Abstract
Objective
To determine the free radical scavenging potentials pytochemical constituents of ethanol leaves extracts of Allamanda cathartica (A. cathartica) and Bixa orellana (B. orellana) and thus their effects in antimalarial activities.
Methods
Both ethanol extracted plant samples were administered at 50 mg/mL, 100 mg/mL and 200 mg/mL to Albino rats and then administered with CCl4 at 1 mL/kg body weight, in liquid paraffin (1:1, v/v) for 2 days (negative control) and compared with 5% Tween 80 (placebo) and vitamin E (positive control) pretreatments. Thiobarbituric acid reactive substances (TBARS), glutathione (GSH) and catalase (CAT) activities in blood and liver tissues were assessed.
Results
In CCl4 treated rats, TBARS levels significantly increased, while decreased GSH and CAT levels were recorded for both plant extracts. Generally, higher TBARS and GSH values were recorded for blood than for liver homogenates; with reverse trend observed for CAT level. Increased concentrations of A. cathartica extract recorded significant antioxidant levels similar to tocopherol (vitamin E). Reducing sugars, saponins, flavonoids were recorded for both species; alkaloids in A. cathartica and terpenoids in B. orellana.
Conclusions
A. cathartica, possess phytochemicals that recorded significant antioxidative defense activities for blood and liver tissues with increasing concentration. However B. orellana did not record similar results.
Keywords: In vivo, Anti-malaria, Anti-oxidant, Allamanda cathartica, Bixa orellana
1. Introduction
An estimated 3.3 billion people were at risk of malaria in 2010, with 216 million cases recorded in 2010; 81% of which are in Africa, with six African countries accounting for 60% of the estimated 655 000 death record annually and 86% of these victims are under 5 years of age. People living in the poorest countries are the most vulnerable to malaria and are most dependent on plant resources for malaria management, especially in the population of West Africa[1],[2]. However, do all the plants employed by the local population have therapeutic prowess for treating Malaria? This question become pertinent, when it is considered that some of the emerging plants are known for treating other conditions even by the same or other natives. With the increasing resistance of the malaria parasite to a number of currently administered anti-malaria drugs[3], the need to search for new drug materials becomes imperative. And those plants hitherto unemployed for malaria treatment may present promising attributes.
Free radical induced lipid peroxidation is believed to be one of the major causes of cell membrane damage leading to a number of pathological situations and affecting a wide range of tissues and organs[4]–[6],[7]. Plant materials and herbal extracts have been recorded to protect organs against oxidative stress created by industrial solvents and carbon tetrachloride (CCl4), a potent environmental hepatotoxin through changing the levels of increased lipid peroxidation[8],[9], enhancing the decreased activities of antioxidant enzymes, like superoxide dismutase, catalase and glutathione-S-transferase and increasing reduced glutathione level in liver[10],[11].
Allamanda cathartica L. (A. cathartica, golden trumpet) is a widely cultivated ornamental plant of the family Apocynaceae. It has been used as a purgative or emetic, febrifuge, as well as for the treatment of coughs, headaches, jaundice and enlarged spleen resulting from malaria. The milky sap is also known to possess antibacterial and possibly anticancer properties[12],[13]. Bixa orellana L. (Bixaceae) (B. orellana) is a tropical shrub, with a bright red heart shaped, bristly fruit and seeds embedded in orange-red pulp. It could be used to produce a common dye employed for a variety of purpose. It has a long history as a medicinal plant for the treatment of varied conditions such as feverish infections like gonorrhoea, dysentery and hepatitis, as well as for cough, snakebites, intestinal parasites, skin toning and for the treatment of diabetes in traditional medicine systems[14].
Measuring the reactive oxidative species scavenging status of plants has become a good point to commence research on ascertaining their therapeutic potentials. The present study seek to determine the pytochemical constituents and the free radical scavenging potentials of A. cathartica and B. orellana and hence their relevance in the treatment of malaria.
2. Material and methods
2.1. Plant and animal samples
Plants samples were collected in Southern Nigeria, during an ethnomedicinal survey, covering 8 states; extending from June-July 2010. Albino rats with average weight of 100-170 g were acclimatized with normal rat feed (Laymore Concentrate) and water ad libitum.
2.2. Preparation of plant extracts solution
Plant materials were air dried at ambient temperature (28-32 °C) for two weeks and blended into uniform powder. The ethanol extracts were prepared by soaking 100 g of each of the dry powdered plant materials in 1 L of ethanol at room temperature for 48 h. The extracts were filtered through Whatmann filter paper No. 42 and then through cotton wool. The extracts were dried and concentrated using a rotary evaporator with the water bath set at 40 °C. Extracts solutions were prepared by dissolving 0.2 g of the evaporated extract in 10 mL of 5% Tween 80, to give an effective concentration of 20 mg/mL. The extracts solutions were prepared two days before administration.
2.3. Phytochemical screening
Tests were carried out on the ethanol extracts of the plant samples using standard procedures to identify the chemical contents like alkaloids, tannins, cardiac glycosides, reducing sugars, saponins, flavonoids and terpenoids constituents as described in previous studies[15]–[17].
2.4. Determination of antioxidant activity in vivo
Animals were divided into four groups of five rats per group, including test group and three control groups: positive, negative and placebo. Prior to CCl4 (1 mL/kg body weight) intoxication for 2 days, test group animals were orally administered with 50 mg/kg, 100 mg/kg and 200 mg/kg dose concentrations of aqueous extracts of A. cathartica and B. orellana. The positive control group received vitamin E, and the normal control group was administered with 5% Tween 80 for 7 days. The negative control group received only CCl4 for 2 days. After the final dose of CCl4, the animals were starved overnight and sacrificed under mild anesthesia using chloroform.
2.5. Preparation of liver homogenates and blood
Following Manna et al. procedure with slight modification; 200 mg of harvested liver tissue were homogenized in 10 volume of 100 mmol/L KH2PO4 buffer containing 1 mmol/L ethylene diamine tetraacetic acid, pH 7.4 and centrifuged at 3 500 r/min for 4 minutes at 4 °C[18]. Blood were collected through cardiac puncture and stored in biofreezer.
2.6. Estimation of lipid peroxidation status (TBARS)-MDA assay
Lipid peroxidation as evidenced by the formation of thiobarbituric acid reactive substances (TBARS) was measured using the methods in previous studies[19],[20]. A volume of 0.1 mL of liver tissue homogenate and blood samples were treated separately with 2 mL of mixture [1:1:1 TBA-TCA-HCl reagent (thiobarbituric acid 0.37%), 0.25 N HCl, 10% TCA] and incubated for 45 min in a water bath (100 °C), cooled and centrifuged at room temperature for 10 min at 1 000 r/min. The absorbance of clear supernatant was measured against reference blank at 535 nm. TBARS concentrations were calculated using the MDA extinction co-efficient 1.56 × 105 M−1cm.
2.7. Determination of non-enzymatic antioxidant status-GSH assay
Reduced glutathione (GSH) was determined by the method outlined by Ellman[21]. 10% TCA was added to the liver homogenate and blood samples separately, and were centrifuged. A volume of 1.0 mL of the supernatant was treated with 0.5 mL Ellman's reagent (19.8 mg of dithiobisnitrobenzoic acid in 100 mL of 0.1% sodium nitrate) and 3.0 mL of 0.2 mol/L phosphate buffer (pH 8.0, 6.8 g of KH2PO4 and 17.9% of Na2HPO4.12H2O dissolved in 500 mL distilled water). The absorbance was read at 412 nm and the GSH contents calculated from the standard graphs generated.
2.8. Determination of enzymatic antioxidant status-CAT assay
Catalase (CAT) was assayed colorimetrically at 620 nm and expressed as µmoles of H202 consumed per min/mg protein as described in previous studies[22]. The reaction mixture (1.5 mL) contained 1.0 mL of 0.01 mol/L pH 7.0 phosphate buffer, 0.1 mL of tissue homogenate and blood samples and 0.4 mL of 2 mol/L H2O2. The reaction was stopped by the addition of 1:3, 2.0 mL dichromate and glacial acetic solution.
2.9. Statistical analysis
Graphs plotted and computation for ANOVA and standard deviation (SD) were carried out using Microsoft Excel (2007) for Windows and Cgi-bin (www.physics.csbsju.edu/cgi-bin/stats/anova).
3. Results
3.1. Effect of plant extracts on lipid peroxidation
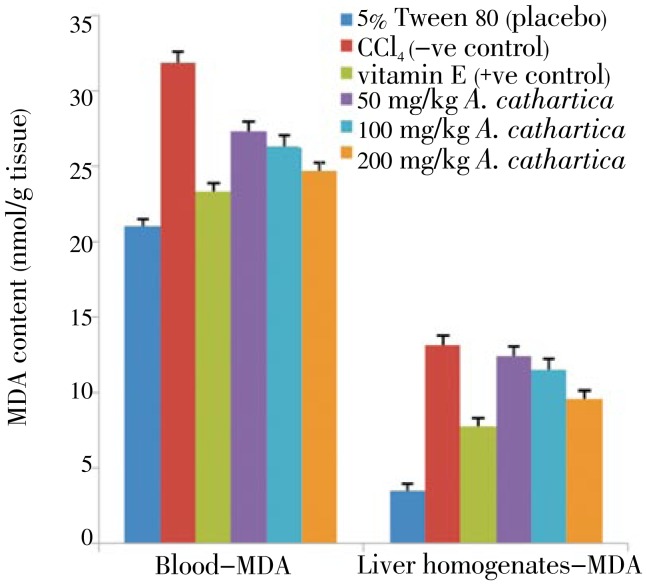
MDA concentrations and expression of TBARS in the liver homogenates and blood samples of all experimental rats is shown in Figures 1 and 2. These parameters were significantly higher in the CCl4 treated (negative control group) animal tissues (blood and liver homogenates) than in other experimental animals. Pre-treatment with A. cathartica extract for 7 days prior 2 days of CCl4 treatment, decreased the MDA levels in the blood and liver tissues; comparable to the vitamin E (positive control) treated MDA levels.
Figure 1. Effect of A. cathartica extract on the MDA level in CCl4 induced damages in the blood (left panel) and liver tissues (right panel) in rats.
5% Tween 80: MDA level in normal rats; CCl4: MDA level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body wt) prior to CCl4 intoxication; 50 to 200 mg/kg A. cathartica: 50 mg/kg, 100 mg/kg, 200 mg/kg of extract prior to CCl4 administration. (Mean±SD) (P<0.05).
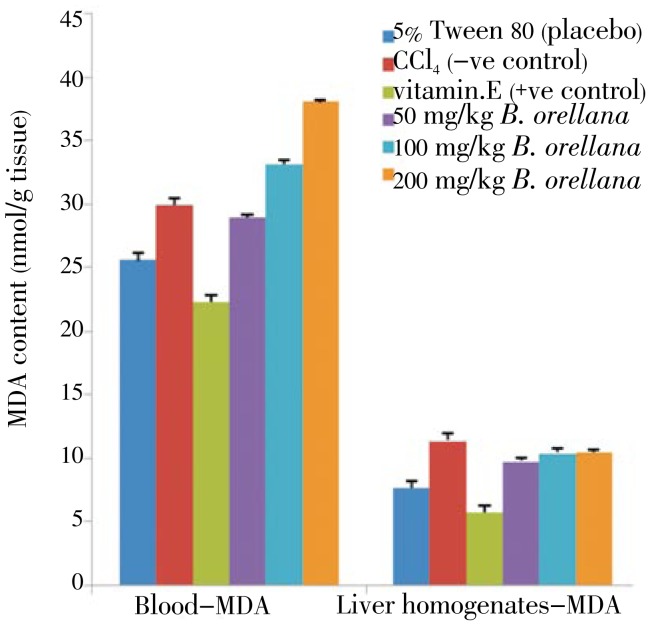
Figure 2. Effect of B. orellana extract on the MDA level in CCl4 induced damages in the blood (left panel) and liver tissues (right panel) in rats.
5% Tween 80: MDA level in normal rats; CCl4: MDA level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body wt) prior to CCl4 intoxication; 50 to 200 mg/kg B. orellana: 50mg/kg, 100mg/kg, 200mg/kg of extract prior to CCl4 administration. (Mean±SD) (P<0.05).
B. orellana pre-treated plasma and liver tissues recorded MDA level that were significantly higher than the vitamin E treated (positive control) rats, particularly for blood tissues. Only the 50 mg/kg treatment recorded level was lower than the levels for the CCl4 treated rats (Figure 3). The MDA levels for the B. orellana pre-treated liver tissues were higher than that recorded for vitamin E treated rats, and compared to the CCl4 treated liver tissues.
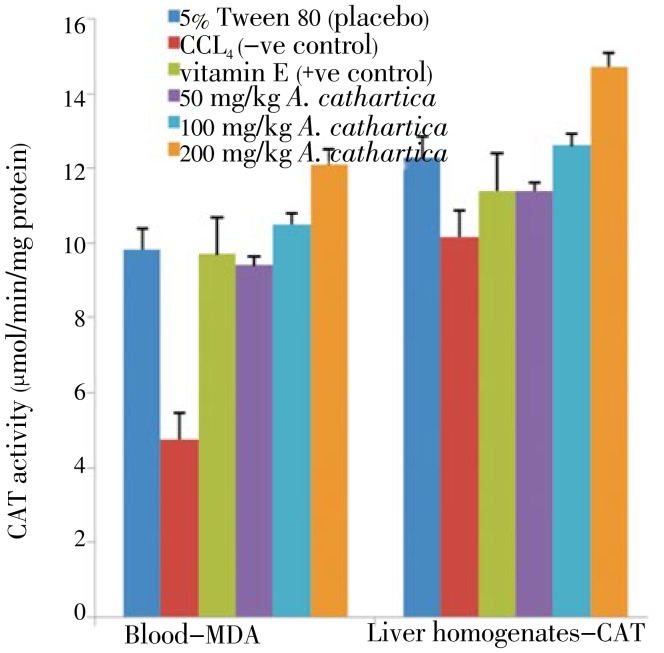
Figure 3. Effect of A. cathartica extract on the CAT level in CCl4 induced damages in the blood (left panel) and liver tissues (right panel) in rats.
5% Tween 80: CAT level in normal rats; CCl4: CAT level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body weight) prior to CCl4 intoxication; 50 to 200 mg/kg A. cathartica: 50 mg/kg, 100 mg/kg, 200 mg/kg of extract prior to CCl4 administration. (Mean±SD) (P<0.05).
3.2. Effect of plant extracts on catalase (CAT) activity
CAT activities in the plasma and liver samples of rats for all experimental groups are shown in the Figures 3 and 4. The CAT activities in the animal tissues of CCl4 treated rats were significantly lower than that of normal and positive controls. In the A. cathartica and B. orellana extracts pretreated animals for 7 days prior to CCl4 intoxication, catalase activity were higher in the plasma and liver tissues than for CCl4 treated rats tissues.
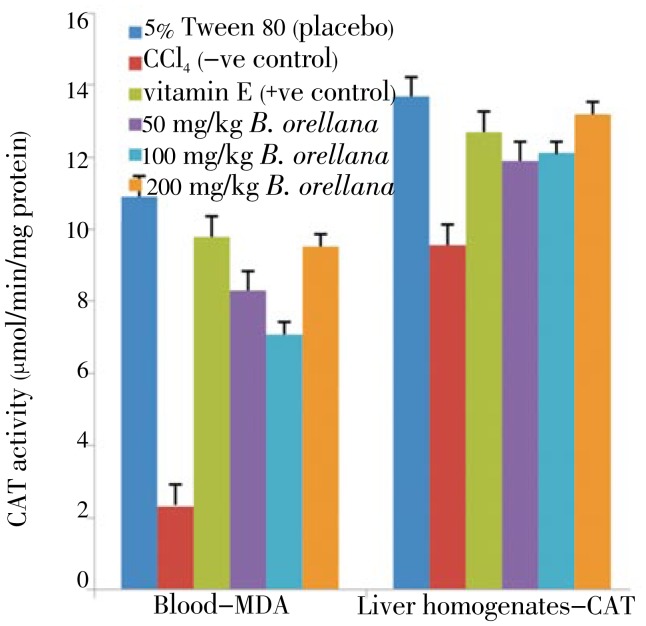
Figure 4. Effect of B. orellana extract on the CAT level in CCl4 induced damages in the blood (left panel) and liver tissues (right panel) in rats.
5% Tween 80: CAT level in normal rats; CCl4: CAT level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body wt) prior to CCl4 intoxication; 50 to 200 mg/kg B. orellana: 50 mg/kg, 100 mg/kg, 200 mg/kg of extract prior to CCl4 administration. (Mean±SD) (P<0.05).
Pretreatment of 200 mg/kg for A. cathartica for 7 days prior to CCl4 treatment showed the highest catalase activity in the blood and liver tissues of the rats. The vitamin. E treatment before CCl4 intoxication showed not significant change from the CAT activities recorded for the placebo (5% Tween 80) group. Similarly, plasma and liver tissues from B. orellana pretreated animals prior to CCl4 intoxication did not record considerable increase in CAT activities above those recorded for the vitamin E and placebo pretreatments (Figure 4).
3.3. Effect of plant extracts on glutathione (GSH) level
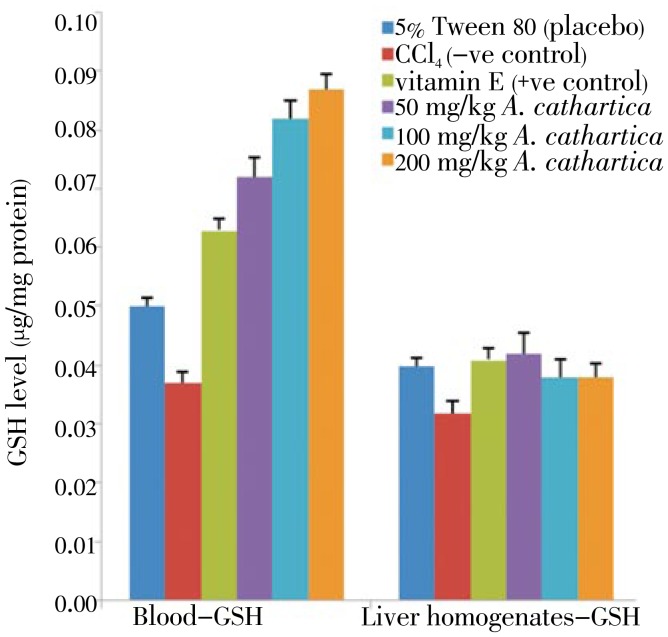
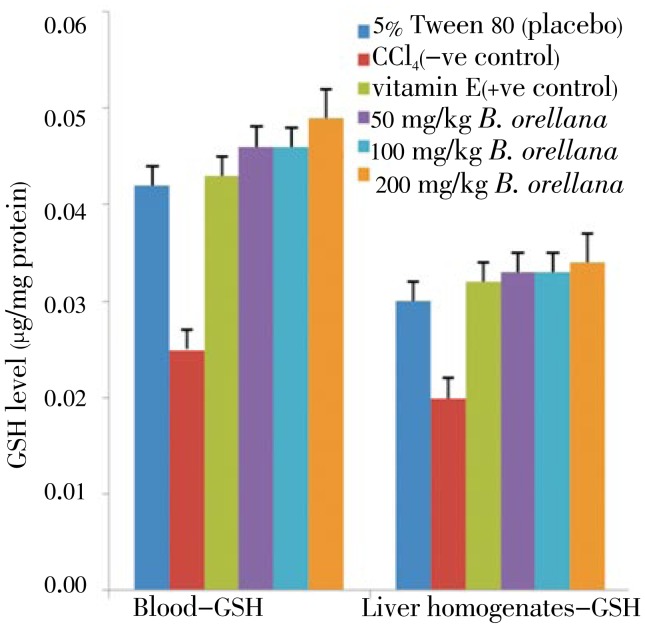
GSH level of plant extract on the experimental groups are shown in Figures 5 and 6. GSH levels in the CCl4 (negative normal) pretreated blood and liver tissues were significantly lower than for the 5% Tween 80 (placebo) and vitamin E (positive normal) groups. A. cathartica extract pretreated groups recorded higher GSH levels. Similarly, B. orellana pretreated group recorded significantly higher GSH levels than the CCl4 intoxicated groups. The GSH levels recorded for blood and liver tissues of plants extracts of pretreated rats were comparable to GSH levels for the vitamin E (positive control) pre-treated rat tissues.
Figure 5. Effect of A. cathartica extract on the GSH level in CCl4 induced damages in the blood (left panel) and liver tissues (right panel) in rats.
5% Tween 80: GSH level in normal rats; CCl4: GSH level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body wt) prior to CCl4 intoxication; 50 to 200 mg/kg A. cathartica: 50mg/kg, 100mg/kg, 200mg/kg of extract prior to CCl4 administration. (Mean±SD) (P<0.05).
Figure 6. Effect of B. orellana extract on the GSH level in CCl4 induced damages in the blood (left panel) and liver tissues (right panel) in rats.
5% Tween 80: GSH level in normal rats; CCl4: GSH level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body wt) prior to CCl4 intoxication; 50 to 200 mg/kg A. cathartica: 50 mg/kg, 100 mg/kg, 200 mg/kg of extract prior to CCl4 administration. (Mean±SD) (P<0.05).
3.4. Dose-dependent preventive activity of A. cathartica and B. orellana extracts
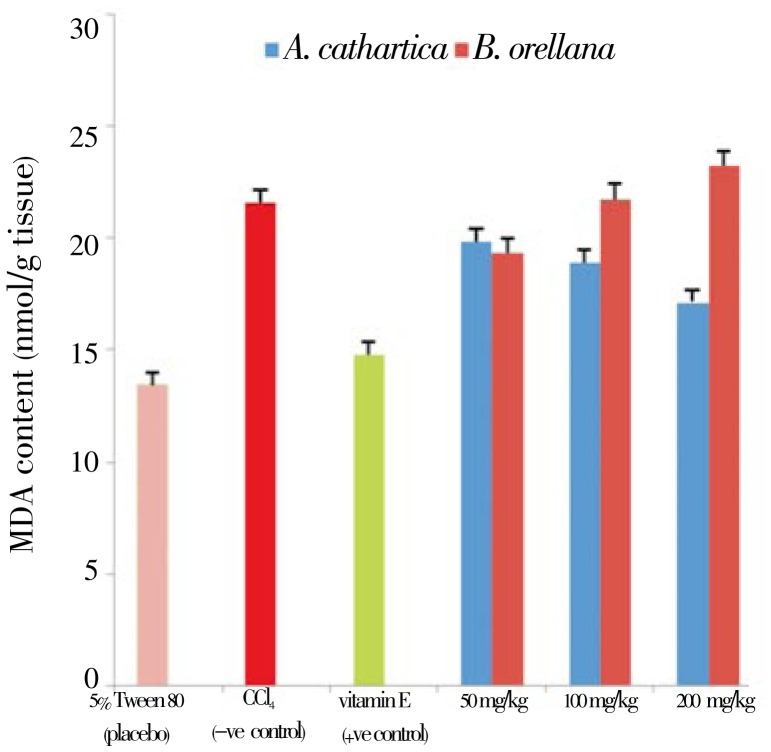
The dose-dependent preventive effects of the plant extracts are shown in Figure 7. Varied dosages of A. cathartica and B. orellana extracts of; 50mg/kg, 100mg/kg and 200mg/kg were administered to three groups of rats, with the bid to ascertain the dose with the most preventive effect. After administration of the extracts for 7 days followed by 2 days of CCl4 intoxication, the dose of 200mg/kg body weight recorded the most preventive effects for A. cathartica. Conversely, B. orellana showed decreasing preventive effect with increase dosage. The dose 50mg/kg body weight recorded the most preventive effect for B. orellana.
Figure 7. Dose dependent profile of ethanol extract of A. cathartica and B. orellana on MDA levels against CCl4 intoxication in blood and liver tissues.
5% Tween 80: MDA level in normal rats; CCl4: MDA level in CCl4 treated rats; vitamin E: vitamin E (200 mg/kg body wt) prior to CCl4 intoxication; 50 to 200 mg/kg: 50 mg/kg, 100 mg/kg, 200 mg/kg of A. cathartica and B. orellana extracts prior to CCl4 administration. (Mean±SD) (P<0.05).
4. Discussion
Plants are generally believed to be rich in a wide variety of secondary metabolites such as alkaloids, flavonoids, terpenoids and saponins. These metabolites, have received attention both as antioxidants such as the numerous phenolic compounds as well as antimalarial[23]. For this reason, research has focused on evaluating the antioxidant properties of plants used in ethnomedicine, in order to relate these properties to their mode of action[24].
Malaria is an intermittent and remittent fever caused by a protozoan parasite that invades the red blood cells and reportedly affects nearly 200 million people[25]. With increased prevalence of malaria and the growing dependence of a sizeable percentage of the population on alternative therapeutic management system such as ethnomedicinal practices, a better understanding of the true nature of the plants and these phytochemical constituents and possible actions has become imperative, as more plants find themselves in the herbal mixture administered for the management of malaria and other diseases.
In the survey towards this study, several plants were recorded as additions to the collections for making ‘Agbo’; some of which have been employed before now for treating other ailments[26]. The need arises for a clarification of the true plant nature of antimalarial status of these plants or if their additions are for accessory effect. During the survey, Saccharum officinale (sugar cane) was listed among the ‘Agbo’ plants. The use of sugar cane as antimalarial is not recorded in literature, rather its sugar content is most prized. However, natives that employs the plant for Agbo formulation claims, “it helps individual get up quickly”. The possible deduction is that it is the sugar content of the sugar cane that is harnessed by the natives, resulting in free reducing sugars in the ‘Agbo’ mixture (personal communication).
In the present study, phytochemical screening of the plants species yielded different constituents, alkaloids, tanins, cardiac glycosides, reducing sugars, saponins, flavanoids and terpenoids. B. orellana did not record alkaloids, tanins and cardiac glycosides; and A. cathartica tested positive only for alkaloids, reducing sugars, saponins and flavanoids. Similarly, Patnaik et al. recorded alkaloids, tannins, triterpenoids, steroids, sterols, saponins, flavonoids but no glycosides, phenolic compounds or anthraquinone from ethanolic, petroleum ether, ethyl acetate and n-butanol B. orellana extracts[27]. Radhika and Nasreen[28] and Tamil et al. recorded carbohydrates, steroids, alkaloids, proteins, flavonoids, terpenoids, phenolics, tannins and glycosides[29]. Aqueous, extract showed alkaloids, flavonoids, saponins and carbohydrates[30]; and petroleum ether, chloroform, ethanol, acetone analysis of phytochemical constituents of A. cathartica flower by Joselin et al. revealed phenolic compounds, flavonoids, saponins, glycosides, terpenoids, steroids, coumarins, quinones, phytosterols, carbohydrates with no traces of alkaloids and proteins[31].
Several compounds including food materials, environmental chemicals, and clinical drugs are known to instigate highly reactive substances such as free radicals, which cause cellular damages in several body tissues and organs and are linked to several diseases conditions. The generation of these free radicals, majority of which are reactive oxygen species creates a condition of oxidative stress in the tissues, which is managed through various cellular enzymatic and non-enzymatic systems. These cellular defensive systems can be bolstered by certain compounds, a lot of which are found in plants and thus are engaged for the management of tissues damages and diseases conditions that results from such injuries.
Lipid peroxidation is determined by the levels of TBARS, measured by the presence of MDA. CCl4 intoxicated animals recorded levels of lipid peroxidation in the plasma and liver tissues higher than for the placebo, positive normal and the extracts pretreated animals. While this confirms the ability of the CCl4 to instigate high levels of lipid peroxidation and thus oxidative stress, the significantly reduced levels of MDA recorded for the plant extracts reflects the potential of the plant extracts at reducing lipid peroxidation in rats tissues. The MDA levels in A. cathartica pre-treated rats' blood and liver tissues compared with the levels recorded for vitamin E, with the 200 mg/kg concentration recording lower levels. However, comparable levels were not recorded in the B. orellana pre-treated plasma and liver tissues. Similarly, higher GSH levels were recorded for the plant extracts than for the normal, with tissues pre-treated with A. cathartica showing increased GSH contents than the tissues pre-treated with B. orellana.
A. cathartica and B. orellana extracts were administered to rats to ascertain the potentials of the plant extracts at bolstering the defensive systems of the animals against oxidative stress created in their organs by the exposure to CCl4 that may eventually lead to tissues damages. A synergistic defence system is formed by the efforts of the enzymes superoxide dismutase, CAT and glutathione-S-transferase against reactive oxygen species created by chemicals like CCl4[7]. Therefore the decrease or increase in the activity or levels of the enzymes point to the degree of lipid peroxidation. Significantly enhanced levels of CAT activities in the liver and blood of the experimental animals recorded for the A. cathartica and B. orellana.
The effect of A. cathartica and B. orellana extracts against lipid peroxidation instigated by CCl4 was further assessed by dose-dependent monitoring of the antioxidant potentials of the plant species, in order to evaluate the protective activity of the plant extracts. The protective effect of A. cathartica increased with dosage, with the 200 mg/kg body weight pre-treatment recording higher protective activity. The B. orellana pre-treatment, however, showed a reverse trend with increased dosage.
The CAT activity of the plants shows the extracts have the potential to protect the antioxidation apparatus of the plasma and hepatic systems of the rats' tissues against oxidative stress and with enhanced GSH levels and reduced MDA levels, representing diminished lipid peroxidation levels. Higher protective activity of A. cathartica extracts were obtained at 200 mg/kg body weight dosage and 50 mg/kg body weight daily for 7 days for B. orellana. While the precise principle(s) and mode of action is unknown, the aqueous extracts of A. cathartica not B. orellana have potentially effective protective attributes against blood and liver injuries caused by CCl4 intoxication.
Acknowledgments
The authors are grateful to Dr. S. Rotimi for his assistance in the lab, and to the management of Covenant University, Ota and the Covenant University Centre for Research & Development (CUCERD) for the seed Grant: CUCERD-0012-34/09.
Comments
Background
Oxidative stress can damage many biomolecules (lipids, proteins and nucleic acids), and the tissue damage that results can lead to a variety of disease states. In some cases, oxidative stress is not a cause but rather a result of tissue damage from some other factors. Leaf extracts which contain antioxidant secondary metabolites can protect the body and are effective in the management of disease conditions resulting from oxidative stress.
Research frontiers
A qualitative analysis of the phytochemical constituents of the ethanolic extract of A. cathartica and B. orellana leaves was carried out. In vivo antioxidant activity of the extract was evaluated by measuring its effect on the levels of rat liver and blood markers of oxidative stress induced by exposure to carbon tetrachloride.
Related reports
Protective effect of the leaf extracts against CCl4 induced oxidative stress observed in this study are in agreement with previous studies by Khan MR, et al., 2012 and Anand T, et al., 2012, which employed extracts of other leaves.
Innovations and breakthroughs
Study presents the secondary metabolite profile of A. cathartica and B. orellana ethanolic extract, and demonstrates its in vivo antioxidant activity and efficacy against CCl4 induced oxidative stress, a condition that underlies many disease states. This may explain its use in preparations for the management of various diseases.
Applications
Findings appear to justify the use of the leaves in the antimalarial portion ‘agbo’. However further studies are required to establish their safety in this application.
Peer review
The interesting study provides a metabolites profile of the leaves; demonstrates the in vivo antioxidant activities in liver and blood of rats and the protective efficacy of the leaf extracts against CCl4 induced oxidative stress, which justifies the use of the leaves in the antimalarial potion ‘agbo’ in Nigeria.
Footnotes
Foundation Project: Supported by Centre for Research & Development (CUCERD), Covenant University (Grant No. CUCERD-0012-34/09).
Conflict of interest statement: We declare that we have no conflict of interest.
References
- 1.WHO . World malaria report 2011-fact sheet. Genava: WHO; 2011. [Online] available from: http://www.who.int/malaria/world_malaria_report_2011/WMR2011_factsheet.pdf. [Accessed on 12 April, 2012]. [Google Scholar]
- 2.Zofou D, Kowa TK, Wabo HK, Ngemenya MN, Tane P, Titanji VP. Hypericum lanceolatum (Hypericaceae) as a potential source of new anti-malarial agents: a bioassay-guided fractionation of the stem bark. Malaria J. 2011;10:167–174. doi: 10.1186/1475-2875-10-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedrich MJ. Resistance to malaria drugs. JAMA. 2011;305(7):663–663. [Google Scholar]
- 4.Krystona TB, Georgieva AB, Pissis P, Georgakilasa AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. doi: 10.1016/j.mrfmmm.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 5.Fahmy SR, Hamdi SAH. Antioxidant effect of the Egyptian freshwater Procambarus clarkii extract in rat liver and erythrocytes. Afr J Pharm Pharmacol. 2011;5(6):776–785. [Google Scholar]
- 6.Ramakrishna S, Geetha KM, Bhaskar Gopal PVVS, Ranjit Kumar P, Charan Madav P, Umachandar L. Effect of Mallotus philippensis Muell. Arg leaves against hepatotoxicity of carbon tetrachloride in rats. Int J Pharma Sci Res. 2011;2(2):74–83. [Google Scholar]
- 7.Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. UK: Oxford University Press; 2000. pp. 148–149. [Google Scholar]
- 8.Khan MR, Marium A, Shabbir M, Saeed N, Bokhari J. Antioxidant and hepatoprotective effects of Oxalis corniculata against carbon tetrachloride (CCl4) induced injuries in rat. Afr J Pharm Pharmacol. 2012;6(30):2255–2267. [Google Scholar]
- 9.Anand T, Gokulakrishnan K. Amelioration of carbon tetrachloride induced oxidative stress in kidney tissues by ethanolic extract of Hybanthus enneaspermus in Wistar rats. Int J Pharm Sci Health Care. 2012;2(2):2249–5738. [Google Scholar]
- 10.Sharma A, Sharma MS, Mishra A, Sharma S, Kumar B, Bhandari A. A review on Thar plants used in liver diseases. Int J Res Pharm Chem. 2011;1(2):224–236. [Google Scholar]
- 11.Blair D, Padilla JJ, Wallace JP. The exercise dose affects oxidative stress and brachial artery flow-mediated dilation in trained men. Eur J Appl Physiol. 2012;112:33–42. doi: 10.1007/s00421-011-1946-8. [DOI] [PubMed] [Google Scholar]
- 12.Kosei Yamauchi K, Mitsunaga T, Batubara I. Isolation, identification and tyrosinase inhibitory activities of the extractives from Allamanda cathartica. Nat Resour. 2011;2:167–172. [Google Scholar]
- 13.Gilman EF. Allamanda cathartica ‘Cherries Jubilee’ cherries jubilee yellow allamanda. FPS30-series of the environmental horticulture, florida cooperative extension service. USA: Institute of Food and Agricultural Sciences, University of Florida; 2011. p. 2. [Google Scholar]
- 14.Chinchilla M, Valerio I, Sanchez R, Mora V, Bagnarello V, Martinez L, et al. et al. In vitro antimalarial activity of extracts of some plants from a biological reserve in Costa Rica. Rev Biol Trop. 2012;60(2):881–891. doi: 10.15517/rbt.v60i2.4024. [DOI] [PubMed] [Google Scholar]
- 15.Madhuri K, Prasad Rao M, Vineela M, Narasimha SV, Kumar BP, Bhogavalli Studies on phytochemical screening and vasoconstrictor activity of leaf extracts of Acalypha indica on frog blood vessels. Ann Biol Res. 2011;2(2):337–340. [Google Scholar]
- 16.Abdulkadir S, Adamu AK, Dangora DB, Alonge SO, Ibrahim G, Abubakar MS, et al. et al. Phytochemical screening and antivenin activity of the methanol extract of Indigofera conferta Gillet (Papilionaceae) in mice. Nigerian J Pharm Sci. 2011;10(1):1–5. [Google Scholar]
- 17.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Int Pharm Sci. 2011;1:98–106. [Google Scholar]
- 18.Manna P, Sinha M, Sil PC. Aqueous extract of Terminalia arjuna prevents carbon tetrachloride induced hepatic and renal disorders. BMC Complement Altern Med. 2006;6:33–43. doi: 10.1186/1472-6882-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beuge JA, Aust SD. The thiobarbituric acid assay. Methods Enzymol. 1978;52:306–307. [Google Scholar]
- 20.Jambunathan N. Determination and detection of reactive oxygen species (ROS), lipid peroxidation, and electrolyte leakage in plants. Methods Mol Biol. 2010;639:292–298. doi: 10.1007/978-1-60761-702-0_18. [DOI] [PubMed] [Google Scholar]
- 21.Ellman GL. Tissue sulphydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 22.Thorsten C. USA: CR-Scientific; 2004. Biochem experiment: Colorimetric assay for enzyme activity. [Google Scholar]
- 23.Wan J, Diaz-Sanchez D. Antioxidant enzyme induction: A new protective approach against the adverse effects of diesel exhaust particles. Inhal Toxico. 2007;19(1):177–182. doi: 10.1080/08958370701496145. [DOI] [PubMed] [Google Scholar]
- 24.Ogbugunafor H, Sofidiya O, Okpuzor J, Kemdilim M, Anajekwe B, Ekechi A. Effect of extracts of Hymenocardia acida Tul (Hymenocardiaceae) on rats. J Am Sci. 2010;6(2):143–146. [Google Scholar]
- 25.Carrasquilla G, Barón C, Monsell EM, Cousin M, Walter V, Lefèvre G, et al. et al. Randomized, prospective, three-arm study to confirm the auditory safety and efficacy of artemether-lumefantrine in colombian patients with uncomplicated Plasmodium falciparum Malaria. Am J Trop Med Hyg. 2012;86:75–83. doi: 10.4269/ajtmh.2012.11-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nwabuisi C. Prophylactic effect of multi-herbal extract ‘Agbo-Iba’ on malaria induced in mice. East Afr Med J. 2002;79:343–346. doi: 10.4314/eamj.v79i7.8836. [DOI] [PubMed] [Google Scholar]
- 27.Patnaik S, Mishra SR, Choudhury GB, Panda SK, Behera M. Phytochemical investigation and simultaneously study on anticonvulsant, antidiabetic activity of different leafy extracts of Bixa orellana Linn. Int J Pharm Biol Arch. 2011;2(5):1497–1501. [Google Scholar]
- 28.Radhika B, Nasreen BSK. Pharmacognostic and preliminary phytochemical evaluation of the leaves of Bixa orellana. Phcog J. 2010;2(10):311–316. [Google Scholar]
- 29.Tamil SA, Dinesh MG, Stayan RS, Chandrasekaran B, Rose C. Leaf and seed extracts of Bixa orellana L. exert anti-microbial activity against bacterial pathogens. J Appl Pharm Sci. 2011;1(9):116–120. [Google Scholar]
- 30.Yeasmin F, Ashrafuzzaman M, Hossain I. Effects of garlic extract, allamanda leaf extract and provax-200 on seed borne fungi of rice. The Agriculturists. 2012;10(1):46–50. [Google Scholar]
- 31.Joselin J, Brintha TSS, Augustian RF, Jeeva S. Screening of select ornamental flowers of the family Apocynaceae for phytochemical constituents. Asian Pac J Biomed. 2012;2:S260–S264. [Google Scholar]