Abstract
Purpose
During the last five decades drug and therapeutics committees (DTCs), have evolved from mainly hospital-based groups of experts in pharmacotherapy and drug logistics into an arena for healthcare professionals employing evidence-based methods of promoting rational drug use. The purpose of this study was to suggest a framework for analysing the structure and activities of DTCs.
Methods
A literature search was carried out in the Medline, Cinahl and Web of Sciences databases for the period 1993–2012.
Results
A total of 207 articles were included. Based on these articles a framework for the analysis of the DTCs based on the role of the DTC, target groups, budget perspective and type of economic decisions could be suggested.
Conclusions
In order to respond to future demands the DTCs will have to develop their skill in pharmacoeconomics. Their processes will have to be standardised and made more transparent in order to be better adapted to evidence-based decision-making. They will also have to embrace the possibilities created by electronic health records in both influencing the decisions of physicians, and in improving quality assurance programmes and longitudinal follow-up of drug therapy and outcomes. They will have to find new ways of interacting with the public and policy makers in order to get the resources needed for their work. Finally, they will have to handle the conflict among national, regional and local decision-making processes and the relationship between formularies and therapeutic guidelines.
Keywords: Drug and therapeutics committee, Pharmacy and therapeutics committee, Prioritization, Cost-effective use of drugs, Pharmacoeconomy, Health economy
Introduction
For the prescriber, time is scarce. There is an overload of information about new treatment options employing old and new drugs, and even new information about old drugs. For providers of healthcare there are new, often more effective, but also more costly, drugs to consider. Meeting the costs of these drugs given local budget constraints and silo budgets is a challenge. At the same time, there may be opportunities to reduce drug costs through procurement, generic substitution, and in some instances therapeutic substitution [1].
Information about drugs and other treatment options is more abundant and easier to access today than ever before. For the patient, there are concerns about the quality of care and access to new treatment options, and about being able to pay for a more expensive drug. In addition, on-going efforts are being made to empower the patient, making him or her more involved in the decision making process in the health-care system.
Some—but not all—of the present day challenges were also present more than half a century ago when the forerunners of today’s drug and therapeutics committees were formed. The first modern drug and therapeutics committee (DTC) in Sweden was formed in 1961 at the Karolinska Hospital in Stockholm [2]. The establishment of this first formal DTC in Sweden coincided with the introduction of clinical pharmacology [3–6] as a medical specialty in the late 1950s, and the DTCs very soon became a hub for the exchange of ideas and knowledge among physicians, clinical pharmacologists and pharmacists.
A drug and therapeutics committee, or as it is also often called, a pharmacy and therapeutics committee, can be defined in different ways. According to the WHO the goal of a DTC is “to ensure that patients are provided with the best possible cost-effective and quality of care through determining what medicines will be available, at what cost, and how they will be used” [7]. The American Society of Health System Pharmacists (ASHP) has stated that “the P&T committee should serve in an evaluative, educational, and advisory capacity to the medical staff and organisational administration in all matters that pertain to the use of medications (including investigational medications)” [8]. The definition of the MeSH term Pharmacy and Therapeutics Committee is narrower: “An advisory group composed primarily of staff physicians and the pharmacist which serves as the communication link between the medical staff and the pharmacy department” [9]. The emphasis in this definition is on hospitals and not on collaboration among different healthcare providers and pharmacies within a geographical area.
Pharmacy and Therapeutics Committee is a term that is also used, especially in the USA, as the name of a committee mandated by law or by local regulation to either decide upon, or to recommend to a deciding body, which of the drugs that are to be made available will also be covered by insurance or a benefit scheme within an organisation or a state. This review focuses on the role of the DTCs within the healthcare system that mainly play a supportive role as defined by the WHO [7].
The purpose of this article is to propose a framework in which the organisation and activities of DTCs can be analysed in different settings. The nature of the framework is based on a narrative review of the evolution of the organisations and goals of DTCs during the last half-century as a consequence of changes both in healthcare systems and in the role of the prescriber.
Materials and methods
A literature search was performed in the Medline, Cinahl and Web of Science databases from 1993 to 2012. For Web of Science and Cinahl all studies with the text “pharmacy and therapeutics committees” OR “drug and therapeutic committees” OR “medicines and therapeutics committees” were included. For Medline, studies indexed under the MeSH terms “pharmacy and therapeutics committee” OR “hospital formularies” were also included.
The search included studies of DTCs published in English, French, Spanish, German and the Scandinavian languages (Swedish, Danish and Norwegian). Studies in other languages with an abstract in English could be eligible for translation and inclusion if they included outcome measures relevant to the review. No study fulfilled these criteria.
Relevant references in published articles fulfilling the search criteria were added. Articles focusing on committees that make decisions or that act as advisors to deciding bodies as regards inclusion in reimbursement schemes and/or insurance coverage were excluded in order to focus on DTCs with a supportive role.
Studies were classified by the author according to type (study, editorial/opinion piece, definition/statement), and focus of the article (goal, organisation of the DTC, interaction with the healthcare organisation, decision-making process, and/or ways of influencing the use of drugs). No independent validation of this selection process was performed.
Results and discussion
The total number of studies identified was 811 in Medline, 269 in Web of Science and 120 in Cinahl. After a manual search of the articles a total of 192 articles—108, 83 and 19 from the different sources – were found relevant to the topic of this review. None of the studies included from Cinahl overlapped with those from the other two sources. In addition 15 relevant articles cited in the reference lists of articles studied were added.
The decision to start a DTC or to expand the activities of an existing DTC can be seen as a multi-modal intervention, often triggered by events in the society that could act as confounders. To start a DTC is a strategic decision in the healthcare setting, often being just one of many different activities initiated at the same time. As a consequence, it is hard to establish a credible control group, or to use historical data as a control, when evaluating the effects of DTCs.
It is thus not surprising that no controlled evaluations of the effects of establishing a DTC on outcomes such as the quality or the cost-effectiveness of health care were found. On the other hand, studies and structured reviews of the effect of various tools used by DTCs in order to influence drug utilisation are common, but these lie outside the scope of this review
Studies of approaches to influencing the behaviour of decision-makers, for instance, prescribers, show that a simple transfer of information will seldom change their behaviour. In order to achieve such changes, the attitudes towards the problem and/or the alternative solutions frequently have to change. This is a well-known issue within health promotion [10–12]. Questions like what kind of problem it is, whose problem it is, what the magnitude of the problem is etc. are important determinants for changed behaviour. Similarly, attitudes towards the solutions such as perception of the size of the effect (including the use of absolute versus relative risk reductions), handling of uncertainty, the perception of drugs as an intervention in a multifactorial disease, etc. are important factors that can influence the decision-making process by physicians.
Many other factors, among them the extent to which the patient is involved in the decision-making process, perceived or real peer pressure, expectations of the patient, the role of the physician in society, marketing, economic incentives for the prescriber, can also influence decision-making. Thus, influencing attitudes among individual and groups of prescribers as regards one or more factors may be seen as avenues leading to changes in the ultimate decision.
Educational outreach visits or academic detailing is a method of knowledge transfer that may be used to influence attitudes among prescribers. A Cochrane review of 69 studies concluded that educational outreach visits appeared to improve the care delivered to patients [13]. Such visits consistently led to small changes in prescribing. For other types of professional practice, such as providing screening tests, outreach visits provided small to moderate changes in practice.
Goal of a DTC
The WHO emphasises the supportive role of a DTC by describing DTCs as forums for the development and implementation of appropriate medicine policies where all the relevant professionals can work together to improve healthcare delivery, whether in hospitals or other health facilities [7]. Even though the WHO promotes the DTC as an integral element in the development of rational guidelines for the use of medicines [14, 15], the number of reports on DTCs in the developing world appearing in the scientific databases searched is still small.
The goals set for DTCs have changed over time. At first the goal was simply to find solutions to basic logistic problems; now, there can be many goals including technology assessment, cost-effectiveness analysis, and the addressing of issues concerning patient safety [16]. Different needs arise in different contexts; in one setting the primary goal can be cost containment, in another the setting of priorities in healthcare systems [17–19]. If setting priorities is a primary goal then healthcare providers, physicians and patients must have criteria for responding to the decisions or recommendations from a DTC. A common criterion for setting priorities in healthcare is “fairness”. The question “How is fairness to be evaluated” must then be dealt with. An example of this is the concept of “accountability for reasonableness” developed by US Health Maintenance Organizations to specify the conditions for operationalising the concept of fairness [19].
Studies of the role and goals of a DTC have shown a wide range of views, sometimes even within one country [20–22]. This is to be expected depending on the choice of the target group, which might in one setting be the healthcare provider and in another the needs of a specific patient population.
Organisation: structure and mandate
A literature review of studies published between 1997 and 2008 on the structure and operation of hospital-based DTCs in nine western countries was identified. Some studies were concerned with the structure and operation of DTCs (n = 9) and others with factors that influence decisions made by DTCs (n = 8) [23]. This review is concerned only with hospital-based DTCs in six countries (USA, The Netherlands, UK, Canada, Germany and Belgium) [24–32]. The structure and operating procedures of the DTCs in these countries were similar. With the exception of Germany, nearly all hospitals had both a DTC and a formulary. In the USA 84 % of hospitals had therapeutic interchange programs [1, 33, 34] while the corresponding figure for Germany was only 36 %. In a study from Spain that was not included in the review 71 % of the hospitals reported having a therapeutic interchange program [35].
Hospital-based or population-focused
Misunderstandings and problems with drug treatment often occur at the interface between different healthcare providers. Many patients receive healthcare, and thus also drugs, from different providers. Drugs initiated in specialist units are continued in primary care, while drugs prescribed from primary care are continued during treatment episodes at hospitals. The need for coordination between different healthcare providers has led to a population-focused goal, taking responsibility for all people in a defined geographic area, for DTCs, for instance, in the Nordic countries [2], and to the development of specific coordinating committees such as Area Prescribing and Medicine Management Committees (APC) in the UK [36]. An APC is essentially an intermediary committee between national/regional decision makers and local hospital-based DTCs with the goal of ensuring a consistent health community approach to medicines management.
The structure of DTCs
A typical DTC consists of physicians (specialised in internal medicine or any other drug-intensive specialty), nurses and pharmacists [23]. The base from which members can be recruited has widened over time as formularies have evolved from very limited lists of drugs into comprehensive documents or programs describing complete systems of medication use policies intended to ensure safe, appropriate, and cost-effective use of pharmaceuticals in patient care [37]. A DTC can now include representatives from administration, quality assurance groups, other healthcare professionals and also representatives from user organisations [38, 39].
In DTCs with a population-focused approach (i.e. having responsibility for improving the use of drugs for all people in a defined geographic area) general practitioners are often included both in the DTC itself and in relevant subcommittees. In addition, other professionals such as clinical pharmacologists, epidemiologists, ethicists, administrators and/or health economists may be included [40]. Representatives from user organisations may also be a part of a DTC in order to capture patient perspectives on drug treatment, provide for better interaction with patient groups about decision making, and to increase the credibility of the DTC among patients and politicians.
Even though pharmacoeconomic considerations have long been described as important for DTCs [40–44] there are several factors that have impeded the application of pharmacoeconomic methods, not least of which is a lack of training in health economics and in the availability of health economists [40, 45].
Two particularly important professional groups represented in a DTC are the pharmacists and the clinical pharmacologists. A statement from American Society of Health System Pharmacists describes in detail the pharmacist’s responsibilities and roles in managing the formulary system in partnership with other healthcare professionals, but with a focus on DTCs in hospitals [37].
The clinical pharmacologist is a physician who has had systematic training in the evaluation of drug therapy and drug products. The International Union of Basic and Clinical Pharmacology, IUPHAR, has stated that participation in DTCs is a key role for clinical pharmacologists, since these bodies provide a basis for implementing the principles of the rational prescribing of drugs. According to the IUPHAR, clinical pharmacologists also have a responsibility for training DTC members in critical drug evaluation and ensuring that drug recommendations are based on scientific evidence and medical needs as assessed by independent drug experts in various pharmaco-therapeutic areas [3].
Inter-professional collaboration
One of the strengths of a DTC is that it facilitates a multi-professional approach through which physicians, nurses, pharmacists and administrators can meet to discuss drug-related questions and policies from different perspectives. This approach can lead to both satisfaction and frustration depending on how the inter-professional relationships develop and are handled by the organisation. The addition of specialists in epidemiology, logistics and/or health economists, as well as patient representatives, may enrich but also complicate the work process. A study of inter-professional relationships in DTCs in the USA showed that the relationships tended to be rated positively and that members tended to be quite willing to cooperate with one another. Low levels of frustration and anger were reported [46].
In 1993 the National Corporation of Swedish Pharmacies commissioned a survey of the attitudes towards different sources of drug information among general practitioners in five counties in Sweden [47]. Among general practitioners, information from representatives of pharmaceutical companies was the only kind of information about drugs that was ranked lower than the information provided by the DTCs. One Swedish DTC, the Örebro County DTC was, however, identified as being particularly successful in providing information. An in-depth analysis showed that among the success factors of this DTC was the organisational structure with a network consisting of therapeutic subcommittees, general practitioners represented as members on all levels, evidence-based recommendations, emphasis on the supportive role of the recommendations, and academic detailing [48]. Several DTCs in Sweden were reorganised as a consequence of the report.
Mandatory or voluntary
Local and regional DTCs, including APCs, have developed out of a defined local need to collaborate in order to improve the practice of drug procurement and logistics, always with the goal of supporting cost minimisation and safer handling of drugs. These committees, which more or less fulfil the WHO definition of a DTC, are normally non-mandatory as opposed to mandatory national (or in some instances regional) committees with the goal of deciding whether or not a specific drug should be included in a formulary and thus be covered by the pharmaceutical benefit scheme, or by a specific health insurance scheme.
In 1997 the Swedish Drug Reform Act included a law making it mandatory for each of the 21 counties to have at least one DTC with a population-focused approach that would support hospital specialists and general practitioners, as well as physicians at outpatient clinics regardless of the type of provider organisation. As a consequence of the law the DTCs were not only given proper resources for performing analyses and interacting with prescribers, they were also given an official role within the healthcare organisations.
The counties are also required to provide a budget to support the activities of the DTC. In the most progressive counties the budget constitutes approximately 1 % of the total cost of the pharmaceutical benefit scheme [2]. The law specifies that expertise from both medicine and the pharmaceutical area is to be drawn upon by the DTC and that the DTC should have access to detailed drug utilisation statistics through the National Corporation of Swedish Pharmacies. Since July 2010 the counties, and thus also the DTCs, have by law access to a monthly file of all of the drugs dispensed to the inhabitants of each county. An encrypted personal identification number makes it possible to link the dispensation of drugs to data from electronic health records and/or administrative databases for quality assurance purposes without the specific consent from ethical committees for research purposes.
National, regional or local
The need for implementation of mandatory and properly financed DTCs in Sweden has led to a discussion in society concerning the risks that might arise from multi-level decision-making regarding introduction of new drugs. The pharmaceutical industry and representatives from the national authorities have questioned the need for the current legislation, claiming that local decision-making processes simply waste resources and lead to inequalities in the provision of healthcare.
The counter-arguments from the healthcare system, and from the DTCs themselves, have been that local structures in the society and the healthcare system, and the absence of unbiased information will always lead to inequalities. Since DTCs are important actors in detecting and addressing such situations within the field of pharmaco-therapy the argument goes that they are in fact needed to counter such inequalities. In addition, in order to be effective in changing behaviour in a desired direction it is argued that some of the most crucial success factors are the existence of a local affiliation and a clear mandate with proper resources allocated.
Similar discussions have taken place in other countries. The need for rapid action, awareness of local circumstances and local safety problems, local pricing arrangements as a consequence of procurement, affordability for the local organisation as a complement to considerations of marginal cost-effectiveness analysis have all been presented in the UK as reasons for supporting the existence of DTCs with locally adapted formularies [49]. Other arguments, based on the available evidence of the existence of effective methods for changing the behaviour of prescribers, are that dialogue is better than providing a one-way information process, that credibility can be increased through the use of local opinion-leaders, and that the involvement of peers can aid in the adaptation of national guidelines for the local setting.
Another common argument against local DTCs is the perceived waste of time that results if the same evidence is assessed twice, both nationally and locally. The counter-argument from local organisations is that local specialists in a disease have to study national guidelines and the evidence on which these guidelines are based, whether or not they participate in a local expert subcommittee. For this reason, it makes sense to give them the possibility of participating in the dissemination of knowledge and these new guidelines.
Decision-making process
Evidence-based medicine
Promoting rational prescribing is in itself a process that depends on an evidence-based approach both to the decision-making process and to the interventions employed in order to change the behaviour of physicians [50]. Evidence-based medicine (EBM) has been promoted as a very important part of the work of a DTC [14]. Still, the process itself is not always as transparent and rigorous as is commonly associated with the term EBM. There might be many reasons for this, such as time constraints and lack of resources, but the absence of a specific process for decision-making may contribute to these concerns. In a qualitative study of the decision-making process in two DTCs in the UK it was found that EBM, while used in decision making, was supplemented by local knowledge about other factors, although decisions were framed in the language of scientific rationality [51].
Pharmacoeconomics
The cost of drugs has been a core issue from the time of the first DTCs. Over time there has been an increasing demand for the full consequences of drug use to be described, not only in terms of cost minimisation, but also in cost-effectiveness as a consequence of changing goals and roles for the DTCs. A thorough knowledge of health economics is needed in order to assess the marginal benefits of one drug versus the alternatives, and to compare this with the marginal net cost. Studies have indicated that evaluation of cost-effectiveness in decision-making has been limited by committee members’ lack of specific training, and by the difficulty of applying pharmacoeconomic studies to actual clinical practice [40, 52].
One important aspect of pharmacoeconomics is the perspective of the analysis, whether the analysis is made from a societal perspective, taking into account all relevant benefits and costs in society, or from a narrower perspective, such as that of the payer or the person responsible for the hospital budget. The choice of perspective depends on the role of the DTC (Table 1), where the trend over time has been a movement from cost minimisation in the healthcare system in the upper-left corner towards marginal cost-effectiveness from a societal perspective in the lower-right corner.
Table 1.
A simple 3-by-3 table illustrating the different roles of a Drug and Therapeutics Committee (DTC) for different groups of prescribers (and thus also organisations)
| Restrictive role | Supportive but with some restrictive tasks | Supportive role | |
|---|---|---|---|
| In-patient specialist care | |||
| Out-patient specialist care | |||
| General practice |
Changing behaviour
A DTC can be primarily supportive, for instance by giving advice, or restrictive, i.e. making decisions that are more or less compulsory for physicians and nurses to follow. It is more likely that they will be a combination of both. In addition, the target group for one DTC can differ from that of another, Table 2. Even in a healthcare setting with similar goals for the DTCs, factors such as whether drug budgets are devolved or not can result in different DTCs taking different positions. In a case study of a county in Sweden with a fully devolved drug budget it was shown that the DTC had positioned itself as purely supportive by developing indicators, but abstaining from setting direct targets for prescribing. Instead, the DTC required each department to set its own targets together with prescribers, arguing that each unit was responsible for the cost-effectiveness and quality of its own prescribing. In another county without devolved drug budgets the DTC was active in setting prescribing targets linked to economic incitements, and thus to a degree restricting physicians’ freedom to make their own decisions [53].
Table 2.
A combination of different perspectives and methods can be employed in pharmacoeconomics. Some of the most important for analysing the role of a DTC are depicted
| Budget perspective | ||
|---|---|---|
| Healthcare | Societal | |
| Cost-minimisation | ||
| Cost-effectiveness | ||
| Marginal cost-effectiveness | ||
So-called “soft regulations” are often used in order to influence the use of drugs. These interventions can typically be categorised as being based on education, engineering, economics and enforcement, or the four “Es” [10, 53]. There are several studies and reviews analysing the effectiveness, or lack of effect, of different interventions aiming at changing the behaviour of physicians, but reviewing these studies is outside the scope of this article.
Education
Providing information, including educational outreach, is a mainstay in the efforts of most DTCs to influence behaviour. Providing traditional information in the form of newsletters and group workshops is still considered a natural basis for other more advanced interventions even though providing traditional information is not deemed effective per se. More interactive strategies such as academic detailing, also called educational outreach, have been shown to be an effective way of influencing prescribing behaviour [13]. One important decision for the DTC is to decide how to balance between “push” and “pull” in their activities. One option for the DTC is to try to change attitudes, and thus behaviour, by providing information or by initiating activities, that is, by “pushing”. Another option is to support making structured information generally available, setting up quality assurance projects, and developing clinical decision support systems to be used when deemed necessary by physicians, that is by “pulling”.
One example of the push–pull dilemma is the handling of drug statistics. Drug statistics often focus on reports with feedback on performance including prescribing targets. Another option is for the organisation to construct a system where the prescribing physicians can self-monitor their prescribing habits, bench-mark themselves relative to their chosen peers, and analyse their prescribing themselves. The balance between the push and pull alternatives depends on whether or not there are other structures, such as, for instance, tradition or devolved drug budgets, that might stimulate action from the prescribers and thus make “pull” more efficient.
Physicians are influenced by patients’ perceptions about, and interest in, new drugs. One way for the DTCs to use this is to engage in patient-orientated educational activities designed to help patients develop a better understanding of the benefits and risks of treatment alternatives, thus influencing the physicians.
Engineering
Formularies, especially formularies directly linked to generic substitution, therapeutic interchange or procurement and thus availability of drugs, can be the basis for strong interventions, since they are easy to communicate and follow up, and since in some instances they are mandatory by law. An important measure in order to make full use of such an intervention in the hospital setting is a strategy for developing a drug logistics program that will ensure continued availability of recommended drugs and making it easier for the physicians to be compliant.
Factors that influence the physician at the point of decision-making are in general also effective interventions. Two such factors are strategies for seeing to it that the physician uses computerised order entry systems (CPOE) and availability of computerised clinical decision support systems. These can interact with the results of procurement of drugs, drug logistics and guidance. Using electronic health records can ensure that data can be collected in quality registers and be used for specific local quality indicators. Horizon-scanning for changes in the drug market is important in order to estimate what resources will be needed to handle the introduction of new drugs. An ordered introduction based on the results from horizon-scanning can make it easier for truly innovative and valuable new drugs to be introduced to, and used by, the right patients. This can also help restrict the use of drugs that are neither innovative nor cost-effective.
Limiting the marketing activities of pharmaceutical companies is a common intervention, but can slow down diffusion of innovation. Thus, if limits are to be placed on information activities provided by drug companies then systems for providing unbiased information about drugs must be developed and given proportionately greater support.
Economics
Setting targets for prescribing (ratios or volumes) is a common intervention done by DTC. The aim can be either cost-minimisation or cost-effectiveness, but it may also include setting quality indicators as part of a quality assurance project [54, 55].
Other examples of interventions by DTCs within economics are assistance in the procurement of drugs, and support in drug budget devolution.
Enforcement
One example of enforcement is the monitoring of prescribing of restricted drugs against agreed guidance with additional interventions if required. Other examples are deciding on mandatory guidelines for drug handling at wards.
Conclusions: the future of the DTC
The DTCs have managed to evolve in response to different demands from healthcare by adopting and endorsing evidence-based medicine, pharmacoeconomics, pharmacoepidemiology and employing evidence-based methods of influencing physicians in order to achieve a more rational drug use. At the same time they have in many cases managed to change the physicians’ perceptions of drug formularies from being “cook-book medicine” to an evidence-based starting point for choosing the right drug for the right patient. This has been made possible through the creation of an arena where different professions involved in the safety, effectiveness and cost-effectiveness of drug therapy can interact. In Sweden, Professor Folke Sjöqvist has been both a catalyst and facilitator for the development of the DTCs and for developing a clear role for clinical pharmacology in their processes and his career has spanned the whole development of the current Swedish DTCs.
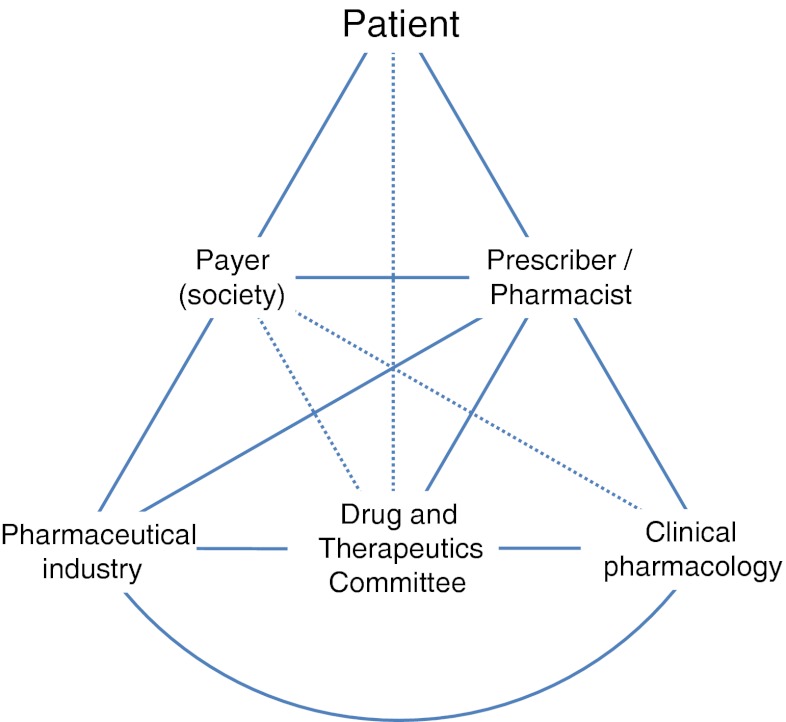
In order to respond to future demands the DTCs will have to develop their skill in pharmacoeconomics and implement it in their processes. Their processes will have to be standardised and made more transparent in order to be better adapted to evidence-based decision-making. They will also have to embrace the possibilities created by electronic health records in both influencing the decisions of physicians and improving quality assurance programs and longitudinal follow-up of drug therapy and outcomes with the assistance of pharmacoepidemiology. They will benefit from finding new ways of interacting with the public and policy makers in order to get the resources needed for their work (Fig. 1). Finally, they will have to handle the conflict among national, regional and local decision-making processes and the relationship between formularies and therapeutic guidelines.
Fig. 1.
Established relations between the Drug and Therapeutics Committee (DTC) and different actors. Dotted lines represent additional, less frequently described, interactions
Acknowledgments
Conflicts of interests
The author acted as a chairman of a local DTC between 2001 and 2007. Since 2007 he has had no affiliation to any DTC, and from 2010 to any county council. There are no other conflicts of interests to declare.
Abbreviations
- APC
Area Prescribing and Medicine Management Committees
- ASHP
The American Society of Health System Pharmacists
- CPOE
Computerised order entry systems
- DTC
Drug and Therapeutics Committee
- EBM
Evidence-based medicine
- IUPHAR
The International Union of Basic and Clinical Pharmacology
- MeSH
Medical subjects heading
- P&T committee
Pharmacy and Therapeutics Committee
Fact sheet
The goal of a DTC according to WHO [7] is to ensure that patients are provided with the best possible cost-effective and quality of care through determining what medicines will be available, at what cost, and how they will be used. In order to achieve this goal a DTC will have the following objectives:
To develop and implement an efficient and cost-effective formulary system that includes consistent standard treatment protocols, a formulary list and formulary manual
To ensure that only efficacious, safe, cost-effective and good quality medicines are used
To ensure the best possible drug safety through monitoring, evaluating and thereby preventing, as far as possible, adverse drug reactions (ADRs) and medication errors
To develop and implement interventions to improve medicine use by prescribers, dispensers and patients; this will require the investigation and monitoring of medicine use.
References
- 1.Holmes ACCF/AHA 2011 health policy statement on therapeutic interchange and substitution (vol 124, pg 1290, 2011) Circulation. 2011;124:E364–E364. doi: 10.1161/CIR.0b013e318234a5f6. [DOI] [PubMed] [Google Scholar]
- 2.Sjöqvist F. Drug and therapeutics committees: a Swedish experience. WHO Drug Inf. 2002;16:207–213. [Google Scholar]
- 3.Birkett D, Brosen K, Cascorbi I, Gustafsson LL, Maxwell S, Rago L, Rawlins M, Reidenberg M, Sjoqvist F, Smith T, Thuerman P, Walubo A, Orme M, Sjoqvist F. Clinical pharmacology in research, teaching and health care: considerations by IUPHAR, the International Union of Basic and Clinical Pharmacology. Basic Clin Pharmacol Toxicol. 2010;107:531–559. doi: 10.1111/j.1742-7843.2010.00602.x. [DOI] [PubMed] [Google Scholar]
- 4.Modell W. Symposium on clinical drug evaluation and human pharmacology. Introduction. Clinical pharmacology: a definition. Clin Pharmacol Ther. 1962;3:235–238. [PubMed] [Google Scholar]
- 5.Sjoqvist F. The past, present and future of clinical pharmacology. Eur J Clin Pharmacol. 1999;55:553–557. doi: 10.1007/s002280050672. [DOI] [PubMed] [Google Scholar]
- 6.Wang RI. The definition and scope of clinical pharmacology. JAMA: J Am Med Assoc. 1980;243:1901–1902. doi: 10.1001/jama.1980.03300450015011. [DOI] [PubMed] [Google Scholar]
- 7.Holloway K, Green T, Carandang E, Hogerzeil H, Lee RL. Drug and therapeutics committees. A practical guide. Geneva: Department of Essential Drugs and Medicines Policy, World Health Organization; 2003. [Google Scholar]
- 8.Tyler LS, Cole SW, May JR, Millares M, Valentino MA, Vermeulen LC, Jr, Wilson AL. ASHP guidelines on the pharmacy and therapeutics committee and the formulary system. Am J Health-Syst Pharm: AJHP: Off J Am Soc Health-Syst Pharmacists. 2008;65:1272–1283. doi: 10.2146/ajhp080086. [DOI] [PubMed] [Google Scholar]
- 9.MeSH-term Pharmacy and Therapeutics Committee. http://www.ncbi.nlm.nih.gov.lt.ltag.bibl.liu.se/mesh?term=pharmacyandtherapeuticscommittee. Accessed 12 January 2013
- 10.Public Communication Campaigns (2001). 3rd edn. SAGE Publications Inc., London, UK
- 11.NICE public health guidance: PH6 Behaviour change (2007) National Institute for Health and Clinical Excellence
- 12.Bettinghaus EP. Health promotion and the knowledge-attitude-behaviour continuum. Prev Med. 1986;15:475–491. doi: 10.1016/0091-7435(86)90025-3. [DOI] [PubMed] [Google Scholar]
- 13.O’Brien M, Rogers S, Jamtvedt G, Oxman A, Odgaard-Jensen J, Kristoffersen D, Forsetlund L, Bainbridge D, Freemantle N, Davis D, Haynes R, Harvey E (2007) Educational outreach visits: effects on professional practice and health care outcomes. Cochrane Database Syst Rev (4). doi:10.1002/14651858.CD000409.pub2 [DOI] [PMC free article] [PubMed]
- 14.Promoting Rational Use of Medicines: Core Components - WHO Policy Perspectives on Medicines (2002), vol 5. World Health Organization
- 15.Laing RO, Hogerzeil HV, Ross-Degnan D. Ten recommendations to improve use of medicines in developing countries. Health Policy Plan. 2001;16:13–20. doi: 10.1093/heapol/16.1.13. [DOI] [PubMed] [Google Scholar]
- 16.Mehr SR. The evolutionary role of the pharmacy and therapeutics committee in technology assessment. Manag Care Interface. 2006;19:42–45. [PubMed] [Google Scholar]
- 17.Garpenby P. Medical experts groups and choice of medications. A fair prioritization process guarantees the reliability of the choice of medications. Lakartidningen. 2004;101:2480–2482. [PubMed] [Google Scholar]
- 18.Lucena Gonzalez MI, Andrade Bellido RJ, Rodriguez-Mendizabal M, Sanchez de la Cuesta F. Drug selection in hospitals. A model of reference in self-management of resources. An Med Interna. 1996;13:505–510. [PubMed] [Google Scholar]
- 19.Martin DK, Hollenberg D, MacRae S, Madden S, Singer P. Priority setting in a hospital drug formulary: a qualitative case study and evaluation. Health Policy. 2003;66:295–303. doi: 10.1016/S0168-8510(03)00063-0. [DOI] [PubMed] [Google Scholar]
- 20.Bjorkman IK, Schmidt IK, Holmstrom I, Bernsten CB. Developing the role of the drug and therapeutics committees: perceptions of chairs. Int J Health Care Qual Assur. 2007;20:161–178. doi: 10.1108/09526860710731843. [DOI] [PubMed] [Google Scholar]
- 21.Tan EL, Day RO, Brien JA. Prioritising drug and therapeutics committee (DTC) decisions: a national survey. Pharm World Sci. 2007;29:90–96. doi: 10.1007/s11096-006-9074-y. [DOI] [PubMed] [Google Scholar]
- 22.Weekes LM, Brooks C. Drug and Therapeutics Committees in Australia: expected and actual performance. Br J Clin Pharmacol. 1996;42:551–557. doi: 10.1111/j.1365-2125.1996.tb00048.x. [DOI] [PubMed] [Google Scholar]
- 23.Duran-Garcia E, Santos-Ramos B, Puigventos-Latorre F, Ortega A. Literature review on the structure and operation of Pharmacy and Therapeutics Committees. Int J Clin Pharm. 2011;33:475–483. doi: 10.1007/s11096-011-9501-6. [DOI] [PubMed] [Google Scholar]
- 24.Cooke J, Mason AR, Drummond MF, Towse AK. Medication management in English National Health Service hospitals. Am J Health-Syst Pharm: AJHP: Off J Am Soc Health-Syst Pharmacists. 2005;62:189–195. doi: 10.1093/ajhp/62.2.189. [DOI] [PubMed] [Google Scholar]
- 25.Fijn R, Brouwers JR, Knaap RJ, De Jong-Van Den Berg LT. Drug and Therapeutics (D & T) committees in Dutch hospitals: a nation-wide survey of structure, activities, and drug selection procedures. Br J Clin Pharmacol. 1999;48:239–246. doi: 10.1046/j.1365-2125.1999.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fijn R, de Jong-van den Berg LTW, Brouwers J. Rational pharmacotherapy in The Netherlands: formulary management in Dutch hospitals. Pharm World Sci. 1999;21:74–79. doi: 10.1023/A:1008654609916. [DOI] [PubMed] [Google Scholar]
- 27.Mannebach MA, Ascione FJ, Gaither CA, Bagozzi RP, Cohen IA, Ryan ML. Activities, functions, and structure of pharmacy and therapeutics committees in large teaching hospitals. Am J Health-Syst Pharm. 1999;56:622–628. doi: 10.1093/ajhp/56.7.622. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen CA, Schneider PJ, Santell JP. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing-2001. Am J Health-Syst Pharm. 2001;58:2251–2266. doi: 10.1093/ajhp/58.23.2251. [DOI] [PubMed] [Google Scholar]
- 29.Pedersen CA, Schneider PJ, Scheckeloff DJ. ASHP national survey of pharmacy practice in hospital settings: prescribing and transcribing—2004. Am J Health-Syst Pharm. 2005;62:378–390. doi: 10.1093/ajhp/62.4.0378. [DOI] [PubMed] [Google Scholar]
- 30.Shalansky SJ, Virk R, Ackman M, Jackevicius C, Kertland H, Tsuyuki R, Humphries K. Access to new cardiovascular therapies in Canadian hospitals: a national survey of the formulary process. Can J Cardiol. 2003;19:173–179. [PubMed] [Google Scholar]
- 31.Thürmann PA, Harder S, Steioff A. Structure and activities of hospital drug committees in Germany. Eur J Clin Pharmacol. 1997;52:429–435. doi: 10.1007/s002280050315. [DOI] [PubMed] [Google Scholar]
- 32.Willems L, Raymakers A, Sermeus W, Vleugels A, Laekeman G. Survey of hospital pharmacy practice in Flemish-speaking Belgium. Am J Health Syst Pharm. 2005;62:321–324. doi: 10.1093/ajhp/62.3.321. [DOI] [PubMed] [Google Scholar]
- 33.Gray T, Bertch K, Galt K, Gonyeau M, Karpiuk E, Oyen L, Sudekum MJ, Vermeulen LC. Guidelines for therapeutic interchange—2004. Pharmacotherapy. 2005;25:1666–1680. doi: 10.1592/phco.2005.25.11.1666. [DOI] [PubMed] [Google Scholar]
- 34.Mahoney CD. Issues in formulary management: therapeutic interchange. The value, cost, and quality of therapeutic interchange. Hosp Formul. 1992;27:2–3. [PubMed] [Google Scholar]
- 35.Puigventos F, Santos-Ramos B, Ortega A, Duran-Garcia E. Structure and procedures of the pharmacy and therapeutic committees in Spanish hospitals. Pharm World Sci. 2010;32:767–775. doi: 10.1007/s11096-010-9435-4. [DOI] [PubMed] [Google Scholar]
- 36.Picton C (2007) Managing medicines across a health community—Making area prescribing committees fit for purpose. National Prescribing Centre, NHS
- 37.ASHP ASHP statement on the Pharmacy and Therapeutics Committee and the formulary system. Am J Health Syst Pharm. 2008;65:2384–2386. doi: 10.2146/ajhp080413. [DOI] [PubMed] [Google Scholar]
- 38.Loorand-Stiver L. Hospital-based Pharmacy and Therapeutics Committees: evolving responsibilities and membership. Ottawa: CADTH: Environmental Scan; 2011. [Google Scholar]
- 39.Mittmann N, Knowles S. A survey of Pharmacy and Therapeutic committees across Canada: scope and responsibilities. Can J Clin Pharmacol. 2009;16:e171–e177. [PubMed] [Google Scholar]
- 40.Anell A, Svarvar P. Pharmacoeconomics and clinical practice guidelines. A survey of attitudes in Swedish formulary committees. Pharmacoeconomics. 2000;17:175–185. doi: 10.2165/00019053-200017020-00006. [DOI] [PubMed] [Google Scholar]
- 41.Aspinall SL, Good CB, Glassman PA, Valentino MA. The evolving use of cost-effectiveness analysis in formulary management within the Department of Veterans Affairs. Med Care. 2005;43:20–26. doi: 10.1097/01.mlr.0000170004.17480.49. [DOI] [PubMed] [Google Scholar]
- 42.Hatoum HT, Freeman RA. The use of pharmacoeconomic data in formulary selection. Top Hosp Pharm Manage. 1994;13:47–53. [PubMed] [Google Scholar]
- 43.Hughes D, Reynolds DJ. Pharmacoeconomics: principles and relevance to the activities of drug and therapeutics committees. Clin Med. 2009;9:490–492. doi: 10.7861/clinmedicine.9-5-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson JA, Bootman JL. Pharmacoeconomic analysis in formulary decisions: an international perspective. Am J Hosp Pharm. 1994;51:2593–2598. [PubMed] [Google Scholar]
- 45.Suh DC, Okpara IR, Agnese WB, Toscani M. Application of pharmacoeconomics to formulary decision making in managed care organizations. Am J Manag Care. 2002;8:161–169. [PubMed] [Google Scholar]
- 46.Bagozzi RP, Ascione FJ, Mannebach MA. Inter-role relationships in hospital-based pharmacy and therapeutics committee decision making. J Health Psychol. 2005;10:45–64. doi: 10.1177/1359105305045347. [DOI] [PubMed] [Google Scholar]
- 47.Läkemedelsinformation—en kvalitetsstudie av 8 läkemedelsinformationskällor. Stockholm
- 48.Hedlund F. Framgångsfaktorer för en läkemedelskommitté. Stockholm: En undersökning i Örebro; 1994. [Google Scholar]
- 49.Reynolds DJM, Fajemisin O, Wilds S. Local formularies. Br J Clin Pharmacol. 2012;74:640–643. doi: 10.1111/j.1365-2125.2012.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hogerzeil HV. Promoting rational prescribing: an international perspective. Br J Clin Pharmacol. 1995;39:1–6. doi: 10.1111/j.1365-2125.1995.tb04402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenkings KN, Barber N. What constitutes evidence in hospital new drug decision making? Soc Sci Med (1982) 2004;58:1757–1766. doi: 10.1016/S0277-9536(03)00373-3. [DOI] [PubMed] [Google Scholar]
- 52.Walkom E, Robertson J, Newby D, Pillay T. The role of pharmacoeconomics in formulary decision-making—Considerations for hospital and managed care pharmacy and therapeutics committees. Formulary. 2006;41:374–376. [Google Scholar]
- 53.Godman B, Wettermark B, Hoffmann M, Andersson K, Haycox A, Gustafsson LL. Multifaceted national and regional drug reforms and initiatives in ambulatory care in Sweden: global relevance. Expert Rev Pharmacoecon Outcomes Res. 2009;9:65–83. doi: 10.1586/14737167.9.1.65. [DOI] [PubMed] [Google Scholar]
- 54.Campbell SM, Kontopantelis E, Hannon K, Burke M, Barber A, Lester HE. Framework and indicator testing protocol for developing and piloting quality indicators for the UK quality and outcomes framework. BMC Fam Pract. 2011;12:85. doi: 10.1186/1471-2296-12-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wettermark B, Bergman U, Krakau I. Using aggregate data on dispensed drugs to evaluate the quality of prescribing in urban primary health care in Sweden. Public Health. 2006;120:451–461. doi: 10.1016/j.puhe.2005.10.011. [DOI] [PubMed] [Google Scholar]