Abstract
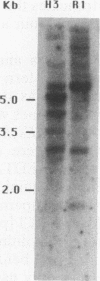
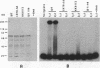
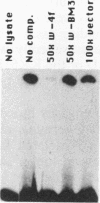
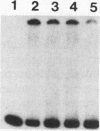
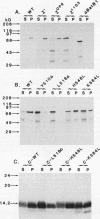
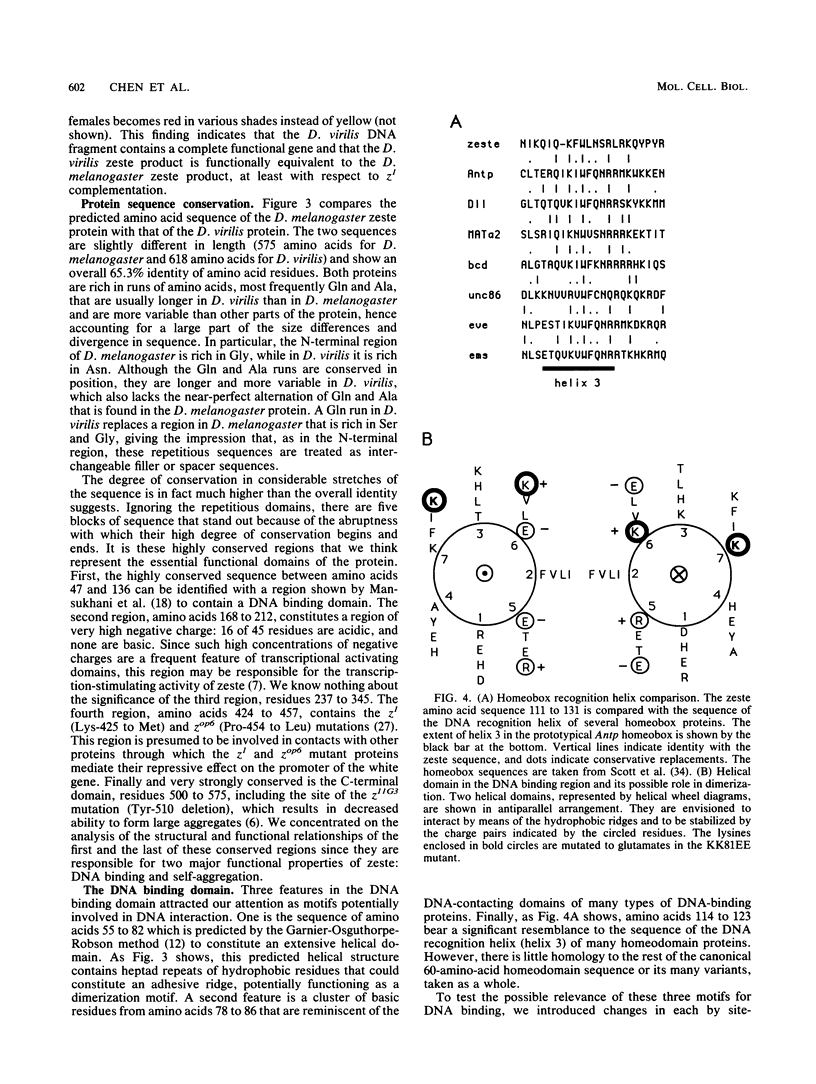
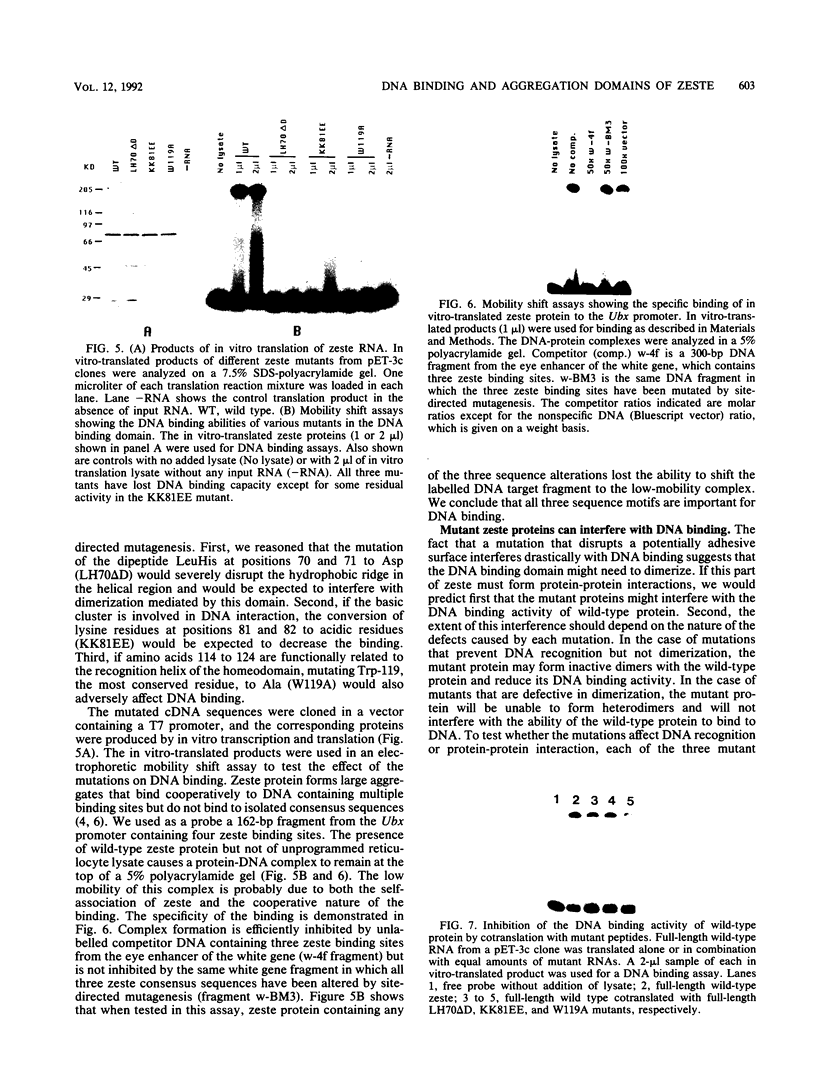
The zeste gene product is involved in two types of genetic effects dependent on chromosome pairing: transvection and the zeste-white interaction. Comparison of the predicted amino acid sequence with that of the Drosophila virilis gene shows that several blocks of amino acid sequence have been very highly conserved. One of these regions corresponds to the DNA binding domain. Site-directed mutations in this region indicate that a sequence resembling that of the homeodomain DNA recognition helix is essential for DNA binding activity. The integrity of an amphipathic helical region is also essential for binding activity and is likely to be responsible for dimerization of the DNA binding domain. Another very strongly conserved domain of zeste is the C-terminal region, predicted to form a long helical structure with two sets of heptad repeats that constitute two long hydrophobic ridges at opposite ends and on opposite faces of the helix. We show that this domain is responsible for the extensive aggregation properties of zeste that are required for its role in transvection phenomena. A model is proposed according to which the hydrophobic ridges induce the formation of open-ended coiled-coil structures holding together many hundreds of zeste molecules and possibly anchoring these complexes to other nuclear structures.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler P. N., Charlton J., Brunk B. Genetic interactions of the suppressor 2 of zeste region genes. Dev Genet. 1989;10(3):249–260. doi: 10.1002/dvg.1020100314. [DOI] [PubMed] [Google Scholar]
- Alani E., Subbiah S., Kleckner N. The yeast RAD50 gene encodes a predicted 153-kD protein containing a purine nucleotide-binding domain and two large heptad-repeat regions. Genetics. 1989 May;122(1):47–57. doi: 10.1093/genetics/122.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M., Pirrotta V. The Drosophila zeste protein binds cooperatively to sites in many gene regulatory regions: implications for transvection and gene regulation. EMBO J. 1988 Dec 1;7(12):3907–3915. doi: 10.1002/j.1460-2075.1988.tb03277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson M., Pirrotta V. The product of the Drosophila zeste gene binds to specific DNA sequences in white and Ubx. EMBO J. 1987 May;6(5):1387–1392. doi: 10.1002/j.1460-2075.1987.tb02379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Wilson A. C. Molecular evolution in Drosophila and the higher Diptera II. A time scale for fly evolution. J Mol Evol. 1984;21(1):1–13. doi: 10.1007/BF02100622. [DOI] [PubMed] [Google Scholar]
- Bickel S., Pirrotta V. Self-association of the Drosophila zeste protein is responsible for transvection effects. EMBO J. 1990 Sep;9(9):2959–2967. doi: 10.1002/j.1460-2075.1990.tb07488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Bickel S., Benson M., Pirrotta V., Tjian R. Zeste encodes a sequence-specific transcription factor that activates the Ultrabithorax promoter in vitro. Cell. 1988 Jun 3;53(5):713–722. doi: 10.1016/0092-8674(88)90089-x. [DOI] [PubMed] [Google Scholar]
- Burtis K. C., Thummel C. S., Jones C. W., Karim F. D., Hogness D. S. The Drosophila 74EF early puff contains E74, a complex ecdysone-inducible gene that encodes two ets-related proteins. Cell. 1990 Apr 6;61(1):85–99. doi: 10.1016/0092-8674(90)90217-3. [DOI] [PubMed] [Google Scholar]
- Colby W. W., Chen E. Y., Smith D. H., Levinson A. D. Identification and nucleotide sequence of a human locus homologous to the v-myc oncogene of avian myelocytomatosis virus MC29. Nature. 1983 Feb 24;301(5902):722–725. doi: 10.1038/301722a0. [DOI] [PubMed] [Google Scholar]
- Diffley J. F., Stillman B. Transcriptional silencing and lamins. Nature. 1989 Nov 2;342(6245):24–24. doi: 10.1038/342024a0. [DOI] [PubMed] [Google Scholar]
- Garnier J., Osguthorpe D. J., Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978 Mar 25;120(1):97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- Hartley D. A., Xu T. A., Artavanis-Tsakonas S. The embryonic expression of the Notch locus of Drosophila melanogaster and the implications of point mutations in the extracellular EGF-like domain of the predicted protein. EMBO J. 1987 Nov;6(11):3407–3417. doi: 10.1002/j.1460-2075.1987.tb02664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S. Unidirectional digestion with exonuclease III creates targeted breakpoints for DNA sequencing. Gene. 1984 Jun;28(3):351–359. doi: 10.1016/0378-1119(84)90153-7. [DOI] [PubMed] [Google Scholar]
- Kidd S., Baylies M. K., Gasic G. P., Young M. W. Structure and distribution of the Notch protein in developing Drosophila. Genes Dev. 1989 Aug;3(8):1113–1129. doi: 10.1101/gad.3.8.1113. [DOI] [PubMed] [Google Scholar]
- Locke J., Kotarski M. A., Tartof K. D. Dosage-dependent modifiers of position effect variegation in Drosophila and a mass action model that explains their effect. Genetics. 1988 Sep;120(1):181–198. doi: 10.1093/genetics/120.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansukhani A., Crickmore A., Sherwood P. W., Goldberg M. L. DNA-binding properties of the Drosophila melanogaster zeste gene product. Mol Cell Biol. 1988 Feb;8(2):615–623. doi: 10.1128/mcb.8.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Miesfeld R., Rusconi S., Godowski P. J., Maler B. A., Okret S., Wikström A. C., Gustafsson J. A., Yamamoto K. R. Genetic complementation of a glucocorticoid receptor deficiency by expression of cloned receptor cDNA. Cell. 1986 Aug 1;46(3):389–399. doi: 10.1016/0092-8674(86)90659-8. [DOI] [PubMed] [Google Scholar]
- PATERSON H. E. Sex-linked and sexlimited mutation of the fly Muscina stabulans (Fall.) (Muscidae). Nature. 1958 Mar 29;181(4613):932–933. doi: 10.1038/181932b0. [DOI] [PubMed] [Google Scholar]
- Paro R. Imprinting a determined state into the chromatin of Drosophila. Trends Genet. 1990 Dec;6(12):416–421. doi: 10.1016/0168-9525(90)90303-n. [DOI] [PubMed] [Google Scholar]
- Pedersen K., Devereux J., Wilson D. R., Sheldon E., Larkins B. A. Cloning and sequence analysis reveal structural variation among related zein genes in maize. Cell. 1982 Jul;29(3):1015–1026. doi: 10.1016/0092-8674(82)90465-2. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bickel S., Mariani C. Developmental expression of the Drosophila zeste gene and localization of zeste protein on polytene chromosomes. Genes Dev. 1988 Dec;2(12B):1839–1850. doi: 10.1101/gad.2.12b.1839. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Manet E., Hardon E., Bickel S. E., Benson M. Structure and sequence of the Drosophila zeste gene. EMBO J. 1987 Mar;6(3):791–799. doi: 10.1002/j.1460-2075.1987.tb04821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S., Capovilla M., Pirrotta V. The bx region enhancer, a distant cis-control element of the Drosophila Ubx gene and its regulation by hunchback and other segmentation genes. EMBO J. 1991 Jun;10(6):1415–1425. doi: 10.1002/j.1460-2075.1991.tb07662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulski M., McGinnis N., Chadwick R., McGinnis W. Developmental and molecular analysis of Deformed; a homeotic gene controlling Drosophila head development. EMBO J. 1987 Mar;6(3):767–777. doi: 10.1002/j.1460-2075.1987.tb04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushlow C. A., Hogan A., Pinchin S. M., Howe K. M., Lardelli M., Ish-Horowicz D. The Drosophila hairy protein acts in both segmentation and bristle patterning and shows homology to N-myc. EMBO J. 1989 Oct;8(10):3095–3103. doi: 10.1002/j.1460-2075.1989.tb08461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saint R., Kalionis B., Lockett T. J., Elizur A. Pattern formation in the developing eye of Drosophila melanogaster is regulated by the homoeo-box gene, rough. Nature. 1988 Jul 14;334(6178):151–154. doi: 10.1038/334151a0. [DOI] [PubMed] [Google Scholar]
- Schneuwly S., Kuroiwa A., Baumgartner P., Gehring W. J. Structural organization and sequence of the homeotic gene Antennapedia of Drosophila melanogaster. EMBO J. 1986 Apr;5(4):733–739. doi: 10.1002/j.1460-2075.1986.tb04275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Shepard S. B., Broverman S. A., Muskavitch M. A. A tripartite interaction among alleles of Notch, Delta, and Enhancer of split during imaginal development of Drosophila melanogaster. Genetics. 1989 Jun;122(2):429–438. doi: 10.1093/genetics/122.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spena A., Viotti A., Pirrotta V. A homologous repetitive block structure underlies the heterogeneity of heavy and light chain zein genes. EMBO J. 1982;1(12):1589–1594. doi: 10.1002/j.1460-2075.1982.tb01360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. A transposable P vector that confers selectable G418 resistance to Drosophila larvae. EMBO J. 1985 Jan;4(1):167–171. doi: 10.1002/j.1460-2075.1985.tb02332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H., Pirrotta V. P transposons controlled by the heat shock promoter. Mol Cell Biol. 1986 May;6(5):1640–1649. doi: 10.1128/mcb.6.5.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward R. Dorsal, an embryonic polarity gene in Drosophila, is homologous to the vertebrate proto-oncogene, c-rel. Science. 1987 Oct 30;238(4827):692–694. doi: 10.1126/science.3118464. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Vaessin H., Grell E., Wolff E., Bier E., Jan L. Y., Jan Y. N. prospero is expressed in neuronal precursors and encodes a nuclear protein that is involved in the control of axonal outgrowth in Drosophila. Cell. 1991 Nov 29;67(5):941–953. doi: 10.1016/0092-8674(91)90367-8. [DOI] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Goldberg M. L. The Drosophila zeste gene and transvection. Trends Genet. 1989 Jun;5(6):189–194. doi: 10.1016/0168-9525(89)90074-7. [DOI] [PubMed] [Google Scholar]
- Wu C. T., Jones R. S., Lasko P. F., Gelbart W. M. Homeosis and the interaction of zeste and white in Drosophila. Mol Gen Genet. 1989 Sep;218(3):559–564. doi: 10.1007/BF00332424. [DOI] [PubMed] [Google Scholar]
- Wu L. C., Fisher P. A., Broach J. R. A yeast plasmid partitioning protein is a karyoskeletal component. J Biol Chem. 1987 Jan 15;262(2):883–891. [PubMed] [Google Scholar]