Background: How endothelial cells (ECs) could acquire exogenous neutrophil myeloperoxidase (MPO) is unknown.
Results: ECs acquired enzymatically active MPO directly from neutrophils, via β2 integrin-mediated cell-cell contact independent of extracellular MPO release.
Conclusion: Neutrophils directly transfer MPO to ECs by cell-cell contact.
Significance: Direct delivery of MPO to ECs may contribute to the vascular pathology and dysfunction seen in atherosclerosis and vasculitides.
Keywords: Cell-Cell Interaction, Endothelium, Integrins, Neutrophil, Vascular Biology, Myeloperoxidase
Abstract
Atherosclerosis and vasculitis both feature inflammation mediated by neutrophil-endothelial cell (EC) contact. Neutrophil myeloperoxidase (MPO) can disrupt normal EC function, although the mechanism(s) by which MPO is transferred to ECs are unknown. We tested the hypothesis that close, β2 integrin-dependent neutrophil-EC contact mediates MPO transfer from neutrophils to ECs. We used sensitive MPO assays and flow cytometry to detect MPO in ECs and demonstrate that ECs acquired MPO when contacted by neutrophils directly but not when ECs and neutrophils were separated in Transwells. The transfer was dependent on neutrophil number, exposure time, and incubation temperature. Transfer occurred in several EC types, increased with endotoxin, was not accompanied by MPO release into the medium, and was not abrogated by inhibiting degranulation to secretagogues. Confocal microscopy showed MPO internalization by ECs with cytoplasmic and nuclear staining. Neutrophils and ECs formed intimate contact sites demonstrated by electron microscopy. Blocking CD11b or CD18 β2 integrin chains, or using neutrophils from CD11b gene-deleted mice, reduced MPO transfer. EC-acquired MPO was enzymatically active, as demonstrated by its ability to oxidize the fluorescent probe aminophenyl fluorescein in the presence of a hydrogen peroxide source. The data suggest an alternative to EC uptake of soluble MPO, namely the cell contact-dependent, β2 integrin-mediated transfer from neutrophils. The findings could be of therapeutic relevance in atherosclerosis and vasculitis.
Introduction
The heme protein myeloperoxidase (MPO)2 is one of the most abundant proteins in neutrophils, accounting for ∼1–2% of the cell protein by dry weight (1). Enzymatically active MPO has the unique capacity to oxidize chloride ion and utilizes hydrogen peroxide formed during the respiratory burst to generate hypochlorous acid (HOCl), a potent microbicide that contributes to host defense against bacterial and fungal infection (2, 3). An increasing body of clinical and experimental findings implicates MPO in the initiation and promotion of cardiovascular disease. Clinical studies suggest that an elevated plasma level of MPO serves as an independent marker of vascular inflammation and is linked to increased cardiovascular morbidity and mortality (4–8). The basis for MPO as a biomarker for cardiovascular disease may reflect its capacity to adversely influence important vascular functions, including regulation of nitric oxide (NO)-mediated vascular relaxation (9), production of oxidants and bradykinin (10), promotion of atherosclerosis (11), recruitment of neutrophils (12), and acceleration of cardiac fibrosis (13). Furthermore, MPO may promote endothelial cell damage in the setting of systemic necrotizing vasculitis because it can serve as a major target-antigen for anti-neutrophil cytoplasmic autoantibodies (14).
Precisely how ECs acquire active MPO from neutrophils is not yet fully understood. It is widely assumed that ECs capture soluble MPO released into the bloodstream by activated neutrophils. However, two features of MPO and its release suggest that the prevailing assumption may not be the complete explanation. MPO in plasma is bound in an inactive state to ceruloplasmin, a copper-containing protein in the α2-globulin fraction of human serum (15, 16), leaving only a fraction of circulating MPO free. In addition, compared with other populations of neutrophil granules, MPO-containing azurophilic granules are the least prone to extracellular release in vitro and in vivo (17, 18). Taken together, these observations suggest that there would be very little free active MPO for ECs to acquire directly from the circulation. Given that alternative mechanisms for MPO transfer merit consideration, we tested the hypothesis that neutrophils transport MPO directly to ECs during close cell-cell contact. Our data demonstrate that intimate neutrophil-EC contact can efficiently transfer enzymatically active MPO to ECs, as might occur under mild inflammatory conditions. Addition of blocking antibody against β2 integrins and β2 integrin deficiency abrogated MPO transfer, indicating that the required cell-cell contact was integrin-dependent and providing a potential therapeutic strategy to decrease MPO acquisition by ECs.
EXPERIMENTAL PROCEDURES
Preparation of Human and Mouse Neutrophil
Neutrophils from human donors and from mice, and human umbilical vein endothelial cells (HUVECs) were obtained after due approval by Charité and governmental authorities and, in the case of humans, after written, informed consent. Standards correspond to those of the American Physiological Society and adhere to the Helsinki Accord. We isolated neutrophils and assessed cell viability as outlined previously (19). Cell viability was assessed by trypan blue exclusion and found to be >99%. The percentage of neutrophils after isolation was >95% by Wright-Giemsa staining and by light microscopy.
Degranulation Assay
Human neutrophils (2 × 106) in 300 μl of Hanks' buffered saline solution were incubated for 30 min with buffer control, 5 μm ionophore A23187, 100 ng/ml LPS, 20 ng/ml GM-CSF, 100 nm IL-8, 10−6 m formylmethionylleucylphenylalanine (fMLF), 5 μg/ml cytochalasin B (cytoB), a combination of cytoB and fMLF, and 2 ng/ml TNFα, respectively. Cell-free supernatants were collected by centrifugation, and MPO activity was assayed by ABTA (Sigma-Aldrich) or isoluminol-amplified chemiluminescence (20) as described previously.
Endothelial Cell Culture and Co-incubation with Neutrophils
The EC line ECV304 and the HUVEC lines EAhy926 and SGHec-7 as well as primary HUVECs after two to four passages were used. Confluent ECs were washed with PBS and incubated either with 300 μl of cell-free neutrophil supernatant or with 1 × 106 human or 2.5 × 106 mouse neutrophils in 300 μl of Hanks' buffered saline solution/10% FCS for 60 min if not otherwise specified. After the indicated co-incubation time, ECs were washed with ice-cold PBS/0.5 mm EDTA, harvested by trypsinization, washed twice, and subjected to the assays. In some parallel experiments trypsin was omitted, and cells were harvested by scraping to assess possible trypsin effects on detection of transferred MPO.
Flow Cytometry for Assessment of MPO and Additional Granule Protein Acquisition by ECs
For the extracellular flow cytometry staining, ECs were incubated with 5 μg/ml primary monoclonal antibody to MPO (clone 2C7; Abcam) on ice followed by staining with a phycoerythrin-labeled secondary F(ab′)2 antibody (Dako) or with a direct phycoerythrin-labeled anti-MPO Ab (clone MPO-7, Dako). Additional primary monoclonal antibodies to proteinase 3 (HISS Diagnostics, Freiburg, Germany), human neutrophil elastase (Thermo Scientific) and bactericidal/permeability-increasing protein (Abcam) were used. For intracellular staining ECs were fixed (0.5% paraformaldehyde, 20 min, ice) and permeabilized (0.2% saponin/1% BSA in PBS). Samples were assayed using a FACSCalibur (BD Biosciences). Using light scatter, the acquisition gate was set on EC, and 10,000 events/sample were collected. For analysis, ECs were tightly gated, and MPO staining was assessed as mean fluorescence intensity (MFI) and the percentage of positively stained cells. Isotype control staining was set at ∼10 MFI, and these values were subtracted.
Cell Lysis, SDS-PAGE, and Immunoblot Analysis
Cell lysis, SDS-PAGE, and immunoblotting were performed as described previously (21). The blots were developed with the polyclonal anti-MPO antibody (Calbiochem) or an anti-actin antibody (Santa Cruz Biotechnology) and visualized by an enhanced chemiluminescence detection system (Thermo Scientific).
Confocal Microscopy
Confluent ECV cells were grown on glass coverslips and co-incubated with human neutrophils for the indicated time points. ECV cells were washed and fixed in 4% paraformaldehyde (15 min, room temperature), washed, and stained for cell membrane with wheat germ agglutinin-Alexa Fluor 555 conjugate (Invitrogen; 10 μg/ml, 20 min, room temperature). Cells were blocked and permeabilized in 10% goat serum in Hanks' buffered saline solution containing 0.1% Triton X-100) for 1 h at room temperature, incubated with 5 μg/ml polyclonal rabbit anti-MPO (Calbiochem) followed by 4 μg/ml goat-anti-rabbit IgG-Alexa Fluor 488 secondary antibody (Molecular Probes). Nuclei were stained with DAPI (Invitrogen; 20 μg/ml, 15 min, room temperature). Cells were mounted in ProLong Gold antifade reagent (Invitrogen). Triple fluorescence images were acquired at a laser scanning confocal microscope Zeiss LSM700 using a 63× NA1.4 PL APO oil immersion objective. Each cell was imaged by 20–30 confocal Z-sections with a section thickness of 0.24 μm. The pixel size was set to 63 nm. The pinhole was set to 40 μm. To improve axial resolution of three-dimensional data sets deconvolution of the Z-stacks was performed using Huygens Professional 7.2 software (SVI). Imaris software (Bitplane) was used for the orthogonal slices to visualize the cytoplasmic localization of internalized MPO and for the maximal intensity projection.
Electron Microscopy
Endothelial cells were co-cultured with human neutrophils for 20 min, washed, and fixed in 2.5% glutaraldehyde in 0.1 m phosphate buffer for 24 h. Samples were treated with 1% osmium tetroxide in 0.1 m phosphate buffer for 2 h, dehydrated in ethanol, and embedded in Poly/Bed® 812 (Polysciences). Ultrathin sections were stained with uranyl acetate and lead citrate and examined with a Zeiss EM 910. Digital images were taken with a 1kx1k high speed, slow scan CCD camera (Proscan) and the iTEM software (Olympus Soft Imaging Solutions, Münster).
Inhibition of MPO Transfer
For blocking neutrophil surface proteins, 5 × 105 neutrophils were incubated with 20 μg/ml mAbs to CD18 (clone 7E4; Immunotech, Marseille, France), CD11b (clone 2LPM19c; Santa Cruz Biotechnology), β1 integrin (clone P4C10; Millipore), β3 integrin (clone SZ21; Beckman Coulter), and isotype control, respectively. After 30 min, pretreated cells were added to confluent ECs in 24-well plates for 15 min. For blocking EC molecules, ECs were preincubated with 20 μg/ml anti-ICAM-1 mAb (CD54; eBioscience, Hatfield, UK) or 15 μg/ml panCK (Abcam), 5 μg/ml cytochalasin B, or 4 μg/ml Pronase (Merck) for 30 min. 5 × 105 neutrophils were added for 15 min. After allowing for MPO transfer, ECs were washed with ice-cold PBS/0.5 mm EDTA, harvested by trypsinization, washed twice, and subjected to flow cytometry. For agitation experiments, plates with neutrophil-EC co-cultures were subjected to shaking with 110 rpm at 37 °C.
Assessment of MPO Activity Using Aminophenyl Fluorescein, Flow Cytometry, and Fluorescence Microscopy
The flow cytometry assay was performed as described recently (22). ECs or neutrophils were loaded for 30 min with 200 μl of aminophenyl fluorescein (APF) (final concentration 5 μm). Diphenyleneiodonium chloride (10 μm final) or 4-aminobenzoic acid hydrazide (4-ABAH, 100 μm final) from Sigma or buffer control was added, respectively, 5 min before APF loading. 1 × 106 neutrophils were activated with TNFα/fMLF (2 ng/ml and 1 × 106 M final concentration, respectively) in polypropylene tubes for 60 min. ECs were cultured in 24-well plates. 100 μl of exogenous MPO (12.3 nm final) or 100 μl of neutrophils (1 × 106) were added to APF-loaded ECs followed by 30 μl of glucose (5 mm final) and glucose oxidase (2 μg/ml final), respectively. We documented that glucose with glucose oxidase generated H2O2 as expected (data not shown). After keeping samples for 30 min at 37 °C in the incubator, cells were washed, harvested, and assayed by flow cytometry. For microscopy, neutrophil-EC incubation was performed on coverslips, and cells were examined using a Zeiss LSM700 and a 63× NA1.4 PL APO oil immersion objective.
Statistical Analysis
Results are given as mean ± S.E. Comparisons between multiple groups were done using ANOVA and appropriate post hoc tests. Comparisons between two groups were done by a two-sided paired t test. Differences were considered significant if p < 0.05.
RESULTS
MPO Degranulation Requires Robust Neutrophil Activation and Is Not Detected with More Physiologic Stimuli
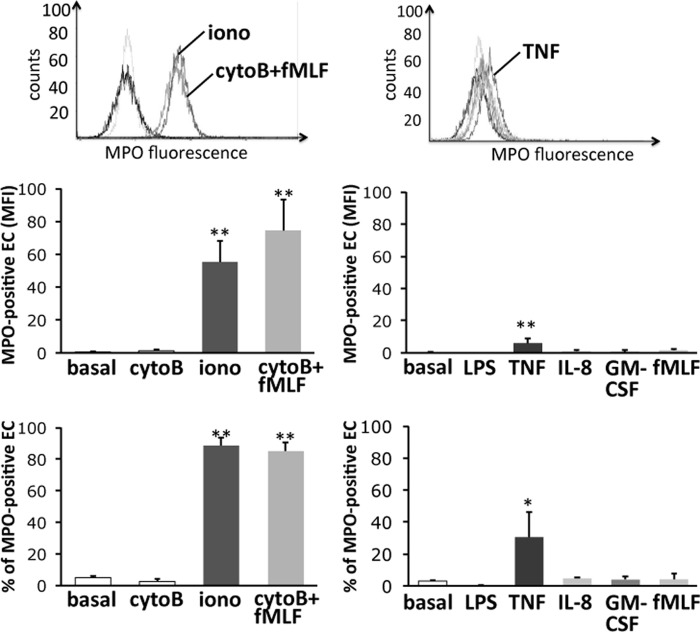
We first determined the relative extent of MPO release from neutrophils in response to different agonists, pharmacologic as well as physiologic. Cells were incubated with varied stimuli, and MPO activity in the supernatant was measured. A 30 min treatment with ionophore A23187 or with a combination of cytoB and fMLF potently triggered MPO degranulation. In contrast, exposure to more physiologic agonists such as LPS, TNFα, GM-CSF, IL-8, and fMLF in the absence of cytoB induced significantly less MPO release (Fig. 1). A second independent assay of MPO release using isoluminol-amplified chemiluminescence gave similar results (data not shown).
FIGURE 1.
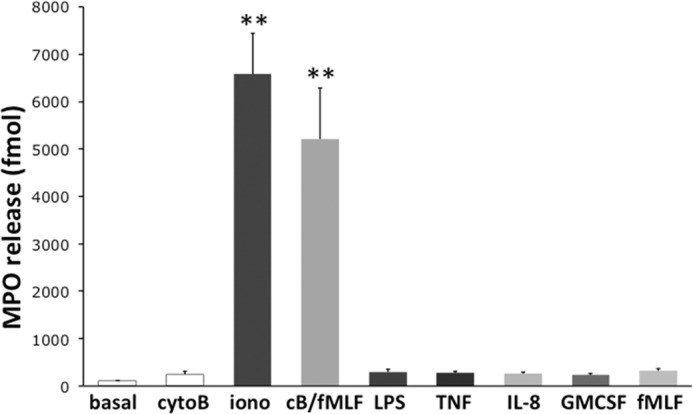
MPO degranulation by human neutrophils stimulated with several different agonists. Neutrophils were incubated with buffer (basal) or cytoB before 5 μm ionophore A23187 (iono), cytoB/fMLF 10−6 m (cB/fMLF), 100 ng/ml LPS, 2 ng/ml TNFα (TNF), 100 nm IL-8 (IL-8), 20 ng/ml GM-CSF (GMCSF), and 10−6 m fMLF (fMLF) were added for further 30 min, respectively. The amount of MPO in the cell-free supernatant was quantitated using an ABTA assay. The assay indicates a significant MPO release in response to nonphysiologic stimuli, but not to physiologic mediators (n = 5). **, p < 0.01. Error bars, S.E.
MPO Transfer to the ECs Does Not Occur with Supernatants from LPS-, GM-CSF-, and IL-8-stimulated Neutrophils
We next used cell-free supernatants from untreated and stimulated neutrophils to study MPO transfer to the ECs by flow cytometry (Fig. 2). We assessed both MPO fluorescence intensity and the percentage of ECs that became MPO-positive. After a 60-min incubation, supernatants from ionophore A23187- and cytoB/fMLF-treated neutrophils effectively transferred MPO to the ECs. In contrast, supernatants from unstimulated, cytoB-, fMLF- (in the absence of cytoB), LPS-, GM-CSF-, and IL-8-treated cells did not transfer MPO to the ECs. TNFα had a moderate, but statistically significant, effect that varied among the experiments.
FIGURE 2.
MPO acquisition by ECs from cell-free neutrophil supernatants. Neutrophils were treated with the indicated stimuli as in Fig. 1 for 30 min. Cell-free supernatants were transferred to EC cultures. After 60 min ECs were assessed for MPO acquisition using flow cytometry (n = 5). *, p < 0.05; **, p < 0.01. Error bars, S.E.
MPO Transfer to the ECs Occurs with Direct Neutrophil-EC Contact
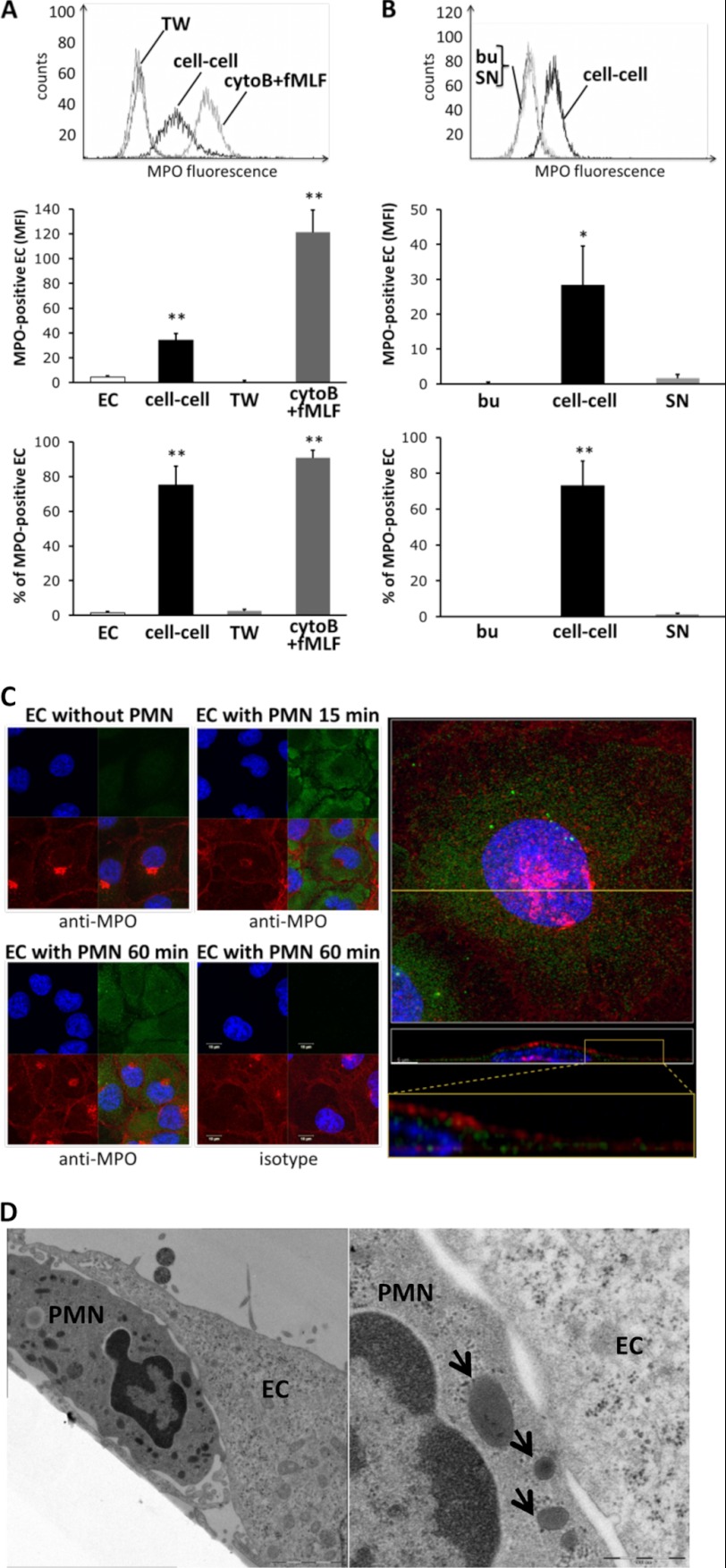
We next explored alternative mechanisms for transfer of MPO to ECs. We compared EC acquisition of MPO in neutrophil-EC co-cultures under two different conditions: when neutrophils and ECs were in direct contact and when neutrophils were incubated in the upper chamber of a Transwell device and separated by a filter from the ECs. As a positive control experiment performed in parallel, ECs were incubated with supernatants from cytoB/fMLF-treated neutrophils of the same donor. By flow cytometry assessing live cells we observed strong MPO acquisition by ECs that had direct cell contact with neutrophils, but not when ECs and neutrophils were separated in Transwells (Fig. 3A).
FIGURE 3.
MPO transfer from the neutrophil to the ECs in co-culture, Transwells, and with supernatants from degranulated neutrophils, respectively. A, MPO acquisition by ECs was examined under three different conditions: neutrophils were co-cultured on ECs (cell-cell) or added to the upper chamber of a Transwell (TW) device preventing cell-cell contact, or ECs were incubated with degranulated neutrophil supernatant after cytoB/fMLF treatment, respectively. MPO transfer was assessed by flow cytometry without fixation or permeabilization by flow cytometry. Direct neutrophil-EC contact resulted in MPO transfer, whereas separation of cell types in Transwells did not (n = 5). B, endothelial MPO staining was assessed by flow cytometry after EC incubation with buffer (bu), neutrophil-EC co-culture (cell-cell), and incubation in cell-free supernatant from neutrophil-EC co-cultures (SN), respectively. Direct neutrophil-EC contact resulted in significant MPO transfer. C, confocal images of MPO staining are shown. ECs were incubated either without or with neutrophils (PMN) for the indicated time points. Cells were fixed, permeabilized, and subsequently stained with the anti-MPO Ab (green). Cell membranes were visualized with wheat germ agglutinin (red) and nuclei counterstained with DAPI (blue). MPO staining of ECV was appreciated after 15 and 60 min. In high resolution Z-stacks of EC images co-incubated with neutrophils for 60 min, maximum intensity projections of MPO (green), membrane (red), and nucleus (blue) fluorescence are shown (upper right panel). The yellow line indicates the slice for orthogonal projection (x-z, middle right panel) from the three-dimensional reconstructed Z-stack. Higher magnification shows MPO localized in the cytoplasm and to some degree in the nucleus (magnification, bottom of the right panel). Error bars, S.E. D, neutrophil-EC co-cultures were analyzed by electron microscopy. ECs were washed after neutrophil co-incubation and processed for conventional plastic embedding. One representative image is shown, indicating intimate cell contacts with formation of synapses-like structures. Peroxidase-positive granules are indicated (arrows). Original magnification, ×3,150 (left) and ×8,000 (right).
To characterize in greater detail the importance of direct cell-to-cell contact for MPO transfer to ECs, we measured MPO acquisition by ECs co-incubated for 60 min with unstimulated neutrophils (Fig. 3B). Under these conditions, ECs acquired MPO efficiently even in the absence of detectable degranulation and when the amount of MPO in supernatants was similar to what we had observed for unstimulated cells in suspension (136 ± 38 fmol of MPO). Standard EC harvesting with trypsin or omitting the enzyme and harvesting by scraping resulted in slightly higher MPO transfer to the EC surface when trypsin was absent (21.2 ± 4.0 MFI with trypsin and 28.2 ± 4.1 MFI with scraping, n = 5, p = 0.035). Because our data suggested that the use of an anti-MPO mAb together with flow cytometry provided higher sensitivity for MPO detection (Fig. 2), we also assessed MPO transfer with cell-free supernatant from the neutrophil-EC co-culture (Fig. 3B). Even with this more sensitive assay, no significant MPO transfer was detected when ECs were incubated with cell-free supernatant from the neutrophil-EC co-culture, whereas experiments done in parallel with neutrophils and ECs in direct cell contact confirmed MPO transfer. To examine further the possibility that neutrophils transferred MPO directly to ECs, we co-incubated ECs with neutrophils pretreated with the degranulation inhibitor adenosine (23). We confirmed that adenosine inhibited degranulation as it reduced MPO release from cytoB/fMLF-stimulated neutrophils to 42 ± 11% (n = 4, p < 0.05). Under these conditions, we observed no reduction in MPO acquisition by ECs. MPO MFI was 59 ± 18 without adenosine and 57 ± 16 with adenosine, whereas the percentage of ECs that acquired MPO was 72 ± 8 and 69 ± 9 (n = 4, n.s.). Taken together, these results strongly suggest that MPO transfer from neutrophils to ECs occurred in a cell contact-dependent fashion that is distinct from EC uptake of soluble MPO released during degranulation.
To visualize the intracellular location of MPO acquired by the ECs after cell-cell contact, we co-incubated neutrophils and ECV304 for 15 and 60 min, fixed the cells, and then permeabilized for intracellular MPO staining (Fig. 3C). Intracellular immunostaining was specific for MPO, predominantly cytoplasmic with some nuclear staining, and dependent on the presence of neutrophils. Using electron microscopy to visualize close neutrophil-EC contacts, we found multiple intimate contact sites where membranes from both cell types had formed synapse-like structures (Fig. 3D).
To determine whether MPO transfer by cell-cell contact is a peculiar trait of ECV304, we tested the capacity of other EC types to acquire MPO from neutrophils. We first probed lysates of ECV, EAHy, SGHec-7, HUVECs, and human neutrophils in immunoblots for the presence of endogenous MPO and confirmed that none of the different EC types expressed endogenous MPO, whereas neutrophils showed a strong signal (Fig. 4A). Flow cytometry demonstrated that all of the studied EC types acquired significant amounts of MPO when co-incubated with human neutrophils for 60 min (Fig. 4B). Thus, the ability of ECs to acquire MPO by cell-to-cell contact with neutrophils was not a unique feature of ECV304 but a general property of endothelial cells.
FIGURE 4.
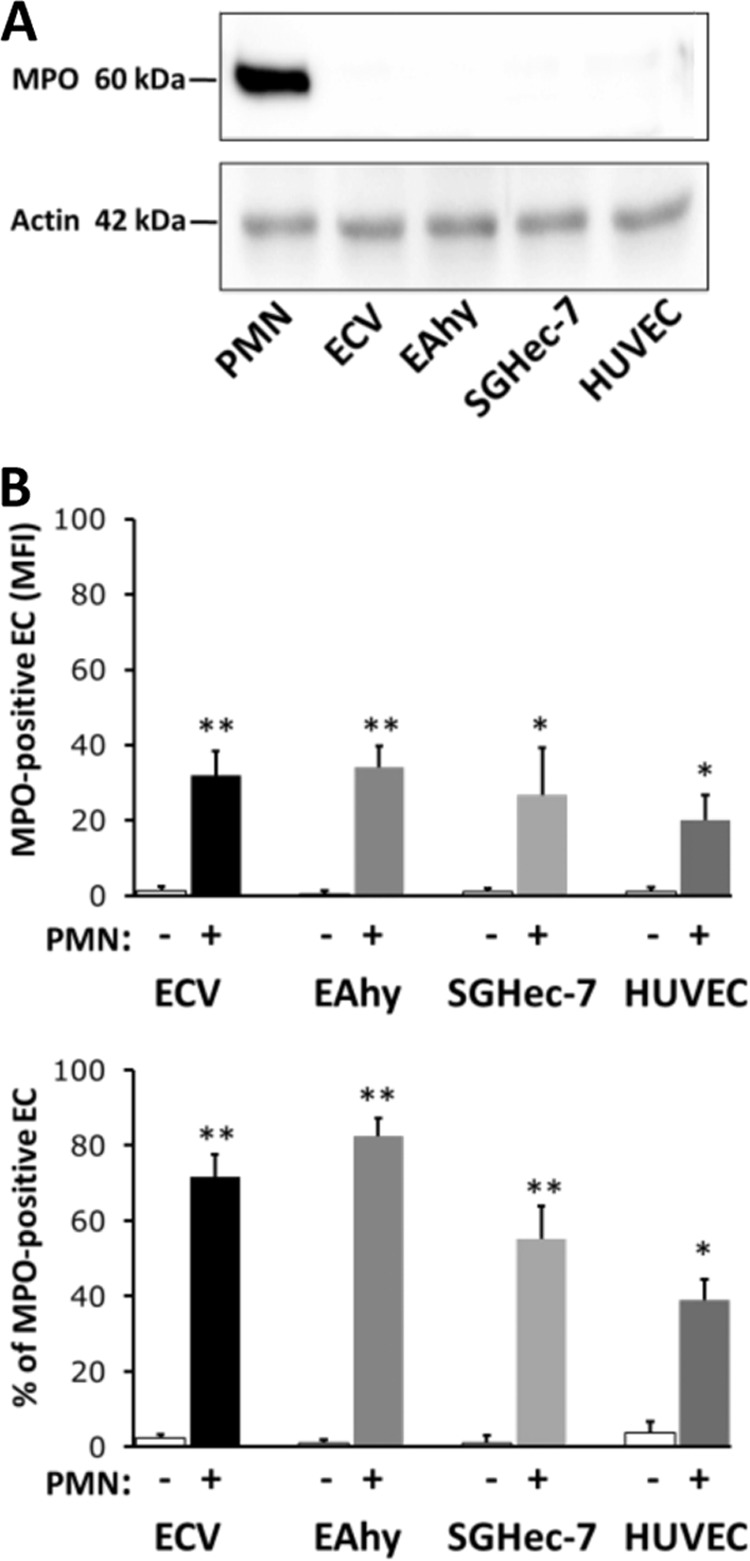
MPO acquisition in different EC types. A, assessment of MPO expression in neutrophils (PMN), ECV, EAhy, SGHec-7, and HUVECs by immunoblotting. The MPO heavy chain was only detected in neutrophils, but not in any of the ECs. The amount of actin in each lane indicates equal loading of all samples. B, neutrophils (PMN+) or buffer control (PMN−) co-cultured on ECVs (n = 5), EAhy (n = 5), SGHec-7 (n = 5), and HUVECs (n = 4) for 60 min. MPO transfer was assessed by flow cytometry without cell fixation or permeabilization. *, p < 0.05; **, p < 0.01. Error bars, S.E.
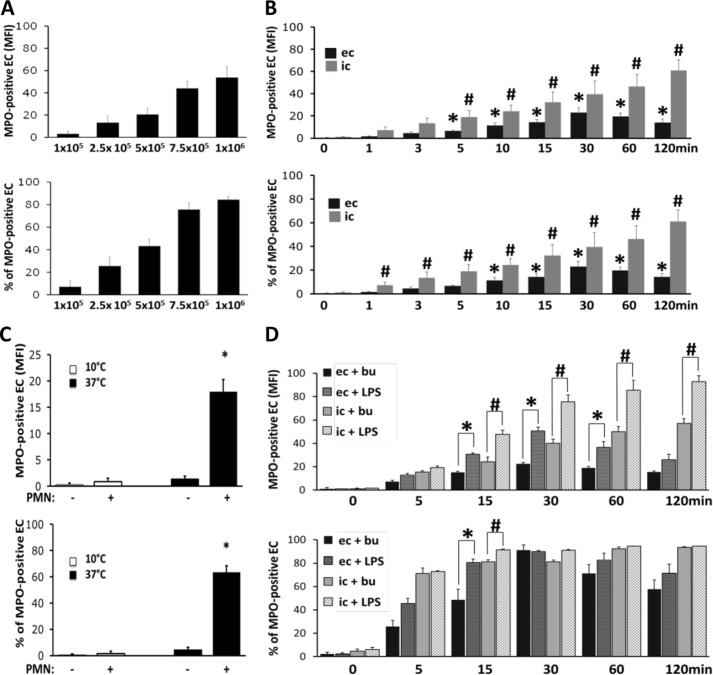
MPO Acquisition by ECs Co-cultured with Neutrophils Depends on Cell Concentration, Time, and Temperature and Increases with LPS
ECs incubated for 60 min with varied numbers of neutrophils demonstrated a dose-dependent transfer of MPO to the EC surface over a range from 1 × 105 to 1 × 106 neutrophils (Fig. 5A). Furthermore, EC acquisition of MPO was dependent on the duration that neutrophils and ECs were co-cultured. MPO transfer to the EC surface and intracellular MPO accumulation occurred rapidly and reached statistical significance already after 5 min. Surface MPO peaked at ∼30 min and slowly decreased thereafter. In contrast, intracellular MPO progressively accumulated over the entire study time (Fig. 5B). Thus, the extent of MPO transfer depended on the amount of MPO available, reflected in the number of neutrophils in the system, and the duration of cell-cell contact. When we co-cultured neutrophils and ECs at 37 °C and 10 °C in parallel, we found that MPO transfer to the EC surface was abrogated at the lower temperature without apparent cell damage as judged by light microscopy (Fig. 5C).
FIGURE 5.
MPO acquisition by ECs is dose-, time-, and temperature-dependent and increases with LPS. A, increasing amounts of neutrophils were co-incubated with ECs for 60 min. Samples were stained for MPO and assessed by flow cytometry. Higher neutrophil to EC ratios resulted in increased MPO transfer to the ECs (n = 3). B, ECs were incubated with 1 × 106 neutrophils for up to 120 min. Samples were harvested at the indicated time points, and MPO was assessed either in unpermeabilized (extracellular, ec) or permeabilized (intracellular, ic) ECs by flow cytometry. MPO acquisition by ECs was time-dependent, surface MPO peaking at 30 min and intracellular MPO accumulating over the entire 120-min period (n = 5). *, p < 0.05 for surface MPO acquisition compared with ECs at time 0 min; #, p < 0.05 for intracellular MPO accumulation compared with ECs at time 0 min. C, 1 × 106 neutrophils (PMN+) or buffer control (PMN−) were cultured on ECs at either 37 °C or 10 °C for 60 min. The lower temperature condition abrogated MPO transfer (n = 3). D, 1 × 106 neutrophils were co-cultured with ECs for up to 120 min. Buffer control (+bu) or 100 ng/ml LPS (+LPS) was added for the indicated incubation time. Flow cytometry assessing unpermeabilized (extracellular) or permeabilized (intracellular) ECs shows that MPO acquisition was increased in the presence of LPS (n = 5). *, significant differences in extracellular MPO; #, significant intracellular MPO differences between buffer control and LPS treatment, respectively. Error bars, S.E.
We reasoned that not only the number but also the functional state of neutrophils would influence how readily transfer occurred. Furthermore, we considered that endotoxemia would represent a situation in which the pathophysiologic consequences of EC-associated MPO would be clinically relevant. Although LPS had not caused MPO degranulation (Fig. 1) or supernatant-mediated transfer to the ECs (Fig. 2), co-incubation of ECs with 5 × 105 neutrophils in the presence of LPS for up to 120 min resulted in greater transfer of MPO both to the surface and to the intracellular compartment of ECs compared with that seen in the presence of buffer alone. The augmented acquisition was significant for surface MPO and for intracellular MPO accumulation after 15 min (Fig. 5D).
We tested whether or not transfer to the EC surface or intracellular acquisition was observed for neutrophil granule proteins other than MPO. We found three different transfer patterns, namely granule proteins that were transferred and detected on the EC surface and intracellularly (MPO), granule proteins that were transferred and detected intracellularly, but barely on the surface (proteinase 3, human neutrophil elastase), and granule proteins that were not transferred at all (bactericidal/permeability-increasing) (supplemental Fig. 1). In the latter experiments, positive staining was obtained in permeabilized neutrophils providing a positive control (data not shown).
MPO Acquisition by ECs Co-cultured with Neutrophils Depends on β2 Integrins
We confirmed that an anti-cytokeratin 1 antibody decreased EC acquisition of soluble MPO added to ECs, as reported previously (10). Anti-cytokeratin 1 antibody pretreatment of ECs reduced the MPO MFI under these conditions from 12.3 ± 2.7 to 3.4 ± 1.6 and the percentage of MPO-positive cells from 58 ± 11 to 17 ± 9 (n = 5, p < 0.01). However, the same antibody blocking cytokeratin 1 had no effect on cell contact-mediated MPO transfer, demonstrating again that different mechanisms were responsible for cell contact-dependent transfer versus uptake of soluble MPO by ECs.
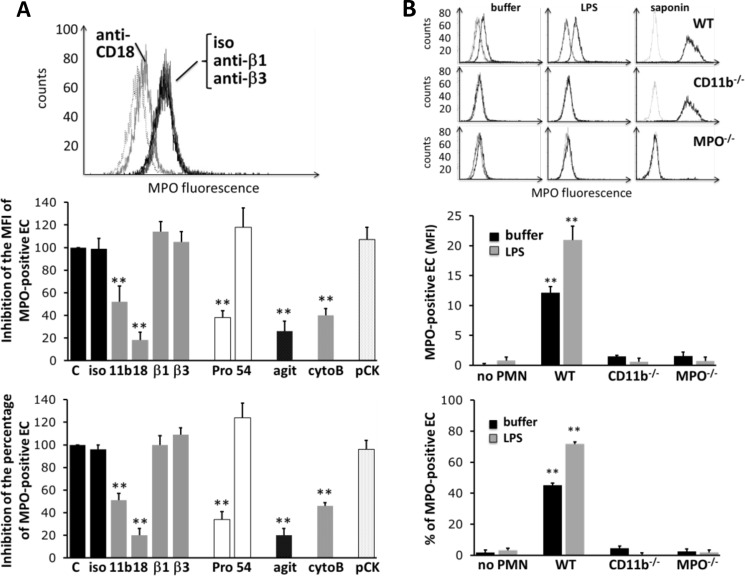
We observed that MPO transfer was significantly decreased when cell-cell contact was reduced by agitating the plate during co-incubation. When we treated the ECs with Pronase to proteolytically remove surface receptors, we observed a strong reduction of MPO transfer. Transfer was also reduced when neutrophils were preincubated with blocking CD18 and CD11b antibodies or with cytoB (Fig. 6A). In contrast, blocking antibodies to β1 and β3 integrins had no effect on transfer. In addition, a blocking CD54 antibody was without effect, suggesting that endothelial ICAM-1 was not involved but that contact-dependent transfer of MPO to ECs was supported by β2 integrins on neutrophils.
FIGURE 6.
Inhibition of MPO transfer from the neutrophil to the ECs. A, EC MPO acquisition after neutrophil incubation with blocking antibodies to CD11b, CD18, and β1 and β3 integrins, respectively. In parallel, ECs were pretreated with Pronase (Pro) to proteolytically remove EC surface receptors or with blocking antibodies to ICAM-1 (CD54). Furthermore, cell-cell contact was prevented by agitation (agit), the cytoskeleton disrupted by cytoB, or ECs were pretreated with a blocking Ab to cytokeratin-1 (pCK). A typical histogram is shown for the integrin-blocking antibodies. The inhibition of the MPO MFI and percentage of MPO-positive ECs was quantified for all experimental conditions. B, MPO acquisition by ECs co-incubated for 30 min in the presence or absence of LPS with murine neutrophils from wild-type (WT) or MPO (MPO−/−)- or CD11b (CD11b−/−)-deficient mice. Histograms of a typical experiment are shown. MPO acquisition by ECs (with buffer or LPS) is depicted in the left and middle histogram columns. Intracellular MPO staining in saponin-permeabilized neutrophils was performed to ensure similar MPO content in WT and CD11b-deficient mice and lacking MPO in the MPO−/− mice and is shown in the right histogram column. The corresponding statistics for MPO MFI and percentage of MPO-positive ECs is shown in the column graphs (n = 5). **, p < 0.01. Error bars, S.E.
To examine in greater detail the role of β2 integrins in MPO transfer, we used neutrophils from CD11b knock-out mice (Fig. 6B). Significantly less MPO was transferred from CD11b−/− neutrophils compared with that from WT neutrophils. Thus, transfer of MPO from neutrophils to ECs required cell-cell contact mediated, in part, by β2 integrins on the neutrophil surface. Intracellular staining in saponin-permeabilized neutrophils by flow cytometry demonstrated WT and CD11b−/− cells had similar cellular MPO content.
MPO Acquired by ECs Is Enzymatically Active as Shown Using APF
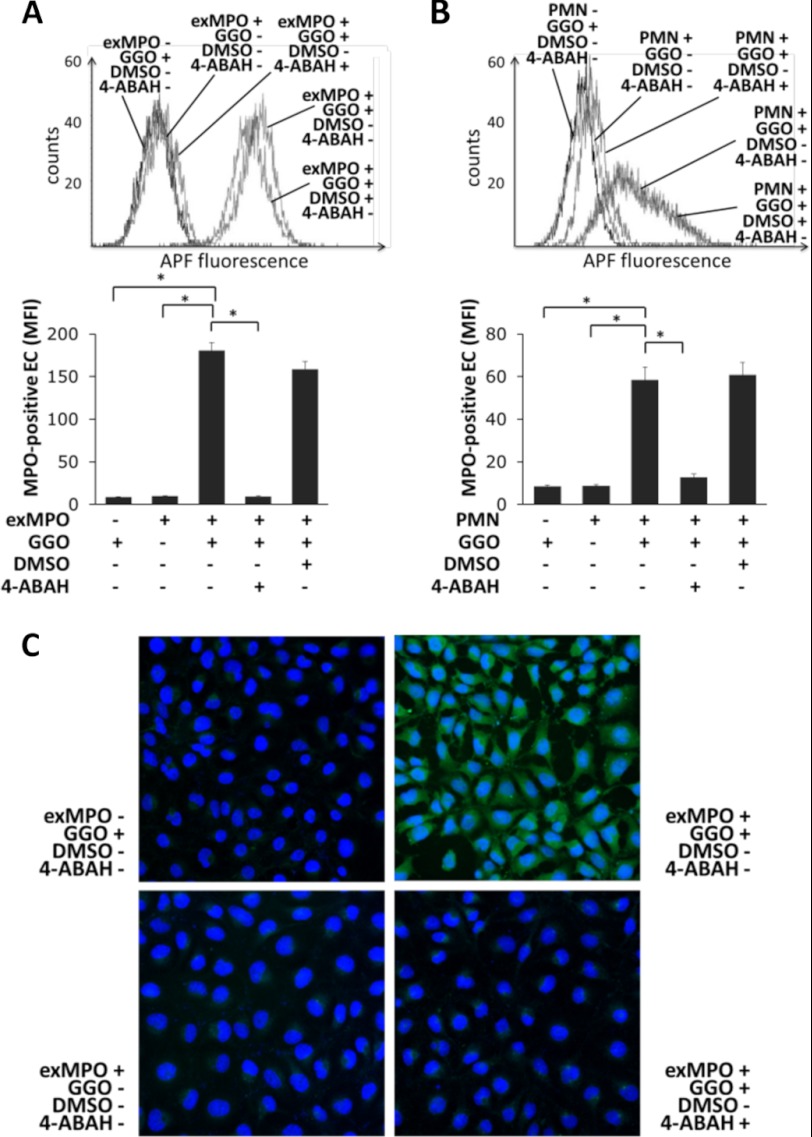
Finally, we studied whether or not MPO transferred from the neutrophil to the ECs was enzymatically active. Active MPO is unique in generating HOCl in the presence of H2O2. APF detects HOCl in living cells, as HOCl oxidizes the nonfluorescent substrate into fluorescent APF (22). First, we verified the APF detection system using human neutrophils (n = 5). Neutrophil activation by TNF/fMLF increased APF fluorescence from 9.5 ± 0.5 to 140.3 ± 25.2 MFI. This effect required endogenous oxidant generation, as indicated by APF MFI reduction to 18.7 ± 2.9 with the NADPH oxidase inhibitor diphenyleneiodonium chloride (n = 4, p < 0.01). APF fluorescence also depended on the presence of enzymatically active MPO, as the MPO inhibitor 4-ABAH reduced APF MFI to 42.6 ± 7.4 (n = 5, p < 0.01).
Having established that intracellular fluorescence of APF reflected the presence of enzymatically active MPO, we next applied the analytical approach to ECs exposed to exogenous MPO or to human neutrophils. When we added active exogenous MPO and glucose/glucose oxidase as a H2O2 source, endothelial APF fluorescence increased significantly (Fig. 7A). APF fluorescence of ECs required H2O2 and active peroxidase, as omitting the H2O2 source or blocking MPO activity abrogated APF fluorescence. Similar data were obtained using neutrophil-EC co-cultures where neutrophils provided the MPO that was subsequently acquired by ECs (Fig. 7B). As with the cell-free sources of oxidant and MPO, fluorescence of APF in ECs required oxidants and enzymatically active MPO. These findings are illustrated by fluorescence microscopy (Fig. 7C). These data establish that MPO that was transferred from neutrophils to ECs during cell-cell contact remained indeed enzymatically active.
FIGURE 7.
MPO acquired by ECs is enzymatically active. ECs were loaded with APF and incubated with exogenous MPO (exMPO) or with 1 × 106 neutrophils for 60 min before EC APF fluorescence was measured by flow cytometry (A and B) and fluorescence microscopy (C). A, APF fluorescence increased when exogenous MPO and glucose/glucose oxidase (GGO) were added to ECs, but not when either one of the reagents was added alone. 4-ABAH, but not the corresponding dimethyl sulfoxide (DMSO) control, significantly reduced APF fluorescence. B, EC incubation with neutrophils (PMN) and glucose/glucose oxidase increased APF fluorescence. 4-ABAH, but not the corresponding dimethyl sulfoxide control, significantly reduced APF fluorescence. Typical flow cytometry results from five independent experiments are depicted. Error bars, S.E. C, microscopy images of EC APF fluorescence. EC APF fluorescence increased when exogenous MPO was added to ECs incubated with glucose/glucose oxidase. APF fluorescence did not increase when either glucose/glucose oxidase was omitted or MPO activity was blocked by 4-ABAH. *, p < 0.05.
DISCUSSION
MPO is the most abundant neutrophil protein, and its long known microbicidal activity supports optimal host defense against a variety of bacterial and fungal challenges (2, 3). In contrast to its protective effect, MPO has more recently been shown to play also a detrimental role during inflammation, mainly by undermining normal cardiovascular functions when MPO is deposited in the vessel wall. Eiserich et al. described that intravenous challenge of rodents with LPS promotes deposition of MPO in the endothelium, causing local oxidant generation that leads to rapid NO consumption and profoundly reduced vascular relaxation (9). Additional MPO-triggered cardiovascular effects were described thereafter, including urate radical (24) and bradykinin generation (10), oxidation of low density lipoproteins (25), high density lipoproteins (26), and α1-proteinase inhibitor (27), accelerated atherosclerosis (11), and promotion of cardiac fibrosis and atrial fibrillation (13). Moreover, MPO also attracts (12) and activates (28) neutrophils, thereby further accelerating inflammation. Collectively, these data demonstrate the potential pathophysiologic consequences when ECs acquire active MPO.
Previous studies of EC-associated MPO presumed that ECs captured soluble MPO that had been released into the circulation by stimulated neutrophils. Accordingly, in vitro experiments recapitulated EC acquisition of MPO by adding purified MPO to cultured ECs. Although we confirmed these findings, we hypothesized that intimate cell contact between neutrophils and ECs could also mediate MPO transfer. In parallel experiments, we observed MPO transfer when neutrophils and ECs were allowed to form close contacts, but not when cells were separated in a Transwell device. The cell contact-mediated transfer was dose- and time-dependent, accelerated by LPS treatment, but not reduced by inhibiting degranulation. When visualized by transmission electron microscopy, contact sites between neutrophils and ECs appeared as synapse-like structures, similar to those described previously between neutrophils and antibody-coated tumor cells, presumably involving Fc receptors (29) and CD11b/CD18 (30). It is conceivable that fusion at these intimate sites promotes cytoplasmic communication and MPO transfer. Membrane fusion is temperature-dependent, and we observed a significant inhibition of MPO transfer when we performed the co-cultures at lower temperatures. Furthermore, MPO transfer was not associated with detectable amounts of MPO in the medium of neutrophil-EC co-cultures, thereby suggesting that the communication sites were tightly sealed, a feature reminiscent of protected zones formed between blood cells, including neutrophils, and extracellular matrices in an integrin-dependent fashion (31–33). MPO transfer was not a property of selected ECs because it occurred similarly in three different endothelial cell lines as well as in primary HUVECs. Furthermore, the MPO transfer pattern was unique compared with proteinase 3, human neutrophil elastase, and bactericidal/permeability-increasing protein, all azurophilic granule proteins. Whereas MPO was transferred both to the EC surface and accumulated intracellularly, proteinase 3, human neutrophil elastase, and bactericidal/permeability-increasing protein either accumulated only intracellularly without significant surface staining or were not transferred at all (supplemental Fig. 1). Although the molecular basis for these disparate transfer patterns remains undetermined, the data demonstrate efficient transfer of MPO into the cytoplasm of ECs.
We have strong evidence that β2 integrins participate in the formation of intimate contacts between neutrophils and ECs and thereby facilitate MPO acquisition by ECs. Our experimental evidence indicates that CD11b/CD18 is an important neutrophil adhesion molecule mediating this process, based on data both with blocking antibodies and with CD11b-deficient murine neutrophils. Pronase treatment of ECs significantly reduced MPO transfer, suggesting that ligands on the EC surface cooperated with CD11b/CD18 on neutrophils to support transfer, although we have not yet identified the specific ligand for CD11b/CD18 on ECs that is responsible. However, cytokeratin 1, shown to mediate the acquisition of exogenously added MPO by ECs (10), did not contribute to CD11/18-dependent cell contact-mediated MPO transfer. We envision that CD11/18-mediated adhesion processes occur in vivo under inflammatory conditions, as simulated in vitro by the addition of LPS to our experimental system. The in vivo requirement for activation of neutrophils and ECs to promote cell-cell engagement is bypassed in vitro, where interactions are enforced merely by co-culturing neutrophils and ECs.
In summary, we demonstrate the presence of two distinct mechanisms by which ECs can acquire enzymatically active MPO. Consistent with prior reports, robust neutrophil activation resulted in azurophilic granule exocytosis and subsequent capture of released MPO from cell-free supernatant by ECs. In addition, we observed a previously unrecognized mechanism that could be operative under milder inflammatory conditions, when little or no degranulation occurs. Under those conditions, direct β2 integrin-dependent neutrophil-EC contacts that result in intimate contact sites supported the transfer of enzymatically active MPO from neutrophils to ECs without measurable extracellular release of MPO. Thus, the type and strength of the inflammatory response may dictate the relative contribution of each mechanism to EC acquisition of MPO. The former might participate under conditions where robust activation of neutrophils occurs, as we achieved experimentally with nonphysiologic treatments such as ionophore A23187 and fMLF in combination with a cytoskeleton-disrupting reagent. Degranulation of MPO-containing azurophilic granules under these conditions was sufficient to support EC uptake of MPO from the cell-free supernatants. In contrast, MPO degranulation and transfer were not detected with supernatants from cells stimulated with more physiologic stimuli, such as LPS, GM-CSF, IL-8, and fMLF in the absence of cytoB. Thus, we have identified an alternative MPO transfer mechanism that can occur even under mild inflammatory conditions and would contribute to vascular damage in clinical entities with low grade inflammation, such as atherosclerosis and vasculitides. Anti-integrin-directed therapies could, at least in part, exert their anti-inflammatory effects by reducing MPO transfer to the endothelium.
Acknowledgments
We thank Anje Sporbert and Zoltan Cseresnyes (Confocal and Two Photon Microscopy core facility, Max-Delbrück Center for Molecular Medicine) for the technical support.
This work was supported, in whole or in part, by National Institutes of Health Grants AI 70958 and AI 044642 (to W. M. N.). This work was also supported by Deutsche Forschungsgemeinschaft Grant KE 576/7-1 (to R. K. and F. C. L.), the Experimental and Clinical Research Center (to R. K. and F. C. L.), and the Veterans Affairs Medical Center, Iowa City (to W. M. N.).

This article contains Supplemental Fig. 1.
- MPO
- myeloperoxidase
- 4-ABAH
- 4-aminobenzoic acid hydrazide
- ABTA
- 2′2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid
- APF
- aminophenyl fluorescein
- cytoB
- cytochalasin B
- EC
- endothelial cell
- fMLF
- formylmethionylleucylphenylalanine
- HUVEC
- human umbilical vein endothelial cell
- MFI
- mean fluorescence intensity.
REFERENCES
- 1. Agner K. (1941) Verdoperoxidase: a ferment isolated from leukocytes. Acta Physiol. Scand. 2 (Suppl. 8), 1–62 [Google Scholar]
- 2. Klebanoff S. J., Kettle A. J., Rosen H., Winterbourn C. C., Nauseef W. M. (2013) Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 93, 185–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nauseef W. M. (2007) How human neutrophils kill and degrade microbes: an integrated view. Immunol. Rev. 219, 88–102 [DOI] [PubMed] [Google Scholar]
- 4. Brennan M. L., Penn M. S., Van Lente F., Nambi V., Shishehbor M. H., Aviles R. J., Goormastic M., Pepoy M. L., McErlean E. S., Topol E. J., Nissen S. E., Hazen S. L. (2003) Prognostic value of myeloperoxidase in patients with chest pain. N. Engl. J. Med. 349, 1595–1604 [DOI] [PubMed] [Google Scholar]
- 5. Buffon A., Biasucci L. M., Liuzzo G., D'Onofrio G., Crea F., Maseri A. (2002) Widespread coronary inflammation in unstable angina. N. Engl. J. Med. 347, 5–12 [DOI] [PubMed] [Google Scholar]
- 6. Karakas M., Koenig W., Zierer A., Herder C., Rottbauer W., Baumert J., Meisinger C., Thorand B. (2012) Myeloperoxidase is associated with incident coronary heart disease independently of traditional risk factors: results from the MONICA/KORA Augsburg study. J. Intern. Med. 271, 43–50 [DOI] [PubMed] [Google Scholar]
- 7. Kalantar-Zadeh K., Brennan M. L., Hazen S. L. (2006) Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 48, 59–68 [DOI] [PubMed] [Google Scholar]
- 8. Wang A. Y., Lam C. W., Chan I. H., Wang M., Lui S. F., Sanderson J. E. (2010) Prognostic value of plasma myeloperoxidase in ESRD patients. Am. J. Kidney Dis. 56, 937–946 [DOI] [PubMed] [Google Scholar]
- 9. Eiserich J. P., Baldus S., Brennan M. L., Ma W., Zhang C., Tousson A., Castro L., Lusis A. J., Nauseef W. M., White C. R., Freeman B. A. (2002) Myeloperoxidase, a leukocyte-derived vascular NO oxidase. Science 296, 2391–2394 [DOI] [PubMed] [Google Scholar]
- 10. Astern J. M., Pendergraft W. F., 3rd, Falk R. J., Jennette J. C., Schmaier A. H., Mahdi F., Preston G. A. (2007) Myeloperoxidase interacts with endothelial cell-surface cytokeratin 1 and modulates bradykinin production by the plasma kallikrein-kinin system. Am. J. Pathol. 171, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McMillen T. S., Heinecke J. W., LeBoeuf R. C. (2005) Expression of human myeloperoxidase by macrophages promotes atherosclerosis in mice. Circulation 111, 2798–2804 [DOI] [PubMed] [Google Scholar]
- 12. Klinke A., Nussbaum C., Kubala L., Friedrichs K., Rudolph T. K., Rudolph V., Paust H. J., Schröder C., Benten D., Lau D., Szocs K., Furtmüller P. G., Heeringa P., Sydow K., Duchstein H. J., Ehmke H., Schumacher U., Meinertz T., Sperandio M., Baldus S. (2011) Myeloperoxidase attracts neutrophils by physical forces. Blood 117, 1350–1358 [DOI] [PubMed] [Google Scholar]
- 13. Rudolph V., Andrié R. P., Rudolph T. K., Friedrichs K., Klinke A., Hirsch-Hoffmann B., Schwoerer A. P., Lau D., Fu X., Klingel K., Sydow K., Didié M., Seniuk A., von Leitner E. C., Szoecs K., Schrickel J. W., Treede H., Wenzel U., Lewalter T., Nickenig G., Zimmermann W. H., Meinertz T., Böger R. H., Reichenspurner H., Freeman B. A., Eschenhagen T., Ehmke H., Hazen S. L., Willems S., Baldus S. (2010) Myeloperoxidase acts as a profibrotic mediator of atrial fibrillation. Nat. Med. 16, 470–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao H., Heeringa P., Hu P., Liu Z., Zhao M., Aratani Y., Maeda N., Falk R. J., Jennette J. C. (2002) Antineutrophil cytoplasmic autoantibodies specific for myeloperoxidase cause glomerulonephritis and vasculitis in mice. J. Clin. Invest. 110, 955–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Segelmark M., Persson B., Hellmark T., Wieslander J. (1997) Binding and inhibition of myeloperoxidase (MPO): a major function of ceruloplasmin? Clin. Exp. Immunol. 108, 167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chapman A. L., Mocatta T. J., Shiva S., Seidel A., Chen B., Khalilova I., Paumann-Page M. E., Jameson G. N., Winterbourn C. C., Kettle A. J. (2013) Ceruloplasmin is an endogenous inhibitor of myeloperoxidase. J. Biol. Chem. 288, 6465–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sengeløv H., Kjeldsen L., Borregaard N. (1993) Control of exocytosis in early neutrophil activation. J. Immunol. 150, 1535–1543 [PubMed] [Google Scholar]
- 18. Sengeløv H., Follin P., Kjeldsen L., Lollike K., Dahlgren C., Borregaard N. (1995) Mobilization of granules and secretory vesicles during in vivo exudation of human neutrophils. J. Immunol. 154, 4157–4165 [PubMed] [Google Scholar]
- 19. Essin K., Gollasch M., Rolle S., Weissgerber P., Sausbier M., Bohn E., Autenrieth I. B., Ruth P., Luft F. C., Nauseef W. M., Kettritz R. (2009) BK channels in innate immune functions of neutrophils and macrophages. Blood 113, 1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uriarte S. M., Rane M. J., Luerman G. C., Barati M. T., Ward R. A., Nauseef W. M., McLeish K. R. (2011) Granule exocytosis contributes to priming and activation of the human neutrophil respiratory burst. J. Immunol. 187, 391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. von Vietinghoff S., Tunnemann G., Eulenberg C., Wellner M., Cristina Cardoso M., Luft F. C., Kettritz R. (2007) NB1 mediates surface expression of the ANCA antigen proteinase 3 on human neutrophils. Blood 109, 4487–4493 [DOI] [PubMed] [Google Scholar]
- 22. Flemmig J., Zschaler J., Remmler J., Arnhold J. (2012) The fluorescein-derived dye aminophenyl fluorescein is a suitable tool to detect hypobromous acid (HOBr)-producing activity in eosinophils. J. Biol. Chem. 287, 27913–27923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bouma M. G., Jeunhomme T. M., Boyle D. L., Dentener M. A., Voitenok N. N., van den Wildenberg F. A., Buurman W. A. (1997) Adenosine inhibits neutrophil degranulation in activated human whole blood: involvement of adenosine A2 and A3 receptors. J. Immunol. 158, 5400–5408 [PubMed] [Google Scholar]
- 24. Meotti F. C., Jameson G. N., Turner R., Harwood D. T., Stockwell S., Rees M. D., Thomas S. R., Kettle A. J. (2011) Urate as a physiological substrate for myeloperoxidase: implications for hyperuricemia and inflammation. J. Biol. Chem. 286, 12901–12911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zouaoui Boudjeltia K., Moguilevsky N., Legssyer I., Babar S., Guillaume M., Delree P., Vanhaeverbeek M., Brohee D., Ducobu J., Remacle C. (2004) Oxidation of low density lipoproteins by myeloperoxidase at the surface of endothelial cells: an additional mechanism to subendothelium oxidation. Biochem. Biophys. Res. Commun. 325, 434–438 [DOI] [PubMed] [Google Scholar]
- 26. Bergt C., Pennathur S., Fu X., Byun J., O'Brien K., McDonald T. O., Singh P., Anantharamaiah G. M., Chait A., Brunzell J., Geary R. L., Oram J. F., Heinecke J. W. (2004) The myeloperoxidase product hypochlorous acid oxidizes HDL in the human artery wall and impairs ABCA1-dependent cholesterol transport. Proc. Natl. Acad. Sci. U.S.A. 101, 13032–13037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shock A., Baum H. (1988) Inactivation of α1-proteinase inhibitor in serum by stimulated human polymorphonuclear leucocytes: evidence for a myeloperoxidase-dependent mechanism. Cell Biochem. Funct. 6, 13–23 [DOI] [PubMed] [Google Scholar]
- 28. Lau D., Mollnau H., Eiserich J. P., Freeman B. A., Daiber A., Gehling U. M., Brümmer J., Rudolph V., Münzel T., Heitzer T., Meinertz T., Baldus S. (2005) Myeloperoxidase mediates neutrophil activation by association with CD11b/CD18 integrins. Proc. Natl. Acad. Sci. U.S.A. 102, 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Horner H., Frank C., Dechant C., Repp R., Glennie M., Herrmann M., Stockmeyer B. (2007) Intimate cell conjugate formation and exchange of membrane lipids precede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. J. Immunol. 179, 337–345 [DOI] [PubMed] [Google Scholar]
- 30. van Spriel A. B., Leusen J. H., van Egmond M., Dijkman H. B., Assmann K. J., Mayadas T. N., van de Winkel J. G. (2001) Mac-1 (CD11b/CD18) is essential for Fc receptor-mediated neutrophil cytotoxicity and immunologic synapse formation. Blood 97, 2478–2486 [DOI] [PubMed] [Google Scholar]
- 31. Campbell E. J., Senior R. M., McDonald J. A., Cox D. L. (1982) Proteolysis by neutrophils: relative importance of cell-substrate contact and oxidative inactivation of proteinase inhibitors in vitro. J. Clin. Invest. 70, 845–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rice W. G., Weiss S. J. (1990) Regulation of proteolysis at the neutrophil-substrate interface by secretory leukoprotease inhibitor. Science 249, 178–181 [DOI] [PubMed] [Google Scholar]
- 33. Loike J. D., Silverstein R., Wright S. D., Weitz J. I., Huang A. J., Silverstein S. C. (1992) The role of protected extracellular compartments in interactions between leukocytes, and platelets, and fibrin/fibrinogen matrices. Ann. N.Y. Acad. Sci. 667, 163–172 [DOI] [PubMed] [Google Scholar]