Background: The role of cofactors in regulating the TCA cycle is poorly understood. The source(s) of NADPH contributing to reductive carboxylation (RC) in the mitochondrion are unknown.
Results: Knockdown of nicotinamide nucleotide transhydrogenase (NNT) decreases RC and stimulates glucose catabolism in the TCA cycle.
Conclusion: NNT produces NADPH for RC and modulates glucose catabolism.
Significance: NNT coordinates glutamine and glucose metabolism in the TCA cycle.
Keywords: Glutamine, Metabolic Tracers, Mitochondria, Pyruvate Carboxylase, Redox Regulation, NADPH, NNT, Reductive Carboxylation
Abstract
Cancer and proliferating cells exhibit an increased demand for glutamine-derived carbons to support anabolic processes. In addition, reductive carboxylation of α-ketoglutarate by isocitrate dehydrogenase 1 (IDH1) and 2 (IDH2) was recently shown to be a major source of citrate synthesis from glutamine. The role of NAD(P)H/NAD(P)+ cofactors in coordinating glucose and glutamine utilization in the tricarboxylic acid (TCA) cycle is not well understood, with the source(s) of NADPH for the reductive carboxylation reaction remaining unexplored. Nicotinamide nucleotide transhydrogenase (NNT) is a mitochondrial enzyme that transfers reducing equivalents from NADH to NADPH. Here, we show that knockdown of NNT inhibits the contribution of glutamine to the TCA cycle and activates glucose catabolism in SkMel5 melanoma cells. The increase in glucose oxidation partially occurred through pyruvate carboxylase and rendered NNT knockdown cells more sensitive to glucose deprivation. Importantly, knocking down NNT inhibits reductive carboxylation in SkMel5 and 786-O renal carcinoma cells. Overexpression of NNT is sufficient to stimulate glutamine oxidation and reductive carboxylation, whereas it inhibits glucose catabolism in the TCA cycle. These observations are supported by an impairment of the NAD(P)H/NAD(P)+ ratios. Our findings underscore the role of NNT in regulating central carbon metabolism via redox balance, calling for other mechanisms that coordinate substrate preference to maintain a functional TCA cycle.
Introduction
Cancer and proliferating cells largely depend on glycolysis but also switch to a glutamine-maintained tricarboxylic acid (TCA)2 cycle to meet the needs of accelerated growth and proliferation (1–3). Such metabolic reprogramming maximizes the utilization of glucose for glucose-specific anabolic processes, while ensuring a functional TCA cycle for pyrimidine, protein, and lipid synthesis (4, 5). In this context, elucidating the signaling pathways and allosteric mechanisms that coordinate glucose and glutamine utilization is key to identify metabolic dependencies of cancer cells. Recent efforts in cancer biology have elucidated how transcription factors, oncogenes, and environmental cues not only regulate glucose and glutamine uptake but also determine their intracellular fate (6–9). Myc is an established oncogenic activator of glutaminolysis in glioma cells (6), whereas glucose-derived anaplerosis via pyruvate carboxylase is required for glutamine-independent growth (10). In addition, glucose uptake and glutamine uptake are coupled through the hexosamine biosynthetic pathway (11) and Mondo A transcriptional activation (12). Also, hypoxia occurs often in the tumor microenvironment, and the stabilization of hypoxia-inducible factors (HIFs) is known to divert glucose to lactate production through the trans-activation of glycolytic enzymes, PKM2, lactate dehydrogenase A, and PDK-1 (13–16). Moreover, hypoxia was recently shown to concurrently activate reductive carboxylation (RC) of α-ketoglutarate by isocitrate dehydrogenase 1 (IDH1) and 2 (IDH2) to support citrate production (7, 17–19), whereby NADPH donates the hydride ion (H−) that reduces α-ketoglutarate into isocitrate (20–22). The mechanisms by which hypoxia promotes RC were under debate until recently. We showed that hypoxia-inducible factors (HIFs) are necessary and sufficient to promote RC through regulation of citrate levels (46). However, HIF expression did not increase NADPH levels, and the sources of this cofactor for the reverse reaction of IDH enzymes have not been investigated.
It is recognized that the TCA cycle is inherently an oxygen-dependent pathway that oxidizes metabolic intermediates to produce NADH, the reducing equivalents necessary for ATP production. Thus, the mitochondrial NADH/NAD+ ratio is an allosteric regulator of the TCA cycle activity via coupling to oxidative phosphorylation (23), but its role in coordinating glucose and glutamine entry in the TCA cycle is not well understood from a carbon flux distribution point of view. Given the requirement of a functional TCA cycle for a cell to proliferate, it is possible that shifting the NADH/NAD+ to a more oxidized or reduced state may differently affect glucose and glutamine utilization in the TCA cycle.
The enzyme nicotinamide nucleotide transhydrogenase (NNT), residing in the inner membrane of the mitochondrion, catalyzes the transfer of hydride ion equivalents (H−) from NADH to NADPH using the proton gradient (xH+ out → xH+ in) as follows: NADH + NADP+ + xH+(out) ↔NAD+ + NADPH + xH+(in). NNT is regarded as a major source of NADPH in the mitochondrion and reduced glutathione (24–26). It is therefore conceivable that NNT activity generates the NADPH necessary for the RC reaction in the mitochondrion, a hypothesis that has been speculated before (27), but has never been experimentally tested. Moreover, given the connection to the NADH/NAD+ balance, NNT may also coordinate glucose and glutamine catabolism in the TCA cycle. Here, using 13C isotopic tracers, we elucidate the function of NNT in central carbon metabolism, in particular its contribution to RC and regulation of glucose catabolism.
EXPERIMENTAL PROCEDURES
Cell Culture and Metabolic Labeling
SkMel5 and 786-O cell lines were obtained from ATCC. Cells were maintained in DMEM (Life Technologies, 25 mm glucose, 4 mm glutamine, 1 mm pyruvate) containing 10% fetal bovine serum (FBS; HyClone) supplemented with penicillin-streptomycin at 5% CO2-containing incubators. Metabolic labeling was described as before (7). In brief, cells were seeded in 6-well plates and labeled with glucose- and glutamine-free DMEM (basal DMEM, Sigma), containing 10% dialyzed FBS (HyClone), supplemented with 4 mm [13C]glutamine tracer and 25 mm unlabeled glucose or 25 mm [13C]glucose tracer and 4 mm unlabeled glutamine. All tracers were purchased from Cambridge Isotope Laboratories. Metabolic labeling was conducted for 24 h before metabolite extraction. For cell viability assays, cells were seeded in 12-well plates and cultured for 24 h in basal DMEM supplemented with 5 mm glucose, 1 mm glutamine, and 10% dialyzed FBS (control condition). Cell viability was assayed by crystal violet staining; quantification of cell viability was determined by the absorbance of crystal violet-retaining cells at 595 nm.
Generation of NNT Knockdown and Overexpressing Cells
Stable cell populations of Sk-Mel5 and 786-O cells with decreased NNT expression were generated by infection with lentiviral particles containing NNT-targeting pLKO.1 lentiviral vectors that had shRNA sequences of 5′-CCGGCCCTATGGTTAATCCAACATTCTCGAGAATGTTGGATTAACCATAGGGTTTTT-3′ (NNTA; TRCN0000028541, Sigma) and 5′-CCGGCGAGAAGCTAATAGCATTATTCTCGAGAATAATGCTATTAGCTTCTCGTTTTT-3′ (NNTD; TRCN0000028507, Sigma). We used a nontargeting shRNA sequence of CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT (scramble control; SHC002, Sigma) as control. Suitable lentiviral-packaging plasmids were cotransfected with each of the pLKO.1 vectors into HEK293T cells, and supernatants containing lentiviruses were collected after 48 h, filtered, and used for infection. Cells were selected with 2 μg/ml puromycin. For the exogenous expression of NNT, we transfected Sk-Mel5 cells with a pCMV6-Entry vector containing a full-length cDNA clone of NNT (RC224002; OriGene) together with TurboFectin 8 transfection reagent (OriGene). A polyclonal population was generated by selecting the SkMel5 cells with 0.75 mg/ml neomycin (G418) for 2–3 weeks.
Metabolite Extractions and GC-MS Analysis
The method was described previously (7, 28). In brief, at 70–80% of cellular confluence, metabolic activity was quenched with 1 volume of −80 °C methanol, 1 volume of water was added, cells were detached by scraping the plate, and biphasic extracts were obtained by adding 2 volumes of −20 °C chloroform. The polar phase containing organic and amino acids was evaporated, dissolved in 30 μl of 2% methoxyamine hydrochloride in pyridine (Pierce) at 37 °C for 1.5 h, and derivatized by adding 45 μl of N-methyl-N-(tert-butyldimethylsilyl)trifluoroacetamide (MTBSTFA) + 1% tert-butyldimethylchlorosilane (TBDMCS; Pierce) at 60 °C for 1 h prior to injection on the GC-MS. GC-MS analysis was performed using an Agilent 6890 GC equipped with a 30-m DB-35MS capillary column interfaced to an Agilent 5975B MS operating under electron impact ionization at 70 eV. One μl of sample was injected at 270 °C, using helium as the carrier gas at a flow rate of 1 ml × min−1. The GC oven temperature was held at 100 °C for 3 min and increased to 300 °C at 3.5° min−1. The detector was operated in scanning mode, recording mass-to-charge ratio spectra in the range of 100–605 m/z.
NAD(P)H/NAD(P)+ Measurements
Cellular NADPH/NADP+ and NADH/NAD+ levels were measured using a fluorescence-based assay kit, according to the manufacturer's instructions (Cell Technology). Standard deviations of mean ratios were calculated using a first-order Taylor approximation of averaged ratios obtained from triplicate measurements. One representative experiment was shown. Cellular NAD(P)H/NAD(P)+ ratios were also measured by LC-MS, employing a methanol extraction, as described previously (29).
Analysis of Spent Medium
Cells were seeded in 6-well plates at low density in basal DMEM (Sigma) supplemented with 25 mm glucose and 4 mm glutamine, spent medium was collected at the end of a 72-h growth period (in triplicate), and glucose, lactate, glutamine, and glutamate concentrations were measured using a Yellow Springs Instruments YSI 7100. The specific growth rate (μ, h−1) was estimated using a linearized equation of cell growth, Ln(X) = Ln(X0) + μt, and the uptake/secretion fluxes (pmol/cell/hour) were calculated by multiplying the normalized consumption/secretion coefficient (Δn/ΔX, difference in moles consumed/secreted divided by difference in cell density) by the estimated μ.
Western Blotting
Proteins were extracted with a 5% SDS aqueous solution to maximize lysis of mitochondrial proteins. Cells were then scraped, and proteins were denatured at 95 °C for 10 min. Cell lysates were clarified by centrifugation, and the proteins were resolved in SDS-PAGE gels, transferred to PVDF membrane (Bio-Rad), and detected by immunoblotting using the following antibodies: mouse monoclonal anti-NNT (Invitrogen, 1: 2,000) and mouse monoclonal anti-actin (NeoMarkers, 1:30,000).
Animal Studies
All protocols conformed to institutional regulations. 5 × 106 NNT knockdown and control SkMel5 cells were injected subcutaneously in the left and right flank of nu/nu mice, respectively. At day 20, animals were sacrificed by cervical dislocation, and tumors were collected and weighed.
Statistical Analysis
Error bars represent S.E. unless otherwise noted. Statistical significance was determined using two-tailed Student's t test, comparing NNT knockdown (NNTA and NNTD) or NNT-overexpressing cells (Ex-NNT) to scramble vector cells, unless otherwise noted. # denotes p < 0.05, and * denotes p < 0.001.
RESULTS
Knockdown of NNT Decreases the Contribution of Glutamine into the TCA Cycle
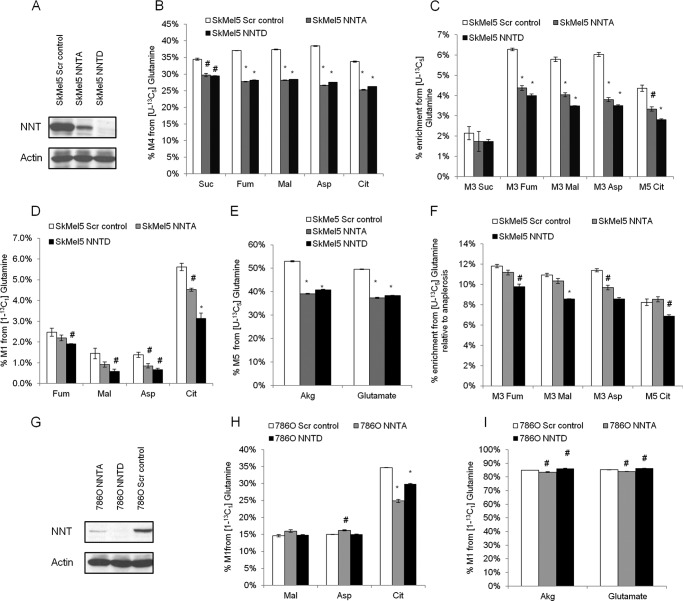
We hypothesized that disturbances in the mitochondrial NAD(P)H/NAD(P)+ balance due to knockdown of NNT would affect glucose and glutamine utilization in the TCA cycle. To investigate the role of NNT in central carbon metabolism, we knocked down its translation and employed different [13C]glucose and [13C]glutamine isotopic tracers to determine the enrichment of TCA cycle metabolites by GC-MS analysis. We infected SkMel5 melanoma cell lines with lentiviruses containing NNT-targeting shRNA sequences and generated NNT knockdown polyclonal cell populations (Fig. 1A). The SkMel5 cell line highly expresses NNT at the RNA level, along with other melanomas, leukemia, and some renal cell carcinoma cells, according to the NCI-60 panel (dtp.nci.nih.gov/mtargets/mt_search.html). To trace the contribution of glutamine to the TCA cycle, we cultured the SkMel5 cells in the presence of the [U-13C5]glutamine tracer. Knockdown of NNT decreased the contribution of glutamine oxidation to the TCA cycle, as determined by the level of M4 enrichment of TCA cycle metabolites (Fig. 1B). We observed that the enrichment of M5 citrate, M3 fumarate, M3 malate, and M3 aspartate was also significantly decreased in NNT knockdown SkMel5 cells when compared with control cells infected with scramble vector shRNA (Fig. 1C), which indicates a decreased contribution of reductive carboxylation to the TCA cycle. We also tested the effect of NNT knockdown on RC by culturing the cells with the [1-13C1]glutamine tracer, which specifically transfers the 13C-labeled carbon to the TCA cycle metabolites only through RC. Knocking down NNT decreased the percentage of M1 TCA cycle metabolites in SkMel5 cells (Fig. 1D). We observed that the anaplerotic contribution from glutamine was also reduced in NNT knockdown SkMel5 cells (Fig. 1E), suggesting a switch in substrate preference (from glutamine to glucose) for the production of α-ketoglutarate/glutamate. Nevertheless, we observed a specific inhibition of RC in SkMel5 cells under NNT knockdown conditions when accounting for the anaplerotic contribution from glutamine (Fig. 1F). We also tested the role of NNT on RC activity in a VHL-deficient renal cell carcinoma cell line, which relies heavily on this reductive pathway (7). To this end, we knocked down NNT in VHL-deficient 786-O cells (Fig. 1G) and cultured them in the presence of [1-13C1]glutamine. Scramble vector-infected 786O cells exhibited a high RC activity when compared with Sk-Mel5 cells (Fig. 1H). Knockdown of NNT decreased the enrichment of M1 citrate (Fig. 1H), whereas it only modestly affected glutamine-derived anaplerosis in 786-O cells (Fig. 1I), as determined by comparing the M1 enrichment between α-ketoglutarate and glutamate. This difference in the anaplerotic response to NNT knockdown may be due to the specific allosteric effects of NAD(P) on glutamate dehydrogenase (see “Discussion”). Interestingly, we did not observe changes in the M1 enrichment of fumarate, malate, and aspartate, downstream metabolites of the IDH2 reaction that are formed in the cytosol. Therefore, we cannot rule out a compensatory role of the cytosolic IDH1 reaction in cells that largely rely on this pathway and may require a constant supply of NADPH. Taken together, these data show that NNT, strictly involved in cofactor balance, contributes to glutamine oxidation and reductive carboxylation in the mitochondrion.
FIGURE 1.
Effect of NNT knockdown on glutamine catabolism. A, validation of NNT knockdown in SkMel5 cells. B–F, effect of NNT knockdown on glutamine metabolism in SkMel5 cells. B and C, contribution of glutamine oxidation (B) and reductive carboxylation (C) to the TCA cycle, from [U-13C5]glutamine. Scr control, scramble control; Suc, succinate; Fum, fumarate; Mal, malate; Asp, aspartate; Cit, citrate. D, contribution of reductive carboxylation to the TCA cycle using the [1-13C1]glutamine tracer. E, contribution of glutamine anaplerosis to α-ketoglutarate (Akg) formation. F, normalized contribution of reductive carboxylation in the panel of SkMel5 cells, from [U-13C5]glutamine. G, validation of NNT knockdown in 786-O cells. H, contribution of reductive carboxylation to the TCA cycle in NNT knockdown 786-O cells. I, contribution of glutamine anaplerosis to α-ketoglutarate (Akg) formation in 786-O cells.
Knockdown of NNT Stimulates Glucose Catabolism in the TCA Cycle
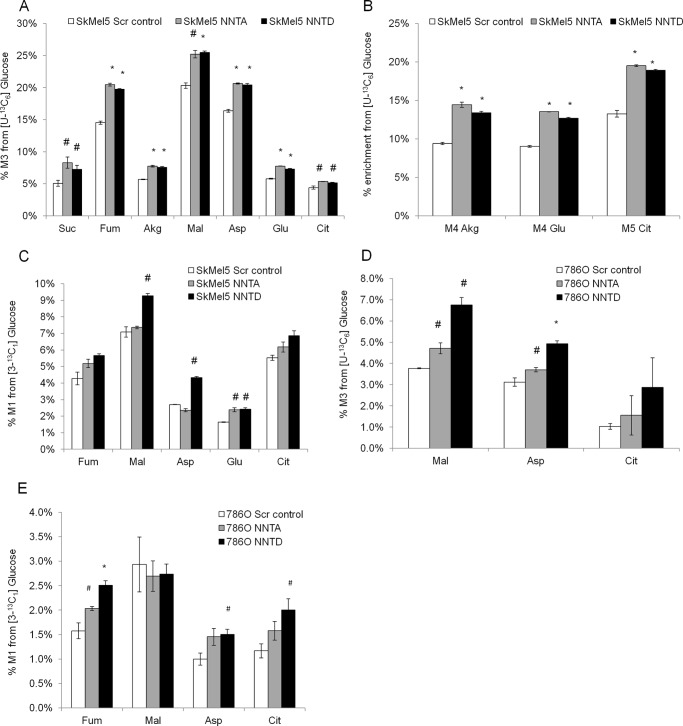
To study the role of NNT in glucose metabolism, we labeled the SkMel5 cells with [U-13C6]glucose for 24 h and determined its contribution to the formation of TCA cycle metabolites by GC-MS analysis. The [U-13C6]glucose tracer transfers two 13C units to TCA cycle metabolites via pyruvate dehydrogenase, whereas pyruvate carboxylase (PC) activity incorporates three 13C atoms in oxaloacetate, which then propagate to other TCA cycle metabolites. As such, M3 mass isotopomers of TCA cycle metabolites are associated with PC activity, whereas M5 citrate, M4 α-ketoglutarate, and M4 glutamate reflect the overall contribution of glucose carbons via pyruvate dehydrogenase and PC. Although knockdown of NNT decreased glutamine oxidation in SkMel5 cells, it concurrently activated glucose catabolism in the TCA cycle, as seen by the level of enriched TCA cycle metabolites from [U-13C6]glucose (Fig. 2, A and B), suggesting that knockdown of NNT switches substrate preference to a glucose-derived TCA cycle. We did not observe changes in the M2 isotopomers of these metabolites, indicating that the specific contribution of pyruvate dehydrogenase (relative to PC-derived anaplerosis) was not significantly affected under NNT knockdown conditions (data not shown). Because M3 mass isotopomers can also be formed by continued TCA cycling, we cultured the SkMel5 cells with [3-13C1]glucose to trace the specific contribution of glucose-derived anaplerosis to the TCA cycle, in which the labeled carbon in pyruvate is either retained in oxaloacetate via PC or lost as CO2 through pyruvate dehydrogenase. PC activity was stimulated in NNT knockdown SkMel5 (Fig. 2C). Of note, the increase in the M1 enrichment of TCA cycle metabolites in NNT knockdown cells was not as pronounced as that observed in the M3 and M5 isotopomers from [U-13C6]glucose. Because the M1 oxaloacetate (in the one-carbon form) from [3-13C1]glucose leads to M1 citrate (in the six-carbon form), the 13C label is partially lost during oxidative TCA cycle, possibly diluting the propagation of the PC-derived 13C label during subsequent TCA cycling. Similar results were obtained in 786-O cells (Fig. 2, D and E), although the effect was not as pronounced as that observed in SkMel5 cells. These observations support our results using [U-13C5]glutamine, which show that glutamine oxidation is decreased in SkMel5 cells but is not significantly affected in 786-O cells upon NNT knockdown.
FIGURE 2.
Effect of NNT knockdown on glucose catabolism. A and B, contribution of glucose oxidation to the TCA cycle in the panel of SkMel5 cells, determined by the enrichment of M3 (A) and M4/M5 (B) TCA cycle metabolites, from [U-13C6]glucose. Scr control, scramble control; Suc, succinate; Fum, fumarate; Akg, α-ketoglutarate; Mal, malate; Asp, aspartate; Cit, citrate. C, specific contribution of pyruvate carboxylase to the formation of TCA cycle metabolites in NNT knockdown and control SkMel5 cells, using the [3-13C1]glucose tracer. D and E, contribution of glucose oxidation to the TCA cycle in the panel of 786-O cells, from [U-13C6]glucose (D) and [3-13C1]glucose (E).
NNT Is Sufficient to Stimulate RC and Suppress Glucose Catabolism in the TCA Cycle
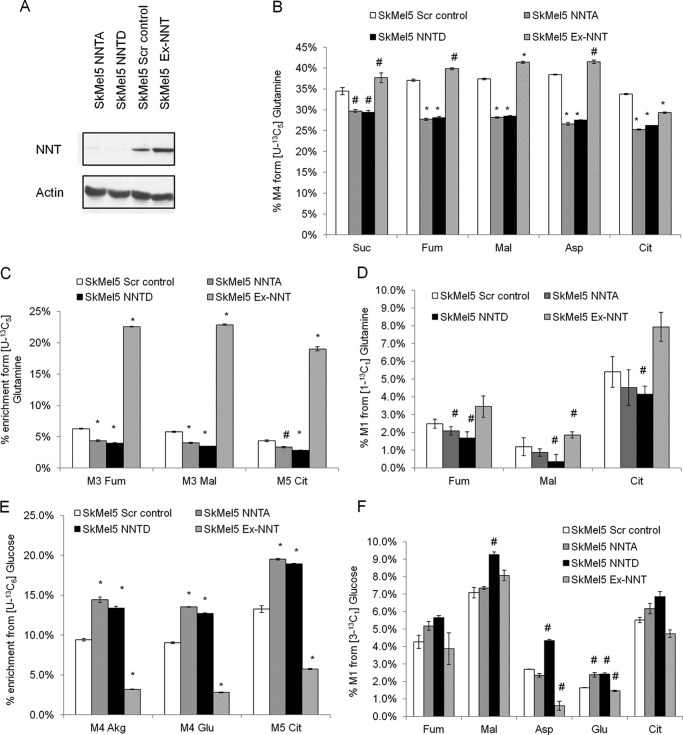
Because knockdown of NNT affected glucose and glutamine utilization in the TCA cycle, we investigated whether overexpression of NNT could be sufficient to induce a complementary metabolic response, in particular in the contribution of reductive carboxylation (RC). To this end, we stably transfected SkMel5 cells with an NNT-coding cDNA plasmid and generated a polyclonal cell population that overexpressed NNT (Ex-NNT) when compared with their scramble vector counterparts (Fig. 3A). Overexpressing NNT simulated the contribution of glutamine oxidation (Fig. 3B) and RC (Fig. 3, C and D) to the TCA cycle. Conversely, PC-derived anaplerosis was inhibited in NNT-overexpressing cells when compared with the scramble vector cells (Fig. 3, E and F). These data suggest that NNT is sufficient to coordinate glutamine and glucose utilization in the TCA cycle.
FIGURE 3.
Metabolic effects of NNT overexpression in SkMel5 cells. A, NNT protein levels in the panel of SkMel5 cells (Ex-NNT, lane 4). B–D, effect of NNT overexpression on glutamine catabolism. B and C, contribution of glutamine oxidation (B) and reductive carboxylation (C) from [U-13C5]glutamine. Scr control, scramble control; Suc, succinate; Fum, fumarate; Mal, malate; Asp, aspartate; Cit, citrate. D, effect of NNT overexpression on reductive carboxylation, using the [1-13C1]glutamine tracer. E and F, contribution of glucose oxidation to the TCA cycle in the panel of SkMel5 cells, from [U-13C6]glucose (E) and [3-13C1]glucose (F). Akg, α-ketoglutarate.
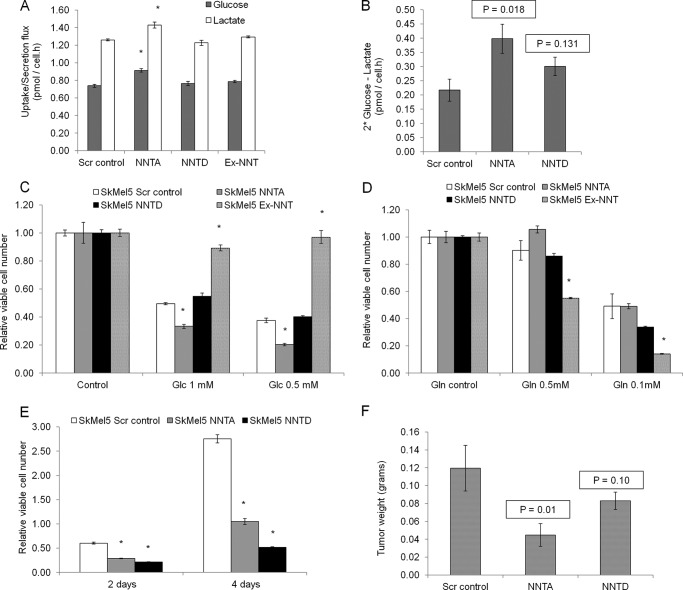
Knockdown of NNT Decreases Cell Proliferation and Sensitizes SkMel5 Melanoma Cells to Glucose Deprivation
Because knockdown of NNT switched the substrate preference in the TCA cycle from glutamine to glucose carbons, we hypothesized that NNT knockdown cells would be more dependent on glucose for proliferation. To test this, we first measured the glucose uptake and lactate secretion rates in the panel of SkMel5 cells over a period of 72 h. NNT knockdown SkMel5 cells exhibited higher glucose uptake and lactate secretion when compared with control cells (Fig. 4A). The net intake of glucose was also higher in NNT knockdown cells when compared with control cells (Fig. 4B), compatible with an overall higher demand of glucose carbons to fuel the TCA cycle. We did not observe significant differences in glutamine consumption and glutamate production rates in the panel of SkMel5 cells (data not shown). Next, to exploit the dependence on glucose carbons, we cultured the panel of SkMel5 cell lines for 24 h in low glucose medium. Loss of viability was more pronounced in NNT knockdown cells, with NNT-overexpressing cells being almost insensitive to low glucose concentrations (Fig. 4C). Conversely, NNT-overexpressing cells were more sensitive to glutamine deprivation when cultured in low glutamine-containing medium (Fig. 4D). We also observed that NNT knockdown SkMel5 cells exhibited decreased proliferation when compared with the control cells (Fig. 4E), with knockdown of NNT partially suppressing the growth of SkMel5 cells growing as xenograft tumors in nu/nu mice (Fig. 4F). In agreement with the isotopic studies, these findings show that low expression of NNT partially rewires glucose and glutamine catabolism in the TCA cycle, rendering cells sensitive to glucose deprivation.
FIGURE 4.
Effect of NNT knockdown on cell proliferation and sensitivity to glucose carbons. A, glucose uptake and lactate secretion rates in the panel of SkMel5 cells used. Scr control, scramble control. B, net intake of glucose carbons as determined by the difference between twice the glucose consumption and lactate production rates. C and D, cell proliferation under low glucose (C) and low glutamine (D) conditions. Cell proliferation is normalized to the corresponding control cell type grown in 5 mm glucose- and 1 mm glutamine-containing medium. E, effect of NNT knockdown on the proliferation of SkMel5 cells. F, effect of NNT knockdown of the proliferation of SkMel5 cells growing as xenograft tumors in nu/nu mice. Student's t test compared the proliferation under low glucose- or glutamine-containing medium to correspondent controls in panels C and D. Statistical significance was determined using one-tailed Student's t test in panel F, n = 5 or more.
Effect of NNT Knockdown on Glucose and Glutamine Catabolism Is Mediated by a Disturbed NAD(P)H/NAD(P)+ Balance
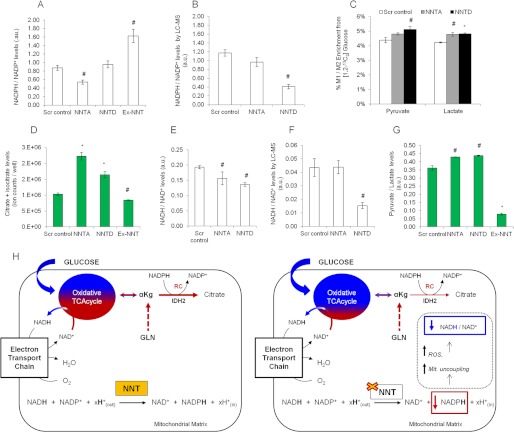
Knockdown of NNT decreased the contribution of reductive carboxylation (RC) in the TCA cycle, whereas NNT overexpression stimulated this pathway. To test whether the contribution of NNT to RC is related to contributing to RC is related to its ability to produce NADPH, we quantified the cellular ratios of the NADP couple using a fluorescent enzymatic assay and LC-MS. As shown in Fig. 5, A and B, the cellular ratio of NADPH/NADP+ was lower in NNT knockdown than in scramble control SkMel5 cells, whereas NNT-overexpressing cells exhibited a higher ratio (Fig. 5A). The NADPH and NADP+ levels and the effect of each NNT knockdown construct on this ratio varied based on the quantification method, possibly reflecting the different extraction procedures. To examine the compensatory role of glucose in providing cytosolic NADPH, we assessed the activity of the pentose phosphate pathway (PPP) under NNT knockdown conditions. To this end, we labeled the panel of SkMel5 cells with [1,2-13C2]glucose, which transfers one labeled carbon to CO2 through oxidative PPP but conserves both 13C atoms via glycolysis (30). Knocking down NNT mildly increased the PPP activity relative to glycolysis in SKMel5 cells, as determined by the ratio of labeled carbon (M1) to dually labeled (M2) carbons of pyruvate and lactate (Fig. 5C). This result indicates that the observed changes in the NADPH/NADP+ ratio and RC in NNT knockdown cells are marginally compensated by PPP activity in the cytosol. The ratio of the mixed pool of isocitrate/citrate to α-ketoglutarate was not significantly affected in NNT knockdown cells (data not shown). This suggests that the IDH2 reaction may be at nonequilibrium under NNT knockdown conditions, wherein changes in the NADPH/NADP+ ratio are not accompanied by an altered ratio of the correspondent reduced (isocitrate/citrate) and oxidized (α-ketoglutarate) metabolites (31). Nevertheless, the citrate and isocitrate levels were elevated in NNT knockdown cells (Fig. 5D), suggesting that knockdown of NNT may indirectly affect the mass action of the IDH2 reaction by modulation of citrate levels.
FIGURE 5.
Cofactor levels in the panel of SkMel5 cells. A, NADPH/NADP+ levels, assayed using an enzymatic kit according to the manufacturer's instructions. Scr control, scramble control; AU, arbitrary units. B, NADPH/NADP+ levels, determined by LC-MS. C, contribution of PPP to pyruvate and lactate production, relative to glycolysis. D, citrate and isocitrate levels, determined by GC-MS analysis. E, NADH/NAD+ levels assayed using an enzymatic kit according to the manufacturer's instructions. F, NADH/NAD+ levels, determined by LC-MS. G, cellular pyruvate-to-lactate ratio, determined by GC-MS analysis. H, proposed model for the role of NNT in regulating the TCA cycle activity. Left diagram, NNT catalysis contributes to reductive carboxylation (RC) in the mitochondrion via production of NADPH. Under NNT activated conditions, cells exhibit an adequate balance between the contribution of glucose (blue)- and glutamine (red)-derived carbons to the oxidative TCA cycle. αKg, α-ketoglutarate. Right diagram, NNT loss of function decreases the NADPH/NADP+ ratio, which inhibits the formation of RC-derived citrate and increases reactive oxygen species (ROS) levels and mitochondrial uncoupling (Mit. uncoupling). This decreases the NADH/NAD+ ratio thereby stimulating the contribution of glucose catabolism into the TCA cycle, depicted as an increased percentage of the blue color in the oxidative TCA cycle. Error bars represent 95% confidence intervals in panels A and E.
We observed a decreased cellular NADH/NAD+ ratio in NNT knockdown cells (Fig. 5, E and F), in agreement with the findings of Yin et al. (26), which showed that knockdown of NNT decreased the cellular NADH/NAD+ ratio in PC12 cells. Nevertheless, this result is opposite to what would be expected based on the enzymatic reaction catalyzed by NNT. It is known that knockdown of NNT induces mitochondrial uncoupling and decreases the mitochondrial membrane potential (26, 32), which leads to a decreased mitochondrial NADH/NAD+ ratio. Conversely, because the majority of mitochondrial NADH is in the bound form (33) and mitochondria comprise up to 20% of the cellular volume (34), the observed NADH/NAD+ ratio represents mostly the ratio of the cytosolic compartment. To further examine the effect of NNT knockdown on the NADH/NAD+ balance, we determined the levels of pyruvate and lactate, which reflect changes in the cytosolic NADH/NAD+ ratio (31). NNT knockdown cells exhibited a higher pyruvate/lactate ratio than control cells (Fig. 5G), which indicates a lower cytosolic NADH/NAD+ ratio.
The NADH/NAD+ cofactor measurements support the model that knockdown of NNT stimulates the contribution of glucose oxidation to the TCA cycle as a response to a lower NADH/NAD+ ratio. Conversely, the NADPH/NADP+ measurements corroborate our 13C-labeled glutamine studies, showing that the role of NNT in contributing to RC lies in its ability to produce NADPH in the mitochondrion (Fig. 5H).
DISCUSSION
NNT is recognized as a main generator of mitochondrial NADPH (24). NNT has also been speculated to regulate insulin secretion and glucose homeostasis, and some studies show that NNT mutant C57BL/6J mice exhibit glucose intolerance and impaired insulin secretion (32, 35), whereas other studies have challenged this hypothesis (36, 37). Being involved in NADPH production and proton translocation, NNT has been viewed mostly through the lens of reactive oxygen species detoxification and stress response (24), with mutations in NNT being reported in individuals with familial glucocorticoid deficiency (38). NNT was also shown to modulate the inflammatory response of macrophages (39), again underscoring its role as a free radical detoxifier in the mitochondrion. NNT is strictly a cofactor-modulating enzyme, transferring the reducing equivalents (the hydride ion) from NADH to NADP+, which produces the NADPH necessary to drive some anabolic reactions. In line with this perspective, the function of NNT in regard to central carbon metabolism has not been studied before. Our 13C isotopic studies elucidated the outcome of a disturbed cofactor balance on the TCA cycle activity.
Our work showed that RC by IDH2 can be modulated at the mass action level through the supply of NADPH. Knockdown of NNT inhibited the contribution of RC to the TCA cycle in SkMel5 and 786-O cells, whereas overexpressing NNT was sufficient to stimulate this reaction. There are other candidate enzymes that may contribute to mitochondrial NADPH production. The malic enzyme 3 (ME3) is localized in the mitochondrion and couples the production of pyruvate to reduction of NADP+ (40). We did not assess the expression levels of ME3 in SkMel5 cells or 786-O cells or its contribution to RC. However, ME3 is a carbon-metabolizing enzyme, and genetic approaches such as shRNA-mediated knockdown of overexpression will perturb not only cofactor balance but also carbon distribution. Thus, the delineation of the role of NADPH supply from that of carbon redistribution requires strategies to rule out metabolic effects that are NADPH-independent. The knockdown of NNT also inhibited glutamine oxidation in SkMel5 cells, which can be partially accounted for by a decreased anaplerosis from glutamine. Nevertheless, the contribution of RC was decreased in NNT knockdown SkMel5 cells when normalized to the anaplerotic contribution from glutamine. Conversely, the [U-13C5]glutamine and [1-13C1]glutamine studies in 786-O cells showed only a modest effect of NNT knockdown at the level of glutamine-derived anaplerosis. The conversion of glutamate into α-ketoglutarate (anaplerosis) can be mediated by glutamate dehydrogenase 1 (GLUD1) or aspartate transaminase (GOT1 and GOT2). In addition, the GLUD1 reaction can be NADP+-or NAD+-dependent toward the deamination of glutamate (41). As such, it is conceivable that the different anaplerotic response of SkMel5 and 786-O cells to NNT knockdown may relate to the different activity of GLUD1 and GOT1/2 and/or specific requirements of GLUD1 for NADP+ and NAD+ in the different cell lines. Together, our findings highlight the role of NNT in contributing to RC via NADPH production.
We showed that knockdown of NNT concomitantly increases glucose catabolism in the TCA cycle. The increase in glucose oxidation partially occurred via PC. This brings attention to the role of cofactors in coordinating glucose and glutamine utilization in the TCA cycle and supports existing literature showing that PC activity is required for the growth of tumor cells in the absence of glutamine (10). NNT knockdown SkMel5 cells exhibited a higher glucose uptake and appeared more sensitive to glucose deprivation than control cells, whereas NNT-overexpressing cells failed to do so and proliferated less under low glutamine conditions. Although the increased contribution of PC to the TCA cycle under NNT knockdown conditions was not as pronounced as that reported in “glutamine-independent cells” (10), these observations shed light into the metabolic plasticity of cancer cells in response to a disturbed cofactor balance. Of note, although NNTD resulted in a better knockdown at the protein level and TCA cycle response, we observed significant differences in the net intake of glucose, response to glucose deprivation, and in vivo proliferation only for NNTA (Fig. 4, B, C, and F). This discrepancy may reflect the metabolic burden of NNTA knockdown SkMel5 cells that, even though they do not rewire their intracellular metabolism to the same extent as NNTD cells, are forced to increase their net glucose uptake to maintain an adequate flux of glucose carbons into the TCA cycle.
It is of particular interest to discuss the observed changes in the NADH/NAD+ ratio and the expected outcome in central carbon metabolism. The knowledge on the enzymatic reaction of NNT predicts that the mitochondrial NADH/NAD+ ratio should be elevated under NNT knockdown conditions. We observed a decreased cellular NADH/NAD+ ratio in NNT knockdown cells, which is in agreement with the findings of Yin et al. (26) and opposite to the predicted trend. In this context, it is reported that knockdown of NNT leads to mitochondrial uncoupling, low ATP levels, and decreased mitochondrial membrane potential (24, 26), which is mechanistically associated with a lower mitochondrial NADH/NAD+ ratio. Therefore, the expected initial increase in mitochondrial NADH/NAD+ ratio upon NNT knockdown is most likely offset by the indirect effect on mitochondrial coupling and activity of the electron transport chain. In fact, Yin et al. (26) noted that knockdown of NNT led to an initial increase in the cellular NADH/NAD+ ratio followed by a decrease in this ratio. In addition, from the allosteric standpoint, a more reduced mitochondrion (higher NADH/NAD+ ratio) should inhibit the TCA cycle, which would be hard to reconcile with the increased glucose oxidation observed in NNT knockdown cells. However, because PC is an ATP-dependent enzyme, it is still conceivable that a higher mitochondrial NADH/NAD+ may equally activate this reaction to maintain a functional TCA cycle. Investigating the contribution of PC to the TCA cycle in more reduced or oxidized mitochondria is an open question. Overall, our isotopic studies showed that knockdown of NNT increases the contribution of glucose catabolism to the TCA cycle partially through PC, as a response to a lower NADH/NAD+ ratio.
Similarly to the study of Yin et al. (26), we measured the cellular NADH/NAD+ ratio, represents mostly the ratio of the cytosolic compartment. Indeed, the pyruvate/lactate ratio and pyruvate levels were higher in NNT knockdown cells when compared with control cells. In this context, the malate-aspartate shuttle (MAS) transfers reducing equivalents from the cytosol to the mitochondrion. The MAS activity is regulated by the mitochondrial redox state (42) and can be assessed by the pyruvate/lactate ratio (43–45). Because MAS is expected to be activated by mitochondrial NADH oxidation, this raises the possibility that NNT knockdown may activate the MAS in response to a lower mitochondrial NADH/NAD+ ratio. Conversely, MAS depends on the mitochondrial energetic state, with the addition of mitochondrial uncouplers inhibiting its activity (44). Because NNT knockdown is known to increase mitochondrial uncoupling, it is unclear how NNT knockdown may impact on the MAS activity. The effect of NNT knockdown on the kinetics of reducing equivalents transport between cytosol and mitochondrion is open to debate and will require direct measurement of the compartmentalized pools of these cofactors.
Collectively, our results show the implications of a disturbed NAD(P)H/NAD(P)+ balance in central carbon metabolism, highlighting the unexplored role of NNT in coordinating glucose and glutamine utilization in the TCA cycle. These findings underline the need for cancer biologists to consider cofactors and allosterism when studying the metabolic outcomes of growth signals and transduction pathways.
Acknowledgments
We thank Ana M. Metelo for insights on the xenograft experiments, Eric L. Bell for help with the NNT cDNA plasmid, Taylor Murphy for help on extracellular flux analyses, Thomas Wasylenko for feedback on cofactor measurements, and Christian M. Metallo for scientific discussions. This work was also supported by the Massachusetts General Hospital (MGH) ProtonBeam Federal Share Project.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK075850 (to G. S.) and R01 CA122591 (to O. I.). This work was also supported by an Award from Astra-Zeneca (to O. I.) and the Foundation for Science and Technology (FCT), Portugal (to P. A. G.).
This article was selected as a Paper of the Week.
- TCA
- tricarboxylic acid
- NNT
- nicotinamide nucleotide transhydrogenase
- IDH
- isocitrate dehydrogenase
- PC
- pyruvate carboxylase
- ME3
- malic enzyme 3
- GLUD1
- glutamate dehydrogenase 1
- GOT
- aspartate transaminase
- MAS
- malate-aspartate shuttle
- RC
- reductive carboxylation
- PPP
- pentose phosphate pathway
- HIFs
- hypoxia-inducible factors.
REFERENCES
- 1. Newsholme E. A., Crabtree B., Ardawi M. S. (1985) The role of high rates of glycolysis and glutamine utilization in rapidly dividing cells. Biosci. Rep. 5, 393–400 [DOI] [PubMed] [Google Scholar]
- 2. Brand K. (1985) Glutamine and glucose metabolism during thymocyte proliferation. Pathways of glutamine and glutamate metabolism. Biochem. J. 228, 353–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. DeBerardinis R. J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C. B. (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. U.S.A. 104, 19345–19350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dang C. V. (2010) Glutaminolysis: Supplying carbon or nitrogen, or both for cancer cells? Cell Cycle 9, 3884–3886 [DOI] [PubMed] [Google Scholar]
- 5. Wise D. R., Thompson C. B. (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wise D. R., DeBerardinis R. J., Mancuso A., Sayed N., Zhang X.-Y., Pfeiffer H. K., Nissim I., Daikhin E., Yudkoff M., McMahon S. B., Thompson C. B. (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. U.S.A. 105, 18782–18787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Metallo C. M., Gameiro P. A., Bell E. L., Mattaini K. R., Yang J., Hiller K., Jewell C. M., Johnson Z. R., Irvine D. J., Guarente L., Kelleher J. K., Vander Heiden M. G., Iliopoulos O., Stephanopoulos G. (2012) Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mullen A. R., Wheaton W. W., Jin E. S., Chen P. H., Sullivan L. B., Cheng T., Yang Y., Linehan W. M., Chandel N. S., DeBerardinis R. J. (2012) Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. DeBerardinis R. J., Lum J. J., Hatzivassiliou G., Thompson C. B. (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 [DOI] [PubMed] [Google Scholar]
- 10. Cheng T., Sudderth J., Yang C., Mullen A. R., Jin E. S., Matés J. M., DeBerardinis R. J. (2011) Pyruvate carboxylase is required for glutamine-independent growth of tumor cells. Proc. Natl. Acad. Sci. U.S.A. 108, 8674–8679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wellen K. E., Lu C., Mancuso A., Lemons J. M., Ryczko M., Dennis J. W., Rabinowitz J. D., Coller H. A., Thompson C. B. (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev. 24, 2784–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaadige M. R., Looper R. E., Kamalanaadhan S., Ayer D. E. (2009) Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc. Natl. Acad. Sci. U.S.A. 106, 14878–14883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lum J. J., Bui T., Gruber M., Gordan J. D., DeBerardinis R. J., Covello K. L., Simon M. C., Thompson C. B. (2007) The transcription factor HIF-1α plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 21, 1037–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim J.-w., Tchernyshyov I., Semenza G. L., Dang C. V. (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: A metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 [DOI] [PubMed] [Google Scholar]
- 15. Luo W., Hu H., Chang R., Zhong J., Knabel M., O'Meally R., Cole R. N., Pandey A., Semenza G. L. (2011) Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell 145, 732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Firth J. D., Ebert B. L., Pugh C. W., Ratcliffe P. J. (1994) Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3′ enhancer. Proc. Natl. Acad. Sci. U.S.A. 91, 6496–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wise D. R., Ward P. S., Shay J. E., Cross J. R., Gruber J. J., Sachdeva U. M., Platt J. M., DeMatteo R. G., Simon M. C., Thompson C. B. (2011) Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of α-ketoglutarate to citrate to support cell growth and viability. Proc. Natl. Acad. Sci. U.S.A. 108, 19611–19616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Filipp F. V., Scott D. A., Ronai Z. A., Osterman A. L., Smith J. W. (2012) Reverse TCA cycle flux through isocitrate dehydrogenases 1 and 2 is required for lipogenesis in hypoxic melanoma cells. Pigment Cell Melanoma Res. 25, 375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Scott D. A., Richardson A. D., Filipp F. V., Knutzen C. A., Chiang G. G., Ronai Z. A., Osterman A. L., Smith J. W. (2011) Comparative metabolic flux profiling of melanoma cell lines: beyond the Warburg effect. J. Biol. Chem. 286, 42626–42634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siebert G., Carsiotis M., Plaut G. W. (1957) [The enzymatic properties of isocitric dehydrogenase]. J. Biol. Chem. 226, 977–991 [PubMed] [Google Scholar]
- 21. Dalziel K., Londesborough J. C. (1968) The mechanisms of reductive carboxylation reactions. Carbon dioxide or bicarbonate as substrate of nicotinamide-adenine dinucleotide phosphate-linked isocitrate dehydrogenase and malic enzyme. Biochem. J. 110, 223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Leonardi R., Subramanian C., Jackowski S., Rock C. O. (2012) Cancer-associated isocitrate dehydrogenase mutations inactivate NADPH-dependent reductive carboxylation. J. Biol. Chem. 287, 14615–14620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreno-Sánchez R., Hogue B. A., Hansford R. G. (1990) Influence of NAD-linked dehydrogenase activity on flux through oxidative phosphorylation. Biochem. J. 268, 421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rydström J. (2006) Mitochondrial NADPH, transhydrogenase, and disease. Biochim. Biophys. Acta 1757, 721–726 [DOI] [PubMed] [Google Scholar]
- 25. Sheeran F. L., Rydström J., Shakhparonov M. I., Pestov N. B., Pepe S. (2010) Diminished NADPH transhydrogenase activity and mitochondrial redox regulation in human failing myocardium. Biochim. Biophys. Acta 1797, 1138–1148 [DOI] [PubMed] [Google Scholar]
- 26. Yin F., Sancheti H., Cadenas E. (2012) Silencing of nicotinamide nucleotide transhydrogenase impairs cellular redox homeostasis and energy metabolism in PC12 cells. Biochim. Biophys. Acta 1817, 401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sazanov L. A., Jackson J. B. (1994) Proton-translocating transhydrogenase and NAD- and NADP-linked isocitrate dehydrogenases operate in a substrate cycle which contributes to fine regulation of the tricarboxylic acid cycle activity in mitochondria. FEBS Lett. 344, 109–116 [DOI] [PubMed] [Google Scholar]
- 28. Yoo H., Antoniewicz M. R., Stephanopoulos G., Kelleher J. K. (2008) Quantifying reductive carboxylation flux of glutamine to lipid in a brown adipocyte cell line. J. Biol. Chem. 283, 20621–20627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan M., Breitkopf S. B., Yang X., Asara J. M. (2012) A positive/negative ion-switching, targeted mass spectrometry-based metabolomics platform for bodily fluids, cells, and fresh and fixed tissue. Nat. Protoc. 7, 872–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vizan P., Boros L. G., Figueras A., Capella G., Mangues R., Bassilian S., Lim S., Lee W.-N. P., Cascante M. (2005) K-ras codon-specific mutations produce distinctive metabolic phenotypes in human fibroblasts. Cancer Res. 65, 5512–5515 [DOI] [PubMed] [Google Scholar]
- 31. Veech R. L., Eggleston L. V., Krebs H. A. (1969) The redox state of free nicotinamide-adenine dinucleotide phosphate in the cytoplasm of rat liver. Biochem. J. 115, 609–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Freeman H., Shimomura K., Horner E., Cox R. D., Ashcroft F. M. (2006) Nicotinamide nucleotide transhydrogenase: A key role in insulin secretion. Cell Metab. 3, 35–45 [DOI] [PubMed] [Google Scholar]
- 33. Blinova K., Carroll S., Bose S., Smirnov A. V., Harvey J. J., Knutson J. R., Balaban R. S. (2005) Distribution of mitochondrial NADH fluorescence lifetimes: steady-state kinetics of matrix NADH interactions. Biochemistry 44, 2585–2594 [DOI] [PubMed] [Google Scholar]
- 34. Alberts B., Johnson A., Lewis J., Raff M., Roberts K., Walter P. (2002) Molecular Biology of the Cell, Chapter 14. Energy Conversion: Mitochondria and Chloroplasts, 4th Ed., Garland Science, New York [Google Scholar]
- 35. Parker N., Vidal-Puig A. J., Azzu V., Brand M. D. (2009) Dysregulation of glucose homeostasis in nicotinamide nucleotide transhydrogenase knockout mice is independent of uncoupling protein 2. Biochim. Biophys. Acta 1787, 1451–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wong N., Blair A. R., Morahan G., Andrikopoulos S. (2010) The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology 151, 96–102 [DOI] [PubMed] [Google Scholar]
- 37. Nicholson A., Reifsnyder P. C., Malcolm R. D., Lucas C. A., MacGregor G. R., Zhang W., Leiter E. H. (2010) Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity 18, 1902–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meimaridou E., Kowalczyk J., Guasti L., Hughes C. R., Wagner F., Frommolt P., Nürnberg P., Mann N. P., Banerjee R., Saka H. N., Chapple J. P., King P. J., Clark A. J., Metherell L. A. (2012) Mutations in NNT encoding nicotinamide nucleotide transhydrogenase cause familial glucocorticoid deficiency. Nat. Genet. 44, 740–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ripoll V. M., Meadows N. A., Bangert M., Lee A. W., Kadioglu A., Cox R. D. (2012) Nicotinamide nucleotide transhydrogenase (NNT) acts as a novel modulator of macrophage inflammatory responses. FASEB J. 26, 3550–3562 [DOI] [PubMed] [Google Scholar]
- 40. Sauer L. A., Dauchy R. T., Nagel W. O., Morris H. P. (1980) Mitochondrial malic enzymes: mitochondrial NAD(P)+-dependent malic enzyme activity and malate-dependent pyruvate formation are progression-linked in Morris hepatomas. J. Biol. Chem. 255, 3844–3848 [PubMed] [Google Scholar]
- 41. Williamson D. H., Lund P., Krebs H. A. (1967) The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem. J. 103, 514–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Eto K., Tsubamoto Y., Terauchi Y., Sugiyama T., Kishimoto T., Takahashi N., Yamauchi N., Kubota N., Murayama S., Aizawa T., Akanuma Y., Aizawa S., Kasai H., Yazaki Y., Kadowaki T. (1999) Role of NADH shuttle system in glucose-induced activation of mitochondrial metabolism and insulin secretion. Science 283, 981–985 [DOI] [PubMed] [Google Scholar]
- 43. Kauppinen R. A., Sihra T. S., Nicholls D. G. (1987) Aminooxyacetic acid inhibits the malate-aspartate shuttle in isolated nerve terminals and prevents the mitochondria from utilizing glycolytic substrates. Biochim. Biophys. Acta 930, 173–178 [DOI] [PubMed] [Google Scholar]
- 44. Cederbaum A. I., Lieber C. S., Beattie D. S., Rubin E. (1973) Characterization of shuttle mechanisms for the transport of reducing equivalents into mitochondria. Arch Biochem. Biophys. 158, 763–781 [DOI] [PubMed] [Google Scholar]
- 45. Greenhouse W. V., Lehninger A. L. (1976) Occurrence of the malate-aspartate shuttle in various tumor types. Cancer Res. 36, 1392–1396 [PubMed] [Google Scholar]
- 46. Gameiro P. A., Yang J., Metelo A. M., Pérez-Carro R., Baker R., Wang Z., Arreola A., Rathmell W. K., Olumi A., López-Larrubia P., Stephanopoulos G., Iliopoulos O. (2013) In vivo HIF-mediated reductive carboxylation is regulated by citrate levels and sensitizes VHL-deficient cells to glutamine deprivation. Cell Metab. 17, 372–385 [DOI] [PMC free article] [PubMed] [Google Scholar]