106 cells/15-cm dish × 11 dishes), which were co-infected with freshly amplified baculoviral stocks expressing its four subunits (p261, FLAG-p59, p17, and p12). 48 h after infection cells were harvested, washed with ice-cold PBS, and resuspended in 4 ml of hypotonic buffer (20 mm Hepes-NaOH, pH 7.5, 5 mm KCl, 1.5 mm MgCl2, 10% glycerol, 1 mm DTT, 0.01% Nonidet P-40, and 2 mm PMSF) containing a protease-inhibitors mixture and 30 units/ml benzonase (Sigma). To guarantee a complete cell disruption, the sample was also subjected to four sonication cycles and incubated for 30 min at room temperature with gentle shaking. After addition of NaCl (at 150 mm) and EDTA (at 1 mm), the sample was centrifuged at 65,000 × g for 30 min. The resulting supernatant was incubated with α-FLAG M2 beads (1 ml) for 2 h at 4 °C with shaking. The beads were washed with washing buffer (20 mm Hepes-NaOH, pH 7.5, 5 mm KCl, 1.5 mm MgCl2, 10% glycerol, 1 mm DTT, 0.01% Nonidet P-40), containing 300 mm NaCl. Elution was carried out with 4 ml of the same buffer containing 150 mm NaCl at 150 mm and the FLAG peptide (200 μg/ml). The fractions containing DNA polymerase ϵ were pooled, and the pool was dialyzed against PBS buffer, concentrated, and stored in aliquots at −80 °C.
DNA Polymerase Assays
DNA polymerase assays on poly(dA)-oligo(dT) were carried out in a volume of 25 μl using [methyl-3H]dTTP as radioactive precursor, as described previously (32). Each DNA polymerase was titrated in the respective assay buffer, and the effect of Tim-Tipin (or Tim or Tipin alone) was measured at an amount of enzyme that gave 15–30% of the maximal activity (DNA polymerase α at 15 nm, DNA polymerase δ at 8 nm, and DNA polymerase ϵ at 0.54 nm). The resulting level of incorporated dTTP was measured with a scintillation counter, and the results were reported as the stimulation -fold of each DNA polymerase against the concentrations of Tim, Tipin, or Tim-Tipin used in the assays. The average values of stimulation and the S.D. values reported in the graphs were obtained from three independent experiments.
Primer extension assays were carried out using a 40-/80-mer DNA duplex as a primer/template. The 40-mer synthetic primer (5′-AGCTCCTAGGGTTACAAGCTTCACTAGGGTTGTCCTTAGG-3′) was labeled at the 5′-end with [γ-32P]ATP by T4 polynucleotide kinase (Roche Applied Science) and purified from the unincorporated radionuclide by passage over a Micro Bio-Spin 30 column (Bio-Rad). Then, the purified primer was annealed to a 2-fold molar excess of a cold complementary 80-mer synthetic oligonucleotide that acted as the template (5′-GCTGATCAACCCTACATGTGTAGGTAACCCTAACCCTAACCCTAAGGACAACCCTAGTGAAGCTTGTAACCCTAGGAGCT-3′). The activity of human DNA polymerase ϵ was titrated in mixtures (10 μl) containing the above 40-/80-mer primer/template (at 10 nm) and the four dNTPs (each at 100 nm) in the respective assay (32). The effect of Tim-Tipin (or Tim full-length and Tim 5, or Tipin alone) was analyzed using an amount of DNA polymerase ϵ (0.2 nm) that gave a low level of primer elongation. The reactions were carried out at 37 °C for 30 min, stopped with 2 μl of stop solution (98% formamide, 0.1% bromphenol blue, and 0.1% xylene cyanol). The samples were denatured at 95 °C for 5 min, chilled on ice, and then run on a 12%-polyacrylamide/bisacrylamide (19:1) gel containing 7 m urea in 0.5× TBE buffer (1× TBE had the following composition: 89 mm Tris, 89 mm boric acid, 2 mm EDTA, pH 8.3). Then, the gel was dried, exposed to a phosphorimaging screen, and analyzed using a Typhoon Imager (GE Healthcare). The elongated primers were quantified using the ImageQuant software (GE Healthcare), and the resulting values were reported as stimulation -fold of each DNA polymerase against the concentration of Tim, Tipin, or Tim-Tipin used in the assays. The reported plots contain average values of stimulation with S.D. values derived from three independent experiments.
Surface Plasmon Resonance Measurements
Dynamic interactions of the Tim and Tipin proteins with recombinant DNA polymerase ϵ were analyzed by using the surface plasmon resonance biosensor system Biacore2000 (Biacore). Tim (7700 resonance units) and Tipin (6000 resonance units) were immobilized on a CM5 sensor chip in 10 mm sodium acetate buffer (pH 4.0 and pH 3.5, respectively), according to the manufacturer's instructions. To collect the sensorgrams, increasing concentrations of human DNA polymerase ϵ (1.2, 2.4, 6, 12, 24, and 48 nm) in PBS buffer were fluxed over the sensor chip surface at a flow rate of 20 μl/min. Recorded sensorgrams were normalized to a base line of 0 resonance units, and the relative dissociation constants (KD) values were calculated using the BIA Evaluation software (version 3.2).
Immunoprecipitation Experiments
Mixtures (30 μl) containing purified Tim (2 μg) and/or recombinant DNA polymerase ϵ (2 μg) in PBS buffer were incubated for 1 h at 4 °C with shaking. 30 μl of protein A-agarose beads (Roche Applied Science) were mixed with 0.6 μg of polyclonal anti-Tim antibodies (Abcam) in 300 μl of PBS buffer for 1 h at 4 °C with shaking. After an extensive wash with PBS buffer to remove the unbound antibodies, the beads were resuspended in 300 μl of PBS buffer. 100 μl of beads was mixed with the binding mixtures containing Tim and/or DNA polymerase ϵ and incubated for additional 2 h at 4 °C. The beads were then washed with 3 × 600 μl of PBS buffer containing 0.1% Nonidet P-40 and resuspended in 30 μl of SDS-PAGE loading buffer (50 mm Tris-HCl, pH 6.8, 10% glycerol, 200 mm β-mercaptoethanol, 0.5% SDS, 0.01% blue bromphenol). Samples were subjected to electrophoresis through 8% polyacrylamide/bisacrylamide (29:1) gel that was also loaded with aliquots of purified recombinant Tim and DNA polymerase ϵ (0.7 and 0.4 μg, respectively) and the unbound fractions (one third of the total).
Immunoprecipitation analyses were carried out with recombinant DNA polymerase ϵ (1 μg) and purified FLAG-Tim 5 (0.21 μg) or full-length FLAG-Tim (2 μg) in the conditions described above. Bound proteins were washed with 3 × 600 μl of PBS containing 0.2% Nonidet P-40. Aliquots of DNA polymerase ϵ, full-length Tim, and FLAG-Tim 5 (0.5, 0.35 and 0.21 μg, respectively) and the immunoprecipitated proteins were separated through a precast denaturing gel (Any kDa Mini Protean gel; Bio-Rad Laboratories).
The gels were electroblotted onto a PVDF membrane, and proteins on the blot were detected with a monoclonal horseradish peroxidase-conjugated anti-FLAG antibody (Abcam) or with a mouse monoclonal α-p261 antibody (Santa Cruz Biotechnology) using the ECL+ detection system (GE Healthcare).
Immunoprecipitation Analysis from Cell Extracts
HEK 293T cells (1.5 × 106/10-cm dish) were transiently transfected with plasmids expressing full-length human Tim, the truncated versions of Tim (18), and the empty vector as a control, using the procedure described previously (28). Cells were harvested 18 h after transfection, washed with ice-cold PBS, and resuspended in 300 μl of immunoprecipitation buffer (20 mm Hepes-NaOH, pH 7.2, 150 mm KCl, 0.1% Triton X-100, 1 mm DTT, 2 mm EDTA, 1 mm Na3VO4, 5% glycerol, protease, and phosphatase inhibitors (Roche Applied Science)). Cells were disrupted with 5 cycles of sonication and centrifuged at 16,000 × g for 30 min at 4 °C. Cell extracts were clarified with 10 μl of protein G-agarose for 1 h at 4 °C, with shaking, and the supernatants were further incubated with 10 μl of α-FLAG-agarose M2 (Sigma) for 2 h at 4 °C. The beads were washed extensively with immunoprecipitation buffer, and the bound proteins were resuspended in SDS-PAGE loading buffer. Samples were separated through an 8% polyacrylamide/bisacrylamide (29:1) gel, electroblotted onto a polyvinylidene difluoride (PVDF) membrane, and analyzed by Western blotting, as above described.
Electrophoretic Mobility Shift Assays (EMSAs)
A DNA ligand made of a 40-mer DNA oligonucleotide annealed to a complementary 80-mer oligonucleotide was prepared as described above. The DNA shift assay mixtures (10 μl) had the following composition: 20 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 0.7 mm β-mercaptoethanol, 10 nm radiolabeled DNA ligand, recombinant Tim or Tipin or Tim-Tipin complex (either at 50 or 200 nm). Where indicated, DNA polymerase ϵ, purified from HeLa cells, was added to a final concentration of 0.1 nm. The samples were incubated for 20 min at 25 °C, and the protein-DNA complexes were separated by electrophoresis through a 5% polyacrylamide/bisacrylamide gel (37.5:1) in 0.5× TBE buffer. Gels were dried and analyzed by phosphorimaging.
RESULTS
The Tim-Tipin Complex (and Tim Alone) Specifically Stimulates the Synthetic Activity of DNA Polymerase ϵ
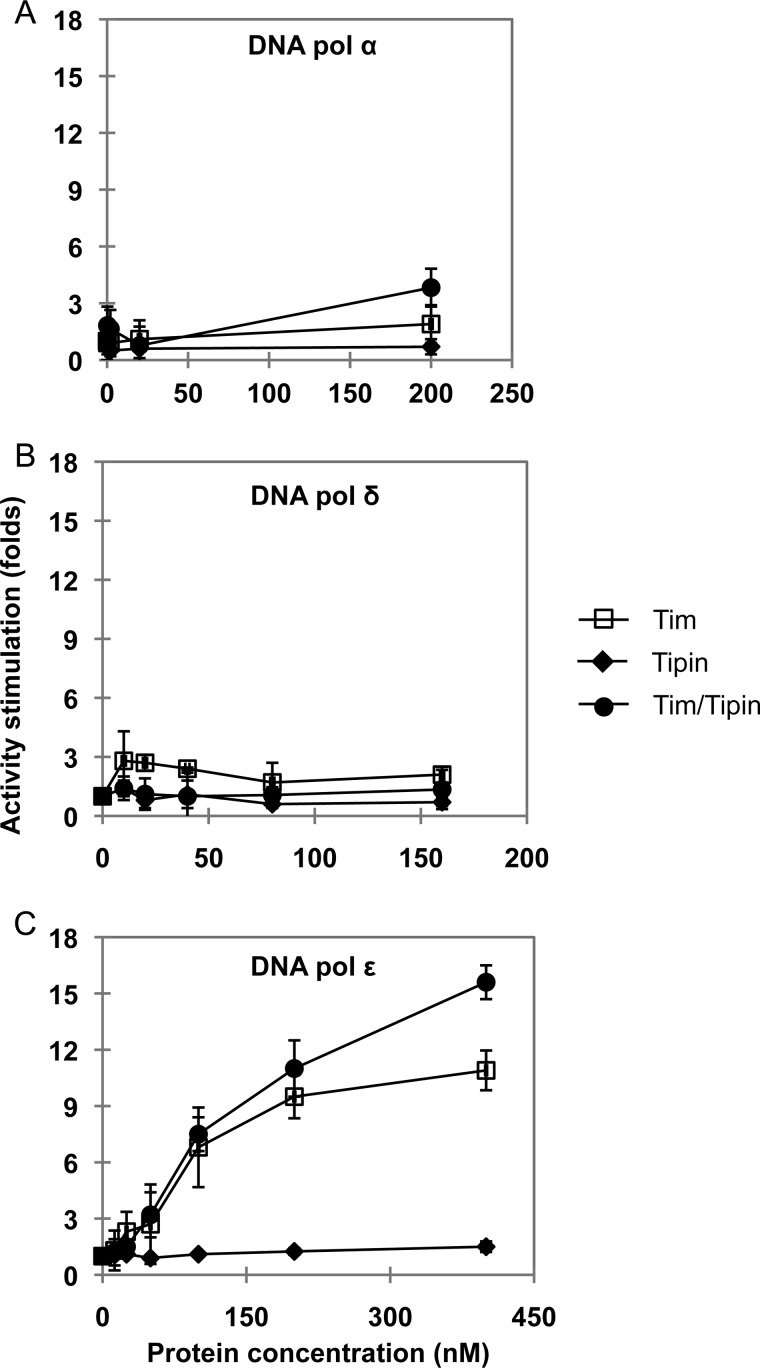
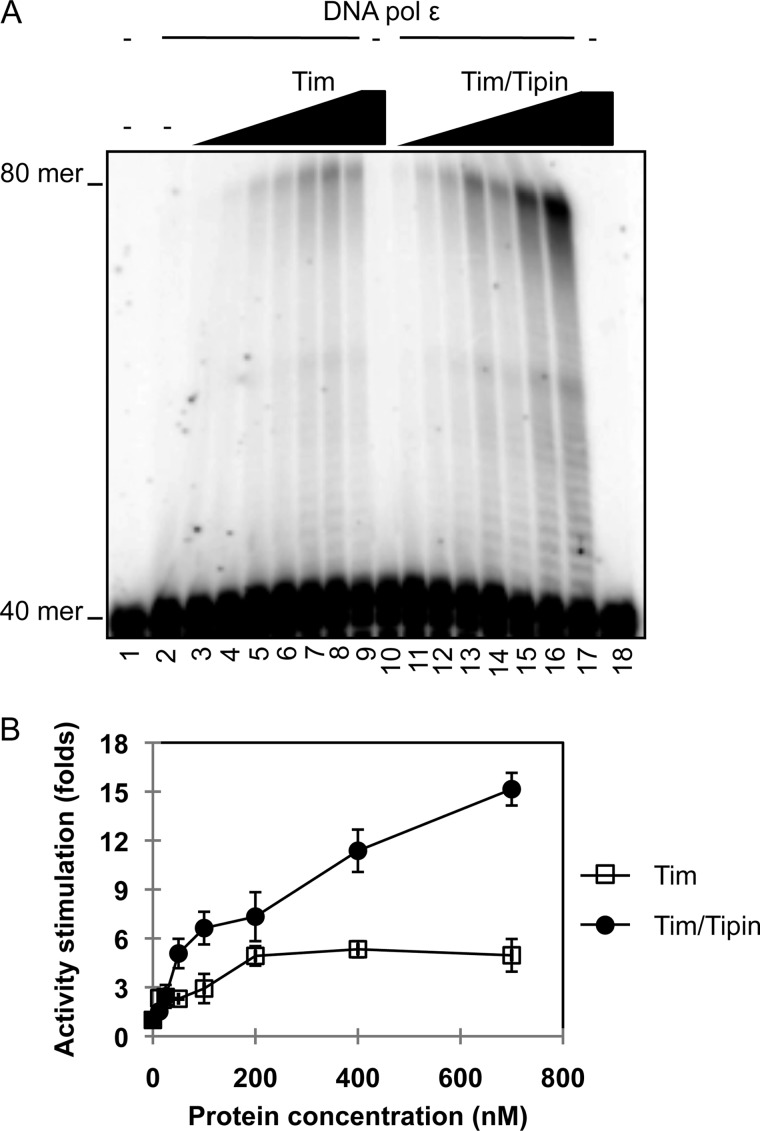
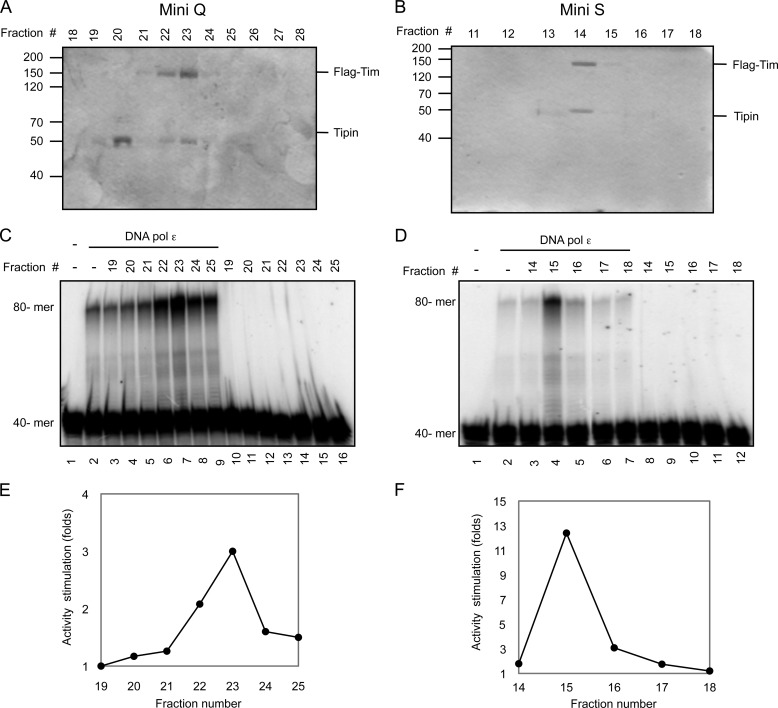
The Tim-Tipin complex was proposed to act as a functional tether between the replicative DNA polymerases and the DNA helicase at the replication fork. In fact, immunoprecipitation experiments carried out on extracts from synchronized HEK 293T cell cultures revealed that epitope-tagged Tim and Tipin associate with the endogenous replication fork proteins, such as Mcm2 and DNA polymerase ϵ and δ, during S phase (19). All that considered, we decided to investigate whether any functional interaction could take place in vitro between human recombinant Tim-Tipin (or the isolated Tim and Tipin proteins) and the three replicative DNA polymerases. In an initial set of experiments we analyzed whether the Tim-Tipin complex (or Tim and Tipin alone) could exert any effect on the synthetic activity of DNA polymerase α, δ, and ϵ. The recombinant proteins used in this study are shown in Fig. 1. The human Tim-Tipin complex was purified from mammalian cells transiently co-transfected with plasmids expressing His6-Tim-HA and His6-Tipin-3×FLAG, as recently described (28, 29). A FLAG-tagged version of human Tim was produced separately in insect cells infected with a recombinant baculovirus, whereas human Tipin was expressed separately in bacterial cells as a fusion with GST at the N terminus (cleavable with the site-specific endoprotease thrombin) and a polyhistidine tag at the C terminus and purified as described under “Experimental Procedures.” After having titrated the synthetic activity of the human replicative DNA polymerases using poly(dA)-oligo(dT) as a primer/template in the specific reaction conditions, we found that the Tim-Tipin complex and Tim alone could dose-dependently and specifically stimulate the synthetic activity of DNA polymerase ϵ, as shown in Fig. 2. We observed that incorporation of radiolabeled dTTP by DNA polymerase ϵ was increased by >15-fold in the presence of Tim-Tipin at concentrations that were >100 nm. These values represent a molar excess of >200-fold over the DNA polymerase concentration used in the assays (0.6 nm). The high molar ratio between Tim-Tipin (or Tim alone) and DNA polymerase ϵ required for the maximal stimulation might depend on the fact that only a fraction of the recombinant Tim-Tipin complex retains a putative cell cycle-specific post-translational modification required for its full biological activity. On the other hand, we observed a modest stimulation of DNA polymerase δ and α synthetic activity in the presence of the Tim-Tipin complex (or Tim alone). No effect was detected on all the three replicative DNA polymerases by increasing amounts of the purified recombinant Tipin protein. Moreover, Tim-Tipin (or Tim alone) did not affect the synthetic function of DNA polymerase δ also in the presence of the sliding clamp proliferating cell nuclear antigen (data not shown). Next, we carried out an additional set of assays to visualize the products synthesized by the DNA polymerases in the presence of Tim-Tipin (or Tim alone). This analysis was performed using a DNA duplex made of a 40-mer primer annealed to a complementary 80-mer template as the substrate. As shown in Fig. 3, we found that the synthetic activity of DNA polymerase ϵ was markedly enhanced on this primer/template in the presence of Tim-Tipin (up to 15-fold). Instead, no effect was detected on DNA polymerase α and δ on the same substrate (data not shown), even when we carried out the enzymatic assays at the same polymerase:Tim molar ratio used to obtain the maximal stimulation of the DNA polymerase ϵ activity (data not shown). To rule out the possibility that our purified samples of Tim-Tipin (and Tim alone) were contaminated by any polymerase activity, in all sets of experiments we performed control assays where the maximal amount of these proteins was used without adding the tested DNA polymerase (i.e. see lanes 10 and 18 of the gel in Fig. 3A). It could be also questioned that the stimulation of DNA polymerase ϵ is not due to the Tim-Tipin complex but to any contaminating protein from the HeLa cell extract that remains associated with it during the purification procedure. To address this issue, we produced human Tim-Tipin using the insect cell-baculovirus expression system, purified the recombinant protein complex by a procedure that, in addition to the FLAG-agarose affinity step, included two high-resolution chromatographies on ion-exchange columns (Mini Q and Mini S), and found that the DNA polymerase ϵ stimulatory activity tracked with the Tim-Tipin protein peaks eluted from these columns with salt gradients (Fig. 4).
FIGURE 1.
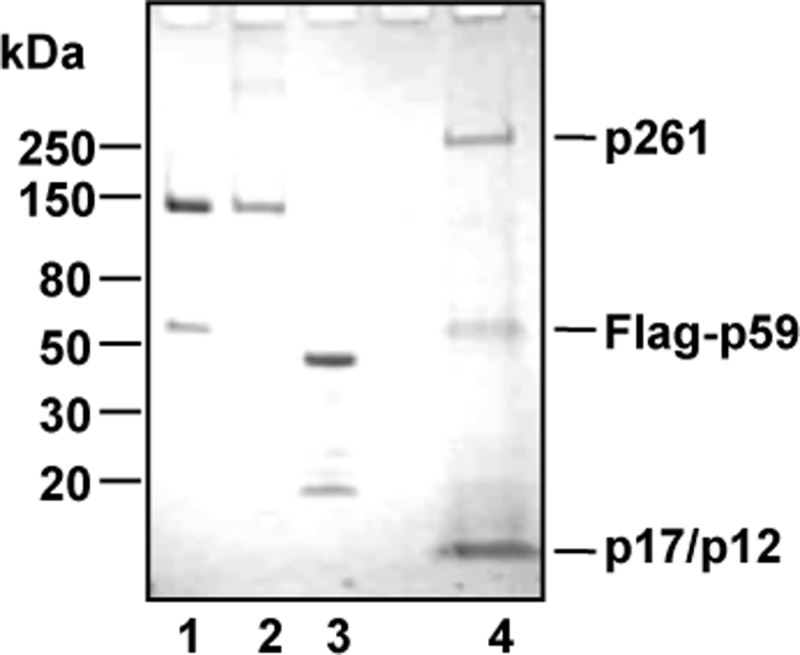
Purified recombinant proteins. His6-Tim-HA/His6-Tipin-3FLAG complex (5 μg, lane 1), FLAG-Tim (4 μg, lane 2), Tipin-His6 (5 μg, lane 3, extra bands are due to proteolytic degradation of the protein), and DNA polymerase ϵ complex (10 μg, lane 4) were run on a 4–15% polyacrylamide gradient-SDS gel, which was stained with Coomassie Blue. The DNA polymerase ϵ subunits (p261, FLAG-p59, p17, and p12) are indicated on the right. Migration of marker proteins run on a parallel lane is indicated on the left.
FIGURE 2.
Effect of human Tim-Tipin, Tim, and Tipin on replicative DNA polymerases synthetic activity. The amount of [methyl-3H]dTTP incorporated into a poly(dA)-oligo(dT) substrate by DNA polymerase α (15 nm, A), δ (8 nm, B), and ϵ (0.54 nm, C) were analyzed in the presence of increasing concentration of Tim-Tipin (circles) or Tim alone (squares) or Tipin alone (diamonds). The activities were normalized to the values calculated in the absence of Tim-Tipin, Tim, and Tipin alone.
FIGURE 3.
Primer elongation capability of DNA polymerase ϵ is enhanced by Tim-Tipin and Tim. A, elongation activity of DNA polymerase ϵ was measured on a 40-/80-mer as the primer/template, as described under “Experimental Procedures.” A fixed amount of DNA polymerase ϵ (0.2 nm) was assayed alone (lane 2) or in the presence of increasing concentrations of recombinant Tim or Tim-Tipin complex (12.5, 25, 50, 100, 200, 400, 700 nm; lanes 3–9 and lanes 11–17, respectively). Control assays were carried out which contained only Tim or Tim-Tipin (at 700 nm) in the absence of DNA polymerase ϵ (lanes 10 and 18, respectively). All of the reactions, including the blank sample (lane 1), were stopped and loaded on a 12% polyacrylamide/bisacrylamide (19:1) gel containing 7 m urea in 0.5× TBE buffer. The electrophoretic run was carried out in the same buffer at 30 watts. B, the radioactivity of the elongated products was quantified using the ImageQuant software (GE Healthcare). The intensity of the signal in each lane was normalized to the value calculated for the reaction carried out by DNA polymerase ϵ alone. In the plot average values are reported with error bars from three independent experiments carried out in the presence of Tim (squares) or Tim-Tipin (circles).
FIGURE 4.
Analysis of the recombinant human FLAG-Tim-Tipin complex purified from insect cells. An aliquot of the indicated fractions eluted with a salt gradient from Mini Q (20 μl; A) and Mini S (10 μl; B) columns was analyzed by a denaturing 8% polyacrylamide/bisacrylamide (29:1) gel stained with Coomassie Blue. Migration of marker proteins run on a parallel lane is indicated on the left. An aliquot (2 μl) of the indicated fractions eluted from Mini Q (C) or Mini S (D) was analyzed for the presence of DNA polymerase ϵ stimulatory activity using a 40-/80-mer as the primer/template, as described under “Results.” The assays were carried out in the presence of a fixed amount of DNA polymerase ϵ (C, lanes 2–9; D, lanes 2–7) or without DNA polymerase ϵ as control reactions (C, lanes 10–16; D, lanes 8–12). All of the reactions, including the blank sample (lane 1 of each gel), were stopped and loaded on a 12% polyacrylamide/bisacrylamide (19:1) gel containing 7 m urea in 0.5× TBE buffer. The electrophoretic run was carried out in the same buffer at 30 watts. E and F, the radioactivity of the elongated products was quantified using the ImageQuant software. The intensity of the signal in each lane was normalized to the value calculated for the reaction carried out by DNA polymerase ϵ alone.
To analyze the effect of Tim-Tipin on the polymerase processivity we carried out assays at a low enzyme to primer/template ratio (1:20) for shorter incubation times to ensure that each product would correspond to a single DNA polymerase ϵ-DNA elongation event (single-hit conditions (33)). This analysis revealed that in the presence of Tim-Tipin (and Tim alone) length distribution of the products synthesized by DNA polymerase ϵ did not change (data not shown).
Taken together, these data suggest that the Tim-Tipin complex (or Tim alone) enhances the synthetic function of DNA polymerase ϵ in a dose-dependent and specific manner on various kinds of DNA primer/template molecules, whereas Tipin alone does not exert any stimulatory effect on any of the three replicative DNA polymerases.
Tim and Tipin Interact Directly with DNA Polymerase ϵ
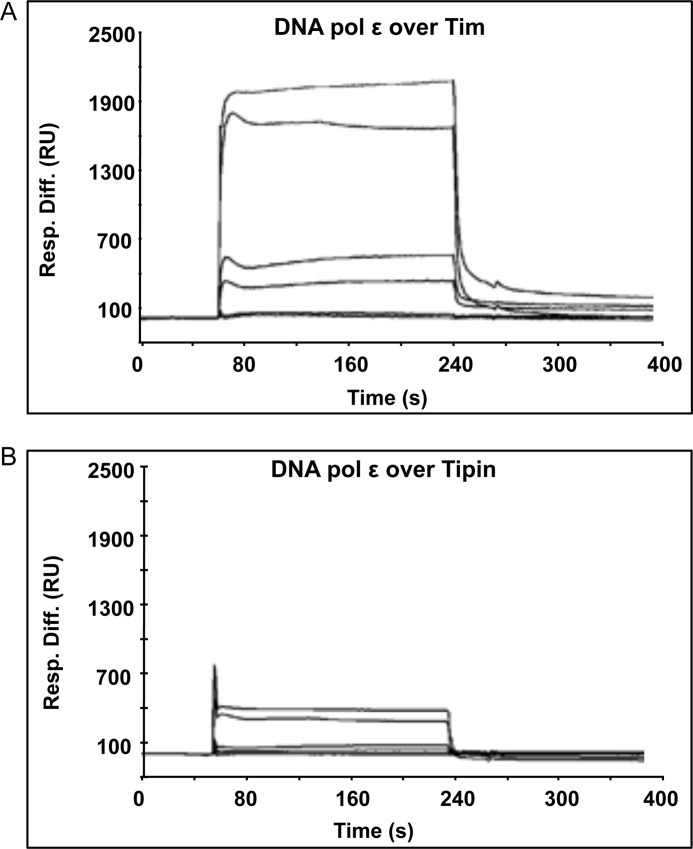
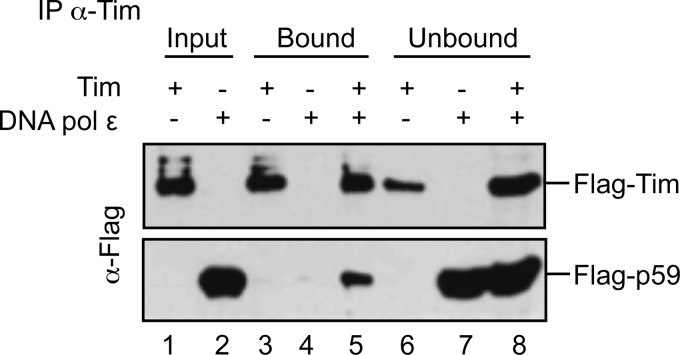
The finding that Tim-Tipin (or Tim alone) specifically stimulated DNA polymerase ϵ prompted us to investigate whether a direct physical interaction could be detected between these proteins in vitro by using the surface plasmon resonance technique. For these analyses the heterotetrameric DNA polymerase ϵ (purified from baculovirus-infected insect cells; Fig. 1, lane 4) was fluxed over a sensor chip on which recombinant Tim (or Tipin) was immobilized. Fig. 5 shows the overlaid sensorgrams obtained by testing six increasing concentrations of human DNA polymerase ϵ (from 1 to 50 nm) either on Tim- or Tipin-immobilized sensor chips (panel A and B, respectively). These results indicated that DNA polymerase ϵ physically interacted with both Tim and Tipin, although with different binding affinity (KD of 6.8 × 10−8 m for Tim and 5.3 × 10−6 m for Tipin). These data were confirmed by co-immunoprecipitation of the recombinant proteins (Fig. 6). In these experiments we used protein A-agarose beads conjugated with anti-Tim antibodies on mixtures containing recombinant heterotetrameric DNA polymerase ϵ (with a FLAG-tagged version of the p59 subunit) and FLAG-tagged Tim. The results of this analysis revealed that the recombinant human DNA polymerase ϵ directly interacted with Tim.
FIGURE 5.
Dynamic interaction of DNA polymerase ϵ with immobilized Tim and Tipin. The recombinant DNA polymerase ϵ complex was fluxed at increasing concentrations (1.2, 2.4, 6, 12, 24, and 48 nm, lower to upper curves) over a Tim- and Tipin-immobilized sensor chip using a Biacore 2000 instrument (A and B, respectively). An overlaid plot of sensorgrams was obtained corresponding to the recorded resonance units versus time (seconds).
FIGURE 6.
DNA polymerase ϵ and Tim co-immunoprecipitate. Immunoprecipitation experiments were carried out using protein A-agarose beads conjugated with anti-Tim antibodies and mixtures of purified recombinant FLAG-Tim and DNA polymerase ϵ, as described under “Experimental Procedures.” The samples were subjected to SDS-PAGE and Western blot analysis with anti-FLAG antibodies to detect recombinant FLAG-Tim and the FLAG-p59 subunit of the recombinant DNA polymerase ϵ, as indicated. Aliquots of FLAG-Tim and DNA polymerase ϵ (0.7 and 0.4 μg, lanes 1 and 2, respectively) as input, and the bound (lanes 3–5) and unbound proteins (lanes 6–8) present in each sample were analyzed.
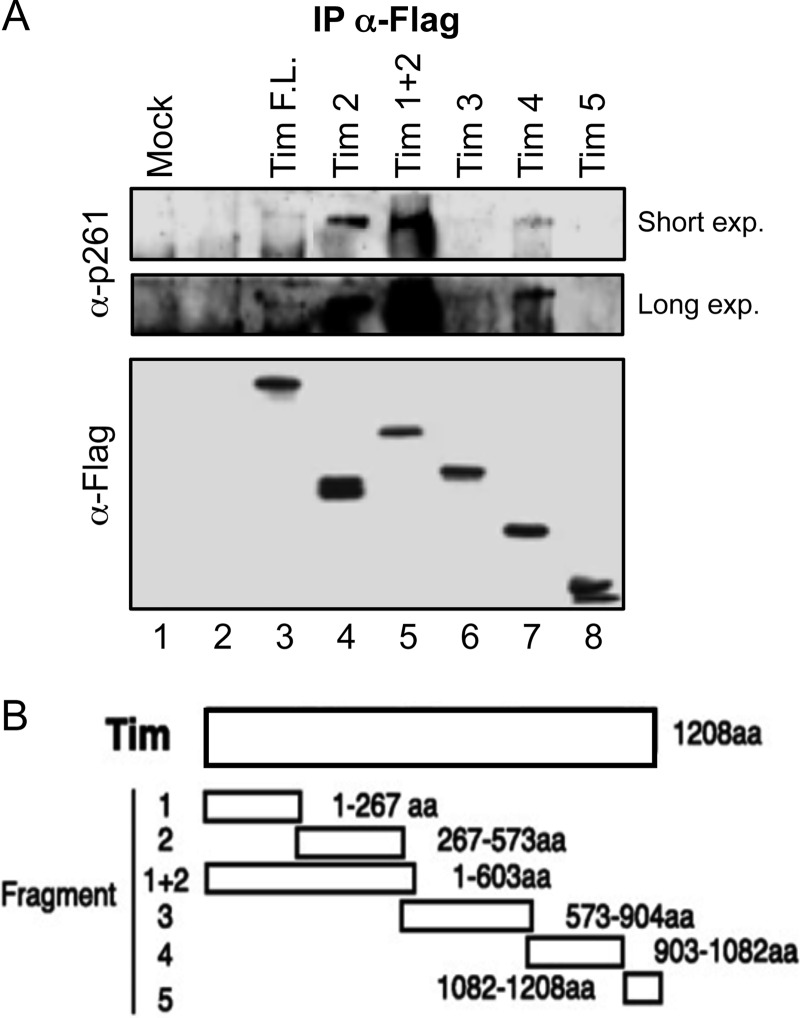
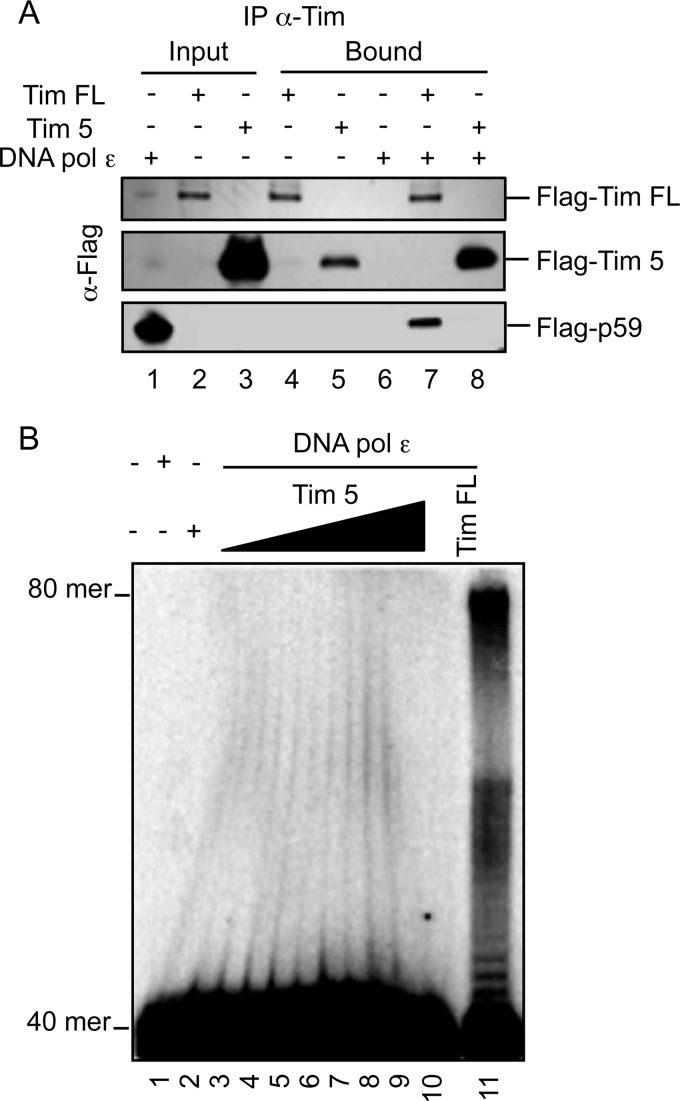
Next, we analyzed the physical interaction of DNA polymerase ϵ with many Tim truncated forms already described (18). Plasmids expressing FLAG-tagged versions of these Tim truncated forms were transiently transfected into HEK 293T cells. Interaction of these proteins with the endogenous DNA polymerase ϵ was tested by co-immunoprecipitation experiments carried out on extracts of the transfected cells. FLAG-Tim 5 (which includes residues 1082–1208 of Tim polypeptide chain) was found to be unable to interact with the endogenous DNA polymerase ϵ, whereas all the other tested Tim deleted forms co-immunoprecipitated with DNA polymerase ϵ although to a different extent (Fig. 7). These findings suggest that an extended surface of the Tim polypeptide chain might be involved in the interaction with DNA polymerase ϵ. Then, we purified the FLAG-Tim 5 protein from HEK 293T transfected cells by the same procedure used for the Tim-Tipin complex. We found that FLAG-Tim 5 did not directly interact with human recombinant DNA polymerase ϵ, as indicated by co-immunoprecipitation assays with anti-Tim antibodies (Fig. 8A). Besides, FLAG-Tim 5 was unable to enhance the synthetic activity of DNA polymerase ϵ on a 40-mer/80-mer primer/template (Fig. 8B). These results provide further evidence that the observed stimulatory effect on DNA polymerase ϵ is truly due to Tim-Tipin and not to any contaminating activity of our protein preparations.
FIGURE 7.
Interaction of Tim truncated forms expressed in mammalian cells with endogenous DNA polymerase ϵ. A, extracts of HEK 293T cells not transfected (lane 1), or transfected with empty vector (lane 2) or with plasmids expressing full-length FLAG-Tim or its truncated derivatives (lanes 3–8) were subjected to co-immunoprecipitation experiments with α-FLAG-agarose beads, as indicated under “Experimental Procedures.” Bound proteins were analyzed by immunoblotting and detected with monoclonal α-FLAG (Abcam) and α-p261 (Santa Cruz) antibodies. B, schematic represents the Tim truncated forms used in A, as described previously (18).
FIGURE 8.
Physical and functional interaction of purified Tim 5 with DNA polymerase ϵ. A, immunoprecipitation experiments were carried out on a mixture of purified DNA polymerase ϵ, full-length Tim, or Tim 5 with α-Tim antibodies, previously immobilized on protein A-agarose, as indicated under “Experimental Procedures.” DNA polymerase ϵ (0.5 μg), full-length Tim (0.35 μg) and Tim 5 (0.21 μg), and bound proteins (lanes 1–8) were analyzed by Western blotting using α-FLAG antibodies. B, the elongation capability of a fixed amount of DNA polymerase ϵ (0.2 nm) alone (lane 2) or in the presence of increasing concentrations of purified Tim 5 (12.5, 25, 50, 100, 200, 400, 700 nm, lanes 4–10, respectively) or Tim-Tipin (700 nm, lane 11) was measured on a 40-/80-mer substrate, as indicated under “Experimental Procedures.” Control assays were carried out with Tim 5 alone (700 nm, lane 3).
DNA Binding Activity of Tim-Tipin and DNA Polymerase ϵ
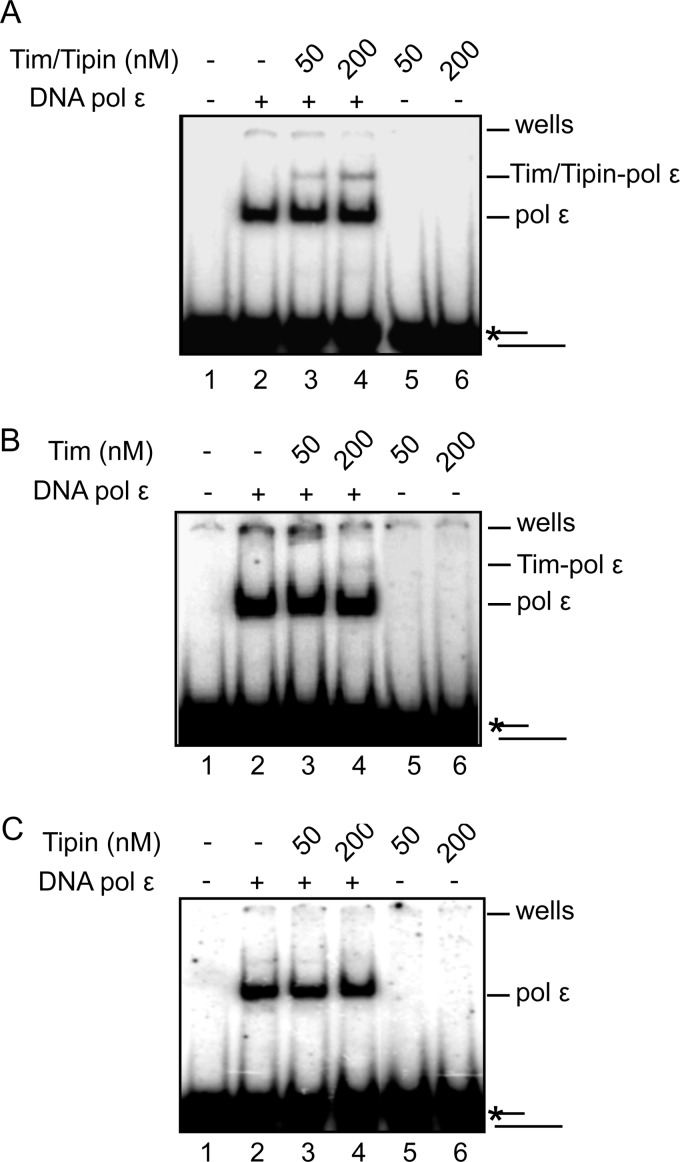
We analyzed whether DNA polymerase ϵ affected DNA binding of the Tim-Tipin complex and Tim and Tipin alone. It was observed previously that S. cerevisiae DNA polymerase ϵ prefers primer/template DNA molecules having a duplex region of at least 40 base pairs as a substrate in enzymatic assays in vitro (33). Based on this observation, we performed band shift assays of human DNA polymerase ϵ in the absence and presence of Tim-Tipin (or Tim and Tipin alone) on the same 40-/80-mer utilized as the primer/template in the polymerase assays (Fig. 9). A fixed concentration of DNA polymerase ϵ (1 pmole in 10 μl) was used in the presence of two concentrations of Tim-Tipin (and Tim or Tipin alone). We found that DNA polymerase ϵ alone (lane 2 in each gel) formed a single protein-DNA complex. Addition of Tim-Tipin (at 50 and 200 nm in lanes 3 and 4, respectively, of the gel shown in panel A of Fig. 9) resulted in the formation of a supershifted band. This is likely to correspond to a DNA-DNA polymerase ϵ-Tim-Tipin ternary complex because the same amounts of Tim-Tipin in the absence of DNA polymerase ϵ did not produce any appreciable DNA-shift (see lanes 5 and 6 in Fig. 9A). Similarly, in the presence of Tim a supershifted band was detected in addition to the one due to the DNA binding activity of DNA polymerase ϵ alone (lanes 2–4 in the gel reported in Fig. 9B). This is likely to correspond to a ternary complex containing DNA, DNA polymerase ϵ, and Tim, because the same amounts of Tim did not result in any appreciable DNA shift, when DNA polymerase ϵ was absent (lanes 5 and 6 in the gel in Fig. 9B). In contrast, in similar assays Tipin did not bind DNA either alone or in the presence of DNA polymerase ϵ (see gel shown in Fig. 9C). Overall, these EMSAs suggested that Tim-Tipin and Tim alone interacted with DNA polymerase ϵ bound to a 40-/80-mer DNA primer/template.
FIGURE 9.
EMSAs with DNA polymerase ϵ and Tim-Tipin (and Tim or Tipin alone). DNA band shift assays were carried out using a radiolabeled 40-/80-mer DNA ligand (10 nm) and DNA polymerase ϵ either alone (lane 2 of each gel) or in the presence of the indicated concentrations of Tim-Tipin, Tim, and Tipin alone (lanes 3 and 4 of A–C, respectively). The DNA binding activity of Tim-Tipin, Tim, and Tipin alone was also assayed (lanes 5 and 6 of each gel). Samples were run on native polyacrylamide/bisacrylamide (37.5:1) gels in 0.5× TBE. After electrophoresis gels were dried and analyzed by phosphorimaging, as described under “Experimental Procedures.” Unbound DNA, wells, and protein-DNA complexes are indicated on the right side of each gel.
DISCUSSION
Replication checkpoint pathways respond to DNA damage and to various stress conditions during S phase by delaying cell cycle progression, stabilizing stalled forks, and promoting DNA repair. An evolutionarily conserved set of proteins (Tof1/Swi1/Tim, Csm3/Swi3/Tipin, Ctf4/Mcl1/And1, and Mrc1/Claspin) plays a critical role in this process. It moves with the replication forks in normal S phase both in yeasts and in metazoans and is thought to stabilize the replisome even during unperturbed replication in a way that is independent of its checkpoint function (5, 14, 19). This mechanism reduces the extension of single-stranded DNA regions due to the uncoupling of the replicative DNA polymerases from the DNA unwinding machinery at the paused replication forks and ensures that components of the replisome are held together ready to resume their activity (DNA synthesis and unwinding) after the obstacle has been removed (3). Coordination of the replicative DNA polymerases and the DNA helicase (CMG complex (27)) takes place even during normal S phase, and in the absence of these proteins all replication forks move at only half the normal rate in yeast cells (12, 15).
In Xenopus laevis and in human cells Tim and Tipin were demonstrated to be replication fork-associated factors, but their function is not essential for genome duplication (19–21). Nonetheless, these proteins appeared to be critical for efficient cell growth and/or organism development because knock-out of mouse Tim provoked embryonic lethality (34), whereas its down-regulation affected the correct lung and kidney morphogenesis (35, 36). The exact biochemical functions played by Tim, Tipin, and/or the Tim-Tipin complex in mammals have not been elucidated so far, although they are clearly implicated in S phase checkpoint, DNA replication, and in chromosome cohesion (16–19, 37, 38). Furthermore, whereas a number of genetic interactions were described in yeast for Tof1/Swi1 and Csm3/Swi3, only a few direct protein/protein interactions involving the mammalian counterparts of these factors were identified so far. In fact, human Tipin was demonstrated in two-hybrid screens to directly interact with the 34-kDa subunit of replication protein A and peroxiredoxin2 (19), and Xenopus Tipin was found to directly interact with the replication/cohesion factor And1 (Ctf4 in S. cerevisiae) and the p180 catalytic subunit of DNA polymerase α (22). On the other hand, only Claspin, in addition to Tipin, was reported so far to be a direct binding partner of Tim (39).
Our analysis revealed that the human Tim-Tipin complex (and Tim alone) was able to enhance the synthetic activity of DNA polymerase ϵ in vitro in the absence of other replication factors (such as replication protein A, replication factor C, and proliferating cell nuclear antigen). Therefore, the observed stimulation appears to derive from a direct action of Tim-Tipin (or Tim alone) on DNA polymerase ϵ without the intervention of additional replication factors. In fact, we demonstrated that both Tim and Tipin directly interacted with DNA polymerase ϵ by surface plasmon resonance measurements and by co-immunoprecipitation experiments. Furthermore, the results of EMSAs suggested that the Tim-Tipin complex (and Tim alone, even though to a lesser extent) associated with DNA polymerase ϵ bound to DNA, as revealed by the presence of a supershifted DNA band when Tim-Tipin (or Tim alone) was added to the mixtures (Fig. 9). In addition, we believe it is unlikely that Tim-Tipin and Tim alone stimulated DNA polymerase ϵ in an unspecific way, simply by covering inhibitory sites on the single-stranded DNA template. In fact, we found that both Tim-Tipin and Tim alone bound the 40-/80-mer primer/template with very low affinity in EMSAs (Fig. 9),3 and, moreover, the stimulatory effect was exerted specifically on DNA polymerase ϵ, but not on the two other replicative DNA polymerases.
The results of this work suggest that the stimulation of human DNA polymerase ϵ by Tim-Tipin (or Tim alone) does not derive from an increase of the enzyme processivity, and we can postulate that it could be due to improved primer/template utilization whose molecular bases require a more thorough investigation to be clarified. However, it is worth noting that this effect was specifically observed for DNA polymerase ϵ, the enzyme acting on the leading strand, whereas the synthetic activity of the lagging strand DNA polymerases (DNA polymerase α and δ) was not affected in the presence of Tim-Tipin (or either Tim or Tipin alone). It should be mentioned that the yeast Mrc1 factor was found to directly interact with the catalytic subunit of DNA polymerase ϵ (26) and to stably associate with Tof1/Csm3 (and Swi1/Swi3) (5, 6). Furthermore, human Claspin was found to stably associate with the replisome and to interact with Tim and DNA polymerase ϵ (28, 39). On the other hand, in the Xenopus egg extracts system and in mammalian cells, it was demonstrated that Tipin and the replication factor And1 (Ctf4 in yeast) interact with each other and independently bind the p180 catalytic subunit of DNA polymerase α to facilitate its loading and stable association with chromatin (22, 40). Our unpublished results3 indicated that the Tim-Tipin complex directly interacted with recombinant purified Mcm2–7 complex and Cdc45, which are components of the CMG complex, the replicative DNA helicase (27). The yeast and Xenopus counterparts of these factors together with Mrc1/Claspin were found to associate with the advancing replisome and to interact in vivo with the CMG complex (14, 20, 26).
Overall, biochemical analyses carried out in yeasts and metazoans suggest the existence of an evolutionarily conserved mechanism to ensure replication fork stability and coordination of DNA unwinding and synthesis. This mechanism is based on the interaction of Mrc1/Claspin and Tof1/Swi1/Tim with DNA polymerase ϵ on the leading strand and of Ctf4/Mcl1/And1 and Csm3/Swi3/Tipin with DNA polymerase α on the lagging strand (3). It would be interesting to analyze whether Claspin in collaboration with Tim-Tipin affects the synthetic activity of the replicative DNA polymerases and/or the unwinding function of the CMG complex. In fact, Claspin is an additional component of the multimolecular assembly responsible for the safeguard of the replisome and coordination of its enzymatic functions.
Acknowledgments
We thank Jerard Hurwitz (Memorial Sloan-Kettering Cancer Center, New York, NY) for the baculoviruses expressing the human DNA polymerase ϵ in insect cells and Anthony Gotter (Merck Research Laboratories, Boston, MA) for the anti-Tipin and anti-Tim antibodies used in the initial phases of this work.
This work was supported by Associazione Italiana per la Ricerca sul Cancro Grant IG 9087 (to F. M. P.), European Molecular Biology Organization Short Term Fellowship ASTF 239.00-2009 (to V. A.), Academy of Finland Grants 123082 and 251576 (to J. E. S.), Swiss National Science Foundation and University of Zürich grants (to B. v. L. and U. H.), and Ministry of Education, Culture, Sports, Science, and Technology of Japan Grant-in-aid for Scientific Research A 14208079 and Grant-in-aid for Scientific Research on Priority Area Chromosome Cycle 17080014 (to S. U. and H. M.).
V. Aria and F. M. Pisani, unpublished results.
- CMG
- Cdc45-Mcm2–7-GINS.
REFERENCES
- 1. Melo J., Toczyski D. (2002) A unified view of the DNA damage checkpoint. Curr. Opin. Cell Biol. 14, 237–245 [DOI] [PubMed] [Google Scholar]
- 2. Branzei D., Foiani M. (2005) The DNA damage response during DNA replication. Curr. Opin. Cell Biol. 17, 568–575 [DOI] [PubMed] [Google Scholar]
- 3. Errico A., Costanzo V. (2012) Mechanisms of replication fork protection: a safeguard for genome stability. Crit. Rev. Biochem. Mol. Biol. 47, 222–235 [DOI] [PubMed] [Google Scholar]
- 4. Tercero J. A., Longhese M. P., Diffley J. F. (2003) A central role for DNA replication forks in checkpoint activation and response. Mol. Cell 11, 1323–1336 [DOI] [PubMed] [Google Scholar]
- 5. Bando M., Katou Y., Komata M., Tanaka H., Itoh T., Sutani T., Shirahige K. (2009) Csm3, Tof1, and Mrc1 form a hetero-trimeric mediator complex that associates with DNA replication forks. J. Biol. Chem. 284, 34355–34365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tanaka T., Yokoyama M., Matsumoto S., Fukatsu R., You Z., Masai H. (2010) Fission yeast Sw1-Swi3 complex facilitates DNA binding of Mrc1. J. Biol. Chem. 285, 39609–39622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noguchi E., Noguchi C., McDonald W. H., Yates J. R., 3rd, Russell P. (2004) Swi1-Swi3 are components of a replication fork protection complex in fission yeast. Mol. Cell. Biol. 24, 8342–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Katou Y., Kanoh Y., Bando M., Noguchi H., Tanaka H., Ashikari T., Sugimoto K., Shirahige K. (2003) S-phase checkpoint proteins Tof1 and Mrc1 form a stable replication-pausing complex. Nature 424, 1078–1083 [DOI] [PubMed] [Google Scholar]
- 9. Nedelcheva M. N., Roguev A., Dolapchiev L. B., Shevchenko A., Taskov H. B., Shevchenko A., Stewart A. F., Stoynov S. S. (2005) Uncoupling of unwinding from DNA synthesis implies regulation of MCM helicase by Tof1/Mrc1/Csm3 checkpoint complex. J. Mol. Biol. 347, 509–521 [DOI] [PubMed] [Google Scholar]
- 10. Tanaka K., Russell P. (2001) Mrc1 channels the DNA replication arrest signal to checkpoint kinase Cds1. Nat. Cell Biol. 3, 966–972 [DOI] [PubMed] [Google Scholar]
- 11. Foss E. J. (2001) Tof1p regulates DNA damage responses during S phase in Saccharomyces cerevisiae. Genetics 157, 567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tourrière H., Versini G., Cordón-Preciado V., Alabert C., Pasero P. (2005) Mrc1 and Tof1 promote replication fork progression and recovery independently of Rad53. Mol. Cell 19, 699–706 [DOI] [PubMed] [Google Scholar]
- 13. Calzada A., Hodgson B., Kanemaki M., Bueno A., Labib K. (2005) Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev. 19, 1905–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gambus A., Jones R. C., Sanchez-Diaz A., Kanemaki M., van Deursen F., Edmondson R. D., Labib K. (2006) GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell Biol. 8, 358–366 [DOI] [PubMed] [Google Scholar]
- 15. Hodgson B., Calzada A., Labib K. (2007) Mrc1 and Tof1 regulate DNA replication forks in different ways during normal S phase. Mol. Cell. Biol. 18, 3894–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chou D. M., Elledge S. J. (2006) Tipin and Timeless form a mutually protective complex required for genotoxic stress resistance and checkpoint function. Proc. Natl. Acad. Sci. U.S.A. 103, 18143–18147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Unsal-Kaçmaz K., Chastain P. D., Qu P. P., Minoo P., Cordeiro-Stone M., Sancar A., Kaufmann W. K. (2007) The human Tim-Tipin complex coordinates an intra-S checkpoint response to UV that slows replication fork displacement. Mol. Cell. Biol. 27, 3131–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshizawa-Sugata N., Masai H. (2007) Human Tim/Timeless-interacting protein, Tipin, is required for efficient progression of S phase and DNA replication checkpoint. J. Biol. Chem. 282, 2729–2740 [DOI] [PubMed] [Google Scholar]
- 19. Gotter A. L., Suppa C., Emanuel B. S. (2007) Mammalian TIMELESS and Tipin are evolutionarily conserved replication fork-associated factors. J. Mol. Biol. 366, 36–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Errico A., Costanzo V., Hunt T. (2007) Tipin is required for stalled replication forks to resume DNA replication after removal of aphidicolin in Xenopus egg extracts. Proc. Natl. Acad. Sci. U.S.A. 104, 14929–14934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee J., Gold D. A., Shevchenko A., Shevchenko A., Dunphy W. G. (2005) Roles of replication fork-interacting and Chk1-activating domains from Claspin in a DNA replication checkpoint response. Mol. Biol. Cell 16, 5269–5282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Errico A., Cosentino C., Rivera T., Losada A., Schwob E., Hunt T., Costanzo V. (2009) Tipin/Tim/And1 protein complex promotes Pol α chromatin binding and sister chromatid cohesion. EMBO J. 28, 3681–3692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee J., Kumagai A., Dunphy W. G. (2003) Claspin, a Chk1-regulatory protein, monitors DNA replication on chromatin independently of RPA, ATR, and Rad17. Mol. Cell 11, 329–340 [DOI] [PubMed] [Google Scholar]
- 24. Lin S. Y., Li K., Stewart G. S., Elledge S. J. (2004) Human Claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 101, 6484–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petermann E., Helleday T., Caldecott K. W. (2008) Claspin promotes normal replication fork rates in human cells. Mol. Biol. Cell 19, 2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lou H., Komata M., Katou Y., Guan Z., Reis C. C., Budd M., Shirahige K., Campbell J. L. (2008) Mrc1 and DNA polymerase ϵ function together in linking DNA replication and S phase checkpoint. Mol. Cell 32, 106–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ilves I., Petojevic T., Pesavento J. J., Botchan M. R. (2010) Activation of the MCM2–7 helicase by association with Cdc45 and GINS proteins. Mol. Cell 37, 247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Uno S., Masai H. (2011) Efficient expression and purification of human replication fork-stabilizing factor, Claspin, from mammalian cells: DNA-binding activity and novel protein interactions. Genes Cells 16, 842–856 [DOI] [PubMed] [Google Scholar]
- 29. Uno S., You Z., Masai H. (2012) Purification of replication factors using insect and mammalian cell expression systems. Methods 57, 214–221 [DOI] [PubMed] [Google Scholar]
- 30. Syväoja J., Suomensaari S., Nishida C., Goldsmith J. S., Chui G. S., Jain S., Linn S. (1990) DNA polymerases α, δ, and ϵ: three distinct enzymes from HeLa cells. Proc. Natl. Acad. Sci. U.S.A. 87, 6664–6668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Podust V. N., Chang L. S., Ott R., Dianov G. L., Fanning E. (2002) Reconstitution of human DNA polymerase δ using recombinant baculoviruses: the p12 subunit potentiates DNA polymerizing activity of the four-subunit enzyme. J. Biol. Chem. 277, 3894–3901 [DOI] [PubMed] [Google Scholar]
- 32. Weiser T., Gassmann M., Thömmes P., Ferrari E., Hafkemeyer P., Hübscher U. (1991) Biochemical and functional comparison of DNA polymerases α, δ, and ϵ from calf thymus. J. Biol. Chem. 266, 10420–10428 [PubMed] [Google Scholar]
- 33. Asturias F. J., Cheung I. K., Sabouri N., Chilkova O., Wepplo D., Johansson E. (2006) Structure of Saccharomyces cerevisiae DNA polymerase ϵ by cryo-electron microscopy. Nat. Struct. Mol. Biol. 13, 35–43 [DOI] [PubMed] [Google Scholar]
- 34. Gotter A. L., Manganaro T., Weaver D. R., Kolakowski L. F., Jr., Possidente B., Sriram S., MacLaughlin D. T., Reppert S. M. (2000) A time-less function for mouse Timeless. Nat. Neurosci. 3, 755–756 [DOI] [PubMed] [Google Scholar]
- 35. Li Z., Stuart R. O., Qiao J., Pavlova A., Bush K. T., Pohl M., Sakurai H., Nigam S. K. (2000) A role for Timeless in epithelial morphogenesis during kidney development. Proc. Natl. Acad. Sci. U.S.A. 97, 10038–10043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xiao J., Li C., Zhu N. L., Borok Z., Minoo P. (2003) Timeless in lung morphogenesis. Dev. Dyn. 228, 82–94 [DOI] [PubMed] [Google Scholar]
- 37. Chan R. C., Chan A., Jeon M., Wu T. F., Pasqualone D., Rougvie A. E., Meyer B. J. (2003) Chromosome cohesion is regulated by a Clock gene paralogue TIM-1. Nature 423, 1002–1009 [DOI] [PubMed] [Google Scholar]
- 38. Smith-Roe S. L., Patel S. S., Simpson D. A., Zhou Y. C., Rao S., Ibrahim J. G., Kaiser-Rogers K. A., Cordeiro-Stone M., Kaufmann W. K. (2011) Timeless functions independently of the Tim-Tipin complex to promote sister chromatid cohesion in normal human fibroblasts. Cell Cycle 10, 1618–1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Serçin O., Kemp M. G. (2011) Characterization of functional domains in human Claspin. Cell Cycle 10, 1599–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu W., Ukomadu C., Jha S., Senga T., Dhar S. K., Wohlschlegel J. A., Nutt L. K., Kornbluth S., Dutta A. (2007) Mcm10 and And-1/CTF4 recruit DNA polymerase α to chromatin for initiation of DNA replication. Genes Dev. 21, 2288–2299 [DOI] [PMC free article] [PubMed] [Google Scholar]