Abstract
Processive DNA synthesis by the αεθ core of the Escherichia coli Pol III replicase requires it to be bound to the β2 clamp via a site in the α polymerase subunit. How the ε proofreading exonuclease subunit influences DNA synthesis by α was not previously understood. In this work, bulk assays of DNA replication were used to uncover a non-proofreading activity of ε. Combination of mutagenesis with biophysical studies and single-molecule leading-strand replication assays traced this activity to a novel β-binding site in ε that, in conjunction with the site in α, maintains a closed state of the αεθ–β2 replicase in the polymerization mode of DNA synthesis. The ε–β interaction, selected during evolution to be weak and thus suited for transient disruption to enable access of alternate polymerases and other clamp binding proteins, therefore makes an important contribution to the network of protein–protein interactions that finely tune stability of the replicase on the DNA template in its various conformational states.
Keywords: beta sliding clamp, DNA polymerase III, DNA replication, Escherichia coli , proofreading exonuclease
Introduction
The Escherichia coli replicase provides a well-characterized system to discover design principles for evolution of structure and function of Nature’s dynamic molecular machines. At its heart is the DNA polymerase III holoenzyme (Pol III HE), a complex of at least 17 subunits that include two (or three; McInerney et al, 2007) αεθ cores, two (or three) β2 sliding clamps, and a δδ′τ3ψχ clamp loader assembly in which one (or non-functionally, two or three) of the τ subunits may be substituted by a C-terminally truncated form called γ (McHenry, 2011). The Pol III replicase is dynamic in that many of its subunits change conformation and even binding partners as it carries out coordinated synthesis of both DNA strands at replication forks.
Work over the past two decades (reviewed by Johnson and O’Donnell, 2005; Schaeffer et al, 2005; Hamdan and Richardson, 2009; McHenry, 2011) has resulted in (i) determination of static high resolution structures of essentially all of the replicase components, (ii) identification of many pairwise protein–protein interactions that show a finely tuned hierarchy of binding energies, (iii) demonstration that many of the dynamic protein–protein interactions are mediated by intrinsically unstructured segments of subunits that become structured upon interaction with partner proteins (e.g., see Ozawa et al, 2005, 2008; Jergic et al, 2007), and (iv) revelation that many of these interactions occur at sites at which binding partners change places in a particular order during the replication cycles, especially during Okazaki fragment synthesis on the lagging strand. These attributes, when combined with irreversible chemical steps involving dNTP incorporation and ATP hydrolysis, provide the underlying design rules for replicase function as a dynamic machine. In particular, the many weak interactions within the Pol III replicase allow it to transit rapidly from one conformational state to another by breaking and remaking of interactions, without risk of the whole complex dissociating from the template DNA. The proximity effects of nearby interactions effectively reduce the apparent dissociation constants (KD) of protein–protein complexes in the context of the whole replisome relative to values for pairwise interactions. It is thus more likely to uncover weak but nonetheless important interactions using functional assays, rather than by biophysical studies of protein–protein interactions in a pairwise manner.
Here, we focus on the complex of the αεθ replicase core with the β2 sliding clamp on primer-template DNA, required for both leading- and lagging-strand synthesis. This complex is expected to have at least two (and probably more) major conformational states, one of which ensures the efficiency and processivity of DNA synthesis (polymerization mode) and the other contributes to fidelity by exonucleolytic editing of polymerase insertion errors (proofreading mode). Protein interactions that affect the transition between these two modes are not currently understood, and are the focus of this article.
The β2 sliding clamp is a ring-shaped dimer (Kong et al, 1992) that has to be opened by interactions with a clamp loader complex to be loaded onto a primer-template DNA in a first step to initiate primer extension DNA synthesis (reviewed by Bloom, 2009). The clamp ensures replicase processivity by interaction with the α polymerase subunit of the core. In the polymerase mode, α interacts at only one of the two symmetry-related protein-binding sites in the β dimer (Dohrmann and McHenry, 2005) via a short peptide motif. Related clamp binding motifs (CBMs) occur in disordered segments or loops in the many β-binding proteins (Dalrymple et al, 2001). Thus, having two equivalent sites in the β2 ring enables it to bind two different proteins at the same time. This is suggested to be important for reversible handover of a primer-template from α to a repair polymerase (e.g., Pol II, IV, or V) during bypass of a lesion in the template DNA (López de Saro et al, 2003a; Indiani et al, 2005).
The Pol III core contains the polymerase subunit α (1160 residues in E. coli), the proofreading 3′–5′ exonuclease ε (243 residues), and the small θ subunit of unknown function (McHenry and Crow, 1979); α interacts with ε but not with θ, while θ interacts only with ε (Studwell-Vaughan and O’Donnell, 1993). The E. coli α subunit contains two β-binding sites, a conserved internal CBM used for processive DNA synthesis (Dohrmann and McHenry, 2005) and a C-terminal site (Kim and McHenry, 1996b) that may have a role in polymerase recycling from the ends of completed Okazaki fragments (López de Saro et al, 2003b). X-ray crystal structures of a large N-terminal portion of E. coli α (that terminates just before the internal CBM at residue 917; Lamers et al, 2006), of the closely related full-length Thermus aquaticus (Taq) α by itself (Bailey et al, 2006) and bound to primer-template DNA (Wing et al, 2008), and of β2 bound separately to CBM peptides (e.g., Georgescu et al, 2008a) and double-stranded (ds) DNA (Georgescu et al, 2008b) allow construction of a plausible model of the α–β2–DNA complex in the polymerization mode (Wing et al, 2008).
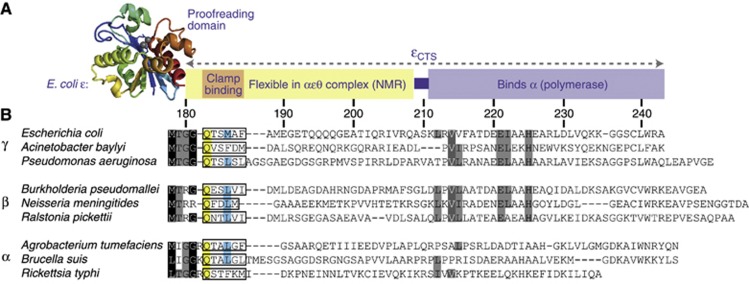
The precise location of the ε proofreading subunit in the replicase is uncertain. It has two domains (Figure 1A): its N-terminal exonuclease domain (residues 2–180; Hamdan et al, 2002) interacts with θ (Pintacuda et al, 2006), and residues following Ala209 in its intrinsically unstructured C-terminal segment (εCTS, Gly181–Ala243) interact with the N-terminal PHP domain of α (Wieczorek and McHenry, 2006; Ozawa et al, 2008). The location of the proofreader is a little more clearly defined in the PolC replicase of Firmicutes, where it is integrated as an insertion into the PHP domain, but was removed for PolC structure determination (Evans et al, 2008).
Figure 1.
A clamp-binding motif (CBM) is located in the C-terminal segment of the ε subunit of Pol III (εCTS). (A) Two-domain organization of ε. The flexible εCTS that interacts with α (Ozawa et al, 2008) extends from the N-terminal exonuclease domain. (B) Sequence alignment of εCTS from representative α-, β-, and γ-proteobacteria shows conservation of the CBM. Residue numbering is based on the E. coli sequence, and boxes denote putative CBMs.
We envisaged that a useful strategy to uncover new protein–protein interactions in the replicase would be to challenge it to make DNA under difficult conditions, so that even the weakest interactions become essential. For example, there has not previously been an assay that depends absolutely on the presence of ε in the replicase; ε was observed to stimulate the rate (Kim and McHenry, 1996a) and processivity (Studwell and O’Donnell, 1990) of DNA synthesis in replication assays under conditions where proofreading is not expected to be limiting, and had more subtle effects on coupled leading- and lagging-strand synthesis by full replisomes (Marians et al, 1998). This is in spite of genetic evidence that the very poor growth phenotype of disruption of the chromosomal dnaQ gene (encoding ε) can be rescued by suppressor mutations in dnaE (encoding α) like spq2 (αV832G) that do not relieve the dnaQ mutator phenotype. It was argued that this indicates an additional role for ε in stabilizing the replicase that does not depend on its proofreading capability (Lancy et al, 1989; Lifsics et al, 1992; Slater et al, 1994).
Here, we report situations where replication of DNA templates by replisomes assembled in vitro becomes highly dependent on ε, but do not require it to be active as an exonuclease. This non-proofreading activity is traced to a relatively weak interaction of a CBM we identify in ε with one of the protein-binding sites in β2. We show using single-molecule (SM) replication experiments that it also makes an important contribution to both rate and processivity in helicase-coupled leading-strand synthesis, without affecting the lifetimes of active replisomes. We conclude that the ε–β interaction is maintained in the polymerization mode of DNA synthesis by the full replicase and that it is disrupted in transitions to other conformational states.
Results
Efficient strand-displacement synthesis by Pol III HE requires ε
We set up a simplified assay for DNA synthesis by Pol III HE on oligonucleotide-primed circular single-stranded (ss) M13 DNA (6.4 kb); the products were separated on an agarose gel and stained with a dye that detects both ss and dsDNA. In addition to the expected strand-extension synthesis of the fully ds circular product (TFII), we observed robust helicase-independent synthesis of products greater than unit length (Figure 2A). These long products arise from strand-displacement (SD) DNA synthesis, a process studied by Yuan and McHenry (2009). Although the conditions we used are somewhat different (e.g., physiological ionic strength), we confirmed that SD synthesis requires high concentrations of dNTPs, works also with isolated pre-filled TFII, and is dependent on ssDNA-binding protein (SSB) with an intact C-terminal protein-binding motif. It also requires loading of β2 on the primer template and interaction of αεθ with at least one τ subunit in a clamp loader that also contains ψ and χ for interaction with SSB.
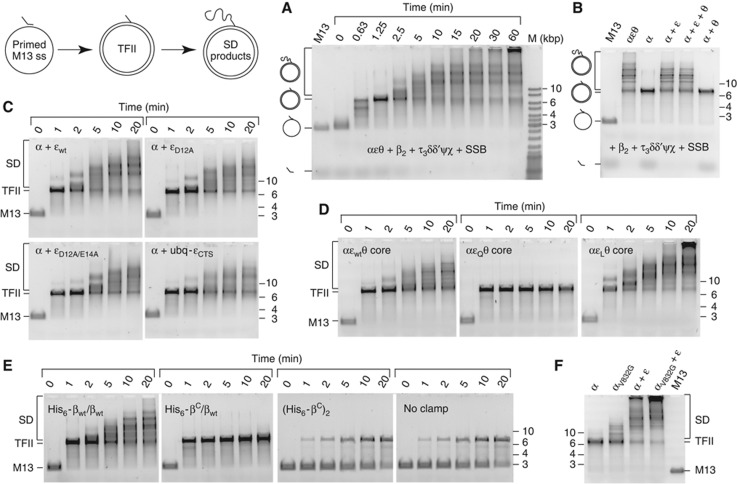
Figure 2.
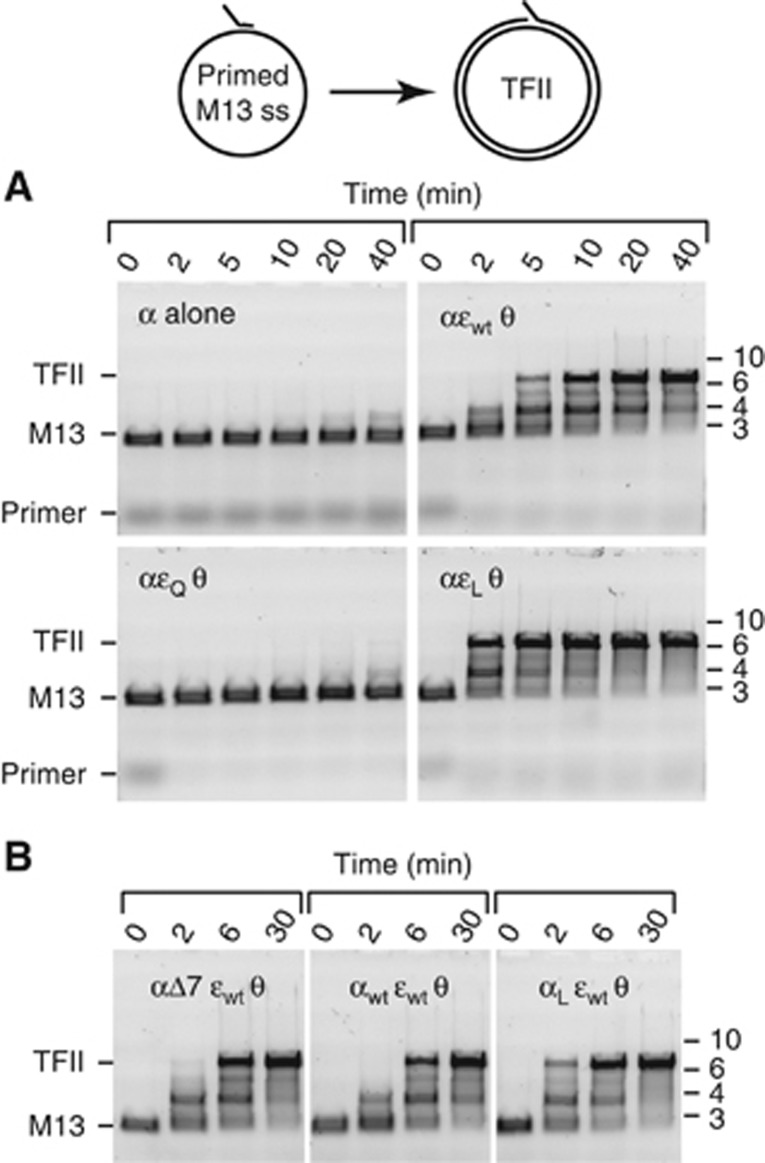
Pol III strand-displacement (SD) DNA synthesis provides functional evidence for the ε–β interaction. (A) Efficient SSB-dependent SD DNA synthesis by the complete Pol III HE under standard conditions (see Materials and methods). Time course of flap-primer extension on M13 ssDNA shows larger than unit length dsDNA (tailed form II, TFII) products produced by SD synthesis. (B) The ε subunit, but not θ, contributes to SD synthesis. Assays (20 min) used Pol III HE with purified αεθ or assembled in situ with α±ε±θ. (C) The ε contribution to SD synthesis does not require proofreading and determinants of it are located in the εCTS. SD DNA synthesis by Pol III HE containing indicated core sub-complexes assembled in situ. (D) Mutations within the β-binding motif of ε affect SD synthesis. Time course of DNA synthesis by Pol III HE with purified wild-type or mutated αεθ cores; εQ does not bind significantly to β while εL has a strengthened binding site. (E) Both protein interaction sites in β2 are engaged during SD synthesis. Assays had wild-type (βwt)2 substituted by modified clamps His6-βwt/βwt (no site occluded), His6-βC/βwt (one site occluded) or (His6-βC)2 (both sites occluded), or no clamp. (F) The spq2 suppressor (αV832G) polymerase is capable of more efficient SD synthesis than wild-type α. Assays (20 min) were under standard conditions except that NaCl was at 100 mM, with versions of Pol III HE assembled in situ. All panels show photographic negative images of gels that had been stained with SYBR gold nucleic acid stain (Invitrogen).
The SD reaction is demanding in terms of its requirement for all but one of the HE subunits. Indeed, hoping to detect a novel function of the θ subunit, we examined the dependence of SD synthesis on the Pol III core subunits. Although we found θ to be dispensable under our standard conditions, SD synthesis was absolutely dependent on the ε proofreading subunit (Figure 2B). This is the first report of a replication assay that is so strongly dependent on ε.
SD synthesis does not require the exonuclease domain of ε
The ε subunit contains a binuclear Mn2+ or Mg2+ metallocentre at its active site coordinated by carboxylates of Asp12, Glu14, and Asp102 (Hamdan et al, 2002), and its D12A and D12A/E14A mutants have no residual 3′–5′ exonuclease (proofreading) activity (Fijalkowska and Schaaper, 1996). Nevertheless, we found that both mutants were capable of sustaining extensive SD synthesis (Figure 2C), indicating that proofreading is not required for this process.
To determine if the εCTS (Figure 1A), decoupled from the exonuclease domain, is itself sufficient to support SD synthesis, we fused it to the C-terminus of human ubiquitin (as a small soluble tag) to produce ubq-εCTS (Supplementary Figure S1A). Although ubq-εCTS was partly proteolysed during expression in E. coli (Supplementary Figure S1B), the intact protein in this preparation still interacted strongly with α (Supplementary Figure S1C), and a mixture of α and ubq-εCTS could still sustain robust SD synthesis (Figure 2C). This provides clear evidence for a non-proofreading role of ε in DNA replication, dependent only on residues within the εCTS.
A potential clamp-binding site in the C-terminal segment of ε
Sequence alignment of the ε subunit in species of the α-, β-, and γ-proteobacteria shows conservation of the structured nuclease domain, but much greater variability in the εCTS. An exception is a short moderately conserved region immediately following the structured domain (Figure 1B) that resembles a CBM in other proteins (Dalrymple et al, 2001); CBMs are either penta- (optimally QLS/DLF) or hexapeptides (QxxΦxΦ, where x is any residue and Φ is hydrophobic). The regions in the ε subunits of various species mostly resemble the hexapeptide (QTSMAF in E. coli), but some also have pentapeptide motifs.
Studies of binding of many synthetic peptides to β2 enable reliable prediction of binding strengths of CBMs (Wijffels et al, 2004, 2011). Thus, we made two mutants of wild-type ε (εwt) that we call εQ, in which the conserved first residue (Gln182) of the motif is changed to Ala, and εL, with the motif changed to QLSLPL. The εQ motif is predicted to bind more weakly to β2 than the εwt motif, while the εL motif is one of the tighter binding CBMs, from the replication initiation factor Hda (Wijffels et al, 2004). We confirmed that both εQ and εL interact with α as expected, and could be used to assemble stable isolable αεθ core complexes.
These isolated Pol III cores containing εQ, εL, and wild-type εwt were compared for their ability to support Pol III SD DNA synthesis (Figure 2D). The results were consistent with the predicted ε–β binding strength; the αεQθ complex could no longer sustain SD synthesis, while αεLθ promoted more extensive synthesis than the wild-type complex. Therefore, the putative CBM in ε is required for SD DNA synthesis.
Efficient SD DNA synthesis by Pol III HE requires two β-binding sites
We predicted that SD synthesis by the Pol III HE would require that the two protein interaction sites in the β dimer be occupied simultaneously by a CBM of α and the newly discovered site in ε. To test this, we prepared a hemi-mutant β dimer made up of one native subunit that contains an intact protein interaction site and one that does not (βC, a mutant lacking five C-terminal residues that comprise part of the CBM-binding cleft). Scouten Ponticelli et al (2009) had earlier made a hemi-mutant βC/βwt dimer in vivo, isolated and characterized it. They showed that it could be efficiently loaded on a primer-template DNA by the clamp loader, and was proficient for DNA strand extension. We used subunit exchange and chromatography to isolate a similar His6-βC/βwt hemi-mutant dimer, and its composition and absence of contamination by (βwt)2 was confirmed by electrospray ionization mass spectrometry (ESI-MS) under native conditions (Supplementary Figure S2). The hemi-mutant β was inactive in the Pol III SD reaction (Figure 2E), showing that both protein interaction sites in β2 are utilized, presumably being bound simultaneously to the CBMs in α and ε. In contrast, and consistent with previous work showing that a single binding cleft in the clamp is sufficient to stimulate replication by Pol III both in vitro (Scouten Ponticelli et al, 2009) and in vivo (Sutton et al, 2010), the hemi-mutant β was still able to support efficient primer extension to form TFII (Figure 2E).
The αV832G mutation suppresses the requirement for ε in SD DNA synthesis
The phenotype of the spq2 mutation in dnaE (αV832G) argues for an important non-proofreading function of ε in vivo, so we studied the activity of αV832G in SD synthesis (Figure 2F) and found that (i) it is still active even in the absence of ε and (ii) its activity is stimulated by ε to a level higher than wild-type α. These observations suggest that αV832G forms more stable interactions with proteins or DNA that can partially compensate for the absence of ε, and that ε normally makes an important contribution to the protein and DNA interaction network that stabilizes the wild-type polymerase on the DNA template.
Physical evidence for interaction of β2 with ε in the αεθ core complex
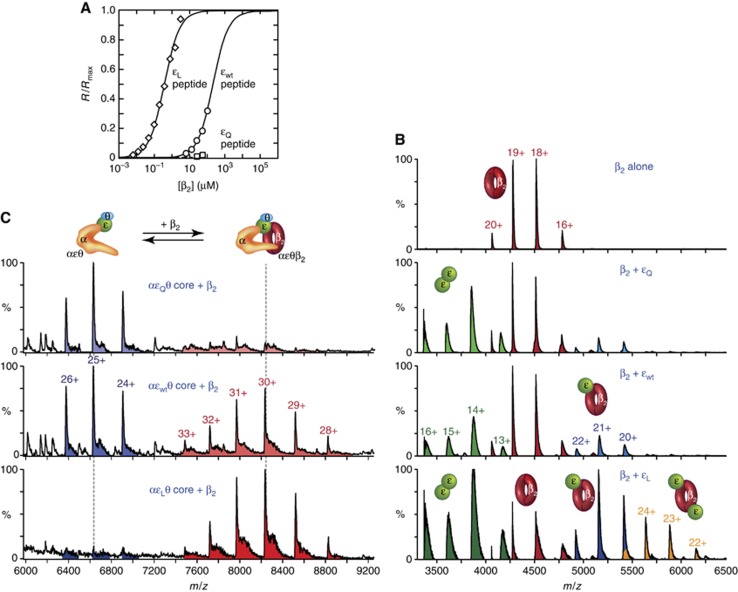
We next used surface plasmon resonance (SPR) to confirm the predicted strengths of interactions of β2 with the εwt, εQ, and εL peptides. Synthetic biotinylated peptides were immobilized on a streptavidin-coated SPR chip, and solutions of β2 were made to flow over it. Binding isotherms (Figure 3A; sensorgrams in Supplementary Figure S3A) showed β2 bound the εL peptide with KD=0.38±0.04 μM, consistent with previous data (0.38–0.45 μM) for very similar peptides (Wijffels et al, 2004). The εwt peptide was bound 550-fold more weakly, with KD=210±50 μM, while essentially no binding was detected with εQ (KD>2 mM).
Figure 3.
Physical interaction occurs between β2 and the clamp-binding motif (CBM) of ε in the αεθ–β2 complex. (A) β2 binds to a peptide containing the εwt CBM. SPR binding isotherms (R/Rmax) for the interaction of β2 with immobilized decapeptides containing CBMs from εL (diamonds), εwt (circles), and εQ (squares) are shown; sensorgrams are in Supplementary Figure S3A. Fits to data using a 1:1 binding model (εL and εwt peptides) are shown as solid lines. The small responses with the εQ peptide at the highest [β2] indicate KD>2 mM (see Supplementary Figure S3A). (B) The CBM in εL is accessible to β2. NanoESI mass spectra of 1 μM β2 alone or with 20 μM εwt, εQ, or εL show that εL interacts more strongly with β2 than does εwt. Proteins were in 140 mM NH4OAc, and ions due to free monomeric ε (1700–3400 m/z) have been omitted for clarity. (C) Wild-type ε contacts β2 in the αεθ–β2 complex, shown by a shift in the αεθ: αεθβ2 equilibrium (in excess β2) with progressive increase in ε–β binding strength. NanoESI-MS of 2.8 μM β2 with 1.8 μM purified αεθ cores in 140 mM NH4OAc. Ions due to free β2 (4000–5000 m/z) are not shown.
Because of the proximity of the putative CBM to the structured domain of ε (Figure 1A), we thought it might not be accessible to a protein as large as β2. Gel filtration of a mixture of β2 with εL (Supplementary Figure S3B) confirmed CBM accessibility since much of the εL eluted in a peak coincident with β2. In the same conditions, however, neither εwt nor εQ showed much evidence of complex formation. To characterize the weak ε–β interaction with the native proteins, we turned to electrospray-ionization mass spectrometry (ESI-MS) in ammonium acetate (NH4OAc) buffers at neutral pH.
While it is difficult to determine accurate KD values of protein complexes in solution by ESI-MS because different species ionize with different efficiencies, it can be used reliably to detect and rank the stabilities of complexes containing similar species in solution, for example, mutant proteins (Kapur et al, 2002). This follows from the argument: For two proteins A and B at equilibrium, KD=[A][B]/[AB]. If [A] is kept constant and in excess of [B], then the ratio of observed ions corresponding to B and AB is related to the KD of AB by a constant (c) determined by [A] and their relative ionization efficiencies, KD=c × [B]/[AB]. Thus, provided the reasonable assumption is made that mutations in B do not greatly affect its ionization efficiency or that of the AB complex, KD values of similar complexes can be ranked using the ratios of ions corresponding to free B and AB.
The ESI-mass spectra of equilibrium mixtures of excess εQ or εwt with β2 showed only small amounts of εβ2 complexes (Figure 3B), providing little evidence for a significant ε–β interaction. In contrast, the εL protein, engineered to contain a strong β-binding site, shows clear evidence of species that contain one or two εL bound to one or both CBM-binding sites in the β dimer. However, although consistent with the gel filtration data, these data do not yet show that εwt interacts with β2.
To demonstrate this, we used mixtures of β2 (in excess) with isolated αεθ complexes containing the three ε variants. The results (Figure 3C) clearly show the existence of an αεwtθ–β2 complex (ratio of ions αεwtθ–β2/αεwtθ is ∼1); the role of the putative CBM in εwt in this interaction over and above the α–β2 interaction (see middle panel in Figure 4A) is further demonstrated by the relative absence of complex formation with αεQθ, and the nearly quantitative formation of an apparently stable αεLθ–β2 complex.
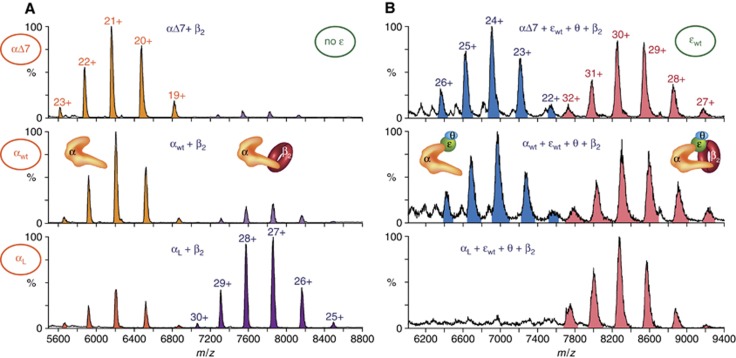
Figure 4.
Physical evidence that the ε subunit and the internal CBM in α synergistically sequester β2 in the αεθ–β2 complex. (A) Of the two CBMs in α, the internal site interacts preferentially with β2. NanoESI-MS of 2 μM β2 and 0.9 μM αΔ7, αwt, or αL in 400 mM NH4OAc. Ions due to free β2 (4000–5000 m/z) are not shown. (B) Both ε and the internal CBM in α bind β2 in the αεθ–β2 complex. NanoESI-MS of 2 μM β2 and cores assembled in situ with 0.9 μM αΔ7, αwt, or αL, 2 μM εwt and 5 μM θ in 400 mM NH4OAc. Ions due to excess ε (1700–3400), θ (1000–3000), β2 (4000–5000), and the β2εθ complex (4800–5800 m/z) are not shown.
β2 in the αεθ–β2 complex interacts with ε and the internal CBM in α
Essentially identical ESI-MS results were obtained when αεθ cores were assembled in situ from mixtures of separately dialysed subunits (middle panels in Figure 4B and Supplementary Figure S4A and B, cf. Figure 3C), which enabled study of β2 binding to αεθ cores with mutations in both α and ε. E. coli α contains two CBMs, but only the conserved internal motif is required for processive DNA synthesis (Dohrmann and McHenry, 2005). We used three variants of α to probe which of these sites binds β2 in the αεθ–β2 complex: αwt, and the mutants αΔ7, which misses the last seven residues that comprise the C-terminal CBM (López de Saro et al, 2003a), and one we term αL that has a strengthened internal CBM (QADMF changed to QLDLF; Dohrmann and McHenry, 2005). ESI-MS of binding of these α variants to (excess) β2 showed clear evidence of an interaction with αL, but only weak binding to αwt and αΔ7 (Figure 4A). We then assembled αεθ cores in situ with all combinations of αwt, αΔ7, or αL with εwt, εQ, or εL, and mixed them with excess β2. Regardless of which ε variant was used, ESI-MS showed that αwtεθ and αΔ7εθ cores bound β2 with very similar efficiency, while αLεθ formed a much stronger complex (Figure 4B; Supplementary Figure S4). This clearly shows that only the internal CBM of α is required for formation of the αεθ–β2 complex detected by ESI-MS, and that β2 in this complex is sequestered by simultaneous interactions with two CBMs—a stronger one in α and a weaker one in ε.
Role of ε–β interaction in strand extension by the Pol III core
While the data clearly show (i) that ε contains a CBM in the intrinsically unstructured region immediately following its exonuclease domain (Figure 1A) and (ii) that interaction of β2 at this site promotes SD synthesis by Pol III HE (Figure 2), they do not yet establish if this interaction occurs during regular DNA synthesis. That ε increases the processivity of α in a β2- and SSB-dependent synthesis reaction (Studwell and O’Donnell, 1990) suggests this to be true, but this effect could be mediated solely through stabilization of α through its contacts with εCTS.
To further demonstrate the significance of ε–β interaction in the αεθ–β2–DNA complex, we used an additional DNA synthesis assay, under ‘difficult’ conditions. We found salt conditions (130 mM NaCl) where primer extension by Pol III core is strongly dependent on both β2 (Supplementary Figure S5A) and the CBM of ε (Figure 5A); proofreading activity is again not required (Supplementary Figure S5B). In this assay, β2 is loaded onto primed ssDNA by the minimal γ3δδ′ clamp loader complex, thus disabling contacts between α and τ necessary for processivity. Moreover, SSB, required to melt secondary structures in the DNA, is omitted. Synthesis by the αεwtθ core shows stalls (appearing as bands in the gel analysis) and it is slow, taking about 5 min before half of the template strands are fully replicated (Figure 5A, αεwtθ core). Use of α alone or the αεQθ core results in very little primer extension, while >5-fold more extensive synthesis (fully replicated strands in <2 min) is achieved by strengthening the CBM using εL (Figure 5A, αεLθ core). Consistent with the ESI-MS studies, this τ-independent strand-extension reaction involves simultaneous interactions of β2 with ε and the internal CBM in α, since αΔ7εwtθ is as active as wild-type αεθ (Figure 5B); DNA synthesis is also stimulated by use of an αLεwtθ core, with the strengthened internal CBM in α. These data indicate that in the absence of SSB, the α–β2 complex is poorly processive and stalls readily on encounter with secondary structures in ssDNA, whereas the additional stabilization contributed by the ε–β interaction in the core–β2 complex allows it to progress more efficiently.
Figure 5.
Functional evidence that ε and the internal (but not the external) CBM in α synergistically bind β2 in the αεθ–β2 complex. Interactions of the CBM in ε (A) and the internal (but not the external) CBM in α (B) with β2 stimulate primer extension DNA synthesis. Time courses of primer extension ‘under difficult conditions’, using the γ3δδ′ clamp loader in the absence of SSB, with 200 nM β2 and 150 nM α or isolated αεθ complexes, as indicated. Both panels show photographic negative images of gels that had been stained with SYBR gold nucleic acid stain (Invitrogen).
The ε–β interaction stabilizes the Pol III replicase in the polymerization mode
An important question is whether the ε–β interaction contributes to DNA synthesis by the replicase in the polymerization, as opposed to the proofreading mode, since this would contribute to understanding of structures of the αεθ–β2–DNA complex during processive DNA synthesis, and lead to new hypotheses. To answer this question, we used SM observations of DnaB helicase-dependent leading-strand synthesis (Tanner et al, 2008; Tanner and van Oijen, 2009) to monitor replication in the context of a complete replisome. Since DNA synthesis must pause transiently (likely for periods of a few ms) during proofreading, progressive strengthening of the ε–β interaction should generate higher synthesis rates if this interaction was important in the polymerization mode, while the converse would be the case if the ε-binding site in β was more important for proofreading.
In the SM assays, one strand of oligonucleotide-primed phage λ dsDNA is tethered between a glass surface at one end and a 2.8-μm bead at the other, and the DNA is extended in a laminar buffer flow (Figure 6A). At the force generated (∼3 pN), the DNA shortens during conversion of the surface-tethered strand from ds to ssDNA by Pol III HE-dependent DnaB activity, thereby displacing the bead in the direction opposite the flow. The position of the bead is tracked in real time; trajectories (Figure 6B; Supplementary Figure S6) reveal rates, extents, and durations of leading-strand synthesis events. By combining data from many such trajectories recorded using Pol III HE containing the αεwtθ core, we previously obtained an average rate of synthesis of 417±8 bp/s and processivity of 10.5±0.9 kb (Tanner et al, 2008). These numbers were in accord with bulk assays with the same reagents, and reasonably reflected synthesis by an authentic fully constituted replicase.
Figure 6.
Single-molecule DNA replication assays demonstrate that the ε–β interaction stabilizes the replicase in the polymerization mode. (A) Schematic representation of the experimental set-up to study leading-strand replication. All proteins are present continuously in the buffer flow. (B) Example single-molecule trajectories showing pauses (dashed lines) during DNA synthesis by isolated αεwtθ core at 20 or 60 nM. (C) Exchange of αεwtθ cores from solution occurs during pauses, since pause times (N events fit with a single exponential) are inversely related to αεwtθ concentration. (D) However, cores do not exchange from solution during single events, since processivity (event size; N events fit with a single exponential) is unaffected by αεwtθ concentration (8.4±1.1 and 8.7±0.4 kb at 20 and 60 nM core, respectively). (E) Average DNA synthesis rate with the wild-type αεθ core is 890±50 bp/s (N events fit to a Gaussian distribution). (F) Termination of individual replication events with the αεθ core is a first-order process with a lifetime τ=10.3±1.1 s. (G) Progressive strengthening of the ε–β interaction increases both rates and processivities of DNA synthesis, but does not affect the lifetime of active replisomes. Derived processivities (left), rates (centre), and lifetimes (right) of replicases containing α or isolated αεθ cores. Fit values of parameters are given, with standard errors in parentheses (see Supplementary Figure S8).
In the current study (Figure 6D and E), we observed similar processivity with the αεwtθ core (8.7±0.4 kb), but higher rates (890±50 bp/s) that closely approach estimates of those that occur in vivo. In 61% of the replicated molecules, we also observed long pauses (defined as being ≥3 s, and often more than one), followed by further synthesis (Figure 6B; Supplementary Figure S6). Note that all proteins are present continuously in the buffer flow, and replication events on any single template DNA commence at random times during these experiments. This reflects the low efficiency of DnaC-dependent loading of DnaB in this situation where the template has no free 5′ end and no replication origin (Tanner et al, 2008; Ribeck et al, 2010). Thus, DnaB must remain bound at the fork while another replisomal component dissociates during pausing. That, at a constant concentration of the clamp loader, the mean duration of these long pauses was inversely proportional to the concentration of αεwtθ, whereas processivity remained constant (Figure 6C and D), indicates that it is the core that dissociates and re-associates during pauses. Therefore, we treated synthesis between pauses as discrete events. The higher rates we measured compared with the previous study (Tanner et al, 2008) seem to be due to use of a preformed and isolated (DnaB6)DnaC6 complex for helicase loading, rather than an approximately stoichiometric mixture of the two proteins (as used by Tanner et al (2008); see Supplementary Figure S7 cf. Figure 6D and E). The basis for inhibition of helicase activity by small amounts of free DnaC in this assay is a topic for further investigation (cf. Allen and Kornberg, 1991).
Having characterized the system, we were able to study leading-strand synthesis by replisomes containing α alone or αεθ cores made with εQ, εwt, and εL, thus progressively strengthening the ε–β interaction (Figure 6; Supplementary Figure S8). We found that although both the measured rates and processivities increased as the ε–β interaction was strengthened, the lifetime (τ=1/kobs) describing dissociation of cores from the replisome, as measured from the duration of events, remained constant at about 10 s (Figure 6G). This constant lifetime must therefore reflect a common rate-limiting step in dissociation of α and the cores, which clearly must involve protein–protein or protein–DNA interactions that occur even in the absence of ε and are much stronger than that between ε and β (e.g., the α–τ interaction; Jergic et al, 2007).
We propose that on the timescale of these measurements, the replicase oscillates rapidly between two (or more) conformational states without the core dissociating from the DNA template, where one of these is a closed state that involves ε–β interaction and is responsible for DNA synthesis in the polymerization mode, and the other is an open inactive state in which this contact is broken. When the ε–β interaction is strengthened, the replicase therefore spends a greater proportion of time in the active mode, leading to increased rate and processivity. Known structures of the various components (discussed below) can be used to derive models of the open and closed states, as shown in Figure 7.
Figure 7.
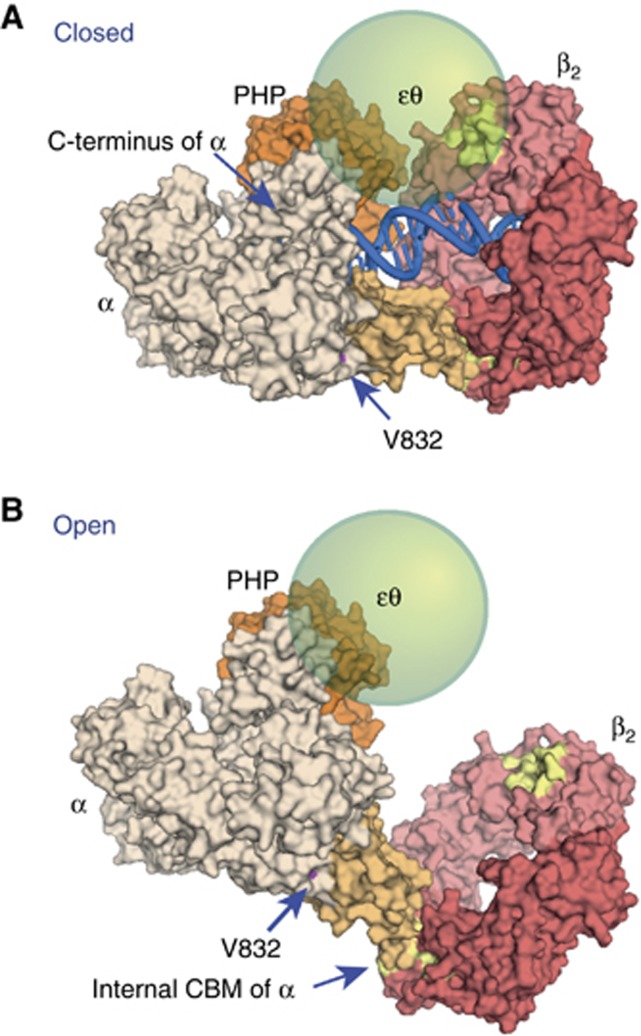
Structural models of α–β2 complexes. (A) Binding of E. coli β2 to the β-binding domain of Taq α (tan) in the ‘closed’ DNA-bound form, modelled on the structure of the ternary complex of Taq α with DNA and dNTP (as described in Wing et al, 2008). Space exists to accommodate εθ between the ε-binding site in β2 (yellow) and the PHP domain of α (orange), spanned by residues 188–210 of ε that remain flexible in the αεθ complex (Ozawa et al, 2008). The location of Leu888 in Taq α (Val832 in E. coli) is shown. (B) Model of the ‘open’ complex (DNA not shown) derived by rigid body transformation of the β-binding domain–β2 moiety in (A) to superimpose on the β-binding domain in the structure of Taq α without DNA (Bailey et al, 2006). Figures were produced using PyMOL (DeLano Scientific, San Carlos CA).
Discussion
Genetic studies by the Maurer group 20 years ago identified an essential non-proofreading role of the ε subunit of the Pol III core in DNA replication. That a suppressor mutation (spq2) in dnaE (encoding αV832G) rescued the severe growth defect but not the mutator phenotype of disruption of dnaQ (encoding ε) prompted the hypothesis that ε stabilizes the replisome through additional contacts with the β sliding clamp or clamp loader subunits (Lancy et al, 1989; Lifsics et al, 1992). Here, we identified an ε–β contact as an important contributor to the network of protein–protein interactions essential for stabilization of the replicase on its template DNA; the absence of this contact in the cell can be compensated by a mutation in α that enables regain of lost stability of the replicase–DNA complex to re-establish replicative competence.
We were able to detect a clear requirement for ε in DNA synthesis, independent of its proofreading activity, initially with an assay that detects helicase-independent SD DNA synthesis by the complete chromosomal replicase (Figure 2). We traced this by mutagenesis to a binding site (CBM) for the sliding clamp that is conserved in bacterial proofreading subunits (Figure 1B). This hexapeptide CBM is in the unstructured region of ε just following the nuclease domain, preceding the region that interacts with the polymerase subunit α. Mutants of ε engineered to contain stronger or weaker CBMs affect, in predictable ways, not only SD synthesis but also replicase rates and processivities in SM leading-strand replication assays (Figure 6), as well as net DNA synthesis in a primer extension assay under difficult conditions (without the benefit of α–τ or SSB–ssDNA interactions; Figure 5A). Moreover, the αV832G mutation was able to suppress the requirement for ε in SD synthesis (Figure 2F). We are therefore able fully to explain the earlier suggestion of a proofreading-independent role of ε in chromosomal DNA synthesis.
We were also able to confirm weak binding of β2 (KD ∼200 μM) to the predicted CBM in ε using the same series of mutant ε proteins (or corresponding peptides) in SPR and ESI-MS experiments (Figure 3), and to show that both protein-binding sites in β2 are utilized for the Pol III SD reaction (Figure 2E).
Our data strongly suggest that the two sites in the β dimer are occupied simultaneously by ε and the internal CBM of α during processive DNA replication. There are at least two situations where synthesis by α might stall to signal a conformational switch to break just the ε–β contact without the polymerase dissociating from the DNA template, or at least change the location of the ε active site in the replicase complex. These are (i) during lesion bypass or repair synthesis by the alternate polymerases Pol II, IV, or V (Indiani et al, 2005; Furukohri et al, 2008; Heltzel et al, 2009) and (ii) during proofreading. It has been suggested that in PolC (Evans et al, 2008), entry of alternate polymerases occurs via transition from the closed primer template-bound structure (similar to the model in Figure 7A) to an open one reminiscent of α in the absence of DNA (Figure 7B). This transition would require that the ε–β contact be broken, providing access of the CBM of the incoming polymerase to β2.
There are two separate models for how primer-template DNA is switched from α to an alternate polymerase (reviewed by Sutton, 2010). In the toolbelt model (Pagès and Fuchs, 2002; López de Saro et al, 2003a; Indiani et al, 2005), a repair or lesion-bypass polymerase would trap the replicase in the open state by temporarily replacing ε at its binding site in β2 to access the primer terminus while α remains attached at the other; ε would remain tethered to α through a flexible linker (Ozawa et al, 2008), enabling it to re-establish contact with β2 when processive synthesis by α is resumed. The second model, demonstrated with Pol IV, involves switching of polymerases at the same protein binding site on the β2 ring (Heltzel et al, 2009), and requires a secondary contact between Pol IV and β. Evidence for this model is that Pol III/IV switching can still occur efficiently on a βC/βwt heterodimer that has only one functional protein-binding site. In this more desperate situation, the Pol III core is apparently able to disengage from β2 while still remaining in the replicase through its contacts with τ. This may not be an unusual situation, since exchange of τ-bound Pol III cores between different clamps certainly occurs during their recycling to new primer termini on completion of lagging-strand Okazaki fragment synthesis. In this process, contacts of both α and ε with β2 must be broken. The differences, if any, among replicase stalling signals in these various situations and how they affect transactions of CBMs on the sliding clamp is an area where we still have much to learn.
Proofreading also involves replicase stalling and requires transfer of a mismatched primer template from the active site of α to that of ε. It has been suggested (Evans et al, 2008; Wing et al, 2008) that this might also require at least partial opening of the closed DNA-bound structure (as in Figure 7A) to pull the primer template from the polymerase site to access the exonuclease site of ε. There is some evidence for this opening. The Pol III replicase can be stalled in a stable complex at a primer terminus when only two of the four dNTPs are present, where it undergoes futile cycles of nucleotide misincorporation and proofreading. In this situation, it has been found to be more prone to exchange with an alternate polymerase than when it is actively replicating DNA (Indiani et al, 2005; Furukohri et al, 2008; Heltzel et al, 2009). This would be nicely explained if breakage of the ε–β contact occurred during proofreading to allow easier access of the incoming polymerase.
It is instructive to reflect on why the ε–β interaction remained undetected for so long. The first reason is that knowledge of the functions, and especially structures, of replicase components can now guide us to find and understand new protein–protein contacts. Pairwise interaction between ε and β2 is weak, and is not easily observable by classical biophysical techniques. Previous measurements (Stukenberg et al, 1991; Kim and McHenry, 1996b; Dohrmann and McHenry, 2005) suggest only up to a 4-fold enhancement by εθ of the strength of the α–β interaction, which could be thought to be due simply to stabilization of α through its interactions with the εCTS. However, the structures of Taq α (Bailey et al, 2006; Wing et al, 2008) suggest that in the absence of primer-template DNA, the polymerase has an open structure that closes by an ∼20° rotation of the β-binding domain to a closed structure in the DNA-bound form. Curiously, Val832 (Gly in the spq2 mutant; Leu888 in Taq α) is at the base of this domain (Figure 7), where it could modulate this structural transition. In the corresponding open structure of the αεθ core, the two CBMs, one at the tip of the β-binding domain of α and the other just following the exonuclease domain of ε, are probably too distant to be bound simultaneously at the two sites on a single β2 ring (Figure 7B). In the closed structure of the α(εθ)–β2–DNA complex (modelled in Figure 7A), the free protein-binding site in β2 would be located proximal to the εCTS-binding PHP domain of α in a position that would favour more optimal ε–β contact, with a gap between subunits still large enough to accommodate the structured part of εθ. Thus, primer-template DNA interactions would be expected to increase the significance of the ε–β interaction in the context of a functioning replicase, and allow its strength to be modulated by conformational change in α.
Both processivities and rates of leading-strand synthesis, as measured in our SM experiments, are affected similarly by changes in the strength of the ε–β interaction, while the lifetime of the complex is determined by a different and stronger replisomal interaction (Figure 6). This is consistent with the idea that the ε–β contact shifts a rapid equilibrium towards the closed state of the replicase, that is, the state where the chemistry of DNA synthesis happens in the polymerization mode.
Inspection of the putative CBMs in other bacterial ε subunits (e.g., in Figure 1B) shows that they have been preserved as weak sites during evolution. This is as expected for a situation where the ε–β interaction needs to be sufficiently strong to maintain the replicase in the polymerization mode, but weak enough that it can be easily disrupted during a structural change necessary for transition to other conformational states. Evolutionary fine tuning of strengths of pairwise interactions between components of dynamic molecular machines is emerging as a key aspect of functional importance. The ε–β interaction in the bacterial replicase is a clear example of this.
A second reason that the ε–β contact was not detected earlier is that the majority of in vitro studies of replicase functions are carried out at low (<20 mM) or moderate (<100 mM) ionic strength conditions where requirements for weak contacts are masked by the relative strengthening of others. For example, primer extension assays in the absence of SSB show no synthesis by α alone in the presence of β2 and γ3δδ′ at higher ionic strengths (130 mM NaCl+10 mM MgCl2). However, as expected based on the literature (e.g., Studwell and O’Donnell, 1990), α alone is capable of relatively efficient synthesis at low ionic strength (10 mM MgCl2; not shown). While the apparent strengthening of replisomal protein–protein and protein–DNA interactions in the low salt conditions of biochemical assays can provide efficient systems to dissect roles of the many actors, it can mask requirements for weak interactions that only become apparent under more physiological conditions.
Materials and methods
Replication proteins
Mutations were introduced into the dnaE and dnaQ genes in plasmids that direct expression of the Pol III α and ε subunits by standard methods, and the proteins were isolated as for the wild-type versions (Scheuermann and Echols, 1984; Wijffels et al, 2004). Described methods (see Supplementary data) were used to isolate other Pol III subunits and sub-assemblies: β2 (Oakley et al, 2003); αεθ and τ3δδ′ψχ complexes (Tanner et al, 2008); PriA, PriB, and DnaT (Marians, 1995); SSB (Mason et al, 2013). New methods to make the ubq-εCTS construct, the modified β2 homo- and heterodimers, the γ3δδ′ clamp loader, and the DnaB6(DnaC)6 complex are given in Supplementary data.
Bulk DNA replication assays
The flap-primed ssDNA template was made by annealing M13 ssDNA to a 30-fold excess of a primer consisting of a 33-Nt complementary segment preceded by a 36-Nt non-complementary flap. The standard coupled strand extension and Pol III SD reaction contained 2.5 nM primed DNA template, 1 mM ATP, 0.5 mM of each dNTP, 30 nM τ3δδ′ψχ, 90 nM αεθ, 200 nM β2, and 750 nM SSB4 in 25 mM Tris–HCl pH 7.6, 10 mM MgCl2, 10 mM dithiothreitol and 130 mM NaCl, in a final volume of 13 μl. Assays containing some core complexes assembled in situ (Figure 2B, C) contained 100 nM α or αV832G+350 nM ε, εD12A, εD12A,E14A or ubq-εCTS±1 μM θ. Primer extension assays under ‘difficult’ conditions were done identically, except that SSB was omitted, 40 nM γ3δδ’ clamp loader was used in place of τ3δδ’ψχ and α or the αεθ cores were present at 150 nM. Components (except DNA) were mixed and treated for 5 min at room temperature, cooled in ice and DNA added. Reactions were initiated at 30°C, and quenched after 20 min (unless indicated otherwise) by addition of EDTA to ∼100 mM and SDS to ∼1%. Products were separated by agarose gel electrophoresis and stained with SYBR Gold (Invitrogen, Carlsbad, CA, USA).
SM leading-strand replication assays
Leading-strand SM assays were essentially as described (Tanner et al, 2008), with a few modifications. Replication proteins were introduced in 50 mM HEPES–KOH pH 7.9, 80 mM KCl, 12 mM Mg(OAc)2, 2 mM MgCl2, 5 mM dithiothreitol, 0.1 mg/ml BSA, 1 mM ATP, and 195 μM of each dNTP, and were present continuously in the flow at the following concentrations: 60 nM α or αεθ, 30 nM τ3δδ′χψ, 30 nM β2, 30 nM DnaB6(DnaC)6, 20 nM PriA, 40 nM PriB, and 480 nM DnaT. Experiments were at 32–34°C. Data were acquired and treated as before except that pauses were defined as a minimum of six data points (images at 2 Hz) with amplitude fluctuations less than three times the standard deviation of the noise.
SPR analysis of εpep–β2 interactions
A ProteOn XPR-36 SPR with an NLC sensor chip (Bio-Rad) was used at 20°C. Solutions of εLpep, εWTpep, and εQpep in SPR buffer (50 mM Tris–HCl pH 7.6, 50 mM NaCl, 0.5 mM TCEP, 0.2 mM EDTA, 0.005% surfactant P20) were used to immobilize ∼50, 70, and 60 RU, respectively, across six interaction spots. After rotation of the chip, three different serially diluted concentration series of β2 in SPR buffer were injected sequentially at 100 μl/min for 60 s followed by SPR buffer for 300 s. The final sensorgrams were generated by double reference subtraction and equilibrium responses at different [β2] used to generate binding isotherms to determine values of KD(εpep–β2) as described in Supplementary data.
Assessment of ε–β 2 interactions by ESI-MS
Protein samples were dialysed against NH4OAc buffers at pH 7.6, 1 mM in β-mercaptoethanol (as specified in figure legends). ESI-mass spectra of ε or purified αεθ variants with β2 were acquired in positive ion mode using nanoelectrospray ionization (nanoESI) on a Waters (Wythenshawe, UK) extended mass range Q-ToF Ultima spectrometer fitted with a Z-spray ESI source under optimized conditions, while interactions between β2 and in-situ assembled αεθ complexes similarly used nanoESI on a Waters Synapt HDMS spectrometer with a Z-spray source. Spectra were acquired over a m/z range of 500–15 000; 100–150 acquisitions were combined and spectra were baseline subtracted and smoothed using the Savitzky-Golay algorithm.
Supplementary Material
Acknowledgments
We thank Michelle Blayney and Linda Jessop for preliminary ESI-MS data. This work was supported by grants from the Australian Research Council, including Fellowships to KO, TH, and NED, and by a KAUST Faculty Initiated Collaborative grant to SMH and NED.
Author contributions: SJ, JLB, AMvO, SMH, and NED conceived the experiments; SJ, NPH, MME, CEM, TU, KO, JMMG, YW, and XP performed the experiments; SJ, NPH, MME, CEM, AR, JLB, AMvO, TH, SMH, and NED analysed the data; and SJ, SMH, and NED wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Allen GC Jr, Kornberg A (1991) Fine balance in the regulation of DnaB helicase by DnaC protein in replication in Escherichia coli. J Biol Chem 266: 22096–22101 [PubMed] [Google Scholar]
- Bailey S, Wing RA, Steitz TA (2006) The structure of T. aquaticus DNA polymerase III is distinct from eukaryotic replicative DNA polymerases. Cell 126: 893–904 [DOI] [PubMed] [Google Scholar]
- Bloom LB (2009) Loading clamps for DNA replication and repair. DNA Repair (Amst) 8: 570–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple BP, Kongsuwan K, Wijffels G, Dixon NE, Jennings PA (2001) A universal protein-protein interaction motif in the eubacterial DNA replication and repair systems. Proc Natl Acad Sci USA 98: 11627–11632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohrmann PR, McHenry CS (2005) A bipartite polymerase-processivity factor interaction: only the internal β binding site of the α subunit is required for processive replication by the DNA polymerase III holoenzyme. J Mol Biol 350: 228–239 [DOI] [PubMed] [Google Scholar]
- Evans RJ, Davies DR, Bullard JM, Christensen J, Green LS, Guiles JW, Pata JD, Ribble WK, Janjic N, Jarvis TC (2008) Structure of PolC reveals unique DNA binding and fidelity determinants. Proc Natl Acad Sci USA 105: 20695–20700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fijalkowska IJ, Schaaper RM (1996) Mutants in the Exo I motif of Escherichia coli dnaQ: defective proofreading and inviability due to error catastrophe. Proc Natl Acad Sci USA 93: 2856–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukohri A, Goodman MF, Maki H (2008) A dynamic polymerase exchange with Escherichia coli DNA polymerase IV replacing DNA polymerase III on the sliding clamp. J Biol Chem 283: 11260–11269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu RE, Kim SS, Yurieva O, Kuriyan J, Kong XP, O’Donnell M (2008b) Structure of a sliding clamp on DNA. Cell 132: 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu RE, Yurieva O, Kim SS, Kuriyan J, Kong XP, O'Donnell M (2008a) Structure of a small-molecule inhibitor of a DNA polymerase sliding clamp. Proc Natl Acad Sci USA 105: 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdan S, Carr PD, Brown SE, Ollis DL, Dixon NE (2002) Structural basis for proofreading during replication of the Escherichia coli chromosome. Structure 10: 535–546 [DOI] [PubMed] [Google Scholar]
- Hamdan SM, Richardson CC (2009) Motors, switches, and contacts in the replisome. Annu Rev Biochem 78: 205–243 [DOI] [PubMed] [Google Scholar]
- Heltzel JMH, Maul RW, Scouten Ponticelli SK, Sutton MD (2009) A model for DNA polymerase switching involving a single cleft and the rim of the sliding clamp. Proc Natl Acad Sci USA 106: 12664–12669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indiani C, McInerney P, Georgescu R, Goodman MF, O'Donnell M (2005) A sliding-clamp toolbelt binds high- and low-fidelity DNA polymerases simultaneously. Mol Cell 19: 805–815 [DOI] [PubMed] [Google Scholar]
- Jergic S, Ozawa K, Su X-C, Scott DD, Williams NK, Hamdan SM, Crowther JA, Otting G, Dixon NE (2007) The unstructured C-terminus of the τ subunit of Escherichia coli DNA polymerase III holoenzyme is the site of interaction with the α subunit. Nucleic Acids Res 35: 2813–2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A, O’Donnell M (2005) Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem 74: 283–315 [DOI] [PubMed] [Google Scholar]
- Kapur A, Beck JL, Brown SE, Dixon NE, Sheil MM (2002) Use of electrospray ionization mass spectrometry to study binding interactions between a replication terminator protein and DNA. Protein Sci 11: 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DR, McHenry CS (1996a) In vivo assembly of overproduced DNA polymerase III. Overproduction, purification, and characterization of the α, α-ε, and α-ε-θ subunits. J Biol Chem 271: 20681–20689 [DOI] [PubMed] [Google Scholar]
- Kim DR, McHenry CS (1996b) Identification of the β-binding domain of the α subunit of Escherichia coli polymerase III holoenzyme. J Biol Chem 271: 20699–20704 [DOI] [PubMed] [Google Scholar]
- Kong X-P, Onrust R, O’Donnell ME, Kuriyan J (1992) Three-dimensional structure of the β subunit of E. coli DNA polymerase III holoenzyme: a sliding DNA clamp. Cell 69: 425–437 [DOI] [PubMed] [Google Scholar]
- Lamers MH, Georgescu RE, Lee SG, O’Donnell M, Kuriyan J (2006) Crystal structure of the catalytic α subunit of E. coli replicative DNA polymerase III. Cell 126: 881–892 [DOI] [PubMed] [Google Scholar]
- Lancy ED, Lifsics MR, Kehres DG, Maurer R (1989) Isolation and characterization of mutants with deletions in dnaQ, the gene for the editing subunit of DNA polymerase III in Salmonella typhimurium. J Bacteriol 171: 5572–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifsics MR, Lancy ED Jr, Maurer R (1992) DNA replication defect in Salmonella typhimurium mutants lacking the editing (ε) subunit of DNA polymerase III. J Bacteriol 174: 6965–6973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Saro FJ, Georgescu RE, Goodman MF, O’Donnell ME (2003a) Competitive processivity-clamp usage by DNA polymerases during DNA replication and repair. EMBO J 22: 6408–6418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López de Saro FJ, Georgescu RE, O’Donnell ME (2003b) A peptide switch regulates DNA polymerase processivity. Proc Natl Acad Sci USA 100: 14689–14694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marians KJ (1995) ϕX174-type primosomal proteins: purification and assay. Methods Enzymol 262: 507–521 [DOI] [PubMed] [Google Scholar]
- Marians KJ, Hiasa H, Kim DR, McHenry CS (1998) Role of the core DNA polymerase III subunits at the replication fork: α is the only subunit required for processive replication. J Biol Chem 273: 2452–2457 [DOI] [PubMed] [Google Scholar]
- Mason CE, Jergic S, Lo ATY, Wang Y, Dixon NE, Beck JL (2013) Escherichia coli single-stranded DNA-binding protein: Salt-modulated subunit exchange and DNA binding transactions monitored by nanoESI-MS. J Am Soc Mass Spectrom 24: (doi:10.1007/s13361-012-0552-2) [DOI] [PubMed] [Google Scholar]
- McHenry CS (2011) DNA replicases from a bacterial perspective. Annu Rev Biochem 80: 403–436 [DOI] [PubMed] [Google Scholar]
- McHenry CS, Crow W (1979) DNA polymerase III of Escherichia coli. Purification and identification of subunits. J Biol Chem 254: 1748–1753 [PubMed] [Google Scholar]
- McInerney P, Johnson A, Katz F, O’Donnell M (2007) Characterization of a triple DNA polymerase replisome. Mol Cell 27: 527–538 [DOI] [PubMed] [Google Scholar]
- Oakley AJ, Prosselkov P, Wijffels G, Beck JL, Wilce MCJ, Dixon NE (2003) Flexibility revealed by the 1.85-Å crystal structure of the β sliding-clamp subunit of Escherichia coli DNA polymerase III. Acta Crystallogr D 59: 1192–1199 [DOI] [PubMed] [Google Scholar]
- Ozawa K, Jergic S, Crowther JA, Thompson PR, Wijffels G, Otting G, Dixon NE (2005) Cell-free protein synthesis in an autoinduction system for NMR studies of protein-protein interactions. J Biomol NMR 32: 235–241 [DOI] [PubMed] [Google Scholar]
- Ozawa K, Jergic S, Park AY, Dixon NE, Otting G (2008) The proofreading exonuclease subunit ε of Escherichia coli DNA polymerase III is tethered to the polymerase subunit α via a flexible linker. Nucleic Acids Res 36: 5074–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagès V, Fuchs RPP (2002) How DNA lesions are turned into mutations within cells? Oncogene 21: 8957–8966 [DOI] [PubMed] [Google Scholar]
- Pintacuda G, Park AY, Keniry MA, Dixon NE, Otting G (2006) Lanthanide labeling offers fast NMR approach to 3D structure determinations of protein–protein complexes. J Am Chem Soc 128: 3696–3702 [DOI] [PubMed] [Google Scholar]
- Ribeck N, Kaplan DL, Bruck I, Saleh OA (2010) DnaB helicase activity is modulated by DNA geometry and force. Biophys J 99: 2170–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer PM, Headlam MJ, Dixon NE (2005) Protein-protein interactions in the eubacterial replisome. IUBMB Life 57: 5–12 [DOI] [PubMed] [Google Scholar]
- Scheuermann RH, Echols H (1984) A separate editing exonuclease for DNA replication: the ε subunit of Escherichia coli DNA polymerase III holoenzyme. Proc Natl Acad Sci USA 81: 7747–7751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scouten Ponticelli SK, Duzen JM, Sutton MD (2009) Contributions of the individual hydrophobic clefts of the Escherichia coli β sliding clamp to clamp loading, DNA replication, and clamp recycling. Nucleic Acids Res 37: 2796–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater SC, Lifsics MR, O’Donnell M, Maurer R (1994) holE, the gene coding for the θ subunit of DNA polymerase III of Escherichia coli: characterization of a holE mutant and comparison with a dnaQ (ε subunit) mutant. J Bacteriol 176: 815–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studwell PS, O’Donnell ME (1990) Processive replication is contingent on the exonuclease subunit of DNA polymerase III holoenzyme. J Biol Chem 265: 1171–1178 [PubMed] [Google Scholar]
- Studwell-Vaughan PS, O'Donnell M (1993) DNA polymerase III accessory proteins. V. θ encoded by holE. J Biol Chem 268: 11785–11791 [PubMed] [Google Scholar]
- Stukenberg PT, Studwell-Vaughan PS, O’Donnell M (1991) Mechanism of the sliding β-clamp of DNA polymerase III holoenzyme. J Biol Chem 266: 11328–11334 [PubMed] [Google Scholar]
- Sutton MD (2010) Coordinating DNA polymerase traffic during high and low fidelity synthesis. Biochim Biophys Acta 1804: 1167–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MD, Duzen JM, Scouten Ponticelli SK (2010) A single hydrophobic cleft in the Escherichia coli processivity clamp is sufficient to support cell viability and DNA damage-induced mutagenesis in vivo. BMC Mol Biol 11: 102–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner NA, Hamdan SM, Jergic S, Loscha KV, Schaeffer PM, Dixon NE, van Oijen AM (2008) Single-molecule studies of fork dynamics in Escherichia coli DNA replication. Nat Struct Mol Biol 15: 170–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner NA, van Oijen AM (2009) Single-molecule observation of prokaryotic DNA replication. Methods Mol Biol 521: 397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieczorek A, McHenry CS (2006) The NH2-terminal php domain of the α subunit of the E. coli replicase binds the ε proofreading subunit. J Biol Chem 281: 12561–12567 [DOI] [PubMed] [Google Scholar]
- Wijffels G, Dalrymple BP, Prosselkov P, Kongsuwan K, Epa VC, Lilley PE, Jergic S, Buchardt J, Brown SE, Alewood PF, Jennings PA, Dixon NE (2004) Inhibition of protein interactions with the β2 sliding clamp of Escherichia coli DNA polymerase III by peptides derived from β2-binding proteins. Biochemistry 43: 5661–5671 [DOI] [PubMed] [Google Scholar]
- Wijffels G, Johnson WM, Oakley AJ, Turner K, Epa VC, Briscoe SJ, Polley M, Liepa A, Hofmann A, Buchardt J, Christensen C, Prosselkov P, Dalrymple BP, Alewood PF, Jennings PA, Dixon NE, Winkler DA (2011) Binding inhibitors of the bacterial sliding clamp by design. J Med Chem 54: 4831–4838 [DOI] [PubMed] [Google Scholar]
- Wing RA, Bailey S, Steitz TA (2008) Insights into the replisome from the structure of a ternary complex of the DNA polymerase III α-subunit. J Mol Biol 382: 859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, McHenry CS (2009) Strand displacement by DNA polymerase III occurs through a τ−ψ−χ link to SSB coating the lagging strand template. J Biol Chem 284: 31672–31679 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.