Abstract
Objective
To isolate and analyse the chemical composition in the essential oils and free radical scavenging activity of different crude extracts from the fresh and dry leaves of vegetable plants of Lactuca sativa L. (L. sativa).
Methods
The essential oils and volatile chemical constituents were isolated from the fresh and dry leaves of L. sativa (lettuce) grown in Sultanate of Oman by hydro distillation method. The antioxidant activity of the crude extracts was carried out by well established free radical scavenging activity (DPPH) method.
Results
About 20 chemical compounds of different concentration representing 83.07% and 79.88% respectively were isolated and identified by gas chromatography-mass spectroscopy in the essential oils isolated from the fresh and dry leaves as α-pinene (5.11% and 4.05%), γ-cymene (2.07% and 1.92%), thymol (11.55% and 10.73%), durenol (52.00% and 49.79%), α-terpinene (1.66% and 1.34%), thymol acetate (0.99% and 0.67%), caryophyllene (2.11% and 1.98%), spathulenol (3.09% and 2.98%), camphene (4.11% and 3.65%), limonene (1.28% and 1.11%) representing these major chemical compounds. However, some other minor chemical constituents were also isolated and identified from the essential oil of lettuce including β-pinene, α-terpinolene, linalool, 4-terpineol, α-terpineol, o-methylthymol, L-alloaromadendrene and viridiflorene.
Conclusions
The chemical constituents in the essential oils from the locally grown lettuce were identified in the following classes or groups of chemical compounds such as monoterpenes, sesquiterpenes volatile organic compounds and their oxygenated hydrocarbons. Therefore, the essential oils and the crude extracts from Omani vegetable species of lettuce are active candidates which would be used as antioxidant, antifungal or antimicrobial agents in new drugs preparation for therapy of infectious diseases.
Keywords: Lactuca sativa, Omani lettuce, Clevenger apparatus, Essential oil, Durenol, Thymol, Soxhlet extractor, Organic crude extracts, Antioxidant activity, GC-MS analyses
1. Introduction
Almost all the medicinal plants available in the world have great potential sources for discovery as well as production of new drugs benefit to mankind. Presently, there are lot of approaches available to search for new biologically active ingredients in the medicinal plants for the preparation of safe drugs. Scientifically many works have been expended to evaluate and discover new antioxidant, antimicrobial and antifungal ingredients from different kinds of natural sources like soil, microorganisms, animals and plants. Different types of folk medicine or herbal medicine are among the most important resources. Systematic screening of these available traditional herbs may result in the discovery of novel effective bioactive compounds for the formulation of drugs[1].
These specific essential oils are made up of many different types of terpenoids and volatile organic compounds and have been shown to possess antimicrobial, antifungal and anti-bacteridal properties[2]–[4]. The essential oils and organic plant crude extracts are of particular interest because of their safety and their wide acceptance by consumers and their uses for potential multi-purpose functional uses[2]–[4]. However, the essential oils and organic plant crude extracts from the medicinal plants are one of the most promising bioactive groups of natural compounds for the preparation of safer anti-bacterial agents, antifungal and antioxidant agents.
Lettuce is a vegetable plant belonging to Asteraceae family. It is often grown everywhere as a leaf vegetable. This leaf vegetable was first cultivated by the Egyptians. After first cultivation during 16th to 18th century Europeans first saw and found many varieties and species of lettuce. By the mid-18th century varieties were being described. Many varieties and species can still be found in gardens in the 21st century. The market demand of lettuce was first fulfilled by the Europe and North America. But nowadays the consumption of lettuce has spread tremendously throughout the world due to their medicinal importance. Lettuce is easily cultivated in relative low temperatures countries. However, currently it is cultivated in tropical and subtropical countries with special nursing. Now it is commercially cultivated worldwide as one of the most popular food items. Lettuce is most often used for salads, but also in other kinds of food like soups, sandwiches and wraps. It contains different kinds of essential elements for humans such as vitamin A and potassium, sodium and calcium as well as minor source for several other vitamins and nutrients[5]–[8].
Most of the scientific and academic data dealing with this subject refers to the effects from the content of potassium, inositol and lipophilic flavones in Lactuca sativa (L. sativa) leaves. In addition, these above mentioned chemical components, saponins, sterols, polyphenols, rosmarinic acid and ursolic acid have also been detected and reported in these plant species[3]–[5],[9],[10]. Sometimes, this essential oil is the reason for diuretic effects of plant drugs. But the reason is unknown and not yet described in detail. However, there is no report available in the literature on the detailed analyses by gas chromatography-mass spectrometer (GC-MS) of essential oil of lettuce grown in Sultanate of Oman. Therefore, the aim of this present study is to isolate and examine the chemical composition of the essential oils and antioxidant activity of the crude extracts from the fresh and dry leaves of lettuce cultivated in Sultanate of Oman by spectroscopic methods.
2. Materials and methods
2.1. Sample collection
The fresh green leaves and stems of lettuce were collected from Al Modibi and Al Sharqia areas, Sultanate of Oman. The plant samples were collected in the morning at 10:00 am on February 13, 2012 and identified by morphological features and data base present in the website. The samples were instantly packed in the plastic bags and stored in freeze until the isolation of essential oil. The plant samples were dried under shade. About 50 g of dried leaves were ground using a grinder (Jaipan, Super Deluxe, India) for 20 seconds.
2.2. Isolation of the essential oil
The air-dried and fresh plant material (100 g) was subjected to hydrodistillation individually for 3 h using a Clevenger type apparatus. The isolated essential oils were re-extracted using organic solvent dichloromethane. Finally, the essential oil from fresh and dry leaves samples were dried over anhydrous sodium sulphate and preserved in a sealed vial at 4 °C until further analysis.
2.3. Radical scavenging activity using DPPH method
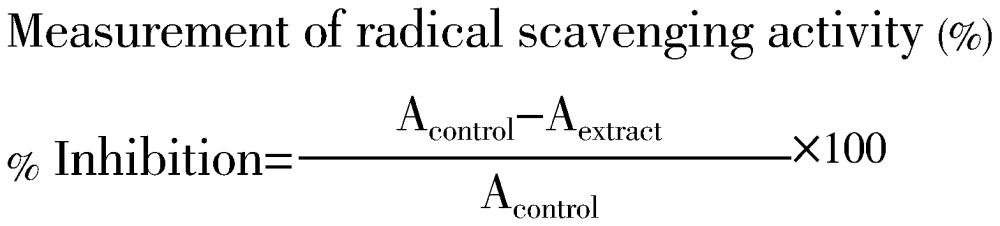
The free radical scavenging activity of the dry plant crude extracts of lettuce was evaluated as described by Blois[10]. The different concentrations of plant crude extracts were prepared manually and taken in different test tubes (12.5, 25.0, 50.0, 100.0 and 200.0 ppm equivalent to 12.5, 25.0, 50.0, 100.0 and 200.0 µg, respectively). Each sample containing test tubes was shaken vigorously by adding one milliliter of 0.1 mmol/L DPPH (2,2-diphenyl-1-picrylhydrazyl) solution. After 5 minutes shaking, all the test tubes were allowed to stand at room temperature in a dark place for 45 min. The control (blank) was prepared accordingly without any extract. The differences of absorbance of the tested samples were measured by using UV spectroscopy at the fixed wavelength 517 nm. Radical scavenging activity of the tested different plant crude extract samples was estimated as an inhibition percentage (%) and the experimental result was calculated by using the following formula:
 |
2.5. GC-MS analysis
The GC-MS analysis of the two essential oils isolated from the fresh and dry leaves of locally grown lettuce was performed using a Perkin Elmer Clarus 600 GC-MS system (equipped with an 30 m×0.25 i.d., film thickness 0.25 µm) coupled with a Perkin Elmer Clarus 600C MS. The fused silica capillary column (model Rtx®-5MS) was used for separation of essential oils. For the detection of gas chromatography-mass spectroscopic data an electron ionization system with ionization energy of 70 eV was used. Inert gas helium was used as a carrier gas at a constant flow rate of 1 mL/min. Mass transfer line and injector temperatures were set at 220 °C and 290 °C, respectively. The oven temperature was programmed from 60 °C (hold 2 min) to 270 °C at 4 °C/min, then held isothermal for 20 min and finally raised to 290 °C at 10 °C/min. One micro litre oil sample was injected into the column with split mode condition. The split ratio was 200:1. The calculative percentage of the chemical constituents in the crude essential oil was expressed as a percentage by peak area.
2.6. Identification of essential oil
The chemical compounds of essential oils were identified based on the retention time on silica capillary column and the matching of mass spectra with the standard library such as NIST 2005 v.2.0 and Wiley Access Pak v.7, 2003. Whenever possible, the mass spectra was matched by co-injection with the authentic compounds[11].
3. Results
3.1. Chemical composition of essential oil
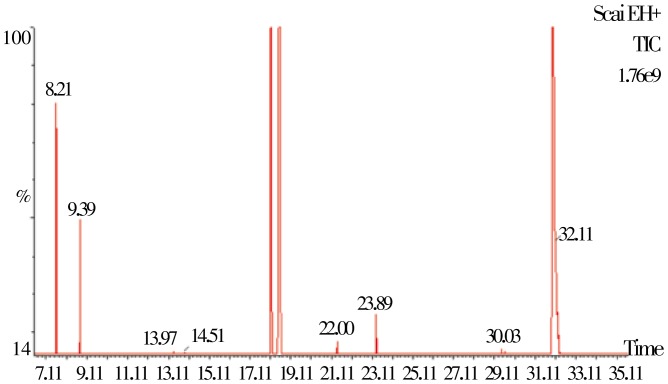
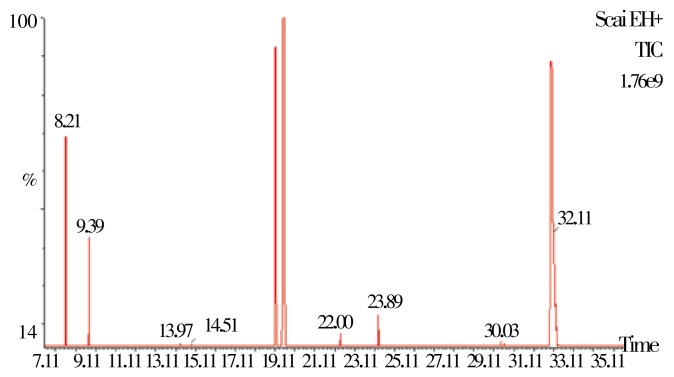
The total ion chromatogram spectra (TIC) of the isolated essential oils from the fresh and dry leaves of lettuce by gas chromatography-mass spectrometry are shown in Figure 1 and Figure 2.
Figure 1. A typical chromatogram of the constituents of essential oil from the fresh lettuce.
Figure 2. A typical chromatogram of the constituents of essential oil from the dry lettuce.
The TIC chromatogram showed that most of the peak heights are higher in essential oil from fresh lettuce than the essential oil from the dry lettuce. The analysis of the chemical constituents in both essential oils by using GC-MS had led to the identification of 20 different classes or groups and different concentration of volatile terpenoids and organic compounds; representing 83.07% and 79.88% of the total essential oils isolated from fresh and dry leaves samples of lettuce, respectively. The identified chemical compounds in the isolated essential oil by GC-MS are listed in Table 1 according to their elution order on the fused silica capillary column (model Rtx®-5MS). Both the essential oil contain a mixture of compounds consisting of mainly oxygenated mono and sesquiterpene hydrocarbons and their derivatives. The major volatile organic compounds detected in the fresh and dry leaves oils, respectively were α-pinene (5.11% and 0.62%), p-Cymene (2.07% and 1.92%), thymol (11.55% and 10.73%), durenol (52.00% and 49.97%), α-terpinene (1.34% and 1.34%), thymol acetate (0.99% and 0.67%), caryophyllene (2.11% and 1.98%), linalool (3.09% and 2.98%), camphene (4.11% and 3.65%), limonene (1.28% and 1.11%) as the major compounds. In this present study, there are some minor compounds that were also isolated and identified from fresh and dry lettuce such as β-pinene, α-terpinolene, linalool, 4-terpineol, α-terpineol, o-methylthymol, L-alloaromadendrene, viridiflorene (Table 1). Almost all the chemical constituents identified in both the essential oil isolated from the fresh and dry lettuce exhibited very high potent biological activity.
Table 1. Percentage composition of the volatile fresh and dry lettuce oils.
| Compound name | M.W. | Molecular formula | Retention time (min) | Fresh leaves (%) | Dry leaves (%) |
| α-Pinene | 136 | C10H16 | 5.600 | 5.11 | 0.62 |
| 3-Octanone | 128 | C8H16O | 6.960 | 0.12 | 0.12 |
| β-Pinene | 136 | C10H16 | 7.110 | 0.22 | 0.22 |
| α-Terpinolene | 136 | C10H16 | 7.950 | 0.31 | 0.31 |
| p-Cymene | 134 | C10H14 | 8.190 | 2.07 | 1.92 |
| α-Terpinene | 136 | C10H16 | 9.390 | 1.35 | 1.35 |
| Linalool | 154 | C10H18O | 10.862 | 3.09 | 2.98 |
| 4-Terpineol | 154 | C10H18O | 13.968 | 0.46 | 0.46 |
| α-Terpineol | 154 | C10H18O | 14.513 | 0.46 | 0.46 |
| O-Methylthymol | 164 | C11H16O | 16.699 | 0.30 | 0.30 |
| Thymol | 150 | C10H14O | 18.750 | 11.55 | 10.73 |
| Durenol | 150 | C10H14O | 19.195 | 52.00 | 49.98 |
| Thymol acetate | 192 | C12H16O2 | 22.001 | 0.99 | 0.67 |
| Caryophyllene | 204 | C15H24 | 23.892 | 2.11 | 1.98 |
| L-Alloaromadendrene | 204 | C15H24 | 24.673 | 0.29 | 0.29 |
| Viridiflorene | 204 | C15H24 | 26.904 | 0.17 | 0.17 |
| α-Pinene | 220 | C15H24O | 30.035 | 0.54 | 0.54 |
| 3-Octanone | 220 | C15H24O | 30.240 | 0.62 | 0.62 |
| Limonene | 137 | C10H17 | 32.700 | 1.28 | 1.11 |
| Camphene | 136.2 | C10H16 | 32.710 | 4.11 | 3.65 |
| Total | 83.07 | 79.88 |
The free radicals i.e. α,α-diphenyl-β-picrylhydrazyl scavenging activity of plant crude extracts of lettuce were tested by using DPPH method and the results were evaluated in Table 2. The antioxidant principle in their interaction depends on oxidative free radicals. The main mechanism of DPPH method is that the bioactive compounds react with a the stable free radical i.e., α,α-diphenyl-β-picrylhydrazyl. It is converted to α,α-diphenyl-β-picrylhydrazine with colour change. The gradually colour change indicates the scavenging activities of the plant crude sample due to bioactive compounds such as phenolic compounds, flavonoids, terpenoids and derivatives [3]–[5],[10],[12]. In the present study, five different plant crude extracts extracted from Omani lettuce were able to decolorize DPPH. The free radical scavenging activities of the plant crude extracts were found to be in the order of hexane>butanol>chloroform>ethyl acetate>methanol. From the experiment, it has been observed that all aromatic and aliphatic compounds such as aromatic hydrocarbon, cysteine, glutathione, ascorbic acid, tocopherol, polyhydroxy and other aromatic amines such as p-phenylene diamine, p-aminophenol etc., reduce the change of colour slowly by α,α-diphenyl-β-picrylhydrazyl helpful their free radical ability[8],[9],[13]. In this study it appears that perhaps the five different plant crude extracts by organic solvent from Omani lettuce possess strong hydrogen donating capabilities to act as natural antioxidants.
Table 2. Free radical scavenging activity of letuce crude extracts by DPPH methods.
| Crude extract | Concentration (mg/L) | DPPH (% Inhibition) |
| Methanol extract | 12.5 | 58.80 |
| 25.0 | 62.07 | |
| 50.0 | 65.24 | |
| 100.0 | 72.19 | |
| 200.0 | 81.90 | |
| Ethyl acetate extract | 12.5 | 56.87 |
| 25.0 | 57.56 | |
| 50.0 | 61.92 | |
| 100.0 | 68.23 | |
| 200.0 | 74.90 | |
| Hexane extract | 12.5 | 39.90 |
| 25.0 | 42.05 | |
| 50.0 | 49.17 | |
| 100.0 | 51.67 | |
| 200.0 | 60.99 | |
| Chloroform extract | 12.5 | 51.07 |
| 25.0 | 55.12 | |
| 50.0 | 58.98 | |
| 100.0 | 65.77 | |
| 200.0 | 69.23 | |
| Butanol extract | 12.5 | 44.88 |
| 25.0 | 50.55 | |
| 50.0 | 53.48 | |
| 100.0 | 57.17 | |
| 200.0 | 61.56 |
4. Discussion
The essential oils from the fresh and dry lettuce by hydrodisstillation were yellowish in colour and the main chemical constituents in the essential oils were oxygenated monoterpenes, sesquiterpenes, hydrocarbons volatile organic compounds and their derivatives. The majority of the researchers have reported that the major chemical constituents in the essential oils from the medicinal plant origin are monoterpenes and sesquiterpenes hydrocarbons and their oxygenated derivatives. These monoterpenes and sesquiterpenes hydrocarbons and their oxygenated derivatives have low molecular weight organic volatiles and enormous potential to strongly exhibit microbial pathogens [3]–[5],[12]. But in most of cases the antimicrobial ingredients in the essential oils are terpene derivatives, which are almost phenolic in character. It would seem reasonable that their antimicrobial and antifungal mode of action might be related to that of other chemical compounds.
The medicinal effects of plant materials typically result from the combinations of secondary metabolic products or compounds present in the plant origin. These secondary metabolic products or compounds are not essential for cell structure and maintenance of life but often involved in plant protection against biotic or abiotic stresses. Natural products, as essential oils, as pure compounds or as standardized extracts, provide unlimited opportunities for the preparation of new drug discoveries because of the unmatched availability of chemical diversity inside the sources[7]–[9],[11]–[13].
This paper reports the isolation and GC-MS analysis of the essential oils from the fresh and dry leaves of lettuce grown in Sultanate of Oman. The chemical compounds of essential oils from this vegetable plant have been identified for the first time. The composition of the essential oil of lettuce differs, depending on where it is grown as well as the environmental conditions. The differences in chemical composition, which can affect the biological activities and pharmaceutical applications, are probably due to the differences in the climatic and geographical conditions (temperature, rainfall, altitude, hours of sunshine, etc)[5],[7],[9],[11],[13],[15]–[18]. Only a few chemical constituents were present in both essential oils. May be it is due to the environmental conditions such as temperature, rainfall, altitude, hours of sunshine, etc. Most of the chemical constituents have low molecular weight and they are easily volatile at room temperature. So, during the harvesting and processing of samples most of the low volatile chemical constituents evaporated[5],[7],[9],[11],[13].
Thus, lettuce could become an alternative to synthetic bactericides for using in agro industries and also to screen and develop such novel types of selective and natural bactericides in the treatment of many microbial phytopathogens causing severe destruction to crops, vegetables and ornamental plants[5],[12],[14]. Therefore, the essential oils isolated from this species are potential active candidates to be used as antimicrobial and antifungal agents in new drugs preparation for therapy of infectious diseases. Further advance research on toxicological and clinical studies are required to prove the safety of the oil as a medicine[5],[7],[9],[11],[13],[19]–[21].
Acknowledgments
The authors are grateful to Prof. Dr. Nafsiah Binti Shamsudin, Dean, College of Pharmacy and Nursing, University of Nizwa, Sultanate of Oman for her continuous encouragement during the work and all laboratory facilities. The authors are also grateful to University of Nizwa, Nizwa, Sultanate of Oman for providing all chemicals and other expenses from their internal research fund to carry out this project. Thanks go to Khaloud Ali Said Al-Alawi and AhlamRashed Alabri, Lab Technicians, Natural Product Lab, University of Nizwa for their continuous help during the experiment. Thanks also go to Mr. Thomas Hughes, Writing Center, University of Nizwa for his help to correct the manuscript.
The authors wish to express sincere gratitude to the Central Instrument Laboratory, College of Agriculture and Marine Sciences, Sultan Qaboos University, Sultanate of Oman where the tests were performed (Grant No. 507/SOP/OB/1/2013).
Comments
Background
Lettuce is a kind of leafy vegetable belonging to Asteraceae family. It is often grown everywhere as a leaf vegetable. This leaf vegetable was first cultivated by the Egyptians. After first cultivation during 16th to 18th century the Europeans found that many varieties and species of lettuce. By the mid-18th century different varieties were being described. Many varieties and species can still be found in gardens in the 21st century. Now it is commercially cultivated worldwide as one of the most popular food items. Lettuce is most often used for salads, but also in other kinds of food like soups, sandwiches and wraps. But the reason is unknown and not yet described in detail. However, there is no report available in the literature on the detailed analyses by GC-MS of essential oil of lettuce grown in Sultanate of Oman. Therefore, the aim of this present study is to isolate and examine the chemical composition of the essential oils and antioxidant activity of the crude extracts from the fresh and dry leaves of lettuce cultivated in Sultanate of Oman by spectroscopic methods.
Research frontiers
The present study is being performed in order to detect and identify the presence of essential oil from the leaves of lettuce by GC-MS. The sample was collected from the Sultanate of Oman and compares the chemical constituents in the essential oil.
Related reports
There is no report available in the literature on the detailed analyses by GC-MS of essential oil of lettuce grown in Sultanate of Oman.
Innovations and breakthroughs
Although the experimental work conducted by the authors is routine analysis but it gives the new information and data for the scientific community for further necessary studies.
Applications
According this paper presented by the authors, there is a presence of so many bioactive materials that can be used directly as traditional medicine as well as the main raw materials of different types of medicine based on their biological activity.
Peer review
This study on chemical composition and the anti oxidant activity of the leaves of Lettuce is giving the valuable and useful information of the medicinal value of plant.
Footnotes
Foundation Project: Supported by Central Instrument Laboratory, College of Agriculture and Marine Sciences, Sultan Qaboos University. Grant No. 507/SOP/OB/1/2013.
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.Nagesh L, Sivasamy S, Muralikrishna KS, Kishore Bhat G. Antibacterial potential of gall extract of Quercus infectoria against Enterococcus faecalis–an in vitro study. Pharmacognosy J. 2012;4(30) doi: 10.5530/pj.2012.30.9. [DOI] [Google Scholar]
- 2.Singh N, Singh RK, Bhunia AK, Stroshine RL. Essential oil or a sequential washing in killing Escherichia coli O157:H7 on lettuce and baby carrots. LWT-Technol. 2010;35:720–729. [Google Scholar]
- 3.Gutierrez J, Bourke P, Lonchamp J. Impact of plant essential oil on microbiological and quality markers of minimally processed vegetables. Food Sci Environ Health. 2009;10(2):195–202. [Google Scholar]
- 4.Hossain MA, Salehuddin SM, Ismail Z. Rosmarinic acid and methyl rosmarinate from Orthosiphon stamineus, Benth. J Bangladesh Acad Sci. 2006;30(2):167–171. [Google Scholar]
- 5.Gülten TG, Brendan AN, Şahika AG, Mehmet K. Antimicrobial activity of oregano oil on Iceberg lettuce with different attachment conditions. J Food Sci. 2012;77(7):412–415. doi: 10.1111/j.1750-3841.2012.02759.x. [DOI] [PubMed] [Google Scholar]
- 6.Mishra AK, Sahu N, Mishra A, Ghosh AK, Jha S, Chattopadhyay P. Phytochemical screening and antioxidant activity of essential oil of Eucalyptus leaf. Pharmacognosy J. 2010;2(16):21–24. [Google Scholar]
- 7.Shah VN, Shah MB, Bhatt PA. In vivo and in vitro antioxidant and hepatoprotective effects of Classical ayurvedic formulation Punarnavashtak kwath against ethanol induced hepatotoxicity. Pharmacognosy J. 2010;2(16):38–47. [Google Scholar]
- 8.Khater HF, Hanafy A, Abdel-Mageed AD, Ramadan MY, El-Madawy RS. Control of the myiasis-producing fly, Lucilia sericata, with Egyptian essential oils. Int J Dermatol. 2011;50(2):187–194. doi: 10.1111/j.1365-4632.2010.04656.x. [DOI] [PubMed] [Google Scholar]
- 9.Cláudia AM, Erlan T, Antonio S, Carlos O, José C, José F. Chemical composition and allelopathyc activity of essential oil of Lippia sidoides Cham. Chilean J Agric Res. 2012;72(1) doi: 10.4067/S0718-58392012000100025. [DOI] [Google Scholar]
- 10.Berrie AMM, Parker W, Knights BA, Hendrie MR. Studies on lettuce seed germination-I. Coumarin induced dormancy. Phytochemistry. 2011;7(4):567–574. [Google Scholar]
- 11.Hossain MA, Siddique AB, Mizanur Rhaman SM, Hossain AM. Chemical composition of the essential oils of Stevia rebaudiana Bertoni leaves. Asian J Trad Med. 2010;5(2):56–61. [Google Scholar]
- 12.Cosa P, Vlietinck AJ, Berghe DV, Maes L. Antiinfective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J Ethnopharmacol. 2006;106:290–302. doi: 10.1016/j.jep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Cho WK, Chen XY, Uddin NM, Rim Y, Moon J, Jung JH, et al. et al. Comprehensive proteome analysis of lettuce latex using multidimensional protein-identification technology. Phytochemistry. 2009;70(5):570–578. doi: 10.1016/j.phytochem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Saraswathy A, Shakila R, Sunilkumar KN. HPTLC fingerprint profile of some Cinnamomum species. Pharmacognosy J. 2010;2(8):211–215. [Google Scholar]
- 15.Hossain MA, Ismail Z, Rahman A, Kang SC. Chemicalcomposition and antifungal properties of the essential oil and crude extracts of Orthosiphon stamineus. Ind Crops Prod. 2008;27:328–334. [Google Scholar]
- 16.Adinortey MB, Sarfo JK, Quayson ET, Weremfo A, Adinortey CA, Ekloh A, et al. et al. Phytochemical screening, proximate and mineral composition of Launaea taraxacifolia leaves. Res J Med Plants. 2012;6(2):171–179. [Google Scholar]
- 17.Ribas-Agustí A, Gratacós-Cubarsí M, Sárraga G, José-Antonio G, Castellari M. Analysis of eleven phenolic compounds including novel p-Coumaroyl derivatives in Lettuce (Lactuca sativa L.) by Ultra-high-performance Liquid Chromatography with photodiode prray and mass spectrometry detection. Phytoche Anal. 2011;22(6):555–563. doi: 10.1002/pca.1318. [DOI] [PubMed] [Google Scholar]
- 18.Pan C, Chen YG, Ma XY, Jiang JH, He F. Phytochemical constituents and pharmacological activities of plants from the genus Adiantum: A review. Trop J Pharma Res. 2011;10(5):681–692. [Google Scholar]
- 19.Altunkaya A, Becker EM, Gökmen V. Antioxidant activity of lettuce extract (Lactuca sativa) and synergism with added phenolic antioxidants. Food Chem. 2009;115:163–168. doi: 10.1016/j.foodchem.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, Kang DH, Kim JK, Ha YG, Hwang JY, Kim T, et al. et al. Antimicrobial activity of plant extracts against Salmonella typhimurium, Escherichia coli O157:H7, and Listeria monocytogenes on fresh lettuce. J Food Sci. 2011;76(1):46–50. doi: 10.1111/j.1750-3841.2010.01926.x. [DOI] [PubMed] [Google Scholar]
- 21.Gündüz GT, Niemira BA, Gönül SA, Karapinar M. Antimicrobial activity of oregano oil on Iceberg lettuce with different attachment conditions. J Food Sci. 2011;77(7):412–413. doi: 10.1111/j.1750-3841.2012.02759.x. [DOI] [PubMed] [Google Scholar]