Background: The role endogenously synthesized hyaluronan plays in myogenesis is not yet known.
Results: Hyaluronan synthase genes were expressed during skeletal muscle growth and regeneration; inhibiting these synthases prevents myoblast differentiation and fusion.
Conclusion: Endogenous hyaluronan synthesis is required for myogenic differentiation.
Significance: The necessity for hyaluronan in myogenesis has implications when considering promoting muscle growth or regeneration.
Keywords: Differentiation, Extracellular Matrix, Glycobiology, Hyaluronate, Skeletal Muscle, Hyaluronan Synthase, Myoblast, Pericellular Matrix
Abstract
Exogenous hyaluronan is known to alter muscle precursor cell proliferation, migration, and differentiation, ultimately inhibiting myogenesis in vitro. The aim of the current study was to investigate the role of endogenous hyaluronan synthesis during myogenesis. In quantitative PCR studies, the genes responsible for synthesizing hyaluronan were found to be differentially regulated during muscle growth, repair, and pathology. Although all Has genes (Has1, Has2, and Has3) were differentially regulated in these models, only Has2 gene expression consistently associated with myogenic differentiation. During myogenic differentiation in vitro, Has2 was the most highly expressed of the synthases and increased after induction of differentiation. To test whether this association between Has2 expression and myogenesis relates to a role for Has2 in myoblast differentiation and fusion, C2C12 myoblasts were depleted of Has2 by siRNA and induced to differentiate. Depletion of Has2 inhibited differentiation and caused a loss of cell-associated hyaluronan and the hyaluronan-dependent pericellular matrix. The inhibition of differentiation caused by loss of hyaluronan was confirmed with the hyaluronan synthesis inhibitor 4-methylumbelliferone. In hyaluronan synthesis-blocked cultures, restoration of the pericellular matrix could be achieved through the addition of exogenous hyaluronan and the proteoglycan versican, but this was not sufficient to restore differentiation to control levels. These data indicate that intrinsic hyaluronan synthesis is necessary for myoblasts to differentiate and form syncytial muscle cells, but the hyaluronan-dependent pericellular matrix is not sufficient to support differentiation alone; additional hyaluronan-dependent cell functions that are yet unknown may be required for myogenic differentiation.
Introduction
Hyaluronan is an acidic polysaccharide composed of repetitive units of N-acetylglucosamine and glucuronic acid (1). Hyaluronan is classified as a glycosaminoglycan; however, unlike the glycosaminoglycan-containing proteoglycans, it lacks a protein core. The molecular mass of hyaluronan can vary from 20 kDa to 10 MDa, depending on the chain length, and different cellular functions are ascribed to these different molecular masses (2). Hyaluronan can be found associated with cells in a pericellular coat surrounding cells as well as mobile in solution. The physical properties, including the hygroscopic nature of hyaluronan, can convey mechanical support within connective tissue, which is especially important within synovial joints. Broadly, hyaluronan is linked to numerous tissue functions, including wound healing, inflammation, angiogenesis, and fibrosis (3).
In postnatal tissues, hyaluronan is found throughout the body; however, it is predominantly localized within the skin (∼50% of total hyaluronan), skeleton (∼30% of total hyaluronan), and skeletal muscle (∼10% of total hyaluronan) (4). In rats, hyaluronan was found in all muscles that were examined, and it was located in the connective tissue layers surrounding myofibers (the endomysium, perimysium, and epimysium) (5, 6) and also within the mechanoreceptor spindle fibers (7). Hyaluronan is also associated with the functions of motility, migration, proliferation, and differentiation of cells from the mesenchymal lineage, including osteoblasts (8), chondrocytes (9), fibroblasts (10), and adipocytes (11), as well as the skeletal muscle-forming myoblasts (12–14).
The majority of studies examining the effect of hyaluronan on the differentiation and fusion of chicken-derived myoblasts concluded that myotubes were not as readily formed when myoblasts were cultured on a substratum consisting of hyaluronan compared with a collagen-I substratum (12, 14, 15). Subsequent studies suggested that myoblasts lose their hyaluronan-dependent pericellular matrix when fusing into myotubes and that this is necessary to allow membranes of adjacent myoblasts to fuse (16). Together, this work suggested that myoblast differentiation and fusion can be modulated by hyaluronan, where excess hyaluronan inhibits differentiation and a reduction in hyaluronan is required for fusion into syncytial muscle cells. A bimodal change in myoblast motility and decrease in the ability of newt myoblasts to differentiate has also been observed when cultured on a hyaluronan substratum (17). Although these studies suggest that exogenous hyaluronan can inhibit myoblast fusion, contrasting studies have found that a hyaluronan substratum coated on Chronoflex (polycarbonate polyurethane) can enhance myogenic differentiation and fusion compared with uncoated Chronoflex (18). Nevertheless, although these few studies have examined myoblast responses to exogenous hyaluronan, none have examined how endogenously synthesized hyaluronan influences myoblast behavior.
Hyaluronan synthase 1 (HAS1), 2 (HAS2), and 3 (HAS3) are the three enzymes found in mammals that synthesize hyaluronan (19, 20). The different synthases have been suggested to produce different size hyaluronan chains such that HAS2 can typically produce the largest chains, often greater than 2 MDa, whereas HAS1- and HAS3-synthesized hyaluronan chains are often shorter and range from 100 kDa to 2 MDa (21, 22). Based on mouse knock-out studies of the three isoforms, it is apparent that Has2 is a crucial developmental gene. Cardiac morphogenesis of embryos lacking Has2 expression was abnormal, and cardiac endothelial cell migration and transition into mesenchyme were prevented (23). Has2−/− mice did not survive beyond embryonic day 10 (E10),2 whereas the individual and combined Has1−/− and Has3−/− genotypes were not lethal. This indicates that Has2 is crucial for development and cannot be compensated for by the other HAS enzymes.
Increased Has2 expression has been observed in the longissimus dorsi muscles of sheep with the Callipyge mutation when compared with wild type (24). The longissimus dorsi muscles of Callipyge sheep display a high degree of hypertrophy compared with wild type, which appears to occur postnatally. Has2 up-regulation occurs neonatally, prior to detection of hypertrophy, suggesting that Has2 could be associated with the cause of hypertrophy in Callipyge muscles. Very recent studies have also reported increases in hyaluronan synthesis in a model of compensatory hypertrophy (25). Increased expression of Has1 and Has2 mRNAs was observed in this synergistic ablation model of hypertrophy, supporting the possibility of HAS2 mediating muscle growth and hypertrophy.
Herein we examine Has expression during muscle development, postnatal regeneration following injury, dystrophic pathology, and in vitro myogenesis. We hypothesize that Has2 may be the primary Has gene involved in syncytial muscle cell formation and muscle growth and repair. The requirement for hyaluronan synthesis during in vitro myogenesis is investigated with the hyaluronan synthesis inhibitor 4-methylumbelliferone and siRNA-mediated knockdown of Has2 in C2C12 myoblasts induced to differentiate into syncytial muscle cells. We also contrast myogenic differentiation studies abolishing endogenously synthesized hyaluronan with those adding exogenous hyaluronan in order to provide a comprehensive evaluation of the role hyaluronan and, more specifically, Has2 play in the formation of muscle cells.
EXPERIMENTAL PROCEDURES
Mice
Mouse experiments were carried out in strict accordance with National Health and Medical Research Council NHMRC guidelines for the care and use of animals in research under the approval of the Animal Welfare Committee, Deakin University, ethics number A41/2009 and the Faculty of Veterinary Science AEC, University of Melbourne, approval number 081090. Six-week-old C57/Bl6J (Animal Resources Centre, Western Australia) female mice were mated with male mice after 1700 h, and conception was confirmed before 0900 h the next morning by observation of vaginal mucus. The morning of conception was designated as gestational age 0.5 days (E0.5). Timed embryos were acquired at E12.5, E13.5, E14.5, E15.5, E16.5, and neonatal (day of birth, 21 days) time points, whereby proximal hind limbs of embryonic ages E12.5–E14.5 or proximal hind limb muscles of older embryos (dissected away from hard tissue) were collected and stored in TRIzol at −80 °C until further processing.
Injections of notexin (obtained from Latoxan, France) into the tibialis anterior muscles of adult mice were performed as described previously (26). In brief, anesthetized C57BL/6 mice received a single intramuscular injection of 50 μl of 10 μg/ml notexin parallel along the tibia into the tibialis anterior muscle. Following notexin injection, at 1, 3, 5, 7, 10, and 14 days, the tibialis anterior muscles were sampled. Five mice were also sampled without notexin injection as an uninjured control. Tibialis anterior muscles were snap-frozen in liquid nitrogen in order to perform RNA extraction for quantitative PCR (qPCR) studies. The diaphragm muscles of C57BL10/mdx and C57BL10/ScSn mice at 2, 3, 6, 12, and 24 weeks of age were excised and snap-frozen to perform qPCR. Histology samples were obtained by freezing tragacanth-mounted muscles in liquid nitrogen-cooled isopentane and cutting 10-μm-thick sections with a cryostat onto glass slides.
Cell Culture and Reagents
C2C12 myoblast lines were obtained from the American Type Culture Collection and maintained in growth medium consisting of 10% fetal bovine serum in Dulbecco's modified Eagle's medium (DMEM). To induce differentiation as cells were approaching confluence, growth medium was replaced with differentiation medium containing 2% horse serum in DMEM. Differentiation medium was replenished every 24 h until the end point of the experiment.
4-Methylumbelliferone (4MU) was obtained from Sigma-Aldrich and dissolved in DMSO at a concentration of 1 mm and subsequently diluted out into medium to the described concentrations. 4MU treatments in differentiation experiments were performed by adding 4MU to the growth medium, which was then incubated with myoblasts for 24 h prior to the induction of differentiation, at which point the medium was removed and replaced with 4MU-containing differentiation medium that was subsequently replenished every 24 h until the end of the experiment.
Hyaluronan was obtained from Sigma-Aldrich (H5388) and was derived from rooster comb, for which the supplier expects an average molecular mass in the range of 1–4 MDa. Hyaluronan was reconstituted in PBS and added to culture medium at the described concentrations with dilutions of PBS as vehicle control.
RNA Extraction, Reverse Transcription, and Quantitative PCR
RNA was extracted from developing proximal hind limb muscles as per the manufacturer's protocol using TRIzol (Invitrogen), and equal amounts of total RNA (up to 1 μg) were reverse-transcribed with either the Superscript III reverse transcriptase kit (Invitrogen) or iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was performed using iQ SYBR Green Supermix (Bio-Rad) and relevant primers for the genes of interest. The Quant-iT OliGreen ssDNA assay kit (Invitrogen) was used to quantitate total cDNA as per the manufacturer's protocol.
RNA was extracted from snap-frozen adult muscle tissue and cells by homogenizing in 1 ml of Tri-reagent, followed by the addition of chloroform to retrieve the RNA-containing aqueous layer. The aqueous layer was added in equal volume to a solution comprising one part 90% ethanol and one part SV lysis solution from the SV total RNA isolation system (Promega). The resulting solution was then passed through the SV columns and DNase-treated. The RNA was eluted from the columns, and 1 μg of RNA per sample was reverse transcribed with Moloney murine leukemia virus reverse transcriptase (Promega) to produce cDNA. Real-time qPCRs were performed on a LightCycler 480 (Roche Applied Science), and the cycle thresholds (Ct) were calculated. Expression data were derived from the Ct and normalized to the housekeeper hypoxanthine-guanine phosphoribosyltransferase (Hprt) for the in vivo studies and peptidylprolyl isomerase A (Ppia) for in vitro studies. These were the most appropriate housekeepers because they possessed the least variability across all samples within the study compared with other housekeepers tested, including glyceraldehyde-3-phosphate dehydrogenase (Gapdh) and β-actin (Actb), using the “Bestkeeper” Excel-based tool (27). Oligonucleotide primer sequences used are presented in Table 1 along with the PubMed Gene ID number for identification.
TABLE 1.
Oligonucleotide primers
| Gene name | Gene ID | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|---|
| Bgn | 12111 | CACCTGGACCACAACAAAA | TCCGAATCTGATTGTGACCTA |
| Cd44 | 12505 | CGCTACAGCAAGAAGGGCGAGT | GCAATGGTGGCCAAGGTGCTC |
| Cdkn1a | 12575 | GCAGACCAGCCTGACAGATTC | TTCAGGGTTTTCTCTTGCAGAAG |
| Col1a1 | 12842 | CGGAGAAGAAGGAAAACGAG | CTTCACCAGGAGAACCTTTGG |
| Dcn | 13179 | CGCATCTCAGACACCAACAT | TTGGTGATCTTGTTGCCATC |
| Fn1 | 14268 | GAGCGCCCTAAAGATTCCAT | CTCCACTTGTCGCCAATCTT |
| Has1 | 15116 | TTCCACTGTGTGTCCTGCAT | TGTACCAGGCCTCCAAGAAC |
| Has2 | 15117 | GGGACCTGGTGAGACAGAAG | ATGAGGCAGGGTCAAGCATA |
| Has3 | 15118 | TCCCCAAGTAGGAGGTGTTG | TTGCACACAGCCAAAGTAGG |
| Hprt | 15452 | GATTAGCGATGATGAACCAGGTT | TCCAAATCCTCGGCATAATGAT |
| Hyal1 | 15586 | CCGTAATGCCCTACGTCCAG | GCCTGGCATGATTCCTTGGTA |
| Hyal2 | 15587 | AGCCGCAACTTTGTCAGTTT | GAGTCCTCGGGTGTATGTGG |
| Lama2 | 16773 | ACGCCAAAGATGATGAGGTC | GCACTTGGTCTCCCATTGAT |
| Myf5 | 17877 | CTGTCTGGTCCCGAAAGAAC | AGCTGGACACGGAGCTTTTA |
| Myf6 | 17878 | GGCTGGATCAGCAAGAGAAG | CCTGGAATGATCCGAAACAC |
| Myod1 | 17927 | TACAGTGGCGACTCAGATGC | TAGTAGGCGGTGTCGTAGCC |
| Myog | 17928 | AGTGAATGCAACTCCCACAG | ACGATGGACGTAAGGGAGTG |
| Pcna | 18538 | GGGCGTGAACCTCACCAGCA | CGTGCAAATTCACCCGACGGC |
| Ppia | 268373 | GGCCGATGACGAGCCC | TGTCTTTGGAACTTTGTCTGCAA |
| Vcan | 13003 | ACCAAGGAGAAGTTCGAGCA | CTTCCCAGGTAGCCAAATCA |
In histograms, qPCR data are displayed as the mean of normalized expression in order to show linear levels of transcripts of interest normalized to the housekeeper using the equation, normalized expression = (Ax)/(By), where A represents the efficiency of amplification of the housekeeper, B is the efficiency of the gene of interest, x is the housekeeper Ct, and y is the gene of interest Ct, respectively.
Immunofluorescence
A biotinylated hyaluronan-binding protein (G1 domain of aggrecan) was kindly provided by Amanda Fosang (Murdoch Children's Research Institute) and was used to label hyaluronan as described previously (28). Cultures and tissue sections were initially fixed in 4% formaldehyde containing 1% hexadecylpyridinium chloride monohydrate (Sigma-Aldrich) for 20 min at room temperature. They were then washed three times with PBS. The hyaluronan-binding protein (HABP) was diluted in PBS to 5 μg/ml and added to the cultures for 60 min at room temperature. After this incubation, samples were washed with three changes of PBS. The HABP was detected with an AlexaFluor-594 streptavidin conjugate diluted 1:500 in PBS (Invitrogen). Samples were counterstained with DAPI. Incubation with 200 units/ml hyaluronidase from Streptomyces hyalurolyticus for 3 h at 37 °C was performed to demonstrate the specificity of the binding protein for hyaluronan. For detection of laminin and desmin, the rat anti-laminin α-2 (4H8-2) antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and mouse anti-desmin (DE-U-10) antibody (Sigma-Aldrich) were used.
The fusion index in differentiated C2C12 cultures, which is the percentage of nuclei contained within syncytial myotubes, was determined by immunofluorescence for desmin, a protein highly enriched in syncytial myotubes. Cells were fixed with 2% (w/v) paraformaldehyde, washed, and subsequently incubated with the primary antibody, mouse anti-desmin, and subsequently probed with an anti-mouse AlexaFluor-488 secondary antibody.
Transfection of C2C12 Myoblasts
C2C12 cells were transfected with 50 pmol of Has2 Stealth Select RNAi siRNA (MSS236746; Invitrogen) in 50 μl of Opti-MEM and 1 μl of Lipofectamine 2000 (Invitrogen) for 6 h in serum- and antibiotic-free medium (0.5 ml in 24-well plates), and then an additional 0.5 ml of growth medium was added to each culture. Cells were cultured overnight before all medium was removed and replaced with normal growth medium (10% FBS). Controls were instead transfected with Stealth RNAi siRNA Negative Control Lo-GC (12935-200, Invitrogen).
C2C12 cells were transfected with the versican construct, which was kindly provided by Suneel Apte, by a similar method. A ratio of 0.5 μg of plasmid DNA to 1 μl of Lipofectamine 2000 in 50 μl of Opti-MEM was used to transfect each well of a 24-well plate by seeding 10,000 cells directly into the DNA and Lipofectamine 2000 mix-containing wells.
Creatine Kinase Activity Assay
Creatine kinase activity from lysed myoblast cultures was determined as described previously (29). In brief, cultures were lysed with 150 μl of lysis buffer containing 40 mm MES buffer, 50 mm Trizma base, 1% (v/v) Triton X-100, and protease inhibitors. Insoluble material in the cellular lysate was pelleted, and 10 μl of the supernatant was combined with 200 μl of CK-NAC reagent (Thermo Scientific). Creatine kinase activity was measured by the change in absorbance at 340 nm over 3 min (20-s intervals) at 37 °C. The change in absorbance was converted to activity in units/liter based on the molar absorption coefficient of NADH at 340 nm and normalized to DNA content.
Total DNA content was assayed using SYBR Safe DNA gel stain (10,000×) (Invitrogen) diluted 1:500 with water. Diluted SYBR Safe (1 μl) was combined with 19 μl of cell lysate or genomic DNA standards (0.78–100 μg/ml) into a black 384-well plate, and the SYBR fluorescence was quantitated on the Paradigm Detection Platform (Beckman Coulter).
Pericellular Exclusion Assay
The size of the pericellular matrix surrounding live C2C12 myoblasts was determined by a particle exclusion assay similar to that described previously (30, 57). C2C12 myoblasts transfected to express GFP were seeded at a density of 5,000 cells/well in 6-well plates, and after 2 days of either 4-methylumbelliferone or Has2 siRNA, the assay was performed. Lyophilized sheep erythrocytes (Sigma-Aldrich) were resuspended in 10 ml of PBS, postfixed with 1.5% (w/v) paraformaldehyde in PBS, washed, and finally resuspended again in 10 ml of PBS. An aliquot (30 μl) of this cell suspension (containing ∼2 × 107 erythrocytes) was added to the cultures, and the erythrocytes were allowed to settle for ∼10 min. The pericellular exclusion area was visualized by imaging the GFP-positive C2C12 cells and the red autofluorescent erythrocytes on an inverted microscope. Binary masks of these images were generated in ImageJ in order to measure the area occupied by the C2C12 cells plus the pericellular space excluding erythrocytes (from which the exclusion area was derived). The size of the myoblasts and the space between the cell membrane of the myoblast and the erythrocytes surrounding them were measured in a blinded fashion with 50 randomly selected cells measured per treatment group. Hyaluronidase digestion was also performed in cultures with 0.5 unit/ml hyaluronidase from S. hyalurolyticus (Sigma-Aldrich) in serum-free medium for 1 h prior to the exclusion assay as a control.
Statistical Analysis
GraphPad Prism software was used for statistical analysis. Student's t tests were performed when comparing a single treatment with control. All histograms represent the mean and the S.E. One-way analysis of variance was used to compare multiple treatments with control, and two-way analysis of variance was used when additional variables were considered. A 95% confidence interval was accepted, where p ≤ 0.05 was deemed significant and is denoted on histograms with an asterisk. The Relative Expression Software Tool was used to analyze statistical differences between Ct values from qPCR (31).
RESULTS
Hyaluronan Synthase Gene Expression Is Regulated during Myogenesis
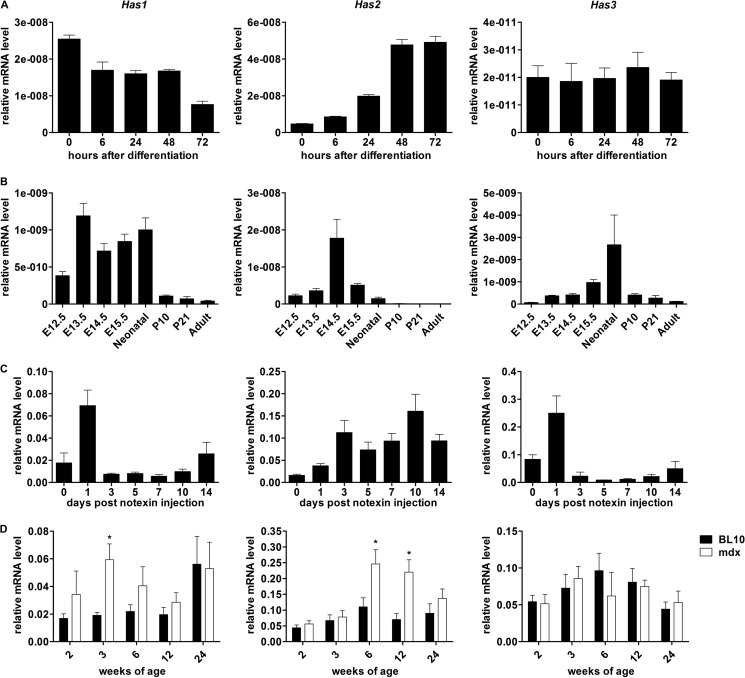
C2C12 myoblasts were induced to differentiate and fuse into multinucleate myotubes over a period of 72 h in differentiation medium. During this time, mRNA was extracted, and Has transcript levels examined by qPCR (Fig. 1A). Has1 expression was at a maximum in undifferentiated nearly confluent myoblasts and decreased as differentiation and fusion proceeded. Has2 expression demonstrated an inverse trend, where expression was lowest in undifferentiated myoblasts but increased as differentiation and fusion proceeded. Has3 expression did not appear to fluctuate throughout the time course.
FIGURE 1.
Has gene expression during skeletal muscle growth and remodeling. Mean normalized expression of relative mRNA levels of Has1, Has2, and Has3 in differentiating C2C12 myoblasts after the addition of differentiation medium (A), developing and adult limb muscles (B), notexin-injured tibialis anterior (C), and mdx and wild type Bl10 diaphragms (D). *, p < 0.05 for comparisons between genotypes in D (n = 8). Error bars, S.E.
Has mRNA expression was examined in the developing (E12.5–E15.5), neonatal, and postnatal (P10, P21, and adult) mouse limb muscles (Fig. 1B). Has1 transcript appeared to have increased at E13.5 through birth in comparison with E12.5. Has2 transcript displayed a sharp increase, peaking at E14.5, and decreased subsequently to very low levels in adult muscle. Has3 transcript demonstrated a peak in neonatal muscle compared with developing and adult muscle. Low levels of all three transcripts were seen in adult muscle relative to neonatal (Has3) and developing muscle (Has1 and Has2), indicating potential roles in muscle development. Has2 was the most highly expressed gene relative to housekeeper, however, and showed the most dramatic increase at E14.5, coincident with the approximate onset of secondary myogenesis.
Notexin is frequently used as a model of postnatal muscle regeneration because it consistently and reproducibly generates whole muscle changes associated with muscle breakdown, necrosis, inflammation, myoblast growth, differentiation, and the regeneration of syncytial muscle cells (32), processes that can be distinguished temporally in this model. Has1, Has2, and Has3 gene expression were all increased following notexin injection compared with uninjured control (day 0; Fig. 1C). Both Has1 and Has3 expression followed a strikingly similar trend, with a large peak at day 1, which is typically coincident with large numbers of neutrophils and increased cytokine expression in the regenerating muscle. Has2 expression, however, increased progressively following notexin injection, coincident with increasing myoblast fusion and myotube maturation with a peak around day 10.
The expression of Has genes was also examined in the diaphragms of mdx mice, a model of human dystrophinopathies (Fig. 1D). Compared with the wild type (C57BL10/ScSn or BL10), mdx mice demonstrated significantly increased mRNA levels of Has1 at 3 weeks of age and Has2 at 6 and 12 weeks of age. No significant differences in Has3 expression between genotypes were observed.
Localization of Hyaluronan in Skeletal Muscle Tissue and Cultured Myoblasts
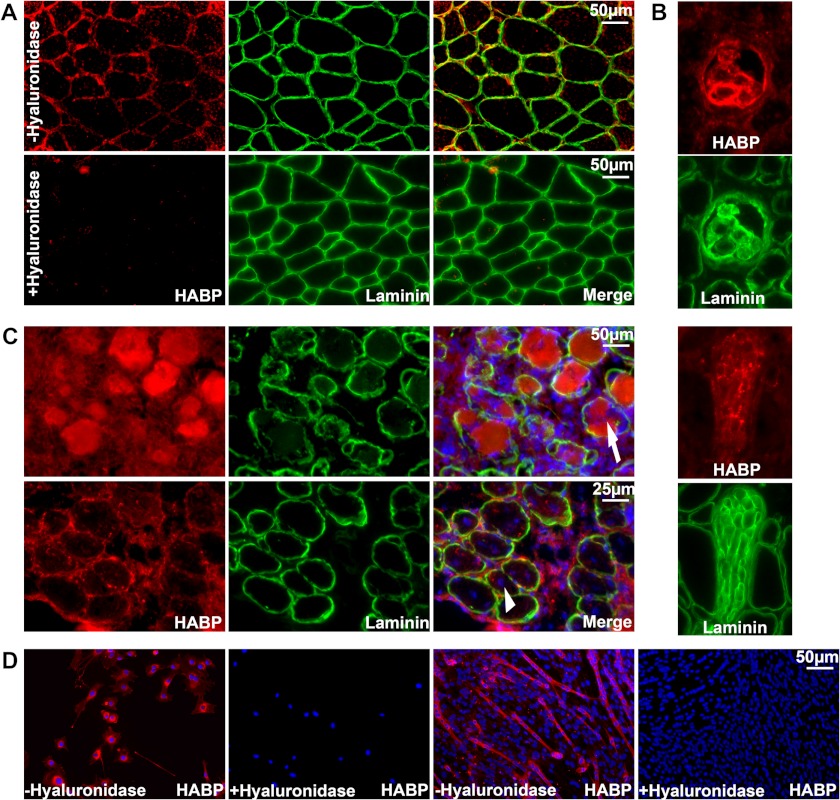
We used the previously described hyaladherin G1 domain of aggrecan to investigate the presence and localization of hyaluronan in normal and notexin-injured muscle as well as in cultured C2C12 myoblasts. A biotinylated G1 domain, herein referred to as HABP, bound to hyaluronan in tissue and culture samples and was detected with streptavidin fluorescence and co-stained with DAPI and laminin to highlight nuclei and the endomysium of myofibers. As expected, hyaluronan appeared to localize to the extracellular spaces surrounding myofibers in normal uninjured muscle. HABP fluorescence co-localized with laminin in the endomysium (Fig. 2A) but was also present in the perimysium and epimysium (data not shown). Control sections incubated with hyaluronidase displayed no specific fluorescence, highlighting the specificity of HABP fluorescence for detection of hyaluronan. Concordant with previous descriptions, we also found intense HABP reactivity within muscle spindle fibers (7) but also intermittently throughout nerve bundles, which could be easily determined by their unique histological appearance (Fig. 2B).
FIGURE 2.
Localization of hyaluronan in skeletal muscle tissue and cultured myoblasts. Hyaluronan was detected by biotinylated HABP probed with red fluorescent conjugated streptavidin. Laminin α-2 immunofluorescence was also performed in green to highlight the endomysium surrounding muscle fibers (A–C). A, localization of hyaluronan or laminin surrounding the fibers in normal adult muscle without (top) or with hyaluronidase pretreatment (bottom) to demonstrate the specificity of the reactivity for hyaluronan. B, strong HABP reactivity in muscle spindle fibers (top) and nerve bundles (bottom). C, localization of hyaluronan in muscle following notexin injection at 3 days (top) in necrotic fibers invaded by inflammatory cells (indicated with an arrow) and 7 days (bottom) surrounding the central nuclei of nascent myotubes (indicated by an arrowhead). D, cell-associated hyaluronan was detected in myoblasts (left) and myotubes (right).
In notexin-injured muscle, however, intense HABP reactivity was observed 3 days after notexin injection in necrotic fibers, which were being invaded by numerous inflammatory cells, and was also present in the surrounding mononucleated cells (Fig. 2C, top). Following the formation of nascent myotubes 7 days after notexin injection, HABP reactivity was present around the centrally nucleated nascent myotubes as well as surrounding the mononucleated cells between fibers, indicating that the mononucleated myoblasts that will subsequently fuse into myotubes are likely to be surrounded by a hyaluronan-containing matrix (Fig. 2C, bottom).
Hyaluronan was indeed associated with mononucleated myoblasts in culture. HABP reactivity in C2C12 cultures was found associated with myoblasts; this was subsequently lost by incubation with hyaluronidase (Fig. 2D, left). Hyaluronan was also associated with long multinucleate myotubes formed by culturing myoblasts to confluence and switching to a low serum differentiation-inducing medium (Fig. 2D, right).
Knockdown of Has2 during in Vitro Myogenesis Reduces Hyaluronan Production and Abolishes the Pericellular Matrix
All three HAS proteins are expressed by myoblasts in skeletal muscle (25), but the consistent relationship observed herein between increased Has2 transcript expression and myogenesis both in vivo and in vitro suggested that Has2 is intimately involved in the process by which myoblasts differentiate and fuse to form syncytial muscle cells. To test this hypothesis, we performed Has2 knockdown in C2C12 myoblasts that were induced to differentiate. The subsequent knockdown during the period of differentiation was monitored by qPCR alongside changes in cell-associated hyaluronan and the pericellular matrix to determine the efficacy of Has2 knockdown and whether this subsequently induced hyaluronan loss.
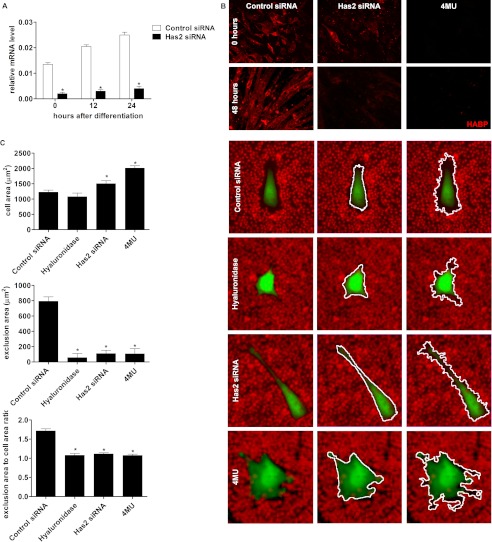
Transfections with the Has2 and negative control siRNA were performed 3 days before the myogenic cultures were induced to differentiate; mRNA was collected at the time of and after induction of differentiation for qPCR analysis. Transfection with Has2 siRNA significantly down-regulated Has2 mRNA compared with negative control siRNA immediately prior to the induction of differentiation (72 h after transfection; 0 h after differentiation), with an 89% decrease in transcript levels (Fig. 3A). This decrease was maintained through to 24 h after differentiation.
FIGURE 3.
Knockdown of Has2 and reduction in levels of hyaluronan. A, relative mRNA levels of Has2 following C2C12 transfection of Has2 siRNA and induction of differentiation. An 89% reduction in Has2 mRNA compared with control was achieved at day 0, and similar magnitude reductions persisted for 24 h following the addition of differentiation medium. Statistical analysis was performed by the Relative Expression Software Tool. *, p < 0.05, n = 4. B, HABP reactivity in control and Has2 siRNA- and 4MU-treated C2C12 cells in nearly confluent undifferentiated cultures (0 h after the addition of differentiation medium) and 48 h after the addition of differentiation medium. Representative images of HABP immunofluorescence with Has2 siRNA and 4MU treatment demonstrated a decrease in hyaluronan content compared with control; in the vast majority of 4MU-treated cells, there was a near absence of HABP reactivity. C, representative images of the pericellular erythrocyte exclusion assay, demonstrating reduced area of the exclusion zone surrounding myoblasts with hyaluronidase, Has2 siRNA, and 4MU treatment compared with control. An increase in cell size and altered morphology with Has2 siRNA and 4MU is also evident. White outlines highlight the binary masks generated for the area measured of the cell (middle) and exclusion area (right). Quantitation of the areas measured for the cells and the exclusion areas alone and the ratio of the exclusion area plus cell size divided by the cell size are presented below. *, p < 0.05, n = 4. Error bars, S.E.
In Has2 siRNA-treated cultures, there was also a clear reduction in the reactivity of HABP associated with myoblasts and myotubes at 0 and 48 h after differentiation, respectively (Fig. 3B), indicating that the knockdown of Has2 led to a decrease in hyaluronan accumulation that persisted throughout the time course. 4MU is a well documented inhibitor of hyaluronan synthesis; it depletes cellular UDP-glucuronic acid, one of the substrates for hyaluronan synthesis, and can also directly reduce hyaluronan synthase gene transcript levels (33) with little to no effect on proteoglycan synthesis (34). Treatment with 4MU, at a concentration of 1 mm in the growth medium 24 h prior to inducing differentiation and also in the differentiation medium reduced cell-associated HABP reactivity in both the undifferentiated and differentiated cultures, but to a greater degree than Has2 siRNA. Together, this suggested that both treatments had reduced the synthesis of hyaluronan, yet 4MU was the most effective, almost completely ablating the detection of hyaluronan with the HABP.
Hyaluronan is essential for the formation of the pericellular matrix, which is particularly large and well described in chondrocytes. The pericellular matrix is largely composed of hyaluronan stabilized by proteoglycans and is capable of excluding cells and other particles from the area that it occupies. It is typically the first point of contact between a cell and the external environment. Previous reports have suggested that Has2 is critical for the formation of the cell-associated matrix in human articular chondrocytes (35). Therefore, we investigated whether Has2 knockdown affects the maintenance of the myoblast pericellular matrix because this could relate to the ability of myoblasts to come into close contact with one another and fuse into syncytial muscle cells.
The pericellular matrix was visualized using the pericellular erythrocyte exclusion assay in C2C12 cells transfected with Has2 or control siRNA or treated with 1 mm 4MU. Representative images of the cells and the exclusion zones are presented in Fig. 3C, with the binary mask outline highlighted for the myoblast (middle) and the exclusion zone (bottom). Control myoblasts demonstrated a moderate sized exclusion zone on average 800 μm2 in area; however, Has2 siRNA- and 4MU-treated cells had on average an area less than 100 μm2. The hyaluronidase-treated control group also demonstrated an almost complete lack of exclusion zone with an average area less than 100 μm2.
Interestingly, both Has2 siRNA- and 4MU-treated myoblasts demonstrated atypical cell morphology, with increased numbers of particularly slender membrane extensions and overall increased cell size as shown in the representative images. There was a significant increase in the area measured for the Has2 siRNA-treated cells, and this was further exaggerated in the 4MU treatment group. When considering the ratio of the size of the exclusion area plus cell size to cell size alone, in order to account for differences in cell size, the decrease in exclusion area was maintained. An average ratio of 1.6 was observed for control myoblasts, indicating an exclusion area equivalent to 60% of the cell size, whereas these ratios were only marginally higher than 1.0 for cells treated with hyaluronidase, Has2 siRNA, and 4MU, indicating almost no pericellular matrix relative to cell size. Together with HABP detection of hyaluronan, this finding suggested that knockdown of Has2 reduced hyaluronan and ablated the hyaluronan-dependent pericellular matrix, as did 4MU treatment.
Has2 Knockdown and Loss of Hyaluronan Inhibits Myogenesis
The fusion index, which is the proportion of myoblasts that had fused 24 h after differentiation in Has2 siRNA-treated cultures, was significantly reduced compared with control (Fig. 4A). The fusion index measurement is normalized and expressed as a proportion of the total number of cells and thus is not biased by potential differences in cell density, which did not appear to exist in the cultures examined. Creatine kinase activity (a marker of myogenic differentiation), which is normalized per μg of genomic DNA to account for differences in cell density, was significantly decreased in Has2 siRNA-transfected cultures compared with control cultures at 1, 2, and 3 days after induction of differentiation (Fig. 4B). This inhibition would best be described as a delay in differentiation to the maximum extent possible for these cultures, because by 5 days after differentiation, creatine kinase activities were not significantly different. This could be due to the acute nature of siRNA-mediated knockdown; however, it would not be possible to address whether differentiation would remain inhibited if the siRNA was replenished due to the challenges involved in transfecting differentiating myogenic cultures. Nevertheless, these data demonstrated that loss of the Has2 gene does impair differentiation and therefore is required during this process.
FIGURE 4.
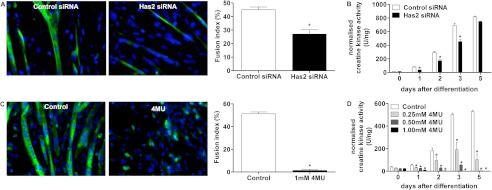
Has2- and 4MU-mediated loss of hyaluronan inhibits myogenic differentiation. A, desmin immunofluorescence for fused cultures (24 h) following both control and Has2 siRNA transfection and the quantitated fusion index on the right. B, creatine kinase activity demonstrating reduced differentiation with Has2 siRNA. A greater inhibition of differentiation was observed with 4MU treatment by fusion index (C) and creatine kinase activity (D). *, p < 0.05, n = 4. Error bars, S.E.
To test whether inhibition of myogenic differentiation could be linked to loss of hyaluronan, the effect of 4MU on differentiation was examined. As demonstrated in Fig. 3, 4MU treatment abolished hyaluronan synthesis; 4MU treatment also abolished myogenic differentiation. In 4MU-treated cultures, almost no myotubes were observed by immunofluorescent staining of desmin (Fig. 4C). Myoblasts were treated with 1 mm 4MU for 24 h in growth medium prior to the induction of differentiation as well as in the differentiation medium, which was replenished every day. Interestingly, although very few desmin-positive syncytia could be seen, there were numerous mononucleated desmin-positive cells, indicating that although 4MU prevents fusion, some degree of the myogenic program relating to the regulation of desmin expression is conserved. Creatine kinase activity from 4MU-treated cultures was significantly reduced at 1, 2, 3, and 5 days after differentiation at a variety of concentrations compared with vehicle control (Fig. 4D). These observations indicated that loss of hyaluronan synthesis resulting from Has2 knockdown was highly likely to have caused inhibition of myogenic differentiation and furthermore suggested that endogenous hyaluronan synthesis by myoblasts is essential to myogenic differentiation and fusion, because the formation of myotubes and increases in creatine kinase activity could not occur with 4MU treatments at concentrations that prevented hyaluronan synthesis. In these experiments, 4MU was consistently replenished with medium changes to maintain inhibition of hyaluronan synthesis; however, if 4MU-containing medium was washed out and cultures were returned to control medium, fusion was able to occur eventually (results not shown), which suggested that 4MU completely blocks fusion and that this effect is reversible when hyaluronan synthesis can be regained by removal of 4MU.
Hyaluronan Synthesis Inhibition Induces Alterations in Expression of Myogenic and Extracellular Matrix Transcripts
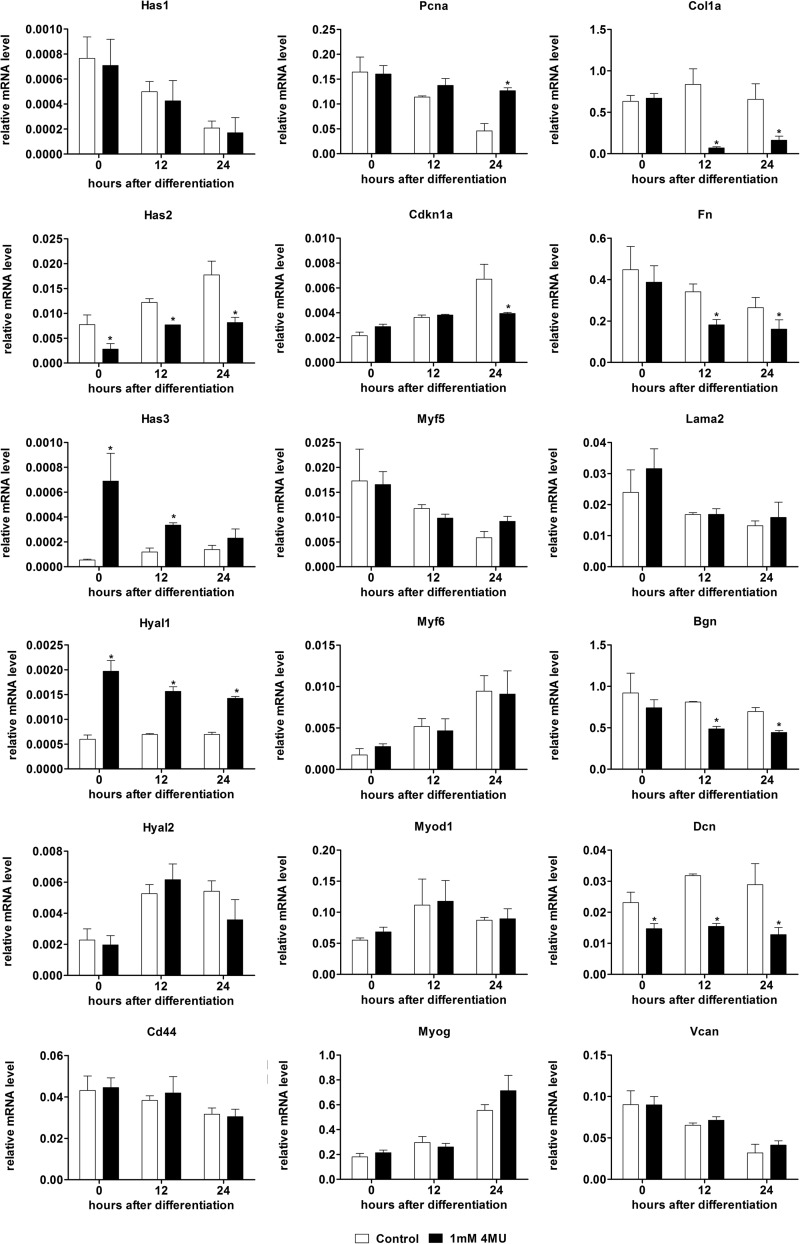
Across this same time course of differentiation for which Has2 knockdown was performed, we investigated transcriptional changes relating to hyaluronan, myogenesis, and the extracellular matrix in order to provide insights into the downstream effects of decreased Has2 transcript levels and loss of Has2-mediated hyaluronan synthesis (Fig. 5).
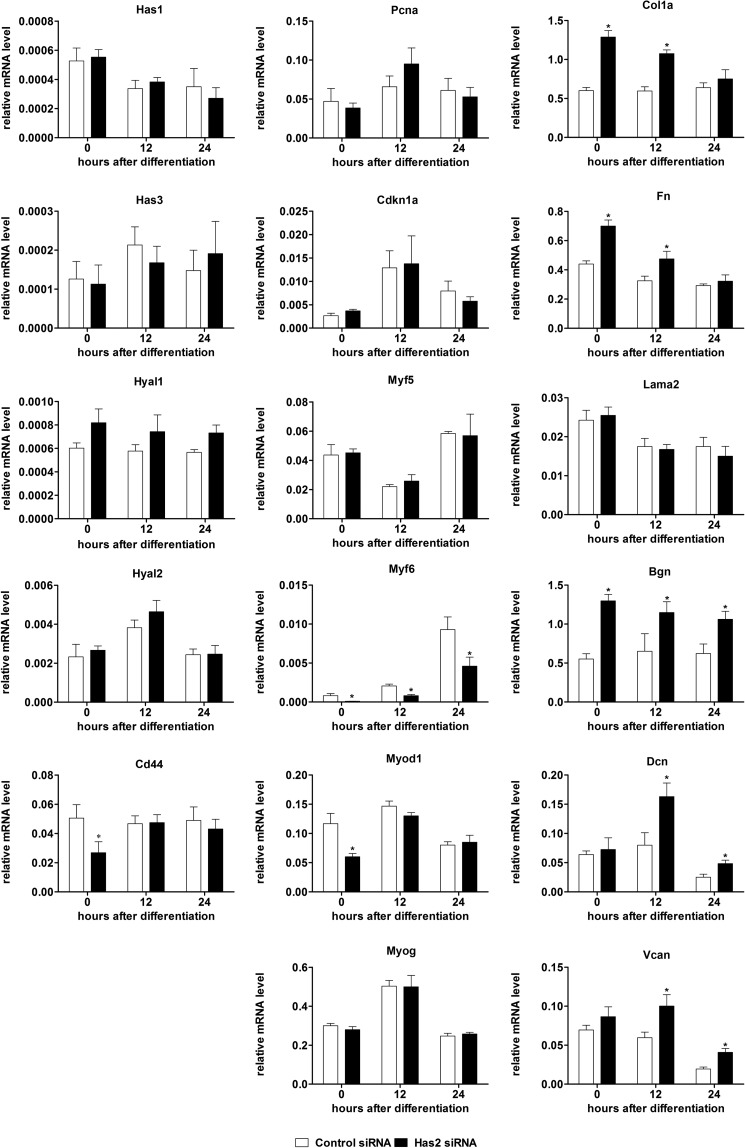
FIGURE 5.
The effect of Has2 knockdown on myogenic and extracellular protein transcripts. Relative mRNA levels of genes related to hyaluronan synthesis and degradation (Has1, Has3, Cd44, Hyal1, and Hyal2), cell proliferation (Pcna), cell cycle withdrawal (Cdkn1a), MRFs (Myf5, Myod1, Myf6, and Myog), and extracellular matrix (Col1a1, Fn1, Lama2, Vcan, Bgn, and Dcn) by qPCR following transfection with Has2 and control siRNA and after the induction of differentiation in C2C12 myoblasts. *, p < 0.05, n = 4. Error bars, S.E.
Genes related to hyaluronan anabolism, catabolism, and receptors were examined. Transcripts for the Has1 and Has3 genes were not significantly altered with Has2 siRNA, suggesting that there is no compensation for loss of Has2 by hyaluronan anabolic mechanisms. Hyaluronidase-2 (Hyal2) in conjunction with Cd44 is capable of catabolizing high molecular weight hyaluronan produced by HAS2 that subsequently undergoes endocytosis from the cell surface (2, 36). No significant differences in Hyal2 expression were observed; however, Cd44 expression was significantly decreased at the time of induction of differentiation (72 h after siRNA transfection). No differences in the expression of the less well characterized hyaluronan receptor gene, hyaluronan-mediated motility receptor (Hmmr), also known as RHAMM, were observed (results not shown). Hyal1 has also recently been suggested to enhance myogenic differentiation by degrading chondroitin sulfate and allowing myoblast fusion (37); no changes in Hyal1 transcripts were observed with Has2 siRNA treatment.
Transcripts for the proliferative marker, proliferating cell nuclear antigen (Pcna), and the cell cycle inhibitor p21 (Cdkn1a) were not significantly altered in the Has2 siRNA group (Fig. 5). This suggested that Has2 knockdown did not affect proliferation or the cessation of proliferation necessary for myogenic differentiation. Of the four myogenic regulatory factors (MRFs) that control myogenic differentiation, only Myf6 (Herculin/MRF4) and Myod1 were significantly down-regulated by Has2 siRNA; Myog and Myf5 transcripts were not significantly altered. This coordinated down-regulation of a specific subset of MRFs (Myf6 and MyoD) is probably the result of loss of Has2-mediated hyaluronan synthesis and is consistent with the observation that Has2 knockdown inhibits myogenic differentiation.
Because hyaluronan is an important part of the extracellular matrix, we also investigated how loss of Has2 and hyaluronan synthesis influenced extracellular matrix transcripts, including interstitial, basement membrane, and proteoglycan components. Although transcripts for the myofiber basement membrane protein laminin α-2 (Lama2) were not significantly changed with Has2 knockdown, the interstitial proteins collagen α-1 (Col1a1) and fibronectin (Fn1) were significantly up-regulated with Has2 knockdown at 0 and 12 h compared with control. Biglycan (Bgn) transcripts followed a similar trend, with significant up-regulation at 0, 12, and 24 h, and the other proteoglycans examined, versican (Vcan) and decorin (Dcn), followed a different trend, only significantly up-regulated at 12 and 24 h. Expression changes that were altered at 0 h relative to differentiation (72 h after siRNA transfection), Col1a1, Fn1, and Bgn, would appear to be the direct result of Has2 knockdown, whereas the changes in Vcan and Dcn expression, which only occurred after the initiation of differentiation, were probably the results of inhibited or delayed myogenesis and not an immediate response to Has2 and hyaluronan loss.
We also examined the changes in gene expression caused by 4MU along the same time course of differentiation (Fig. 6). Although 4MU is suggested to act primarily by limiting the availability of UDP-glucuronic acid to cells for glycosaminoglycan synthesis, it was reported that 4MU can cause decreases in transcripts for hyaluronan synthase genes (33). Similarly, we found that 4MU significantly decreased transcripts for Has2 but interestingly increased transcripts for Has3. Hyal1 mRNA was also significantly increased with 4MU treatment; however, Hyal2 and Cd44 were not.
FIGURE 6.
The effect of 4MU on myogenic and extracellular protein transcripts. Relative mRNA levels of genes related to hyaluronan synthesis and degradation (Has1, Has3, Cd44, Hyal1, and Hyal2), cell proliferation (Pcna), cell cycle withdrawal (Cdkn1a), MRFs (Myf5, Myod1, Myf6, and Myog), and extracellular matrix (Col1a1, Fn1, Lama2, Vcan, Bgn, and Dcn) by qPCR following treatment with 4MU and control (DMSO vehicle) after the induction of differentiation in C2C12 myoblasts. *, p < 0.05, n = 4. Error bars, S.E.
Although no MRFs were significantly altered, Pcna and Cdkn1a were both significantly different at 24 h of differentiation in the opposite directions. 4MU increased Pcna and decreased Cdkn1a, suggesting that 4MU-treated myoblasts were more likely to be proliferative and unable to exit the cell cycle compared with control myoblasts. Matrix transcripts were also altered; Col1a, Fn, Bgn, and Dcn were all significantly down-regulated at certain points with 4MU treatment compared with control, suggesting an inability to produce matrix components.
Restoration of the Pericellular Matrix to Hyaluronan Synthesis-blocked Myoblasts Does Not Rescue Myogenic Differentiation
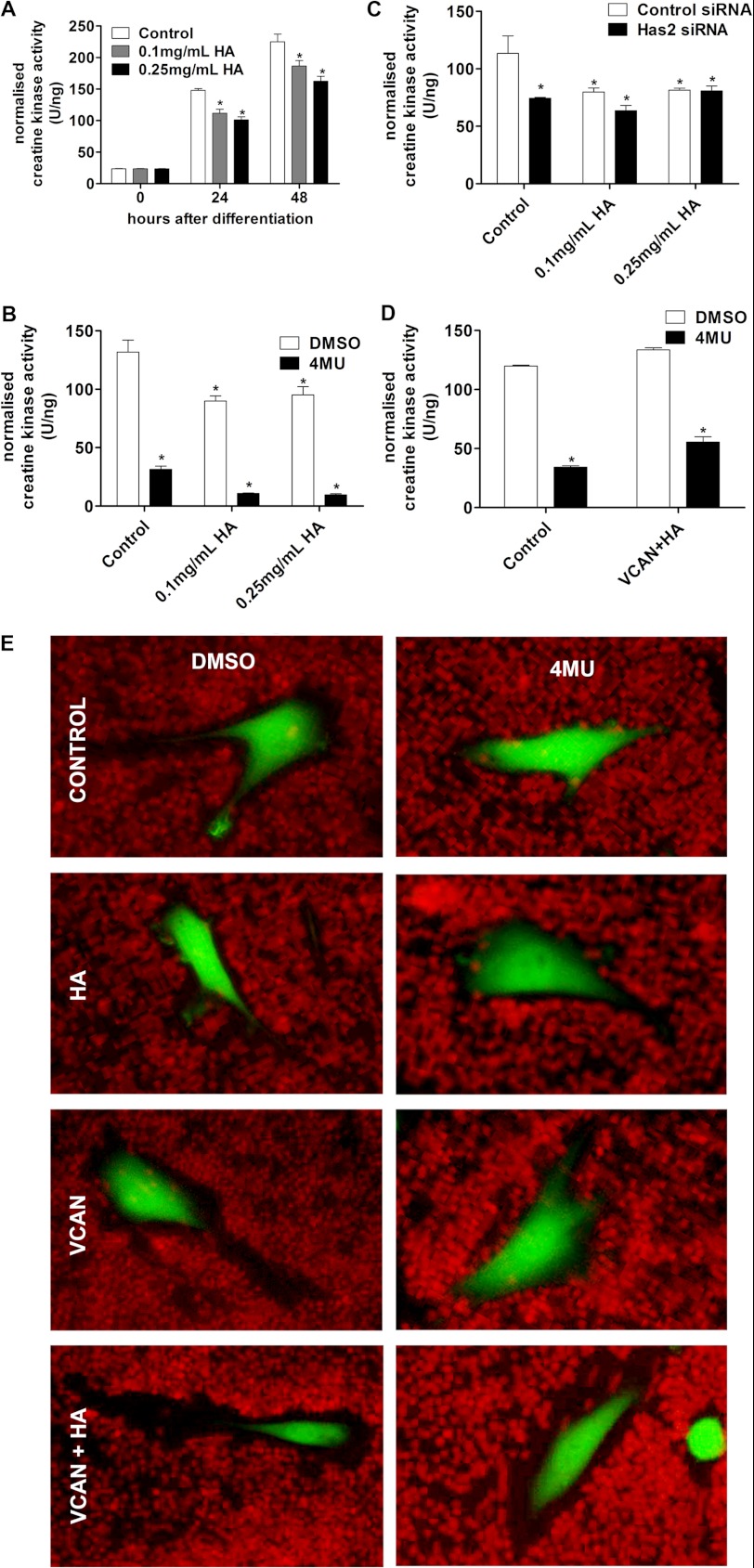
We examined whether the addition of high molecular mass hyaluronan from rooster comb (which, according to the supplier, is expected to be in the range of 1–4 MDa), which corresponds closely with the molecular mass of hyaluronan produced by HAS2, could in any way compensate for Has2 knockdown or loss of hyaluronan by 4MU and allow myogenic differentiation.
Hyaluronan was added to cultures in the growth medium for 24 h prior to inducing differentiation and also in the differentiation medium. The addition of exogenous hyaluronan by itself mildly inhibited differentiation at 24 and 48 h; there was significant reduction in creatine kinase activity at 24 and 48 h with both 0.1 and 0.25 mg/ml hyaluronan compared with vehicle control (Fig. 7A). This agreed with previous reports that exogenous hyaluronan inhibits myogenic differentiation (12–14). However, the addition of hyaluronan to 4MU-treated cultures did not restore creatine kinase activity to normal vehicle control levels (Fig. 7B). In fact the inhibition of myogenic differentiation caused by 4MU and hyaluronan were additive. Has2 siRNA-mediated inhibition of myogenic differentiation was also not restored with exogenous hyaluronan addition (Fig. 7C). This indicates that there is an important functional difference between exogenous hyaluronan and endogenously synthesized hyaluronan; whereas exogenous hyaluronan may inhibit myogenic differentiation, endogenous hyaluronan is absolutely critical for myogenic differentiation.
FIGURE 7.
Inhibition of differentiation by blocking hyaluronan synthesis cannot be rescued by restoration of the pericellular matrix. A, creatine kinase activity in C2C12 cultures that were incubated with hyaluronan-containing medium prior to and during differentiation show a minor reduction in differentiation. B, the addition of hyaluronan does not increase creatine kinase activity in 4MU-treated cultures 24 h after differentiation. C, the addition of hyaluronan does not increase creatine kinase activity in Has2 siRNA-treated cultures 24 h after differentiation. D, hyaluronan addition in conjunction with versican expression does not restore creatine kinase levels to control 24 h after differentiation. E, hyaluronan addition in conjunction with versican expression restores the erythrocyte-excluding pericellular matrix to 4MU hyaluronan synthesis-blocked cells, but neither hyaluronan nor versican could achieve this independently. *, p < 0.05, n = 4. Error bars, S.E.
To determine if the exogenous hyaluronan addition could restore the pericellular matrix lost with 4MU treatment, we once again utilized the pericellular erythrocyte exclusion assay (Fig. 7E). We found that the addition of hyaluronan by itself did not restore the pericellular matrix. Previous studies have demonstrated that recapitulation of a pericellular matrix requires both a hyaluronan-binding proteoglycan, such as aggrecan or versican, in conjunction with hyaluronan (38). Therefore, we examined the addition of hyaluronan to C2C12 cells transfected with either a control (GFP) or versican (V1 variant) construct following 4MU inhibition of hyaluronan synthesis. C2C12 myoblasts were transfected with either the versican construct or a GFP reporter control construct and allowed to grow for 3 days, after which time they were incubated with 0.25 mg/ml hyaluronan in growth medium or control growth medium for 24 h with or without 1 mm 4MU. Individually, hyaluronan and versican did not restore the pericellular matrix; however, when combined, large pericellular matrices could be seen even in cells that were treated with 4MU.
Restoration of the pericellular matrix in this fashion, however, did not restore differentiation in 4MU-treated cells, as measured by creatine kinase activity, to levels similar to that without 4MU treatment (Fig. 7D). In this experiment, C2C12 myoblasts were transfected with either the versican construct or a GFP reporter control construct and allowed to grow for 3 days, after which time they were incubated with 0.25 mg/ml hyaluronan in growth medium or control growth medium for 24 h with or without 1 mm 4MU; following this, they were then induced to differentiate for 24 h with differentiation medium either with or without hyaluronan and 4MU. No appreciable myotube formation could be seen in 4MU-treated cultures that also received hyaluronan and versican treatments. This suggested that although hyaluronan is essential to myogenic differentiation, it is not the hyaluronan- and proteoglycan-dependent pericellular matrix that is necessary to allow myoblast differentiation and fusion.
DISCUSSION
Hyaluronan and Has genes have been studied extensively biochemically and in certain contexts, such as the embryo, developing heart, metastases, and adult articular cartilage, yet few studies have focused specifically on hyaluronan and synthase gene functions in skeletal muscle, a tissue rich in hyaluronan. Recent reports have indicated almost ubiquitous expression of all three HAS enzymes on myoblasts (M-cadherin+) and fibroblasts (FSP1+) as well as synthase-dependent expression in other cell types, such as endothelial (CD31+) and monocytic (F4/80+) cells, in the context of compensatory skeletal muscle hypertrophy (25). We present herein the first findings that describe changes in Has gene expression in skeletal muscle during differentiation, development, and skeletal muscle remodeling in response to notexin-induced injury and dystrophic pathology. We discovered gene expression profiles of Has1 and Has3, which temporally associated with muscle necrosis and inflammation, and we established a clear link between Has2 and myogenic differentiation in vivo and in vitro. Importantly, we demonstrate for the first time that hyaluronan synthesis is essential for myogenic differentiation and that Has2 mediates myogenic differentiation, muscle cell hyaluronan synthesis, and maintenance of a pericellular matrix around myoblasts.
Has1 and Has3 Expression Is Correlated with Inflammation
The pattern of Has1 expression in vivo and in vitro was not in keeping with a role for Has1 in myogenesis. Has1 expression in injured muscle did, however, coincide well with the acute inflammatory response induced by notexin that we have described previously (39); moreover, Has1 expression was significantly up-regulated in mdx compared with wild type diaphragms at 3 weeks of age, which is typically considered the time of onset of pathology, when necrosis and inflammation first become visible histologically. These observations strongly suggest that Has1 may be involved in the initial inflammatory response to necrosis in skeletal muscle.
As for Has1, changes in Has3 transcript expression were not particularly indicative of a role in myogenesis. Has3 expression was not altered during C2C12 myoblast differentiation or by pathology in the mdx diaphragm; however, there was an increase in expression neonatally compared with embryonic and postnatal time points. Most striking, however, was the similarity in expression patterns between Has3 and Has1 following notexin injection. Both genes showed a very similar trend with a peak at 1 day after notexin injection associated with the acute inflammatory response.
It was therefore very interesting to observe a large accumulation of hyaluronan in necrotic fibers being invaded by inflammatory cells not long after injection of notexin. This strongly suggests that hyaluronan could have a role in phagocytosis of necrotic myofibers by leukocytes and could promote the migration and adhesion of leukocytes to necrotic fibers. Numerous studies have suggested that hyaluronan is linked with inflammation, including the ability of small hyaluronan fragments to induce chemokine expression (40). Studies by Calve et al. (25) have indicated that hyaluronan synthesis was increased in a model of compensatory skeletal muscle hypertrophy induced by tenectomy. Indeed Calve et al. (25) demonstrated increased hyaluronan synthase gene expression associated with inflammation and expression of Has1, Has2, and Has3 co-localized with non-resident inflammatory macrophages. In addition, very recent studies have indicated that overexpression of Has1 promotes dermal regeneration by decreasing the number of inflammatory cells in the healing excision wound (41). Combined with our studies presented herein, these findings suggest that hyaluronan synthases may regulate inflammation associated with remodeling in skeletal muscle.
Has2 Expression Is Correlated with Myogenesis
A strong positive correlation between Has2 mRNA expression and myogenesis was observed in all four models examined, suggesting that Has2 is involved in the formation and regeneration of muscle fibers.
The formation of skeletal muscle myofibers during mouse development occurs in several phases. Following the formation of the muscle anlage, primary myogenesis (when embryonic myoblasts begin to differentiate and fuse into the primary syncytial muscle fibers) occurs around E11–E14 and is followed subsequently by fetal myoblast fusion in the secondary wave of myogenesis beginning around E14–E16 (42, 43). The data presented here show that in developing skeletal muscle, Has2 expression peaks at E14.5, a time that is associated with the onset of fetal myoblast fusion.
Has2 transcript expression was also up-regulated following notexin injection into the tibialis anterior. The expression profile of Has2 associated best with macrophage accumulation or myoblast differentiation, myotube formation, and maturation (39).
Increased expression of Has2 at 6 and 12 weeks of age in mdx compared with wild type diaphragms was during a phase that also corresponds with both myogenic differentiation and inflammation. Taken together with the observed up-regulation of Has2 during C2C12 myoblast differentiation, these observations constitute a substantial body of evidence implicating Has2 in fetal myoblast differentiation and also potentially in postnatal myoblast differentiation and myotube formation. These expression profiles suggested a function for Has2 in muscle growth and regeneration potentially relating to myogenesis. Therefore, we examined the involvement of Has2 in the process of myogenesis in culture by siRNA-mediated knockdown.
Has2 Knockdown and Loss of Hyaluronan Inhibit Myogenesis
Has2 transcript was successfully knocked down in C2C12 myoblasts induced to differentiate, and no change in Has1, Has3, Hyal1, or Hyal2 expression was observed, which, in conjunction with HABP reactivity, suggested that hyaluronan levels were greatly reduced as a consequence of knockdown without compensation toward hyaluronan anabolism or inhibition of catabolism. The reduction in HABP reactivity caused by Has2 knockdown, however, was not as complete as that achieved with 4MU, suggesting residual hyaluronan synthesis even with Has2 knockdown. Indeed, 4MU and Has2 siRNA caused distinct changes in transcriptional profiles, indicating that although these treatments can cause similar but different decreases in hyaluronan production and the pericellular matrix, which is useful for comparison of the effects of differentiation, these treatments should not be viewed as the same.
The hypothesis for this series of experiments was that the knockdown of Has2 would inhibit differentiation due to the positive correlation between Has2 expression and myogenesis. Myogenic differentiation was indeed inhibited by Has2 knockdown, as assessed by changes in morphology, creatine kinase activity, fusion index, and MRF gene expression. This inhibition of differentiation was mimicked by the hyaluronan synthesis inhibitor 4MU. The prevention of differentiation with 4MU was so pervasive that it suggests that hyaluronan synthesis is critical to myogenic differentiation. Because of the importance of hyaluronan in epithelial-mesenchymal transitions (44), we cannot exclude a more generalized cellular effect of 4MU, whereby loss of hyaluronan shifts C2C12 cells to a more epithelial rather than mesenchymal-like phenotype incapable of differentiating toward the myogenic lineage. There are many possible reasons for why and how loss of Has2 and hyaluronan could inhibit differentiation, and those investigated in this study are discussed.
CD44, the extracellular receptor for hyaluronan, has previously been reported as a protein necessary for the differentiation of myoblasts (45). However, these studies do not indicate whether the loss of CD44 may prevent differentiation through hyaluronan-dependent mechanisms. Has2 and Cd44 expression frequently appear to be linked, where higher expression of Has2 typically yields higher expression of Cd44 and vice versa (46–50). Thus, the decrease in Cd44 expression with Has2 knockdown could potentially be mediating inhibition of differentiation or reflect a dysregulation of CD44 and hyaluronan interactions, which could even more broadly lead to a dysregulation of cell and extracellular matrix interactions.
Loss of the extracellular matrix has previously been demonstrated to inhibit differentiation of myoblasts in culture. Inhibition of collagen synthesis reduced MRF expression and prevented myogenic differentiation in a manner that could be reversed with exogenous collagen in Matrigel (51, 52). Similarly, the loss of proteoglycans in the extracellular matrix by synthesis inhibitors inhibited differentiation and could be rescued by exogenous extracellular matrix administration (53–55). Hyaluronan is an important constituent of the extracellular matrix and therefore could be equally important for differentiation as collagens and proteoglycans and explain why Has2 knockdown and 4MU inhibit myogenic differentiation. 4MU treatment produced conspicuous decreases in transcripts for several matrix components, including collagen-I, fibronectin, and the proteoglycans biglycan and decorin, suggesting that 4MU could limit production of the extracellular matrix.
Loss of the pericellular matrix was considered due to the importance of cell-cell adhesion in mediating myoblast fusion and the role of hyaluronan in the initial contact between cells prior to integrin-mediated adhesion (56). It has been speculated that it could be necessary to remove the hyaluronan-rich pericellular coat to allow myoblast fusion (16), presumably through allowing closer contact between cell membranes and associated proteins. This mechanism has been suggested recently as a method by which proteoglycan cleavage can prevent retention of an overwhelmingly large pericellular matrix and thus allow cell-cell contact and differentiation/fusion (57). Both Has2 knockdown and 4MU abolished the hyaluronidase-sensitive pericellular matrix and also inhibited myogenic differentiation. Despite both treatments abolishing the pericellular matrix, 4MU caused a considerably greater inhibition of differentiation relative to Has2 knockdown. Consideration of the above findings and the inability of hyaluronan synthesis-inhibited cells to differentiate even after restoration of the pericellular matrix would suggest that the inhibition of differentiation caused by loss of hyaluronan occurs independently of pericellular matrix ablation. In support of this, we found that although both Has2 knockdown and 4MU treatment abolished pericellular matrix formation, hyaluronan could still be detected in or around Has2 knockdown cells but not with 4MU treatment by HABP. This indicated a difference in total hyaluronan production between Has2 knockdown and 4MU treatments, where 4MU appears to completely prevent hyaluronan synthesis, whereas Has2 knockdown reduces hyaluronan synthesis sufficiently to prevent pericellular matrix formation but not to completely prevent hyaluronan production. Therefore, hyaluronan functions independent from extracellular/pericellular functions may also be regulating myogenic differentiation.
Hyaluronan has recently come to be appreciated as a factor that can also be present and act intracellularly, mediating cell homeostasis by associating with intracellular hyaluronan-binding proteins that regulate RNA transcription and cell cycle progression, such as Cdc37, IHABP4, and p32 (58–60). Intracellular hyaluronan functions, such as binding Cdc37, could provide an alternative explanation for hyaluronan-mediated differentiation, because Cdc37 interacts with the myogenic transcription factor MyoD (61).
Altogether, this work demonstrates that hyaluronan synthase regulation occurs during skeletal muscle growth, injury, and regeneration. These changes in transcriptional regulation can be associated with different phases involved in regeneration, which can differ, depending on the synthase considered; Has1 and, to a lesser extent, Has3 associate well with an acute inflammatory response, and Has2 associates best with myoblast differentiation and myotube formation. Furthermore, this work demonstrates the requirement for Has2 and for hyaluronan synthesis in general for myogenic differentiation. This work has important implications for the field of skeletal muscle and potentially tissue engineering.
Acknowledgments
We thank Kitipong Uaesoontrachoon and Charles Pagel for providing oligonucleotide primer sequences for the genes Myod1 and Myog, and we thank Su Toulson for contributions toward the sampling of mice and subsequent processing of those samples. We also thank Amanda Fosang for providing the biotinylated hyaluronan-binding protein. We also thank Suneel Apte for providing the versican expression construct.
This work was supported by the Muscular Dystrophy Association of Australia (MDA) and the Victorian Government's Operational Infrastructure Support Program.
- En
- embryonic day n
- Pn
- postnatal day n
- qPCR
- quantitative PCR
- 4MU
- 4-methylumbelliferone
- HABP
- hyaluronan-binding protein
- MRF
- myogenic regulatory factor.
REFERENCES
- 1. Laurent T. C. (1970) Chemistry and Molecular Biology of the Intercellular Matrix, Vol. 2, pp. 703–732, Academic Press, Inc., New York [Google Scholar]
- 2. Stern R. (2003) Devising a pathway for hyaluronan catabolism. Are we there yet? Glycobiology 13, 105R–115R [DOI] [PubMed] [Google Scholar]
- 3. Laurent T. C., Fraser J. (1992) Hyaluronan. FASEB J. 6, 2397–2404 [PubMed] [Google Scholar]
- 4. Reed R. K., Lilja K., Laurent T. C. (1988) Hyaluronan in the rat with special reference to the skin. Acta Physiol. Scand. 134, 405–411 [DOI] [PubMed] [Google Scholar]
- 5. Laurent C., Johnson-Wells G., Hellström S., Engström-Laurent A., Wells A. F. (1991) Localization of hyaluronan in various muscular tissues. A morphological study in the rat. Cell Tissue Res. 263, 201–205 [DOI] [PubMed] [Google Scholar]
- 6. Piehl-Aulin K., Laurent C., Engström-Laurent A., Hellström S., Henriksson J. (1991) Hyaluronan in human skeletal muscle of lower extremity. Concentration, distribution, and effect of exercise. J. Appl. Physiol. 71, 2493–2498 [DOI] [PubMed] [Google Scholar]
- 7. Pedrosa-Domellöf F., Hellström S., Thornell L. E. (1998) Hyaluronan in human and rat muscle spindles. Histochem. Cell Biol. 110, 179–182 [DOI] [PubMed] [Google Scholar]
- 8. Huang L., Cheng Y. Y., Koo P. L., Lee K. M., Qin L., Cheng J. C., Kumta S. M. (2003) The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mater. Res. A 66, 880–884 [DOI] [PubMed] [Google Scholar]
- 9. Kujawa M. J., Carrino D. A., Caplan A. I. (1986) Substrate-bonded hyaluronic acid exhibits a size-dependent stimulation of chondrogenic differentiation of stage 24 limb mesenchymal cells in culture. Dev. Biol. 114, 519–528 [DOI] [PubMed] [Google Scholar]
- 10. Meran S., Thomas D., Stephens P., Martin J., Bowen T., Phillips A., Steadman R. (2007) Involvement of hyaluronan in regulation of fibroblast phenotype. J. Biol. Chem. 282, 25687–25697 [DOI] [PubMed] [Google Scholar]
- 11. Allingham P. G., Brownlee G. R., Harper G. S., Pho M., Nilsson S. K., Brown T. J. (2006) Gene expression, synthesis and degradation of hyaluronan during differentiation of 3T3-L1 adipocytes. Arch. Biochem. Biophys. 452, 83–91 [DOI] [PubMed] [Google Scholar]
- 12. Elson H. F., Ingwall J. S. (1980) The cell substratum modulates skeletal muscle differentiation. J. Supramol. Struct. 14, 313–328 [DOI] [PubMed] [Google Scholar]
- 13. Yoshimura M. (1985) Change of hyaluronic acid synthesis during differentiation of myogenic cells and its relation to transformation of myoblasts by Rous sarcoma virus. Cell Differ. 16, 175–185 [DOI] [PubMed] [Google Scholar]
- 14. Kujawa M. J., Pechak D. G., Fiszman M. Y., Caplan A. I. (1986) Hyaluronic acid bonded to cell culture surfaces inhibits the program of myogenesis. Dev. Biol. 113, 10–16 [DOI] [PubMed] [Google Scholar]
- 15. Kujawa M. J., Tepperman K. (1983) Culturing chick muscle cells on glycosaminoglycan substrates. Attachment and differentiation. Dev. Biol. 99, 277–286 [DOI] [PubMed] [Google Scholar]
- 16. Orkin R. W., Knudson W., Toole B. P. (1985) Loss of hyaluronate-dependent coat during myoblast fusion. Dev. Biol. 107, 527–530 [DOI] [PubMed] [Google Scholar]
- 17. Calve S., Odelberg S. J., Simon H.-G. (2010) A transitional extracellular matrix instructs cell behavior during muscle regeneration. Developmental Biology 344, 259–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Petillo O., Margarucci S., Peluso G., Barbarisi A., Melone M. A., Ambrosio L., Nicolais L. (1999) Modulation of in vitro myogenesis induced by different polymer substrates. J. Mater. Sci. Mater. Med. 10, 595–600 [DOI] [PubMed] [Google Scholar]
- 19. Spicer A. P., Olson J. S., McDonald J. A. (1997) Molecular cloning and characterization of a cDNA encoding the third putative mammalian hyaluronan synthase. J. Biol. Chem. 272, 8957–8961 [DOI] [PubMed] [Google Scholar]
- 20. Watanabe K., Yamaguchi Y. (1996) Molecular identification of a putative human hyaluronan synthase. J. Biol. Chem. 271, 22945–22948 [DOI] [PubMed] [Google Scholar]
- 21. Itano N., Sawai T., Yoshida M., Lenas P., Yamada Y., Imagawa M., Shinomura T., Hamaguchi M., Yoshida Y., Ohnuki Y., Miyauchi S., Spicer A. P., McDonald J. A., Kimata K. (1999) Three isoforms of mammalian hyaluronan synthases have distinct enzymatic properties. J. Biol. Chem. 274, 25085–25092 [DOI] [PubMed] [Google Scholar]
- 22. Spicer A. P., McDonald J. A. (1998) Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J. Biol. Chem. 273, 1923–1932 [DOI] [PubMed] [Google Scholar]
- 23. Camenisch T. D., Spicer A. P., Brehm-Gibson T., Biesterfeldt J., Augustine M. L., Calabro A., Jr., Kubalak S., Klewer S. E., McDonald J. A. (2000) Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Invest. 106, 349–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vuocolo T., Byrne K., White J., McWilliam S., Reverter A., Cockett N. E., Tellam R. L. (2007) Identification of a gene network contributing to hypertrophy in callipyge skeletal muscle. Physiol. Genomics 28, 253–272 [DOI] [PubMed] [Google Scholar]
- 25. Calve S., Isaac J., Gumucio J. P., Mendias C. L. (2012) Hyaluronic acid, HAS1, and HAS2 are significantly upregulated during muscle hypertrophy. Am. J. Physiol. Cell Physiol. 303, C577–C588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hunt L. C., Anthea Coles C., Gorman C. M., Tudor E. M., Smythe G. M., White J. D. (2011) Alterations in the expression of leukemia inhibitory factor following exercise. Comparisons between wild-type and mdx muscles. PLoS Curr. 3, RRN1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pfaffl M. W., Tichopad A., Prgomet C., Neuvians T. P. (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity. BestKeeper. Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515 [DOI] [PubMed] [Google Scholar]
- 28. Fosang A. J., Hey N. J., Carney S. L., Hardingham T. E. (1990) An ELISA plate-based assay for hyaluronan using biotinylated proteoglycan G1 domain (HA-binding region). Matrix 10, 306–313 [DOI] [PubMed] [Google Scholar]
- 29. Hunt L. C., Upadhyay A., Jazayeri J. A., Tudor E. M., White J. D. (2011) Caspase-3, myogenic transcription factors and cell cycle inhibitors are regulated by leukemia inhibitory factor to mediate inhibition of myogenic differentiation. Skelet. Muscle 1, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hattori N., Carrino D. A., Lauer M. E., Vasanji A., Wylie J. D., Nelson C. M., Apte S. S. (2011) Pericellular versican regulates the fibroblast-myofibroblast transition. A role for ADAMTS5 protease-mediated proteolysis. J. Biol. Chem. 286, 34298–34310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Plant D. R., Colarossi F. E., Lynch G. S. (2006) Notexin causes greater myotoxic damage and slower functional repair in mouse skeletal muscles than bupivacaine. Muscle Nerve 34, 577–585 [DOI] [PubMed] [Google Scholar]
- 33. Kultti A., Pasonen-Seppänen S., Jauhiainen M., Rilla K. J., Kärnä R., Pyöriä E., Tammi R. H., Tammi M. I. (2009) 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp. Cell Res. 315, 1914–1923 [DOI] [PubMed] [Google Scholar]
- 34. Nakamura T., Takagaki K., Shibata S., Tanaka K., Higuchi T., Endo M. (1995) Hyaluronic-acid-deficient extracellular matrix induced by addition of 4-methylumbelliferone to the medium of cultured human skin fibroblasts. Biochem. Biophys. Res. Commun. 208, 470–475 [DOI] [PubMed] [Google Scholar]
- 35. Nishida Y., Knudson C. B., Nietfeld J. J., Margulis A., Knudson W. (1999) Antisense inhibition of hyaluronan synthase-2 in human articular chondrocytes inhibits proteoglycan retention and matrix assembly. J. Biol. Chem. 274, 21893–21899 [DOI] [PubMed] [Google Scholar]
- 36. Harada H., Takahashi M. (2007) CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 282, 5597–5607 [DOI] [PubMed] [Google Scholar]
- 37. Mikami T., Koyama S., Yabuta Y., Kitagawa H. (2012) Chondroitin sulfate is a crucial determinant for skeletal muscle development/regeneration and improvement of muscular dystrophies. J. Biol. Chem. 287, 38531–38542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Knudson C. B. (1993) Hyaluronan receptor-directed assembly of chondrocyte pericellular matrix. J. Cell Biol. 120, 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hunt L. C., Upadhyay A., Jazayeri J. A., Tudor E. M., White J. D. (2013) An anti-inflammatory role for leukemia inhibitory factor receptor signaling in regenerating skeletal muscle. Histochem. Cell Biol. 139, 13–34 [DOI] [PubMed] [Google Scholar]
- 40. McKee C. M., Penno M. B., Cowman M., Burdick M. D., Strieter R. M., Bao C., Noble P. W. (1996) Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J. Clin. Invest. 98, 2403–2413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Caskey R. C., Allukian M., Lind R. C., Herdrich B. J., Xu J., Radu A., Mitchell M. E., Liechty K. W. (2013) Lentiviral-mediated over-expression of hyaluronan synthase-1 (HAS-1) decreases the cellular inflammatory response and results in regenerative wound repair. Cell Tissue Res. 351, 117–125 [DOI] [PubMed] [Google Scholar]
- 42. Kelly A. M. (1983) Emergence of specialization in skeletal muscle. In Handbook of Physiology (Peachy L. D., Adrian R., Geiger S. R., eds) Section 10, pp. 507–537, American Physiological Society, Bethesda, MD [Google Scholar]
- 43. Buckingham M., Bajard L., Chang T., Daubas P., Hadchouel J., Meilhac S., Montarras D., Rocancourt D., Relaix F. (2003) The formation of skeletal muscle. From somite to limb. J. Anat. 202, 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Toole B. P., Zoltan-Jones A., Misra S., Ghatak S. (2005) Hyaluronan. A critical component of epithelial-mesenchymal and epithelial-carcinoma transitions. Cells Tissues Organs 179, 66–72 [DOI] [PubMed] [Google Scholar]
- 45. Mylona E., Jones K. A., Mills S. T., Pavlath G. K. (2006) CD44 regulates myoblast migration and differentiation. J. Cell. Physiol. 209, 314–321 [DOI] [PubMed] [Google Scholar]
- 46. Nishida Y., D'Souza A. L., Thonar E. J., Knudson W. (2000) Stimulation of hyaluronan metabolism by interleukin-1α in human articular cartilage. Arthritis Rheum. 43, 1315–1326 [DOI] [PubMed] [Google Scholar]
- 47. Nishida Y., Knudson C. B., Eger W., Kuettner K. E., Knudson W. (2000) Osteogenic protein 1 stimulates cells-associated matrix assembly by normal human articular chondrocytes. Up-regulation of hyaluronan synthase, CD44, and aggrecan. Arthritis Rheum. 43, 206–214 [DOI] [PubMed] [Google Scholar]
- 48. Tammi R., Pasonen-Seppänen S., Kolehmainen E., Tammi M. (2005) Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J. Invest. Dermatol. 124, 898–905 [DOI] [PubMed] [Google Scholar]
- 49. Udabage L., Brownlee G. R., Nilsson S. K., Brown T. J. (2005) The over-expression of HAS2, Hyal-2 and CD44 is implicated in the invasiveness of breast cancer. Exp. Cell Res. 310, 205–217 [DOI] [PubMed] [Google Scholar]
- 50. Wang H., Zhan Y., Xu L., Feuerstein G. Z., Wang X. (2001) Use of suppression subtractive hybridization for differential gene expression in stroke. Discovery of CD44 gene expression and localization in permanent focal stroke in rats. Stroke 32, 1020–1027 [DOI] [PubMed] [Google Scholar]
- 51. Nandan D., Clarke E. P., Ball E. H., Sanwal B. D. (1990) Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation. Evidence for an essential role of collagen. J. Cell Biol. 110, 1673–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Saitoh O., Periasamy M., Kan M., Matsuda R. (1992) cis-4-Hydroxy-l-proline and ethyl-3,4-dihydroxybenzoate prevent myogenesis of C2C12 muscle cells and block MyoD1 and myogenin expression. Exp. Cell Res. 200, 70–76 [DOI] [PubMed] [Google Scholar]
- 53. Melo F., Carey D. J., Brandan E. (1996) Extracellular matrix is required for skeletal muscle differentiation but not myogenin expression. J. Cell. Biochem. 62, 227–239 [DOI] [PubMed] [Google Scholar]
- 54. Osses N., Brandan E. (2002) ECM is required for skeletal muscle differentiation independently of muscle regulatory factor expression. Am. J. Physiol. Cell Physiol 282, C383–C394 [DOI] [PubMed] [Google Scholar]
- 55. Osses N., Casar J. C., Brandan E. (2009) Inhibition of extracellular matrix assembly induces the expression of osteogenic markers in skeletal muscle cells by a BMP-2 independent mechanism. BMC Cell Biol. 10, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cohen M., Joester D., Sabanay I., Addadi L., Geiger B. (2007) Hyaluronan in the pericellular coat. An additional layer of complexity in early cell adhesion events. Soft Matter 3, 327–332 [DOI] [PubMed] [Google Scholar]
- 57. Stupka N., Kintakas C., White J. D., Fraser F. W., Hanciu M., Aramaki-Hattori N., Martin S., Coles C., Collier F., Ward A. C. (2013) Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and -15 facilitates myoblast fusion. J. Biol. Chem. 288, 1907–1917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grammatikakis N., Grammatikakis A., Yoneda M., Yu Q., Banerjee S. D., Toole B. P. (1995) A novel glycosaminoglycan-binding protein is the vertebrate homologue of the cell cycle control protein, Cdc37. J. Biol. Chem. 270, 16198–16205 [DOI] [PubMed] [Google Scholar]
- 59. Hascall V. C., Majors A. K., De La Motte C. A., Evanko S. P., Wang A., Drazba J. A., Strong S. A., Wight T. N. (2004) Intracellular hyaluronan. A new frontier for inflammation? Biochim. Biophys. Acta 1673, 3–12 [DOI] [PubMed] [Google Scholar]
- 60. Huang L., Grammatikakis N., Yoneda M., Banerjee S. D., Toole B. P. (2000) Molecular characterization of a novel intracellular hyaluronan-binding protein. J. Biol. Chem. 275, 29829–29839 [DOI] [PubMed] [Google Scholar]
- 61. Yun B. G., Matts R. L. (2005) Differential effects of Hsp90 inhibition on protein kinases regulating signal transduction pathways required for myoblast differentiation. Exp. Cell Res. 307, 212–223 [DOI] [PubMed] [Google Scholar]