Abstract
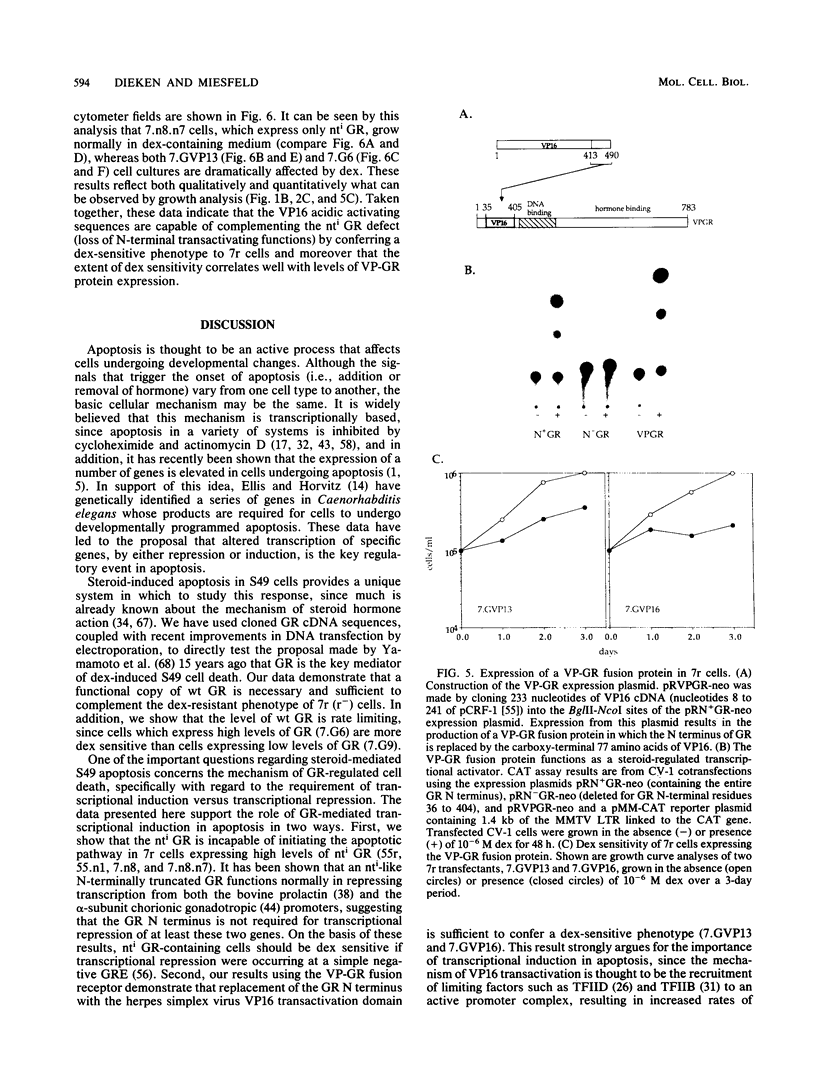
Genetic studies have suggested that transcriptional regulation of specific target genes (by either induction or repression) is the molecular basis of glucocorticoid-mediated lymphocyte apoptosis. To examine the role of transcriptional regulation more directly, we developed a complementation assay utilizing stable transfection of wild-type (wt) and mutant (nti) glucocorticoid receptor (GR) cDNA constructs into a GR-deficient S49 murine cell line (7r). Our data confirm that the level of functional GR is rate limiting for S49 apoptosis and moreover that the GR amino terminus (N terminus), which as been deleted from the nti GR, is absolutely required for complementation in this system. Surprisingly, we found that at physiological levels of receptor, expression of the nti GR in cells containing wt GR results in enhanced dexamethasone sensitivity rather than a dominant negative phenotype. One interpretation of these data is that DNA binding by wt-nti heterodimers may be functionally similar to that of wt-wt homodimers, indicating that GRE occupancy by at least one transactivation domain may be sufficient to induce the hormonal response. To determine whether acidic activating sequences such as those localized to the GR N terminus are important in the induction of lymphocyte apoptosis, we tested the activity of a chimeric receptor in which we replaced the entire GR N terminus with sequences from the herpes simplex virus VP16 protein. Our results demonstrate that 7r cells expressing VP-GR fusions are indeed steroid sensitive, strongly supporting the idea that S49 apoptosis is dependent on transcriptional regulation of specific genes which respond to acidic activating domains, implying that induction, rather than repression, may be the critical initiating event.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baughman G., Harrigan M. T., Campbell N. F., Nurrish S. J., Bourgeois S. Genes newly identified as regulated by glucocorticoids in murine thymocytes. Mol Endocrinol. 1991 May;5(5):637–644. doi: 10.1210/mend-5-5-637. [DOI] [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Correlation between glucocorticoid receptor and cytolytic response of murine lymphoid cell lines. Cancer Res. 1979 Nov;39(11):4749–4751. [PubMed] [Google Scholar]
- Bourgeois S., Newby R. F. Diploid and haploid states of the glucocorticoid receptor gene of mouse lymphoid cell lines. Cell. 1977 Jun;11(2):423–430. doi: 10.1016/0092-8674(77)90060-5. [DOI] [PubMed] [Google Scholar]
- Briehl M. M., Miesfeld R. L. Isolation and characterization of transcripts induced by androgen withdrawal and apoptotic cell death in the rat ventral prostate. Mol Endocrinol. 1991 Oct;5(10):1381–1388. doi: 10.1210/mend-5-10-1381. [DOI] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Jonklaas J., Ringold G. M. Domains of the glucocorticoid receptor involved in specific and nonspecific deoxyribonucleic acid binding, hormone activation, and transcriptional enhancement. Mol Endocrinol. 1987 Nov;1(11):816–822. doi: 10.1210/mend-1-11-816. [DOI] [PubMed] [Google Scholar]
- Danielsen M., Northrop J. P., Ringold G. M. The mouse glucocorticoid receptor: mapping of functional domains by cloning, sequencing and expression of wild-type and mutant receptor proteins. EMBO J. 1986 Oct;5(10):2513–2522. doi: 10.1002/j.1460-2075.1986.tb04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFranco D., Yamamoto K. R. Two different factors act separately or together to specify functionally distinct activities at a single transcriptional enhancer. Mol Cell Biol. 1986 Apr;6(4):993–1001. doi: 10.1128/mcb.6.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Dieken E. S., Meese E. U., Miesfeld R. L. nti glucocorticoid receptor transcripts lack sequences encoding the amino-terminal transcriptional modulatory domain. Mol Cell Biol. 1990 Sep;10(9):4574–4581. doi: 10.1128/mcb.10.9.4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distelhorst C. W., Kullman L., Wasson J. Characterization of glucocorticoid receptors in S49 mouse lymphoma cells by affinity labeling with [3H]dexamethasone 21-mesylate. J Steroid Biochem. 1987 Jan;26(1):59–65. doi: 10.1016/0022-4731(87)90031-8. [DOI] [PubMed] [Google Scholar]
- Ellis H. M., Horvitz H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986 Mar 28;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Gaido M. L., Cidlowski J. A. Identification, purification, and characterization of a calcium-dependent endonuclease (NUC18) from apoptotic rat thymocytes. NUC18 is not histone H2B. J Biol Chem. 1991 Oct 5;266(28):18580–18585. [PubMed] [Google Scholar]
- Galili U., Leizerowitz R., Moreb J., Gamliel H., Gurfel D., Polliack A. Metabolic and ultrastructural aspects of the in vitro lysis of chronic lymphocytic leukemia cells by glucocorticoids. Cancer Res. 1982 Apr;42(4):1433–1440. [PubMed] [Google Scholar]
- Gametchu B. Glucocorticoid receptor-like antigen in lymphoma cell membranes: correlation to cell lysis. Science. 1987 Apr 24;236(4800):456–461. doi: 10.1126/science.3563523. [DOI] [PubMed] [Google Scholar]
- Gehring U., Mugele K., Ulrich J. Cellular receptor levels and glucocorticoid responsiveness of lymphoma cells. Mol Cell Endocrinol. 1984 Jun;36(1-2):107–113. doi: 10.1016/0303-7207(84)90089-3. [DOI] [PubMed] [Google Scholar]
- Gehring U., Tomkins G. M. A new mechanism for steroid unresponsiveness: loss of nuclear binding activity of a steroid hormone receptor. Cell. 1974 Nov;3(3):301–306. doi: 10.1016/0092-8674(74)90145-7. [DOI] [PubMed] [Google Scholar]
- Godowski P. J., Picard D., Yamamoto K. R. Signal transduction and transcriptional regulation by glucocorticoid receptor-LexA fusion proteins. Science. 1988 Aug 12;241(4867):812–816. doi: 10.1126/science.3043662. [DOI] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Harris A. W. Differentiated functions expressed by cultured mouse lymphoma cells. I. Specificity and kinetics of cell responses to corticosteroids. Exp Cell Res. 1970 Jun;60(3):341–353. doi: 10.1016/0014-4827(70)90527-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Ingles C. J., Shales M., Cress W. D., Triezenberg S. J., Greenblatt J. Reduced binding of TFIID to transcriptionally compromised mutants of VP16. Nature. 1991 Jun 13;351(6327):588–590. doi: 10.1038/351588a0. [DOI] [PubMed] [Google Scholar]
- Ip M. M., Shea W. K., Sykes D., Young D. A. The truncated glucocorticoid receptor in the P1798 mouse lymphosarcoma is associated with resistance to glucocorticoid lysis but not to other glucocorticoid-induced functions. Cancer Res. 1991 Jun 1;51(11):2786–2796. [PubMed] [Google Scholar]
- Iwata M., Hanaoka S., Sato K. Rescue of thymocytes and T cell hybridomas from glucocorticoid-induced apoptosis by stimulation via the T cell receptor/CD3 complex: a possible in vitro model for positive selection of the T cell repertoire. Eur J Immunol. 1991 Mar;21(3):643–648. doi: 10.1002/eji.1830210316. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin Y. S., Green M. R. Mechanism of action of an acidic transcriptional activator in vitro. Cell. 1991 Mar 8;64(5):971–981. doi: 10.1016/0092-8674(91)90321-o. [DOI] [PubMed] [Google Scholar]
- Martin D. P., Schmidt R. E., DiStefano P. S., Lowry O. H., Carter J. G., Johnson E. M., Jr Inhibitors of protein synthesis and RNA synthesis prevent neuronal death caused by nerve growth factor deprivation. J Cell Biol. 1988 Mar;106(3):829–844. doi: 10.1083/jcb.106.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miesfeld R. L. Molecular genetics of corticosteroid action. Am Rev Respir Dis. 1990 Feb;141(2 Pt 2):S11–S17. [PubMed] [Google Scholar]
- Miesfeld R. L. The structure and function of steroid receptor proteins. Crit Rev Biochem Mol Biol. 1989;24(2):101–117. doi: 10.3109/10409238909086395. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Godowski P. J., Maler B. A., Yamamoto K. R. Glucocorticoid receptor mutants that define a small region sufficient for enhancer activation. Science. 1987 Apr 24;236(4800):423–427. doi: 10.1126/science.3563519. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Okret S., Wikström A. C., Wrange O., Gustafsson J. A., Yamamoto K. R. Characterization of a steroid hormone receptor gene and mRNA in wild-type and mutant cells. Nature. 1984 Dec 20;312(5996):779–781. doi: 10.1038/312779a0. [DOI] [PubMed] [Google Scholar]
- Nazareth L. V., Harbour D. V., Thompson E. B. Mapping the human glucocorticoid receptor for leukemic cell death. J Biol Chem. 1991 Jul 15;266(20):12976–12980. [PubMed] [Google Scholar]
- Norman M. R., Thompson E. B. Characterization of a glucocorticoid-sensitive human lymphoid cell line. Cancer Res. 1977 Oct;37(10):3785–3791. [PubMed] [Google Scholar]
- Northrop J. P., Gametchu B., Harrison R. W., Ringold G. M. Characterization of wild type and mutant glucocorticoid receptors from rat hepatoma and mouse lymphoma cells. J Biol Chem. 1985 May 25;260(10):6398–6403. [PubMed] [Google Scholar]
- Oliviero S., Struhl K. Synergistic transcriptional enhancement does not depend on the number of acidic activation domains bound to the promoter. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):224–228. doi: 10.1073/pnas.88.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheim R. W., Prevette D., Tytell M., Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Dev Biol. 1990 Mar;138(1):104–113. doi: 10.1016/0012-1606(90)90180-q. [DOI] [PubMed] [Google Scholar]
- Oro A. E., Hollenberg S. M., Evans R. M. Transcriptional inhibition by a glucocorticoid receptor-beta-galactosidase fusion protein. Cell. 1988 Dec 23;55(6):1109–1114. doi: 10.1016/0092-8674(88)90255-3. [DOI] [PubMed] [Google Scholar]
- PUCK T. T., MARCUS P. I., CIECIURA S. J. Clonal growth of mammalian cells in vitro; growth characteristics of colonies from single HeLa cells with and without a feeder layer. J Exp Med. 1956 Feb 1;103(2):273–283. doi: 10.1084/jem.103.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson M. K., Jr Measurement of growth and viability of cells in culture. Methods Enzymol. 1979;58:141–152. doi: 10.1016/s0076-6879(79)58132-4. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Perlmann T., Eriksson P., Wrange O. Quantitative analysis of the glucocorticoid receptor-DNA interaction at the mouse mammary tumor virus glucocorticoid response element. J Biol Chem. 1990 Oct 5;265(28):17222–17229. [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Rabindran S. K., Danielsen M., Stallcup M. R. Glucocorticoid-resistant lymphoma cell variants that contain functional glucocorticoid receptors. Mol Cell Biol. 1987 Dec;7(12):4211–4217. doi: 10.1128/mcb.7.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehmus E. H., Howard K. J., Janiga K. E., Distelhorst C. W. Immunochemical comparison of mutant glucocorticoid receptors and wild type receptor fragments produced by neutrophil elastase and chymotrypsin. J Steroid Biochem. 1987 Aug;28(2):167–177. doi: 10.1016/0022-4731(87)90373-6. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Identification of regulatory elements of cloned genes with functional assays. Methods Enzymol. 1987;152:704–720. doi: 10.1016/0076-6879(87)52075-4. [DOI] [PubMed] [Google Scholar]
- Rundlett S. E., Wu X. P., Miesfeld R. L. Functional characterizations of the androgen receptor confirm that the molecular basis of androgen action is transcriptional regulation. Mol Endocrinol. 1990 May;4(5):708–714. doi: 10.1210/mend-4-5-708. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ma J., Triezenberg S., Ptashne M. GAL4-VP16 is an unusually potent transcriptional activator. Nature. 1988 Oct 6;335(6190):563–564. doi: 10.1038/335563a0. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz L. M., Kosz L., Kay B. K. Gene activation is required for developmentally programmed cell death. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6594–6598. doi: 10.1073/pnas.87.17.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley C. H., Tomkins G. M. Isolation of lymphoma cell variants resistant to killing by glucocorticoids. Cell. 1974 Aug;2(4):213–220. doi: 10.1016/0092-8674(74)90013-0. [DOI] [PubMed] [Google Scholar]
- Simons S. S., Jr, Schleenbaker R. E., Eisen H. J. Activation of covalent affinity labeled glucocorticoid receptor-steroid complexes. J Biol Chem. 1983 Feb 25;258(4):2229–2238. [PubMed] [Google Scholar]
- Simons S. S., Jr, Thompson E. B. Dexamethasone 21-mesylate: an affinity label of glucocorticoid receptors from rat hepatoma tissue culture cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3541–3545. doi: 10.1073/pnas.78.6.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Carlstedt-Duke J., Weigel N. L., Dahlman K., Gustafsson J. A., Tsai M. J., O'Malley B. W. Molecular interactions of steroid hormone receptor with its enhancer element: evidence for receptor dimer formation. Cell. 1988 Oct 21;55(2):361–369. doi: 10.1016/0092-8674(88)90059-1. [DOI] [PubMed] [Google Scholar]
- Vanderbilt J. N., Miesfeld R., Maler B. A., Yamamoto K. R. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987 Jan;1(1):68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- Wrange O., Eriksson P., Perlmann T. The purified activated glucocorticoid receptor is a homodimer. J Biol Chem. 1989 Mar 25;264(9):5253–5259. [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Gehring U., Stampfer M. R., Sibley C. H. Genetic approaches to steroid hormone action. Recent Prog Horm Res. 1976;32:3–32. doi: 10.1016/b978-0-12-571132-6.50008-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]
- de Cuevillas F., Lachman H. Transfection of a T-cell line with neo increases dexamethasone cytotoxicity. Leuk Res. 1990;14(7):623–627. doi: 10.1016/0145-2126(90)90017-4. [DOI] [PubMed] [Google Scholar]