Background: Mechanisms of endogenous PKC signal termination remain to be fully characterized.
Results: Activated endogenous PKCα undergoes dynamin-dependent and -independent endocytic uptake and traffics through early and late endosomes for processing by lysosomes.
Conclusion: Lysosomal degradation represents a novel mechanism of desensitizing PKC-mediated signaling.
Significance: Multiple degradation mechanisms ensure strict control of the duration of PKC signaling in cells.
Keywords: Caveolae, Endocytosis, Lipid Raft, Lysosomes, Protein Degradation, Protein Kinase C (PKC), PKCα Degradation, Lysosomal
Abstract
Protein kinase C (PKC) isozymes undergo down-regulation upon sustained stimulation. Previous studies have pointed to the existence of both proteasome-dependent and -independent pathways of PKCα processing. Here we demonstrate that these down-regulation pathways are engaged in different subcellular compartments; proteasomal degradation occurs mainly at the plasma membrane, whereas non-proteasomal processing occurs in the perinuclear region. Using cholesterol depletion, pharmacological inhibitors, RNA interference, and dominant-negative mutants, we define the mechanisms involved in perinuclear accumulation of PKCα and identify the non-proteasomal mechanism mediating its degradation. We show that intracellular accumulation of PKCα involves at least two clathrin-independent, cholesterol/lipid raft-mediated pathways that do not require ubiquitination of the protein; one is dynamin-dependent and likely involves caveolae, whereas the other is dynamin- and small GTPase-independent. Internalized PKCα traffics through endosomes and is delivered to the lysosome for degradation. Supportive evidence includes (a) detection of the enzyme in EEA1-positive early endosomes, Rab7-positive late endosomes/multivesicular bodies, and LAMP1-positive lysosomes and (b) inhibition of its down-regulation by lysosome-disrupting agents and leupeptin. Only limited dephosphorylation of PKCα occurs during trafficking, with fully mature enzyme being the main target for lysosomal degradation. These studies define a novel and widespread mechanism of desensitization of PKCα signaling that involves endocytic trafficking and lysosome-mediated degradation of the mature, fully phosphorylated protein.
Introduction
Members of the protein kinase C (PKC) family of serine/threonine kinases are central players in signal transduction pathways involved in regulation of cell proliferation, differentiation, apoptosis/survival, migration, and invasion as well as receptor trafficking and gene expression (1–8). PKCα, a member of the conventional, Ca2+-dependent class of PKC isozymes (that also includes PKCβI, -βII, and -γ), has been implicated in both negative and positive regulation of cell proliferation, survival, and motility in normal cells and tissues (1), and alterations in PKCα signaling/expression have been associated with the transformed phenotype in a variety of tumor types (9–14). Aberrant degradation of PKCα has also been associated with various pathologies, including Huntington and Alzheimer (15, 16). Strict control of PKCα function is achieved by three coordinated mechanisms: (a) phosphorylation on three priming sites in the C-terminal domain (activation loop (Thr497), turn motif (Thr638), and hydrophobic motif (Ser657)), which is required for catalytic competence (17, 18); (b) binding of Ca2+ and diacylglycerol, which promotes membrane targeting and conformational activation (18); and (c) acute and long term desensitization mechanisms (7, 19). Acute termination of PKCα signaling may occur through a process termed “reverse translocation,” involving return of the enzyme to the cytosol after its translocation and activation at the membrane (20, 21). Ligand-induced PKC activity may also be terminated through multisite dephosphorylation by cellular phosphatases such as protein phosphatase 2A and PH domain leucine-rich repeat protein phosphatase (PHLPP) (22–25). Other studies have indicated that PKCα can be acutely inactivated by oxidative mechanisms (26).
It has long been recognized that PKC/PKCα signaling can also undergo long term desensitization through down-regulation (27, 28). Prolonged activation of PKC isozymes by physiological activators (e.g. hormones, growth factors, G protein-coupled receptor agonists; Refs. 23 and 28–30) or pharmacological agonists (e.g. phorbol esters, bryostatin 1 (Bryo)2) leads to degradation of these proteins (25, 27, 31–33). Although the steps involved in maturation, activation, and acute desensitization of PKC isozymes have been extensively studied, the pathways underlying long term desensitization of these molecules remain poorly understood (19, 34, 35). Mechanisms that have been put forward include (a) conformation-dependent proteolysis by Ca2+-dependent proteases (calpains; Ref. 36, 37, and 38), (b) vesicle trafficking-dependent multisite dephosphorylation (23, 39) and ubiquitin/proteasome-dependent degradation of the dephosphorylated species (29–32, 40–42), and (c) caspase-mediated cleavage. It has been further proposed that the kinase domain is required for recognition by the degradation machinery (25, 32, 39, 40) and that kinase activity may be important for modification of the enzyme itself, increased vesicle trafficking for delivery to a degradation compartment, and activation of protease activity. Together, the available data have been combined to generate the following working model for long term PKCα desensitization (19): (a) trafficking of the active enzyme to a perinuclear compartment (19, 39, 42), (b) dephosphorylation of priming sites by protein phosphatase 2A-like activity (24) and/or by PHLPP1/2 (22), and (c) ubiquitination of the dephosphorylated protein and degradation through a proteasomal pathway (19, 40).
Although this model may reflect one pathway of PKC degradation, several lines of evidence from our laboratory and others indicate that there is considerable complexity in the regulation of PKCα desensitization processes (35) and that the proposed pathway likely combines aspects of more than one distinct down-regulation mechanism. One source for this confusion may be the widespread use of overexpression strategies to study PKC processing. Our previous studies on endogenous PKCα in Bryo-treated IEC-18 intestinal epithelial cells have indicated that at least two mechanisms of PKCα degradation can be engaged at the same time. In addition to proteasomal degradation, Bryo induces a novel non-proteasomal, calpain-independent mechanism of PKCα processing. In the current study we further characterize this non-proteasomal mechanism of PKCα desensitization. We demonstrate that it involves trafficking of activated PKCα from the plasma membrane through cholesterol/lipid raft-mediated endocytic pathways that can be dynamin-dependent or -independent, with eventual degradation of the protein via a lysosomal mechanism. Intracellular trafficking of PKC does not require ubiquitination, and the mature, phosphorylated species appears to be the predominant target for lysosomal processing. A better understanding of PKC desensitization mechanisms has important implications for the development of novel approaches to target these kinases in disease (35, 43).
MATERIALS AND METHODS
Cell Culture
IEC-18 rat intestinal crypt-like cells (ATCC CRL-1589) were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 4 mm l-glutamine, and 5 μg/ml insulin. U-87 human glioblastoma cells and HeLa human cervical cancer cells were cultured in DMEM supplemented with 10% FBS and 2 mm l-glutamine; HeLa cells were maintained in the presence of penicillin/streptomycin. L929 mouse fibroblasts were grown in DMEM, 5% FBS. Experiments with genistein were carried out in serum-free DMEM with no additives.
Drugs and Reagents
PKCα was activated by treatment with 100 nm bryostatin (Biomol) or 100 nm phorbol 12-myristate 13-acetate (PMA) (Sigma). Sources of other chemicals were as follows: chloroquine diphosphate, bafilomycin A1, cycloheximide, U18666A (Sigma); leupeptin (A. G. Scientific); concanamycin A (VWR); bisindolylmaleimide I, dynasore, nystatin, genistein, N-acetyl-Leu-Leu-Nle-CHO (ALLN; Nle is norleucine) and MG132 (Calbiochem); epoxomicin (Enzo); lactacystin (Dr. E. J. Corey, Harvard University); 4[4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester (UBEI-41, BioGenova); Clostridium difficile Toxin B (EMD Biosciences); cholesterol (Krackeler). Drugs were dissolved in DMSO except for Bryo, PMA, lactacystin, and concanamycin A, which were dissolved in ethanol, C. difficile Toxin B, chloroquine, and leupeptin, which were dissolved in sterile water, and nystatin, which was prepared as a 50 mm stock solution in ethanol and diluted 1:2 in DMSO before cell treatments. Equal volumes of the relevant solvent were used as vehicle controls (concentrations of individual solvents remained below 0.2% v/v and did not affect the localization or degradation of PKCα). Inhibitors were added 30 min before PKC agonist treatment, with the exception of leupeptin and genistein, which were added 24 h or 0.5–1 h before agonist treatment, respectively.
Antibodies
Primary antibodies for immunofluorescence and Western blotting were: rabbit anti-C-terminal PKCα (Epitomics), mouse anti-clathrin heavy chain (BD Biosciences), rabbit anti-β-actin (Sigma), mouse anti-LAMP1 (Santa Cruz), mouse anti-EEA1 (BD Biosciences), and mouse anti-HA tag (Covance). Secondary antibodies were Cy3-conjugated goat anti-mouse (Jackson ImmunoResearch Laboratories), Alexa Fluor 488-conjugated donkey anti-rabbit (Invitrogen), TRITC-conjugated donkey anti-rabbit (Jackson ImmunoResearch Laboratories), horseradish peroxidase-conjugated goat anti-rabbit IgG (Millipore), and horseradish peroxidase-conjugated goat anti-mouse IgG (Bio-Rad).
Western Blotting
Preparation of whole cell lysates, Western blotting, and signal detection with SuperSignal West Pico ECL reagents (ThermoScientific) and GeneMate Blue Lite Autoradiography Film (BioExpress) were performed using standard protocols as we have described (31, 44). Nitrocellulose membranes were routinely stained with 0.1% Fast Green (Sigma) to confirm equal loading and even transfer. Antibody dilutions were as follows: anti-C-terminal PKCα (1:20,000), anti-β-actin (1:10,000), anti-clathrin heavy chain (1:1,000), horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,000), and horseradish peroxidase-conjugated goat anti-mouse IgG (1:2,000). Estimates of band intensity were obtained by analysis of scanned blots using ImageJ software, and data are presented relative to control (mean ± S.E.). Two-tailed Student's t tests were performed using Microsoft Excel software.
Immunofluorescence Analysis
Cells grown on glass coverslips were treated as indicated, fixed in 2% formaldehyde/PBS for 15 min at room temperature, and processed for immunofluorescence microscopy as we have described (31). Saponin (0.2%) or Triton X-100 (0.2%) was used for cell permeabilization. Primary antibody dilutions were: rabbit anti-C-terminal PKCα (1:500), mouse anti-EEA1 (1:100), mouse anti-clathrin heavy chain (1:100), and mouse anti-HA tag (1:2,000). TRITC-conjugated anti-rabbit secondary antibody was used at 1:100, anti-mouse-Cy3 was used at 1:2,000, and anti-rabbit-Alexa Fluor 488 was used at 1:800. Cells were viewed with a Zeiss Axioskop epifluorescence microscope using a 63× Plan Apochromat (1.4 NA) objective lens. Images were obtained using a Hamamatsu C7780 digital camera. Superimposed images were generated using Adobe Photoshop software.
Confocal images are 0.5 μm thick optical sections acquired on a Zeiss LSM 510 Meta Confocal Laser Scanning Microscope using a 63× Plan-Apo/1.4 NA oil objective and Zeiss AIM and LSM Image Browser software. Alexa Fluor 488 was visualized using an argon laser at 488 nm, with detection through a 505–550-nm band pass filter. Cy3 was visualized using a HeNe laser at 543 nm and a 575–615-nm band pass filter.
Adenoviral Overexpression
IEC-18 cells on glass coverslips were infected with LacZ-adenovirus or PKCα-adenovirus at a multiplicity of infection of 5 for 16 h in IEC-18 minimal medium: DMEM supplemented with 2% FBS, 4 mm l-glutamine, and 5 μg/ml insulin. The medium was then replaced with IEC-18 complete medium (DMEM supplemented with 5% FBS, 4 mm l-glutamine, and 5 μg/ml insulin), and cells were grown to near confluence before treatments.
Plasmids and Transfection
Dominant negative mutant constructs T22N-Rab7-GFP, T17N-Cdc42-GFP, and T27N-Arf6-HA were purchased from Addgene. The dominant negative K44A-dynamin-HA plasmid was a kind gift from Dr. Jeffrey Benovic (Thomas Jefferson University). IEC-18 cells were grown at low confluency on glass coverslips, and transfections were performed using a 3:1 ratio of FuGENE® 6 to plasmid DNA as recommended by the manufacturer (Roche Applied Science). Drug treatments and analysis were performed 24–48 h after transfection.
siRNA
IEC-18 cells were transfected with 100 nm clathrin heavy chain siRNA (Dharmacon) or with 100 nm Silencer Select negative control #1 siRNA (Invitrogen) using Lipofectamine 2000 transfection reagent (Invitrogen) according to manufacturer protocols (Invitrogen). Medium was changed, and transfection with siRNA was repeated after 72 h to ensure full depletion of clathrin heavy chain. Analysis was performed 72 h after the second transfection.
Transferrin and Cholera Toxin Uptake Assay
Transferrin and cholera toxin uptake was examined using previously published protocols (45, 46). Briefly, cells were washed twice with PBS to remove all residual serum and incubated 1 h in serum-free DMEM containing 20 mm l-glutamine and 2 nm insulin. 25 μg/ml Alexa Fluor 488-conjugated transferrin or 10 μg/ml Alexa Fluor 488-conjugated cholera toxin (Invitrogen) were added to the treated cells for 15 min at room temperature, and the medium was then replaced with 37 °C medium containing endocytic pathway inhibitors and PKC agonists as indicated. Cells were processed for immunofluorescence after 15–30 min. Where indicated, dynasore was added 30 min before Alexa Fluor 488-conjugated transferrin.
RESULTS
Differential Effects of Agonist Treatment on Endogenous and Overexpressed PKCα in IEC-18 Cells
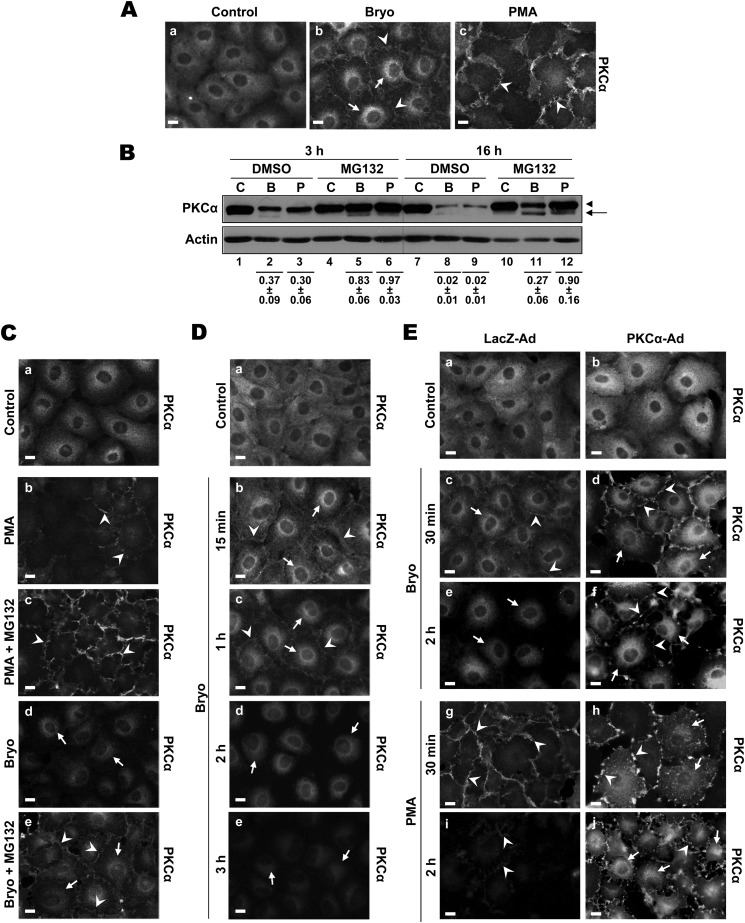
The PKC agonists Bryo and PMA potently activate and then down-regulate PKCα in a variety of cell types, and both agents promote subcellular redistribution of the enzyme. Our previous studies demonstrated that these agonists have different effects on the distribution of endogenous PKCα in IEC-18 intestinal epithelial cells; Bryo induces both membrane translocation and perinuclear accumulation of the enzyme, whereas PMA predominantly induces plasma membrane association (Fig. 1A) (31). As shown in Fig. 1B, both Bryo and PMA significantly down-regulate endogenous PKCα in IEC-18 cells (p < 0.001 for Bryo/PMA versus control). Down-regulation primarily occurs through a proteasome-dependent pathway (31), as indicated by the inhibitory effects of MG132 at early times of treatment (e.g. 3 h; Fig. 1B, p < 0.001 for effects of MG132 on down-regulation by Bryo or PMA). Immunofluorescence analysis demonstrated that plasma membrane-associated PKCα is the main target for proteasomal degradation, as proteasome inhibition protected the enzyme in this compartment while not affecting levels of internalized protein (Fig. 1C, compare panels b and c and panels d and e). Bryo also engages a slower, non-proteasomal mechanism of endogenous PKCα down-regulation, evident after prolonged incubation in the presence of proteasome inhibitors (Fig. 1B, compare lanes 4–6 and 10–12; p = 0.005 for the difference between MG132 plus Bryo and MG132 plus PMA at 16 h). The failure of proteasome inhibition to affect the down-regulation of internalized protein seen at 2 h (Fig. 1C, panels d and e, and Fig. 1D, compare panels b and d) together with the delayed kinetics of down-regulation of internalized protein relative to membrane-associated enzyme (Fig. 1D) point to the perinuclear region as the site for this slower, non-proteasomal mechanism of agonist-induced degradation.
FIGURE 1.
Differential effects of Bryo and PMA on the subcellular distribution and expression of endogenous and overexpressed PKCα. A, immunofluorescence analysis of the effects of Bryo and PMA on the subcellular distribution of PKCα is shown. IEC-18 cells were grown on glass coverslips and treated with vehicle (Control) (a), 100 nm Bryo (b), or 100 nm PMA (c) for 30 min. Cells were then fixed and immunostained for PKCα. Arrows, perinuclear staining; arrowheads, membrane staining. Magnification bars, 10 μm. B, shown is an immunoblot analysis of the effects of Bryo and PMA on PKCα expression. Bryo induces proteasomal and non-proteasomal degradation of PKCα, whereas PMA only induces proteasomal degradation of the enzyme. IEC-18 cells were treated for 3 or 16 h with vehicle (C), 100 nm Bryo (B), or 100 nm PMA (P) in the presence or absence of 20 μm MG132, and whole cell lysates were subjected to Western blot analysis for PKCα and β-actin. Arrows, faster migrating, non-phosphorylated PKCα; arrowheads, mature phosphorylated PKCα. Numbers below lanes represent PKCα band intensity in that lane relative to the corresponding control. Values are the mean ± S.E., n = 7 (3 h) or 6 (16 h). C, proteasome inhibition protects plasma membrane-associated PKCα but does not affect levels of the internalized enzyme in PKC agonist-treated IEC-18 cells. Cells grown on glass coverslips were treated for 2 h with vehicle (Control) (a), 100 nm PMA (b and c), or 100 nm Bryo (d and e) in the presence or absence of 20 μm MG132 as indicated and processed for PKCα immunofluorescence. Arrows and arrowheads are as in A. Magnification bars, 10 μm. D, time course of down-regulation of membrane-associated and perinuclear PKCα in Bryo-treated IEC-18 cells is shown. Cells were treated with vehicle (Control, 2 h) (a) or with 100 nm Bryo for the indicated times (b–d) and processed for PKCα immunofluorescence. Arrows and arrowheads are as in A. Magnification bars, 10 μm. E, shown is a comparison of the effects of Bryo and PMA on the subcellular distribution and down-regulation of endogenous and overexpressed PKCα in IEC-18 cells. Cells infected with 5 multiplicity of infection of LacZ- or PKCα-adenovirus were treated for 30 min or 2 h with Bryo (c–f) or PMA (g–j) as indicated and processed for PKCα immunofluorescence. Arrows and arrowheads are as in A. Magnification bars, 10 μm. All data are representative of at least three independent experiments.
In contrast to our findings, studies using overexpressed exogenous PKCα have pointed to a model in which PKC agonists, including PMA, target internalized enzyme for proteasomal degradation (19, 42). The inconsistency between our results and this model raised the possibility that overexpression leads to an altered response of PKCs to agonist treatment. To determine if this is the case, we compared the effects of Bryo and PMA on the subcellular localization of PKCα in IEC-18 cells infected with control LacZ- or PKCα-expressing adenovirus. As expected, Bryo induced translocation of endogenous PKCα to both the plasma membrane and the perinuclear region in LacZ-adenovirus infected cells (Fig. 1E, panel c, arrowheads and arrows, respectively), whereas PMA targeted the enzyme exclusively to the plasma membrane (Fig. 1E, panel g, arrowheads). Plasma membrane staining was markedly diminished by 2 h (Fig. 1E, panels e and i), consistent with the previously observed rapid proteasomal degradation of endogenous protein at this location (Fig. 1C) (31). In contrast, the perinuclear staining seen in Bryo-treated cells was still clearly evident at 2 h (Fig. 1E, panel e, arrows), reflecting the slower, likely non-proteasomal, degradation of internalized protein (31). The fact that both the relocalization and kinetics of degradation paralleled those seen in non-infected cells indicates that adenoviral infection does not in itself affect agonist-induced processing of PKCα. Bryo similarly promoted plasma membrane and perinuclear accumulation of overexpressed PKCα (Fig. 1E, panel d, arrowheads and arrows). However, membrane staining was still evident at 2 h (Fig. 1E, panel f), suggesting saturation of the translocation and/or degradation systems by the overexpressed protein. Notably, the subcellular distribution of overexpressed protein in PMA-treated cells was markedly different from that seen for the endogenous protein. In addition to translocation to the plasma membrane (Fig. 1E, panels h and j, arrowheads), exogenous PKCα also localized to the perinuclear region (Fig. 1E, panels h and j, arrows). This intracellular accumulation was evident by 30 min, a time at which agonist-induced down-regulation of the endogenous protein is minimal and remained prominent at 2 h, by which time the endogenous protein was extensively down-regulated in PMA- treated cells. Because overexpression alters both the kinetics of degradation and the intracellular trafficking of activated PKCα, it would appear that the normal agonist-induced processing mechanisms for PKCα are easily saturated and that overexpressed protein can be channeled into alternate pathways.
Collectively, these data indicate that, although overexpression systems have yielded valuable information regarding the regulation of PKCs, a true understanding of the mechanisms involved in signal termination necessitates analysis of the endogenous protein. As discussed above, this approach has revealed the existence of a previously unrecognized non-proteasomal mechanism of agonist-induced PKCα degradation that appears to occur in a perinuclear compartment. However, the following questions regarding this pathway remained unanswered; what is the mechanism of Bryo-induced PKCα perinuclear accumulation? and what is the mechanism of proteasome-independent agonist-induced PKCα degradation?
Mechanisms of Bryo-induced PKCα Perinuclear Accumulation
Bryostatin-induced PKCα Perinuclear Accumulation Involves Endocytic Trafficking
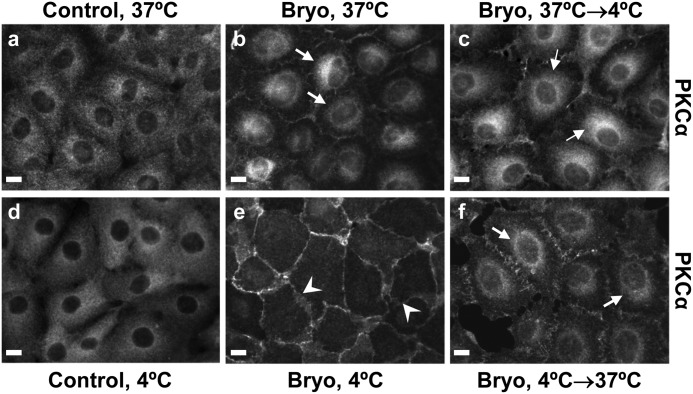
Two possible mechanisms could explain the perinuclear accumulation of PKCα seen after Bryo treatment: (a) simultaneous targeting of PKCα to the plasma membrane and a perinuclear compartment or (b) initial targeting of the enzyme to the plasma membrane followed by engagement of an endocytic pathway and translocation to the interior of the cell (39, 47). Because all endocytic pathways are temperature-sensitive, we manipulated the incubation temperature to explore the role of endocytosis in perinuclear compartmentalization of PKCα. As shown in Fig. 2, treatment of IEC-18 cells with Bryo at 4 °C for 2 h resulted in recruitment of the entire pool of cellular PKCα to the plasma membrane (see Fig. 2, panel e). Perinuclear accumulation of the enzyme was only observed after the cells were shifted to 37 °C (Fig. 2, panel f). Incubation at 4 °C did not affect the diffuse cytoplasmic distribution of PKCα in control cells (Fig. 2, panel d), and the enzyme did not relocalize to the plasma membrane when the cells were treated with Bryo at 37 °C before being shifted to 4 °C (Fig. 2, panel c), excluding a direct effect of low temperature on PKCα localization. These data support a mechanism in which PKCα is first recruited to the plasma membrane and then translocated to a perinuclear compartment via an endocytic pathway.
FIGURE 2.
Bryo targets PKCα to the cell periphery and subsequently promotes temperature-dependent perinuclear accumulation of the enzyme. IEC-18 cells on glass coverslips were treated as follows: panel a, DMSO (Control) for 1 h at 37 °C; panel b, Bryo (100 nm) for 1 h at 37 °C; panel c, Bryo for 30 min at 37 °C followed by 2 h at 4 °C; panel d, DMSO for 2 h at 4 °C; panel e, Bryo for 2 h at 4 °C; panel f, Bryo for 2 h at 4 °C followed by 1 h at 37 °C. Cells were then fixed and processed for PKCα immunofluorescence. Arrows indicate perinuclear staining, and arrowheads point to membrane staining. Magnification bars, 10 μm. Data are representative of at least three independent experiments.
Bryostatin-induced PKCα Internalization Is Clathrin-independent but Dependent on Cholesterol and Sensitive to Genistein
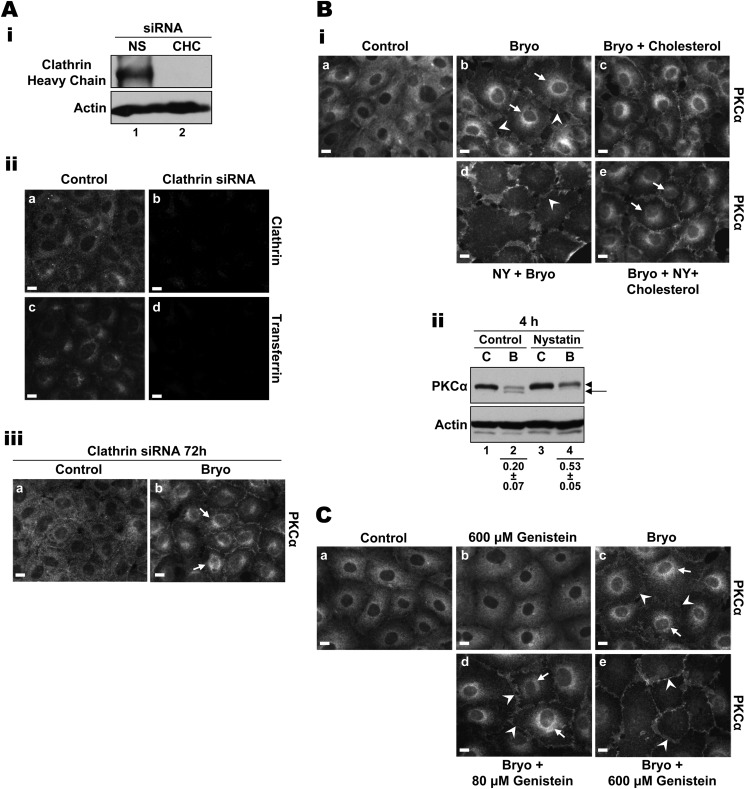
Internalization occurs via two main pathways, clathrin-dependent and clathrin-independent lipid raft-dependent endocytosis (48, 49) (Table 1). Previous studies of overexpressed (7, 39) or endogenous (31, 42) protein have indicated that PKC intracellular trafficking can involve clathrin-dependent (7, 42) or lipid raft/caveolae-dependent pathways (31, 39). To determine whether Bryo-induced intracellular trafficking of PKCα involves a clathrin-dependent pathway, clathrin heavy chain expression was silenced in IEC-18 cells using siRNA. Knockdown of clathrin heavy chain, which potently disrupts clathrin-dependent endocytosis (50), was confirmed by Western blotting and immunofluorescence analysis (Fig. 3A, i and ii, panels a and b). As shown in Fig. 3Aii, panels c and d), siRNA-mediated knockdown of clathrin heavy chain completely abrogated clathrin-dependent uptake of transferrin (51, 52); however, clathrin deficiency failed to inhibit perinuclear accumulation of PKCα in Bryo-treated cells (Fig. 3Aiii, arrows). Consistent with this finding, inhibition of clathrin-dependent intracellular trafficking using potassium depletion (53, 54) also had no effect on Bryo-induced internalization (data not shown).
TABLE 1.
Characteristics of known endocytic pathways
| Endocytic Pathway | Clathrin-dependent | Clathrin-independent, lipid raft-dependent |
||||
|---|---|---|---|---|---|---|
| Caveolae- mediated | RhoA-regulated | Cdc42-regulated | Arf6-regulated | Non-caveolar, small GTPase- independent | ||
| Temperature-sensitive | + | + | + | + | + | + |
| Dependent on clathrin | + | − | − | − | − | − |
| Sensitive to cholesterol binding agents, e.g. nystatin | − | + | + | + | + | + |
| Sensitivity to genistein | − | + | + | + | + | + |
| Dynamin-dependent | + | + | + | − | − | − |
| RhoA-regulated | − | − | + | − | − | − |
| Cdc42-regulated | − | − | − | + | − | − |
| Arf6-regulated | + | − | − | − | + | − |
FIGURE 3.
Bryo-induced perinuclear accumulation of PKCα is clathrin-independent, cholesterol-dependent, and sensitive to genistein. A, clathrin knockdown does not inhibit Bryo-induced PKCα internalization. i, IEC-18 cells, transfected with non-targeting control siRNA (NS) or clathrin heavy chain-targeted siRNA (CHC), were subjected to anti-clathrin heavy chain immunoblotting. ii, IEC-18 cells, transfected with clathrin heavy chain siRNA (Clathrin siRNA) or mock-transfected (Control), were subjected to Alexa Fluor 488-transferrin uptake assays and immunostained for clathrin. Panels a and b show clathrin staining; panels c and d confirm that clathrin knockdown prevents clathrin-dependent transferrin uptake in IEC-18 cells. iii, siRNA-transfected, clathrin-deficient IEC-18 cells were treated with DMSO (Control, a) or 100 nm Bryo (b) for 30 min and processed for PKCα immunofluorescence. Arrows show perinuclear staining for PKCα. Bi, Bryo-induced PKCα internalization requires cholesterol. IEC-18 cells were treated with DMSO (Control, a), 100 nm Bryo (b), 100 nm Bryo plus 0.5 mm cholesterol (c), 100 nm Bryo plus 100 μm nystatin (NY) (d), or 100 nm Bryo plus 100 μm nystatin and 0.5 mm cholesterol (e), for 30 min. Cells were then fixed and processed for PKCα immunofluorescence. Arrows, perinuclear staining; arrowheads, membrane staining. ii, inhibition of PKCα internalization with nystatin protects the enzyme from Bryo-induced down-regulation. IEC-18 cells were treated with 100 nm Bryo (B) for 4–6 h in the presence or absence of 100 μm nystatin, and whole cell lysates were subjected to Western blot analysis for PKCα and β-actin. C, control. Arrows, faster migrating, non-phosphorylated PKCα; arrowheads, mature phosphorylated PKCα. Numbers below the lanes represent PKCα band intensity in that lane relative to the corresponding control. Values are the mean ± S.E., n = 5. C, Bryo-induced perinuclear accumulation of PKCα is genistein-sensitive. IEC-18 cells were treated with DMSO (Control, a), 600 μm genistein (b), 100 nm Bryo (c), 100 nm Bryo plus 80 μm genistein (d), or 100 nm Bryo plus 600 μm genistein (e) for 30 min. Cells were then processed for PKCα immunofluorescence. Arrows, perinuclear staining; arrowheads, membrane staining. Magnification bars, 10 μm. All data are representative of at least three independent experiments.
Having excluded a role for a clathrin-dependent pathway, we next explored the involvement of clathrin-independent, lipid raft-dependent mechanisms (48, 49). Because cholesterol is known to be an important component of clathrin-independent endocytic pathways (55–57) (Table 1), we tested the cholesterol dependence of Bryo-induced PKCα intracellular trafficking by cholesterol depletion and rescue experiments. At concentrations of ≤100 μm, the cholesterol sequestering agent nystatin has been shown to block lipid raft-dependent endocytosis while not affecting clathrin-coated pits or clathrin-mediated endocytosis (58–61). This selectivity was confirmed in IEC-18 cells by the inability of nystatin to affect the uptake of transferrin (data not shown). Our previous studies had determined that Bryo-induced internalization of PKCα is nystatin-sensitive (31); however, the dependence of this effect on cholesterol was not examined. As seen previously, nystatin treatment completely inhibited Bryo-induced perinuclear accumulation of PKCα (Fig. 3Bi, panel d). The ability of excess cholesterol (0.5 mm) to rescue the effect (Fig. 3Bi, panel e) established the cholesterol dependence of PKCα internalization. Notably, nystatin treatment also resulted in partial inhibition of Bryo-induced degradation of the enzyme (Fig. 3Bii, p = 0.004), indicating that the cholesterol-dependent internalization pathway directly contributes to PKCα degradation after Bryo treatment. Together, the data indicate that Bryo-induced PKCα trafficking and subsequent degradation involves a clathrin-independent, lipid raft-dependent mechanism(s) (48, 49).
Clathrin-independent/lipid raft-dependent endocytic pathways have also been shown to be sensitive to the isoflavonoid genistein (48, 56–61) (Table 1), and this agent has been widely used to differentiate between clathrin-dependent and -independent uptake mechanisms (cf. Refs. 48, 49, 62, and 63–66). As shown in Fig. 3C, concentrations of genistein as low as 40–80 μm caused a reduction in Bryo-induced translocation of PKCα from the plasma membrane. However, consistent with the concentration of 400 μm required for optimal inhibition of clathrin-independent uptake of lactosylceramide in a variety of cell types (48), full blockade of perinuclear accumulation required 300–600 μm genistein. Together, these data indicate that Bryo-induced PKCα intracellular trafficking is sensitive to cholesterol depletion and genistein treatment and are, thus, consistent with the involvement of a clathrin-independent/lipid raft-dependent pathway(s).
Clathrin-independent Endocytosis of Endogenous PKCα Occurs via Dynamin-dependent and -independent Pathways and Is Independent of Small GTPase Activity
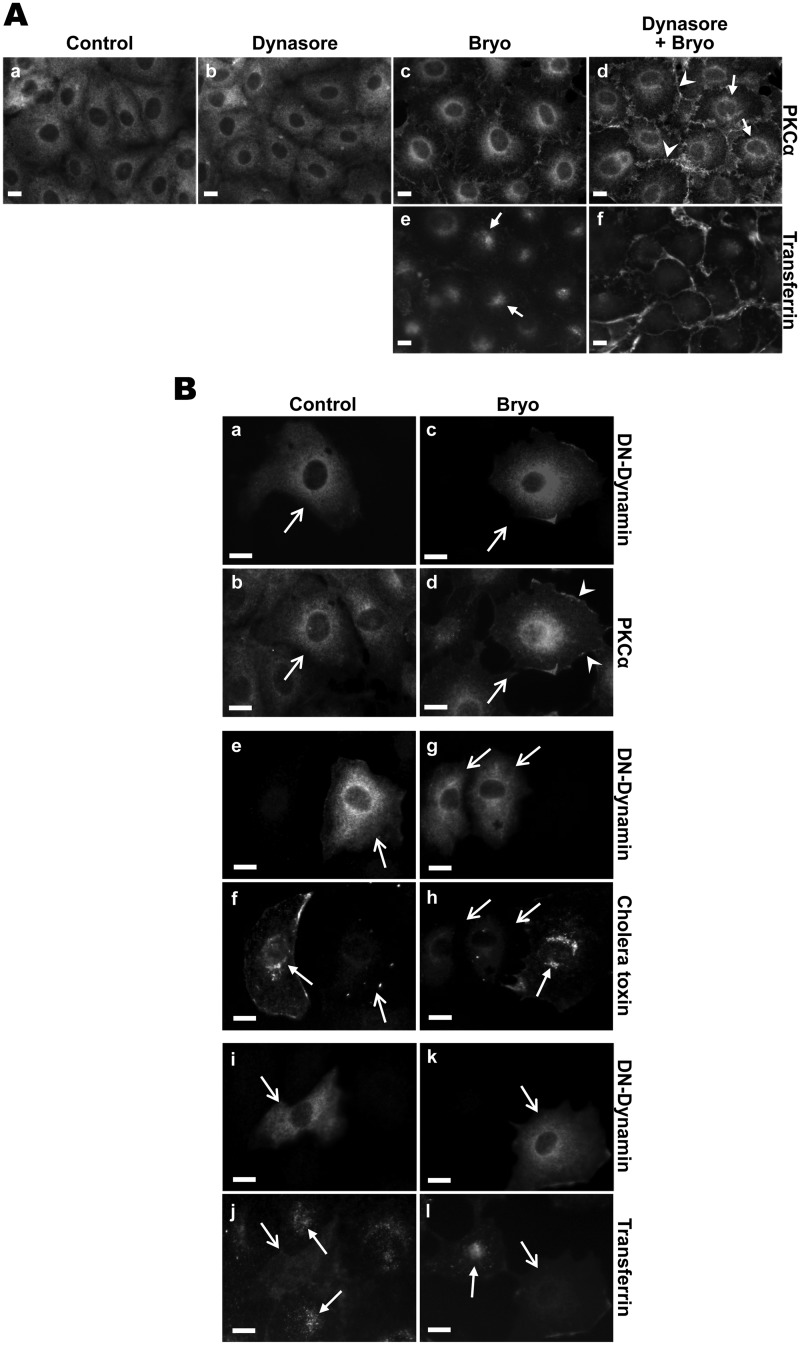
Clathrin-independent, lipid raft-dependent pathways can be classified based on (a) their requirement for dynamin, which is responsible for scission of vesicles from the plasma membrane during endocytosis (67) and (b) the involvement of the small GTPases, RhoA, ADP-ribosylation factor-6 (Arf6) or Cdc42 (68) (Table 1). Clathrin-independent, dynamin-dependent pathways include caveolae-mediated endocytosis and RhoA-regulated endocytosis; however, Cdc42- and Arf6-regulated pathways are dynamin-independent (48, 49). Two approaches were used to determine the dynamin dependence of PKCα internalization: (a) pharmacological inhibition of dynamin activity with dynasore (69, 70) and (b) expression of a dominant-negative K44A dynamin mutant (67). The effectiveness of these strategies in blocking dynamin-dependent processes was confirmed by their ability to completely block clathrin-mediated uptake of transferrin and/or caveolar uptake of cholera toxin (Fig. 4A, panels e and f, and B, panels e–l), both of which are dynamin-dependent (45, 69, 71). Treatment of IEC-18 cells with dynasore consistently, albeit only partially, reduced Bryo-induced PKCα internalization. As shown in Fig. 4A, panel d, levels of PKCα at the plasma membrane were higher in the presence of dynasore (arrowheads), but perinuclear staining was still evident (arrows). Transfection of cells with the K44A dynamin mutant also led to a partial inhibition of PKCα internalization, as indicated by increased retention of the protein at the plasma membrane (Fig. 4B, arrowheads). Because the dynamin-dependent uptake was completely blocked under these conditions, these data indicate that Bryo-induced PKCα intracellular trafficking involves both dynamin-dependent and -independent mechanisms.
FIGURE 4.
Bryostatin-induced PKCα trafficking is partially dynamin-dependent. A, the dynamin inhibitor dynasore partially inhibits Bryo-induced PKCα internalization. IEC-18 cells were treated with vehicle control (a), 80 μm dynasore (b), or 100 nm Bryo for 30 min in the absence (c) or presence (d) of 80 μm dynasore and processed for PKCα immunofluorescence. Arrows, perinuclear staining; arrowheads, membrane staining. Alexa Fluor 488-tranferrin uptake assays confirm inhibition of dynamin by 80 μm dynasore in IEC-18 cells (e and f). Arrows, internalized transferrin. B, partial blockade of perinuclear accumulation of PKCα by dominant-negative mutant dynamin is shown. IEC-18 cells were transfected with a HA-tagged K44A-dynamin mutant construct and treated with vehicle control (a and b) or 100 nm Bryo (c and d) for 30 min. Cells were then immunostained for HA (a and c) or PKCα (b and d). Inhibition of dynamin by K44A-dynamin mutant protein was confirmed by blockade of dynamin-dependent Alexa Fluor 488-cholera toxin uptake (panels e–h) and Alexa Fluor 488-transferrin uptake (panels i–l). Open arrows indicate transfected cells, arrowheads point to membrane-associated PKCα, and closed arrows indicate internalized cholera toxin or transferrin. Magnification bars, 10 μm. Data are representative of at least three independent experiments.
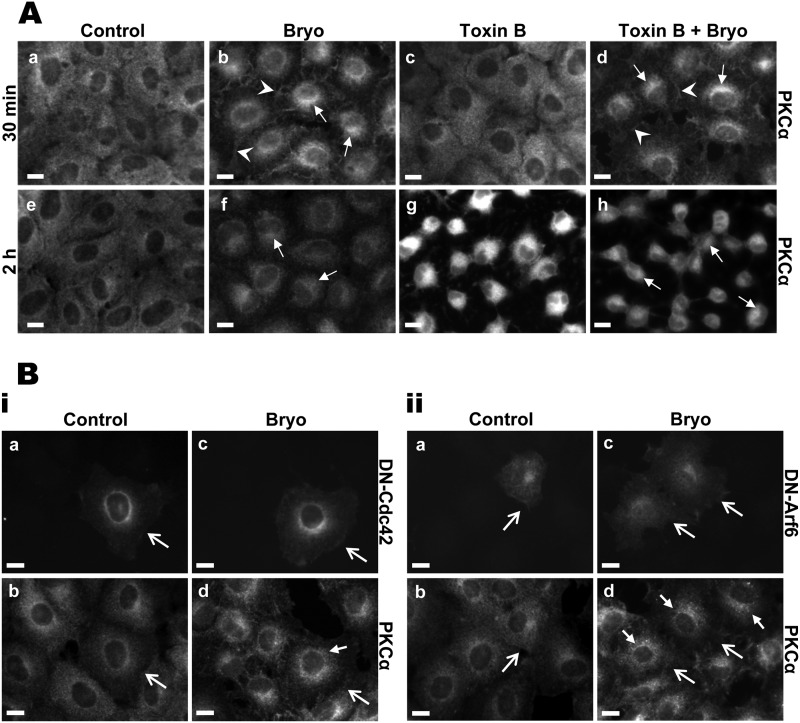
The dependence of PKCα trafficking on small GTPases was determined using C. difficile Toxin B, which inhibits RhoA, Rac1, and Cdc42 function through monoglucosylation of a threonine residue in their nucleotide binding domain (72–76). Functional blockade of these molecules results in actin condensation and consequent cell retraction and rounding (76, 77). These effects could be seen in IEC-18 cells, with concentrations as low as 10 ng/ml inducing cell retraction by 30 min and extensive rounding by 2 h (e.g. Fig. 5A, panels c and g). As shown in Fig. 5A, up to 100 ng/ml Toxin B did not inhibit Bryo-induced plasma membrane translocation (panel d, arrowheads) or down-regulation (panel h) of PKCα in IEC-18 cells. Furthermore, small GTPase inhibition failed to affect Bryo-induced trafficking of PKCα from the membrane to the perinuclear region (Fig. 5A, panels d and h, arrows), thus excluding RhoA, Rac1, and Cdc42 as mediators of Bryo-induced perinuclear accumulation of the enzyme. A role for Cdc42 and Arf6 was further excluded by the inability of dominant negative mutants of these proteins (T17N-Cdc42, T27N-Arf6; Ref. 78) to affect the internalization of enzyme (Fig. 5B, small closed arrows).
FIGURE 5.
Bryostatin-induced perinuclear accumulation of PKCα is not regulated by small GTPases RhoA, Cdc42, or Arf6. A, C. difficile Toxin B does not inhibit redistribution of PKCα. IEC-18 cells were pretreated with vehicle (Control) or 100 ng/ml Toxin B before the addition of vehicle or 100 nm Bryo for 30 min (a–d) or 2 h (e–h) as indicated. Cells were then fixed and immunostained for PKCα. Arrowheads, plasma membrane staining; arrows, perinuclear staining. B, dominant negative Cdc42 or Arf6 do not affect PKCα intracellular trafficking. i, IEC-18 cells were transfected with a dominant negative T17N Cdc42-GFP mutant construct and treated with vehicle (a and b) or 100 nm Bryo (c and d) for 30 min. After fixation, cells were analyzed for GFP fluorescence (a and c) or by PKCα immunofluorescence (b and d). Open arrows, cells expressing exogenous protein; closed arrow, perinuclear PKCα staining. ii, procedures were as in i, except that cells were transfected with a construct expressing HA-tagged T27N Arf6 mutant and processed for immunofluorescence analysis of HA tag (a and c) or PKCα (b and d). Magnification bars, 10 μm. Data are representative of at least three independent experiments.
Taken together, the data indicate that Bryo promotes perinuclear accumulation of PKCα via at least two clathrin-independent, lipid raft-dependent pathways: a dynamin-dependent pathway, which likely involves caveolae, and a non-caveolar, small GTPase-independent pathway (cf. Table 1).
Bryostatin-induced PKCα Intracellular Trafficking and Non-proteasomal Degradation Does Not Require Ubiquitination
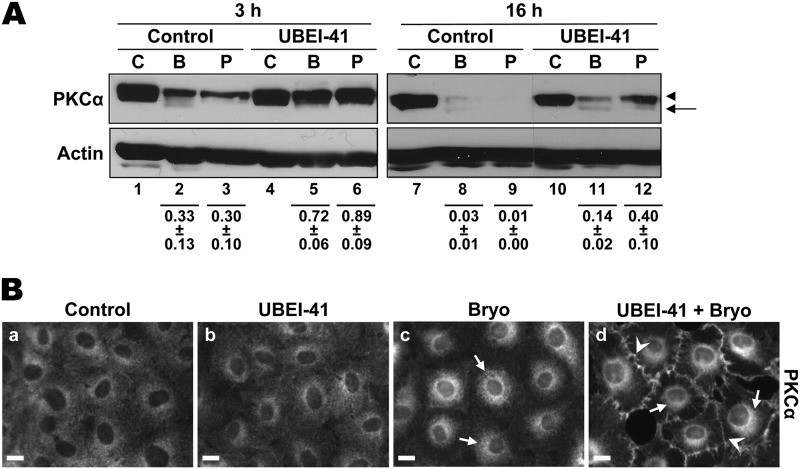
Recent studies have indicated that monoubiquitination of proteins can provide a signal for endocytosis and intracellular trafficking (79). To determine if Bryo-induced PKCα internalization is dependent on ubiquitination, we tested the effects of the E1 ubiquitin activating enzyme inhibitor, UBEI-41 (80). UBEI-41 effectively blocked ubiquitination in these cells, as indicated by its ability to prevent the proteasomal degradation of PKCα induced by PMA and short-term Bryo treatment (Fig. 6A, 3 h; p < 0.05 for the effects of UBEI-41 on Bryo- and PMA-induced down-regulation). As discussed above, proteasomal degradation of activated PKCα occurs at the plasma membrane, and inhibition of the proteasome leads to accumulation of PKCα at the cell periphery in agonist-treated cells (Fig. 1, B and C). Thus, consistent with its ability to block proteasomal processing of PKCα, UBEI-41 also increased the levels of the enzyme at the plasma membrane in Bryo-treated cells (Fig. 6B, panel d, arrowheads). Notably, however, inhibition of ubiquitination failed to prevent Bryo-induced PKCα intracellular trafficking after 2 h of treatment (Fig. 6B, panel d, arrows). Although UBEI-41 showed considerable toxicity with prolonged exposure, it also failed to block the eventual degradation of PKCα after long-term Bryo treatment (Fig. 6A, 16 h). The fact that similar levels of PKCα were localized to the perinuclear region in the presence and absence of UBEI-41 (Fig. 6B, panels c and d, arrows) confirms that trafficking of PKCα is not noticeably affected by the absence of ubiquitination.
FIGURE 6.
E1 ubiquitin activating enzyme activity is not required for Bryo-induced PKCα internalization or proteasome-independent down-regulation. A, Bryo-induced PKCα degradation does not require ubiquitin conjugation. IEC-18 cells were treated for 3 or 16 h with DMSO vehicle (C), 100 nm Bryo (B), or 100 nm PMA (P) in the absence or presence of 25 μm UBEI-41. Whole cell lysates were subjected to Western blot analysis for PKCα and β-actin. Panels show data from a single blot with dotted lines indicating where lanes have been rearranged for clarity. Numbers below the lanes represent PKCα band intensity in that lane relative to the corresponding control. Values are the mean ± S.E., n = 3. B, ubiquitin conjugation is not required for Bryo-induced PKCα internalization. IEC-18 cells were treated with DMSO (a and b) or Bryo (c and d) for 2 h in the absence (a and c) or presence (b and d) of 25 μm UBEI-41. Cells were fixed and analyzed for PKCα subcellular distribution. Arrows, perinuclear staining; arrowheads, membrane staining. Magnification Bars, 10 μm. All data are representative of at least three independent experiments.
Identification of an Endolysosomal Mechanism of Degradation for Endogenous PKCα
Bryostatin Induces Trafficking of PKCα through the Endolysosomal Pathway
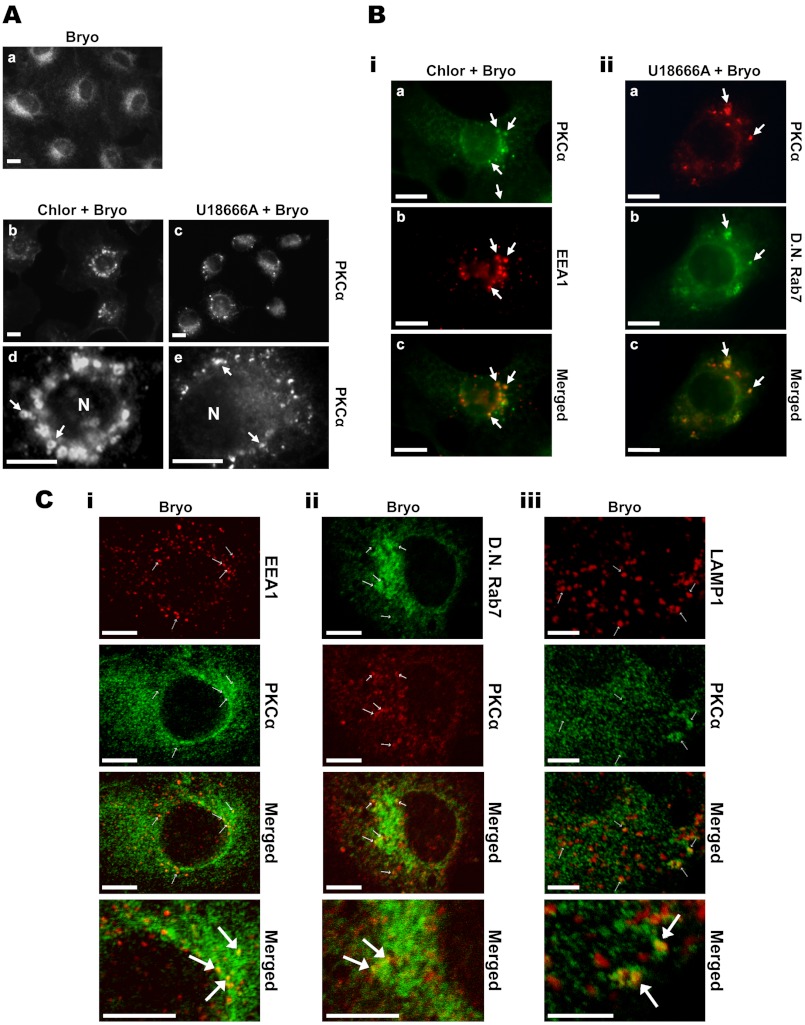
Bryo engages at least two pathways of PKCα degradation in IEC-18 cells; that is, a proteasomal pathway that predominates at earlier times of treatment (<4 h) and a non-proteasomal pathway that is clearly evident in the presence of inhibitors of the proteasome or of ubiquitination (see Figs. 1 and 6). A major mechanism of protein degradation in cells is the endolysosomal pathway. Based on the finding that Bryo promotes endocytosis of PKCα, endosome/lysosome disrupting agents were used to examine the role of this pathway in non-proteasomal processing of the protein. In initial studies, we tested (a) the lysosomotropic agent chloroquine, a weak base that inhibits endosomal/lysosomal function by raising the pH of acidic vesicular compartments and inducing vesicle swelling (81–86) and (b) the amphiphilic amine U18666A, which promotes cholesterol accumulation and swelling in late endosomes and lysosomes, thus altering the trafficking of multivesicular body-associated proteins (87–89). Immunofluorescence analysis of PKCα localization in cells treated with Bryo in the presence of chloroquine clearly demonstrated association of the enzyme with the membrane of swollen vesicles, indicating that internalized endogenous PKCα is targeted to the endolysosomal pathway (Fig. 7A, panels b and d, arrows). Similarly, Bryo-stimulated PKCα was detected in swollen vesicular structures in U18666A-treated cells (Fig. 7A, panels c and e, arrows).
FIGURE 7.
Involvement of the endolysosomal pathway in Bryo-induced PKCα degradation. A, activated PKCα associates with acidic vesicles/late endosomes in IEC-18 cells. Cells were treated for 2 h with 100 nm Bryo in the absence (a) or presence of 50 μm chloroquine (Chlor, b, and d) or 50 μm U18666A (c and e). Cells were then fixed and analyzed by PKCα immunofluorescence. Arrows, PKCα-associated perinuclear vesicles; N, nucleus. B, bryostatin promotes PKCα trafficking through early and late endosomes. i, IEC-18 cells on glass coverslips were treated with Bryo for 2 h in the presence of 50 μm chloroquine. Cells were fixed and immunostained for PKCα (Alexa Fluor 488-conjugated secondary antibody) and the early endosomal marker EEA1 (Cy3-conjugated secondary antibody). Images in a and b are from the same field of cells, and a merged image is shown in c. Arrows, vesicular structures with colocalization of PKCα and EEA1. ii, IEC-18 cells were transfected with dominant negative (D.N.) Rab7-GFP to mark late endosomes and treated with Bryo in the presence of 50 μm U18666A for 1 h. After fixation, cells were analyzed by PKCα immunofluorescence using a TRITC-conjugated secondary antibody (a) or for dominant negative Rab7-GFP fluorescence (b). Images in a and b are from the same field; these images are merged in c. Arrows, vesicular structures with colocalization of PKCα and dominant negative Rab7-GFP. C, confocal analysis of PKCα trafficking through EEA1-, Rab7-, and LAMP1-decorated vesicles in Bryo-treated IEC-18 cells. Untransfected (i and iii) or dominant negative Rab7-GFP- transfected (ii) IEC-18 cells were treated with Bryo for 1 h and processed for PKCα, EEA1, and LAMP1 immunofluorescence as indicated. Secondary antibodies were as follows: PKCα, Alexa Fluor 488-conjugated (i and iii) or TRITC-conjugated (ii) antibody; EEA1 and LAMP1, Cy3-conjugated antibody (i and iii). Images are 0.5-μm optical sections collected using a Zeiss LSM 510 Meta Confocal Laser Scanning Microscope. Arrows point to colocalization of PKCα with endolysosomal markers. Magnification bars, 10 μm. Data are representative of three independent experiments.
To identify the PKCα-associated structures in chloroquine- and U18666A-treated cells, dual localization experiments were performed using various markers of specific endolysosomal compartments. EEA1 identifies early endosomes, whereas Rab7 is a marker for late endosomes and multivesicular bodies (90, 91). Co-localization of PKCα and EEA1 was detected in chloroquine-treated cells (Fig. 7Bi, arrows), indicating that Bryo induces trafficking of PKCα to early endosomes. Similarly, colocalization with transfected dominant negative Rab7-GFP (T22N-Rab7-GFP) demonstrated that Bryo-stimulated PKCα accumulates in late endosomes/multivesicular bodies in the presence of U18666A (Fig. 7Bii). The association of PKCα with early and late endosomes was confirmed by confocal microscopy, which detected colocalization of PKCα with EEA1 and T22N-Rab7-GFP in cells that had not been treated with either chloroquine or U18666A (Fig. 7C, i and ii). Notably, confocal analysis also detected association of PKCα with lysosomes, as indicated by colocalization of the enzyme with the lysosomal marker LAMP1 (Fig. 7Ciii). Taken together, these findings indicate that Bryo induces trafficking of PKCα through early and late endosomal compartments and that the enzyme is ultimately delivered to lysosomes in the perinuclear region.
Bryostatin Induces Endolysosomal Processing of PKCα in a Variety of Cell Types
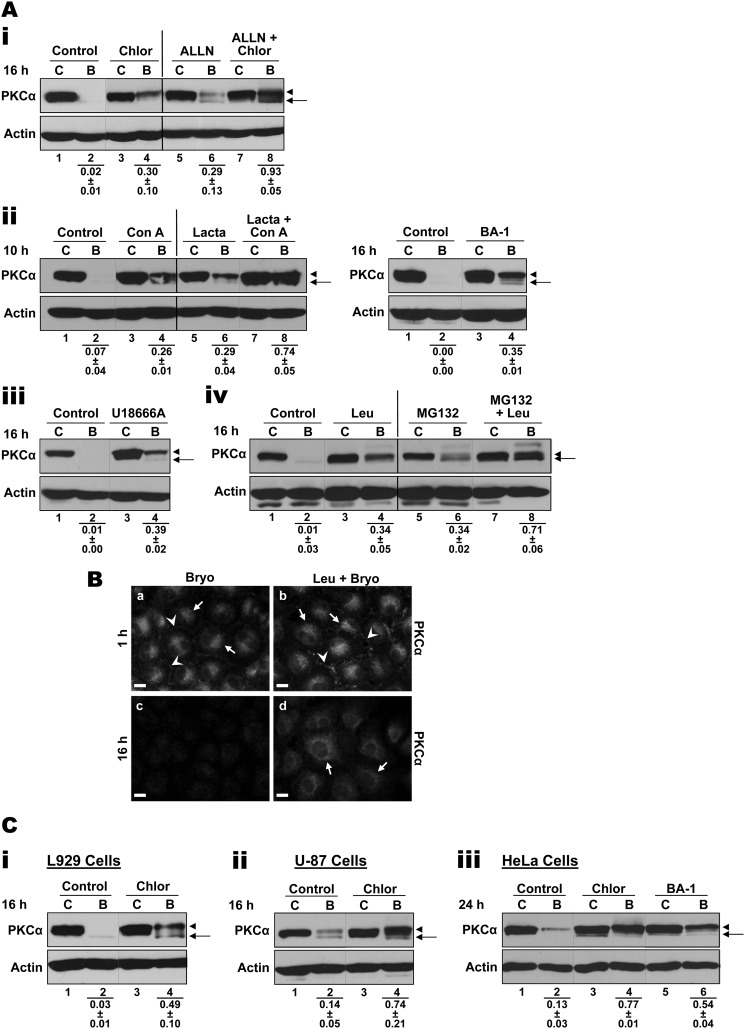
Treatment of IEC-18 cells with a panel of agents that disrupt endolysosomal proteolytic function conferred partial protection from Bryo-induced degradation of endogenous PKCα (Fig. 8A; p < 0.05 for the effects of all inhibitors), providing further evidence that internalized PKCα is delivered to the lysosome for degradation. In addition to the lysosomotropic agent chloroquine and U18666A (see Fig. 8A, panels i and iii), significant protection was observed with (a) the macrolide antibiotics concanamycin A and bafilomycin A1, which potently and specifically inhibit vacuolar proton-ATPases but do not cause vesicular swelling (92, 93), and (b) leupeptin, which inhibits lysosomal peptidases (84, 94, 95) (Fig. 8, A, panels ii and iv, and B). This disruption cannot be attributed to blockade of the intracellular trafficking of PKCα as observed in RBL-2H3 cells (42), as the protein was translocated to the perinuclear region in cells treated with chloroquine, U18666A, and leupeptin (Figs. 7B and 8B). Furthermore, it cannot be attributed to indirect inhibition of proteasomal degradation because lysosomal agents significantly (p < 0.02) protect PKCα from Bryo-induced degradation in cells treated with proteasome inhibitors: e.g. chloroquine with ALLN (Fig. 8A, panel i), concanamycin A with lactacystin (panel ii), and leupeptin with MG132 (panel iv). Finally, lysosomal degradation appears to be a major mechanism of PKCα processing in the perinuclear region, as indicated by the ability of leupeptin to rescue perinuclear PKCα from degradation while having no protective effects on plasma membrane-associated enzyme (Fig. 8B).
FIGURE 8.
Endolysosomal pathway inhibitors protect PKCα from Bryo-induced degradation. Ai, IEC-18 cells were treated for 16 h with vehicle (Control, C) or Bryo (B) in the absence or presence of 50 μm chloroquine (Chlor) and/or 150 μm ALLN as indicated. Whole cell lysates were subjected to Western blot analysis for PKCα and β-actin. Arrowhead, mature phosphorylated PKCα; arrow, faster migrating species of PKCα lacking priming site phosphorylation. ii–iv, procedures were as in i, except that IEC-18 cells were treated with vehicle (Control, C) or Bryo in the absence or presence of 100 nm concanamycin A (Con A), 100 nm bafilomycin A1 (BA-1), 50 μm lactacystin, 100 μm U18666A, 200 μm leupeptin (Leu), and/or 20 μm MG132 for 10 or 16 h as indicated. Numbers below the lanes represent PKCα band intensity in that lane relative to the corresponding control. Values are the mean ± S.E., n = 3 (i and ii, left panel, iv) or n = 2 (ii, right panel, iii). B, IEC-18 cells on glass coverslips were treated with Bryo for 1 h (a and b) or 16 h (c and d) in the presence or absence of 200 μm leupeptin (Leu) before being processed for PKCα immunofluorescence. Arrows and arrowheads indicate perinuclear and membrane staining of PKCα, respectively. Magnification bars, 10 μm. Data are representative of at least three independent experiments. C, endolysosomal pathway disrupting agents inhibit Bryo-induced PKCα degradation in a variety of cell types. L929 mouse fibroblasts (i), U-87 human glioblastoma cells (ii), and HeLa human cervical cancer cells (iii) were treated for 16 or 24 h with DMSO (vehicle control, C) or Bryo (B) in the absence or presence of chloroquine (Chlor) or bafilomycin A1 (BA-1) as indicated. Cell lysates were subjected to Western blot analysis for PKCα and β-actin. Arrowhead, mature phosphorylated PKCα; arrow, faster migrating species of PKCα lacking priming site phosphorylation. Numbers below the lanes represent PKCα band intensity in that lane relative to the corresponding control. Values are the mean ± S.E., n = 3. All Western blot panels show data from a single blot, with dotted or solid lines indicating where lanes have been rearranged for clarity. Solid lines are also included for clarity.
In previous studies we demonstrated that Bryo induces limited accumulation of faster migrating species of PKCα in the perinuclear region, which result from a combination of delayed maturation and dephosphorylation of the protein (Figs. 1, 3Bii, and 8A, arrows, and Ref. 31). As shown in Fig. 3Bii, formation of this species is inhibited by nystatin, which prevents intracellular accumulation of the enzyme. Although this faster migrating species is a target for proteasomal degradation (23), as indicated by its protection by proteasome inhibitors (Fig. 8A), lysosomal agents predominantly protect the slower migrating, fully phosphorylated form of the enzyme (Fig. 8A, arrowheads), indicating that this is a major target for lysosomal degradation. Furthermore, the degree of protection provided by endolysosomal disrupting agents in cells treated with proteasome inhibitors indicates that lysosomal mechanisms represent the predominant mechanism of non-proteasomal Bryo-induced degradation of PKCα.
To exclude the possibility that endolysosomal processing of PKCα in IEC-18 cells is a cell type-specific effect, the ability of endolysosomal disrupting agents to protect PKCα from Bryo-induced degradation was explored in L929 murine fibroblasts, U-87 human glioblastoma cells, and HeLa human cervical cancer cells. Immunofluorescence analysis revealed that Bryo induces perinuclear accumulation of PKCα in all of these cell lines (data not shown). Importantly, lysosome disrupting agents such as chloroquine and bafilomycin were capable of significantly (p < 0.02) protecting endogenous PKCα (predominantly the mature form) in each of these cell types. Indeed, in some cases protection was more effective than in IEC-18 cells (Fig. 8C). Thus, both lysosomal and proteasomal pathways of PKCα degradation exist in different cell types, and the extent to which they are engaged by PKC agonists appears to be cell type-dependent as well as agonist-dependent.
DISCUSSION
Although it has long been recognized that prolonged agonist stimulation leads to degradation of PKC isozymes, understanding of the molecular mechanisms involved remains limited (19, 34). Furthermore, much of our knowledge has come from the use of overexpression systems, which can result in channeling of PKCs into alternate pathways (Fig. 1E). In previous studies of the effects of agonists on endogenous PKCα (31), we established that at least two pathways of degradation of the activated protein can co-exist in cells and demonstrated that a single PKC activator can engage both mechanisms at the same time (e.g. Bryo). These enzyme processing pathways involve proteasome-dependent and -independent mechanisms. In the current study we advance the understanding of PKC signal termination by demonstrating that (a) proteasome-dependent and -independent PKCα degradation largely occur in different cellular compartments, (b) activated PKCα is directed to the perinuclear region of cells via dynamin-dependent and -independent lipid raft-mediated pathways, and (c) perinuclear PKCα is mainly degraded in lysosomes. Our data also indicate that caution should be exercised in interpreting findings from studies using overexpressed enzyme.
The current study clearly demonstrates that plasma membrane-associated PKCα is a substrate for proteasomal processing. PMA and Bryo both target the entire pool of PKCα to the plasma membrane, and the activated enzyme undergoes rapid ubiquitination and proteasome-mediated degradation in this compartment (Figs. 1C and 6B). Blockade of ubiquitination using the E1 ubiquitin-activating enzyme inhibitor UBEI-41 or of the proteasome with proteasome inhibitors rescues the membrane-bound enzyme (Figs. 1 and 6). In IEC-18 cells, this is the sole pathway engaged by PMA and is the predominant processing mechanism at early times of Bryo treatment.
The second pathway, engaged by Bryo but not PMA in IEC-18 cells, involves temperature-sensitive and cholesterol-dependent trafficking of active PKCα to a perinuclear compartment. This slower pathway is clearly evident in the context of proteasome inhibition, which does not affect internalization of the enzyme. Use of a panel of endolysosome disrupting agents and lysosome protease inhibition provided the first demonstration that PKCα can undergo endolysosomal processing, a finding that was further supported by immunofluorescence detection of the enzyme in EEA1-, Rab7-, and LAMP1-positive vesicles (Figs. 7, B and C). Several lines of evidence support an intracellular location for non-proteasomal degradation of PKCα. First, cholesterol depletion, which inhibits perinuclear accumulation of PKCα, delays its down-regulation (Fig. 3Bii). Second, the slower kinetics of degradation of perinuclear PKCα are consistent with involvement of slower lysosomal processing. Unlike plasma membrane-associated enzyme, which is largely down-regulated by 1 to 2 h of PKC agonist treatment, intracellular PKCα persists for longer than 3 h (Fig. 1D). Finally, lysosomotropic agents and leupeptin protect perinuclear PKCα from degradation while having no effect on plasma membrane-associated enzyme. Endolysosomal processing does not appear to be a secondary pathway that operates only when the proteasome is impaired or inhibited as (a) endolysosomal blockade could partially rescue PKCα from Bryo-induced down-regulation in the presence of a functional proteasome, and (b) combined use of endolysosomal and proteasome inhibitors provided enhanced protection. Rather, the proteasomal and endolysosomal mechanisms represent independent pathways of PKCα processing that largely occur at different cellular locations, i.e. the plasma membrane and perinuclear region, respectively.
Importantly, lysosomal processing appears to be a common mechanism for degradation of endogenous PKCα, as indicated by the protective effects of endolysosomal inhibitors in a variety of cell types (Fig. 8C). Furthermore, in several cell types this pathway seems to be a major mechanism of activation-induced down-regulation of endogenous PKCα. Although PKC signaling has been implicated in regulation of endolysosomal processing of other proteins (e.g. Refs. 96 and 97–100), to our knowledge this is the first report of a direct role for lysosomal degradation in agonist-induced down-regulation of PKCα itself. The failure to detect this pathway in other studies may be attributed to (a) the fact that it is masked at early time points by more rapid proteasomal mechanisms (Fig. 1), (b) the common use of overexpressed, exogenous proteins in studies of PKC down-regulation, which may divert the protein into alternate pathways, (c) agonist-specific differences in the engagement of different pathways, and/or (d) cell type differences.
Further insight into the mechanisms controlling lysosomal processing of activated PKCα came from analysis of the pathways mediating Bryo-induced perinuclear accumulation of the enzyme. Immunofluorescence analysis demonstrated that PKCα is targeted to EEA1-, Rab7-, and LAMP1-decorated vesicles in cells treated with Bryo; thus, perinuclear accumulation of the enzyme involves trafficking through early and late endosomes/multivesicular bodies, with ultimate delivery to lysosomes. Consistent with this finding, Bryo-induced internalization of PKCα is distinct from the previously described intracellular targeting of this isozyme to a recycling (Rab11-positive) compartment termed the pericentrion (7); whereas the latter is clathrin-dependent, siRNA-mediated knockdown of clathrin heavy chain had no effect on Bryo-induced perinuclear translocation of PKCα (Fig. 3A). Furthermore, Bryo-induced PKCα trafficking was blocked by cholesterol depletion and genistein treatment, pointing to a clathrin-independent, lipid raft-dependent mechanism(s) (48, 49). Use of a combination of pharmacological inhibitors and dominant negative mutants led to the identification of two distinct pathways of PKCα trafficking that differ in their dependence on dynamin (Fig. 4). Because involvement of RhoA was excluded (Fig. 5), the dynamin-dependent pathway likely involves caveolae (48, 49). Although this caveolar pathway bears similarities to the dynamin-dependent pathway of PKCα translocation and degradation previously identified in PMA-treated PKCα-overexpressing MCF7 cells (39), a Rab7-positive late endosomal compartment was not implicated in that study. This difference could reflect the involvement of distinct caveaolar pathways in the two systems or to delivery of PKCα to late endosomes by the dynamin-independent pathway identified in IEC-18 cells. Although dynamin-independent endocytic pathways remain poorly characterized, the recently identified flotillin-associated pathway (101) represents a candidate mechanism for perinuclear accumulation of PKCα. The potential involvement of the enzyme in flotillin-associated events is supported by reports that (a) PKCα and flotillin are colocalized on mature phagosomes (102), (b) flotillin-1 is required for PKC-mediated endocytosis of the dopamine transporter (103), and (c) both PKCα and flotillin have been implicated in regulation of β1 integrin trafficking (104, 105). Although the precise molecular characteristics of dynamin-independent PKCα internalization remain to be fully characterized, our data collectively indicate that Bryo-induced trafficking of PKCα to the perinuclear region in IEC-18 cells (a) involves transit through early and late endosomes and (b) occurs via at least two pathways, caveolar endocytosis and a lipid raft-mediated, non-caveolar, dynamin- and small GTPase-independent mechanism. To our knowledge this is the first time that dynamin-independent mechanisms have been implicated in PKC isozyme intracellular trafficking.
A number of studies have pointed to a model of PKC/PKCα down-regulation involving dephosphorylation and proteasomal degradation of internalized protein (e.g. Refs. 19, 34, 39, and 42). Consistent with this model, Bryo does induce some dephosphorylation of PKCα (31) after its accumulation in the perinuclear region, as confirmed by the ability of nystatin to inhibit the generation of a dephosphorylated species in IEC-18 cells (Fig. 3Bii). This dephosphorylated species is slightly protected by proteasome inhibitors (Fig. 1B), indicating that the internalization → dephosphorylation → proteasome-mediated degradation pathway is operative in IEC-18 cells. However, the failure of chloroquine or U18666A to promote significant accumulation of a non-phosphorylated species indicates that the internalized protein remains phosphorylated at least as far as late endosomes/multivesicular bodies (Figs. 7 and 8). Furthermore, there is minimal accumulation of non-phosphorylated PKCα in the absence or presence of proteasome and lysosome inhibitors even after prolonged Bryo treatment (e.g. 16–24 h; Figs. 1 and 8), with these inhibitors predominantly protecting the mature, phosphorylated species (e.g. Fig. 8). Thus, it is the phosphorylated form of PKCα that is targeted for endosomal trafficking to the lysosome. A role for fully phosphorylated PKCα as a target for degradation is consistent with findings with other PKC isozymes (e.g. PKCδ; Ref. 106) and with the observation that PMA induces internalization of phosphorylated PKCα in MCF7 cells (39).
In summary, our findings (a) provide additional evidence for the existence of multiple pathways of PKCα desensitization that can occur in different cellular compartments, (b) identify a novel pathway of PKCα internalization that is clathrin-independent and does not involve caveolae, and (c) show that activated PKCα traffics through early and late endosomal compartments to be degraded by the lysosomal machinery.
Acknowledgments
We thank Kathryn J. Curry for expert technical assistance and Drs. Xinjiang Wang and Srikumar Raja for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK60632, DK54909, CA16056, and CA036727.
- Bryo
- bryostatin 1
- ALLN
- N-acetyl-Leu-Leu-Nle-CHO (Nle is norleucine)
- EEA1
- early endosome antigen 1
- PMA
- phorbol 12-myristate 13-acetate
- Toxin B
- C. difficile Toxin B
- UBEI-41
- 4 [4-(5-nitro-furan-2-ylmethylene)-3,5-dioxo-pyrazolidin-1-yl]-benzoic acid ethyl ester
- TRITC
- tetramethylrhodamine isothiocyanate
- Arf6
- ribosylation factor-6.
REFERENCES
- 1. Black J. D. (2000) Protein kinase C-mediated regulation of the cell cycle. Front. Biosci. 5, D406–D423 [DOI] [PubMed] [Google Scholar]
- 2. Clemens M. J., Trayner I., Menaya J. (1992) The role of protein kinase C isoenzymes in the regulation of cell proliferation and differentiation. J. Cell Sci. 103, 881–887 [DOI] [PubMed] [Google Scholar]
- 3. Gutcher I., Webb P. R., Anderson N. G. (2003) The isoform-specific regulation of apoptosis by protein kinase C. Cell. Mol. Life Sci. 60, 1061–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fields A. P., Regala R. P. (2007) Protein kinase Cι. Human oncogene, prognostic marker, and therapeutic target. Pharmacol. Res. 55, 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kermorgant S., Parker P. J. (2005) c-Met signaling. Spatio-temporal decisions. Cell Cycle 4, 352–355 [DOI] [PubMed] [Google Scholar]
- 6. Dempsey E. C., Newton A. C., Mochly-Rosen D., Fields A. P., Reyland M. E., Insel P. A., Messing R. O. (2000) Protein kinase C isozymes and the regulation of diverse cell responses. Am. J. Physiol. Lung Cell Mol. Physiol. 279, L429–L438 [DOI] [PubMed] [Google Scholar]
- 7. Alvi F., Idkowiak-Baldys J., Baldys A., Raymond J. R., Hannun Y. A. (2007) Regulation of membrane trafficking and endocytosis by protein kinase C. Emerging role of the pericentrion, a novel protein kinase C-dependent subset of recycling endosomes. Cell. Mol. Life Sci. 64, 263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Caino M. C., von Burstin V. A., Lopez-Haber C., Kazanietz M. G. (2011) Differential regulation of gene expression by protein kinase C isozymes as determined by genome-wide expression analysis. J. Biol. Chem. 286, 11254–11264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Couldwell W. T., Uhm J. H., Antel J. P., Yong V. W. (1991) Enhanced protein kinase C activity correlates with the growth rate of malignant gliomas in vitro. Neurosurgery 29, 880–886; discussion 886–887 [DOI] [PubMed] [Google Scholar]
- 10. da Rocha A. B., Mans D. R., Regner A., Schwartsmann G. (2002) Targeting protein kinase C. New therapeutic opportunities against high-grade malignant gliomas? Oncologist 7, 17–33 [DOI] [PubMed] [Google Scholar]
- 11. Lahn M., Kohler G., Sundell K., Su C., Li S., Paterson B. M., Bumol T. F. (2004) Oncology 67, 1–10 [DOI] [PubMed] [Google Scholar]
- 12. Lahn M., Sundell K., Gleave M., Ladan F., Su C., Li S., Ma D., Paterson B. M., Bumol T. F. (2004) Protein kinase Cα in prostate cancer. BJU Int. 93, 1076–1081 [DOI] [PubMed] [Google Scholar]
- 13. Black J. D. (2001) Protein kinase C isozymes in colon carcinogenesis. Guilt by omission. Gastroenterology 120, 1868–1872 [DOI] [PubMed] [Google Scholar]
- 14. Oster H., Leitges M. (2006) Protein kinase Cα but not PKCζ suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 66, 6955–6963 [DOI] [PubMed] [Google Scholar]
- 15. Favit A., Grimaldi M., Nelson T. J., Alkon D. L. (1998) Alzheimer's-specific effects of soluble β-amyloid on protein kinase Cα and -γ degradation in human fibroblasts. Proc. Natl. Acad. Sci. U.S.A. 95, 5562–5567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zemskov E. A., Nukina N. (2003) Impaired degradation of PKCα by proteasome in a cellular model of Huntington's disease. Neuroreport 14, 1435–1438 [DOI] [PubMed] [Google Scholar]
- 17. Keranen L. M., Dutil E. M., Newton A. C. (1995) Protein kinase C is regulated in vivo by three functionally distinct phosphorylations. Curr. Biol. 5, 1394–1403 [DOI] [PubMed] [Google Scholar]
- 18. Newton A. C. (1995) Protein kinase C. Structure, function, and regulation. J. Biol. Chem. 270, 28495–28498 [DOI] [PubMed] [Google Scholar]
- 19. Carmena D., Sardini A. (2007) Lifespan regulation of conventional protein kinase C isotypes. Biochem. Soc. Trans. 35, 1043–1045 [DOI] [PubMed] [Google Scholar]
- 20. Feng X., Hannun Y. A. (1998) An essential role for autophosphorylation in the dissociation of activated protein kinase C from the plasma membrane. J. Biol. Chem. 273, 26870–26874 [DOI] [PubMed] [Google Scholar]
- 21. Feng X., Zhang J., Barak L. S., Meyer T., Caron M. G., Hannun Y. A. (1998) Visualization of dynamic trafficking of a protein kinase C βII/green fluorescent protein conjugate reveals differences in G protein-coupled receptor activation and desensitization. J. Biol. Chem. 273, 10755–10762 [DOI] [PubMed] [Google Scholar]
- 22. Gao T., Brognard J., Newton A. C. (2008) The phosphatase PHLPP controls the cellular levels of protein kinase C. J. Biol. Chem. 283, 6300–6311 [DOI] [PubMed] [Google Scholar]
- 23. Hansra G., Garcia-Paramio P., Prevostel C., Whelan R. D., Bornancin F., Parker P. J. (1999) Multisite dephosphorylation and desensitization of conventional protein kinase C isotypes. Biochem. J. 342, 337–344 [PMC free article] [PubMed] [Google Scholar]
- 24. Hansra G., Bornancin F., Whelan R., Hemmings B. A., Parker P. J. (1996) 12-O-Tetradecanoylphorbol-13-acetate-induced dephosphorylation of protein kinase Cα correlates with the presence of a membrane-associated protein phosphatase 2A heterotrimer. J. Biol. Chem. 271, 32785–32788 [DOI] [PubMed] [Google Scholar]
- 25. Lee H. W., Smith L., Pettit G. R., Bingham Smith J. (1996) Dephosphorylation of activated protein kinase C contributes to down-regulation by bryostatin. Am. J. Physiol. 271, C304–C311 [DOI] [PubMed] [Google Scholar]
- 26. Ward N. E., Stewart J. R., Ioannides C. G., O'Brian C. A. (2000) Oxidant-induced S-glutathiolation inactivates protein kinase Cα (PKC-α). A potential mechanism of PKC isozyme regulation. Biochemistry 39, 10319–10329 [DOI] [PubMed] [Google Scholar]
- 27. Parker P. J., Bosca L., Dekker L., Goode N. T., Hajibagheri N., Hansra G. (1995) Protein kinase C (PKC)-induced PKC degradation. A model for down-regulation. Biochem. Soc. Trans. 23, 153–155 [DOI] [PubMed] [Google Scholar]
- 28. Olivier A. R., Parker P. J. (1994) Bombesin, platelet-derived growth factor, and diacylglycerol induce selective membrane association and down-regulation of protein kinase C isotypes in Swiss 3T3 cells. J. Biol. Chem. 269, 2758–2763 [PubMed] [Google Scholar]
- 29. Junoy B., Maccario H., Mas J. L., Enjalbert A., Drouva S. V. (2002) Proteasome implication in phorbol ester- and GnRH-induced selective down-regulation of PKC (α,ϵ,ζ) in α T(3)-1 and LβT(2) gonadotrope cell lines. Endocrinology 143, 1386–1403 [DOI] [PubMed] [Google Scholar]
- 30. Faghiri Z., Bazan N. G. (2006) Selective relocalization and proteasomal down-regulation of PKCα induced by platelet-activating factor in retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 47, 397–404 [DOI] [PubMed] [Google Scholar]
- 31. Leontieva O. V., Black J. D. (2004) Identification of two distinct pathways of protein kinase Cα down-regulation in intestinal epithelial cells. J. Biol. Chem. 279, 5788–5801 [DOI] [PubMed] [Google Scholar]
- 32. Lee H. W., Smith L., Pettit G. R., Smith J. B. (1997) Bryostatin 1 and phorbol ester down-modulate protein kinase Cα and -ϵ via the ubiquitin/proteasome pathway in human fibroblasts. Mol. Pharmacol. 51, 439–447 [PubMed] [Google Scholar]
- 33. Maccario H., Junoy B., Poulin B., Boyer B., Enjalbert A., Drouva S. V. (2004) Protein kinase Cδ as gonadotropin-releasing hormone target isoenzyme in the αT3–1 gonadotrope cell line. Neuroendocrinology 79, 204–220 [DOI] [PubMed] [Google Scholar]
- 34. Newton A. C. (2010) Protein kinase C. Poised to signal. Am. J. Physiol. Endocrinol. Metab. 298, E395–E402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gould C. M., Newton A. C. (2008) The life and death of protein kinase C. Curr. Drug Targets 9, 614–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kishimoto A., Mikawa K., Hashimoto K., Yasuda I., Tanaka S., Tominaga M., Kuroda T., Nishizuka Y. (1989) Limited proteolysis of protein kinase C subspecies by calcium-dependent neutral protease (calpain). J. Biol. Chem. 264, 4088–4092 [PubMed] [Google Scholar]
- 37. Hong D. H., Huan J., Ou B. R., Yeh J. Y., Saido T. C., Cheeke P. R., Forsberg N. E. (1995) Protein kinase C isoforms in muscle cells and their regulation by phorbol ester and calpain. Biochim. Biophys. Acta 1267, 45–54 [DOI] [PubMed] [Google Scholar]
- 38. Savart M., Letard P., Bultel S., Ducastaing A. (1992) Induction of protein kinase C down-regulation by the phorbol ester TPA in a calpain/protein kinase C complex. Int. J. Cancer 52, 399–403 [DOI] [PubMed] [Google Scholar]
- 39. Prevostel C., Alice V., Joubert D., Parker P. J. (2000) Protein kinase Cα actively downregulates through caveolae-dependent traffic to an endosomal compartment. J. Cell Sci. 113, 2575–2584 [DOI] [PubMed] [Google Scholar]
- 40. Lee H. W., Smith L., Pettit G. R., Vinitsky A., Smith J. B. (1996) Ubiquitination of protein kinase Cα and degradation by the proteasome. J. Biol. Chem. 271, 20973–20976 [PubMed] [Google Scholar]
- 41. Lu Z., Liu D., Hornia A., Devonish W., Pagano M., Foster D. A. (1998) Activation of protein kinase C triggers its ubiquitination and degradation. Mol. Cell. Biol. 18, 839–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Melnikov S., Sagi-Eisenberg R. (2009) Down-regulating protein kinase Cα. Functional cooperation between the proteasome and the endocytic system. Cell. Signal. 21, 1607–1619 [DOI] [PubMed] [Google Scholar]
- 43. Abrahamsen H., O'Neill A. K., Kannan N., Kruse N., Taylor S. S., Jennings P. A., Newton A. C. (2012) Peptidyl-prolyl isomerase Pin1 controls down-regulation of conventional protein kinase C isozymes. J. Biol. Chem. 287, 13262–13278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pysz M. A., Leontieva O. V., Bateman N. W., Uronis J. M., Curry K. J., Threadgill D. W., Janssen K. P., Robine S., Velcich A., Augenlicht L. H., Black A. R., Black J. D. (2009) PKCα tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp. Cell Res. 315, 1415–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heikkilä O., Susi P., Tevaluoto T., Härmä H., Marjomäki V., Hyypiä T., Kiljunen S. (2010) Internalization of coxsackievirus A9 is mediated by β2-microglobulin, dynamin, and Arf6 but not by caveolin-1 or clathrin. J. Virol. 84, 3666–3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Torgersen M. L., Skretting G., van Deurs B., Sandvig K. (2001) Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114, 3737–3747 [DOI] [PubMed] [Google Scholar]
- 47. Szallasi Z., Du L., Levine R., Lewin N. E., Nguyen P. N., Williams M. D., Pettit G. R., Blumberg P. M. (1996) The bryostatins inhibit growth of B16/F10 melanoma cells in vitro through a protein kinase C-independent mechanism. Dissociation of activities using 26-epi-bryostatin 1. Cancer Res. 56, 2105–2111 [PubMed] [Google Scholar]
- 48. Doherty G. J., McMahon H. T. (2009) Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902 [DOI] [PubMed] [Google Scholar]
- 49. Delva E., Jennings J. M., Calkins C. C., Kottke M. D., Faundez V., Kowalczyk A. P. (2008) Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin- and dynamin-independent mechanism. J. Biol. Chem. 283, 18303–18313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Moskowitz H. S., Yokoyama C. T., Ryan T. A. (2005) Highly cooperative control of endocytosis by clathrin. Mol. Biol. Cell 16, 1769–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Okamoto Y., Ninomiya H., Miwa S., Masaki T. (2000) Cholesterol oxidation switches the internalization pathway of endothelin receptor type A from caveolae to clathrin-coated pits in Chinese hamster ovary cells. J. Biol. Chem. 275, 6439–6446 [DOI] [PubMed] [Google Scholar]
- 52. Sorkina T., Hoover B. R., Zahniser N. R., Sorkin A. (2005) Constitutive and protein kinase C-induced internalization of the dopamine transporter is mediated by a clathrin-dependent mechanism. Traffic 6, 157–170 [DOI] [PubMed] [Google Scholar]
- 53. Hansen S. H., Sandvig K., van Deurs B. (1993) Clathrin and HA2 adaptors. Effects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 121, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vercauteren D., Vandenbroucke R. E., Jones A. T., Rejman J., Demeester J., De Smedt S. C., Sanders N. N., Braeckmans K. (2010) The use of inhibitors to study endocytic pathways of gene carriers. Optimization and pitfalls. Mol. Ther. 18, 561–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sabharanjak S., Sharma P., Parton R. G., Mayor S. (2002) GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev. Cell 2, 411–423 [DOI] [PubMed] [Google Scholar]
- 56. Nichols B. (2003) Caveosomes and endocytosis of lipid rafts. J. Cell Sci. 116, 4707–4714 [DOI] [PubMed] [Google Scholar]
- 57. Lajoie P., Nabi I. R. (2010) Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell Mol. Biol. 282, 135–163 [DOI] [PubMed] [Google Scholar]
- 58. Simons K., Toomre D. (2000) Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39 [DOI] [PubMed] [Google Scholar]
- 59. Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. (1992) Caveolin, a protein component of caveolae membrane coats. Cell 68, 673–682 [DOI] [PubMed] [Google Scholar]
- 60. Schnitzer J. E., Oh P., Pinney E., Allard J. (1994) Filipin-sensitive caveolae-mediated transport in endothelium. Reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell Biol. 127, 1217–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Finger E. C., Lee N. Y., You H. J., Blobe G. C. (2008) Endocytosis of the type III transforming growth factor-β (TGF-β) receptor through the clathrin-independent/lipid raft pathway regulates TGF-β signaling and receptor down-regulation. J. Biol. Chem. 283, 34808–34818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Damm E.-M., Pelkmans L., Kartenbeck J., Mezzacasa A., Kurzchalia T., Helenius A. (2005) Clathrin- and caveolin-1-independent endocytosis. Entry of simian virus 40 into cells devoid of caveolae. J. Cell Biol. 168, 477–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kojic L. D., Joshi B., Lajoie P., Le P. U., Cox M. E., Turbin D. A., Wiseman S. M., Nabi I. R. (2007) Raft-dependent endocytosis of autocrine motility factor is phosphatidylinositol 3-kinase-dependent in breast carcinoma cells. J. Biol. Chem. 282, 29305–29313 [DOI] [PubMed] [Google Scholar]
- 64. van der Aa M. A., Huth U. S., Häfele S. Y., Schubert R., Oosting R. S., Mastrobattista E., Hennink W. E., Peschka-Süss R., Koning G. A., Crommelin D. J. (2007) Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm. Res. 24, 1590–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lajoie P., Nabi I. R. (2007) Regulation of raft-dependent endocytosis. J. Cell Mol. Med. 11, 644–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kojic L. D., Wiseman S. M., Ghaidi F., Joshi B., Nedev H., Saragovi H. U., Nabi I. R. (2008) Raft-dependent endocytosis of autocrine motility factor/phosphoglucose isomerase. A potential drug delivery route for tumor cells. PLoS One 3, e3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Damke H., Baba T., Warnock D. E., Schmid S. L. (1994) Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J. Cell Biol. 127, 915–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mayor S., Pagano R. E. (2007) Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8, 603–612 [DOI] [PubMed] [Google Scholar]
- 69. Nankoe S. R., Sever S. (2006) Dynasore puts a new spin on dynamin. A surprising dual role during vesicle formation. Trends Cell Biol. 16, 607–609 [DOI] [PubMed] [Google Scholar]
- 70. Kirchhausen T., Macia E., Pelish H. E. (2008) Use of dynasore, the small molecule inhibitor of dynamin, in the regulation of endocytosis. Methods Enzymol. 438, 77–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Le P. U., Nabi I. R. (2003) Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J. Cell Sci. 116, 1059–1071 [DOI] [PubMed] [Google Scholar]
- 72. Yanagawa Y., Onoé K. (2003) CCR7 ligands induce rapid endocytosis in mature dendritic cells with concomitant up-regulation of Cdc42 and Rac activities. Blood 101, 4923–4929 [DOI] [PubMed] [Google Scholar]
- 73. Just I., Fritz G., Aktories K., Giry M., Popoff M. R., Boquet P., Hegenbarth S., von Eichel-Streiber C. (1994) Clostridium difficile Toxin B acts on the GTP-binding protein Rho. J. Biol. Chem. 269, 10706–10712 [PubMed] [Google Scholar]
- 74. Hippenstiel S., Schmeck B., N'Guessan P. D., Seybold J., Krüll M., Preissner K., Eichel-Streiber C. V., Suttorp N. (2002) Rho protein inactivation induced apoptosis of cultured human endothelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 283, L830–L838 [DOI] [PubMed] [Google Scholar]
- 75. Genth H., Dreger S. C., Huelsenbeck J., Just I. (2008) Clostridium difficile toxins. More than mere inhibitors of Rho proteins. Int. J. Biochem. Cell Biol. 40, 592–597 [DOI] [PubMed] [Google Scholar]
- 76. Voth D. E., Ballard J. D. (2005) Clostridium difficile toxins. Mechanism of action and role in disease. Clin. Microbiol. Rev. 18, 247–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Fiorentini C., Arancia G., Paradisi S., Donelli G., Giuliano M., Piemonte F., Mastrantonio P. (1989) Effects of Clostridium difficile toxins A and B on cytoskeleton organization in HEp-2 cells. A comparative morphological study. Toxicon 27, 1209–1218 [DOI] [PubMed] [Google Scholar]
- 78. deCathelineau A. M., Bokoch G. M. (2009) Inactivation of rho GTPases by statins attenuates anthrax lethal toxin activity. Infect. Immun. 77, 348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Mukhopadhyay D., Riezman H. (2007) Proteasome-independent functions of ubiquitin in endocytosis and signaling. Science 315, 201–205 [DOI] [PubMed] [Google Scholar]
- 80. Yang Y., Kitagaki J., Dai R. M., Tsai Y. C., Lorick K. L., Ludwig R. L., Pierre S. A., Jensen J. P., Davydov I. V., Oberoi P., Li C. C., Kenten J. H., Beutler J. A., Vousden K. H., Weissman A. M. (2007) Inhibitors of ubiquitin-activating enzyme (E1), a new class of potential cancer therapeutics. Cancer Res. 67, 9472–9481 [DOI] [PubMed] [Google Scholar]
- 81. Styrt B., Klempner M. S. (1986) Inhibition of neutrophil oxidative metabolism by lysosomotropic weak bases. Blood 67, 334–342 [PubMed] [Google Scholar]
- 82. Agostinelli E., Seiler N. (2007) Lysosomotropic compounds and spermine enzymatic oxidation products in cancer therapy (review). Int. J. Oncol. 31, 473–484 [PubMed] [Google Scholar]
- 83. Hatch G. M., Oskin A., Vance D. E. (1993) Involvement of the lysosome in the catabolism of intracellular lysophosphatidylcholine and evidence for distinct pools of lysophosphatidylcholine. J. Lipid Res. 34, 1873–1881 [PubMed] [Google Scholar]
- 84. Seglen P. O., Grinde B., Solheim A. E. (1979) Inhibition of the lysosomal pathway of protein degradation in isolated rat hepatocytes by ammonia, methylamine, chloroquine and leupeptin. Eur. J. Biochem. 95, 215–225 [DOI] [PubMed] [Google Scholar]
- 85. Misinzo G., Meerts P., Bublot M., Mast J., Weingartl H. M., Nauwynck H. J. (2005) Binding and entry characteristics of porcine circovirus 2 in cells of the porcine monocytic line 3D4/31. J. Gen. Virol. 86, 2057–2068 [DOI] [PubMed] [Google Scholar]
- 86. Mellman I., Fuchs R., Helenius A. (1986) Acidification of the endocytic and exocytic pathways. Annu. Rev. Biochem. 55, 663–700 [DOI] [PubMed] [Google Scholar]
- 87. Liscum L., Faust J. R. (1989) The intracellular transport of low density lipoprotein-derived cholesterol is inhibited in Chinese hamster ovary cells cultured with 3-β-[2-(diethylamino)ethoxy]androst-5-en-17-one. J. Biol. Chem. 264, 11796–11806 [PubMed] [Google Scholar]
- 88. de Diego I., Schwartz F., Siegfried H., Dauterstedt P., Heeren J., Beisiegel U., Enrich C., Grewal T. (2002) Cholesterol modulates the membrane binding and intracellular distribution of annexin 6. J. Biol. Chem. 277, 32187–32194 [DOI] [PubMed] [Google Scholar]
- 89. Lu A., Tebar F., Alvarez-Moya B., López-Alcalá C., Calvo M., Enrich C., Agell N., Nakamura T., Matsuda M., Bachs O. (2009) A clathrin-dependent pathway leads to KRas signaling on late endosomes en route to lysosomes. J. Cell Biol. 184, 863–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bucci C., Thomsen P., Nicoziani P., McCarthy J., van Deurs B. (2000) Rab7. A key to lysosome biogenesis. Mol. Biol. Cell 11, 467–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang T., Ming Z., Xiaochun W., Hong W. (2011) Rab7. Role of its protein interaction cascades in endo-lysosomal traffic. Cell. Signal. 23, 516–521 [DOI] [PubMed] [Google Scholar]
- 92. Huss M., Ingenhorst G., König S., Gassel M., Dröse S., Zeeck A., Altendorf K., Wieczorek H. (2002) Concanamycin A, the specific inhibitor of V-ATPases, binds to the V(o) subunit c. J. Biol. Chem. 277, 40544–40548 [DOI] [PubMed] [Google Scholar]
- 93. Yoshimori T., Yamamoto A., Moriyama Y., Futai M., Tashiro Y. (1991) Bafilomycin A1, a specific inhibitor of vacuolar-type H+-ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J. Biol. Chem. 266, 17707–17712 [PubMed] [Google Scholar]
- 94. Chu T., Tran T., Yang F., Beech W., Cole G. M., Frautschy S. A. (1998) Effect of chloroquine and leupeptin on intracellular accumulation of amyloid-β (Aβ) 1–42 peptide in a murine N9 microglial cell line. FEBS Lett. 436, 439–444 [DOI] [PubMed] [Google Scholar]
- 95. Hamer I., Paccaud J. P., Belin D., Maeder C., Carpentier J. L. (1998) Soluble form of complement C3b/C4b receptor (CR1) results from a proteolytic cleavage in the C-terminal region of CR1 transmembrane domain. Biochem. J. 329, 183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Allen L. A., Aderem A. (1995) Protein kinase C regulates MARCKS cycling between the plasma membrane and lysosomes in fibroblasts. EMBO J. 14, 1109–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kamsteeg E. J., Hendriks G., Boone M., Konings I. B., Oorschot V., van der Sluijs P., Klumperman J., Deen P. M. (2006) Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. U.S.A. 103, 18344–18349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Manna P. T., Smith A. J., Taneja T. K., Howell G. J., Lippiat J. D., Sivaprasadarao A. (2010) Constitutive endocytic recycling and protein kinase C-mediated lysosomal degradation control K(ATP) channel surface density. J. Biol. Chem. 285, 5963–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Miranda M., Wu C. C., Sorkina T., Korstjens D. R., Sorkin A. (2005) Enhanced ubiquitylation and accelerated degradation of the dopamine transporter mediated by protein kinase C. J. Biol. Chem. 280, 35617–35624 [DOI] [PubMed] [Google Scholar]
- 100. Tapper H., Sundler R. (1995) Protein kinase C and intracellular pH regulate zymosan-induced lysosomal enzyme secretion in macrophages. J. Leukoc. Biol. 58, 485–494 [DOI] [PubMed] [Google Scholar]
- 101. Glebov O. O., Bright N. A., Nichols B. J. (2006) Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 8, 46–54 [DOI] [PubMed] [Google Scholar]
- 102. Ng Yan Hing J. D., Desjardins M., Descoteaux A. (2004) Proteomic analysis reveals a role for protein kinase Cα in phagosome maturation. Biochem. Biophys. Res. Commun. 319, 810–816 [DOI] [PubMed] [Google Scholar]
- 103. Cremona M. L., Matthies H. J., Pau K., Bowton E., Speed N., Lute B. J., Anderson M., Sen N., Robertson S. D., Vaughan R. A., Rothman J. E., Galli A., Javitch J. A., Yamamoto A. (2011) Flotillin-1 is essential for PKC-triggered endocytosis and membrane microdomain localization of DAT. Nat. Neurosci. 14, 469–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ng T., Shima D., Squire A., Bastiaens P. I., Gschmeissner S., Humphries M. J., Parker P. J. (1999) PKCα regulates β1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 18, 3909–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Vassilieva E. V., Gerner-Smidt K., Ivanov A. I., Nusrat A. (2008) Lipid rafts mediate internalization of β1-integrin in migrating intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 295, G965–G976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Srivastava J., Procyk K. J., Iturrioz X., Parker P. J. (2002) Phosphorylation is required for PMA- and cell cycle-induced degradation of protein kinase Cδ. Biochem. J. 368, 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]