Abstract
Glycerol-3-phosphate acyltransferase (GPAT) is involved in the first step in glycerolipid synthesis and is localized in both the endoplasmic reticulum (ER) and mitochondria. To clarify the functional differences between ER-GPAT and mitochondrial (Mt)-GPAT, we generated both GPAT mutants in C. elegans and demonstrated that Mt-GPAT is essential for mitochondrial fusion. Mutation of Mt-GPAT caused excessive mitochondrial fragmentation. The defect was rescued by injection of lysophosphatidic acid (LPA), a direct product of GPAT, and by inhibition of LPA acyltransferase, both of which lead to accumulation of LPA in the cells. Mitochondrial fragmentation in Mt-GPAT mutants was also rescued by inhibition of mitochondrial fission protein DRP-1 and by overexpression of mitochondrial fusion protein FZO-1/mitofusin, suggesting that the fusion/fission balance is affected by Mt-GPAT depletion. Mitochondrial fragmentation was also observed in Mt-GPAT-depleted HeLa cells. A mitochondrial fusion assay using HeLa cells revealed that Mt-GPAT depletion impaired mitochondrial fusion process. We postulate from these results that LPA produced by Mt-GPAT functions not only as a precursor for glycerolipid synthesis but also as an essential factor of mitochondrial fusion.
Keywords: C. elegans , fusion, glycerol-3-phosphate acyltransferase, lysophosphatidic acid, mitochondria
Introduction
Glycerolipids, which include phospholipids and triacylglycerol, are ubiquitous and important biological components. Phospholipids are the major constituents of biological membranes, playing important roles in multiple cellular processes including maintenance of the cellular permeability barrier, regulation of the activities of proteins associated with the membrane, and regulation of intracellular signalling by serving as precursors of signalling molecules (Dowhan, 1997). Triacylglycerol is a major storage form of energy, as well as being a major component of secreted lipoproteins. The biosynthetic pathways for these glycerolipids have been well established (Kent, 1995; Dowhan, 1997). The initial and rate-limiting step of glycerolipid synthesis is the acylation of glycerol-3-phosphate (G3P) with long-chain fatty acyl-CoA to form lysophosphatidic acid (LPA). This reaction is catalysed by glycerol-3-phosphate acyltransferase (GPAT) (Coleman et al, 2000; Wendel et al, 2009). LPA is further acylated by LPA acyltransferase (LPAAT) located at the ER to form phosphatidic acid (PA), a common precursor for phospholipid and triacylglycerol synthesis (Coleman and Lee, 2004; Takeuchi and Reue, 2009). To date, four mammalian GPATs have been identified and classified into two groups based on sequence homology and subcellular localization (Figure 1A; Gimeno and Cao, 2008; Wendel et al, 2009). GPAT1 and GPAT2 are mitochondrial GPATs that are localized to the mitochondrial outer membrane, and GPAT3 and GPAT4 are microsomal GPATs that are localized to the endoplasmic reticulum (ER) membrane (Gimeno and Cao, 2008; Wendel et al, 2009). All four of these GPATs are members of the AGPAT (1-acyl-sn-glycerol-3-phosphate acyltransferase) family that contain four conserved AGPAT motifs (Neuwald, 1997). Mitochondrial GPAT1 knockout (GPAT1−/−) mice (Hammond et al, 2002) and microsomal GPAT4 knockout (GPAT4−/−) mice (Vergnes et al, 2006) exhibited reduced triacylglycerol content in the liver. GPAT4−/− mice also showed large reductions in the triacylglycerol contents in adipose tissue, subdermal fat, and milk (Beigneux et al, 2006; Vergnes et al, 2006), while GPAT1−/− mice showed increased hepatic fatty acid oxidation and altered insulin resistance when fed a high fat diet (Hammond et al, 2005; Neschen et al, 2005; Yazdi et al, 2008). Significant GPAT activity remains in the mitochondrial and microsomal fractions in the GPAT1−/− and GPAT4−/− mice, respectively, suggesting that GPAT2 and GPAT3 compensate for the functions of mitochondrial and microsomal GPATs in mice (Lewin et al, 2004; Chen et al, 2008; Nagle et al, 2008). However, the effects of knocking out both mitochondrial GPATs (GPAT1 and GPAT2) or both microsomal GPATs (GPAT3 and GPAT4) have not yet been examined.
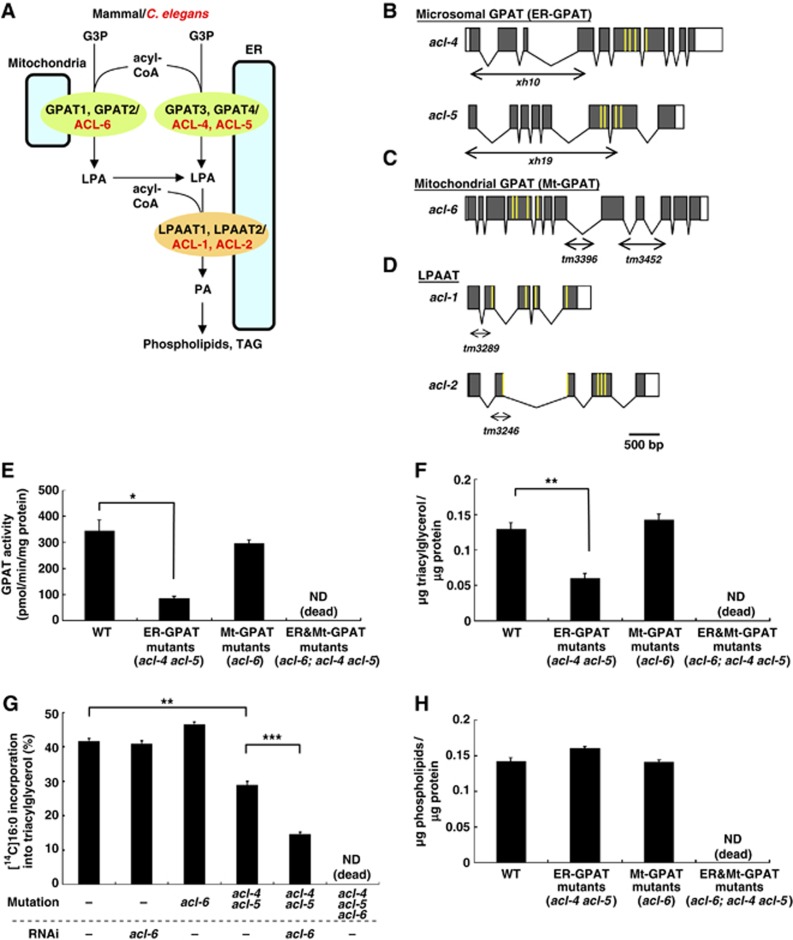
Figure 1.
C. elegans GPATs, acl-4, acl-5, and acl-6, contribute to triacylglycerol synthesis. (A) In mammals and C. elegans, glycerol-3-phosphate acyltransferases (GPATs) are located on both the outer mitochondrial membrane (mitochondrial GPAT) and endoplasmic reticulum (ER) (microsomal GPAT) to produce lysophosphatidic acid (LPA). LPA is further converted into phosphatidic acid (PA), a precursor of triacylglycerol and membrane phospholipids, by LPA acyltransferases (LPAATs) which are localized in the ER membranes. Mammalian mitochondrial GPATs, GPAT1 and GPAT2, correspond to C. elegans ACL-6. Mammalian microsomal GPATs, GPAT3 and GPAT4, correspond to C. elegans ACL-4 and ACL-5. C. elegans LPAATs are ACL-1 and ACL-2. (B–D) Genomic structures of ER-GPAT (acl-4 and acl-5) (B), Mt-GPAT (acl-6) (C) and LPAAT (acl-1 and acl-2) (D). Grey boxes indicate exons and white boxes indicate 5′ and 3′ untranslated regions. The extent of deletion in acl-4(xh10), acl-5(xh19), acl-6(tm3396 and tm3452), acl-1(tm3289), and acl-2(tm3246) are indicated by horizontal double arrows. The positions of the predicted conserved lysophospholipid acyltransferase motifs, characteristic of AGPAT family members, are shown in yellow lines. (E) GPAT activity in the membrane fractions of wild-type and the indicated acl mutants. [14C]Palmitoyl-CoA (40 μM) and glycerol-3-phosphate (G3P) (800 μM) were used. We could not measure GPAT activity of the worms lacking both ER-GPAT and Mt-GPAT double mutants (acl-6;acl-4 acl-5 triple mutants) because they were lethal. (F) Triacylglycerol contents of wild-type and the indicated acl mutants. (G) Incorporation of [14C]palmitic acids into neutral lipid fractions of the living worms. The amount of incorporation was expressed as the percentage of radioactivity incorporated into total lipids. (H) Phospholipids contents of wild-type and the indicated acl mutants. Each bar represents the mean±s.e.m. of at least three independent experiments. *P<0.05, **P<0.01, ***P<0.001 (Student’s t test).
Most of the enzymes of glycerolipid biosynthesis reside in the ER membrane, but GPATs are also located on the mitochondrial outer membrane, which raises a question: Why is LPA synthesized in both mitochondria and ER? To answer this question, a comprehensive mutational analysis of GPATs would be useful. The C. elegans genome contains two microsomal GPATs (acl-4, acl-5), and one mitochondrial GPAT (acl-6) (Figure 1A). To understand the functional differences of the two types of GPAT, we generated C. elegans deletion mutants of mitochondrial and microsomal GPATs.
Mitochondria are essential organelles in most eukaryotic cells that are involved in several metabolic pathways, cell signalling, and apoptosis. Mitochondria undergo dynamic changes in morphology through continual fission and fusion, which is essential for maintenance of mitochondrial function and to adapt mitochondria to cellular needs (Detmer and Chan, 2007). The balance of fission and fusion determines the overall mitochondrial morphology. When mitochondrial fusion is reduced, mitochondria fragment due to ongoing fission; conversely, mitochondria are long and overly interconnected when this balance shifts towards fusion (Smirnova et al, 1998, 2001; Chen et al, 2003; Eura et al, 2003; Santel et al, 2003). Genetic studies in D. melanogaster and S. cerevisiae have led to the identification of components that regulate mitochondrial dynamics (Okamoto and Shaw, 2005; Westermann, 2008; Hoppins and Nunnari, 2009). These include the mitofusin (Mfn), which is involved in mitochondrial fusion, and Drp1, which is involved in mitochondrial fission. Mfn is a large transmembrane GTPase localized in the mitochondrial outer membrane (Santel and Fuller, 2001; Rojo et al, 2002; Chen et al, 2003; Eura et al, 2003; Santel et al, 2003; Ishihara et al, 2004), whereas Drp1 is a cytosolic dynamin-related GTPase that is associated with the mitochondrial outer membrane at sites of fission (Smirnova et al, 1998, 2001). These molecules are evolutionarily conserved in eukaryotic organisms including human and C. elegans.
Here, we show that depletion of mitochondrial GPAT in C. elegans causes mitochondrial fragmentation. This defect is most probably induced by impaired mitochondrial fusion. We also demonstrate that injection of LPA, a direct product of GPAT, into cells rescues the mitochondrial defect in the mitochondrial GPAT mutants. We propose, from these results, that in addition to functioning as a precursor of glycerolipid synthesis, LPA produced by mitochondrial GPAT plays an important role in regulating mitochondrial dynamics.
Results
Identification of microsomal and mitochondrial GPAT in C. elegans
A search of the C. elegans genome for proteins with four conserved AGPAT motifs yielded 14 proteins (acl-1-14) (Imae et al, 2010). Among these genes, acl-4 and acl-5 are homologous to microsomal GPATs (GPAT3 and GPAT4) and show 45–60% identity to human GPAT3 and GPAT4 (Figure 1A; Supplementary Figure S1A). acl-6 is a sole C. elegans homologue of mitochondrial GPATs (GPAT1 and GPAT2) and shows about 30% identity to human GPAT1 and GPAT2 (Figure 1A; Supplementary Figure S1B). As expected from the sequence homology, acl-4 and acl-5 gene products colocalized with an ER marker (calnexin) (Supplementary Figure S2A–F), but not with a mitochondrial marker (MitoTracker) (Supplementary Figure S2J–O) when expressed in mammalian cells. In contrast, the acl-6 gene product colocalized with a mitochondrial marker in mammalian cells (Supplementary Figure S2P–R) and also in C. elegans muscle cells (Supplementary Figure S2S–U).
Generation of GPAT mutants
To address the in vivo functions of LPA-producing enzymes, we isolated deletion mutants of all GPATs by PCR-based screening of UV-TMP-mutagenized libraries. The acl-4(xh10) allele had a deletion that causes a frameshift resulting in a premature stop codon (Figure 1B). The acl-5(xh19) allele lacked the translation initiation codon (Figure 1B). Both alleles lacked the conserved AGPAT motifs that are essential for enzyme activity. Two deletion alleles of acl-6, tm3396, and tm3452, caused a frameshift leading to a premature stop codon (Figure 1C). Both tm3396 and tm3452 appeared to be null or strong loss-of-function alleles because inhibition of acl-6 by RNAi failed to enhance the acl-6 mutant phenotypes described below. Because acl-6(tm3396) and acl-6(tm3452) were phenotypically indistinguishable, we used acl-6(tm3396) mutants in subsequent experiments.
In acl-4 acl-5 double mutants (hereafter, referred to as ‘ER-GPAT mutants’), GPAT activity in the membrane fraction was reduced to 25% of the level in the wild type (Figure 1E) and the triacylglycerol level was reduced to 46% of the level in the wild type (Figure 1F). Incorporation of supplemented [14C]palmitic acid into the triacylglycerol fraction was also reduced in ER-GPAT mutants (Figure 1G). In contrast, no reductions in GPAT activity, the triacylglycerol level, or incorporation of [14C]palmitic acid into triacylglycerol were observed in acl-6 mutants (‘Mt (mitochondrial)-GPAT mutants’) (Figure 1E–G). However, in the ER-GPAT mutant background, knockdown of Mt-GPAT significantly reduced the incorporation of [14C]palmitic acid into triacylglycerol (Figure 1G). These data indicate that ER-GPAT and Mt-GPAT complement each other to maintain triacylglycerol synthesis in C. elegans.
On the other hand, the amount of phospholipids, the other final products of glycerolipid synthesis, was not affected in either ER-GPAT mutants or Mt-GPAT mutants (Figure 1H). Mutants lacking both mitochondrial and microsomal GPATs (acl-6; acl-4 acl-5 triple mutants) did not survive (Table I; Supplementary Table S1A, see Materials and methods for details).
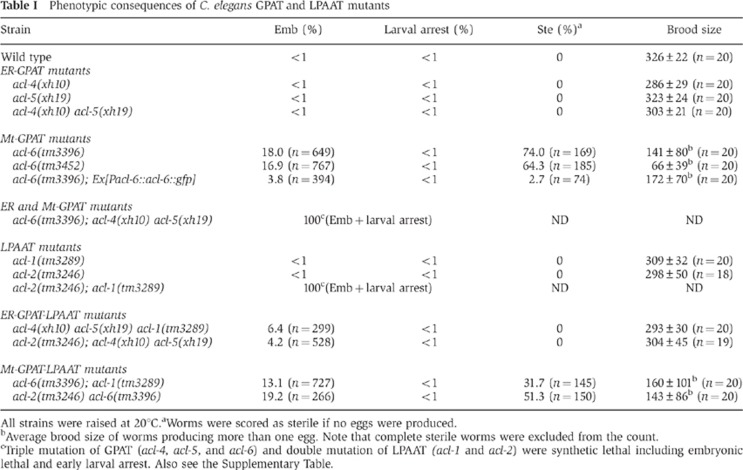
Table 1. Phenotypic consequences of C. elegans GPAT and LPAAT mutants.
All strains were raised at 20°C.
aWorms were scored as sterile if no eggs were produced.
bAverage brood size of worms producing more than one egg. Note that complete sterile worms were excluded from the count.
cTriple mutation of GPAT (acl-4, acl-5, and acl-6) and double mutation of LPAAT (acl-1 and acl-2) were synthetic lethal including embryonic lethal and early larval arrest. Also see the Supplementary Table.
Mitochondrial GPAT, acl-6, is required for oogenesis in C. elegans
Single mutants with acl-4 or acl-5, and acl-4 acl-5 double mutants (ER-GPAT mutants) were viable and showed no detectable abnormalities in viability, growth, morphology, or motility under a dissecting microscope (Table I, ER-GPAT mutants). In contrast, 18% of acl-6 mutants (Mt-GPAT mutants) died during late embryogenesis (Table I, Mt-GPAT mutants, Emb). Hatched Mt-GPAT mutants developed normally to the adult stage; however, about 70% of these animals were sterile (Table I, Mt-GPAT mutants, Ste). The embryonic lethality and sterility of Mt-GPAT mutants were efficiently rescued by expression of acl-6 cDNA under the control of its own promoter (Table I, acl-6(tm3396); Ex[Pacl-6::acl-6::gfp]).
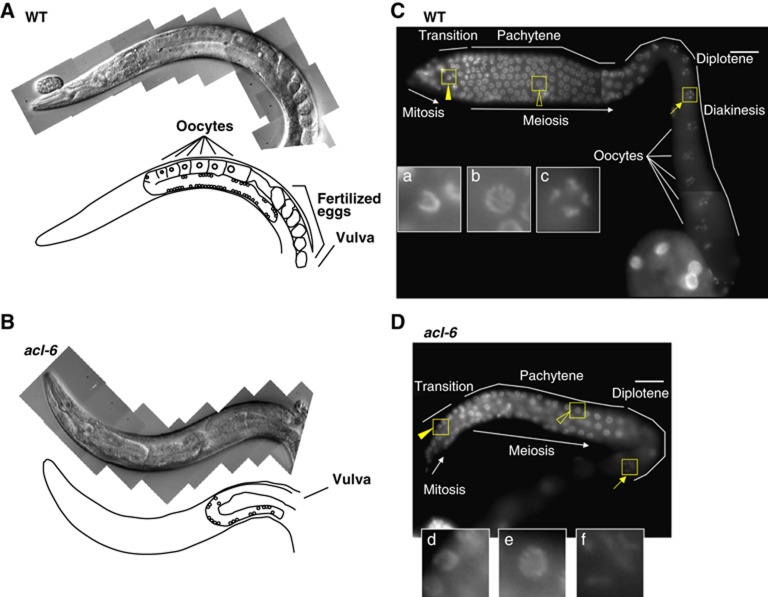
We then focused on the reproductive system of Mt-GPAT mutants. The gonadal arm of the adult hermaphrodite is U shaped, with a distal arm composed of a syncytium of germline nuclei and a proximal arm containing oocytes and fertilized eggs (Figure 2A; Hubbard and Greenstein, 2005; Kimble and Crittenden, 2005). Germ cells proliferate mitotically in the distal-most region, and as they migrate proximally, they enter meiotic prophase in the transition zone (Hubbard and Greenstein, 2005; Kimble and Crittenden, 2005). In Mt-GPAT mutants, the U-shaped gonad was reduced in size and contained no oocytes in the proximal position, while the morphology of the vulva, an egg-laying apparatus, appeared normal (Figure 2B). DAPI staining of Mt-GPAT mutants also revealed a reduction in the number of germ cells in the mitotic and transition zone and an absence of oocytes at the diakinesis stage (Figure 2C and D). These data indicate that mitochondrial GPAT, acl-6, is required for normal oogenesis.
Figure 2.
Mt-GPAT mutants show defects in oogenesis. (A, B) Nomarski images and schematic diagrams of the adult hermaphrodite gonads of wild-type (A) and Mt-GPAT (acl-6) mutant (B). (C, D) DAPI-stained images of adult hermaphrodite gonads of wild-type (C) and Mt-GPAT (acl-6) mutant (D). (Insets) Enlargement of the boxed regions: transition states (a, d), pachytene nuclei (b, e), diplotene nuclei (f), and diakinesis nuclei (c). Scale bars, 20 μm.
Deletion of Mt-GPAT causes abnormal mitochondrial morphology
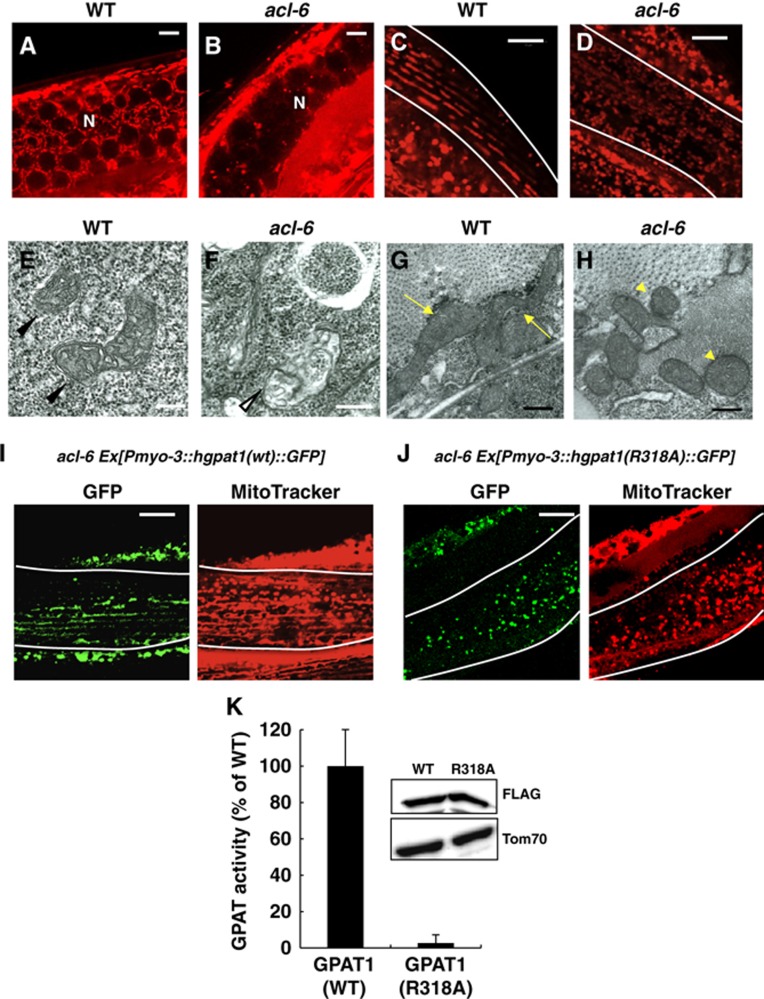
We next examined the mitochondrial morphology of Mt-GPAT mutants by staining with MitoTracker. In wild-type gonads, a meshwork-like structure of mitochondria was observed around the germ cell nuclei (Figure 3A), as reported previously (Labrousse et al, 1999; Pitt et al, 2000). In Mt-GPAT mutant gonads, a meshwork-like structure was lost and the MitoTracker staining was faint (Figures 3B and 4I). Thin-section transmission electron microscopy (TEM) revealed that Mt-GPAT mutants lacked mitochondria with normal morphology, but had swollen and abnormal structures that were not present in wild-type gonads (Figure 3E and F, white arrowhead). Because impaired mitochondrial function causes sterility in C. elegans (Artal-Sanz et al, 2003; Bratic et al, 2009), mitochondrial abnormality in the Mt-GPAT mutants may lead to sterility at the adult stage.
Figure 3.
Mt-GPAT deficiency causes defects in mitochondrial morphology. (A, B) MitoTracker staining of germ cells in wild-type (A) and Mt-GPAT (acl-6) mutant (B). N: nuclei. Scale bars, 5 μm. (C, D) MitoTracker staining of wild-type (C) and Mt-GPAT (acl-6) mutant (D). Body wall muscle cells are outlined with lines. Scale bars, 10 μm. (E, F) Transmission electron micrographs of germ cells in wild-type (E) and Mt-GPAT (acl-6) mutant (F). Arrowheads indicate the mitochondria with normal morphology. A white arrowhead indicates a swollen and abnormal mitochondrial-like structure. Scale bars, 200 nm. (G, H) Transmission electron micrographs of body wall muscle cells in wild-type (G) and Mt-GPAT (acl-6) mutant (H). Arrows indicate long and tubular mitochondria and arrowheads indicate short and round mitochondria. Scale bars, 200 nm. (I, J) Mt-GPAT (acl-6) mutants expressing hGPAT1 (WT):: GFP (I) or hGPAT1 (R318A):: GFP (J) translational fusion protein in body wall muscle cells using a myo-3 promoter were stained with MitoTracker. Body wall muscle cells are outlined with lines. Scale bars, 10 μm. (K) Glycerol-3-phosphate acyltransferase (GPAT) activity in the membrane fractions of HEK293 cells expressing hGPAT1(WT)-FLAG or hGPAT1(R318A)-FLAG. [14C]Palmitoyl-CoA (40 μM) and glycerol-3-phosphate (G3P) (800 μM) were used. Activities of mutant forms are expressed as percentages of wild-type activity. The values represent the mean±s.e.m. of four independent experiments. The insets show immunoblots of HEK 293 cells expressing GPAT1(WT)-FLAG or GPAT1(R318A)-FLAG after fractionation into membrane fraction.
Figure 4.
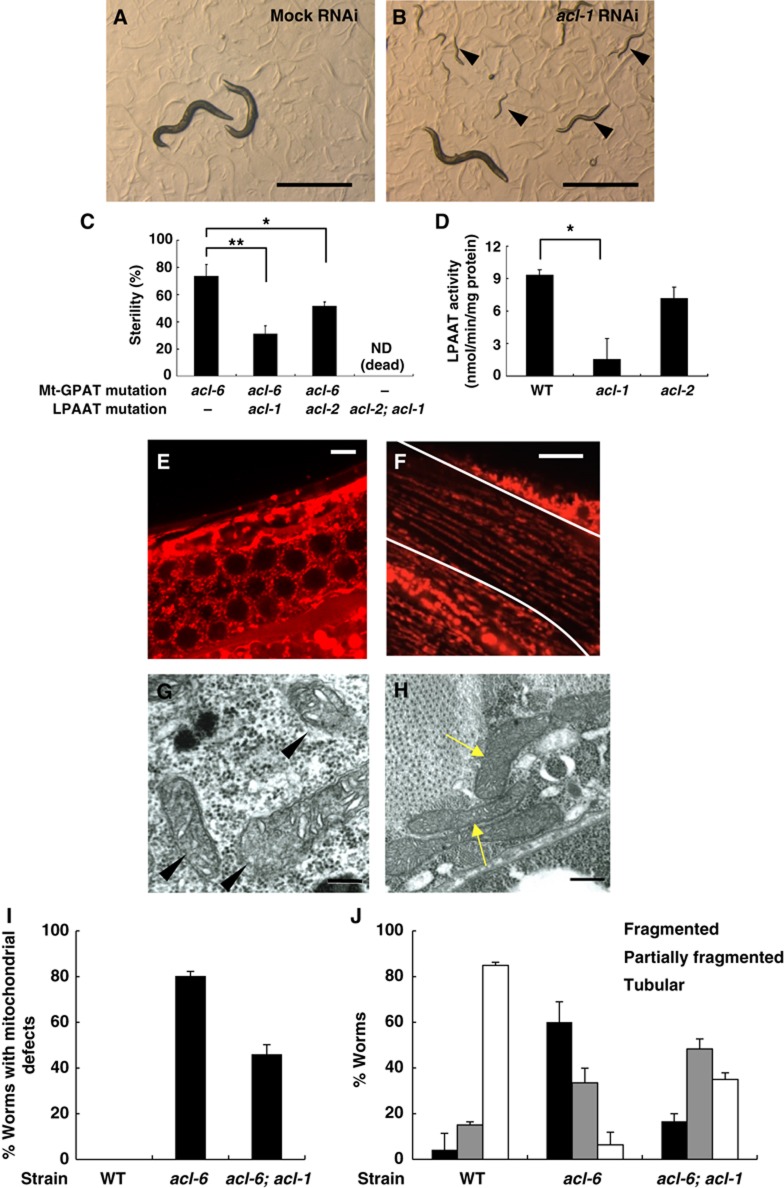
Mitochondrial defects of Mt-GPAT mutants are suppressed by LPAAT (acl-1) mutation. (A, B) Photographs of Mt-GPAT (acl-6) mutants subjected to mock (A) or acl-1 RNAi (B). Note that Mt-GPAT (acl-6) mutants produced progeny when they were subjected to acl-1 RNAi (arrowheads). Scale bars, 1 mm. (C) The percentage of sterile worms in each strain. acl-2; acl-1 double mutants that lack both LPAATs were lethal. Each bar represents the mean±s.e.m. of at least three independent experiments (n>50 for each experiment). *P<0.05, **P<0.01 (Student’s t test). (D) LPA acyltransferase activity in the membrane fractions of wild-type, acl-1 mutants, and acl-2 mutants. 1-oleoyl lysophosphatidic acid (40 μM) and [14C]oleoyl-CoA (12.5 μM) were used. Each bar represents the mean±s.e.m. of at least three independent experiments. *P<0.05 (Student’s t-test). (E, F) MitoTracker staining of germ cells (E) and body wall muscle cells (F) in acl-6; acl-1 double mutants. Body wall muscle cells are outlined with lines. Scale bars, 5 μm (germ cells) and 10 μm (muscle cells). (G, H) Transmission electron micrographs of germ cells (G) and body wall muscle cells (H) in acl-6; acl-1 double mutants. Arrowheads indicate the mitochondria with normal morphology and arrows indicate long and tubular mitochondria. Scale bars, 200 nm. (I) The percentage of worms with mitochondrial defects in germ cells evaluated by MitoTracker stain. The experiment was repeated three times (n>20 for each experiment). Bars, mean±s.e.m. (J) The bar graph shows the relative frequencies of worms with fragmented, partially fragmented or tubular mitochondria in each strain. The experiment was repeated three times (n>20 for each experiment). Bars, mean±s.e.m.
In the wild type, muscle cell mitochondria were tubular and elongated and ran parallel to the myofibrils as previously reported (Figure 3C; Labrousse et al, 1999), while in the Mt-GPAT mutants, they were fragmented and disorganized (Figures 3D and 4J). We also examined the mitochondrial morphology in Mt-GPAT mutants by expressing mitoGFP in the wall muscle cells. Mitochondria observed with mitoGFP, like those observed with MitoTracker, were significantly fragmented in Mt-GPAT mutants (Supplementary Figure S3). The mitoGFP fluorescence mostly matched the Mitotracker stain, indicating that fragmented mitochondria still maintain membrane potential. MitoGFP also detected very small portions of mitochondria that were not detected by Mitotracker (Supplementary Figure S3, arrowhead). TEM analysis showed that mitochondria of the Mt-GPAT mutants were short and round (Figure 3H, arrowheads), whereas those of wild-type muscle were long and tubular (Figure 3G, arrows). We measured the rates of thrashing, readouts for muscle activity, and found that the Mt-GPAT mutation reduced thrashing rates by 78% compared to the wild type (Supplementary Figure S4).
We conducted cell-specific rescue experiments to confirm the cell autonomy of Mt-GPAT (acl-6) function. The fragmented mitochondria in muscle cells were rescued by expression of acl-6 in the muscle cells (under myo-3 promoter), but not by expression in the intestine cells (under ges-1 promoter) (Supplementary Figure S5A–C), indicating that acl-6 functions cell autonomously to maintain mitochondrial morphology.
Next, we determined whether GPAT activity of Mt-GPAT is required for maintaining mitochondrial morphology. Essential amino-acid residues for the catalytic activity have been identified in mammalian GPAT1 (Dircks et al, 1999). Then, we generated transgenic worms expressing GFP-tagged human GPAT1 (acl-6 [Pmyo-3::hgpat1 (WT)::GFP]) or catalytically inactive hGPAT1 (arginine 318 to alanine; R318A) (acl-6 [Pmyo-3::hgpat1 (R318A)::GFP]). As shown in Figure 3K, the membrane fractions of HEK293 cells expressing hGPAT1 (R318A)-FLAG did not show increased GPAT activity compared with those of hGPAT1 (WT). Mitochondrial fragmentation in Mt-GPAT mutants was rescued in hGPAT1 (WT)-expressing cells (Figure 3I), but not in hGPAT1 (R318A)-expressing cells (Figure 3J). Thus, GPAT activity of Mt-GPAT is required for normal mitochondrial morphology.
LPA is required for normal mitochondrial morphology
The finding that GPAT activity was required for normal mitochondrial morphology indicated that LPA, a product of GPAT, or the downstream metabolites in glycerolipids synthesis pathway is involved in mitochondrial morphology. Then, we conducted RNAi knockdown of 80 genes whose homologues are reported to be involved in phospholipid synthesis and lipid transfer in other species (Supplementary Table S2) in Mt-GPAT (acl-6) mutants. Interestingly, we found that inhibition of acl-1 rescued the sterility (Figure 4A–C) and abnormal mitochondrial morphology (Figure 4E–J). acl-1 encodes a homologue of mammalian LPAAT, which catalyses the conversion of LPA to PA (Figure 1A; Supplementary Figure S1C). The acl-1 mutation reduced LPAAT activity to 17% of that in the wild type (Figures 1D and 4D), and caused no detectable abnormalities (Table I, LPAAT mutants). The acl-1 mutation rescued both abnormal MitoTracker staining in the gonad and abnormal mitochondrial morphology in muscle cells of Mt-GPAT mutants (Figure 4E, F). TEM analysis also showed that acl-1 mutation restored normal mitochondrial morphology in Mt-GPAT mutants (Figure 4G and H). Two human LPAAT isoforms have been cloned (Figure 1A), and we identified acl-2 as another homologue of mammalian LPAAT. Although the acl-2 mutation did not significantly reduce the in vitro LPAAT activity (Figures 1D and 4D), double mutations of acl-1 and acl-2 resulted in lethal phenotypes (Table I; Figure 1D; Supplementary Table S1B), suggesting that acl-1 and acl-2 cooperatively function as LPAAT. The acl-2 mutation also rescued the sterility of Mt-GPAT mutants, but to a lesser extent than did the acl-1 mutation (Figure 4C).
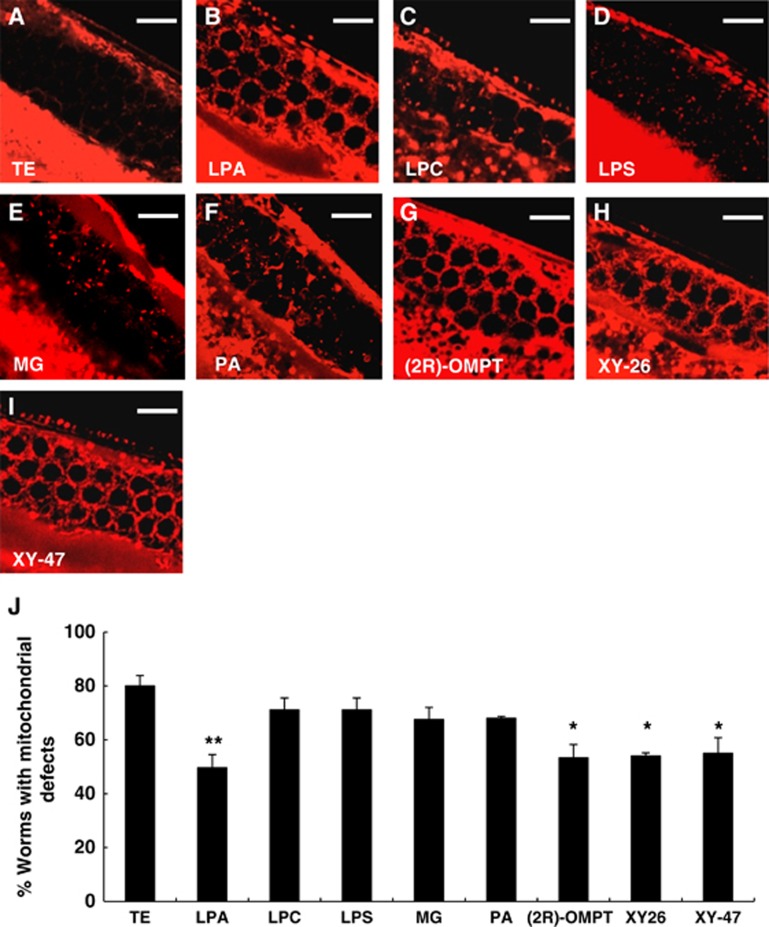
Since reduction in LPAAT activity is assumed to increase the level of LPA in the cells by suppressing the conversion of LPA to PA (Figure 1A), we hypothesized that an increased level of LPA, a direct product of GPAT reaction, could rescue the mitochondrial defects of Mt-GPAT mutants. To test this hypothesis, we injected LPA directly into the gonad of Mt-GPAT mutants. When a solution is injected into the C. elegans gonad, it spreads within the cytoplasm and directly reaches the organelles of germ cells because the gonad is a syncytium where germ nuclei share the cytoplasm (Hubbard and Greenstein, 2005). About 80% of non-treated or vehicle-injected Mt-GPAT mutants showed faint MitoTracker staining in the gonad (Figure 5A and J). By injection of 1-palmitoyl-2-hydroxy-sn-glycerol-3-phosphate, a molecular species of LPA, into the gonad, about half of the Mt-GPAT mutants showed normal meshwork-like MitoTracker staining (Figure 5B and J). Injection of other lyosphospholipids such as lysophosphatidylcholine and lysophosphatidylserine did not rescue the abnormal mitochondrial staining (Figure 5C, D). Since LPA is a metabolically active intermediate of glycerolipid synthesis, it can be hydrolysed at the bond between phosphate and the glycerol backbone to form monoacylglycerol, or it can be acylated at the sn-2 position to form PA. However, these metabolites did not rescue the mitochondrial staining (Figure 4E, F). We also injected metabolically stabilized LPA analogues such as 1-oleoyl-2-O-methyl-glycerophosphothionate (OMPT), XY-26, and XY-47 (Supplementary Figure S6B–E; Xu et al, 2005). OMPT, in which the hydroxy group and the phosphate are converted to an O-methoxy group and a phosphothionate, is an acyltransferase- and phosphatase-resistant analogue. XY-26 is a phosphatase-resistant LPA analogue in which the bridging oxygen in the mono phosphate is replaced by an α-monofluoromethylene (-CF-) moiety. XY-47 is an alkyl LPA in which the sn-1 O-acyl group is replaced by an O-alkyl ether, and is resistant to phospholipase A. We found that all these LPA analogues suppressed the mitochondrial defects (Figure 6G–J). Although these LPA analogues may have ability to suppress LPAAT activity, which also rescues the mitochondrial defects in Mt-GPAT mutants (see Figure 4E), we found that these analogues did not inhibit LPAAT activity (Supplementary Figure S6A). These results, together with the genetic rescue by the LPAAT mutation, strongly suggest that LPA is required for normal mitochondrial morphology.
Figure 5.
Injection of LPA into germ cells suppresses the mitochondrial defects in Mt-GPAT mutants. (A–I) Mt-GPAT (acl-6) mutants were injected with each lipid into the gonad, and stained with MitoTracker. Germ cells stained with MitoTracker are shown. Scale bars, 10 μm. (J) The percentage of worms with mitochondrial defects in germ cells. The experiment was repeated three times (n>20 for each experiment). Bars, mean±s.e.m. *P<0.05, **P<0.01 (Student’s t-test compared to TE injection).
Figure 6.
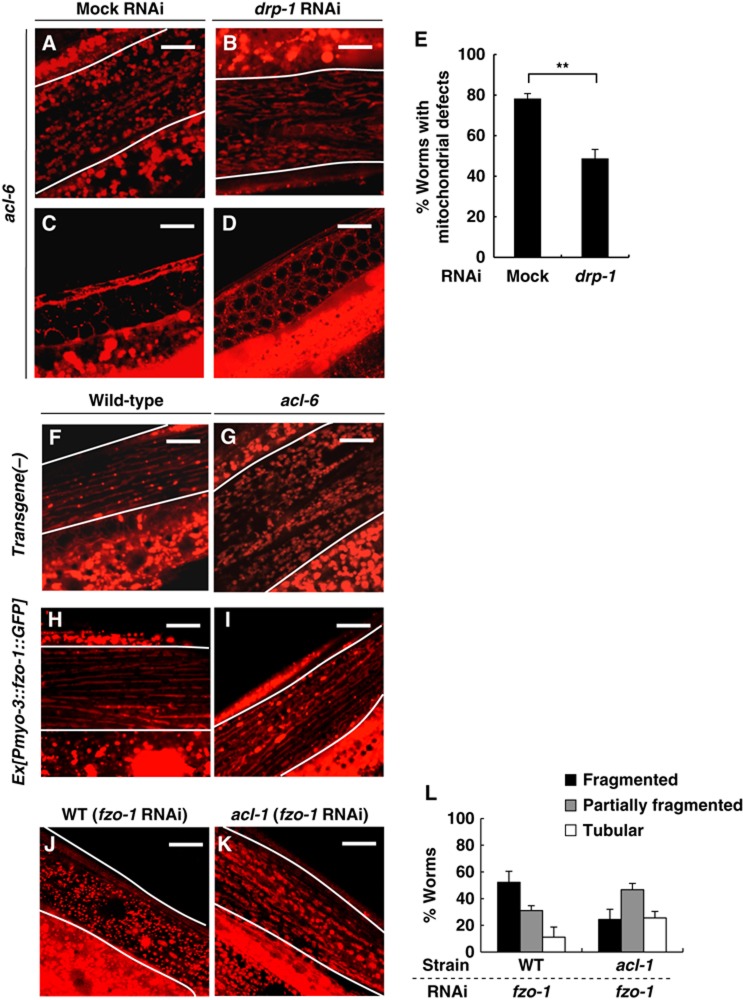
Inhibition of Drp1 rescues the mitochondrial defects in Mt-GPAT mutants. (A–D) MitoTracker staining of body wall muscle cells (A, B) and germ cells (C, D) in Mt-GPAT (acl-6) mutants subjected to mock or drp-1 RNAi. Body wall muscle cells are outlined with lines. Scale bars, 10 μm. (E) The percentage of worms with the mitochondrial defects in germ cells evaluated by MitoTracker stain. Each bar represents the mean±s.e.m. of three independent experiments (n>20 for each experiment). **P<0.01 (Student’s t-test). (F–I) MitoTracker staining of body wall muscle cells in wild-type (F, H) and Mt-GPAT (acl-6) mutants (G, I) expressing the C. elegans mitofusin homologue, FZO-1. FZO-1::GFP was specifically expressed in body wall muscle cells using a myo-3 promoter. Body wall muscle cells are outlined with lines. Scale bars, 10 μm. (J–L) MitoTracker staining of body wall muscle cells in wild-type (J) or acl-1 mutants (K) subjected to fzo-1 RNAi. Body wall muscle cells are outlined with lines. Scale bars, 10 μm. The bar graph shows the relative frequencies of worms with fragmented, partially fragmented or tubular mitochondria in each strain. The experiment was repeated three times (n>30 for each experiment). Bars, mean±s.e.m. (L).
Abnormal mitochondrial morphology in Mt-GPAT mutants is suppressed by drp-1 RNAi
Proper mitochondrial morphology results from a balance between fission and fusion, which compete against each other (Sesaki and Jensen, 1999; Hoppins and Nunnari, 2009). Mitochondrial fragmentation that we observed in Mt-GPAT mutant muscle cells (Figure 3D and H) led us to hypothesize that Mt-GPAT inhibition reduces fusion. Studies in S. cerevisiae and mammals have demonstrated that defects in mitochondrial fusion can be ameliorated by mutations in genes required for mitochondrial fission and vice versa. We knocked down the expression of the pro-fission protein Drp1 in Mt-GPAT mutants. As a control, we checked mitochondrial morphology in mutants of a pro-fusion protein Mfn (fzo-1 mutants). The fzo-1 mutation caused fragmented mitochondria in the muscle cells, which was suppressed by drp-1 RNAi (Supplementary Figure S7A and B), as reported previously (Ichishita et al, 2008). The fzo-1 mutation also reduced MitoTracker staining in the gonad, which was recovered by drp-1 RNAi (Supplementary Figure S7C and D). In Mt-GPAT mutants, drp-1 RNAi significantly suppressed both fragmented mitochondria of muscle cells and MitoTracker staining of the gonad (Figure 6A–E). Furthermore, overexpression of fzo-1 suppressed the fragmented mitochondria of muscle cells in Mt-GPAT mutants (Figure 6F–I). These results indicate that Mt-GPAT inhibition reduces fusion and/or enhances fission, leading to abnormal mitochondrial morphology. drp-1 RNAi did not rescue significantly the oogenesis defect of Mt-GPAT mutants (Supplementary Figure S8). Although mitochondrial morphology is apparently rescued by drp-1 RNAi, mitochondrial function may not be restored enough to rescue the fertility. Alternatively, LPA produced by Mt-GPAT may have an as yet unknown function in oogenesis.
Although acl-1 mutation, which may raise the intracellular LPA level, rescued mitochondrial fragmentation caused by fzo-1 RNAi (Figure 6J–L), it did not rescue mitochondrial fragmentation caused by fzo-1 mutation (Supplementary Figure S7E), suggesting that LPA acts on the fzo-1-dependent mitochondrial fusion.
Knockdown of Mt-GPAT leads to mitochondrial fragmentation in HeLa cells
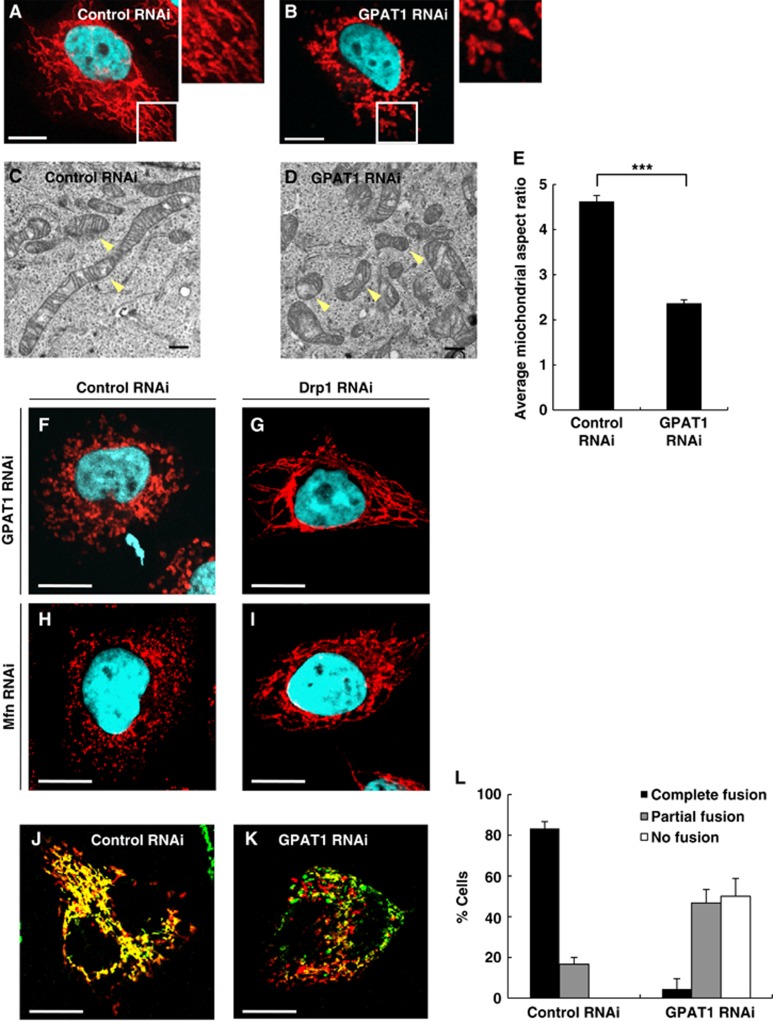
To determine whether Mt-GPAT is required for mitochondrial morphology in mammals, we used HeLa cells in which GPAT1 was a major mitochondrial GPAT isoform (Supplementary Figure S9A). In cells transfected with GPAT1 siRNA duplex, the GPAT1 mRNA level was reduced to 24% of the level in control siRNA-transfected cells at 72 h after transfection (Supplementary Figure S9B). In control cells, mitochondria visualized by MitoTracker were characterized by a network of extended wavy tubules throughout the cytoplasm (Figure 7A). In contrast, mitochondria appeared as punctate structures in GPAT1-depleted cells (Figure 7B). In electron micrographs, mitochondrial networks appeared to be collapsed to form small and fragmented mitochondria in GPAT1-depleted cells, while mitochondria were long and tubular in control cells (Figure 7C and D). Quantitative analysis of electron micrographs revealed that the mitochondrial aspect ratio was smaller in GPAT1-depleted cells than in control cells (Figure 7E). The mitochondrial membrane potential and the ratio of mitochondrial DNA: nuclear DNA were not changed in GPAT1-depleted cells (Supplementary Figure S9C and D). These results suggest that mitochondrial fragmentation in GPAT1-depleted cells was not caused by a loss of mitochondrial function.
Figure 7.
Mt-GPAT is required for mitochondrial fusion in HeLa cells. (A, B) HeLa cells were transfected with control (A) or Mt-GPAT (GPAT1) siRNA (B), and stained with MitoTracker and DAPI. White boxes indicate regions enlarged in insets. Scale bars, 10 μm. (C, D) Transmission electron micrographs of HeLa cells, transfected with control (C) or Mt-GPAT (GPAT1) siRNA (D). Arrowheads indicate mitochondria. Scale bars, 500 nm. (E) Mitochondrial length was determined by analysing the aspect ratio (length of major axes/minor axes). Data are shown as mean±s.e.m. ***P<0.001 (Student’s t-test). (F–I) HeLa cells were treated with siRNA against the indicated genes and stained with MitoTracker and DAPI. Scale bars, 10 μm. (J–L) HeLa cells expressing su9-GFP or su9-RFP (Taguchi et al, 2007) were coplated, fused with PEG1500 and imaged after 8 h. Confocal images of representative cells transfected with control (J) or Mt-GPAT (GPAT1) siRNA (K). Scale bars, 20 μm. The bar graph shows the relative frequencies of full, partial, and almost no fusion for each siRNA-treated cell. The experiment was repeated three times. Bars, mean±s.e.m. (L).
Mitochondrial fragmentation in GPAT1-depleted cells was suppressed by knockdown of either Drp1 or LPAATs (LPAAT1 and LPAAT2) as it was in C. elegans Mt-GPAT mutants (Figure 7F–I; Supplementary Figure S10). To address whether the fragmented mitochondria are the result of reduced fusion of mitochondria, we performed a mitochondrial fusion assay by using a cell fusion technique with polyethylene glycol (PEG) (Chen et al, 2003). When cells expressing mitochondrially targeted GFP were fused with cells expressing mitochondrially targeted RFP by using PEG, most of the fused cells contained extensively fused mitochondria as demonstrated by colocalization of green and red fluorescent signals (Figure 7J). In contrast, GPAT1-depleted cells showed reduced mitochondrial fusion activity, that is, 50% of fused cells possessed predominantly unfused mitochondria and 47% showed partial fusion (Figure 7K and L). These results indicate that mitochondrial GPAT is required for mitochondria fusion in mammals as well as in C. elegans.
Discussion
Mt-GPAT and ER-GPAT are considered as the initial enzymes for glycerolipid synthesis. In this study, we generated both GPAT mutants using C. elegans to elucidate the functional difference between Mt-GPAT and ER-GPAT. Mutants lacking either Mt-GPAT or ER-GPAT could survive but mutants lacking both GPATs could not, indicating that these two GPATs complement each other for synthesizing glycerolipids such as phospholipids, which are essential components of biological membranes. In addition to a role in the synthesis of glycerolipids, we found that Mt-GPAT is required for normal mitochondrial dynamics in both C. elegans and mammals. Mt-GPAT mutants, but not ER-GPAT mutants, showed abnormal mitochondrial morphology. Mitochondrial fragmentation in Mt-GPAT mutants was rescued by wild-type GPAT but not by catalytically inactive GPAT. The mitochondrial defect was also rescued by injection of LPA, a direct product of Mt-GPAT. Furthermore, the mitochondrial defect in Mt-GPAT mutants was rescued by inhibition of LPAAT, which converts LPA to PA. In Mt-GPAT mutants, LPA is normally produced in the ER by ER-GPAT and is further acylated to form PA by LPAAT. When the LPAAT activity is inhibited by an LPAAT mutation, LPA may be accumulated in the ER and fluxed into the mitochondria, which leads to restoration of the mitochondrial defects in Mt-GPAT mutants.
Among mammalian four GPATs, knockout mice for mitochondrial GPAT1 (GPAT1−/− mice) have been reported (Hammond et al, 2007). Mitochondria obtained from the liver of GPAT1−/− mice are more sensitive to Ca2+-induced mitochondrial permeability transition, a common marker of mitochondrial dysfunction, though mitochondrial morphology appears normal. In mammals, GPAT2, another mitochondrial GPAT, may function to produce LPA, which may account for the apparent normal mitochondrial morphology in GPAT1−/− mice. We expect that double knockout of GPAT1 and GPAT2 would induce a mitochondrial fragmentation as observed in HeLa cells.
LPA has been shown to act as an extracellular signalling molecule that evokes a wide variety of cellular processes including cell proliferation and migration, smooth muscle contraction, and actin stress fibre formation (van Meeteren and Moolenaar, 2007; Ye and Chun, 2010). These processes are exerted through interactions with a family of G protein-coupled receptors on the plasma membrane (Aoki et al, 2008; Ye and Chun, 2010). Extracellular LPA is produced either from lysophosphatidylcholine by a plasma phospholipase D, named autotaxin, or from PA by an extracellular phospholipase A1 (Aoki et al, 2008; Ye and Chun, 2010). In the present study, we showed that LPA produced by Mt-GPAT functions at mitochondria in a cell-autonomous manner. First, the mitochondrial defect in muscle cells was rescued by muscle-specific expression of acl-6 (Mt-GPAT), but not by expression in other tissues such as the intestine. Second, the same muscle-specific expression of Mt-GPAT failed to rescue the mitochondrial defect in the gonads (Supplementary Figure S5D–H). Third, Mt-GPAT RNAi caused sterility in the rrf-1 mutant background, in which RNAi works only in the germ cells in the gonads (Supplementary Figure S5I–K; Sijen et al, 2001). These observations indicate that LPA produced by Mt-GPAT is an intracellular bioactive lipid that regulates mitochondrial morphology.
How does Mt-GPAT/LPA affect mitochondrial morphology? In this study, we provide three lines of evidence that Mt-GPAT/LPA is required for mitochondrial fusion. First, inhibition of Mt-GPAT caused mitochondrial fragmentation which is similar to that caused by inhibition of a pro-fusion protein, Mfn (mammals)/FZO-1 (C. elegans). Second, as in fzo-1 mutants, mitochondrial fragmentation in Mt-GPAT mutants was rescued by knockdown of the pro-fission protein DRP-1. Third, in HeLa cells, fusion of mitochondria derived from two different cells after cell fusion was strongly inhibited by Mt-GPAT depletion. Mutation of LPAAT, which may increase the intracellular LPA level by blocking the conversion of LPA to PA, could rescue the mitochondrial fragmentation induced by fzo-1 RNAi but not the mitochondrial fragmentation induced by fzo-1 mutation. This result indicates that LPA cannot rescue the mitochondrial fragmentation unless FZO-1 is expressed. Alternatively, the fzo-1 deletion may lead to an alternative compensatory response that is not influenced by LPA levels.
Mfn is degraded through the ubiquitin proteasome pathway that involves two E3 ligases, MITOL/March5 and Parkin (Cohen et al, 2008; Tanaka et al, 2010). The level of Mfn was not changed significantly in Mt-GPAT-depleted HeLa cells (Supplementary Figure S9E). In addition, the levels of Bax, Bak, Bcl-xL and Mfn-binding protein, MIB, which are known to modulate Mfn-mediated mitochondrial fusion (Eura et al, 2006; Karbowski et al, 2006; Rolland et al, 2009), were not changed in Mt-GPAT-depleted cells (Supplementary Figure S9E). Opa1 is involved in mitochondrial inner membrane fusion, and its function is controlled by proteolytic cleavage (Griparic et al, 2004; Ishihara et al, 2006). As previously reported (Griparic et al, 2007), Opa1 can be resolved into several bands on western blots in HeLa cells, and this band pattern was not altered by Mt-GPAT knockdown (Supplementary Figure S9E). Mfns form multimeric complexes that can be detected by blue native polyacrylamide gel electrophoresis (BN-PAGE). Oligomeric states of Mfns are changed in a GTP-dependent manner (Ishihara et al, 2004; Karbowski et al, 2006), and are modulated by Mfn regulators such as Bax and Bak (Karbowski et al, 2006). We detected endogenous Mfn oligomers from HeLa cells by BN-PAGE and found that their oligomeric states were GTP dependent (Supplementary Figure S11A). However, the oligomeric states were not changed by Mt-GPAT depletion or by addition of LPA in vitro (Supplementary Figure S11B and C). Mfn is a dynamin-like GTPase and its GTPase activity is required for mitochondrial fusion (Santel and Fuller, 2001; Hoppins and Nunnari, 2009). We examined the effect of LPA on the GTPase activity of Mfn by using a recombinant rat Mfn produced by E. coli (Ishihara et al, 2004). Interestingly, addition of LPA to the reaction mixture slightly but significantly stimulated the Mfn1 GTPase activity (Supplementary Figure S11D–F). Lysophosphatidylcholine (LPC), which is inactive in rescuing the mitochondrial defect of Mt-GPAT mutants, did not stimulate GTPase activity (Supplementary Figure S11F). Although the stimulation was small in the detergent (Triton X-100)-containing in vitro assay, LPA produced on the mitochondrial outer membrane may stimulate GTPase activity of Mfn and enhance mitochondrial fusion.
Lipids also have roles in membrane dynamics (Chernomordik and Kozlov, 2008; Furt and Moreau, 2009). LPA is an inverted cone-shaped lipid that causes the membrane to have a positive curvature (Haucke and Di Paolo, 2007). In lipid bilayer fusion, hemifusion structures and fusion pores are characterized as sequences of the intermediate structures (Chernomordik and Kozlov, 2008). Lipids inducing positive curvature facilitate the formation of a fusion pore (Haucke and Di Paolo, 2007; Chernomordik and Kozlov, 2008). Therefore, LPA produced by Mt-GPAT may assist Mfn-mediated mitochondrial outer membrane fusion. Cardiolipin (CL) is predominantly localized in the mitochondrial inner membrane where it is synthesized from phosphatidylglycerol and cytidinediphosphate diacylglycerol. CL is required for optimal activities of many mitochondrial proteins (Hoffmann et al, 1994; Claypool et al, 2008). Loss of CL impacts on a variety of mitochondrial functions including mitochondrial fusion (Jiang et al, 2000; Choi et al, 2006; Gebert et al, 2009; Zhang et al, 2011). In Mt-GPAT mutants, the amount of CL was not affected (Supplementary Table S3), indicating that mitochondrial defect was not due to the decreased CL level in the mitochondria.
Mitochondrial phospholipase D (MitoPLD) has been identified as a key factor of mitochondrial fusion (Choi et al, 2006; Huang et al, 2011). MitoPLD hydrolyses CL on the outer mitochondrial membrane, resulting in the formation of PA. Overexpression of wild-type MitoPLD causes mitochondrial aggregation, whereas overexpression of catalytically inactive MitoPLD or RNAi-mediated knockdown of intrinsic MitoPLD causes mitochondrial fragmentation. Moreover, transient expression of Lipin 1b, one isoform of PA phosphatase, which may degrade MitoPLD-generated PA to diacylglycerol on the mitochondrial surfaces, induces the fission of long mitochondrial tubules into moderately sized fragments. Based on these results, the authors of the above studies postulated that PA facilitates mitochondrial fusion. A PA requirement has often been demonstrated in the SNARE-mediated fusion of secretary vesicles with the plasma membranes during many types of regulated exocytosis and during sporulation in yeast. Nonetheless, the mechanism through which MitoPLD-generated PA controls mitochondrial fusion remains unknown. LPA produced by Mt-GPAT could be acylated to form PA in the outer leaflet of the mitochondrial outer membranes. So far, LPAATs other than LPAAT1, LPAAT2/acl-1, acl-2 in the ER have not been identified in either C. elegans or mammals. Moreover, our findings that the mitochondrial defect of Mt-GPAT mutants was rescued by metabolically stabilized LPA analogues and that the PA level was not decreased in C. elegans Mt-GPAT mutants or in Mt-GPAT-depleted HeLa cells (Supplementary Table S3) suggest that PA production through Mt-GPAT is not responsible for mitochondrial fusion. Thus, the MitoPLD/PA and Mt-GPAT/LPA pathways may work independently in mitochondrial fusion. PA is metabolically active; it can be dephosphorylated to form diacylglycerol, which was substantiated in MitoPLD-overexpressing cells (Huang et al, 2011), and it can be deacylated to form LPA. Another possibility is that MitoPLD-generated PA is further deacylated by an unknown enzyme to form LPA which functions in mitofusin-dependent mitochondrial fusion. The C. elegans genome does not have MitoPLD homologues, so that Mt-GPAT should dominate in supplying LPA for mitochondrial fusion.
Taken together, our results demonstrate that LPA produced by Mt-GPAT functions not only as a precursor for glycerolipid synthesis but also as an essential factor of mitochondrial fusion.
Materials and methods
Materials
[1-14C]palmitic acid, [1-14C]palmitoyl-CoA, and [1-14C]oleoyl-CoA were purchased from American Radiolabeled Chemicals (St Louis, MO, USA). Glycerol-3-phosphate and 1-monopalmitoyl-rac-glycerol were purchased from Sigma. sn-1-palmitoyl lysophosphatidic acid, sn-1-palmitoyl-sn-2-oleoyl phosphatidic acid, sn-1-palmitoyl lysophosphatidylcholine, and sn-1-oleoyl lysophosphatidylserine were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Stabilized LPA analogues, (2R)-1-oleoyl-2-O-methyl-glycerophosphothionate (OMPT), (3S)-1-fluoro-3-hydroxy-4-(linoleoyloxy) butyl-1-phosphonate (XY-26), and 1-palmitoyl alkyl LPA (XY-47), were synthesized and kindly provided by Dr Glenn D Prestwich at the University of Utah.
Acyltransferase assay
Synchronized young adult worms were suspended in 50 mM potassium phosphate buffer (pH 7.0) containing 0.15 M KCl, 1 mM EDTA, 1 mM dithiothreitol, 1 mM phenylmethanesulfonyl fluoride (PMSF) and 0.25 M sucrose (homogenizing buffer) and sonicated three times on ice for 30 s. The C. elegans homogenate was centrifuged at 1000 g for 5 min at 4°C. The resulting supernatant was further centrifuged at 105 000 g for 60 min. The pellet was suspended in homogenizing buffer (without EDTA, dithiothreitol, and PMSF) and used for the enzyme assay described below. HEK293 cells were harvested, washed with ice-cold phosphate-buffered saline, and sonicated three times on ice for 5 s in homogenizing buffer. The membrane fraction of HEK293 cells was prepared as described above. GPAT activity was assayed at 20°C for 30 min (C. elegans) or 37°C for 15 min (HEK293 cells) in a 200-μl reaction mixture containing 75 mM Tris/HCl, pH 7.5, 4 mM MgCl2, 1 mg/ml bovine serum albumin free fatty acids, 8 mM NaF, 800 μM glycerol-3-phosphate, 40 μM [14C]palmitoyl-CoA, and 45 μg (C. elegans) or 10 μg (HEK293 cells) protein of the membrane fraction. LPAAT activity was measured at 20°C for 5 min in a 800 μl reaction mixture containing 100 mM Tris/HCl, pH 7.5, 1 mg/ml bovine serum albumin free fatty acids, 40 μM 1-oleoyl lysophosphatidic acid, 20 μM LPA analogue, 12.5 μM [14C]oleoyl-CoA, and 5 μg protein of the membrane fraction. The acyltransferase reaction was stopped by mixing with methanol. The lipids were extracted by the method of Bligh and Dyer (1959) and separated by TLC in chloroform/methanol/water (65/25/4, v/v) and the amount of radioactivity was analysed with a bioimaging analyzer (BAS-1500, FUJIFILM).
Lipid analysis
Content of phospholipids and triacylglycerol
Lipids of synchronized young adult worms were extracted by the method of Bligh and Dyer. The phosphorus contents of the total lipids were determined by the method of Bartlett (1959). The triacylglycerol contents were measured using enzymatic kit (Wako Triglyceride E-test, Wako Pure Chemical Ltd., Osaka, Japan).
In vivo incorporation of exogenous fatty acids into C. elegans
Incorporation of exogenous fatty acids into C. elegans was analysed as described previously (Lee et al, 2008). Synchronized first-stage larvae (800–1200 animals) were cultured with 1 μCi of [14C]palmitic acid on NGM plates at 20°C until they reached the young adult stage. Lipids of young adult worms were extracted by the method of Bligh and Dyer and separated by one-dimensional TLC on silica gel 60 plates (Merck) in chloroform/ethanol/water/triethylamine (30/35/7/35, v/v). Then, the area of silica gel corresponding to neutral lipids was scraped off the plates, and the lipids was re-extracted and separated by TLC in petroleum ether/ethyl ether/acetic acid (60/40/1, v/v). Incorporation of [14C]palmitic acid into triacylglycerol was expressed as the percentage of radioactivity incorporated into total lipids.
Contents of CL and phosphatidic acid
Lipids of young adult worms and HeLa cells were extracted by the method of Bligh and Dyer and separated by one-dimensional TLC on silica gel 60 plates (Merck) in chloroform/methanol/water/ammonium hydroxide (60:37.5:3:1, v/v). Then, the area of silica gel corresponding to CL or phosphatidic acid was scraped off the plates, and the lipids were re-extracted and the phosphorus contents of each lipid were determined as described above.
MitoTracker staining
C. elegans
Young adult worms were transferred to NGM plates containing 2 μM MitoTracker Red CMXRos (Molecular Probes, Inc., Eugene, OR) and incubated for 2 days in the dark. Worms were washed with M9 buffer, mounted on an agar pad and images were taken by using a confocal fluorescence microscope (LSM510; Carl Zeiss Inc., Thornwood, NY, USA).
HeLa cells
HeLa cells were incubated with 500 nM MitoTracker Red CM-H2XMRos (Molecular Probes, Inc.) for 40 min before fixation and imaged as described above.
Electron microscopy
Synchronized 72-h-old adult worms or HeLa cells were pre-fixed with 4% paraformaldehyde and 1% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4). Samples were then cut into small pieces, fixed again with 2% paraformaldehyde and 2% glutaraldehyde in the same buffer, and post-fixed with 2% osmium tetroxide in phosphate buffer for 4 h. Fixed specimens were dehydrated in a graded series of ethanol and embedded in Quetol 651 epoxy resin. Ultrathin (80 to 90 nm-thick) sections obtained by ultramicrotomy were stained with uranyl acetate for 15 min and with modified Sato’s lead solution for 5 min. TEM observation was performed using a JEOL JEM-1200EX electron microscope. For the quantification of mitochondrial length in HeLa cells, over 350 mitochondria were measured per siRNA condition using ImageJ (NIH, USA).
Injection of lipids into C. elegans germ cells
Each lipid was diluted in TE buffer to 50 μM and was microinjected into the gonads of acl-6 mutants at the L4 stage. After injection, worms were transferred to NGM plates containing 2 μM MitoTracker Red CMXRos (Molecular Probes, Inc.) and incubated for 2 days in the dark.
Mitochondrial fusion assay
Mitochondrial fusion assay was performed as described previously (Cipolat et al, 2004), except that HeLa cells stably expressing su9-GFP and su9-RFP (Taguchi et al, 2007) were used.
Supplementary Material
Acknowledgments
We thank T Taguchi (University of Tokyo) for helpful discussions; H Fukuda for technical support; A Fire (Stanford University School of Medicine) for plasmids; and the Caenorhabditis Genetics Center (University of Minnesota, Minneapolis) for strains. This work was supported by the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (HA), the Program for Promotion of Basic and Applied Research for Innovations in Bio-Oriented Industry (HA), Grants-in-aid from the Japanese Ministry of Education, Culture, Sports, Science, and Technology and the Japanese Ministry of Health, Labor, and Welfare (to HA).
Author contributions: YO, TS, EKN, NHT, and NK carried out the experiments. All authors discussed the results and designed the experimental approaches. TI and HA wrote the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aoki J, Inoue A, Okudaira S (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781: 513–518 [DOI] [PubMed] [Google Scholar]
- Artal-Sanz M, Tsang WY, Willems EM, Grivell LA, Lemire BD, van der Spek H, Nijtmans LG, Sanz MA (2003) The mitochondrial prohibitin complex is essential for embryonic viability and germline function in Caenorhabditis elegans. J Biol Chem 278: 32091–32099 [DOI] [PubMed] [Google Scholar]
- Bartlett GR (1959) Phosphorous assay in column chromatography. J Biol Chem 234: 466–468 [PubMed] [Google Scholar]
- Beigneux AP, Vergnes L, Qiao X, Quatela S, Davis R, Watkins SM, Coleman RA, Walzem RL, Philips M, Reue K, Young SG (2006) Agpat6—a novel lipid biosynthetic gene required for triacylglycerol production in mammary epithelium. J Lipid Res 47: 734–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917 [DOI] [PubMed] [Google Scholar]
- Bratic I, Hench J, Henriksson J, Antebi A, Burglin TR, Trifunovic A (2009) Mitochondrial DNA level, but not active replicase, is essential for Caenorhabditis elegans development. Nucleic Acids Res 37: 1817–1828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YQ, Kuo MS, Li S, Bui HH, Peake DA, Sanders PE, Thibodeaux SJ, Chu S, Qian YW, Zhao Y, Bredt DS, Moller DE, Konrad RJ, Beigneux AP, Young SG, Cao G (2008) AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J Biol Chem 283: 10048–10057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM (2008) Mechanics of membrane fusion. Nat Struct Mol Biol 15: 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SY, Huang P, Jenkins GM, Chan DC, Schiller J, Frohman MA (2006) A common lipid links Mfn-mediated mitochondrial fusion and SNARE-regulated exocytosis. Nat Cell Biol 8: 1255–1262 [DOI] [PubMed] [Google Scholar]
- Cipolat S, de Brito OM, Dal Zilio B, Scorrano L (2004) OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci USA 101: 15927–15932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claypool SM, Oktay Y, Boontheung P, Loo JA, Koehler CM (2008) Cardiolipin defines the interactome of the major ADP/ATP carrier protein of the mitochondrial inner membrane. J Cell Biol 182: 937–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MM, Leboucher GP, Livnat-Levanon N, Glickman MH, Weissman AM (2008) Ubiquitin-proteasome-dependent degradation of a mitofusin, a critical regulator of mitochondrial fusion. Mol Biol Cell 19: 2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Lee DP (2004) Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res 43: 134–176 [DOI] [PubMed] [Google Scholar]
- Coleman RA, Lewin TM, Muoio DM (2000) Physiological and nutritional regulation of enzymes of triacylglycerol synthesis. Annu Rev Nutr 20: 77–103 [DOI] [PubMed] [Google Scholar]
- Detmer SA, Chan DC (2007) Functions and dysfunctions of mitochondrial dynamics. Nat Rev Mol Cell Biol 8: 870–879 [DOI] [PubMed] [Google Scholar]
- Dircks LK, Ke J, Sul HS (1999) A conserved seven amino acid stretch important for murine mitochondrial glycerol-3-phosphate acyltransferase activity: significance of arginine 318 in catalysis. J Biol Chem 274: 34728–34734 [DOI] [PubMed] [Google Scholar]
- Dowhan W (1997) Molecular basis for membrane phospholipid diversity: why are there so many lipids? Annu Rev Biochem 66: 199–232 [DOI] [PubMed] [Google Scholar]
- Eura Y, Ishihara N, Oka T, Mihara K (2006) Identification of a novel protein that regulates mitochondrial fusion by modulating mitofusin (Mfn) protein function. J Cell Sci 119: 4913–4925 [DOI] [PubMed] [Google Scholar]
- Eura Y, Ishihara N, Yokota S, Mihara K (2003) Two mitofusin proteins, mammalian homologues of FZO, with distinct functions are both required for mitochondrial fusion. J Biochem 134: 333–344 [DOI] [PubMed] [Google Scholar]
- Furt F, Moreau P (2009) Importance of lipid metabolism for intracellular and mitochondrial membrane fusion/fission processes. Int J Biochem Cell Biol 41: 1828–1836 [DOI] [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, Rehling P, Meisinger C, Ryan MT, Wiedemann N, Greenberg ML, Pfanner N (2009) Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol 19: 2133–2139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno RE, Cao J (2008) Mammalian glycerol-3-phosphate acyltransferases: newgenes for an old activity. J Lipid Res 49: 2079–2088 [DOI] [PubMed] [Google Scholar]
- Griparic L, Kanazawa T, van der Bliek AM (2007) Regulation of the mitochondrial dynamin-like protein Opa1 by proteolytic cleavage. J Cell Biol 178: 757–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griparic L, van der Wel NN, Orozco IJ, Peters PJ, van der Bliek AM (2004) Loss of the intermembrane space protein Mgm1/OPA1 induces swelling and localized constrictions along the lengths of mitochondria. J Biol Chem 279: 18792–18798 [DOI] [PubMed] [Google Scholar]
- Hammond LE, Albright CD, He L, Rusyn I, Watkins SM, Doughman SD, Lemasters JJ, Coleman RA (2007) Increased oxidative stress is associated with balanced increases in hepatocyte apoptosis and proliferation in glycerol-3-phosphate acyltransferase-1 deficient mice. Exp Mol Pathol 82: 210–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LE, Gallagher PA, Wang S, Hiller S, Kluckman KD, Posey-Marcos EL, Maeda N, Coleman RA (2002) Mitochondrial glycerol-3-phosphate acyltransferase-deficient mice have reduced weight and liver triacylglycerol content and altered glycerolipid fatty acid composition. Mol Cell Biol 22: 8204–8214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond LE, Neschen S, Romanelli AJ, Cline GW, Ilkayeva OR, Shulman GI, Muoio DM, Coleman RA (2005) Mitochondrial glycerol-3-phosphate acyltransferase-1 is essential in liver for the metabolism of excess acyl-CoAs. J Biol Chem 280: 25629–25636 [DOI] [PubMed] [Google Scholar]
- Haucke V, Di Paolo G (2007) Lipids and lipid modifications in the regulation of membrane traffic. Curr Opin Cell Biol 19: 426–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Stockl A, Schlame M, Beyer K, Klingenberg M (1994) The reconstituted ADP/ATP carrier activity has an absolute requirement for cardiolipin as shown in cysteine mutants. J Biol Chem 269: 1940–1944 [PubMed] [Google Scholar]
- Hoppins S, Nunnari J (2009) The molecular mechanism of mitochondrial fusion. Biochim Biophys Acta 1793: 20–22 [DOI] [PubMed] [Google Scholar]
- Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA (2011) piRNA-associated germline nuage formation and spermatogenesis require MitoPLD pro-fusogenic mitochondrial-surface lipid signaling. Dev Cell 20: 376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJA, Greenstein D (2005) Introduction to the germ line. In Worm book The C. elegans Research Community (ed) WormBook, doi/10.1895/wormbook.1.18.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichishita R, Tanaka K, Sugiura Y, Sayano T, Mihara K, Oka T (2008) An RNAi screen for mitochondrial proteins required to maintain the morphology of the organelle in Caenorhabditis elegans. J Biochem 143: 449–454 [DOI] [PubMed] [Google Scholar]
- Imae R, Inoue T, Kimura M, Kanamori T, H.Tomioka N, Kage-Nakadai E, Mitani S, Arai H (2010) Intracellular phospholipase A1 and acyltransferase, which are involved in Caenorhabditis elegans stem cell divisions, determine the sn-1 fatty acyl chain of phosphatidylinositol. Mol Biol Cell 21: 3114–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara N, Eura Y, Mihara K (2004) Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci 117: 6535–6546 [DOI] [PubMed] [Google Scholar]
- Ishihara N, Fujita Y, Oka T, Mihara K (2006) Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J 25: 2966–2977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, Ryan MT, Schlame M, Zhao M, Gu Z, Klingenberg M, Pfanner N, Greenberg ML (2000) Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J Biol Chem 275: 22387–22394 [DOI] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ (2006) Role of Bax and Bak in mitochondrial morphogenesis. Nature 443: 658–662 [DOI] [PubMed] [Google Scholar]
- Kent C (1995) Eukaryotic phospholipid biosynthesis. Annu Rev Biochem 64: 315–343 [DOI] [PubMed] [Google Scholar]
- Kimble J, Crittenden SL (2005) Germline proliferation and its control. In Worm book The C. elegans Research Community (ed) WormBook, doi/; DOI: 10.1895/wormbook.1.13.1, http://www.wormbook.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrousse AM, Zappaterra MD, Rube DA, van der Bliek AM (1999) C. elegans dynamin-related protein DRP-1 controls severing of the mitochondrial outer membrane. Mol Cell 4: 815–826 [DOI] [PubMed] [Google Scholar]
- Lee HC, Inoue T, Imae R, Kono N, Shirae S, Matsuda S, Gengyo-Ando K, Mitani S, Arai H (2008) Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol Biol Cell 19: 1174–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin TM, Schwerbrock NM, Lee DP, Coleman RA (2004) Identification of a new glycerol-3-phosphate acyltransferase isoenzyme, mtGPAT2, in mitochondria. J Biol Chem 279: 13488–13495 [DOI] [PubMed] [Google Scholar]
- Nagle CA, Vergnes L, Dejong H, Wang S, Lewin TM, Reue K, Coleman RA (2008) Identification of a novel sn-glycerol-3-phosphate acyltransferase isoform, GPAT4, as the enzyme deficient in Agpat6-/- mice. J Lipid Res 49: 823–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang WM, Choi CS, Coleman RA, Shulman GI (2005) Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA: glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell Metab 2: 55–65 [DOI] [PubMed] [Google Scholar]
- Neuwald AF (1997) Barth syndrome may be due to an acyltransferase deficiency. Curr Biol 7: R465–R466 [DOI] [PubMed] [Google Scholar]
- Okamoto K, Shaw JM (2005) Mitochondrial morphology and dynamics in yeast and multicellular eukaryotes. Annu Rev Genet 39: 503–536 [DOI] [PubMed] [Google Scholar]
- Pitt JN, Schisa JA, Priess JR (2000) P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev Biol 219: 315–333 [DOI] [PubMed] [Google Scholar]
- Rojo M, Legros F, Chateau D, Lombès A (2002) Membrane topology and mitochondrial targeting of mitofusins, ubiquitous mammalian homologs of the transmembrane GTPase Fzo. J Cell Sci 115: 1663–1674 [DOI] [PubMed] [Google Scholar]
- Rolland SG, Lu Y, David CN, Conradt B (2009) The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1- dependent mitochondrial fusion. J Cell Biol 186: 525–540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santel A, Frank S, Gaume B, Herrler M, Youle R, Fuller MT (2003) Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. J Cell Sci 116: 2763–2774 [DOI] [PubMed] [Google Scholar]
- Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874 [DOI] [PubMed] [Google Scholar]
- Sesaki H, Jensen RE (1999) Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J Cell Biol 147: 699–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijen T, Fleenor J, Simmer F, Thijssen KL, Parrish S, Timmons L, Plasterk RHA, Fire A (2001) On the role of RNA amplification in dsRNA-triggered gene silencing. Cell 107: 465–476 [DOI] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM (2001) Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Shurland DL, Ryazantsev SN, van der Bliek AM (1998) A human dynamin-related protein controls the distribution of mitochondria. J Cell Biol 143: 351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K (2007) Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 282: 11521–11529 [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Reue K (2009) Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab 296: E1195–E1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ (2010) Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol 191: 1367–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meeteren LA, Moolenaar WH (2007) Regulation and biological activities of the autotaxin-LPA axis. Prog Lipid Res 46: 145–160 [DOI] [PubMed] [Google Scholar]
- Vergnes L, Beigneux AP, Davis R, Watkins SM, Young SG, Reue K (2006) Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J Lipid Res 47: 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendel AA, Lewin TM, Coleman RA (2009) Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim Biophys Acta 1791: 501–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B (2008) Molecular machinery of mitochondrial fusion and fission. J Biol Chem 283: 13501–13505 [DOI] [PubMed] [Google Scholar]
- Xu Y, Aoki J, Shimizu K, Umezu-Goto M, Hama K, Takanezawa T, Yu S, Mills GB, Arai H, Qian L, Prestwich GD (2005) Structure-activity relationships of fluorinated lysophosphatidic acid analogues. J Med Chem 48: 3319–3327 [DOI] [PubMed] [Google Scholar]
- Yazdi M, Ahnmark A, William-Olsson L, Snaith M, Turner N, Osla F, Wedin M, Asztely AK, Elmgren A, Bohlooly YM, Schreyer S, Linden D (2008) The role of mitochondrial glycerol-3-phosphate acyltransferase-1 in regulating lipid and glucose homeostasis in highfat diet fed mice. Biochem Biophys Res Commun 369: 1065–1070 [DOI] [PubMed] [Google Scholar]
- Ye X, Chun J (2010) Lysophosphatidic acid (LPA) signaling in vertebrate reproduction. Trends Endocrinol Metab 21: 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Guan Z, Murphy AN, Wiley SE, Perkins GA, Worby CA, Engel JL, Heacock P, Nguyen OK, Wang JH, Raetz CRH, Dowhan W, Dixon JE (2011) Mitochondrial phosphatase PTPMT1 is essential for cardiolipin biosynthesis. Cell Metab 13: 690–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.