Abstract
Advanced polymeric biomaterials continue to serve as a cornerstone of new medical technologies and therapies. The vast majority of these materials, both natural and synthetic, interact with biological matter without direct electronic communication. However, biological systems have evolved to synthesize and employ naturally-derived materials for the generation and modulation of electrical potentials, voltage gradients, and ion flows. Bioelectric phenomena can be interpreted as potent signaling cues for intra- and inter-cellular communication. These cues can serve as a gateway to link synthetic devices with biological systems. This progress report will provide an update on advances in the application of electronically active biomaterials for use in organic electronics and bio-interfaces. Specific focus will be granted to the use of natural and synthetic biological materials as integral components in technologies such as thin film electronics, in vitro cell culture models, and implantable medical devices. Future perspectives and emerging challenges will also be highlighted.
Keywords: biomaterials, conducting polymers, interfaces, medical devices, flexible electronics
1. Introduction
Endogenous bioelectric signals play critical roles in a near-infinite number of ubiquitous biological processes such as energy harvesting, rapid communication, and inter-/intra-cellular synchronization. Specific examples include photosynthesis, vision, carbohydrate metabolism, neurophysiology, wound healing, tissue regeneration, and embryonic development. [1, 2] The foundational role of electronics in biology suggests several intriguing corollaries. First, nature must be able to design and synthesize biologically-derived materials and assemble those species into useful structures that can harvest, sense, transduce, and manipulate electrical signals. Second, synthetic electronic devices may be designed to precisely measure physical aspects of bioelectric processes such as ion flows and voltage gradients in biological systems; quantifying these phenomena provides insight into the underlying physiological mechanisms. Third, pathologies in biological function and deviations from homeostasis can be mitigated by supplying exogenous electrical cues. These assertions provide a framework that motivates the design of numerous technologies ranging from hybrid inorganic-organic electronic devices to electronically active medical implants. Materials selection and the subsequent quality of the biotic-abiotic interface play central roles in the prospective success and impact of many of these emerging technologies. This progress report will highlight recent progress in biomaterials-based electronics with an emphasis on the following topics:
Unique optoelectronic properties of biologically-derived materials.
Novel soft matter for interfacing synthetic devices with cells in vitro.
Electronically active biomaterials for implantable medical devices.
One unifying theme is the continuous interplay between naturally-derived materials and biology with synthetic engineered systems: this mutual interaction catalyzes scientific discoveries and drives technological innovation. This progress report is not intended to be an exhaustive review, but rather a brief summary that highlights recent progress with the specific aforementioned topics.
2. Biologically-Derived Materials as Active Components in Electronic Devices
Millenia of evolution have stimulated living organisms to design, synthesize, and utilize materials with unique optoelectronic properties for a variety of important biological functions. Materials such as pigments and dyes play critical roles in light-harvesting, photo-conversion, charge transport, and free radical scavenging. The specialized function of these compounds mandates specific electronically active properties, which are usually derived from polyconjugated or polyaromatic motifs. Consequently, the unique electronic signatures of highly evolved biological materials can be harnessed into device architectures. Materials that can be isolated from the native environment and repurposed into thin film technologies may serve as useful device technologies (Figure 1).[3] This section presents an emerging theme of recapitulating biologically-derived semiconductors, insulators, and metals as components in electronic devices.
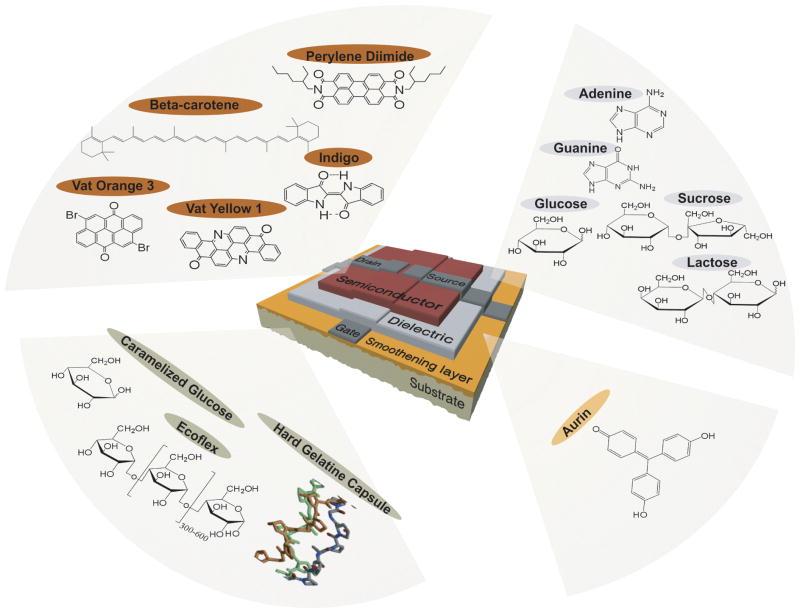
Figure 1.
Naturally-derived biomaterials for organic electronic devices. Virtually all of the essential components of the organic thin film transistors can be composed of naturally-derived biomaterials. Semiconducting active layers can be fabricated from a variety of small molecule dyes and pigments. Hydrophilic biopolymers are perfectly suitable for gate dielectrics while a wide range of polymers, both natural and synthetic, are appropriate for use as flexible device substrates with additional capabilities such as compostability and bioabsorbability. Reproduced from M. Irimia-Vladu, P. A. Troshin, M. Reisinger, L. Shmygleva, Y. Kanbur, G. Schwabegger, M. Bodea, R. Schwödiauer, A. Mumyatov, J. W. Fergus, V. F. Razumov, H. Sitter, N. S. Sariciftci, S. Bauer, Advanced Functional Materials 2010, 20, 4069. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
2.1. Natural Semiconductors
2.1.1. Eumelanins
Eumelanins are a class of optoelectronically active natural molecules synthesized from the oxidative polymerization of 5,6-dihydroxyindoles.[4] The precise structure of eumelanins has yet to be elucidated. Rather, eumelanins are macromolecular species with a high degree of heterogeneity at the molecular and surpramolecular length scales.[5, 6] The broadband absorption and efficient photon-phonon coupling of these pigments suggests that these molecules play critical roles as photo-protective agents against UV irradiation.[7] This photo-protective property is thought to be derived from the primary chemical structure rather than the supramolecular organization into nanometer scale assemblies.[6] Additional speculation on the role of eumelanin as a protective agent is also indirectly supported by studies that identify the ability for eumelanins to bind heavy cationic metals.[8] The interest in melanins, both natural and synthetic, has blossomed since the initial discovery of its amorphous semiconducting behavior that was described in a seminal paper by McGinness et al.[9] The remaining discussion for this article will focus exclusively on eumelanins, which will be referred to as simply “melanins” to eliminate ambiguity. Although the specific mechanism of charge transport in the report by McGinness was not addressed explicitly, recent evidence suggests that hydration-mediated proton transport is the dominant process that governs conductivity in melanins.[10]
Melanins are extremely difficult to process into form factors that might be useful for device applications because of the lack of solubility in many organic solvents. Potential limitations on melanin processing have been resolved by implementing a number of creative techniques. Solution processing of melanin into device-quality films is made possible by using aqueous ammonia preparations.[11] This process can be adapted to fabricate metal-insulator-silicon (MIS) devices that utilize melanin as the insulating component.[12] Melanin-based MIS devices exhibit charge transport mechanisms and charge carriers that depend on the hydration state of the melanin thin films. In air, charge conduction in melanin films is based on electron/hole transport through charge trapping. Under vacuum, charge transport is dominated by the drift of ions. The hydration-dependent charge transport mechanisms could serve as a signal transduction mechanism for use in biosensors.
The preparation of melanin films by electrodeposition represents an alternative fabrication strategy to solution processing.[13] Conformal melanin-like films can be fabricated through the electrochemical polymerization of 5,6-dimethoxyindole-2-carboxylic acid (DMICA), a prevalent monomeric precursor found in naturally-occurring melanin.[14] Thin films composed of poly(5,6-dimethoxyindole-2-carboxylic acid) (PDMICA) exhibit unique properties including an electrochromic effect, a highly porous microstructure, and a degree of crystallinity that has not yet been observed in naturally occurring melanins (Figure 2). Electrochemical processing of melanin-like materials may enable the deposition of these films to non-planar geometries through electroforming. In situ electrochemical processing may be used to integrate melanin films with viable cells and tissues directly.[15, 16]
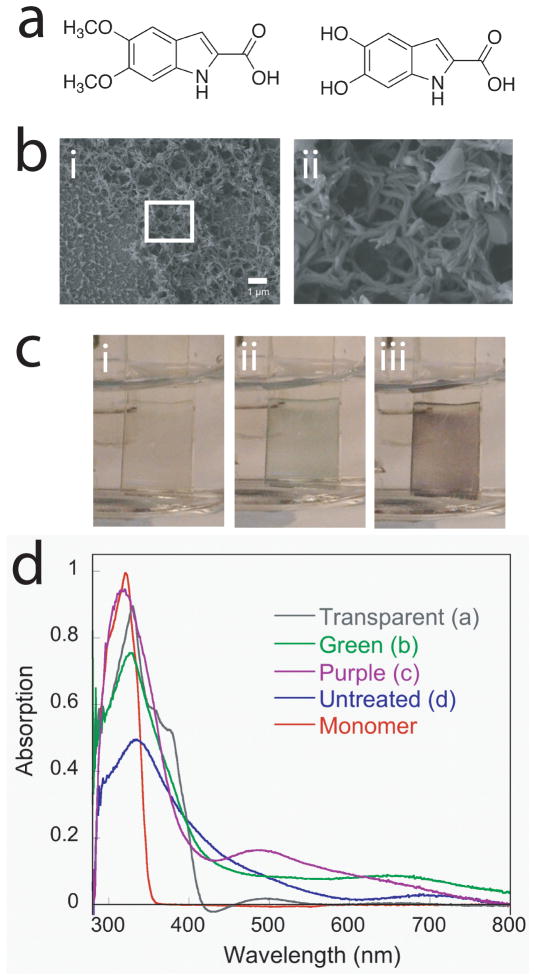
Figure 2.
Melanin-like films exhibit unique optoelectronic properties. (a) The monomer used for the electrochemical synthesis of melanin-like films (5,6-dimethoxyindole-2-carboxylic acid, DMICA, left) closely resembles one of the key components of native eumelanins, 5,6-dihydroxyindole-2-carboxylic acid (DHICA, right). (b) Scanning electron micrographs of melanin-like films indicate nanophase morphology that may be useful as a structure to increase electrode surface area or promote cell adhesion. (c) Electrochromic PDMICA films are transparent when subjected to (i) negative voltages, (ii) green near 0 V, and (iii) purple above 0.5 V, which are confirmed by (d) shifts in the UV-vis spectra. Adapted with permission from L. K. Povlich, J. Le, J. Kim, D. C. Martin, Macromolecules 2010, 43, 3770. Copyright 2010. American Chemical Society.
Melanins may prove to be a critical material in the long-term goal of interfacing viable cells, such as neurons, with synthetic devices. In this context, substrates fabricated using synthetic melanins enhance neuritogenesis in PC12 cells.[17] Melanin films prepared by spin coating promote neurite extension from PC12 cells that exceeds the length of neurites extended from PC12 cells cultured on collagen films. The combination of enhanced biocompatibility, in situ cellular integration, and appreciable electrical conductivities suggests that melanin is advantageous for hybrid devices such as biosensors. Furthermore, melanin films degrade in vivo, which is a potentially attractive attribute for future in vivo applications. [17] Although the precise mechanism of in vivo melanin degradation has yet to be fully elucidated, the likely mechanisms is free radical-mediated decomposition due to exposure to reactive oxygen species.[18, 19]
2.1.2. Other Natural Pigments
Aside from melanin, there are a number of recent reports that have detailed the charge transport in a variety of naturally-derived, small molecule, semiconducting, biological compounds. Carotenoids are poly(acetylene)-like natural materials that are produced by plants and bacteria. The natural functions of carotenoids include protection against oxidative species, pigmentation, and light harvesting for photosynthesis. The polyconjugated structure of this class of compounds suggests that the natural electronic activity of derivatives could be repurposed as an active semiconductor material for organic electronic devices. A variety of carotenoids can be solution processed into thin film transistors including β-carotene, bixin, astacene, torularhodin, and isorenieratene.[20] Devices with active layers consisting of β-carotene and bixin with thicknesses of 20 nm exhibit relatively low hole mobilities of 10−7 and 10−6 cm2-V−1-sec−1, respectively. Although these mobilities are not immediately useful for commercial organic electronic device application, the observation of field-effect responses in naturally-derived small molecule semiconductors is intriguing. Silicon-based photovoltaics can utilize β-carotene as an active layer in hybrid organic-inorganic devices as well.[21]
Indigo is another biomolecule that exhibits adequate performance in biomaterials-based thin film organic transistors. Indigo is a naturally-occurring, small molecule pigment that is produced by plants such as Indigofera tinctoria and Isatis tinctoria. There is a high degree of intermolecular hydrogen bonding, which is responsible in part for the notable high melting point of indigo. While strong aromatic interactions dramatically reduce the solubility and prospects for solution processing, indigo can be incorporated into thin film devices by thermal evaporation. Organic thin film transistors based on indigo active layers are completed using aluminum-oxide dielectrics and gold source-drain electrodes.[22] These devices exhibit ambipolar behavior with hole mobilities and electron mobilities as high as 10−2 cm2-V−1-sec−1 and threshold voltages between −1.5 to −3 V (holes) and 4.5 to 7 V (electrons), respectively. The ambipolar device characteristics are attributed to HOMO/LUMO energy levels of −5.5 eV and −3.8 eV, respectively. The resulting band gap of 1.7 eV can be verified through cyclic voltammetry and optical absorption measurements.
2.1.3. Proteins and Peptides
The vast majority of naturally-occurring proteins are electronic insulators. However, some polypeptide sequences have evolved to exhibit reasonable intrinsic electronic conductivities. Bacteria are known to secrete proteinaceous pilin filaments that are on the order of 3–5 nm in width and up to 10 μm long. These naturally-derived protein-based nanostructures are composed primarily of insulating polypeptides. However, pilin nanofilaments secreted from the bacterium Geobacter sulfurreducens exhibit conductivities of up to 5 mS-cm−1.[23] Furthermore, biofilms composed of this species exhibit significant electrical conductivities across distances of up to 1 cm. Conductive biofilms have also been observed in Shewanella oneidensis,[24] but biofilms produced from other species including Pseudomonas aeruginosa and Escherichia coli are electronic insulators. Another curious observation is that the electrical conductivity of G. sulfurreducens biofilms is strain-dependent. Specifically, strains of G. sulfurreducens that produce higher densities of pilin nanostructures exhibit comparatively higher electrical conductivities. This observation supports the hypothesis that bacterial pili are a dominant contributor to the unique electrical properties of these biofilms. The electrical conductivity within pilin nanofilaments is dependent upon external environmental conditions such as pH. This observation suggests that the structures can be doped with protons and serve as a charge carrier in organic thin film electronics.[25] Furthermore, the charge transport mechanism in biopolymers found within naturally-occurring pili is likely a combination of hopping and intrinsic metallic transport, a conclusion which is supported by temperature-dependent electrical conductivity measurements. The molecular structure of pili nanofilaments in G. sulfurreducens has not yet been fully elucidated. It is hypothesized that the aromatic amino acid residues found in pilin monomers self-assemble to form motifs that minimize the intermolecular distance. These interactions promote charge delocalization, which may ultimately be responsible for the observed metallic-like conductivity. X-ray diffraction studies of purified pili indicate π-orbital overlap, which in turn suggests strong π-π interchain stacking in aromatic residues such as phenylalanine or tyrosine. Bacteria pili with high aspect ratios are known to withstand extreme mechanical stress. Gram-positive bacteria derive this resilience from the coordination of β-sheet subunits that are organized through the intramolecular interactions between two lysine-asparagine isopeptide bonds.[26] Another natural protein that exhibits comparable mechanical strength is spider silk, which exhibits highly ordered defect-free protein structures formed by self-assembly. Highly ordered dragline spider silk has been found to transport phonons efficiently.[27] In fact, spider silk and copper have been found to exhibit comparable thermal conductivities.
Furthermore, applying uniaxial strain further increases the thermal conductivity. Taken together, these data suggest that protein structures may be leveraged for the coordinated manipulation and transport of both electrons and phonons. Furthermore, improving the extent of intramolecular organization and reducing the defect density in organic materials can uniformly increase the mechanical stiffness in conjunction with enhancing the efficiency of electron/phonon transport.
Semiconducting structures found in nature can serve as a template for the design of synthetic electronic components that are comprised of biologically-derived soft matter. The observed π-π stacking motifs in bacterial pili that form by self-assembly are found in a variety of other proteins including amyloid fibrils.[28] Synthetic dipeptides composed of diphenyalanine spontaneously assemble into nanotubes with a hexagonal crystal structure.[29] Furthermore, the self-assembly of diphenyalanine can be modulated to form either 1-dimensional nanotubes or zero-dimensional quantum dots. This process is reversible and is largely dependent upon the extent of allowable hydrogen bonding. In aqueous environments, the formation of extensive hydrogen bond networks biases the arrangement of peptides into nanotube structures. Conversely, exposure of diphenyalanine peptides to methanol abolishes the extensive hydrogen bond network and promotes hydrophobic interactions. Intermolecular coupling of aromatic groups promotes the formation of peptide quantum dots as opposed to complete dissolution. Modulating the extent of hydrogen bonding in polypeptides is a useful technique for controlling the macromolecular and macroscopic properties in protein biomaterials. [30, 31] In the case of diphenyalanine, the zero-dimensional and one-dimensional structures produce unique physical properties including quantum confinement effects. In contrast to diphenyalanine, dipeptides composed of two heteroaromatic residues (phenylalanine-tryptophan) did not form peptide nanotubes or quantum dots by self-assembly.[32] Therefore, the spontaneous formation of these protein nanostructures with unique optoelectronic signatures is highly sensitive to the molecular composition. The self-assembly process can be programmed precisely by engineering the composition of the solvent or the peptide composition of synthetic protein nanomaterials. For example, the physical dimensions of peptide quantum dots can be tuned by adjusting the hydrogen bond density in the peptide solution. Modifying the composition of the peptide chain or altering the profile of the peptide components in the ensemble can also yield zero-dimensional organic quantum dots with a wide range of sizes. From a clinical translation perspective, the use of protein-based quantum dot materials is attractive because of the potential for accelerated approval for use as medical devices for sensing or therapeutic technologies.
The intrinsic optoelectronic activity of many types of biologically-derived soft matter is attributed to aromatic components. For example, aromatic peptides such as tryptophan and tyrosine along with their derivatives serve as the foundation for unique structure-property-relationships in naturally-occurring macromolecular networks. In the case of melanins, the disordered macromolecular superstructure confers broadband optical absorption and photo-protective properties. In the case of bacterial pili and peptide nanomaterials, strong aromatic interactions promote electron delocalization and increased electrical conductivity. Electron delocalization is also present in the conjugated structures found in pigments and dyes. The subsequent optoelectronic properties are critical for many essential biological processes. However, they can also be repurposed to fabricate synthetic electronic devices for specific applications.
2.2. Naturally-derived Insulating Materials
2.2.1 Hydrated Materials
In general, the use of electronically active devices for biomedical applications implies that these systems will be eventually exposed to aqueous environments. From one perspective, this inevitability suggests that device performance must not be adversely affected in a negative manner by hydrated conditions. This recognition motivates the design of materials that can operate stably in aqueous environments. Beyond encapsulation, generally accepted strategies include the fabrication of low-voltage transistors[33] and the synthesis of hydrophobic semiconductors that can resist hydration and subsequent doping from aqueous ionic species.[34] However, another possible strategy may be to embrace the aqueous phase as an active component in device design. For example, the highly polar nature of liquid water may be harnessed for use as a gate dielectric for low-voltage organic thin film transistors.[35] Top gate devices can be fabricated using semiconducting layers composed of either rubrene with gold source-drain electrodes or poly(3-hexylthiophene) (P3HT) with either gold or platinum source-drain electrodes. In both cases, pure water (resistivity of 18 MΩ-cm) comprises the gate dielectric. These devices achieve hole mobilities of up to 6 × 10−2 cm2-V−1-sec−1 with Ion-Ioff ratios of up to 8 × 104, which are a direct consequence of the low frequency capacitance in water-gated thin film transistors (Cwater = 3 μF-cm−2). The observed transistor behavior can be attributed almost exclusively to operation under field-effect as opposed to alternative electrochemical processes. This is because the devices operate at very low voltages (~0.5–0.6 V) that are insufficient for electrolysis (1.23 V). The utilization of aqueous media as a critical device component is indicative of the interest in embracing materials that are ubiquitous in biological systems. The continuation of this trend will work to bridge the gap between synthetic devices and materials that are utilized to support the functions of living organisms.
The ever-present aqueous environment in biological systems presents a convenient source of ions that can be harnessed by active device components. There are a wide range of naturally-derived hydrophilic biopolymers that play critical roles in biological function. Heparin sulfates are essential for natural regulation of thrombogenicity[36] while hyaluronic acid, alginate, dextran, lectins, cellulose, chitin, chitosan, and the corresponding derivatives play instrumental roles in defining the physical properties of the extracellular matrix in various microenvironments.[37–42] Both the extreme hydrophilicity and the ability to process these naturally-derived biopolymers into nanofibers[43–46] suggest that this class of materials may be used as a matrix for ionic conduction. Specifically, when coupled with the intrinsic populations of protonic species at physiological pH, carboyhydrate-based biopolymers may serve as the foundation for active electronic devices. Chitin, the polymeric form of the glucose derivative N-acetylglucosamine, can be processed into thin films composed of nanofiber matrices. [47] Thin films of chitin can be utilized as a proton conducting medium. Furthermore, the degree of protonic transconductance can be modulated by applying an external field in a mode that is analogous to gating of a thin film transistor.[48] Devices are fabricated by processing dilute aqueous solutions of chitin into networks with random nanofiber orientation. Nanofiber matrices can be aligned into ordered superstructures by replica-molding techniques.[49] Devices with a channel length of 8.5 μm exhibit effective proton mobilities of 5 × 10−3 cm2-V−1-sec−1 with maximum drain currents on the order of nA. These characteristics are achievable by operating the device with a source-drain bias of −1 V and a gate bias with a field strength of 5 × 107 V-m−1. The ability to fabricate biopolymers into thin film device architectures suggests that fundamental studies on charge transport may be studied in a comprehensive manner. For example, similar device geometries may elucidate electron transfer mechanisms in DNA; either in isolated single DNA molecules or multi-strand ensembles.[50, 51] The transformation of biologically-derived protonic conductors from the native state into synthetic device architectures may also eventually lead to the fabrication of unique signal transduction modalities for applications in biosensing.
2.2.2. Polypeptides
Electrically insulating biopolymers may be suitable for other device components. The monodisperse nature of isolated proteins suggests that they may be useful for the fabrication of high fidelity dielectric films. Albumin is an ubiquitous protein that is present in large quantities in many organisms. Aqueous albumin solutions isolated from chicken eggs can be rapidly processed into pristine films or crosslinked networks for use as gate dielectrics in thin film transistors.[52] A stepwise thermal annealing procedure induces denaturation and crosslink formation through disulfide coupling. The final films exhibit a dielectric constant of 5.3 to 6.1. Thin film transistors using pentacene or C60 as active layers and albumin dielectrics exhibit hole and electron mobilities that are comparable for the respective semiconducting materials. Leakage currents are relatively low at 10−1 nA at |VGS| = 25 V.
2.2.3 DNA as an Insulator
The application of proteins as biopolymers for dielectric components is advantageous because of the natural abundance. However, there is a virtually infinite amount of electronically insulating biopolymers that may be utilized as biologically-derived dielectric materials. The ideal gate dielectric would balance potential considerations of cost-effectiveness, biocompatibility, and performance. DNA is uniquely positioned as a naturally-occurring dielectric material from a device performance perspective. DNA has been previously used in non-conventional electronics such as an anionic templates for in situ metallization of gold nanowires.[53] However, DNA itself may also be used as a bulk material for the fabrication of thin films that serve as reliable gate dielectrics.[54, 55] Aqueous solutions of DNA can be prepared using hexadecyltrimethylammmonium chloride (CTMA) as a cationic surfactant additive. Thin film insulators are deposited on conductive indium-tin oxide (ITO) substrates using spin coating. DNA-CTMA films can then be crosslinked using poly(phenylisocyanate-co-formaldehyde) (PPIF) to create mechanically robust films that are highly resistant to downstream processes that employ organic solvents. The dielectric constant of crosslinked DNA-CTMA films is 5.4, which is sufficient for many applications in organic electronics.
2.3 Optically-Active Biopolymers
2.3.1. DNA in Optical Devices
The unique electronic structure of DNA also suggests that this class of biopolymers can be utilized as an active component in optical device engineering. Specifically, DNA films can be utilized in the dual role as a combination hole-transporting and electron-blocking layer in optical devices. The unique electron-blocking properties of DNA-CTMA composites are attributed to the relatively large disparity between the energy levels in the HOMO/LUMO with values of −5.6 eV and −0.9 eV, respectively. This energy band level profile can be leveraged to engineer quantum-dot based LED with high efficiencies, increased brightness, and reduced turn-on voltages.[56] DNA-CTMA layers have also been leveraged as hole-transporting/electron-blocking layers in the fabrication of solution processable multilayer white polymer organic LED (OLED).[57] OLED fabricated with DNA-CTMA films exhibit increased brightness and improved white-color stability.
DNA-based biopolymers have demonstrated utility in the fabrication of other optical devices including waveguides. High molecular weight (Mw = 8 000 000 Da, 12 000 base pairs) raw DNA extracts of approximately 4.1 μm in length were sonicated to yield low molecular weight products with lengths of 100 nm. Reducing the molecular weight of the DNA prior to film formation is a critical step in achieving a high degree of device uniformity. DNA-CTMA mixtures can be processed into thin films from solution and crosslinked using PPIF. These films are stable during thermal annealing processes that employ temperatures up to 175 °C and high vacuum. The completed waveguide features a DNA-CTMA core doped with Disperse Red 1. In addition to the compelling intrinsic electronic properties, DNA is an attractive naturally-derived biopolymer for applications in electronic devices from a processing perspective. Namely, DNA is a thermally stable organic compound that can withstand annealing temperatures that are commonly encountered during processing of flexible electronic devices. Furthermore, the composition and subsequent physical properties can be precisely controlled by sequence selection, chemical functionalization, and physical manipulation.
2.3.2. Proteins
Optically transparent biologically-derived materials can be fabricated into optoelectronic materials that may be of interest to the biomaterials community. Silk fibroin, a natural protein isolated from the Bombyx mori, can be processed from aqueous solutions into nanometer-scale structures for use as diffraction gratings or optical fibers.[58, 59] Silk films exhibit several desirable properties from a device fabrication perspective such as the ability to generate replica-molded features with sub-micron resolution and robust mechanical properties.[59] The aqueous stability can be modulated by controlling the extent of hydrogen bonding through solvent exposure. Optical transparency may be a key property of implantable biomaterials in order to facilitate minimally-invasive interrogation. One may envision the application of an implantable device that can be loaded with bioactive proteins that may function as in vivo biosensors. The optical signatures of the embedded proteins may be altered in response to biological processes associated with disease states, for example. The signatures can then be measured in real time using in vivo optical characterization at tissue-transparent near-infrared wavelengths. A variety of bioactive proteins can be stabilized in soft polymeric matrices composed of silk fibroin including glucose oxidase, lipases, and horse radish peroxidase. [60] Collectively, immobilized proteins retain adequate reactivity for over 10 months when stored at 37 °C. Glucose oxidase maintains almost complete activity in this time frame under the described conditions, which may be attributed to renaturation in the complex proteinacious microenvironment. As expected, the resulting activity is highly dependent upon the protein configuration of the silk fibroin matrix. A high fraction of the protein matrix is composed of β-sheet secondary structure, which is present throughout the storage time. The use of biologically-derived optically-active materials as substrates may eventually provide a suitable material framework for the fabrication of in vitro or in vivo biosensing technologies.[61] Natural proteins exhibit many advantages as a material for use in optoelectronically active device components. However, many of these properties are not limited to the existing set of naturally-derived biopolymeric materials. There is a broad range of synthetic polymers that exhibit similar enabling properties such as mechanical resiliency, optical transparency, compatibility with micron- and sub-micron scale fabrication techniques, and biodegradation on clinically relevant time scales.[62–73]
2.3.3. Naturally-Occurring Metals
There is a broad spectrum of metal alloys derived from biological components that may serve as electrical conductors in the fabrication of biomaterials-based electronic implants. Magnesium is a naturally-occurring metal that composes 21 to 28 grams in the average human body. [74, 75] Magnesium is biodegradable and serves as the foundational element in many alloys intended for temporary medical implants including applications in endovascular devices[76] and orthopedics.[77] Magnesium may serve as a useful material for temporary high strength implants including biodegradable stents and orthopedic screws. One drawback to medical devices composed of pure magnesium is the evolution of hydrogen upon degradation. Degradation byproducts can form gas pockets that cause local tissue damage.[78] Although the primary component of the gas pockets has been assumed to be hydrogen, recent evidence suggests that this may not be the case because hydrogen gas can diffuse very rapidly through soft tissues. Regardless of the molecular composition, the evolution of gaseous pockets is not a desirable consequence of metal biodegradation. This has motivated the design of biodegradable metals composed of magnesium alloyed with calcium and zinc.[79, 80] Beyond high strength applications, magnesium may serve as a bioabsorbable material for use in electrically conducting device components such as leads, interconnects, antennae, or electrodes in biodegradable electronic devices.[81, 82] Other biodegradable metals include iron-based alloys, which have been pursued for applications in endovascular devices.[83, 84] In virtually all medical device applications, the biodegradation of metals will transform macroscopic components into nanoscale particles. Given this inevitability, the potential toxicity of metal-oxide nanomaterials must also serve as a consideration for material selection.[85] The use of novel magnesium-based alloys for implantable medical devices has been reviewed extensively elsewhere.[86]
3. Polymeric Biomaterials as In Vitro Interfaces
Biologically-inspired electronic materials can serve as an enabling technological component for studying in vitro models that use bioelectric communication. Such processes extend beyond obvious bioelectric phenomenon such as neuronal signaling and coordination of cardiac function. For example, cell migration in wound healing relies upon the generation of local electric field gradients.[2, 87] Fundamental processes such as cell adhesion, proliferation, migration, and differentiation may be elucidated by fabricating artificial bioelectric cell-material constructs to test emerging hypotheses.[88, 89] Additional insight into disease progression and intracellular signaling may also be facilitated through careful design and implementation of these systems. Electronically active polymers, both natural and synthetic, play a central role in the design, fabrication, and validation of in vitro interfaces for cell culture models.
3.1 Dynamic Cell Culture Models
3.1.1. Bioelectric Control over Adhesion and Migration
Cell adhesion and migration are critical processes that underpin highly complex supercellular progressions involved in embryonic development, wound repair, and tissue pathologies. There are countless examples in which passive materials are used to study cell adhesion and migration including micropatterning of proteins and the manipulation of substrate structure. [65, 90–98] The redox states of conducting polymers can also be used as a method to actively modulate cell adhesion, which may be useful in dynamic patterning of co-cultures.[99] Poly(3,4-ethylenedioxythiophene) (PEDOT) derivatives can be used as electroactive materials for programmed adhesion of cultured mammalian cells.[100] The key innovation in this technique is the ability to utilize redox-responsive polymeric films that disintegrate rapidly to release the super-positioned cells. This process occurs after the application of 1 V to cell-seeded PEDOT films that can liberate cells in the absence of enzymatic treatments. Furthermore, the release process does not negatively impact cell viability or compromise the integrity of the cell membrane. A similar approach may be used for the rapid release of therapeutics from conformal films through the application of voltages that do not reduce the bioactivity of the compounds.
Cell adhesion is also highly sensitive to the ionic species that may be integrated into conducting polymer films. Poly(pyrrole) (PPy) can be used as a substrate for the attachment and maintenance of rat fetal neural stem cells. This capability is directly dependent upon the molecular composition of the counterion that is co-deposited with the PPy films.[101] Neural stem cells cultured on PPy films with dodecylbenzenesulfonate (DBS) produce a morphology and phenotype that best approximates cells cultured on standard tissue culture polystyrene (TCPS). Oxidized PPy films that incorporate alternative anionic species (chloride, perchlorate, tosylate) exhibit poor cell attachment and limited viability, which is likely attributed to differential protein adsorption.[102, 103]
PPy has been extensively used as an electronically active polymer for the electrical stimulation of neuronal lineages. Electrical stimulation of PC12 cells cultured on PPy surfaces enhances in vitro neuritogenesis.[104] The dominant in vitro biocompatibility profiles of PPy also extend to in vivo models. PPy films demonstrate exceptional biocompatibility for potential use as a neural interface material, a topic that will be discussed more in-depth later.[105] Briefly, microfabricated PPy films implanted into the cerebral cortex of a murine model exhibit gliosis and macrophage responses that are comparable to control materials composed of poly(tetrafluoroethylene) films. In addition to exhibiting intrinsic biocompatibility, PPy exhibits unique physical properties that are widely known to the microdevice community.[106] Specifically, PPy has been used as a material in electronic actuators for a variety of applications[107] including medical devices.[108] More recently, the electric actuation of PPy has been repurposed as a method to create dynamic mechanical environments for specific in vitro cell culture experiments. Cell monolayers cultured directly on PPy films can be stimulated in vitro using microscale mechanical perturbations with a high degree of spatial precision. In one such demonstration, renal epithelial cells are cultured on micropatterned PPy substrates and selectively actuated.[109] Substrates with dynamic mechanical actuations increase intracellular concentrations of calcium in Madin-Darby canine kidney (MDCK) epithelial cell lines compared to unstimulated controls. Activation at −1 V vs. Ag/AgCl results in a perpendicular expansion of 1 μm or almost 9% in the z-direction of the 11 μm thick PPy films. A high percentage of the MDCK cells cultured on PPy can be exposed to mechanical stimulation by patterning active PPy areas into linear arrays of strips approximately 100 μm in thickness. This spacing ensured that MDCK cells, which are approximately 50 μm in diameter, are subjected to interfaces on the x-y plane between the static substrate and the electroactive PPy films that expand in the z-axis. This cell culture model is amenable to microfabrication techniques, which enables integration of this stimulation device with other in vitro assay systems.[110] Furthermore, the ability to fabricate large arrays enables simultaneous testing of control conditions. Finally, device arrays may be fabricated into networks with fine tuned spatial control and individually addressable microactuator components. This advancement will unlock a large parameter space for subsequent studies including phenotype-specific stimulation, actuation in homogeneous cell populations, and uniform in vitro culture conditions with variable magnitudes, strain rates, and frequencies.
3.1.2. Electronic Controlled Release
Electronically active polymers may be fabricated into more sophisticated structures that can be used to control the spatiotemporal presentation of potent soluble factors. In essence, this approach employs controlled release strategies that are empowered by sophisticated materials design. Initial demonstrations of this overarching approach can deliver soluble nerve growth factor (NGF) from PPy films using biotin-avidin conjugates.[111] NGF-loaded PPy films stimulated with long duration times release NGF using a mechanism based on ion exchange. Furthermore, the eluted NGF retains bioactivity as assessed by enhanced neuritogenesis in cultured PC12 cells. Other strategies utilize controlled release mechanisms based on devices with distinct structural components. PEDOT can be doped with poly(styrenesulphonate) (PSS) and processed into microstructures that serve as systems to deliver charged small molecule neurotransmitters via electrophoretic transport. Neurotransmitters used in this study include glutamate, aspartic acid, and γ-amino butyric acid (Figure 3).[112] The general device structure is composed of the following components: (1) electrolyte source; (2) electrolyte target; (3) anode; and (4) cathode. These components are fabricated into a variety of geometries and packaged using several widely accepted encapsulation strategies. However, the mechanism for delivery of neurotransmitters through the device is conserved. These devices can produce zero-order release kinetics of small molecule neurotransmitters, the rate of which depends upon the magnitude of the applied voltage along with the mass and net charge of the molecule. The delivery of bioactive small molecule neurotransmitters can be verified by intracellular calcium recordings in vitro. In addition to representative in vitro models, this technology can be potentially utilized as an implantable device therapy. In vivo validation of glutamate controlled release was verified by measuring the auditory brainstem response in the cochlea (Figure 3). Device-based therapies that use electroactive controlled release are particularly attractive because the rate of therapeutic release can be precisely controlled through electrochemical methods. These materials may also be compatible with previously established remote-controlled release implants.[113]
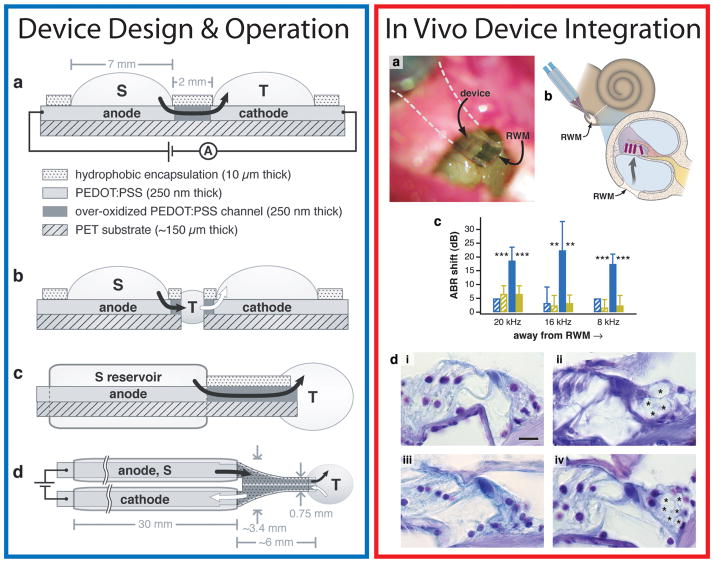
Figure 3.
Microfabricated organic electronic devices for precise delivery of neurotransmitters to modulate mammalian sensory function in vivo. The structure and principle of device operation are detailed at left. (a, left) The side view of the planar device used in small molecule neurotransmitter transport studies is shown. The black arrow indicates the flow of neurotransmitters from the source electrolyte, S, through the anode and over-oxidized channels, and finally out into the target electrolyte, T. (b, left), Side view showing the developmental progression from the planar device to the planar device with intermediate electrolyte (salt bridge). The white arrow indicates the flow of cations from T into the cathodic electrolyte. (c, left) The side view of the encapsulated device is shown with the arrow indicating ion flow. ( d, left) The top view of the encapsulated device, showing both electrolyte chambers and the requisite target system, T. The electrolyte reservoir tubes are 2 mm in outer diameter. The in vivo validation of the device is shown at right. (a-b, right) Briefly, the device is placed on the round window membrane of a guinea pig. The electronically activated release of neurotransmitters produces (c, right) a detectable shift in the auditory brainstem response. (d, right) The stimulated release of neurotransmitters can produce excitotic-induced damage to auditory dendrites (indicated by asterisks, ii, iv) compared to controls (i, iii). Adapted and reprinted by permission from Macmillan Publishers Ltd: Nature Materials ( D. T. Simon, S. Kurup, K. C. Larsson, R. Hori, K. Tybrandt, M. Goiny, E. W. H. Jager, M. Berggren, B. Canlon, A. Richter-Dahlfors, Nature Materials 2009, 8, 742), copyright (2009).
3.2 Hybrid Cell-Device Interfaces
3.2.1. Artificial Synapses
Electronically active conducting polymers have an established record of in vitro biocompatibility with neuronal lineages. The unique intrinsic ability of conducting polymers to create intimate interfaces with cells is a property that can be leveraged in the design of synthetic models for neuronal function. The key structural component of the nervous system is the synapse. The essential characteristics of the natural synapse include: (1) the storage of neurotransmitters that are released at the precise moment of electrical excitation; (2) a high degree of spatial localization across the synapse; (3) the ability to replenish neurotransmitter reservoirs after a brief refractory period. The natural properties of the synapse serve as a blueprint for the design and fabrication of synthetic synapses in vitro. Conducting polymers serve as a key material that can mimic many essential properties of the native synapse. First, conducting polymeric matrices can serve as reservoirs for small molecule neurotransmitters. Second, conducting polymers are amenable to voltage-activated release of embedded therapeutic payloads.[114, 115] Lastly, these materials are compatible with microfabrication techniques which may be used to produce microstructures that are on the same length scale as individual cells. These advantages of synthetic conducting polymers can be recapitulated into devices that function as synthetic interfaces.[116] PEDOT:PSS electrodes can be fabricated into microdevices to measure the release of neurotransmitters from single cells. Micropatterned PEDOT:PSS electrodes are fabricated into planar geometries and patterned using a combination of fluoropolymer and photoresist layers. The final electrode exhibits a surface area of 30 μm2 and can be used as a biosensor to detect norepinephrine release from single chromaffin cells. Oxidation of norepinephrine into the ortho-quinone produces an amperometric signal at the electrode surface, which can be measured accurately. Mechanical stimulation of the soma using a micropipette was used to trigger spontaneous norepinephrine release, which was recorded by the underlying electrodes. Individual neurotransmitter release processes were measured to have currents between 100 pA to 1 nA with time intervals of 10 ms, thereby producing a total charge between 1 and 10 pC per event. The RMS noise for these electrodes is on the order of 15 pA, which produces sufficient signal-to-noise ratios for the proposed application.
Artificial microfabricated synapases could be interesting in the context of designing hybrid biotic-abiotic computing devices. One futuristic idea is the ability to design computational architectures based on traditional CMOS-based integrated circuits that are supplemented with living neuronal cultures. The long-term goal will be to fabricate logic devices that also exhibit advantages of embedded plasticity. Viable neuronal cultures could therefore play an important component in reconfigurable computational networks. Although there are obvious technical hurdles along with challenges in complexity that are associated with the fabrication of practical devices, the notion of a hybrid logic network is intriguing. At the least, such devices could be used to elucidate fundamental computational properties of simple neuronal abstractions in vitro. More practical aspects of artificial electrochemical synapses may be leveraged for use in neural interfaces such as retinal prosthetics.[117] For example, it may be possible to fabricate implantable devices with integrated neurotransmitter reservoirs that can be activated by optical or electrical stimuli. The newly released neurotransmitters can then stimulate the underlying ganglia. Spatiotemporal controlled release of neurotransmitters to the ganglia can serve as a viable strategy to restore vision in diseases of the retina such as macular degeneration or retinitis pigmentosa.[118]
3.2.2. In Vitro Disease Models
Electronically active synthetic biomaterials will continue to play an emerging role in flexible electronics for in vitro models of neurodegenerative diseases. Fabricating flexible electronic components using biocompatible materials will serve as a foundation for next-generation in vitro tissue culture systems. One alluring concept is the ability to monitor the real time neuronal activity in brain slices that undergo dynamic mechanical perturbations. In vitro models of mechanical insults may be engineered to simulate those that are attributed to the onset of traumatic brain injury.[119, 120] Devices used as interfaces for these studies must exhibit a unique intersection of physical properties including maintenance of electrical conductivity under high mechanical strains.[121, 122] Other practical challenges include efficient packaging and encapsulation of electronic components. Fabrication strategies can produce arrays of 28 electrode structures, each with an area of 100 × 100 μm2, and bulk stiffness of approximately 2 MPa. Electrode arrays can be used to both stimulate and measure spike trains in hippocampal tissue slices that are exposed to dynamic biaxial mechanical strains. The frequency of spontaneous activity increases as the equibiaxial strain gradually increases from 0% to 10%. Another notable observation is the presence of a significant hysteresis. Namely, after the strain was reset to 0%, the frequency of the spontaneous firing remained elevated (Figure 4).[123] These data suggest that large mechanical strains may lead to irreversible effects on neuronal function. All polymer flexible electrode arrays can be fabricated using other electrically conductive elastomers including poly(dimethylsiloxane) (PDMS) composites.[124] Microfabricated PDMS membranes can be converted into conducting elastomeric substrates by integrating graphitic carbon or PEDOT:PSS structures into the polymeric network. The resulting microelectrode arrays can be used to record local field potentials that are generated from in vitro cultures of hippocampal brain tissue and cardiomyocytes.
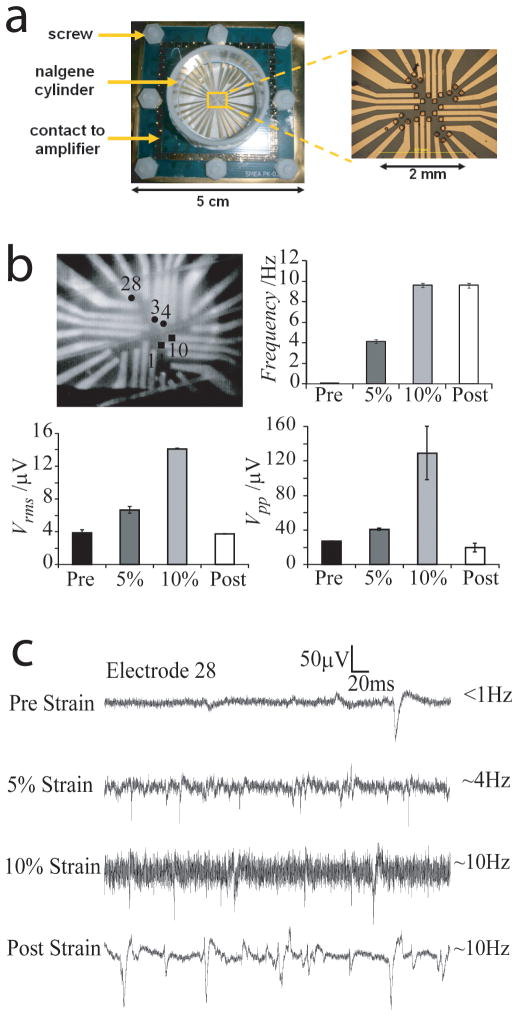
Figure 4.
Elastomeric electronics for in vitro cell-device interfaces. (a) All polymer flexible microelectrode arrays with 28 microelectrodes and two reference electrodes were fabricated using a combination of soft lithography and electrodeposition for use in measuring spontaneous activity in neuronal lineages under biaxial deformation. ( b) Photograph of an organotypic hippocampal tissue slice on microelectrode arrays. Recording electrodes are indicated by circles with the electrode number indicated. Stimulating electrodes are indicated by squares. Frequency of spontaneous activity, Vrms, and Vpp before stretching (Pre), at 5% and at 10% equibiaxial strain, and after relaxation (Post); (c) Representative traces of spontaneous activity recorded on electrode 28 before stretching (Pre), at 5% and at 10% equibiaxial strain, and after relaxation (Post). The frequency of spontaneous activity is indicated. Adapted from O. Graudejus, B. Morrison, C. Goletiani, Z. Yu, S. Wagner, Advanced Functional Materials 2012, 22, 640. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
3.3 Nanomaterials
Perhaps the great challenges in applying flexible electronic devices to the study of in vitro cell culture systems are related to the design of materials that produce robust biotic-abiotic interfaces with high sensitivity. Maintaining a high degree of electrode sensitivity is essential, especially when measuring single unit action potentials with signals on the order of μV. Perhaps just as important is the necessity to preserve the physical characteristics of the electrode materials. Hence, there is a dual mandate in electrode design: (1) to protect electronic devices from the corrosive, high salinity environments encountered both in vitro and in vivo; (2) to maintain an intimate connection with the cells or soft tissue targets that are intended to be measured. These requirements are somewhat antithetical. The paradoxical nature arises from the need to protect devices from biological media, while also promoting invasive integration with viable cells and tissues.
Encapsulation and packaging of active electronic components is an obvious challenge that continues to be incorporated in the design of systems intended for operation both in vitro and in vivo.[125, 126] Long-term electrode stability is another challenge that has motivated the design of novel materials.[127] In the context of medical implants, exotic electrode materials including iridium oxide and conducting polymers can overcome limitations related to charge injection at the interface.[16, 128–132] Recent advances in this specific arena have been discussed in detail elsewhere.[133–135] Electrically conducting polymers and other soft materials may also achieve the dual mandate by promoting tissue-device integration at the biotic-abiotic interface.[136] The continuous trend in device miniaturization may improve the prospects for using synthetic microfabricated devices both in vitro and in vivo. The next generation of in vitro sensors may use electronically active nanomaterials to create stable biotic-abiotic interfaces that are able to measure biological processes including intracellular functions.[135] Passive nanowire arrays fabricated on solid substrates efficiently penetrate cell membranes and serve as an efficient portal for intracellular measurements.[137] This idea can be extended to active device components that are prepared into functional nanowire devices. Silicon nanowires with non-linear kinked geometries can be fabricated by controlling the synthesis conditions during nanowire growth. The probe-like geometry of these nanomaterials makes them especially suitable for measuring transmembrane potential and extracellular ion flows in real time.[138] Nanowire-based materials may also be scaled into larger device arrays for multiple measurements within a single cell.
Nanoscale devices composed of electronically robust materials such as silicon may be able to achieve stable and sensitive biotic-abiotic interactions by significantly reducing the characteristic length scale of the device. In essence, devices with dimensions that are several orders of magnitude smaller than the characteristic dimension of the cell may appear “invisible”. These devices obviate traditional cell-materials interactions and therefore are able to accurately measure intracellular processes without significant perturbation of the system itself. Eventually, scaling these devices into tissue and organ level sensor arrays may prove suitable for accurately measuring biological processes with higher order complexity or monitoring disease states in vitro.[139, 140] Organic nanomaterials such as carbon nanotubes or organic semiconducting nanowires may also prove to be useful as functional materials for in vitro biotic-abiotic interfaces.[141, 142] Other frontiers for in vitro cell-nanomaterial interfaces may involve the fabrication of constructs that can be stably integrated into the membrane. Candidate materials include carbon nanotubes or polymeric constructs that can be integrated with freestanding cells.[143]
4. Soft Materials for In vivo Device Interfaces
Medical devices with electrical sensing and stimulation capabilities have been widely used to restore and enhance functions that have been compromised due to disease or injury. Engineering devices with increased biocompatibility could empower numerous clinically-relevant diagnostic and rehabilitation technologies including brain-machine interfaces (BMI),[144] retinal prostheses,[118] peripheral nerve regeneration,[17] and real time cardiac monitoring.[145] Other potential applications include improved electrode materials for vagus nerve stimulation and systems to measure electrophysiological performance of in vitro tissue models for use in drug discovery.[146] In general, in vivo device interfaces serve as a key foundation for electrical communication by providing a high fidelity channel for electronic communication between medical devices and soft tissue. Stable electronically active biotic-abiotic interfaces are essential components of implantable devices that are intended to sense, monitor, and stimulate tissues in the human body. One global hurdle in the widespread adoption of therapeutic bioelectrical stimulation and measurement including neuromodulation is the ability to fabricate devices using materials that lead to stable and reliable integration with human tissues. Traditional engineering materials used in microelectrical mechanical systems (MEMS) such as noble metals, silicon, quartz, parylene, and polyimide, while suitable for short-term electrical stimulation therapies, fail eventually due to inflammation and scarring. Although the exact mechanism for the injurious reaction to such materials remains unknown, the intrinsic limitations in materials have motivated many investigators to design new systems to ameliorate injurious reactions associated with synthetic implants. Reliable tissue-scale biotic-abiotic interfaces have the potential to transform and empower numerous clinically-relevant technologies including: (1) rehabilitation therapies; (2) biosensors; (3) and devices to augment human performance. There is an urgent and unmet need to design flexible electronic components that are mechanically compliant, conformal, and able to be integrated with soft curvilinear tissue structures such as the brain and the heart (Figure 5).
Figure 5.
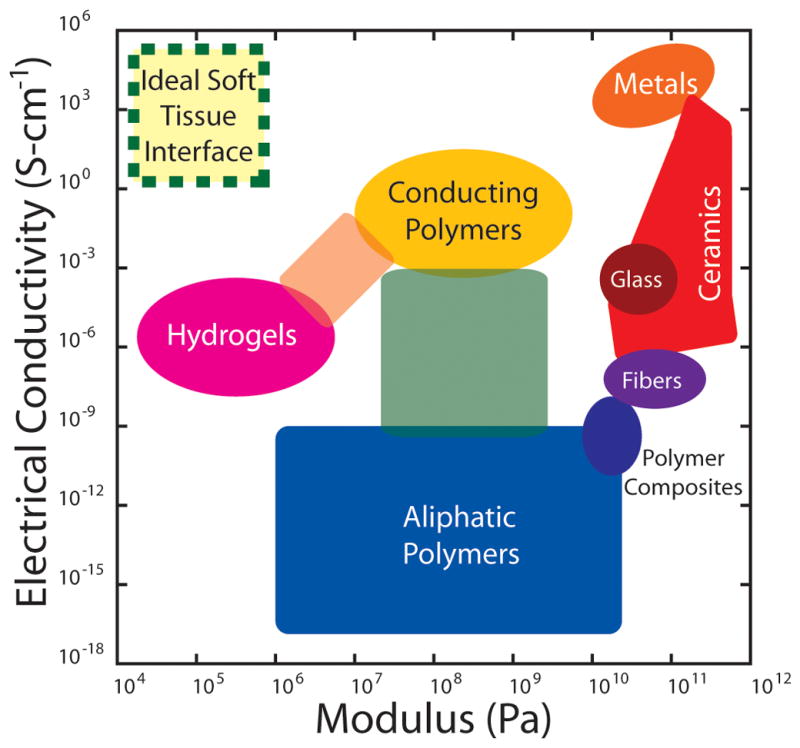
Ashby diagram to identify ideal materials for electrically-active tissue-device interfaces for use in vitro or in vivo. The ideal material to generate reliable biotic-abiotic interfaces with soft tissue will exhibit simultaneous properties of high electrical conductivity and high mechanical compliance (dashed border). This optimal materials domain is currently inaccessible by virtue of hydrogels, polymers, or composites (unlabeled intermediate regions).
4.1 Miniaturization in Biotic-Abiotic Interfaces
The emerging trend of device miniaturization using robust inorganic materials may serve as a key guideline in achieving stable tissue-device interfaces in vivo. Specifically, devices that are designed to reduce macrophage-mediated foreign body responses may be able to better achieve seamless tissue-device integration.[147] It has been widely reported that macrophage and lymphocyte interactions, which dictate the extent of the foreign body reaction and overall biocompatibility, [148] depend on the physical properties and macroscopic geometry of the implant.[147–150] Therefore, one strategy may be to design interfaces with unique geometries that can attenuate the foreign body response.[148, 149] Specifically, the idea of creating “low visibility” implants with significantly reduced surface areas is gaining traction.[151] The guiding principle is to design biomaterial interfaces with reduced surface areas in order to bias macrophages into a phenotype that prevents fusion and foreign body giant cell formation.[149] Smaller device dimensions may reduce foreign body giant cell formation and minimize the onset of gliosis.[152] Electrode interfaces with smaller surface areas induce a less persistent degree of macrophage activation, decreased blood brain barrier leakiness, and reduced neuronal cell loss compared to control implants. Taken together, the overall performance and biocompatibility of tissue-level interfaces may be significantly improved by reducing the characteristic dimensions of the implant.[153] Therefore, device miniaturization is likely to be preserved as a guideline for the design of next-generation in vivo biotic-abiotic interfaces.
4.2 Flexible Electronics
The advent of flexible silicon-based electronics has enabled the fabrication of mechanically compliant biosensors for a variety of medical applications. This technological achievement is made possible by fabricating traditional inorganic semiconductors into planar form factors with nanometer scale thicknesses. The reduced thickness permits mechanical deformations that have not been previously attainable using devices with conventional micron-scale dimensions. Mechanically compliant semiconducting materials[154] can be coupled with interconnect geometries that retain electrical conductivity under high strains.[121, 122] The integration of highly compliant semiconductors and robust metallic interconnects is a potent combination of technologies. Flexible gallium-arsenide LED and epidermal electronics equipped with radiofrequency communication may usher in the next generation of external wearable electronics for non-invasive real time monitoring of vital signs.[155] Comparable device innovations may be leveraged for applications that use more invasive implantable medical devices as well. For example, temperature and pressure sensors can be integrated into endovascular catheters that are used in laparoscopic techniques.[156] These devices can provide real time information regarding cardiovascular function during stenting procedures. Although several MEMS-based sensor technologies have been previously integrated into endovascular devices, [157–159] the utilization of large area format flexible sensors enables real time sensing with increased data acquisition before, during, and after balloon expansion. Active sensors based on silicon devices can also be fabricated into large multiplexed arrays, which have been used to measure the cardiac output in a live beating heart in real time.[145]
4.3 Polymeric Materials for Neural Interfaces
The predominant innovation in flexible silicon-based electronics is the ability to fabricate traditional inorganic device materials into form factors that can confer unique macroscopic mechanical properties. However, it is also possible to incorporate polymeric and biologically-derived materials with hybrid organic-inorganic devices in order to achieve new functionalities. One such demonstration of this strategy utilizes water-soluble proteins as a sacrificial substrate material to promote the intimate integration of sensors arrays for electrocorticography (ECoG). ECoG arrays are subdural neural electrodes that are placed directly on top of the outer portion of the brain. Sensor arrays for ECoG are crucial components in some classes of BMI that measure electrical signals originating from neurons located in the outer cortices of the brain. These signals are transmitted to external hardware where it is recorded and interpreted. There are several critical design criteria that must be met in order to ensure the utility of ECoG arrays as a strategy for BMI. First, the electrodes must be positioned in close proximity to the cortex in order to monitor high frequency single unit action potentials, which are typically on the order of μV in magnitude. Second, electrode arrays for ECoG must be arranged to cover large areas in order to increase the signal density. Traditional microelectronic devices intended for use in ECoG arrays are fabricated with planar form factors that may exhibit some degree of mechanical flexibility. Although elastomeric device arrays may be able to monitor larger tissue areas due to enhanced conformability, flexible electronics alone are not an ideal solution to creating intimate tissue-device interfaces in ECoG arrays. One alternative idea solution may be to utilize sacrificial device components to facilitate integration with curvilinear neural tissues such as the brain or the peripheral nerve. Silk fibroin sheets can be processed into thin films that are suitable for the downstream fabrication of super-positioned electronic components; in this case, isolated gold electrodes are fabricated into rectangular arrays.[160] Sensor arrays were validated in a feline model in which a visual stimulus was presented and the evoked response was measured in the visual cortex in real time. The maximum achievable RMS amplitude of the measured signals is highly dependent upon the extent of integration of the device array with the visual cortex. Microelectrode arrays fabricated on silk films with thicknesses of 76 μm were able to measure a four-fold increase in RMS amplitudes after visual stimulus presentation relative to the resting state. This ratio in measured RMS amplitude increases eight-fold when silk fibroin meshes of 2.5 μm in thickness are used. These results suggest that a higher degree of sensitivity is achievable by engineering films that are more amenable to conformal wrapping around soft tissue. Reducing the thickness of silk fibroin films leads to a significant reduction in the bending stiffness. A reduced bending stiffness, in turn, promotes spontaneous wrapping on curvilinear geometries in aqueous environments. Silk has also been used to promote neuronal outgrowth in interfaces.[161] Guonosine-modified silk films can also be used to directly modulate the conductance in astroglia; a capability that is useful for in vitro electrophysiological characterization.[162] Silk fibroin is an ubiquitous naturally-derived protein biomaterial that has been used in a variety of medical device applications including medical devices for drug delivery, regenerative medicine, and surgical reconstruction.[31, 163, 164] Sacrificial silk fibroin films for neural interfaces exhibit dissolution times on the order of 15–20 min. Next-generation sacrificial layers may employ water-soluble materials such as sucrose,[165] gelatin,[166–168] or poly(vinyl alcohol).[169–173] These materials may accelerate dissolution kinetics due to increased hydrophilicity and reduced molecular weight. Furthermore, the cytotoxicity and biocompatibility profiles are widely known and thoroughly characterized due to the ubiquity in the food processing industry.[169, 170] Materials with increased familiarity and known risk may expedite the regulatory processes for these medical devices as well. Integrating electronics with non-conventional polymeric biomaterials may use previous advances as a guideline to direct future activities. Lessons learned from the fabrication of electronic circuits on paper, foil, and flexible plastics will catalyze progress in this area.[174–177]
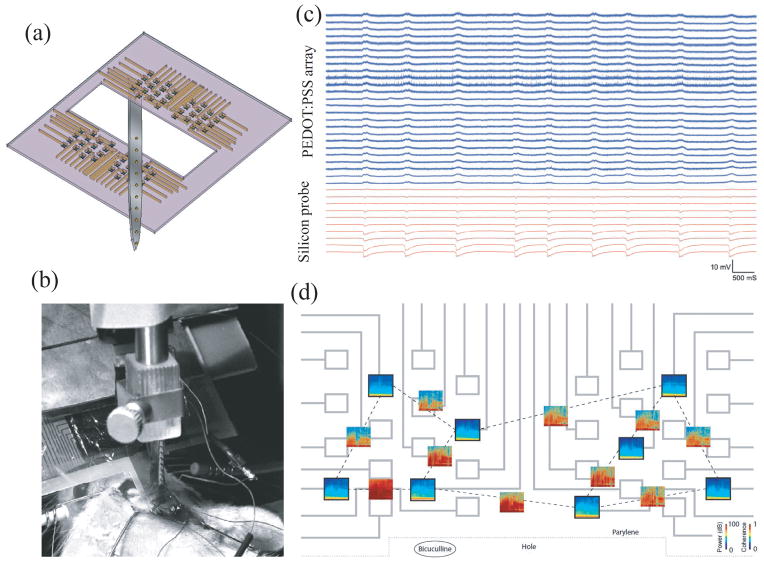
Highly biocompatible and conformable electronic device interfaces can be fabricated using polymeric electrodes as well (Figure 6).[178] Metallic structures can be integrated into a 2 μm-thick parylene C matrix. Gold pads and interconnects can be patterned using a traditional lift-off process followed by solution processing of PEDOT doped with PSS and crosslinked with dilute concentrations of 3-glycidoxypropytrimethoxysilane. Although PEDOT:PSS films are fabricated using solution processing, it may be possible to selectively functionalize gold electrodes with conducting soft matter by virtue of electrochemical processing.[179] The finalized devices have a total thickness of 4 μm with the gold interconnects and PEDOT:PSS electrodes both located at the neutral mechanical plane; the region that is exempt from both compressive or tensile stresses under flexion. Highly compliant mechanical arrays can conform to features with a radius of curvature as small as 2.2 millimeters. Rectangular electrode arrays consist of two sets of 32 electrodes, each with an area of 20 × 20 μm2 and a center-to-center spacing of 60 μm. Polymeric sensor arrays were validated in murine models. Simultaneous recording of action potentials from both polymer arrays and silicon-based cortical implants indicate that intrinsic electrophysiological events could be monitored by both devices. Furthermore, gold electrodes functionalized with PEDOT:PSS films are more sensitive to electrophysiological monitoring than bare gold electrodes. This study demonstrates the additional improvement in device performance that can be established by using conducting polymers as active materials.
Figure 6.
Highly conformal conducting polymer electrodes for in vivo recordings. (a) Schematic of the all polymer electrode arrays used in conjunction with silicon-based probes for in vivo cortical recording and (b) photograph showing the implantation. (c) Representative recordings from 25 electrodes in the polymer array and from 10 electrodes in the silicon probe measured from the surface and interior of the cortex, respectively. (d) Time-frequency (TF) analysis of the signals recorded by electrodes (black frames, x-axis: time, 10 min; y-axis: frequency, 0.1–50 Hz; color coding: power (dB) and their cross-spectrum coherences (open boxes, same axes as TF plots, color coding: coherence). Adapted from D. Khodagholy, T. Doublet, M. Gurfinkel, P. Quilichini, E. Ismailova, P. Leleux, T. Herve, S. Sanaur, C. Bernard, G. G. Malliaras, Advanced Materials 2011, 23, H268. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
4.4 Next-Generation Soft Matter Electronics for Medical Implants
The tissue-electrode interface plays a critical role in the stability of many devices designed to modulate the neuromuscular system.[180] There have been substantial advances in electrode materials including the use of biocompatible low impedance materials (iridium oxide, carbon nanotubes, conducting polymers, etc). Chronic electrode stability remains elusive due to issues such as corrosion and isolation from excessive fibrous capsule formation.[44, 181–183] For example, invasive cortical BMI leads to astrocyte activation and glial scarring. These reactions isolate electrodes and accelerate failure modes in cortical BMI such as low signal-to-noise ratios. Implantable retinal prostheses provide a rapid and viable path to vision restoration in patients suffering from degenerative retinal diseases.[184] For example, patients in ongoing clinical trials that have received retinal prostheses demonstrate sufficient visual acuity for object localization and motion discrimination. A variety of application-specific devices have been designed for this therapy including passive implants based on photodiode arrays[185] and active implants with tissue-integrated microelectrode arrays.[186–188] Intimate contact between the retinal prosthesis electrode (RPE) arrays and retinal ganglia is required to minimize stimulation current. The threshold for safely delivering current to the retina is 0.6 mA for a duration of 0.5 ms. The modulus mismatch between electrode materials (E > 10 MPa) and the retina (E ~ 10 kPa) produces injurious tissue reactions, which increase the retina-electrode displacement. In turn, the increased distance in the tissue-device interface mandates increasing current densities beyond safe levels. Excessive electrical stimulation currents can produce tissue damage. Electrodes used in functional electrical stimulation would also benefit from an intimate tissue-device interface.[182] However, the foreign body response, often manifested through fibrous capsule formation, increases the impedance, which in turn elevates the current required for stimulation. High current densities (>1 μC-mm−2 @ 50 Hz) can lead to tissue damage and place additional burden on power supplies and reduce battery lifetime. There is renewed interest in addressing the role of tissue-materials interactions in the context of improving the biotic-abiotic tissue-device interface.[189] Strategies include reducing the onset of inflammation via controlled release of therapeutics, electrode surface modification using bioactive molecules, and reduced device dimensions as previously described. These approaches, which are partially effective, are not able to fully address the fundamental issues of the monocyte/macrophage-mediated foreign body response. This realization has spawned the next-generation of soft materials electrodes that can obviate failure modes associated with these inevitable interactions.
4.4.1. Hydrogel Electronics
Materials systems with both ultra-low mechanical stiffness and low electrical impedance are elusive, yet critical to engineering chronically stable biotic-abiotic interfaces for tissue-level device integration. Currently available flexible conductors for use in biotic-abiotic interfaces use ubiquitous engineering elastomers as bulk materials and then devise methods to embed conductive structures.[124] This overarching strategy produces elastomeric devices (E ~ 0.1 – 1 MPa).[190] However, the elastic modulus of tissue in the brain and the retina, two key targets of interest for interface technology, can be at least three orders of magnitude smaller than elastomeric conductors.[191–193] The resulting modulus mismatch contributes significantly to the failure of chronic BMI.[194] Low modulus hydrogels play a key role in currently available biotic-abiotic interfaces[195] by serving as coatings, immunoisoloation barriers, and reservoirs for drug delivery.[196, 197] Conducting hydrogels offer a potential material framework to match the mechanical properties of devices with soft tissues.[198, 199] Hydrogel matrices based on poly(vinyl alcohol)/poly(acrylic acid) can be functionalized with conducting polymers via the in situ synthesis of PEDOT and PPy.[179, 197, 200] Hydrogel matrices impregnated with conductive domains produce bulk physical properties that are usually linear combinations of each intrinsic material. Hydrogel-based materials will become a desirable option for biotic-abiotic interfaces matrices if: (1) the intrinsic conductivity is enhanced without impacting the bulk mechanical properties of the network; (2) complex multi-dimensional structures can be microfabricated within hydrogel matrices.
Recent efforts have focused on the synthesis of hydrogel networks that retain their intrinsic ultra-compliant mechanical properties. One approach can fabricate ordered two-dimensional microfabricated PEDOT structures in agarose matrices. This technique employs two electrochemical processes in succession; electropolymerization of PEDOT into hydrated agarose networks followed by electrochemical-actuation-assisted composite delamination. Common two-dimensional soft lithography microfabrication methods for polymeric substrates may use processes such as inkjet printing or microstamping. These processes are difficult to apply to hydrogel substrates due to the high mechanical flexibility and extreme hydration of the underlying material.[179] The alternative fabrication approach begins by patterning a platinum microelectrode on a glass substrate and forming a hydrated agarose gel superior to the surface with the defined platinum film. After gelation, PEDOT films are selectively formed on the platinum electrodes by first allowing monomeric EDOT precursors to diffuse through the agarose network before engaging in electrochemical polymerization on the platinum electrodes beneath the hydrogel. The electrodes exhibit a resistivity of 11 kΩ-square, which is comparable to previously reported values in PEDOT films.[201] Furthermore, printed electrodes can stimulate the contraction of hydrogel networks seeded with C2C12 myotubes. Electrical stimulation produces simultaneous contraction of both the gel and the myotubes. The intrinsic electrical conductivity of PEDOT/agarose composite electrodes may need to be increased for in vivo applications. There are many promising innovative materials-based strategies aimed at dramatically improving the fidelity of in vivo biotic-abiotic interfaces. Tissue-device interfaces based on well-defined nanomaterials improve sensing and modulation of biological matter across length scales ranging from sub-cellular domains to organ systems.[139] Flexible semiconductors enable the integration of active device components.[155] Device deployment can be improved by incorporating biomaterials-based integration strategies.[160] Although pre-fabricated devices typically require invasive implantation procedures, alternative device integration methods may circumvent these issues. In situ fabrication of conducting polymers can be accomplished through in vivo polymerization with living neurons.[15, 202] Although numerous technical challenges persist, the in situ fabrication and integration of biotic-abiotic interfaces is an attainable goal.
4.4.2. Biodegradable Electronics
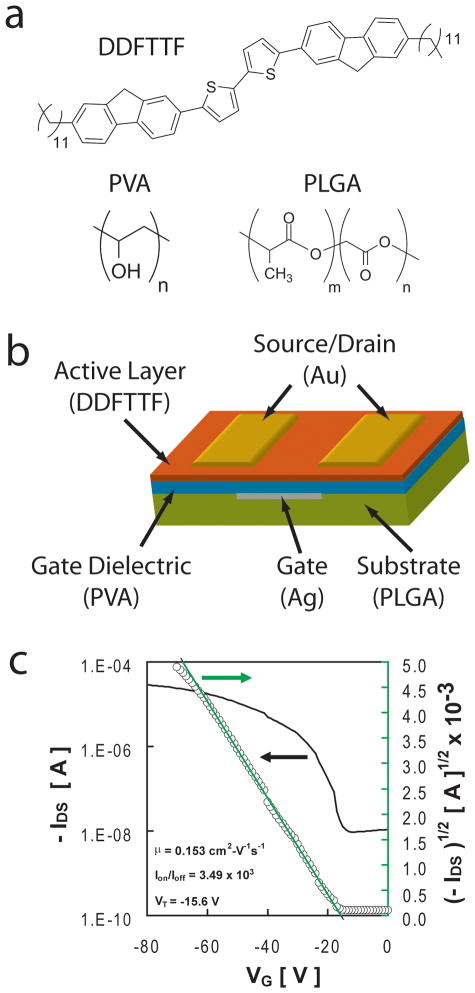
There are a number of future challenges that face BMI including minimally-invasive implantation and long-term device stability in vivo. Biologically-derived materials may continue to overcome obstacles for efficient utilization of neural interfaces. The issue of device stability dominates the design of chronic neural interfaces that serve as a core technology for rehabilitation therapies. However, other applications may not require chronically stable biotic-abiotic interfaces. For example, biodegradable sensor arrays may only need to measure physiological parameters within a finite window. For example, perhaps the concentration of a biomarker or pathogen is known to peak sometime within a 2-month window. Microfabricated controlled release devices[113] may only need to deliver therapeutics for a brief period of time after which the active device components may be absorbed and metabolized within the human body.[203] In vivo electrical stimulation of tissue for regenerative medicine therapies [204–206] may only require an active device lifetime that coincides with a therapeutic window that may be on the order of a few weeks. The potential impact of temporary electronic implants has motivated the exploration of biodegradable electronics. Initial efforts in this area have focused on the synthesis of new materials that are electrically conductive and biodegradable.[207, 208] More recent efforts have demonstrated the fabrication of completely biodegradable electronic devices. [209] Fully biodegradable devices can be fabricated using poly(L-lactide-co-glycolide), poly(vinyl alcohol), 5,5′-bis-(7-dodecyl-9H- uoren-2-yl)-2,2′-bithiophene, and trace metallic contacts at concentrations that are lower than the amount of mercury in tuna fish (Figure 7).[210] These devices exhibit tunable in vitro biodegradation rates and exceptional device performance that is preserved even after direct exposure to water. The concept of biodegradable electronics will ultimately rely heavily on the clever design of packaging strategies that can balance in vivo device operation lifetimes and bioabsorption time lines.
Figure 7.
Biodegradable organic thin film transistors. (a) A combination of bio-inspired small molecule semiconductors and synthetic bioerodible/bioexcretable polymers can be assembled into (b) organic thin film transistor geometries. (c) These devices exhibit transfer characteristics, hole mobilities, and Ion-Ioff ratios that are suitable for a variety of simple operations that would be useful when integrated into temporary medical implants. Adapted from C. J. Bettinger, Z. Bao, Advanced Materials 2010, 22, 651. Copyright Wiley-VCH Verlag GmbH & Co. KGaA. Reproduced with permission.
5. Conclusion
Biomaterials will continue to play an increasingly important role in flexible electronics, biotic-abiotic interfaces, and electronically active medical implants. Recent progress in scientific discovery and technological advancement related to this field will motivate additional investment of resources. Disciplines such as organic electronics, MEMS microfabrication, cell biology, and medical device design will continue to cross-pollinate thereby stoking rapid innovations across a wide range of applications. There are countless opportunities for investigators in the aforementioned fields to become actively engaged in this expanding topic.
5.1. Design and Processing of New Materials
The design of new synthetic polymers and the repurposing of naturally-derived materials will likely continue unabated. Specifically, the confluence of “soft” organic materials and “hard” inorganic materials for medical applications will provide a convenient forum for materials innovation. For example, imparting new electronic functionalities in polymers, lipids, hydrogels, and elastomers may yield composites with ultra-high mechanical compliance and electrical conductivity.[200, 211] In addition to mechanical flexibility, electronic materials may also be conferred medically relevant properties such as biodegradability for temporary implants and shape-memory capabilities for minimally-invasive procedures.[212–214] Nature will continue to serve as an indefatigable source of inspiration for the design of next-generation materials including conducting polymers. The invention of novel materials processing techniques is viewed as an important and distinct, yet related research pursuit. Ultimately, the combination of novel materials and processes will generate intriguing electronic devices based on new forms of soft matter.
5.2. Validation and Expansion of In Vitro Cell-Material Interfaces
The design and application of in vitro device interfaces will continue to be motivated by the intrinsic complexity of biological systems. A key windfall for in vitro device interfaces will be the elucidation of a previously undiscovered biological phenomenon that was made possible solely through a functional synthetic interface. Another new direction will be interfaces that are able to integrate devices with novel optical functionalities.[185] This capability is extremely attractive given the recent emergence of optogenetics.[215] For example, hybrid optical bio-organic interfaces can be fabricated from blends of regio-regular poly(3-hexylthiophene-2,5-diyl) (rr-P3HT) and phenyl-C61-butyric-acid-methyl ester (PCBM), two materials commonly used in the organic photovoltaic industry.[216, 217] Optically active substrates can be used to stimulate action potentials in primary neurons by exposing them to brief pulses of light. Expanding this capability may lead to new neuronal communication and photo-manipulation techniques, thus paving the way for the development of artificial retinas and other neuroprosthetic interfaces based on organic photodetectors.
Controlled tissue regeneration is another attractive avenue for well-defined electronically active in vitro device interfaces. Bulk electrical stimulation of in vitro tissue constructs has been shown to promote increased supercellular organization and a more native-like morphology.[204–206, 218] This phenomenon is especially pronounced in cell populations that use ion flows and voltage gradients for intercellular synchronization and communication such as cardiac tissue. Next-generation interfaces with well-defined electrodes could serve as a key technology in expanding this capability to pattern tissue morphogenesis in space and time.[219] Electronic in vitro interfaces for soft tissue regeneration would also benefit from previously established capabilities of organic electronics such mechanical flexibility, facile microfabrication approaches, and abundant surface modification techniques.[220]
5.3. In Vivo Integration of Soft Electronics
Advancements in organic electronics, biomaterials, and flexible devices will continue to improve the integration of synthetic devices with tissues in vivo. There are many additional opportunities to consider given the inevitability of tissue-biomaterials interactions as a key constraint in the design and fabrication of in vivo interfaces. This paradigm has been most enthusiastically embraced in the arena of neural interfaces. Novel biomaterials used in neural probes can dramatically extend device lifetimes by addressing potential failure modes relating to microtrauma, gliosis, and neurodegeneration. Broad strategies include designing ultra-compliant materials, incorporating the controlled release of therapeutics, and modifying electrode surfaces with bioactive molecules. Similar strategies may be applicable to medical implants designed to sense and stimulate other soft tissues. The generally accepted long-term goal of these strategies is to extend the lifetime of tissue-electrode interfaces to 50 years or longer. It is rare for medical implants to maintain their desired function on this time scale given the caustic in vivo environments and the inevitability of fibrosis, infection risks, and corrosion.[221–226] Even passive devices such as total knee and hip replacements are subject to complex microenvironments that contribute to failure due to mechanisms including loosening and infection.[226] The human body is a dynamic system that is constantly pursuing homeostasis and will continuously respond to external insults arising from either injury or implanted material. Therefore, alternative paradigms may be worth considering. For example, we readily adopt the notion of periodic maintenance in many complex engineering systems such buildings, bridges, vehicles, airplanes, and consumer electronics. Perhaps this analogy should be extended as a criterion in the design of electronically active medical implants. Initial strategies may consider the partial abandonment of the notion that long-term chronic stability is an achievable goal. Rather, electronically active medical implants may be designed with the inherent understanding that these systems will have to undergo periodic maintenance. This proposed mandate should be accommodated by creating novel biomaterials, new device designs, and innovative methods for rapid and efficient in situ tissue integration, maintenance, and repair. Taken together, the rapid emergence of biomaterials-based electronics will continue to catalyze scientific discoveries ranging from fundamentals in charge transport in biopolymers to neuroscience and boundless innovation in medical device technology.
Acknowledgments
The authors would like to first acknowledge the authors whose work was not able to be highlighted due to space constraints. Funding provided by the following organizations: the Berkman Foundation; the American Chemical Society Petroleum Research Fund (ACS PRF #51980-DNI7); the Proctor & Gamble Education Grant Program; and the Carnegie Mellon University School of Engineering.
Biographies
Meredith Muskovich is pursuing her Ph.D. in Materials Science and Engineering at Carnegie Mellon University under the supervision of Prof. Bettinger. Her current research interests involve the design, synthesis, and microfabrication of ultra-compliant electrode arrays for applications in medical devices. She received her B.S. in Materials Science and Engineering from The Pennsylvania State University under the supervision of Prof. Colby.
Prof. Christopher Bettinger is an Assistant Professor in the Departments of Materials Science and Engineering and Biomedical Engineering at Carnegie Mellon University. Current research interests include polymer synthesis for tissue-device interfaces and functional surfaces for studying cell-biomaterial interactions. He has received many distinctions including the National Academy of Sciences Award for Initiatives in Research, the MIT Technology Review TR35, the ACS AkzoNobel Award for Polymer Chemistry, and the Tissue Engineering Society Young Investigator Award. Prof. Bettinger received a Ph.D. in Materials Science from MIT as a Charles Stark Draper Fellow and completed an NIH post-doctoral fellowship at Stanford University.
Contributor Information
Meredith Muskovich, Department of Materials Science & Engineering, 5000 Forbes Avenue, Pittsburgh, PA, 15213.
Prof. Christopher J. Bettinger, Department of Biomedical Engineering, Department of Materials Science & Engineering, 5000 Forbes Avenue, Pittsburgh, PA, 15213.
References
- 1.Levin M. Bio Essays. 2012;34:205. [Google Scholar]
- 2.Zhao M, Song B, Pu J, Wada T, Reid B, Tai G, Wang F, Guo A, Walczysko P, Gu Y, Sasaki T, Suzuki A, Forrester JV, Bourne HR, Devreotes PN, McCaig CD, Penninger JM. Nature. 2006;442:457. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 3.Irimia-Vladu M, Troshin PA, Reisinger M, Shmygleva L, Kanbur Y, Schwabegger G, Bodea M, Schwödiauer R, Mumyatov A, Fergus JW, Razumov VF, Sitter H, Sariciftci NS, Bauer S. Advanced Functional Materials. 2010;20:4017. [Google Scholar]
- 4.Ito S. Pigment Cell Research. 2003;16:230. doi: 10.1034/j.1600-0749.2003.00037.x. [DOI] [PubMed] [Google Scholar]
- 5.Tran ML, Powell BJ, Meredith P. Biophysical Journal. 2006;90:743. doi: 10.1529/biophysj.105.069096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watt AAR, Bothma JP, Meredith P. Soft Matter. 2009;5:3754. [Google Scholar]
- 7.Jastrzebska MM, Isotalo H, Paloheimo J, Stubb H, Pilawa B. Journal of Biomaterials Science, Polymer Edition. 1996;7:781. doi: 10.1163/156856296x00129. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, Hong L, Kempf VR, Wakamatsu K, Ito S, Simon JD. Pigment Cell Research. 2004;17:262. doi: 10.1111/j.1600-0749.2004.00140.x. [DOI] [PubMed] [Google Scholar]
- 9.McGinness J, Corry P, Proctor P. Science. 1974;183:853. doi: 10.1126/science.183.4127.853. [DOI] [PubMed] [Google Scholar]
- 10.Meredith P, Powell BJ, Riesz J, Nighswander-Rempel SP, Pederson MR, Moore EG. Soft Matter. 2006:2. doi: 10.1039/b511922g. [DOI] [PubMed] [Google Scholar]
- 11.Bothma JP, Boor Jd, Divakar U, Schwenn PE, Meredith P. Advanced Materials. 2008;20:3539. [Google Scholar]
- 12.Ambrico M, Ambrico PF, Cardone A, Ligonzo T, Cicco SR, Mundo RD, Augelli V, Farinola GM. Advanced Materials. 2011;23:3332. doi: 10.1002/adma.201101358. [DOI] [PubMed] [Google Scholar]
- 13.Diaz P, Gimeno Y, Carro P, Gonzalez S, Schilardi PL, Benitez G, Salvarezza RC, Creus AH. Langmuir. 2005;21:5924. doi: 10.1021/la0469755. [DOI] [PubMed] [Google Scholar]
- 14.Povlich LK, Le J, Kim J, Martin DC. Macromolecules. 2010;43:3770. [Google Scholar]
- 15.Peramo A, Urbanchek MG, Spanninga SA, Povlich LK, Cederna P, Martin DC. Tissue Engineering. 2008;14:423. doi: 10.1089/tea.2007.0123. [DOI] [PubMed] [Google Scholar]
- 16.Richardson-Burns SM, Hendricks JL, Foster B, Povlich LK, Kim D-H, Martin DC. Biomaterials. 2007;28:1539. doi: 10.1016/j.biomaterials.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bettinger CJ, Bruggeman JP, Misra A, Borenstein JT, Langer R. Biomaterials. 2009;30:3050. doi: 10.1016/j.biomaterials.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napolitano A, Pezzella A, Vincensi MR, Prota G. Tetrahedron. 1995;51:5913. [Google Scholar]
- 19.Borovansky J, Elleder M. Pigment Cell Research. 2003;16:280. doi: 10.1034/j.1600-0749.2003.00040.x. [DOI] [PubMed] [Google Scholar]
- 20.Burch RR, Dong Y-H, Fincher C, Goldfinger M, Rouviere PE. Synthetic Metals. 2004;146:43. [Google Scholar]
- 21.Yakuphanoglu F, Aydin ME, Kiliçoǧlu T. Journal of Physical Chemistry B. 2006;110:9782. doi: 10.1021/jp0610620. [DOI] [PubMed] [Google Scholar]
- 22.Irimia-Vladu M, Głowacki ED, Troshin PA, Schwabegger G, Leonat L, Susarova DK, Krystal O, Ullah M, Kanbur Y, Bodea MA, Razumov VF, Sitter H, Bauer S, Sariciftci NS. Advanced Materials. 2011;24:375. doi: 10.1002/adma.201102619. [DOI] [PubMed] [Google Scholar]
- 23.Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim B-C, Inoue K, Mester T, Covalla SF, Johnson JP, Rotello VM, Tuominen MT, Lovley DR. Nature Nanotechnology. 2011;6:573. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- 24.Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, Dohnalkova A, Beveridge TJ, Chang IS, Kim BH, Kim KS, Culley DE, Reed SB, Romine MF, Saffarini DA, Hill EA, Shi L, Elias DA, Kennedy DW, Pinchuk G, Watanabe K, Ishii Si, Logan B, Nealson KH, Fredrickson JK. Proceedings of the National Academy of Sciences USA. 2006;103:11358. doi: 10.1073/pnas.0604517103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Herlogsson L, Crispin X, Robinson ND, Sandberg M, Hagel OJ, Gustafsson G, Berggren M. Advanced Materials. 2007;19:97. [Google Scholar]
- 26.Kang HJ, Coulibaly Fl, Clow F, Proft T, Baker EN. Science. 2007;318:1625. doi: 10.1126/science.1145806. [DOI] [PubMed] [Google Scholar]
- 27.Huang X, Liu G, Wang X. Advanced Materials. 2012;24:1482. doi: 10.1002/adma.201104668. [DOI] [PubMed] [Google Scholar]
- 28.Gazit E. FASEB Journal. 2002;16:77. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 29.Reches M, Gazit E. Science. 2003;300:625. doi: 10.1126/science.1082387. [DOI] [PubMed] [Google Scholar]
- 30.Jin HJ, Park J, Karageorgiou V, Kim UJ, Valluzzi R, Cebe R, Kaplan DL. Advanced Functional Materials. 2005;15:1241. [Google Scholar]
- 31.Bettinger CJ, Cyr KM, Matsumoto A, Langer R, Borenstein JT, Kaplan DL. Advanced Materials. 2007;19:2847. doi: 10.1002/adma.200602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amdursky N, Molotskii M, Gazit E, Rosenman G. Journal of the American Chemical Society. 2010;132:15632. doi: 10.1021/ja104373e. [DOI] [PubMed] [Google Scholar]
- 33.Klauk H, Zschieschang U, Pflaum J, Halik M. Nature. 2007;445:745. doi: 10.1038/nature05533. [DOI] [PubMed] [Google Scholar]
- 34.Roberts ME, Mannsfeld SCB, Queraltó N, Reese C, Locklin J, Knoll W, Bao Z. Proceedings of the National Academy of Sciences USA. 2008;105:12134. doi: 10.1073/pnas.0802105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kergoat L, Herlogsson L, Braga D, Piro B, Pham M-C, Crispin X, Berggren M, Horowitz G. Advanced Materials. 2010;22:2565. doi: 10.1002/adma.200904163. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson BI, Wille-Jorgensen P, Kalebo P, Mouret P, Rosencher N, Bosch P, Baur M, Ekman S, Bach D, Lindbratt S, Close P. New England Journal of Medicine. 1997;337:1329. doi: 10.1056/NEJM199711063371901. [DOI] [PubMed] [Google Scholar]
- 37.Sahoo S, Chung C, Khetan S, Burdick JA. Biomacromolecules. 2008;9:1088. doi: 10.1021/bm800051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuo CK, Ma PX. Biomaterials. 2001;22:511. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 39.Bencherif SA, Washburn NR, Matyjaszewski K. Biomacromolecules. 2009;10:2499. doi: 10.1021/bm9004639. [DOI] [PubMed] [Google Scholar]
- 40.Freddi G, Romanò M, Massafra MR, Tsukada M. Journal of Applied Polymer Science. 1995;56:1537. [Google Scholar]
- 41.Dinnes DLM, Marçal H, Raftery MJ, Labow RS, Mahler SM. Journal of Chemical Technology & Biotechnology. 2008;83:482. [Google Scholar]
- 42.Yi H, Wu LQ, Bentley WE, Ghodssi R, Rubloff GW, Culver JN, Payne GF. Biomacromolecules. 2005;6:2881. doi: 10.1021/bm050410l. [DOI] [PubMed] [Google Scholar]
- 43.Li D, Wang Y, Xia Y. Nano Letters. 2003;3:1167. [Google Scholar]
- 44.Abidian MR, Martin DC. Advanced Functional Materials. 2009;19:573. [Google Scholar]
- 45.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Biomacromolecules. 2002;3:232. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 46.Bhattarai N, Edmondson D, Veiseh O, Matsen FA, Zhang M. Biomaterials. 2005;26:6176. doi: 10.1016/j.biomaterials.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Ohkawa K, Cha D, Kim H, Nishida A, Yamamoto H. Macromolecular Rapid Communications. 2004;25:1600. [Google Scholar]
- 48.Zhong C, Deng Y, Roudsari AF, Kapetanovic A, Anantram MP, Rolandi M. Nature Communications. 2011;2:476. doi: 10.1038/ncomms1489. [DOI] [PubMed] [Google Scholar]
- 49.Zhong C, Kapetanovic A, Deng Y, Rolandi M. Advanced Materials. 2011;23:4776. doi: 10.1002/adma.201102639. [DOI] [PubMed] [Google Scholar]
- 50.Giese B. Accounts of Chemical Research. 2000;33:631. doi: 10.1021/ar990040b. [DOI] [PubMed] [Google Scholar]
- 51.Xu Zhang, Li Tao. Nano Letters. 2004;4:1105. [Google Scholar]
- 52.Chang J-W, Wang C-G, Huang C-Y, Tsai T-D, Guo T-F, Wen T-C. Advanced Materials. 2011;23:4077. doi: 10.1002/adma.201102124. [DOI] [PubMed] [Google Scholar]
- 53.Deng Z, Mao C. Nano Letters. 2003;3:1545. [Google Scholar]
- 54.Yumusak C, Singh TB, Sariciftci NS, Grote JG. Applied Physics Letters. 2009;95:263304. [Google Scholar]
- 55.Stadler P, Oppelt K, Singh TB, Grote JG, Schwödiauer R, Bauer S, Piglmayer-Brezina H, Bäuerle D, Sariciftci NS. Organic Electronics. 2007;8:648. [Google Scholar]
- 56.Sun Q, Subramanyam G, Dai L, Check M, Campbell A, Naik R, Grote J, Wang Y. ACS Nano. 2009;3:737. doi: 10.1021/nn8009079. [DOI] [PubMed] [Google Scholar]
- 57.Sun Q, Chang DW, Dai L, Grote J, Naik R. Applied Physics Letters. 2008;92:251108. [Google Scholar]
- 58.Lawrence BD, Cronin-Golomb M, Georgakoudi I, Kaplan DL, Omenetto FG. Biomacromolecules. 2008;9:1214. doi: 10.1021/bm701235f. [DOI] [PubMed] [Google Scholar]
- 59.Perry H, Gopinath A, Kaplan DL, Dal Negro L, Omenetto FG. Advanced Materials. 2008;20:3070. [Google Scholar]
- 60.Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, Kaplan DL. Biomacromolecules. 2009;10:1032. doi: 10.1021/bm800956n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tao H, Brenckle MA, Yang M, Zhang J, Liu M, Siebert SM, Averitt RD, Mannoor MS, McAlpine MC, Rogers JA, Kaplan DL, Omenetto FG. Advanced Materials. 2012;24:1067. doi: 10.1002/adma.201103814. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Ameer GA, Sheppard BJ, Langer R. Nature Biotechnology. 2002;20:602. doi: 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- 63.Yang J, Webb AR, Ameer GA. Advanced Materials. 2004;16:511. [Google Scholar]
- 64.Mahdavi A, Ferreira L, Sundback C, Nichol JW, Chan EP, Carter DJD, Bettinger CJ, Patanavanich S, Chignozha L, Ben-Joseph E, Galakatos A, Pryor H, Pomerantseva I, Masiakos PT, Faquin W, Zumbuehl A, Hong S, Borenstein J, Vacanti J, Langer R, Karp JM. Proceedings of the National Academy of Sciences USA. 2008;105:2307. doi: 10.1073/pnas.0712117105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bettinger CJ, Orrick B, Misra A, Langer R, Borenstein JT. Biomaterials. 2006;27:2558. doi: 10.1016/j.biomaterials.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 66.Bettinger CJ, Weinberg EJ, Kulig KM, Vacanti JP, Wang Y, Borenstein JT, Langer R. Advanced Materials. 2006;18:165. doi: 10.1002/adma.200500438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer R. Journal of Biomedical Materials Research. 2008;91A:1077. doi: 10.1002/jbm.a.32306. [DOI] [PubMed] [Google Scholar]
- 68.Bettinger CJ, Bruggeman JP, Borenstein JT, Langer RS. Biomaterials. 2008;29:2315. doi: 10.1016/j.biomaterials.2008.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruggeman JP, Bettinger CJ, Nijst CLE, Kohane DS, Langer R. Advanced Materials. 2008;20:1922. [Google Scholar]
- 70.Bruggeman JP, de Bruin B-J, Bettinger CJ, Langer R. Biomaterials. 2008;29:4726. doi: 10.1016/j.biomaterials.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bettinger CJ. Pure and Applied Chemistry. 2011;83:9. doi: 10.1351/PAC-CON-10-12-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amsden B, Wang S, Wyss U. Biomacromolecules. 2004;5:1399. doi: 10.1021/bm034538j. [DOI] [PubMed] [Google Scholar]
- 73.Amsden BG, Tse MY, Turner ND, Knight DK, Pang SC. Biomacromolecules. 2006;7:365. doi: 10.1021/bm050731x. [DOI] [PubMed] [Google Scholar]
- 74.Wacker WEC, Parisi AF. New England Journal of Medicine. 1968;278:658. doi: 10.1056/NEJM196803212781205. [DOI] [PubMed] [Google Scholar]
- 75.Al-Ghambi SMG, Cameron EC, Sutton RAL. American Journal of Kidney Diseases. 1994;24:737. doi: 10.1016/s0272-6386(12)80667-6. [DOI] [PubMed] [Google Scholar]
- 76.Mario CD, Griffiths H, Goktekin O, Peeters N, Verbist J, Bosiers M, Deloose K, Heublein B, Rohde R, Kasese V, Ilsley C, Erbel R. Journal of Interventional Cardiology. 2004;17:391. doi: 10.1111/j.1540-8183.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- 77.Witte F, Ulrich H, Rudert M, Willbold E. Journal of Biomedical Materials Research Part A. 2007;81A:748. doi: 10.1002/jbm.a.31170. [DOI] [PubMed] [Google Scholar]
- 78.Song G, Song S. Advanced Engineering Materials. 2007;9:298. [Google Scholar]
- 79.Li Z, Gu X, Lou S, Zheng Y. Biomaterials. 2008;29:1329. doi: 10.1016/j.biomaterials.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 80.Zberg B, Uggowitzer PJ, Loffler JF. Nature Materials. 2009;8:887. doi: 10.1038/nmat2542. [DOI] [PubMed] [Google Scholar]
- 81.Metzger RM. Accounts of Chemical Research. 1999;32:950. [Google Scholar]
- 82.Jiang G, Zhou DD, Zhou D, Greenbaum E. In: Implantable Neural Prostheses. Zhou DD, Greenbaum E, editors. Vol. 2. Springer; New York: 2010. p. 27. [Google Scholar]
- 83.Peuster M, Hesse C, Schloo T, Fink C, Beerbaum P, von Schnakenburg C. Biomaterials. 2006;27:4955. doi: 10.1016/j.biomaterials.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 84.Waksman RON, Pakala R, Baffour R, Seabron R, Hellinga D, Tio FO. Journal of Interventional Cardiology. 2008;21:15. doi: 10.1111/j.1540-8183.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 85.Landsiedel R, Ma-Hock L, Kroll A, Hahn D, Schnekenburger J, Wiench K, Wohlleben W. Advanced Materials. 2010;22:2601. doi: 10.1002/adma.200902658. [DOI] [PubMed] [Google Scholar]
- 86.Frank W. Acta Biomaterialia. 2010;6:1680. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 87.Zhao M. Seminars in Cell & Developmental Biology. 2009;20:674. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 88.Gumus A, Califano JP, Wan AMD, Huynh J, Reinhart-King CA, Malliaras GG. Soft Matter. 2010;6:5138. [Google Scholar]
- 89.Rajnicek AM, Foubister LE, McCaig CD. Developmental Biology. 2007;312:448. doi: 10.1016/j.ydbio.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 90.Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Proceedings of the National Academy of Sciences USA. 2005;102:975. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerecht S, Bettinger CJ, Zhang Z, Borenstein J, Vunjak-Novakovic G, Langer R. Biomaterials. 2007;28:4068. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bettinger CJ, Zhang Z, Gerecht S, Borenstein JT, Langer R. Advanced Materials. 2008;20:99. doi: 10.1002/adma.200702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bettinger C, Langer R, Borenstein J. Angewandte Chemie. 2009;48:5406. doi: 10.1002/anie.200805179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Folch A, Jo BH, Hurtado O, Beebe DJ, Toner M. Journal of Biomedical Materials Research. 2000;52:346. doi: 10.1002/1097-4636(200011)52:2<346::aid-jbm14>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 95.Charest JL, Eliason MT, Garcia AJ, King WP, Talin AA, Simmons BA. Journal of Vacuum Science and Technology B. 2005;23:3011. [Google Scholar]
- 96.Fukuda J, Khademhosseini A, Yeh J, Eng G, Cheng J, Farokhzad OC, Langer R. Biomaterials. 2006;27:1479. doi: 10.1016/j.biomaterials.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 97.Khademhosseini A, Bettinger C, Karp JM, Yeh J, Ling Y, Borenstein J, Fukuda J, Langer R. Journal of Biomaterials Science Polymer Edition. 2006;17:1221. doi: 10.1163/156856206778667488. [DOI] [PubMed] [Google Scholar]
- 98.Bettinger CJ, Kulig KM, Vacanti JP, Langer R, Borenstein JT. Tissue Engineering. 2009;15:1321. doi: 10.1089/ten.tea.2008.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Svennersten K, Bolin MH, Jager EWH, Berggren M, Richter-Dahlfors A. Biomaterials. 2009;30:6257. doi: 10.1016/j.biomaterials.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 100.Persson KM, Karlsson R, Svennersten K, Löffler S, Jager EWH, Richter-Dahlfors A, Konradsson P, Berggren M. Advanced Materials. 2011;23:4403. doi: 10.1002/adma.201101724. [DOI] [PubMed] [Google Scholar]
- 101.Lundin V, Herland A, Berggren M, Jager EWH, Teixeira AI. PLoS ONE. 2011;6:e18624. doi: 10.1371/journal.pone.0018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Harder P, Grunze M, Dahint R, Whitesides GM, Laibinis PE. The Journal of Physical Chemistry B. 1998;102:426. [Google Scholar]
- 103.Ostuni E, Chapman RG, Holmlin RE, Takayama S, Whitesides GM. Langmuir. 2001;17:5605. doi: 10.1021/la0015258. [DOI] [PubMed] [Google Scholar]
- 104.Schmidt CE, Shastri VR, Vacanti JP, Langer R. Proceedings of the National Academy of Sciences USA. 1997;94:8948. doi: 10.1073/pnas.94.17.8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.George PM, Lyckman AW, LaVan DA, Hegde A, Leung Y, Avasare R, Testa C, Alexander PM, Langer R, Sur M. Biomaterials. 2005;26:3511. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 106.Lavan DA, George PM, Langer R. Angewandte Chemie. 2003;42:1262. doi: 10.1002/anie.200390323. [DOI] [PubMed] [Google Scholar]
- 107.Hara S, Zama T, Takashima W, Kaneto K. Synthetic Metals. 2004;146:47. [Google Scholar]
- 108.Smela E. Advanced Materials. 2003;15:481. [Google Scholar]
- 109.Svennersten K, Berggren M, Richter-Dahlfors A, Jager EWH. Lab on a Chip. 2011:11. doi: 10.1039/c1lc20436j. [DOI] [PubMed] [Google Scholar]
- 110.Fernandes TG, Diogo MM, Clark DS, Dordick JS, Cabral JMS. Trends in Biotechnology. 2009;27:342. doi: 10.1016/j.tibtech.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.George PM, LaVan DA, Burdick JA, Chen CY, Liang E, Langer R. Advanced Materials. 2006;18:577. [Google Scholar]
- 112.Simon DT, Kurup S, Larsson KC, Hori R, Tybrandt K, Goiny M, Jager EWH, Berggren M, Canlon B, Richter-Dahlfors A. Nature Materials. 2009;8:742. doi: 10.1038/nmat2494. [DOI] [PubMed] [Google Scholar]
- 113.Santini JT, Cima MJ, Langer R. Nature. 1999;397:335. doi: 10.1038/16898. [DOI] [PubMed] [Google Scholar]
- 114.Wadhwa R, Lagenaur CF, Cui XT. Journal of Controlled Release. 2006;110:531. doi: 10.1016/j.jconrel.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 115.Yali L, Neoh KG, Kang ET. Journal of Biomedical Materials Research: Part A. 2005;73A:171. doi: 10.1002/jbm.a.30286. [DOI] [PubMed] [Google Scholar]
- 116.Yang SY, Kim BN, Zakhidov AA, Taylor PG, Lee J-K, Ober CK, Lindau M, Malliaras GG. Advanced Materials. 2011;23:H184. doi: 10.1002/adma.201100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shire DB, Kelly SK, Jinghua C, Doyle P, Gingerich MD, Cogan SF, Drohan WA, Mendoza O, Theogarajan L, Wyatt JL, Rizzo JF. Biomedical Engineering, IEEE Transactions on. 2009;56:2502. doi: 10.1109/TBME.2009.2021401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chader GJ, Weiland J, Humayun MS. Progress in Brain Research. 2009;175:317. doi: 10.1016/S0079-6123(09)17522-2. [DOI] [PubMed] [Google Scholar]
- 119.Morrison B, III, Cater HL, Benham CD, Sundstrom LE. Journal of Neuroscience Methods. 2006;150:192. doi: 10.1016/j.jneumeth.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 120.Morrison B, III, Elkin BS, Dolle J-P, Yarmush ML. Annual Review of Biomedical Engineering. 2011;13:91. doi: 10.1146/annurev-bioeng-071910-124706. [DOI] [PubMed] [Google Scholar]
- 121.Lacour SP, Wagner S, Huang Z, Suo Z. Applied Physics Letters. 2003;82:2404. [Google Scholar]
- 122.Li T, Huang Z, Suo Z, Lacour SP, Wagner S. Applied Physics Letters. 2004;85:3435. [Google Scholar]
- 123.Graudejus O, Morrison B, Goletiani C, Yu Z, Wagner S. Advanced Functional Materials. 2012;22:640. doi: 10.1002/adfm.201102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Blau A, Murr A, Wolff S, Sernagor E, Medini P, Iurilli G, Ziegler C, Benfenati F. Biomaterials. 2011;32:1778. doi: 10.1016/j.biomaterials.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 125.Feili D, Schuettler M, Doerge T, Kammer S, Stieglitz T. Sensors and Actuators A: Physical. 2005;120:101. [Google Scholar]
- 126.Stieglitz T. Microsystem Technologies. 2009;16:723. [Google Scholar]
- 127.Hammerle H, Kobuch K, Kohler K, Nisch W, Sachs H, Stelzle M. Biomaterials. 2002;23:797. doi: 10.1016/s0142-9612(01)00185-5. [DOI] [PubMed] [Google Scholar]
- 128.Cogan SF, Ehrlich J, Plante TD, Smirnov A, Shire DB, Gingerich M, Rizzo JF. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;89B:353. doi: 10.1002/jbm.b.31223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stuart FCaPRTaJEaCMGaTDP. Journal of Neural Engineering. 2007;4:79. [Google Scholar]
- 130.Abidian MR, Corey JM, Kipke DR, Martin DC. Small. 2010;6:421. doi: 10.1002/smll.200901868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cui X, Lee VA, Raphael Y, Wiler JA, Hetke JF, Anderson DJ, Martin DC. Journal of Biomedical Materials Research. 2001;56:261. doi: 10.1002/1097-4636(200108)56:2<261::aid-jbm1094>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 132.Hong-Bo Z, Gang L, Xiao-Na S, Zhuang-Hui Z, Qing-Hui J, Jian-Long Z, Qiu- Shi R. Journal of Microelectromechanical Systems. 2009;18:88. [Google Scholar]
- 133.Grill WM, Norman SE, Bellamkonda RV. Annual Review of Biomedical Engineering. 2009;11:1. doi: 10.1146/annurev-bioeng-061008-124927. [DOI] [PubMed] [Google Scholar]
- 134.Asplund M, Nyberg T, Inganas O. Polymer Chemistry. 2010;1:1374. [Google Scholar]
- 135.Kotov NA, Winter JO, Clements IP, Jan E, Timko BP, Campidelli S, Pathak S, Mazzatenta A, Lieber CM, Prato M, Bellamkonda RV, Silva GA, Kam NWS, Patolsky F, Ballerini L. Advanced Materials. 2009;21:3970. [Google Scholar]
- 136.Shoval A, Adams C, David-Pur M, Shein M, Hanein Y, Sernagor E. Frontiers in Neuroengineering. 2009;2:1. doi: 10.3389/neuro.16.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Duan X, Gao R, Xie P, Cohen-Karni T, Qing Q, Choe HS, Tian B, Jiang X, Lieber CM. Nature Nanotechnology. 2011 doi: 10.1038/nnano.2011.223. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tian B, Cohen-Karni T, Qing Q, Duan X, Xie P, Lieber CM. Science. 2010;329:830. doi: 10.1126/science.1192033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Timko BP, Cohen-Karni T, Yu G, Qing Q, Tian B, Lieber CM. Nano Letters. 2009;9:914. doi: 10.1021/nl900096z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cohen-Karni T, Timko BP, Weiss LE, Lieber CM. Proceedings of the National Academy of Sciences USA. 2009;106:7309. doi: 10.1073/pnas.0902752106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lu F, Gu L, Meziani MJ, Wang X, Luo PG, Veca LM, Cao L, Sun Y-P. Advanced Materials. 2009;21:139. [Google Scholar]
- 142.Briseno AL, Mannsfeld SCB, Reese C, Hancock JM, Xiong Y, Jenekhe SA, Bao Z, Xia Y. Nano Letters. 2007;7:2847. doi: 10.1021/nl071495u. [DOI] [PubMed] [Google Scholar]
- 143.Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Nano Letters. 2008;8:4446. doi: 10.1021/nl802404h. [DOI] [PubMed] [Google Scholar]
- 144.Schwartz AB, Cui XT, Weber Douglas J, Moran DW. Neuron. 2006;52:205. doi: 10.1016/j.neuron.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 145.Viventi J, Kim D-H, Moss JD, Kim Y-S, Blanco JA, Annetta N, Hicks A, Xiao J, Huang Y, Callans DJ, Rogers JA, Litt B. Science Translational Medicine. 2010;2:24ra22. doi: 10.1126/scitranslmed.3000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yang ST, Zhang X, Wen Y. Current Opinion in Drug Discovery and Development. 2008;11:111. [PubMed] [Google Scholar]
- 147.Voskerician G, Shive MS, Shawgo RS, Recum Hv, Anderson JM, Cima MJ, Langer R. Biomaterials. 2003;24:1959. doi: 10.1016/s0142-9612(02)00565-3. [DOI] [PubMed] [Google Scholar]
- 148.Anderson J, McNally A. Seminars in Immunopathology. 2011;33:221. doi: 10.1007/s00281-011-0244-1. [DOI] [PubMed] [Google Scholar]
- 149.Chen S, Jones JA, Xu Y, Low H-Y, Anderson JM, Leong KW. Biomaterials. 2010;31:3479. doi: 10.1016/j.biomaterials.2010.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anderson JM, Rodriguez A, Chang DT. Seminars in Immunology. 2008;20:86. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Skousen SL, Merriam SM, Srivannavit O, Perlin G, Wise KD, Tresco PA. Progress in Brain Research. 2011;194:167. doi: 10.1016/B978-0-444-53815-4.00009-1. [DOI] [PubMed] [Google Scholar]
- 152.Cao H, McHugh K, Chew SY, Anderson JM. Journal of Biomedical Materials Research Part A. 2010;93A:1151. doi: 10.1002/jbm.a.32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Winslow BD, Tresco PA. Biomaterials. 2010;31:1558. doi: 10.1016/j.biomaterials.2009.11.049. [DOI] [PubMed] [Google Scholar]
- 154.Ko HC, Stoykovich MP, Song J, Malyarchuk V, Choi WM, Yu C-J, Geddes JB, III, Xiao J, Wang S, Huang Y, Rogers JA. Nature. 2008;454:748. doi: 10.1038/nature07113. [DOI] [PubMed] [Google Scholar]
- 155.Kim R-H, Kim D-H, Xiao J, Kim BH, Park S-I, Panilaitis B, Ghaffari R, Yao J, Li M, Liu Z, Malyarchuk V, Kim DG, Le A-P, Nuzzo RG, Kaplan DL, Omenetto FG, Huang Y, Kang Z, Rogers JA. Nature Materials. 2010;9:929. doi: 10.1038/nmat2879. [DOI] [PubMed] [Google Scholar]
- 156.Kim D-H, Lu N, Ghaffari R, Kim Y-S, Lee SP, Xu L, Wu J, Kim R-H, Song J, Liu Z, Viventi J, de Graff B, Elolampi B, Mansour M, Slepian MJ, Hwang S, Moss JD, Won S-M, Huang Y, Litt B, Rogers JA. Nature Materials. 2011;10:316. doi: 10.1038/nmat2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ellozy SH, Carroccio A, Lookstein RA, Minor ME, Sheahan CM, Juta J, Cha A, Valenzuela R, Addis MD, Jacobs TS, Teodorescu VJ, Marin ML. Journal of Vascular Surgery. 2004;40:405. doi: 10.1016/j.jvs.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 158.Springer F, Gunther R, Schmitz-Rode T. Cardiovascular and Interventional Radiology. 2008;31:460. doi: 10.1007/s00270-007-9245-9. [DOI] [PubMed] [Google Scholar]
- 159.Milner R, Ruurda JP, Blankensteijn JD. Journal of Endovascular Therapy. 2004;11:372. doi: 10.1583/04-1229.1. [DOI] [PubMed] [Google Scholar]
- 160.Kim D-H, Viventi J, Amsden JJ, Xiao J, Vigeland L, Kim Y-S, Blanco JA, Panilaitis B, Frechette ES, Contreras D, Kaplan DL, Omenetto FG, Huang Y, Hwang K-C, Zakin MR, Litt B, Rogers JA. Nature Materials. 2010;9:511. doi: 10.1038/nmat2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Benfenati V, Stahl K, Gomis-Perez C, Toffanin S, Sagnella A, Torp R, Kaplan DL, Ruani G, Omenetto FG, Zamboni R, Muccini M. Advanced Functional Materials. 2012 doi: 10.1002/adfm.201102310. (in press) [DOI] [PubMed] [Google Scholar]
- 162.Benfenati V, Toffanin S, Capelli R, Camassa LMA, Ferroni S, Kaplan DL, Omenetto FG, Muccini M, Zamboni R. Biomaterials. 2010;31:7883. doi: 10.1016/j.biomaterials.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Needles HL. Journal of Applied Polymer Science. 1967;11:719. [Google Scholar]
- 164.Nazarov R, Jin HJ, Kaplan DL. Biomacromolecules. 2004;5:718. doi: 10.1021/bm034327e. [DOI] [PubMed] [Google Scholar]
- 165.Bellan LM, Kniazeva T, Kim ES, Epshteyn AA, Cropek DM, Langer R, Borenstein JT. Advanced Healthcare Materials. 2012 doi: 10.1002/adhm.201100052. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Normand V, Muller S, Ravey J-C, Parker A. Macromolecules. 2000;33:1063. [Google Scholar]
- 167.Paguirigan A, Beebe DJ. Lab Chip. 2005;6:407. doi: 10.1039/b517524k. [DOI] [PubMed] [Google Scholar]
- 168.Irimia-Vladu M, Sariciftci NS, Bauer S. Journal of Materials Chemistry. 2011:21. doi: 10.1039/c3tb20193g. [DOI] [PubMed] [Google Scholar]
- 169.Burczak K, Gamianb E, Kochman A. Biomaterials. 1996;17:2351. doi: 10.1016/s0142-9612(96)00076-2. [DOI] [PubMed] [Google Scholar]
- 170.Chiellini E, Corti A, D’Antone S, Solaro R. Progress in Polymer Science. 2003;28:963. [Google Scholar]
- 171.Peppas NA, Merrill EW. Journal of Applied Polymer Science. 1977;21:1763. [Google Scholar]
- 172.Mathur AM, Moorjani SK, Scranton AB. Polymer Reviews. 1996;36:405. [Google Scholar]
- 173.Matsumura S, Tomizawa N, Toki A, Nishikawa K, Toshima K. Macromolecules. 1999;32:7753. [Google Scholar]
- 174.Siegel AC, Phillips ST, Dickey MD, Lu N, Suo Z, Whitesides GM. Advanced Functional Materials. 2009;20:28. [Google Scholar]
- 175.Rogers JA, Bao Z, Baldwin K, Dodabalapur A, Crone B, Raju VR, Kuck V, Katz H, Amundson K, Ewing J, Drzaic P. Proceedings of the National Academy of Sciences USA. 2001;90:4835. doi: 10.1073/pnas.091588098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Florian E, Hagen K, Marcus H, Ute Z, Gunter S, Christine D. Applied Physics Letters. 2004;84:2673. [Google Scholar]
- 177.Kelley TW, Baude PF, Gerlach C, Ender DE, Muyres D, Haase MA, Vogel DE, Theiss SD. Chemistry of Materials. 2004;16:4413. [Google Scholar]
- 178.Khodagholy D, Doublet T, Gurfinkel M, Quilichini P, Ismailova E, Leleux P, Herve T, Sanaur S, Bernard C, Malliaras GG. Advanced Materials. 2011;23:H268. doi: 10.1002/adma.201102378. [DOI] [PubMed] [Google Scholar]
- 179.Sekine S, Ido Y, Miyake T, Nagamine K, Nishizawa M. Journal of the American Chemical Society. 2010;132:13174. doi: 10.1021/ja1062357. [DOI] [PubMed] [Google Scholar]
- 180.Wallace GG, Moulton SE, Clark GM. Science. 2009;324:185. doi: 10.1126/science.1168346. [DOI] [PubMed] [Google Scholar]
- 181.Grill WM, Mortimer JT. Journal of Biomedical Materials Research. 2000;50:215. doi: 10.1002/(sici)1097-4636(200005)50:2<215::aid-jbm17>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 182.Plenk H., Jr Artificial Organs. 2011;35:237. doi: 10.1111/j.1525-1594.2011.01221.x. [DOI] [PubMed] [Google Scholar]
- 183.Majji AB, Humayun MS, Weiland JD, Suzuki S, D’Anna SA, de Juan E. Investigative Ophthamology and Visual Science. 1999;40:2073. [PubMed] [Google Scholar]
- 184.Humayun MS, Dorn JD, da Cruz L, Dagnelie G, Sahel J-A, Stanga PE, Cideciyan AV, Duncan JL, Eliott D, Filley E, Ho AC, Santos A, Safran AB, Arditi A, Del Priore LV, Greenberg RJ. Ophthalmology. 2012 doi: 10.1016/j.ophtha.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ghezzi D, Antognazza MR, Dal Maschio M, Lanzarini E, Benfenati F, Lanzani G. Nature Communications. 2011;2:166. doi: 10.1038/ncomms1164. [DOI] [PubMed] [Google Scholar]
- 186.Weiland JD, Humayun MS. Proceedings of the IEEE. 2008;96:1076. [Google Scholar]
- 187.Caspi A, Dorn JD, McClure KH, Humayun MS, Greenberg RJ, McMahon MJ. Archives of Ophthamology. 2009;127:398. doi: 10.1001/archophthalmol.2009.20. [DOI] [PubMed] [Google Scholar]
- 188.Kandagor V, Cela CJ, Sanders CA, Greenbaum E, Lazzi G, Zhou DD, Castro R, Gaikwad S, Little J, Zhou D, Greenbaum E. Implantable Neural Prostheses. Vol. 2. Springer; New York: 2010. p. 139. [Google Scholar]
- 189.Zhong Y, Yu X, Gilbert R, Bellamkonda RV. Journal of Rehabilitation Research and Development. 2001;38:627. [PubMed] [Google Scholar]
- 190.Miller MS, Davidson GJE, Sahli BJ, Mailloux CM, Carmichael TB. Advanced Materials. 2008;20:59. [Google Scholar]
- 191.Green MA, Bilston LE, Sinkus R. NMR in Biomedicine. 2008;21:755. doi: 10.1002/nbm.1254. [DOI] [PubMed] [Google Scholar]
- 192.Chen K, Rowley AP, Weiland JD. Journal of Biomedical Materials Research. 2010;93A:634. doi: 10.1002/jbm.a.32571. [DOI] [PubMed] [Google Scholar]
- 193.Winter JO, Gokhale M, Jensen RJ, Cogan SF, Rizzo JF., III Materials Science and Engineering: C. 2008;28:448. doi: 10.1016/j.msec.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Winslow BD, Christensen MB, Yang W-K, Solzbacher F, Tresco PA. Biomaterials. 2010;31:9163. doi: 10.1016/j.biomaterials.2010.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Anthony G-E. Biomaterials. 2010;31:2701. [Google Scholar]
- 196.Lu Y, Wang D, Li T, Zhao X, Cao Y, Yang H, Duan YY. Biomaterials. 2009;30:4143. doi: 10.1016/j.biomaterials.2009.04.030. [DOI] [PubMed] [Google Scholar]
- 197.Lu Y, Li Y, Pan J, Wei P, Liu N, Wu B, Cheng J, Lu C, Wang L. Biomaterials. 2012;33:378. doi: 10.1016/j.biomaterials.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 198.Ghosh S, Inganäs O. Advanced Materials. 1999;11:1214. [Google Scholar]
- 199.Runge MB, Dadsetan M, Baltrusaitis J, Ruesink T, Lu L, Windebank AJ, Yaszemski MJ. Biomacromolecules. 2010;11:2845. doi: 10.1021/bm100526a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kim D-H, Abidian M, Martin DC. Journal of Biomedical Materials Research Part A. 2004;71A:577. doi: 10.1002/jbm.a.30124. [DOI] [PubMed] [Google Scholar]
- 201.Ouyang J, Chu CW, Chen FC, Xu Q, Yang Y. Advanced Functional Materials. 2005;15:203. [Google Scholar]
- 202.Egeland BM, Urbanchek MG, Peramo A, Richardson-Burns SM, Martin DC, Kipke DR, Kuzon WM, Jr, Cederna PS. Plastic and Reconstructive Surgery. 2010;126:1865. doi: 10.1097/PRS.0b013e3181f61848. [DOI] [PubMed] [Google Scholar]
- 203.Richards-Grayson AC, Choi IS, Tyler BM, Wang PP, Brem H, Cima MJ, Langer R. Nature Materials. 2003;2:767. doi: 10.1038/nmat998. [DOI] [PubMed] [Google Scholar]
- 204.Radisic M, Park H, Shing H, Consi T, Schoen FJ, Langer R, Freed LE, Vunjak-Novakovic G. Proceedings of the National Academy of Sciences USA. 2004;101:18129. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tandon N, Cannizzaro C, Chao P-HG, Maidhof R, Marsano A, Au HTH, Radisic M, Vunjak-Novakovic G. Nature Protocols. 2009;4:155. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Tissue Engineering A. 2009;15:3605. doi: 10.1089/ten.TEA.2008.0689. [DOI] [PubMed] [Google Scholar]
- 207.Rivers TJ, Hudson TW, Schmidt CE. Advanced Functional Materials. 2002;12:33. [Google Scholar]
- 208.Zelikin AN, Lynn DM, Farhadi J, Martin I, Shastri V, Langer R. Angewandte Chemie. 2002;41:141. doi: 10.1002/1521-3773(20020104)41:1<141::aid-anie141>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 209.Bettinger CJ, Bao Z. Polymer International. 2010;59:563. doi: 10.1002/pi.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Bettinger CJ, Bao Z. Advanced Materials. 2010;22:651. doi: 10.1002/adma.200902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.McCullough LA, Dufour B, Matyjaszewski K. Macromolecules. 2009;42:8129. [Google Scholar]
- 212.Shi G, Rouabhia M, Wang Z, Dao LH, Zhang Z. Biomaterials. 2004;25:2477. doi: 10.1016/j.biomaterials.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 213.Neffe AT, Hanh BD, Steuer S, Lendlein A. Advanced Materials. 2009;21:3394. doi: 10.1002/adma.200802333. [DOI] [PubMed] [Google Scholar]
- 214.Lendlein A, Langer R. Science. 2002;296:1673. doi: 10.1126/science.1066102. [DOI] [PubMed] [Google Scholar]
- 215.Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Cell. 2010;141:154. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Zhao J, Swinnen A, Assche GV, Manca J, Vanderzande D, Mele BV. Journal of Physical Chemistry B. 2009;113:1587. doi: 10.1021/jp804151a. [DOI] [PubMed] [Google Scholar]
- 217.Verploegen E, Mondal R, Bettinger CJ, Sok S, Toney MF, Bao Z. Advanced Functional Materials. 2010;20:3519. [Google Scholar]
- 218.Wiesmann H-P, Hartig M, Stratmann U, Meyer U, Joos U. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2001;1538:28. doi: 10.1016/s0167-4889(00)00135-x. [DOI] [PubMed] [Google Scholar]
- 219.Adams D, Levin M. Cell and Tissue Research. 2012:1. doi: 10.1007/s00441-012-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Akkerman HB, Chang AC, Verploegen E, Bettinger CJ, Toney MF, Bao Z. Organic Electronics. 2012;13:235. [Google Scholar]
- 221.Letouzey V, Lavigne JP, Garric X, Coudane J, de Tayrac R, Callaghan DO. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;100B:471. [Google Scholar]
- 222.Bellows CF, Wheatley BM, Moroz K, Rosales SC, Morici LA. PLoS ONE. 2011;6:e21228. doi: 10.1371/journal.pone.0021228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Krouskop TA, Brown HD, Gray K, Shively J, Romovacek GR, Spira M, Runyan RS. Biomaterials. 1988;9:398. doi: 10.1016/0142-9612(88)90003-8. [DOI] [PubMed] [Google Scholar]
- 224.Keselowsky BG, Bridges AW, Burns KL, Tate CC, Babensee JE, LaPlaca MC, García AJ. Biomaterials. 2007;28:3626. doi: 10.1016/j.biomaterials.2007.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.James SJ, Pogribna M, Miller BJ, Bolon B, Muskhelishvili L. Biomaterials. 1997;18:667. doi: 10.1016/s0142-9612(96)00189-5. [DOI] [PubMed] [Google Scholar]
- 226.Goodman SB, Gómez Barrena E, Takagi M, Konttinen YT. Journal of Biomedical Materials Research Part A. 2009;90A:603. doi: 10.1002/jbm.a.32063. [DOI] [PubMed] [Google Scholar]