Background: Age-related macular degeneration (AMD) involves complement activation; however, initiating ligands and essential arms of the complement cascade are unknown.
Results: Phospholipid neoepitopes recognized by natural IgM antibodies triggered lectin pathway in RPE injury models.
Conclusion: A role for neoepitopes in triggering AMD pathology was identified.
Significance: Identifying macular neoepitopes and components of the complement cascade essential for pathology will aid in developing new therapeutic approaches.
Keywords: Antibodies, Complement System, Epithelium, Lipid Peroxidation, Oxidative Stress, Age-related Macular Degeneration, Choroidal Neovascularization, Lectin Complement Pathway, Natural Antibodies, Retinal Pigment Epithelium
Abstract
Uncontrolled activation of the alternative complement pathway (AP) is thought to be associated with age-related macular degeneration. Previously, we have shown that in retinal pigmented epithelial (RPE) monolayers, oxidative stress reduced complement inhibition on the cell surface, resulting in sublytic complement activation and loss of transepithelial resistance (TER), but the potential ligand and pathway involved are unknown. ARPE-19 cells were grown as monolayers on transwell plates, and sublytic complement activation was induced with H2O2 and normal human serum. TER deteriorated rapidly in H2O2-exposed monolayers upon adding normal human serum. Although the effect required AP activation, AP was not sufficient, because elimination of MASP, but not C1q, prevented TER reduction. Reconstitution experiments to unravel essential components of the lectin pathway (LP) showed that both ficolin and mannan-binding lectin can activate the LP through natural IgM antibodies (IgM-C2) that recognize phospholipid cell surface modifications on oxidatively stressed RPE cells. The same epitopes were found on human primary embryonic RPE monolayers. Likewise, mouse laser-induced choroidal neovascularization, an injury that involves LP activation, could be increased in antibody-deficient rag1−/− mice using the phospholipid-specific IgM-C2. In summary, using a combination of depletion and reconstitution strategies, we have shown that the LP is required to initiate the complement cascade following natural antibody recognition of neoepitopes, which is then further amplified by the AP. LP activation is triggered by IgM bound to phospholipids. Taken together, we have defined novel mechanisms of complement activation in oxidatively stressed RPE, linking molecular events involved in age-related macular degeneration, including the presence of natural antibodies and neoepitopes.
Introduction
Age-related macular degeneration (AMD),2 which is characterized by progressive loss of central vision resulting from damage to the photoreceptor cells in the central area of the retina, the macula, is the leading cause of vision loss in the elderly of industrialized nations (1). Although AMD occurs in two forms, neovascular (wet) and atrophic (dry), both are associated with pathological lesions at the retinal pigmented epithelium (RPE)/choroid interface in the macular region (2). Early AMD is characterized by a thickening of Bruch's membrane, which includes basal linear deposits and drusen (3). Additionally, changes in RPE morphology and pigmentation, and deterioration of its function as blood-retina barrier have been reported (4). Advanced AMD is characterized by additional subtype-specific morphological features exacerbating the early pathological damage (5). Dry AMD, or geographic atrophy, results from the loss of RPE followed by the loss of photoreceptors, whereas wet AMD is associated with choroidal neovascularization and leakage of these new vessels.
AMD is a complex disease with both genetic and environmental risk factors. The main environmental risk factor is persistent oxidative stress (6), whether that might be caused by smoking, nutritional deficits, or even light exposure. A main genetic risk factor for the disease is polymorphisms in genes for complement proteins, including complement factor H (CFH), complement factor B, complement component 2 (C2), and complement component 3 (C3) (reviewed in Ref. 7). Discovering complement genes as risk factors was consistent with prior clinical studies, which demonstrated that the complement system activation products were found locally in the eye in all stages of AMD (8). Follow-up experiments in animal models, in particular of wet AMD, further support the hypothesis that inadequate control of complement-driven inflammation may be a major factor in the disease pathogenesis of AMD (e.g. see Refs. 9–11). Although the current understanding of AMD is that chronic oxidative damage over time leads to alterations in photoreceptors, RPE/Bruch's membrane, and the choriocapillaris complex, in particular in the macula, resulting in chronic inflammation and complement activation (12), it is unclear which components of the complement cascade are involved in causing damage and what ligands or age-related changes in these tissues enable complement activation.
The complement cascade, an evolutionarily ancient and highly conserved system, is part of the innate and adaptive immune system, consisting of >40 soluble and membrane bound components (13). Its normal role is to complement the ability of antibodies and phagocytic cells to eliminate pathogens. To spot these microorganisms, pattern recognition molecules complexed to inactive serum proteases circulate in the blood. Upon ligand interaction, the protease becomes activated to initiate the complement cascade. This results in the production of anaphylatoxins to recruit phagocytic cells and of opsonins to tag material for removal, and in the generation of the membrane attack complex to rupture membranes of cells, leading to proinflammatory signaling in the target cell. Self cells are protected by either membrane-bound or soluble complement inhibitors. However, under pathological conditions, complement inhibition might be compromised, resulting in complement activation on self surfaces.
The complement system can be activated by one of three pathways, the classical, lectin, and alternative pathway, each with its unique pattern recognition molecules. The classical pathway (CP) is activated when C1q binds to its ligands, which include C-reactive protein, serum amyloid protein, or IgG and IgM molecules present as immune complexes. The lectin pathway (LP) is activated when mannan-binding lectin (MBL) or ficolin (H-ficolin, L-ficolin, or M-ficolin) binds to specific carbohydrates or acetylated molecules on foreign cells or IgM molecules bound to antigens. Finally, the alternative pathway (AP) is spontaneously continuously activated at a low level in a process called tickover as well as when C3b is generated on cell surfaces by the CP or LP and becomes a substrate for the AP. All three pathways lead to the generation of a pathway-specific C3 convertase that then triggers the common terminal pathway with its above-described biological effects.
In AMD eyes, complement components have been found to be present in drusen and basolaminar deposits. Drusen contain complement components, including CFH (8), and Bruch's membrane and the RPE have been shown to be immunopositive for C3 activation fragments and the membrane attack complex proteins (3, 9, 14–16). In addition, complement-inhibitory protein expression and localization are altered. CFH distribution shifts from the choroidal capillary walls and intercapillary pillars near Bruch's membrane to drusen, and the membrane-bound complement inhibitor CD46, which normally is present on the basal surface of the RPE, is lost altogether (17). This pattern is consistent with the hypothesis that a reduction in complement inhibition at the level of RPE/Bruch's membrane results in persistent complement activation and resulting AMD pathology (18). However, a lack of inhibition does not equate with complement activation in the CP or LP. If we acknowledge that oxidative stress is the earliest event leading to AMD, which known cell surface modification generated by oxidative stress could then be recognized by either pattern recognition molecules of the complement cascade or natural antibodies? Ligands might include, but are not restricted to, the following: (a) C-reactive protein and amyloid, which are both present in drusen (19), and C-reactive protein, which is elevated in AMD eyes at the RPE/choroid interface (20); (b) annexins, which get exposed on the cell surfaces of damaged cells and are present in drusen (19); and (c) finally, phospholipid and neutral lipid, which accumulate in the RPE/Bruch's membrane of AMD eyes (16), as do malondialdehyde (MDA) modifications (21), an end product of lipid peroxidation.
Although a wide variety of animal models for AMD exist, based on the complexity of the human disease, it is not surprising that none exists that replicates all aspects of AMD (22). The most commonly used animal model for the wet form of AMD is the laser-induced choroidal neovascularization (CNV) model. Using this model, we have shown that CNV development involves CP- and LP-initiated complement activation followed by amplification by the AP (11). Although the potential CP and LP ligands are still unknown, because CNV development is associated with oxidative stress (18), similar ligands as in human AMD might be involved.
We have developed an in vitro system to analyze complement activation in oxidatively stressed RPE cells, using either ARPE-19 (23, 24) or primary RPE cells (25) grown as stable monolayers. RPE cells grown as mature monolayers exhibit stable transepithelial resistance (26), are polarized as shown by the apical localization of the Na+K+-ATPase (23), and stain for markers of tight and adherence junctions (27). In these experiments, oxidative stress, generated by exposing cells to non-toxic levels of H2O2, was found to reduce complement inhibition and thereby sensitized the cells to transient or sublytic complement attack. Complement attack was generated by the addition of 25% complement-sufficient normal human serum and confirmed by complement component 7 (C7) depletion/reconstitution experiments (23). This transient complement activation increased both apical and basal vascular endothelial growth factor (VEGF) secretion (23) and mobilized extracellular VEGF from binding sites (25), resulting in a VEGF receptor 2-dependent reduction in barrier facility (23). Thus, the reduction in transepithelial resistance (an indirect measure of barrier facility or leakiness) is a convenient substitute measure to probe the complement pathway.
To facilitate the analysis of complement pathways involved in oxidative stress-mediated RPE cell damage and the identification of potential ligands, we used this RPE cell monolayer system in conjunction with depletion/reconstitution strategies. Based on our results, we propose that oxidative stress generates phospholipid-containing neoepitopes that are recognized by specific natural autoantibodies. These antigen-autoantibody complexes provide the cell-based triggers for the lectin pathway that is subsequently amplified by the alternative pathway to generate the final effect. The same natural antibody was found to identify epitopes in CNV lesions and amplify injury in antibody-deficient rag1−/− mice. Thus, the mechanism whereby the binding of autoantibodies to neoepitopes generated by oxidative stress initiates complement-dependent damage appears to be similar in both human and mouse RPE cells.
MATERIALS AND METHODS
Reagents
The reagents were obtained from the following sources. Pooled normal human serum (NHS) from Quidel Corp. (Santa Clara, CA) was used as a source of complement. To probe the involvement of the classical pathway, complement C2- and C4-depleted serum as well as complement proteins C2 and C4 were purchased from Complement Technology Inc. (Tyler, TX), and C1q-depleted serum was contributed by Deborah Fraser (University of California, Irvine, CA) (28). To probe the involvement of the lectin pathway, recombinant M-ficolin, H-ficolin, and MBL-associated serine protease 2 (MASP-2) (29–31), purified L-ficolin (32), and recombinant MBL (33) were used. Finally, to determine the requirement for the alternative pathway, factor B-depleted serum was purchased from Complement Technology Inc. To analyze the involvement of immunoglobulins (Igs) in triggering the lectin pathway, Ig-depleted serum was obtained from Sunnylab (Sittingbourne, UK) or collected from rag1−/− mice, and reconstitution experiments were performed by adding back antigen-specific (IgM-C2) (34) or control IgMs (F1102; raised against 4-hydroxy-3-nitrophenylacetyl hapten conjugated to keyhole limpet hemocyanin and purified in the same way as IgM-C2). Primary antibodies included a mouse monoclonal anti-MBL from Millipore (Billerica, MA), a rabbit polyclonal anti-MBL from Abcam (Cambridge, MA), a rabbit polyclonal MASP-2, a goat antibody against human C3 (Complement Technology Inc.), and monoclonal antibodies to human H-ficolin, L-ficolin, and M-ficolin from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Species-specific secondary antibodies were from Zymed Laboratories Inc. (Invitrogen).
Mice and Collection of Serum
C57BL/6J (B6) and B6 rag1−/− mice were generated from breeding pairs (Jackson Laboratory, Bar Harbor, ME). For collection of serum, mice were deeply anesthetized (ketamine/xylazine, 80/10 mg/kg). Blood was collected in BD Vacutainer tubes by cardiac puncture, and serum was collected after clot formation (2 h on ice) and centrifugation (1000–1400 relative centrifugal force, 4 °C for 10 min).
Cell Culture
ARPE-19 cells were expanded in Dulbecco's modified Eagle's medium F12 (Invitrogen) with 10% fetal bovine serum (FBS) and antibiotics as described before (23). Human fetal RPE cells were prepared and expanded in minimum essential medium (MEM; Sigma-Aldrich) with 15% FBS, following our published protocol (25). Globes were supplied by Advanced Bioscience Resources (Alameda, CA), and experiments adhered to the Declaration of Helsinki on the ethical principles for medical research involving human materials. All cell culture experiments were performed in duplicate and repeated at least three times; data represent mean ± S.D. of at least six values.
Transepithelial Resistance (TER) Measurements
ARPE-19 cells or human fetal RPE were grown as mature monolayers on 6-well transwell inserts (Corning; 0.4-μm polyester, 24-mm insert) in the presence of 5% FBS for 2–3 weeks (26). For the final 2–3 days prior to the experiments, cells were changed to serum-free media. Complement activation was induced as reported previously (23), exposing cells to 0.5 mm H2O2 in the presence of 10% NHS. Because we have shown previously that sublytic complement activation results in VEGF release, which in turn reduces barrier function (23), TER measurements are a convenient readout for the level of activity in the complement cascade. TER was determined by measuring the resistance across the monolayer with an EVOM voltohmmeter (World Precision Instruments, Sarasota, FL). The value for cell monolayers was determined by subtracting the TER for filters without cells, and the percentage was calculated using the starting value as reference.
Binding Assays
For testing the binding of ficolin, nonspecific IgM, or antigen-specific IgM (IgM-C2) binding, ARPE-19 cells were grown as monolayers in 96-well plates. Cells were changed to serum-free media 2 days before the experiments. To identify ficolin binding, normal human serum was used as a source of ficolins. Cells were incubated with serial dilutions of serum for 1 h at 37 °C, washed, and fixed in PBS containing 4% paraformaldehyde, and nonspecific binding sites were blocked with 1% bovine serum albumin (BSA) in PBS. Bound ficolins were detected with corresponding antibodies followed by alkaline phosphatase-conjugated secondary antibody and color development using the p-nitrophenyl phosphate substrate system (KPL, Gaithersburg, MD). To characterize nonspecific IgM or antigen-specific IgM (IgM-C2) binding to control and oxidatively stressed ARPE-19 cells, neoepitopes were generated by exposing cells to 0.5 mm H2O2 for 10 min prior to incubation with serial dilutions of serum (for detection of IgM binding) or C2 antibody (in PBS). Bound IgMs were detected with alkaline phosphatase-conjugated secondary antibody and color development using the p-nitrophenyl phosphate substrate system.
Depletion of MBL
MBL-depleted human serum was prepared using mannan-agarose (Sigma-Aldrich) as depleting beads according to published protocols (35). A 2.0-ml column of mannan-agarose was prepared and equilibrated with Veronal buffer (Lonza, Allendale, NJ) containing calcium chloride (3 mm) and magnesium chloride (10 mm). Normal human serum was passed through the column, and the flow-through was collected. The depletion of MBL was confirmed by ELISA and Western blot.
MBL ELISA
Microtiter (Immulon2, Dynatech Laboratories, Chantilly, VA) plates were first coated with 10 μg/ml polyclonal rabbit anti-MBL capture antibody overnight at 4 °C. The plates were then washed three times with PBS and blocked with 3% milk in PBS for 1 h at room temperature, followed by exposure to the antigen (normal human serum or MBL-depleted human serum) for 2 h at 37 °C. The plates were again washed and incubated with monoclonal antibody to MBL followed by peroxidase-conjugated secondary antibody and color development using Turbo-TMB ELISA (Pierce).
Characterization of IgM-C2 Epitopes
ELISAs to determine reactivity of the IgM-C2 were performed as described for phospholipids (34). In short, microtiter plates (Immulon 1B) were coated with 100 μl/well 50 μg/ml phosphorylcholine (PC)10-BSA (Biosearch Technologies, Novato, CA) and MDA-BSA (Cell Biolabs, San Diego, CA) in methanol. After the plates were air-dried, the wells were washed with PBS and blocked with 1% BSA. IgM was added to wells, and bound antibody was detected by alkaline phosphatase-conjugated goat anti-mouse IgM (Jackson ImmunoResearch Laboratories, West Grove, PA). Relative units of antibody were calculated by comparing OD at 405 nm for individual titrated serum with a standard curve of OD measurements established previously (34). Binding of IgM-C2 was compared against a control IgM known to cross-react with annexin IV (IgM-B4) (34).
SDS-PAGE and Western Blotting
Protein concentration was determined by the BCATM protein assay according to the manufacturer's instructions (Pierce). For each sample, 40 μg of protein was denatured, subjected to SDS-PAGE, and analyzed by immunoblotting with appropriate antibodies.
Immunofluorescence Staining
Surface exposure of phospholipid-specific epitopes was examined by immunofluorescence microscopy. ARPE-19 cells were grown on 35-mm lysine-coated glass bottom culture dishes (MatTek Corp., Ashland, MA), treated with H2O2 for 10 min, and fixed in PBS containing 4% paraformaldehyde, and nonspecific binding sites were blocked with 1% normal goat serum and 3% BSA in PBS (preabsorption buffer) for 2 h. The cells were incubated overnight at 4 °C with either rabbit anti-MDA polyclonal antibody (1:200 in PBS) followed by incubation for 1 h at room temperature with FITC-conjugated goat anti-rabbit IgG (1:200; Zymed Laboratories Inc., Invitrogen) or with IgM natural antibody (IgM-C2) followed by goat anti-mouse IgM (1:200; Zymed Laboratories Inc., Invitrogen). As a negative control, primary antibodies were omitted. Staining was also performed ex vivo on eyes with CNV lesions (see below). Eye cups were fixed in 4% paraformaldehyde, washed, preabsorbed, and incubated overnight at 4 °C with C2-IgM (1:200), followed by anti-mouse IgM (1:200) described above. Omission of primary antibody staining served as the negative control. Staining of cells and flat mounts was examined by confocal microscopy (Olympus FluoView).
In Vivo CNV Induction and Assessment
B6 rag1−/− and B6 control mice were housed in the Medical University of South Carolina animal care facility under a 12-h/12-h light/dark cycle with access to food and water ad libitum. All experiments were performed in accordance with the Association for Research in Vision and Ophthalmology and were approved by the Institutional Animal Care and Use Committee. CNV lesions (four spots in each eye surrounding the optic nerve) were generated as described previously using argon laser photocoagulation (532 nm; 100-μm spot size; 0.1-s duration; 100 milliwatts) (36). Animals (n = 6–8/treatment group) were treated on days 0, 2, and 4 with C2-IgM or control F1102-IgM (100 μg diluted in 400 μl of PBS) using intraperitoneal injections. Intraperitoneal injections have been shown to be effective for antibody delivery to CNV lesions (37). Relative CNV size was determined in flat mount preparations of RPE-choroid stained with ICAM2 (37). Staining, flat mounting, imaging, and analysis of fluorescence measurements by confocal microscopy were performed as reported previously (36). Data are expressed as mean ± S.E.
Statistics
For data consisting of multiple groups, one-way analysis of variance followed by Fisher's post hoc test (p < 0.05) was used; single comparisons were analyzed by Student's t test analysis (p < 0.05).
RESULTS
Sublytic Complement Activation as a Function of Complement Status
Activation of the complement cascade has been shown to be involved in AMD-related pathology of the RPE. Previously, we have shown that oxidative stress generated by H2O2 significantly reduces complement inhibition at the cell surface of ARPE-19 cells grown in monolayers, resulting in sublytic complement activation (i.e. requiring complement C7 protein but not leading to cell death). This transient level of complement activation results in the secretion and mobilization of VEGF (23, 25), which in turn reduces transepithelial resistance due to its effect on tight junction stability (26). Finally, both secretion of VEGF and TER reduction require the involvement of the alternative pathway (23). Here, we wished to extend these experiments by asking which pathway(s) might be involved in initiating the complement cascade in RPE cell damage by oxidative stress.
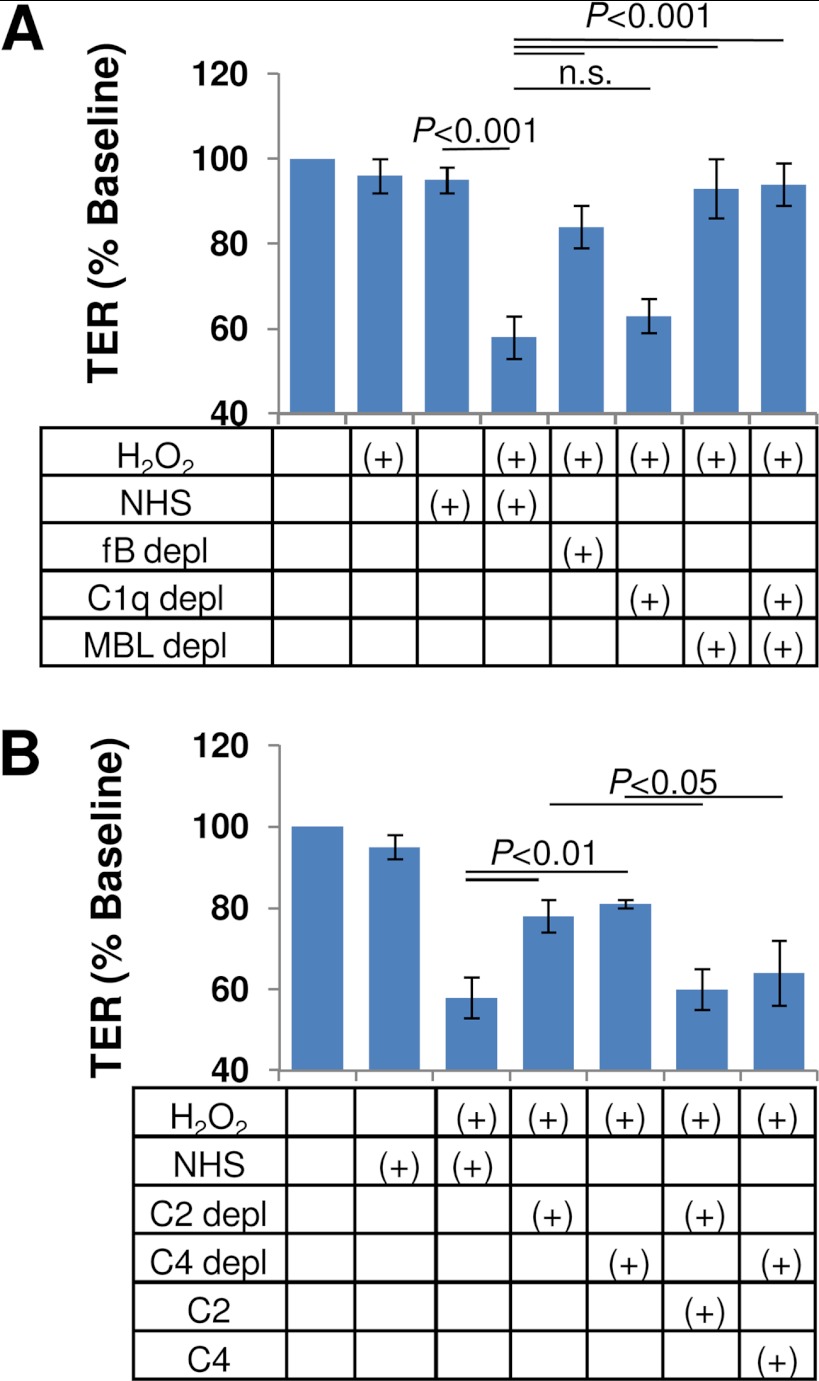
Using a combination of serum depletion strategies, we analyzed the complement activation pathways involved in TER reduction (Fig. 1A). ARPE-19 cells grown as monolayers on Transwell filters develop TER levels of 40–45 ohms cm2, a value that is not affected over the course of a 4-h exposure to 0.5 mm H2O2 or 10% NHS. However, the co-treatment with H2O2 plus NHS reduced TER by >40% (i.e. resulting in <60% base-line TER values; p < 0.001). TER reduction in control serum did not differ significantly from that elicited in the presence of C1q-depleted serum, whereas both MBL- and factor B-depleted serum were found to be ineffective in reducing TER (i.e. resulting in ∼95% base-line TER values after 4 h of exposure; not significant). Taken together, these results allow the conclusion that the lectin pathway is responsible for triggering the complement attack on oxidatively stressed RPE cells, followed by the amplification by the alternative pathway.
FIGURE 1.
Complement pathway analysis in oxidatively stressed ARPE-19 cell monolayers. A, oxidative stress was induced by treating cells with 500 μm H2O2, which sensitizes monolayers to complement attack when treating cells with 10% NHS (23). Pathway analysis was performed using serum depleted of specific complement components. Results shown are the percentage of starting value in the presence of factor B-, C1q-, MBL-, and C1q/MBL-depleted serum, revealing that complement activation on H2O2-treated cells is triggered by the lectin and amplified by the alternative pathway. B, lectin pathway activation was probed in the absence of complement factor C2 or C4 to examine a potential bypass of these components. Elimination of either C2 or C4 from NHS eliminated the effect of H2O2 plus serum on TER, indicating that both components were required for activity. Specificity of the depleted sera was confirmed by reconstituting with purified C2 and C4 protein, respectively. Error bars, S.D.
The typical activity of the lectin pathway serine protease MASP-2 is to split complement C2 and C4 into their respective a- (C2a and C4a) and b-components (C2b and C4b), resulting in the formation of the C3 convertase (C4b2a complex) (38). However, recently, bypass mechanisms have been observed; MASP-2 has been shown to activate the alternative pathway via a C2-bypass pathway (39), and Schwaeble et al. (40) have described a lectin pathway-dependent C4-bypass. Removing C2 or C4 from normal human serum attenuated the effect of H2O2 plus serum on TER (∼80% base-line TER values) (Fig. 1B) although not to the level of MBL-depleted serum (∼95% base-line TER values). The addition of physiological levels of C2 (10 μg/ml) and C4 (400 μg/ml) to their respective depleted serum reconstituted the effect of serum on TER in both C2- and C4-depleted serum (Fig. 1B). Based on the partial effect on TER reduction of the C2- and C4-depleted sera, the results suggest that although activity in the lectin pathway involves the generation of the regular C4b2a complex, a contribution by MASP-2-mediated activation of the alternative pathway cannot be excluded as has been described for MASP-1 and MASP-3 (41).
Pattern Recognition Molecules in the Lectin Pathway
Complement activation requires the binding of a ligand by a pattern recognition molecule. For the lectin pathway, those entry molecules are MBL and the ficolins (H-ficolin, L-ficolin, and M-ficolin), which then activate the MBL-associated serine protease, MASP-2. Here we employed a combination of binding assays and reconstitution experiments to determine which pattern recognition molecule could be employed to recognize ligands on oxidatively stressed RPE cells to activate the lectin pathway.
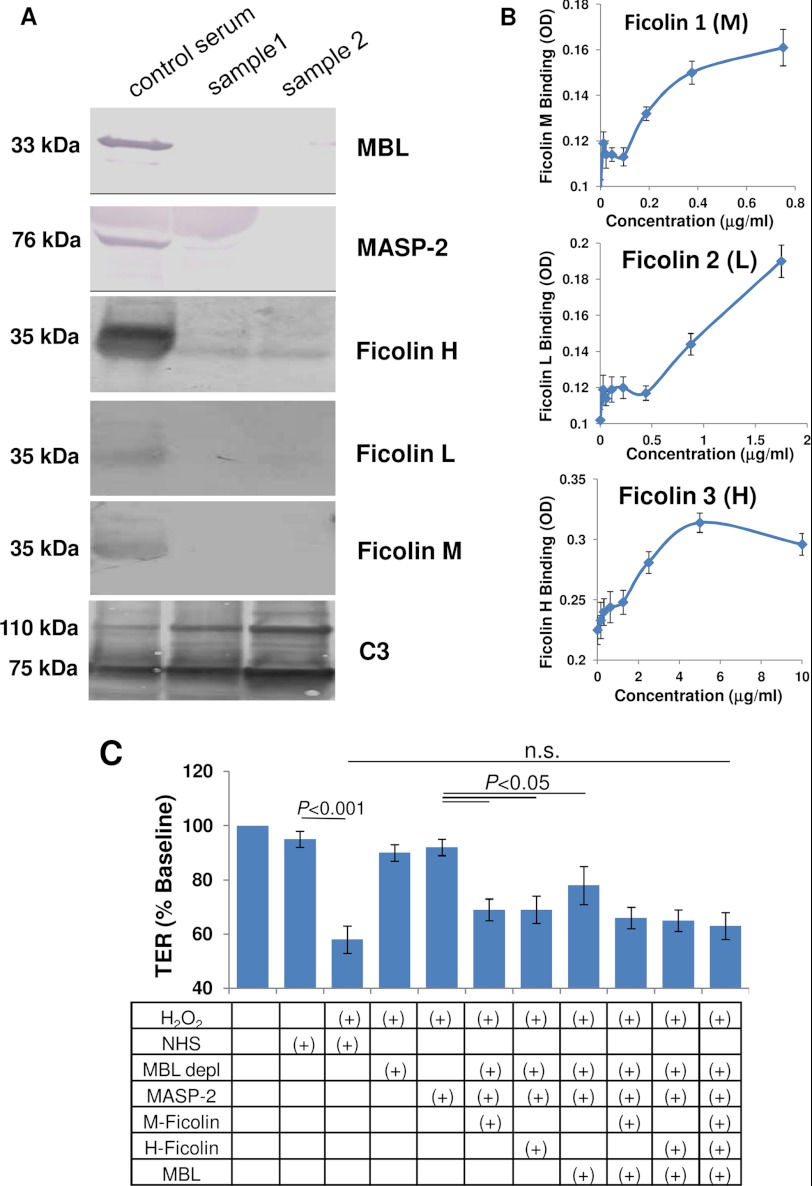
In the blood, MBL and ficolin are both complexed with inactive MASP; thus, if MBL is removed from serum using a mannan-agarose column, MASP levels might also be affected. In addition, L-ficolin has been shown to bind directly to cyanogen-activated Sepharose beads (42), a problem that might also apply to H-ficolin and M-ficolin. Western blot analysis confirmed that serum passed over a mannan-agarose column is depleted of MBL, MASP-2, M-ficolin, and L-ficolin, or levels are below the detection level, whereas H-ficolin levels were drastically reduced (Fig. 2A). As a positive control, complement C3 levels were unaffected by the depletion process (Fig. 2A). To narrow down which ficolins recognize binding sites on ARPE-19 cells, monolayers were exposed to serial dilutions of serum in the presence of 0.5 mm H2O2 (1 h at 37 °C), and bound ficolins were detected using subtype-specific antibodies (Fig. 2B). In oxidatively stressed cells, M- and H-ficolin binding was found to be saturable at physiological concentrations (mean serum concentrations of ficolins are as follows: M-ficolin, 1.1 μg/ml; L-ficolin, 3.3 μg/ml; H-ficolin, 18.4 μg/ml (31)), whereas L-ficolin binding was not saturable but did appear to bind. However, because L-ficolin has been shown to bind nonspecifically to BSA in solid-phase binding assays, a possible contribution of L-ficolin cannot be ruled out completely (43).
FIGURE 2.
Analysis of the pattern recognition receptors involved in lectin pathway activation. A, serum passed over the mannan column was analyzed for components of the lectin pathway. Western blot analysis showed depletion of MBL, MASP-2, and M-, L-, and H-ficolin in two samples (lanes 2 and 3), whereas C3 levels were unaffected. Normal human serum (lane 1) was used as a control. B, ficolin binding to the ARPE-19 cell monolayer was examined using NHS as the source in oxidatively stressed cells, followed by specific antibody binding. Specific, saturable binding could be documented for M-ficolin and H-ficolin, whereas saturable binding was not seen for L-ficolin. Concentration of ficolins was calculated based on known concentrations in human serum. C, reconstitution assays were performed to examine whether ligands on ARPE-19 cells triggering complement activation were recognized by ficolin or MBL, using TER as the readout. TER is reduced by H2O2 plus serum, but is eliminated when serum is passed over a mannan binding column (MBL depl). Reduction in TER is reconstituted by adding MASP-2 together with one of the pattern recognition receptors; adding all three was not found to be additive. n.s., not significant. Error bars, S.D.
Thus, the flow-through from the mannan-agarose column was utilized for reconstitution experiments, comparing MBL with M- and H-ficolin for their ability to reconstitute activity in the TER assay (Fig. 2C). Exposure of monolayers to H2O2 plus MBL-depleted serum resulted in ∼95% base-line TER values after 4 h of exposure, whereas H2O2 plus NHS reduced TER to <60% of base-line values. The addition of MASP-2 (50 ng/ml) in combination with any one of the three pattern recognition molecules at physiological levels increased activity in the TER assay (p < 0.05) to levels that were not significantly different from those of complete normal human serum. Adding MBL to either M- or H-ficolin or both did not further increase the activity. Thus, the lectin pathway can be activated in oxidatively stressed cells by either MBL-MASP or ficolin-MASP.
Activation of the Lectin Pathway by Natural IgM
The lectin pathway has historically been recognized as a pathway that is activated by ficolin-MASP or MBL-MASP recognizing specific carbohydrates or acetylated molecules on pathogen surfaces; however, more recently, it has been shown that IgM molecules recognizing epitopes generated during ischemia reperfusion injury can activate the lectin pathway (44). Thus, here we asked whether ficolin-MASP or MBL-MASP require immunoglobulins and, specifically, natural IgM to initiate complement activation on the cell surface of RPE cells.
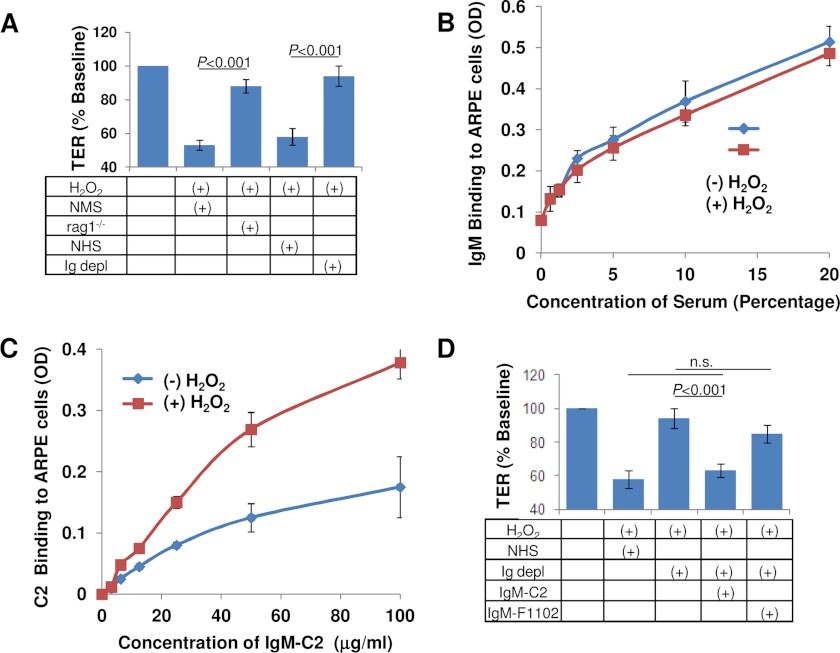
To test this hypothesis, TER reduction was tested in complement-sufficient serum (mouse or human) and compared with serum from which Igs have been eliminated either genetically (rag1−/− mice) or by depletion (human Ig-depleted serum) (Fig. 3A). Both human and mouse complement-sufficient serum reduced TER by 40–50%, whereas Ig-depleted serum was ineffective. If neoepitopes are generated on RPE cell surfaces by oxidative stress, it should be possible to document binding sites for natural IgM antibodies upon H2O2 treatment. To characterize nonspecific IgM binding to control and oxidatively stressed ARPE-19 cells, cells were exposed to serial dilutions of serum followed by detection of bound IgMs using an IgM-specific secondary antibody coupled to alkaline phosphatase. However, just like for the ficolins and MASP, binding under both conditions was indistinguishable (Fig. 3B). We have recently characterized a natural IgM (labeled IgM-C2) that recognizes epitopes in ischemic stroke injury (34). When epitope-specific IgM-C2 binding to control and oxidatively stressed ARPE-19 cells was compared, exposing cells to serial dilutions of IgM-C2 antibody followed by colorimetic detection of bound IgMs, ∼2.5-fold higher binding of IgM-C2 could be documented when comparing control and oxidative stress conditions (Fig. 3C). If IgM-C2 recognizes a physiologically relevant epitope, the addition of IgM-C2 to Ig-depleted serum should reconstitute activity in the TER assay. The addition of the IgM-C2, but not a control antibody (IgM F1102, raised against dinitrophenol), was able to reconstitute activity to levels indistinguishable from normal human serum (p = 0.53; Fig. 3D).
FIGURE 3.
Lectin pathway activation requires natural antibodies. A, TER measurements were performed using serum from which either all antibodies (NHS depleted of all Igs) or IgM and IgD (serum from rag1−/− mice) was used, indicating that antibodies are required for complement activation in this assay. B, IgM binding to ARPE-19 monolayer was examined on ARPE-19 cells cultured as monolayers in 96-well plates, using serum as a source of IgM, followed by colorimetric detection of IgM binding with anti-IgM antibodies. No difference in overall binding of IgM to ARPE-19 cells could be detected when comparing control and oxidatively stressed cells. C, when using an IgM antibody specific for oxidative stress epitopes (IgM-C2), specific binding to cells was observed, which was augmented in the presence of oxidative stress. D, reconstitution assays were performed to determine whether an IgM antibody known to recognize neoepitopes generated by oxidative stress can activate the complement cascade in Ig-depleted serum. Reduction in TER is obliterated in Ig-depleted serum. Ig-depleted human serum used in the presence of IgM natural antibody C2 was found to activate the complement cascade in this assay, whereas the control antibody F1102 was ineffective. n.s., not significant. Error bars, S.D.
Identification of Ligands for Natural IgM on RPE Cells
Our experiments thus far indicate that oxidative stress generates neoepitopes on RPE cells that are recognized by a natural antibody. The IgM-C2 antibody identified here has previously been characterized to recognize a subset of phospholipids that included PC, phosphatidylethanolamine, and cardiolipin (34). However, our cell binding assays have shown that IgM-C2 binding and injury are augmented under oxidative stress conditions (Fig. 3C). Oxidative damage of membrane phospholipids results in the formation of MDA, an end product of lipid peroxidation. Because both phospholipids (16) and MDA modifications (21) accumulate in AMD tissues, we further characterized the natural ligand of IgM-C2 on oxidatively stressed RPE cells.
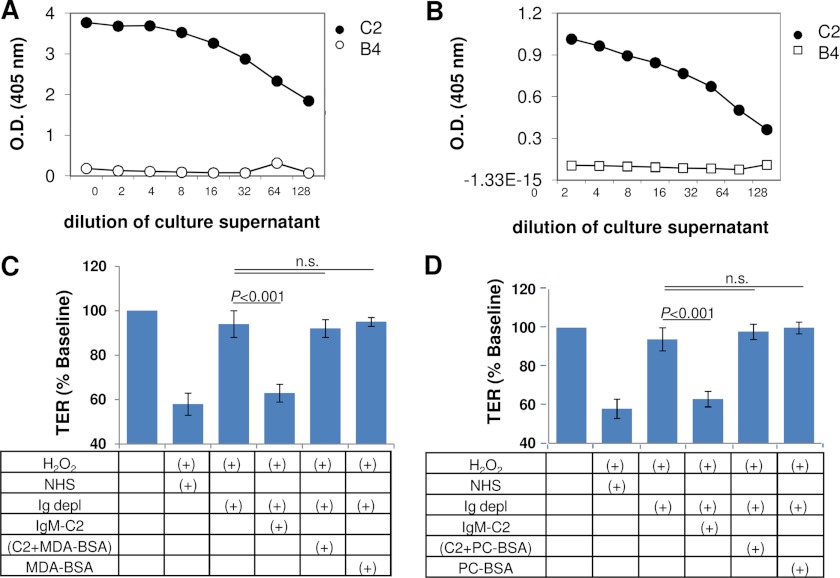
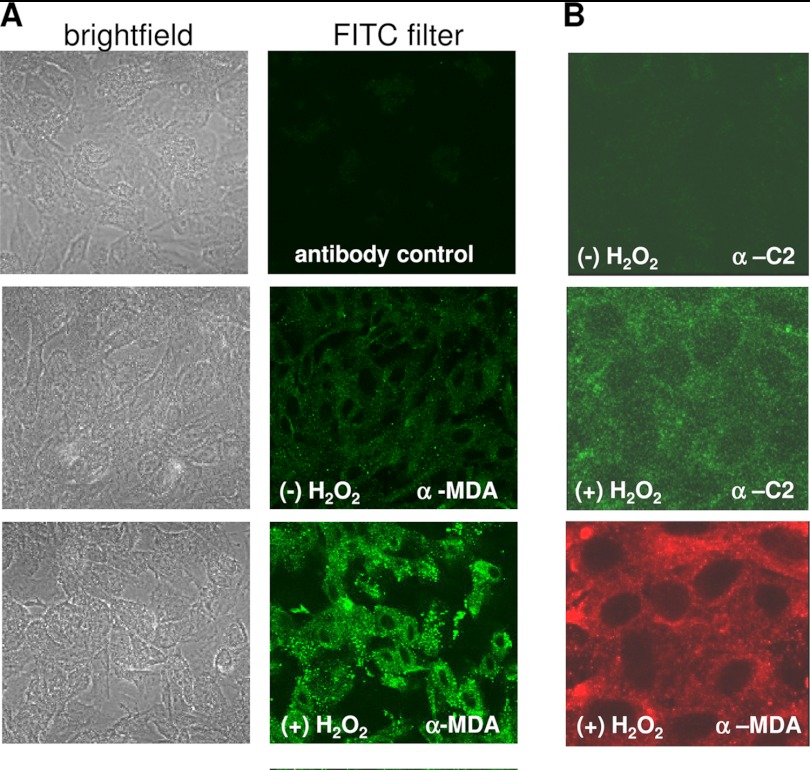
ELISAs to determine reactivity of IgM-C2 to ligands were performed using microtiter plates coated with PC-BSA or MDA-BSA in methanol. IgM-C2 recognized both phospholipid (PC) and MDA (Fig. 4, A and B). To determine which ligand is relevant for IgM-C2 binding to oxidatively stressed RPE cells, IgM-C2 was preabsorbed with either MDA-BSA or PC-BSA. IgM-C2 preabsorbed with either MDA-BSA or PC-BSA completely abolished its ability to reconstitute Ig-depleted serum (Fig. 4, C and D). MDA and C2-IgM neoepitopes could be identified in a punctate fashion on H2O2-treated cells when compared with control cells. Both the anti-MDA antibody and the IgM-C2 antibody recognize epitopes present in puncta across the apical surface of the ARPE-19 cells (Fig. 5).
FIGURE 4.
Epitope analysis of IgM-C2 natural antibody. A, ELISA analysis was performed, coating plates with BSA coupled to PC. Specific binding could be observed for IgM-C2 to this ligand as reported previously (34), whereas the control IgM specific for annexin IV (IgM-B4) showed no binding. B, because MDA is generated on lipids by oxidative stress, we examined whether specific binding of IgM-C2 to MDA-BSA could be documented. ELISAs revealed binding of IgM-C2 to MDA-BSA, albeit possibly at a lower apparent affinity when compared with PC-BSA, or the MDA-BSA wells have less capacity. No binding could be documented for the control IgM (IgM-B4). C and D, to test whether reconstitution of Ig-depleted serum using IgM-C2 antibody is mediated by MDA binding (C) or unmodified lipid binding (D), IgM-C2 was preabsorbed with either BSA-MDA or PC-BSA. BSA-MDA or PC-BSA was added to Ig-depleted serum as control. Reduction in TER can be mediated through binding of the IgM-C2 antibody to either one of the two lipid ligands. n.s., not significant. Error bars, S.D.
FIGURE 5.
MDA neoepitopes are present on oxidatively stressed ARPE-19 cells. A, immunofluorescence staining of ARPE-19 cells using α-MDA in the presence and absence of H2O2. Specific staining was revealed in oxidatively stressed cells when compared with control cells. Incubation without primary antibody was performed as a negative control. B, both the anti-MDA (red; α-MDA) and the IgM-C2 antibody (green; α-C2) recognized epitopes present in a punctate fashion on ARPE-19 cells.
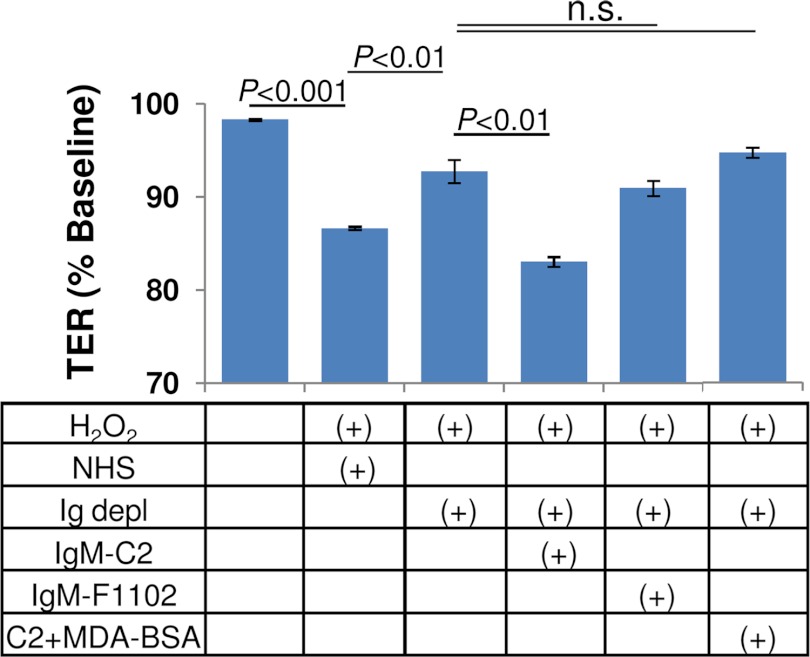
To confirm that the same neoepitopes are generated in primary human RPE cells, primary fetal RPE cells were grown into monolayers with a stable TER level of 250–300 ohms cm2. As demonstrated previously (25), these monolayers are susceptible to complement attack (Fig. 6), albeit not to the same degree as ARPE-19 cells. Note that in our previous study, TER reduction was tested using 25% NHS, which elicited a maximal response of ∼23% (25); here we wished to be consistent with the ARPE-19 studies that use 10% NHS, resulting in a smaller, but consistent and significant reduction (∼12–15%). The combined treatment of H2O2 plus 10% NHS significantly decreased TER (p < 0.001), which was attenuated by the elimination of all immunoglobulins (Ig-depleted serum; p < 0.01). IgM-C2 (p < 0.01), but not IgM-F1102 (not significant), was able to reconstitute Ig-depleted serum, an effect that could be eliminated by preabsorption with MDA-BSA.
FIGURE 6.
MDA neoepitopes are present on oxidatively stressed primary human fetal cells. Primary human fetal RPE cells were grown in monolayers, and TER was assessed in response to 500 μm H2O2 and 10% NHS. Elimination of immunoglobulin (Ig-depleted serum) significantly reduced the drop in TER. The Ig-depleted serum could be reconstituted using the IgM-C2 and not a control (IgM-F1102) antibody. Reconstitution of Ig-depleted serum using IgM-C2 antibody is in part mediated by MDA binding because preabsorbing with BSA-MDA eliminated the effect. Oxidative stress-mediated generation of phospholipid neoepitopes is a more general phenomenon for RPE cells. n.s., not significant. Error bars, S.D.
MDA has recently been shown to bind CFH. In a human acute monocytic leukemia cell line, malondialdehyde-acetaldehyde-BSA elicited a proinflammatory response as determined by IL-8 secretion, which was inhibited by physiological concentrations of CFH. If MDA represents a ligand for CFH in ARPE-19 cells, physiological concentrations of CFH might inhibit complement activation to prevent TER reduction. Because all of the experiments thus far have been executed with 10% NHS, the experiments were repeated with higher NHS concentrations as well as in the presence of exogenous CFH. Average CFH concentration in serum is ∼250 μg/ml (45); however, levels have been reported to vary by 5-fold (116–562 μg/ml) (46), and full reconstitution of factor H-depleted serum requires the addition of 500 μg of factor H/ml of serum. Hence, TER experiments were performed in the presence of 25% NHS (Fig. 7) or 25% NHS supplemented with 375 μg of CFH. Both combinations were unable to prevent TER reduction by H2O2 plus NHS. To ensure that potential modification of CFH by H2O2 does not impair its binding to its ligand on ARPE-19 cells, the supernatant containing H2O2 was removed after 5 min of stimulation, and NHS plus exogenous CFH was added, which also did not prevent TER deterioration. However, CR2-fH (at 10 μg/ml), a CFH mimetic that consists of the complement receptor 2 binding domain for C3bi and C3d coupled to the inhibitory domain of CFH, was able to inhibit TER deterioration induced by H2O2 plus NHS as reported previously (23). Thus, on ARPE-19 cells, using H2O2 as the oxidant stimulus, MDA does not appear to serve as a ligand for CFH.
FIGURE 7.
Neoepitopes generated by oxidative stress do not serve as ligands for CFH on oxidatively stressed ARPE-19 cells. MDA has been postulated to serve as a ligand on cell surfaces to recruit CFH and prevent complement-mediated damage (21). TER measurements were performed in cells treated with 500 μm H2O2 plus 25% NHS or with H2O2 plus 25% NHS supplemented with 375 μg of purified CFH, or in cells treated with H2O2, which was removed prior to the addition of HNH plus exogenous CFH, or with H2O2 plus 25% NHS supplemented with 50 μg of CR2-fH (a targeted inhibitory protein of the alternative pathway that targets the inhibitory domain of CFH to sites of C3d deposition). Only CR2-fH was able to block TER reduction induced by H2O2 plus NHS, suggesting that neoepitopes generated by oxidative stress do not recruit CFH to the cell surface for protection. n.s., not significant. Error bars, S.D.
Identification of Roles for IgM-C2 in Vivo
MDA neoepitopes have been demonstrated in mouse CNV lesions by immunohistochemistry (21), and reactive oxygen species and superoxide radicals have been visualized in both the CNV lesion and the surrounding area (18). However, the functional consequence of the generation of these neoepitopes has not yet been investigated.
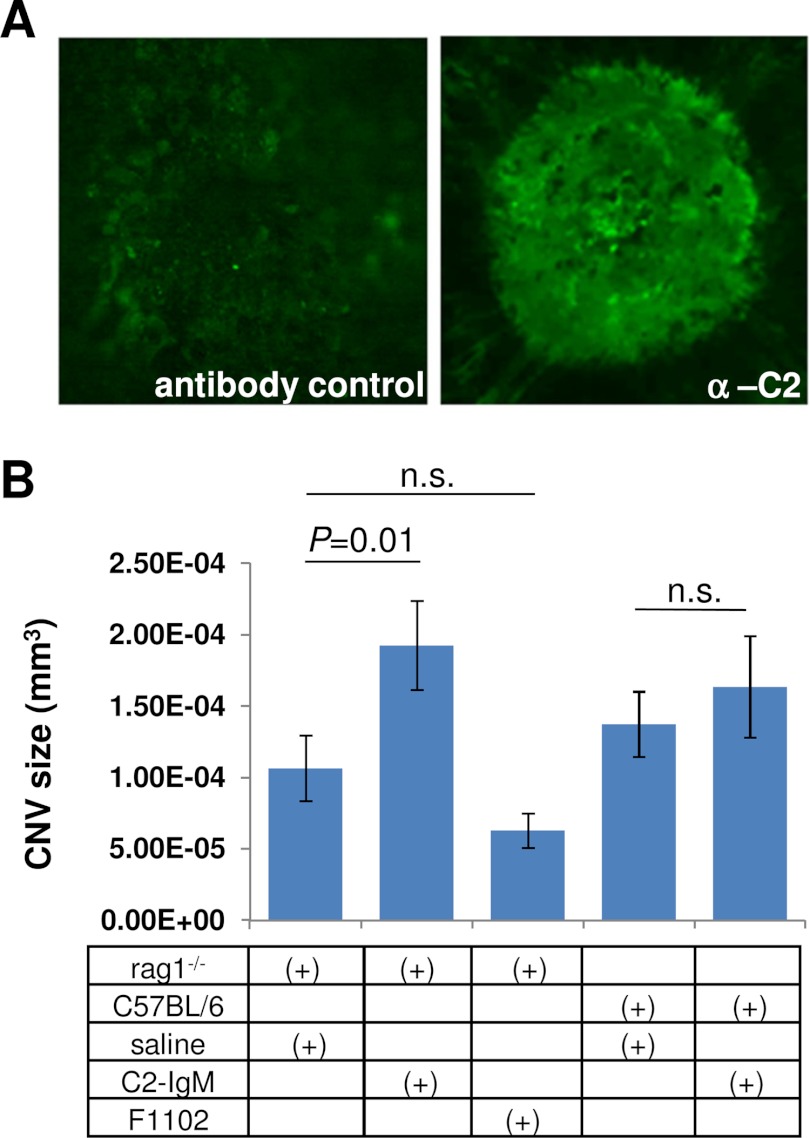
Mouse CNV laser lesions were examined by immunohistochemistry for labeling with the C2-IgM antibody. Specific labeling could be identified in CNV lesions as opposed to the area surrounding the lesion, when compared with the secondary antibody-only control (Fig. 8A). The MDA-specific antibody labeling was indistinguishable from that shown previously (21) (data not shown).
FIGURE 8.
IgM-C2 neoepitopes are generated in CNV lesions and serve to augment CNV growth in antibody-deficient mice reconstituted with C2-IgM. A, immunofluorescence staining of CNV lesions using the IgM-C2 antibody. Specific staining was revealed when compared with controls in which the primary antibody was omitted. B, antibody-deficient rag1−/− mice were reconstituted with three injections of IgM-C2 or control IgM (F1102) during the course of CNV development. IgM-C2 injections resulted in a significant increase in lesion size when compared with the control antibody. CNV lesion size in wild type mice was unaffected. These data are expressed as mean ± S.E. as described under “Materials and Methods.” n.s., not significant; error bars, S.E.
Thus, to address the physiological relevance of the C2-IgM autoantibodies in CNV lesions, we examined whether C2-IgM reconstitution experiments would alter injury in rag1−/− mice. rag1−/− mice, which produce no mature T cells or B cells and are therefore antibody-deficient, have previously been used for reconstitution experiments to test for the involvement of autoantibodies in disease (e.g. see Ref. 34). CNV lesions were examined in rag1−/− mice after three administrations every 48 h of either PBS, the F1102-IgM, or the C2-IgM (100 μg/mouse in 100 μl of PBS). Although the control IgM antibody had no effect on the size of the CNV lesion when compared with PBS-injected animals, CNV lesions were approximately twice the size after C2-IgM injections (p < 0.01). C2-IgM injections had no effect in wild type mice (Fig. 8B).
DISCUSSION
The pathogenesis of and potential therapeutic approaches for age-related macular degeneration are frequently discussed within the context of a number of concepts, including chronic oxidative stress as a central cause, chronic inflammation, mutations in genes belonging to the complement cascade, and the compromised regulation of the complement system by the RPE (12, 18). However, currently, our knowledge is limited as to how oxidative stress might lead to chronic complement activation. The principal findings obtained here, studying complement activation in oxidatively stressed RPE monolayers, can be summarized as follows: 1) oxidative stress generates neoepitopes on RPE cell surfaces that contain phospholipids, including MDA; 2) specific autoantibodies present in normal serum recognize these surface epitopes and consequently trigger the activation of the complement cascade using the lectin pathway; 3) both MBL and ficolin can serve as the pattern recognition receptors for the lectin pathway; 4) this basal activity generated by the lectin pathway is subsequently amplified by the alternative pathway to generate the maximal effect; and finally, 5) the C2-IgM antibody recognizes neoepitopes in mouse CNV lesions and augments CNV development in antibody-deficient rag1−/− mice.
Oxidative stress within a biological system is defined as a condition of imbalance between the production and detoxification of reactive oxygen species, with the toxic effects being generated by the production of oxidizing species, such as peroxides or free radicals, which can damage all essential cellular components, including proteins, lipids, and DNA. The RPE has developed an efficient array of antioxidant and repair systems to combat oxidative stress. However, as oxidative damage increases, either due to aging or due to genetic and epigenetic alterations, and exceeds antioxidant capacity, damage starts to accumulate, and repair systems become impaired. In the RPE, reactive oxygen species can be generated in a number of ways, including the oxidative phosphorylation reaction of the mitochondria, by enzymes capable of producing superoxides, as well as by environmental factors (e.g. smoking, sunlight exposure, diet, etc.) (18, 47). Alterations to the cell surface that occur in response to oxidative stress include the following. 1) Oxidation of membrane proteins and peroxidation of cell membrane lipids may occur. 2) Proteins, such as the annexins, normally present on the inner leaflet of the membrane might flip to the outer leaflet. 3) Oxidative stress can increase exocytosis of damaged intracellular proteins, transiently generating novel epitopes on the cell membrane (e.g. see Refs. 34 and 48). All of these modifications generate neoepitopes that mark the cell as altered. In AMD tissues, the presence of annexins (19, 49) as well as the accumulation of phospholipids (16) and MDA modifications (21) have been documented.
Autoantibodies are antibodies that recognize one or more of the individual's own proteins and are typically produced by B-2 lymphocytes in a T cell-dependent manner. In contrast, natural antibodies are mainly produced by B-1 lymphocytes in mice and humans and are continuously made throughout life (50). Autoantibodies can be of the IgG or IgM type. Indeed, IgG autoantibodies against retinal proteins have been identified in AMD patients (e.g. see Refs. 51 and 52), as have IgG autoantibodies against carboxyethlpyrrole protein adducts (53) and IgG autoantibodies against CFH (54). The observation that mice immunized with carboxyethlpyrrole-modified bovine serum albumin develop AMD-like pathology (55) has led to the suggestion that AMD might have an autoimmune component; however, the relative lack of immunoglobulins in drusen (19) might call into question that hypothesis. No data are yet available on natural antibodies (IgM) and AMD. However, natural IgM antibodies recognizing neoepitopes have been reported to contribute to complement-mediated injury in ischemia reperfusion injury, such as intestinal (56, 57), myocardial (58), and skeletal muscle (59) ischemia reperfusion injury as well as stroke (34). The relevance of IgM natural antibodies has been demonstrated by utilizing rag1−/− mice. These mice, which are devoid of B and T cells and hence cannot produce IgG and IgM molecules, are protected against injury. Target organ damage can be reinstated by providing either the IgM pool isolated from wild type mice or specific IgM subpopulations recognizing certain neoepitopes, as we have done here with IgM-C2 antibodies in CNV lesions (Fig. 8). Likewise, injections of these identified neoepitopes can reduce pathology in wild type mice (34).
Here, oxidative stress was generated by exposing RPE monolayers to non-toxic levels of H2O2. We have shown previously that this treatment results in an increase in intracellular reactive oxygen species but not superoxide levels (25). In addition, it diminishes the effectiveness of complement inhibition at the level of the cell membrane by reducing the surface level of complement inhibitors as well as reducing the effectiveness of alternative pathway inhibition by factor H (23). Together, an environment is created that allows complement activation on the cell surface, resulting in a complement C7-dependent release of VEGF and concomitant reduction of tight junction function measurable as a loss of transepithelial resistance. Here we use TER as a convenient, rapid, and sensitive readout of complement activation. The availability of complement- and Ig-depleted serum as well as purified protein and individual IgMs allowed us to dissect the activation pathway for the terminal complement cascade on these oxidatively stressed RPE cells. Importantly for our study, because the three pathways require unique entry or activator molecules, pathway-specific serum can be generated (C1q-depeted serum no CP; MASP-2-depleted serum no LP, factor B-depleted serum no AP, and C1q-MASP-2-double-depleted serum AP-only) (Fig. 1). The CP was found not be involved in inducing injury, because eliminating C1q from the serum had no effect on TER. However, both the LP and the AP are necessary for generating the loss of TER. Because the AP activation was determined to be necessary but is not alone sufficient for injury, we conclude that the AP only plays a primary role in amplifying, but not in initiating, the complement cascade on oxidatively stressed RPE cells. Because the serum depleted for LP pathway components was found to be depleted for MASP-2, MBL, and all three ficolins (Fig. 2A), reconstitution studies could be performed with the individual pattern recognition molecules. Roles for MBL, M-ficolin, and H-ficolin could be demonstrated utilizing the TER assay. Because specific binding of M- and H-ficolin to RPE cells could be demonstrated in the context of complete serum, this suggested that LP activation in our model requires the presence of a specific antibody-antigen complex for surface activation. Elvington et al. (34) have demonstrated that oxidative stress-specific epitopes are generated and recognized by a natural antibody that recognizes specific phospholipids to trigger the complement cascade in stroke. Although Ig dependence was demonstrated utilizing Ig-depleted serum (Fig. 3A), H2O2-dependent changes in total IgM (Fig. 3B) or total IgG (data not shown) could not be demonstrated. However, H2O2-dependent changes in binding of a phospholipid antigen-specific IgM (IgM-C2) were revealed (Fig. 3C), and in reconstitution assays, IgM-C2 antibodies, but not control antibody F1102, resulted in the restoration of the effect on TER in Ig-depleted serum (Fig. 3D). Control antibody F1102, when tested in binding assays, also showed no saturable binding to RPE cells (data not shown). Finally, antigen specificity for IgM-C2 was further refined by ELISA (Fig. 4A) and TER experiments (Fig. 4, B and C). Because the original characterization of the ligands (34) was performed in the absence of H2O2, but phospholipids can undergo peroxidation, binding was compared between PC (non-oxidized) and MDA (oxidized) coupled to BSA. Specific binding could be documented by ELISA, and preabsorption of the antibody with either MDA-BSA or PC-BSA interfered with activity in the TER assay. H2O2-dependent binding could be documented for both an MDA-specific antibody and the neoepitope-specific IgM on RPE cell surfaces (Fig. 5). The neoepitopes recognizable by IgM-C2 are not only present in ARPE-19 cells, but importantly they are also present on oxidatively stressed primary human fetal RPE cells (Fig. 6) as well as in mouse CNV lesions (Fig. 8A). Finally, in vivo data demonstrated that CNV lesions could be augmented in rag1−/− mice by providing the IgM-C2 subpopulation, whereas in wild type mice, which have those antibodies present, CNV lesion size is unaffected (Fig. 8). It will be of great interest to determine whether injections of the IgM-C2 neoepitopes can reduce pathology in wild type mice, as has been shown for annexin IV in ischemic stroke (34), as well as in other animal models of AMD that present with complement activation (e.g. see Refs. 60–62; reviewed in Ref. 63).
From our current observations together with our previous data, we infer that alterations in barrier function produced by oxidative stress-mediated complement activation require a phospholipid as a ligand, LP initiation molecules, and alternative pathway amplification, followed by activation of the terminal pathway, including transient membrane attack complex activation. This is the first report that identifies a potential ligand, the pattern recognition receptor, and the pathway required for activation in a model relevant for AMD. In an elegant study, Johnson et al. (64) identified a CP-dependent pathway utilizing primary embryonic human RPE cells grown in long term cultures on transwell plates. Their readout involved complement activation associated with basal apolipoprotein E deposition. Surprisingly, the alternative pathway might not play a role in this process, making this the first model in which no amplification by the AP could be documented (65). Malondialdehyde has been examined previously in the context of AMD. Blood levels of MDA are elevated in AMD (66); lipid peroxidation-derived protein modifications, including MDA, impair degradation of photoreceptor outer segments (67), result in lysosomal dysfunction (68), and induce VEGF expression (69). On the other hand, Weismann et al. (21) have suggested that MDA present on RPE/Bruch's membrane might provide a recognition site for CFH, protecting the surfaces from oxidative stress. In biochemical assays, binding of CFH to MDA epitopes was found to stimulate its co-factor activity for factor I, promoting the degradation of C3b into iC3b. In a human acute monocytic leukemia cell line, malondialdehyde-acetaldehyde-modified BSA elicited IL-8 secretion, which could be inhibited by CFH, and in ARPE-19 cells, malondialdehyde-acetaldehyde-modified BSA also resulted in IL-8 secretion, but unfortunately the inhibitory effects of CFH were not examined. This is in contrast to our combined findings, showing that despite the presence of MDA on cell surfaces (see Fig. 5), CFH present in serum or serum supplemented with exogenous CFH did not prevent complement activation at the cell surface in ARPE-19 cell monolayers (Fig. 7), whereas a synthetic form of CFH that relies on the CR2 receptor epitope for binding to the cells, was found to be effective (Fig. 7) (23). On the other hand, co-localization of MDA and CFH in tissues was reported, in particular in pathological structures, such as drusen, and CFH was found to bind cellular debris and membrane blebs (21). Thus, the protective role of CFH might be in preventing the spread of inflammation at the cost of sequestering CFH together with the pathological material into drusen. This mechanism might provide an explanation for the shift of CFH localization observed in AMD (17).
These findings link oxidative stress, phospholipids, possibly including MDA, natural antibodies, and the alternative pathway of complement activation, which have all been associated with the pathogenesis of AMD and further link the aseptic insults generated by oxidative stress in the eye with a pathogenic immune response. Elucidation of this potential complement activation pathway, elucidating molecular events that convert oxidative stress of the RPE layer into an inflammatory response, will open up the field for the development of potential therapies targeted directly at these processes. In this context, it is of interest to note that blocking MASP-2 function using a blocking antibody has been found to significantly reduce tissue damage caused by ischemia-reperfusion injury (40), and the same antibody has been used to reduce choroidal neovascularization in the mouse model of wet CNV (preclinical MASP-2 program at Omeros Corp., Seattle, WA). Identification of neoepitopes, such as MDA, might provide novel mechanisms to target complement inhibitors or other compounds to sites of inflammation. Our experiments utilizing the rag1−/− mice for reconstitution experiments further demonstrated that neoepitopes generated by oxidative stress do exist in the CNV lesions and participate in amplifying damage, presumably through activating the complement system. In particular, if macula-specific epitopes or epitopes associated with different forms of the disease can be identified, such an approach would offer the possibility of ameliorating this progressive disease in a manner precisely tailored to the disease state while minimizing the side effects of chronic therapy.
This work was supported, in whole or in part, by National Institutes of Health Grant R01EY019320. This work was also supported by Department of Veterans Affairs Grant I01 RX000444, Foundation Fighting Blindness, and an unrestricted grant to the Medical University of South Carolina from Research to Prevent Blindness, Inc. (New York). Animal studies were conducted in a facility constructed with support from National Institutes of Health Grant C06 RR015455.
- AMD
- age-related macular degeneration
- AP
- alternative pathway
- C2, C3, C4, and C7
- complement component 2, 3, 4, and 7, respectively
- CFH
- complement factor H
- CNV
- choroidal neovascularization
- CP
- classical pathway
- LP
- lectin pathway
- MASP-2
- MBL-associated serine protease 2
- MBL
- mannan-binding lectin
- MDA
- malondialdehyde
- NHS
- normal human serum
- PC
- phosphorylcholine
- RPE
- retinal pigment epithelium
- TER
- transepithelial resistance.
REFERENCES
- 1. National Advisory Eye Council (1999) Vision Research. A National Plan. 1999–2003. Executive Summary, NEI, National Institutes of Health, Washington, D. C [Google Scholar]
- 2. Nowak J. Z. (2006) Age-related macular degeneration (AMD). Pathogenesis and therapy. Pharmacol. Rep. 58, 353–363 [PubMed] [Google Scholar]
- 3. Hageman G. S., Luthert P. J., Victor Chong N. H., Johnson L. V., Anderson D. H., Mullins R. F. (2001) An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 20, 705–732 [DOI] [PubMed] [Google Scholar]
- 4. McLeod D. S., Taomoto M., Otsuji T., Green W. R., Sunness J. S., Lutty G. A. (2002) Quantifying changes in RPE and choroidal vasculature in eyes with age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 43, 1986–1993 [PubMed] [Google Scholar]
- 5. Bhutto I., Lutty G. (2012) Understanding age-related macular degeneration (AMD). Relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol. Aspects Med. 33, 295–317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Snodderly D. M. (1995) Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am. J. Clin. Nutr. 62, 1448S–1461S [DOI] [PubMed] [Google Scholar]
- 7. Charbel Issa P., Chong N. V., Scholl H. P. (2011) The significance of the complement system for the pathogenesis of age-related macular degeneration. Current evidence and translation into clinical application. Graefes. Arch. Clin. Exp. Ophthalmol. 249, 163–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hageman G. S., Anderson D. H., Johnson L. V., Hancox L. S., Taiber A. J., Hardisty L. I., Hageman J. L., Stockman H. A., Borchardt J. D., Gehrs K. M., Smith R. J., Silvestri G., Russell S. R., Klaver C. C., Barbazetto I., Chang S., Yannuzzi L. A., Barile G. R., Merriam J. C., Smith R. T., Olsh A. K., Bergeron J., Zernant J., Merriam J. E., Gold B., Dean M., Allikmets R. (2005) A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 102, 7227–7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nozaki M., Raisler B. J., Sakurai E., Sarma J. V., Barnum S. R., Lambris J. D., Chen Y., Zhang K., Ambati B. K., Baffi J. Z., Ambati J. (2006) Drusen complement components C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. U.S.A. 103, 2328–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bora P. S., Sohn J. H., Cruz J. M., Jha P., Nishihori H., Wang Y., Kaliappan S., Kaplan H. J., Bora N. S. (2005) Role of complement and complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 174, 491–497 [DOI] [PubMed] [Google Scholar]
- 11. Rohrer B., Coughlin B., Kunchithapautham K., Long Q., Tomlinson S., Takahashi K., Holers V. M. (2011) The alternative pathway is required, but not alone sufficient, for retinal pathology in mouse laser-induced choroidal neovascularization. Mol. Immunol. 48, e1–e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zarbin M. A., Rosenfeld P. J. (2010) Pathway-based therapies for age-related macular degeneration. An integrated survey of emerging treatment alternatives. Retina 30, 1350–1367 [DOI] [PubMed] [Google Scholar]
- 13. Müller-Eberhard H. J. (1988) Molecular organization and function of the complement system. Annu. Rev. Biochem. 57, 321–347 [DOI] [PubMed] [Google Scholar]
- 14. Johnson L. V., Leitner W. P., Staples M. K., Anderson D. H. (2001) Complement activation and inflammatory processes in drusen formation and age-related macular degeneration. Exp. Eye Res. 73, 887–896 [DOI] [PubMed] [Google Scholar]
- 15. Kijlstra A., La Heij E., Hendrikse F. (2005) Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul. Immunol. Inflamm. 13, 3–11 [DOI] [PubMed] [Google Scholar]
- 16. Lommatzsch A., Hermans P., Müller K. D., Bornfeld N., Bird A. C., Pauleikhoff D. (2008) Are low inflammatory reactions involved in exudative age-related macular degeneration? Morphological and immunhistochemical analysis of AMD associated with basal deposits. Graefes. Arch. Clin. Exp. Ophthalmol. 246, 803–810 [DOI] [PubMed] [Google Scholar]
- 17. Fett A. L., Hermann M. M., Muether P. S., Kirchhof B., Fauser S. (2012) Immunohistochemical localization of complement regulatory proteins in the human retina. Histol. Histopathol. 27, 357–364 [DOI] [PubMed] [Google Scholar]
- 18. Rohrer B., Bandyopadhyay M., Kunchithapautham K., Thurman J. M. (2012) Complement pathways and oxidative stress in models of age-related macular degeneration. in Studies on Retinal and Choroidal Disorders (Stratton R. D., Hauswirth W. W., Gardner T. W., ed) pp. 47–64, Springer, New York [Google Scholar]
- 19. Crabb J. W., Miyagi M., Gu X., Shadrach K., West K. A., Sakaguchi H., Kamei M., Hasan A., Yan L., Rayborn M. E., Salomon R. G., Hollyfield J. G. (2002) Drusen proteome analysis. An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 99, 14682–14687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bhutto I. A., Baba T., Merges C., Juriasinghani V., McLeod D. S., Lutty G. A. (2011) C-reactive protein and complement factor H in aged human eyes and eyes with age-related macular degeneration. Br. J. Ophthalmol. 95, 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weismann D., Hartvigsen K., Lauer N., Bennett K. L., Scholl H. P., Charbel Issa P., Cano M., Brandstätter H., Tsimikas S., Skerka C., Superti-Furga G., Handa J. T., Zipfel P. F., Witztum J. L., Binder C. J. (2011) Complement factor H binds malondialdehyde epitopes and protects from oxidative stress. Nature 478, 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zeiss C. J. (2010) Animals as models of age-related macular degeneration. An imperfect measure of the truth. Vet. Pathol. 47, 396–413 [DOI] [PubMed] [Google Scholar]
- 23. Thurman J. M., Renner B., Kunchithapautham K., Ferreira V. P., Pangburn M. K., Ablonczy Z., Tomlinson S., Holers V. M., Rohrer B. (2009) Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 284, 16939–16947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kunchithapautham K., Rohrer B. (2011) Sublytic membrane-attack-complex (MAC) activation alters regulated rather than constitutive vascular endothelial growth factor (VEGF) secretion in retinal pigment epithelium monolayers. J. Biol. Chem. 286, 23717–23724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bandyopadhyay M., Rohrer B. (2012) Matrix metalloproteinase activity creates pro-angiogenic environment in primary human retinal pigment epithelial cells exposed to complement. Invest. Ophthalmol. Vis. Sci. 53, 1953–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ablonczy Z., Crosson C. E. (2007) VEGF modulation of retinal pigment epithelium resistance. Exp. Eye Res. 85, 762–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bailey T. A., Kanuga N., Romero I. A., Greenwood J., Luthert P. J., Cheetham M. E. (2004) Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 45, 675–684 [DOI] [PubMed] [Google Scholar]
- 28. Fraser D. A., Laust A. K., Nelson E. L., Tenner A. J. (2009) C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J. Immunol. 183, 6175–6185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Frederiksen P. D., Thiel S., Larsen C. B., Jensenius J. C. (2005) M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand. J. Immunol. 62, 462–473 [DOI] [PubMed] [Google Scholar]
- 30. Thiel S., Kolev M., Degn S., Steffensen R., Hansen A. G., Ruseva M., Jensenius J. C. (2009) Polymorphisms in mannan-binding lectin (MBL)-associated serine protease 2 affect stability, binding to MBL, and enzymatic activity. J. Immunol. 182, 2939–2947 [DOI] [PubMed] [Google Scholar]
- 31. Zacho R. M., Jensen L., Terp R., Jensenius J. C., Thiel S. (2012) Studies of the pattern recognition molecule H-ficolin. Specificity and purification. J. Biol. Chem. 287, 8071–8081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lacroix M., Dumestre-Pérard C., Schoehn G., Houen G., Cesbron J. Y., Arlaud G. J., Thielens N. M. (2009) Residue Lys57 in the collagen-like region of human L-ficolin and its counterpart Lys47 in H-ficolin play a key role in the interaction with the mannan-binding lectin-associated serine proteases and the collectin receptor calreticulin. J. Immunol. 182, 456–465 [DOI] [PubMed] [Google Scholar]
- 33. Jensenius J. C., Jensen P. H., McGuire K., Larsen J. L., Thiel S. (2003) Recombinant mannan-binding lectin (MBL) for therapy. Biochem. Soc. Trans. 31, 763–767 [DOI] [PubMed] [Google Scholar]
- 34. Elvington A., Atkinson C., Kulik L., Zhu H., Yu J., Kindy M. S., Holers V. M., Tomlinson S. (2012) Pathogenic natural antibodies propagate cerebral injury following ischemic stroke in mice. J. Immunol. 188, 1460–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rajagopalan R., Salvi V. P., Jensenius J. C., Rawal N. (2009) New insights on the structural/functional properties of recombinant human mannan-binding lectin and its variants. Immunol. Lett. 123, 114–124 [DOI] [PubMed] [Google Scholar]
- 36. Rohrer B., Long Q., Coughlin B., Wilson R. B., Huang Y., Qiao F., Tang P. H., Kunchithapautham K., Gilkeson G. S., Tomlinson S. (2009) A targeted inhibitor of the alternative complement pathway reduces angiogenesis in a mouse model of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 50, 3056–3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Campa C., Kasman I., Ye W., Lee W. P., Fuh G., Ferrara N. (2008) Effects of an anti-VEGF-A monoclonal antibody on laser-induced choroidal neovascularization in mice. Optimizing methods to quantify vascular changes. Invest. Ophthalmol. Vis. Sci. 49, 1178–1183 [DOI] [PubMed] [Google Scholar]
- 38. Takahashi M., Mori S., Shigeta S., Fujita T. (2007) Role of MBL-associated serine protease (MASP) on activation of the lectin complement pathway. Adv. Exp. Med. Biol. 598, 93–104 [DOI] [PubMed] [Google Scholar]
- 39. Tateishi K., Matsushita M. (2011) Activation of the alternative complement pathway by mannose-binding lectin via a C2-bypass pathway. Microbiol. Immunol. 55, 817–821 [DOI] [PubMed] [Google Scholar]
- 40. Schwaeble W. J., Lynch N. J., Clark J. E., Marber M., Samani N. J., Ali Y. M., Dudler T., Parent B., Lhotta K., Wallis R., Farrar C. A., Sacks S., Lee H., Zhang M., Iwaki D., Takahashi M., Fujita T., Tedford C. E., Stover C. M. (2011) Targeting of mannan-binding lectin-associated serine protease-2 confers protection from myocardial and gastrointestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 108, 7523–7528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Banda N. K., Takahashi M., Takahashi K., Stahl G. L., Hyatt S., Glogowska M., Wiles T. A., Endo Y., Fujita T., Holers V. M., Arend W. P. (2011) Mechanisms of mannose-binding lectin-associated serine proteases-1/3 activation of the alternative pathway of complement. Mol. Immunol. 49, 281–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tan S. M., Chung M. C., Kon O. L., Thiel S., Lee S. H., Lu J. (1996) Improvements on the purification of mannan-binding lectin and demonstration of its Ca2+-independent association with a C1s-like serine protease. Biochem. J. 319, 329–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Faro J., Chen Y., Jhaveri P., Oza P., Spear G. T., Lint T. F., Gewurz H. (2008) L-ficolin binding and lectin pathway activation by acetylated low-density lipoprotein. Clin. Exp. Immunol. 151, 275–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhang M., Takahashi K., Alicot E. M., Vorup-Jensen T., Kessler B., Thiel S., Jensenius J. C., Ezekowitz R. A., Moore F. D., Carroll M. C. (2006) Activation of the lectin pathway by natural IgM in a model of ischemia/reperfusion injury. J. Immunol. 177, 4727–4734 [DOI] [PubMed] [Google Scholar]
- 45. Hakobyan S., Harris C. L., Tortajada A., Goicochea de Jorge E., García-Layana A., Fernández-Robredo P., Rodríguez de Córdoba S., Morgan B. P. (2008) Measurement of factor H variants in plasma using variant-specific monoclonal antibodies. Application to assessing risk of age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 49, 1983–1990 [DOI] [PubMed] [Google Scholar]
- 46. Esparza-Gordillo J., Soria J. M., Buil A., Almasy L., Blangero J., Fontcuberta J., Rodríguez de Córdoba S. (2004) Genetic and environmental factors influencing the human factor H plasma levels. Immunogenetics 56, 77–82 [DOI] [PubMed] [Google Scholar]
- 47. Jarrett S. G., Boulton M. E. (2012) Consequences of oxidative stress in age-related macular degeneration. Mol. Aspects Med. 33, 399–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gounopoulos P., Merki E., Hansen L. F., Choi S. H., Tsimikas S. (2007) Antibodies to oxidized low density lipoprotein. Epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 55, 821–837 [PubMed] [Google Scholar]
- 49. Rayborn M. E., Sakaguchi H., Shadrach K. G., Crabb J. W., Hollyfield J. G. (2006) Annexins in Bruch's membrane and drusen. Adv. Exp. Med. Biol. 572, 75–78 [PubMed] [Google Scholar]
- 50. Fleming S. D., Shea-Donohue T., Guthridge J. M., Kulik L., Waldschmidt T. J., Gipson M. G., Tsokos G. C., Holers V. M. (2002) Mice deficient in complement receptors 1 and 2 lack a tissue injury-inducing subset of the natural antibody repertoire. J. Immunol. 169, 2126–2133 [DOI] [PubMed] [Google Scholar]
- 51. Morohoshi K., Goodwin A. M., Ohbayashi M., Ono S. J. (2009) Autoimmunity in retinal degeneration. Autoimmune retinopathy and age-related macular degeneration. J. Autoimmun. 33, 247–254 [DOI] [PubMed] [Google Scholar]
- 52. Morohoshi K., Ohbayashi M., Patel N., Chong V., Bird A. C., Ono S. J. (2012) Identification of anti-retinal antibodies in patients with age-related macular degeneration. Exp. Mol. Pathol. 93, 193–199 [DOI] [PubMed] [Google Scholar]
- 53. Gu X., Meer S. G., Miyagi M., Rayborn M. E., Hollyfield J. G., Crabb J. W., Salomon R. G. (2003) Carboxyethylpyrrole protein adducts and autoantibodies, biomarkers for age-related macular degeneration. J. Biol. Chem. 278, 42027–42035 [DOI] [PubMed] [Google Scholar]
- 54. Dhillon B., Wright A. F., Tufail A., Pappworth I., Hayward C., Moore I., Strain L., Kavanagh D., Barlow P. N., Herbert A. P., Schmidt C. Q., Armbrecht A. M., Laude A., Deary I. J., Staniforth S. J., Holmes L. V., Goodship T. H., Marchbank K. J. (2010) Complement factor h autoantibodies and age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 51, 5858–5863 [DOI] [PubMed] [Google Scholar]
- 55. Hollyfield J. G., Bonilha V. L., Rayborn M. E., Yang X., Shadrach K. G., Lu L., Ufret R. L., Salomon R. G., Perez V. L. (2008) Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat. Med. 14, 194–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang M., Austen W. G., Jr., Chiu I., Alicot E. M., Hung R., Ma M., Verna N., Xu M., Hechtman H. B., Moore F. D., Jr., Carroll M. C. (2004) Identification of a specific self-reactive IgM antibody that initiates intestinal ischemia/reperfusion injury. Proc. Natl. Acad. Sci. U.S.A. 101, 3886–3891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kulik L., Fleming S. D., Moratz C., Reuter J. W., Novikov A., Chen K., Andrews K. A., Markaryan A., Quigg R. J., Silverman G. J., Tsokos G. C., Holers V. M. (2009) Pathogenic natural antibodies recognizing annexin IV are required to develop intestinal ischemia-reperfusion injury. J. Immunol. 182, 5363–5373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Haas M. S., Alicot E. M., Schuerpf F., Chiu I., Li J., Moore F. D., Carroll M. C. (2010) Blockade of self-reactive IgM significantly reduces injury in a murine model of acute myocardial infarction. Cardiovasc. Res. 87, 618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weiser M. R., Williams J. P., Moore F. D., Jr., Kobzik L., Ma M., Hechtman H. B., Carroll M. C. (1996) Reperfusion injury of ischemic skeletal muscle is mediated by natural antibody and complement. J. Exp. Med. 183, 2343–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rutar M., Natoli R., Provis J., Valter K. (2012) Complement activation in retinal degeneration. Adv. Exp. Med. Biol. 723, 31–36 [DOI] [PubMed] [Google Scholar]
- 61. Ding J. D., Johnson L. V., Herrmann R., Farsiu S., Smith S. G., Groelle M., Mace B. E., Sullivan P., Jamison J. A., Kelly U., Harrabi O., Bollini S. S., Dilley J., Kobayashi D., Kuang B., Li W., Pons J., Lin J. C., Bowes Rickman C. (2011) Anti-amyloid therapy protects against retinal pigmented epithelium damage and vision loss in a model of age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 108, E279–E287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang A. L., Lukas T. J., Yuan M., Du N., Handa J. T., Neufeld A. H. (2009) Changes in retinal pigment epithelium related to cigarette smoke. Possible relevance to smoking as a risk factor for age-related macular degeneration. PLoS ONE 4, e5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Edwards A. O., Malek G. (2007) Molecular genetics of AMD and current animal models. Angiogenesis 10, 119–132 [DOI] [PubMed] [Google Scholar]
- 64. Johnson L. V., Forest D. L., Banna C. D., Radeke C. M., Maloney M. A., Hu J., Spencer C. N., Walker A. M., Tsie M. S., Bok D., Radeke M. J., Anderson D. H. (2011) Cell culture model that mimics drusen formation and triggers complement activation associated with age-related macular degeneration. Proc. Natl. Acad. Sci. U.S.A. 108, 18277–18282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Holers V. M. (2008) The spectrum of complement alternative pathway-mediated diseases. Immunol. Rev. 223, 300–316 [DOI] [PubMed] [Google Scholar]
- 66. Nowak M., Swietochowska E., Wielkoszyński T., Marek B., Karpe J., Górski J., Głogowska-Szelag J., Kos-Kudła B., Ostrowska Z. (2003) Changes in blood antioxidants and several lipid peroxidation products in women with age-related macular degeneration. Eur. J. Ophthalmol. 13, 281–286 [DOI] [PubMed] [Google Scholar]
- 67. Kaemmerer E., Schutt F., Krohne T. U., Holz F. G., Kopitz J. (2007) Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest. Ophthalmol. Vis. Sci. 48, 1342–1347 [DOI] [PubMed] [Google Scholar]
- 68. Krohne T. U., Kaemmerer E., Holz F. G., Kopitz J. (2010) Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp. Eye Res. 90, 261–266 [DOI] [PubMed] [Google Scholar]
- 69. Bergmann M., Holz F., Kopitz J. (2011) Lysosomal stress and lipid peroxidation products induce VEGF-121 and VEGF-165 expression in ARPE-19 cells. Graefes. Arch. Clin. Exp. Ophthalmol. 249, 1477–1483 [DOI] [PubMed] [Google Scholar]