Psychiatric disorders are increasingly regarded as diseases of the brain, as opposed to diseases of the mind; they are alterations in neurobiological brain circuits that are influenced by genetic and environmental factors [1]. Nonetheless, diagnostic classifications are still largely based on clinical observation and symptom reports by patients, rather than biological evidence. Consequently, treatment is aimed at reducing and managing observable symptoms. This inability to target the causes of disease results in suboptimal response rates and adverse effects of medication. For example, 30–40% of patients with depression do not respond to appropriate drug therapy and treatment, and approximately one-third of those with schizophrenia do not respond to standard treatments [2,3,4,5]. Understanding the real biological causes of psychiatric disorders would therefore help to improve diagnostics and treatment efficiency, and should reduce adverse side effects. It would also help to decrease the health and economic burden of psychiatric diseases, which costs the National Health Service (NHS) in England approximately £22.5 billion per year [6].
Advances in clinical neuroscience, genomics and neuroimaging are increasing our knowledge of brain function and promise to elucidate aetiological factors of neuropsychiatric disorders. Detecting associations between genetic or environmental factors and a given disease, however, requires studies with access to many samples. A biobank that collects biological samples from patients with psychiatric disorders, along with their clinical records and neuroimaging data could, therefore, become an important component for basic and clinical research. It would allow a more precise identification of disease mechanisms, contribute to devising specific and personalized therapies that target relevant neural processes effectively, help to identify individuals for whom particular treatments will be harmful, and hopefully bring us closer to identifying factors that can either prevent or minimize the occurrence of neuropsychiatric disorders. However, to our knowledge, there are no dedicated mental-health biobanks within the NHS.
…diagnostic classifications are still largely based on clinical observation and symptom reports by patients, rather than biological evidence
We therefore conducted a pilot study at three hospitals of the South London and Maudsley NHS Foundation Trust: the Bethlem Royal Hospital, Maudsley Hospital and Lambeth Hospital; to identify a feasible framework for a biobank that addresses the unique ethical and governance challenges associated with mental-health research. It is based on the UK Biobank protocol to ensure that samples are suitable for many types of investigations including genomic, epigenomic, transcriptomic, proteomic and metabonomic analyses, but expands the protocol to respect the particular vulnerabilities of mental-health patients [7]. Important challenges that we faced included the establishment of simple procedures that could be implemented within a busy clinical setting, and addressing the vulnerability of mental-health patients by developing appropriate safeguards that would not place undue limitations on the scope and utility of the collection. This paper focuses on some of the crucial ethical and governance challenges, discusses measures to address these challenges, and proposes a model for a mental-health biobank within a clinical setting.
At the heart of a biobank is consent by participants or patients. Accordingly, there is considerable debate regarding the appropriate type of consent. The two most common options are project-specific consent and broad consent. In the first case, participants agree that their samples and data are used for only one clearly specified research project. Researchers who wish to reuse the samples and data for additional experiments and analyses have to contact participants a second time to ask for new consent. The primary disadvantages of specific consent are that it is costly and time consuming. Furthermore, there is some attrition in participant numbers and the process has the potential to introduce sample selection biases [8,9].
The alternative is broad consent, by which participants give consent for their samples and data to be used for unspecified future research, making the process more efficient. Importantly, studies indicate that members of the public might agree to broad consent because it limits the number of times they will be approached by researchers [8,10,11,12]. Nevertheless, broad consent does raise some ethical issues, as donors have only limited control over the use of their samples and data and are usually unaware of the type of future research that could be carried out. Even so, the prevailing view is that broad consent is acceptable if all proposed changes to existing procedures and protocols are reviewed and approved by a research ethics committee, and if all participants have the right to withdraw their consent at any time [13]. We therefore opted for broad consent with requisite safeguards as outlined below.
Although the consent process for a mental-health biobank is similar to most other biobanks, there are two key differences: first, there is a higher proportion of vulnerable individuals among psychiatric patients, which necessitates a more cautious and considered approach. Second, the stigma that still surrounds mental illness perpetuates a sense of secrecy among sufferers for fear of discrimination. As a consequence of addressing these issues, the process of acquiring samples becomes more elaborate and is harder to integrate into clinical settings in comparison with many other research studies or biobanks. We trialled several implementation options on low-, medium- and high-throughput wards to obtain a clearer idea of what would be feasible and sustainable in each setting. This was combined with obtaining personal views from clinical staff and led to the decision to develop an adaptive and collaborative add-on approach for the implementation on each ward and to use additional personnel—the research team and phlebotomy services—to carry out sample acquisition.
As mentioned above, a particular issue for a mental-health biobank is the capacity, or lack thereof, of participants to provide informed consent. The Mental Capacity Act (MCA) 2005 for England and Wales stipulates that capacity should always be assumed unless there is reason to believe that the individual might not be able to make a particular decision at the time of being asked [14,15]. Briefly, capacity means that the individual is able to understand information about the required decision (the Act calls this “relevant information”), retain that information in the mind, use or weigh that information as part of the decision-making process and communicate their decision [14,15].
However, we noticed during the initial phase of the project that in many cases, it was difficult to establish whether or not an individual had the capacity to consent. This observation was in agreement with a study conducted at the same NHS Trust, which found that approximately 60% of individuals admitted to psychiatric hospitals—especially patients diagnosed with mania or psychosis and patients who are formally detained for treatment under the Mental Health Act—lack the capacity to make decisions about their treatment [16]. By extension of this, it is probable that a high percentage of those admitted to a psychiatric hospital are likely to lack the capacity to make decisions about research participation. Furthermore, several patients would listen attentively and actively engage with the researcher, but at the end of the session, could not say what the research was about or what participation would involve.
Another example involved a young woman with anorexia on an eating disorders ward, who immediately expressed a wish to participate when she was told that taking part would involve donating a blood sample. She was not interested in the details of what her sample would be used for, but focused on the fact that she had an opportunity to provide a blood sample, leaving the researcher with the impression that the young woman felt that providing blood would help her lose weight. Her decision to participate was seemingly not based on the information provided and thus was not an informed one.
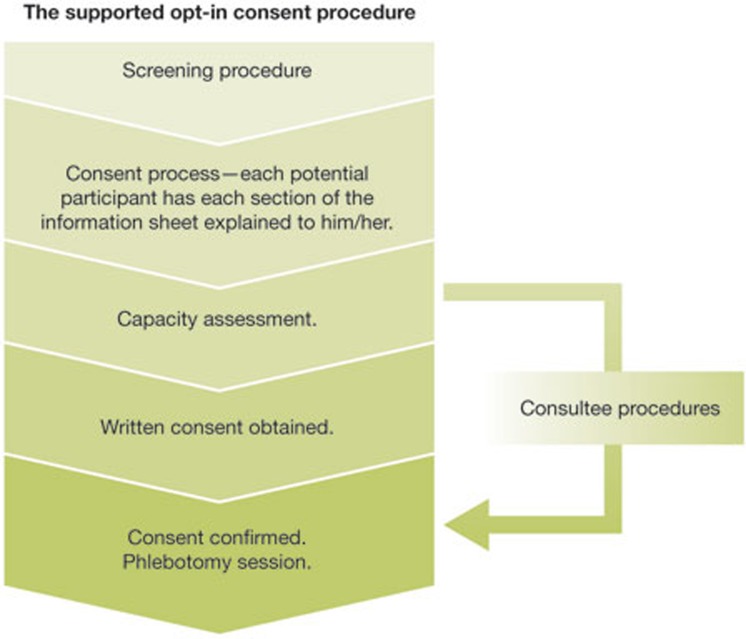
Thus, we needed to ensure that our procedures (Fig 1) would be in accordance with the MCA and all other governance stipulations; we needed to ensure that vulnerable patients would not be exploited or included against their will, and that the autonomy of all potential participants would always be respected; and we needed to ensure that certain individuals or populations who might seem to lack the capacity to consent would not be excluded, as this would result in the inadvertent restriction of research and unfairly stop those who might wish to participate.
Figure 1.
Stages from identifying potential participants to obtaining samples.
We therefore decided to include the safeguard of conducting a brief capacity assessment on all participants before obtaining their consent. The form we used included the four crucial elements of assessing capacity and the evidence for the researcher's finding [17]. This ensured that all participants could make an informed decision at the time of giving consent. To minimize the possibility of certain individuals or groups being excluded, we liaised with clinical teams and reapproached both those who were likely to regain capacity at a later date and implemented consultee procedures for those who were unlikely to regain capacity. We appreciate that some might consider that our decision to conduct capacity assessments on all participants was not strictly in agreement with the MCA. Nevertheless, we felt that on balance, this was the most appropriate course of action given that if we assumed capacity, some individuals who lacked the capacity to make the decision to participate might inadvertently be included, and that a proportion of these individuals might later feel that they had been exploited when they were vulnerable.
A biobank that collects biological samples […] would allow a more precise identification of disease mechanisms…
During the course of the trial, we found that some individuals had comprehension difficulties, possibly owing to impaired brain functioning, low literacy levels, side effects of medication or a combination of these and other factors. Other studies have reported similar findings, including one that found that as many as 75% of those accessing psychiatric services from community settings, such as walk-in clinics, had a reading age below that of a 13-year-old [18,19]. To address this, our information sheets were written in simple language, used the active form and short sentences as much as possible, and included descriptive headings; all measures that have been found to be beneficial in the past [20].
As the pilot was within a clinical setting, it was vital to identify individuals who should not be approached. Therefore, we asked ward doctors to identify individuals who were too unwell to be approached by researchers, who had expressed a wish not to be approached by research staff, who could not tolerate giving blood or simply did not like giving blood, who might be distressed if they were approached by a stranger, or who would require additional support, such as a translator. This screening also highlighted possible risks, such as patients with a propensity to behave aggressively and how the researcher could best deal with the situation if required.
Although the consent process for a mental-health biobank is similar […] there is a higher proportion of vulnerable individuals among psychiatric patients…
Once a patient was cleared to be approached after the screening process, we used a supported opt-in procedure during which the researcher talked through and explained each section of the information sheet. We felt this was necessary to ensure that potential participants were informed of the relevant information, particularly as we were requesting broad consent and because some participants might not consider the information sheet or the consequences of doing so, as they do not perceive informed consent to be important [21,22]. During this process, the researcher also informed potential participants that donated samples and data would only be used for research in mental health, and that they might be invited to participate in future related research, but that they were under no obligation to do so. We reassured them that they could withdraw from the study at any time and that they could either have their data remain in the bank with their name and contact details removed, or have their samples destroyed. We also described the measures to protect participant privacy and briefly outlined our data-sharing policies. Furthermore, we explained to potential participants that their inclusion in the biobank would be a voluntary initiative and that they would not receive anything in return for taking part. The next step was to conduct the brief capacity assessment. If we were satisfied that the patient had the capacity to make an informed decision to participate, we would go through the consent form with the patient and obtain his or her written consent.
The final stage of the process involved a phlebotomy appointment to obtain the samples. This was scheduled at least a day later to make sure that the participants did not feel coerced into participating. It also gave them time to discuss participation with family, friends or their clinician before making a final decision. During the phlebotomy appointment, the participant's consent was confirmed before the samples were collected. We endeavoured to have the same researcher complete the whole process for consistency and because we found that participants would be less likely to withdraw their consent when that was the case (Fig 1).
For those potential participants who lacked the capacity to give informed consent and who were unlikely to regain capacity, consultee procedures were implemented with the proposed participant integrally involved in the selection of a consultee and with subsequent elements. This gave patients the opportunity to participate in the decision-making process to the extent that they were able, and minimized the possibility of them being included against their wishes [23]. If the consultee advised that the proposed participant would want to be involved in the study (if he/she had the capacity to make that decision) the subsequent procedures were identical to the ones described above. However, if the participant appeared to object at any point, the researcher withdrew the individual from the study.
Confidentiality and data security are fundamental to biobanks. Moreover, concerns about these aspects of biobanks are a major reason why people decline to participate [12,24]. This is even more pertinent for mental-health biobanks, given the broad stigma that is associated with mental illnesses. In a survey conducted by the Mental Health Foundation, a leading UK mental-health research, policy and service improvement charity, 56% and 51% of people suffering from mental distress had experienced discrimination from family and friends respectively, and 44% had experienced discrimination from a General Practitioner (GP; doctor) working in a primary healthcare setting. Furthermore, 37% of people suffering from mental distress had experienced discrimination when seeking employment and 47% had experienced discrimination at the workplace [25].
…a young woman with anorexia [left] the researcher with the impression that [she] felt that providing blood would help her lose weight
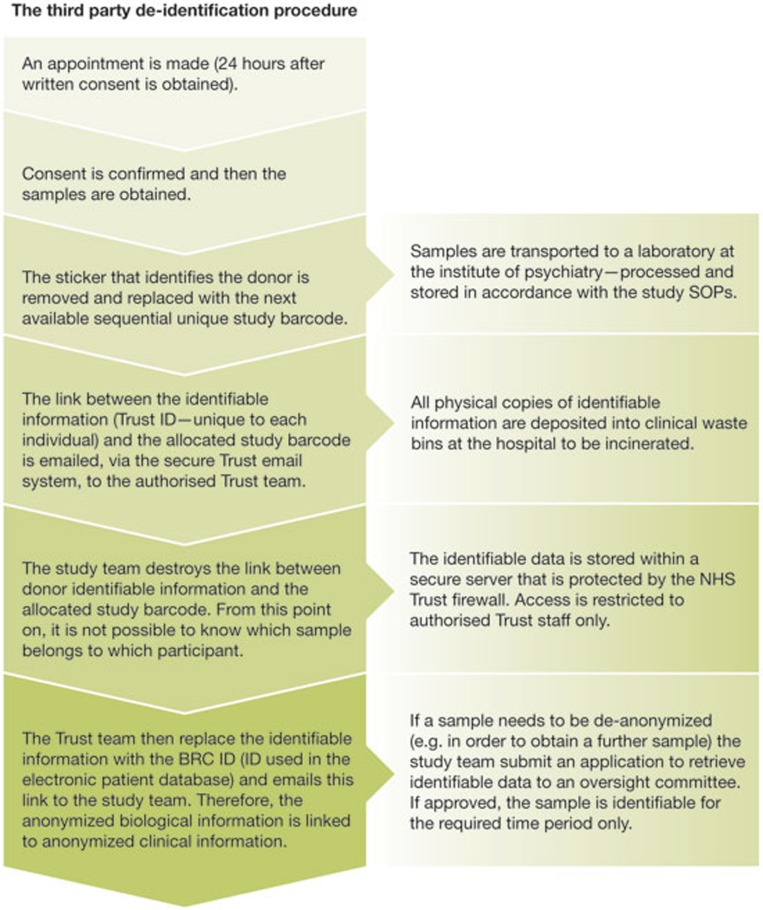
To address concerns about privacy, we needed to develop procedures that would maximize participant confidentiality and data security. The first step was to remove all identifiable information from a sample before it was taken off the NHS Trust site for processing and storage, to minimize the likelihood of the inadvertent loss of personal data. The second step was to pass on to a trusted third party at the hospital, through the Trust secure e-mail system, the link between the individual's hospital reference number and the unique sample collection code allocated to the participant (Fig 2). This third-party anonymization process was approved by the Caldicott guardian who is responsible for protecting patient information within the Trust. It enabled us to link the biological samples with clinical notes and neuroimaging data, where possible, without identifying patients. This was achieved by using an existing electronic patient database, in this instance, the Biomedical Research Centre electronic Case Register Interactive Search system [26]. Importantly, this worked without removing the participant's data from the servers, which are located within the Trust's secure firewall. However, these procedures, which are viable at present, will need to be reviewed and enhanced to ensure that increasing sample numbers can be accommodated. We are also exploring the possibility of developing an automated system that would increase overall efficiency and decrease the potential for human error without compromising data security.
Figure 2.
Stepped procedures developed to safeguard personal information of participants and to maximize data security.
Our third-party anonymization procedure also enables researchers to re-contact patients and ask for additional samples and information for further investigations. We have developed and obtained ethical approval for such procedures to ensure that we do not inadvertently cause distress or grief to family members of participants who might have passed away. If the individual is not accessing hospital services, the team will send the participant an initial neutral invitation letter after checking their clinical records to check for any pertinent information. Once contacted, and if the individual is deemed to have the capacity to make the decision and decides to consent, we offer to provide them with the details of the research team conducting the research. This gives the participant the opportunity to speak directly to the researchers if they wish, but ensures their anonymity if they decide not to do so.
The ability to re-contact specific individuals is of particular importance for mental-health research because it enables a longitudinal approach and the characterization of markers that are potentially predictive for disease course: for predicting symptoms and response to therapy. It also enables us to contact a participant's GP or clinician in case of a clinically significant incidental finding.
There are no clear UK guidelines regarding the reporting of clinically significant pertinent or incidental findings. A study commissioned by the Wellcome Trust and the Medical Research Council revealed that there was “overwhelming support for the return of health-related findings to research participants, particularly where a condition is serious and treatable” [27], which demonstrates that our approach seems to be in agreement with public expectations. The aforementioned report also revealed that participants had indicated a preference for being offered a choice about whether to be re-contacted with regards to incidental findings. Future work will therefore require a structured assessment of stakeholder views, particularly on whether it would influence someone's decision to participate if they knew that in certain situations, researchers would be obliged to inform them of findings, even if they had chosen otherwise.
During the development of a mental-health biobank, we had to consider whether we should compensate participants for their time and effort. This raised the issue, however, of whether some patients would be more motivated to participate if they knew there would be a financial gain. Research indicates that the motivations underlying research participation are varied and complex; it is not just a risk–benefit assessment or a purely altruistic act [28,29].
However, whether or not to compensate participants in a mental-health biobank is a more complex and challenging issue. A central priority should be to ensure that individuals who are cognitively vulnerable, or who might be vulnerable to coercion or undue influence, do not feel compelled to participate owing to a financial incentive [30]. Additionally, our biobank is located within an NHS Trust that serves some of the more disadvantaged regions of London. As such, even a modest financial compensation might be sufficient to influence some individuals to participate. Therefore, we decided to follow a conservative approach of not compensating participants.
We have successfully implemented this pilot project on several in-patient wards. Both participants and clinical staff have accepted the procedures and the feedback has been positive. In terms of participation, a lack of compensation is one of the most common reasons for declining to participate, alongside a fear or dislike of giving blood. Conversely, many individuals expressed satisfaction with or a sense of duty about helping others. We have not conducted a systematic investigation into the differences in participation rates in the different subpopulations as yet, although we have observed some emerging trends. Anecdotally, those with mood disorders have been most interested in participating. Those receiving treatment at psychosis wards have been more reluctant, presumably due to the presence of paranoid, delusional or negative symptoms. Finally, those receiving treatment at forensic units most concerned about confidentiality and data safety.
This pilot project has several unique advantages. It is taking place within an NHS Trust that actively engages in research, receives thousands of referrals each year and has several specialist complex care units that accept referrals from across the UK and provides a wide range of mental-health services. Together, these factors increase the possibility of obtaining samples from various psychiatric populations. Additionally, several individuals will probably access services from the Trust over a long period, which will enable the collection of multiple samples and richer clinical data for longitudinal research. Finally, the geographical location of the Trust also enables the collection of representative sample numbers from different ethnicities.
Our goal was to develop a feasible and practical framework for a mental-health biobank in a clinical setting that considers the unique ethical and governance challenges associated with mental-health research. Our model aims to ensure that the best interests of potential participants are upheld and enables the acquisition of biological and, where possible, neuroimaging data. Furthermore, the structure allows for the targeted recall of participants, and also enables the team to feed back information in the event of a clinically significant pertinent or incidental finding. So far, we have successfully implemented this pilot on wards at the largest mental-health Trust in the UK. The procedures implemented have been accepted by both participants and clinical staff; additionally, most participants have been interested in learning more about research and several participants have also mentioned that they appreciated the opportunity to speak to researchers about the goals of the study and how research has the potential to inform future clinical care. Future work will need to formally evaluate the above procedures in terms of long-term acceptability, feasibility and efficiency. However, we hope that our pilot will encourage the development of other biobanks and sample collections in mental-health research.
Acknowledgments
This work was supported by United Kingdom National Institute for Health Research Biomedical Research Centre Mental Health, the South London and Maudsley NHS Foundation Trust, the Innovative Medicine Initiative Project EU-AIMS (115300-2) and the Medical Research Council Programme Grant ‘Developmental pathways into adolescent substance abuse’ (93558). Further support was provided by the the Swedish Research Council Formas http://www.formas.se/en/.
Footnotes
The authors declare that they have no conflict of interest.
References
- Insel TR, Wang PS (2010) Rethinking mental illness. JAMA 303: 1970–1971 [DOI] [PubMed] [Google Scholar]
- Spear BB, Heath-Chiozzi M, Huff J (2001) Clinical application of pharmacogenetics. Trends Mol Med 7: 201–204 [DOI] [PubMed]
- Fava M (2003) Diagnosis and definition of treatment-resistant depression. Biol Psych 53: 649–659 [DOI] [PubMed] [Google Scholar]
- Souery D, Amsterdam J, de Montigny C, Lecrubier Y, Montgomery S, Lipp O, Racagni G, Zohar J, Mendlewicz J (1999) Treatment resistant depression: methodological overview and operational criteria. Eur Neuropsychopharmacol 9: 83–91 [DOI] [PubMed] [Google Scholar]
- Barry SJE, Gaughan TM, Hunter R (2012) Systematic Review of Schizophrenia. Clinical Evidence. http://clinicalevidence.bmj.com/x/pdf/clinical-evidence/en-gb/systematic-review/1007.pdf. [PMC free article] [PubMed] [Google Scholar]
- McCrone P, Dhanasiri S, Patel A, Knapp M, Lawton-Smith S (2008) Paying the Price: The Cost of Mental Health Care in England to 2026. London, UK: King's Fund. http://www.kingsfund.org.uk/sites/files/kf/Paying-the-Price-the-cost-of-mental-health-care-England-2026-McCrone-Dhanasiri-Patel-Knapp-Lawton-Smith-Kings-Fund-May-2008_0.pdf [Google Scholar]
- Elliott P, Peakman TC, UK Biobank (2008) The UK Biobank sample handling and storage protocol for the collection, processing and archiving of human blood and urine. Int J Epidemiol 37: 234–244 [DOI] [PubMed] [Google Scholar]
- Wendler D (2006) One-time general consent for research on biological samples. BMJ 332: 544–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson MG, Dillner J, Bartram CR, Carlson JA, Helgesson G (2006) Should donors be allowed to give broad consent to future biobank research? Lancet Oncol 7: 266–269 [DOI] [PubMed] [Google Scholar]
- Elger BS, Caplan AL (2006) Consent and anonymization in research involving biobanks. EMBO Rep 7: 661–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biobank UK (2003b) UK Biobank Consultation on the Ethical and Governance Framework. London, UK: People Science & Policy Ltd [Google Scholar]
- Kaufman DJ, Murphy-Bollinger J, Scott J, Hudson KL (2009) Public opinion about the importance of privacy in biobank research. Am J Hum Genet 85: 643–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of Europe Committee of Ministers. Recommendation Rec(2006)4 of the Committee of Ministers to member states on research on biological materials of human origin. Strasbourg, France: Council of Europe
- Mental Capacity Act 2005 c.9 (2005) London, UK: HMSO [Google Scholar]
- Department for Constitutional Affairs. (2007) Mental Capacity Act 2005 Code of practice. London, UK: TSO [Google Scholar]
- Owen GS, Richardson G, David AS, Szmukler G, Hayward P, Hotopf M (2008) Mental capacity to make decisions on treatment in people admitted to psychiatric hospitals: a cross sectional study. BMJ 337: a448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J, McCarney R, Griffin M, Hill K, Fisher P (2008) Participation in dementia research: rates and correlates of capacity to give informed consent. J Med Ethics 34: 167–170 [DOI] [PubMed]
- Christensen RC, Grace GD (1999) The prevalence of low literacy in an indigent psychiatric population. Psychiatr Serv 50: 262–263 [DOI] [PubMed] [Google Scholar]
- Gralton E, Sher M, Lopez CD (2010) Information and readability issues for psychiatric patients: e-learning for users. The Psychiatrist 34: 376–380 [Google Scholar]
- Beskow LM, Friedman JY, Hardy NC, Lin L, Weinfurt KP (2010) Developing a simplified consent form for biobanking. PLoS ONE 5: e13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porteri C, Borry P (2008) A proposal for a model of informed consent for the collection, storage and use of biological materials for research purposes. Patient Educ Couns 71: 136–142 [DOI] [PubMed] [Google Scholar]
- Busby H (2006) Consent, trust and ethics: reflections on the findings of an interview based study with people donating blood for genetic research within the NHS. Clin Ethics 1: 211–215 [Google Scholar]
- Warren JW, Sobal J, Jame MPH, Tenney JH, Hoopes JM, Damron D, Levenson S, DeForge BR, Muncie HL (1986) Informed consent by proxy: an issue in research with elderly patients. N Engl J Med 315: 1124–1128 [DOI] [PubMed] [Google Scholar]
- Goddard KAB, Smith KS, Chen C, McMullen C, Johnson C (2009) Biobank recruitment: motivations for nonparticipation. Biopreserv Biobank 7: 119–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Mental Health Foundation (2000) Pull yourself together! A survey of the stigma and discrimination faced by people who experience mental distress. Updates Volume 2 Issue 4. http://www.mentalhealth.org.uk/content/assets/PDF/publications/pull_yourself_togther_update.pdf?view=Standard
- Stewart R, Soremekun M, Perera G, Broadbent M, Callard F, Denis M, Hotopf M, Thornicroft G, Lovestone S (2009) The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: development and descriptive data. BMC Psychiatry 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Wellcome Trust, Medical Research Council and Opinion Leader (2012) Assessing Public Attitudes to Health Related Findings in Research. London, UK: The Wellcome Trust, Medical Research Council and Opinion Leader [Google Scholar]
- Hallowell N, Cooke S, Crawford G, Lucassen A, Parker M, Snowdon C (2012) An investigation of patients' motivations for their participation in genetics-related research. J Med Ethics 36: 37–45 [DOI] [PubMed] [Google Scholar]
- Gaskell G, Gottweis H (2011) Biobanks need publicity. Nature 471: 159–160 [DOI] [PubMed] [Google Scholar]
- World Medical Association (2008) The Declaration of Helsinki. http://www.wma.net/en/20activities/10ethics/10helsinki/ [Google Scholar]