Abstract
The innate immune cell network detects specific microbes and damages to cell integrity in order to coordinate and polarize the immune response against invading pathogens. In recent years, a cross-talk between microbial-sensing pathways and endoplasmic reticulum (ER) homeostasis has been discovered and have attracted the attention of many researchers from the inflammation field. Abnormal accumulation of proteins in the ER can be seen as a sign of cellular malfunction and triggers a collection of conserved emergency rescue pathways. These signalling cascades, which increase ER homeostasis and favour cell survival, are collectively known as the unfolded protein response (UPR). The induction or activation by microbial stimuli of several molecules linked to the ER stress response pathway have led to the conclusion that microbe sensing by immunocytes is generally associated with an UPR, which serves as a signal amplification cascade favouring inflammatory cytokines production. Induction of the UPR alone was shown to promote inflammation in different cellular and pathological models. Here we discuss how the innate immune and ER-signalling pathways intersect. Moreover, we propose that the induction of UPR-related molecules by microbial products does not necessarily reflect ER stress, but instead is an integral part of a specific transcription programme controlled by innate immunity receptors.
Keywords: dendritic cells, GADD34, interferon, PKR, TLR
Introduction
Cells are constantly subjected to diverse stresses such as nutrient deprivation, radiation, oxidative stress and also infection by microbial pathogens that can lead to damage and cell death. Cells have therefore evolved different mechanisms to cope with these exogenous stresses. As protein synthesis is a fundamental cell function, the control of mRNA translation plays a central role in most stress responses. mRNA translation can be divided into three phases: initiation, elongation and termination. Although all phases are subject to regulatory mechanisms, initiation is regarded as the rate-limiting step (Holcik and Sonenberg, 2005). Much of this control involves different post-translational modifications of initiation factors. Among them, phosphorylation of the α subunit of the eukaryotic protein synthesis initiation factor 2 (eIF2α) provided one of the first examples of the control of eukaryotic protein synthesis by protein phosphorylation (Proud, 2005). This mechanism of protein translation control is triggered by diverse stresses and is conserved from budding yeast to higher mammals (Proud, 2005).
Phosphorylation of eIF2α at serine 51 by eIF2α kinases abolishes the formation of the translation initiation ternary complex (eIF2α/GTP/methionyl tRNA) by inhibiting the GTP exchange factor eIF2B (Holcik and Sonenberg, 2005), leading to translation initiation suppression and promotion of a specific transcriptional response (Harding et al, 2003). Mice bearing a homozygous mutation (S51A) at serine 51 residue die within the first day after birth from severe hypoglycemia, resulting from low plasma insulin levels (Scheuner et al, 2001). These results suggest that aberrant eIF2α phosphorylation, resulting from malfunction or misregulation of eIF2α kinases and phosphatases, could play a role in different cellular pathologies.
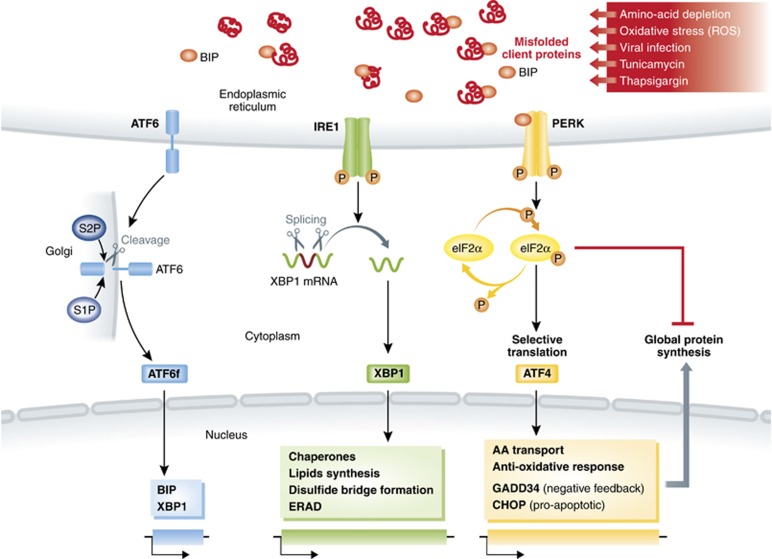
In recent years, the regulation of eIF2α phosphorylation has been implicated in biological processes as diverse as synaptic plasticity, inflammation and metabolic diseases (Deng et al, 2004; Nakamura et al, 2010; Tabas and Ron, 2011; Trinh et al, 2012). Most of the available biochemical and genomic data about eIF2α biology were obtained during the study of the unfolded protein response (UPR) in the endoplasmic reticulum (ER) (Ron and Walter, 2007). The ER is an essential cellular compartment for the synthesis and folding of secreted and transmembrane proteins. Only correctly folded proteins are exported to the Golgi apparatus (Schröder and Kaufman, 2005; Ron and Walter, 2007; Yoshida, 2007). This organelle is also responsible for intracellular calcium homeostasis and lipid biosynthesis, which are required for cell survival and normal cellular functions. Certain environmental conditions induce the accumulation of misfolded/unfolded proteins leading to ER stress. In cells of higher eukaryotes, three major signalling cascades, commonly known as the UPR, connect ER stress detection with the regulation of the transcriptional and translational machineries (Schröder and Kaufman, 2005; Ron and Walter, 2007) (Figure 1). (1) IRE1 (inositol-requiring enzyme 1) cleaves the mRNA encoding for the transcription factor X-box-binding protein-1 (XBP1) (Yoshida et al, 2001; Ron and Walter, 2007). XBP1 activates the expression of a large number of genes regulating ER homeostasis and involved in protein folding, disulphide bond formation, lipid biosynthesis or ER-associated degradation (ERAD) such Bip or ERdj4 (Yoshida et al, 2001; Lee et al, 2003, 2008). (2) Upon ER stress, activating transcription factor 6 (ATF6) is transported to the Golgi and is processed into an active transcription factor (Haze et al, 1999; Chen et al, 2002; Shen et al, 2002). After nuclear translocation, ATF6 induces the transcription of ER chaperone genes, such as Bip, again and several major targets of the mammalian UPR (Haze et al, 1999), including Xbp1 (Wang et al, 2000). (3) PERK (protein kinase RNA (PKR)-like ER kinase) is a kinase that phosphorylates eIF2α inhibiting the flux of neo-synthetized proteins and activating the expression of the transcription factor ATF4, and its downstream targets, including the pro-apoptotic transcription factor C/EBP homologous protein (CHOP/GADD153) and, the growth arrest and DNA-damage-inducible protein 34 (GADD34, also known as PPP1R15a or Myd116), a phosphatase 1 cofactor that functions as a negative-feedback regulator of eIF2α phosphorylation.
Figure 1.
Schematic description of the unfolded-protein response (UPR). Misfolded protein accumulation in the ER activates three distinct sensors: ATF6, inositol-requiring transmembrane kinase/endonuclease 1 (IRE1) and pancreatic ER kinase (PERK). ER stress can be induced by stressors, such as ROS, leading to IRE1-dependent XBP1 mRNA splicing and translation. XBP1 nuclear translocation drives the transcriptional activation of multiple genes involved in ER and molecular chaperones homeostasy. Other UPR transcription factors, such as ATF4, and CHOP are induced upon eIF2α phosphorylation by PERK, which also inhibits translation initiation. The phosphatase 1 cofactor, GADD34, functions in a negative-feedback loop driven by ATF4, which dephosphorylates eIF2α and restores protein synthesis upon stress relief.
Innate sensing is the first line of defense against pathogens and is necessary for efficient activation of adaptive immunity. Microbes detection is mediated by pattern recognition receptors (PRRs), which detect conserved structures of pathogens called pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharides (LPS) or nucleic acids. Toll-like receptors (TLRs) are the most characterized PRRs and their triggering results in the induction of multiple signalling cascades that lead to the expression of genes involved in shaping specific immune responses against infectious pathogens (Kawai and Akira, 2010). Similarities have recently been noted in signalling pathways stemming from innate immune and ER-stress-signalling pathways (Zhang and Kaufman, 2008). Both IRE1 and TLRs can trigger antimicrobial response and engage molecular adaptors to trigger inflammatory responses through NF-kB or mitogen-activated protein kinase activation (Urano et al, 2000; Martinon et al, 2010). Moreover, results from experiments investigating the molecular connections between microbe-sensing and protein synthesis regulation, and in particular the role of GADD34 in this process (Clavarino et al, 2012a, 2012b), indicate that although several key genes including ATF4, CHOP and GADD34 are upregulated both by microbial detection and ER stress, these gene expression programmes are part of distinct transcriptional responses. We propose that this stress response, which is embedded within the larger innate defense gene expression signature driven by microbe recognition, should be considered as microbe specific and not merely a reflection of accentuated ER stress (Leber et al, 2008; Seimon et al, 2010; Hetz, 2012) (Table I).
Table 1. Characteristics of the UPR and the MSR.
| UPR | MSR |
|---|---|
| Induced in response to unfolded protein accumulation in the ER. UPR counteracts the harmful effect of unfolded proteins and promotes ER homeostasis (Schröder and Kaufman, 2005; Ron and Walter, 2007). | Activated in response to PAMPs. MSR counteracts the physiological consequences of infection or microbial detection, while promoting immune defenses (Woo et al, 2009; Goodall et al, 2010; Martinon et al, 2010; Clavarino et al, 2012a, 2012b) |
| UPR comprises three different signal cascades, including the PERK/ATF4, ATF6 and IRE1/XBP1 pathways (Schröder and Kaufman, 2005; Ron and Walter, 2007). | MSR comprises at least the TRIF/ATF4 or PKR/ATF4 pathways, mostly without CHOP protein expression and can display some IRE1 activation in specific cells types (Clavarino et al, 2012a, 2012b) |
| Upregulation of Xbp1, Bip, Ero1, ERdj4 and p58IPK, as well as other ER chaperones, and induction of protein degradation pathways. CHOP transcription and synthesis are strongly induced (Lee et al, 2003; Schröder and Kaufman, 2005; Ron and Walter, 2007). Atf3 and Gadd34 are strongly induced and ATF4 is synthetized. | No upregulation of Bip, Ero1, ERdj4 and p58IPK. Cell-type-dependent limited upregulation of Xbp1 (Martinon et al, 2010). CHOP induction is limited both transcriptionaly and translationaly. Atf3 and Gadd34 are strongly induced and ATF4 is synthetized. |
| Translation is temporarily arrested, but is reinitiated upon eIF2α dephosphorylation by GADD34 (Schröder and Kaufman, 2005; Ron and Walter, 2007). | Protein translation activity is induced and can be regulated independently of eIF2α dephosphorylation (Clavarino et al, 2012a, 2012b). |
| Potential role in sterile inflammatory cytokine transcription, but no demonstrated action on type-I IFN expression (Deng et al, 2004; Hsu et al, 2004). | The ATF4/GADD34 axis regulates cytokine expression both transcriptionally and translationally in a PKR- or TRIF-dependent manner. Correct type-I IFN expression requires GADD34 and XBP1 (Clavarino et al, 2012a, 2012b; Martinon et al, 2010). |
| Can be ROS dependent (Hetz, 2012). | Can be ROS independent (Li et al, 2010; Martinon et al, 2010). |
The negative-feedback-signalling loop of the UPR
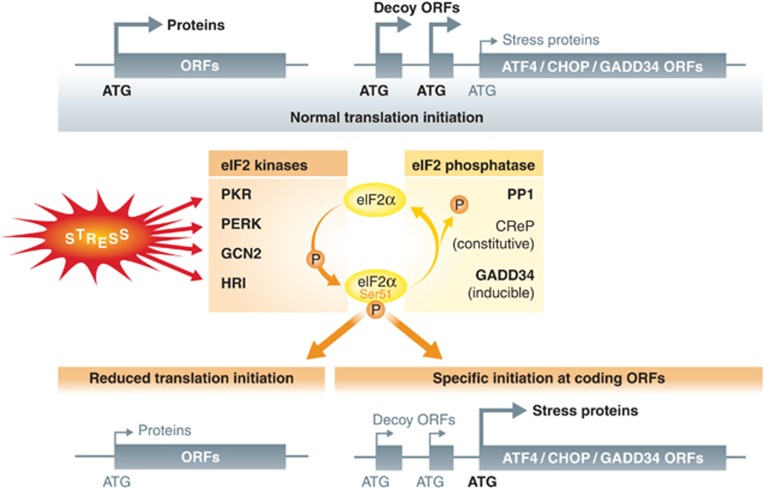
Mammalians possess four different eIF2α kinases (Holcik and Sonenberg, 2005; Proud, 2005; Wek et al, 2006): PKR, PERK, GCN2 (general control non-derepressible-2) and HRI (haem-regulated inhibitor). All of them act via phosphorylation of serine 51 of eIF2α, thereby limiting global protein synthesis (Figure 2). Each kinase is associated with the response to different kinds of stress. HRI is activated under conditions of low haem, as well as by oxidative, osmotic or heat shock (Han et al, 2001). PERK is mostly activated in response to ER stress, through the quenching of the ER-resident HSP70 chaperone BiP by an excess of misfolded client proteins (Figure 1) (Harding et al, 2000b). GCN2 is activated in response to amino-acid starvation and UV irradiation (Berlanga et al, 1999; Deng et al, 2002). GCN2, which mostly senses unloaded tRNAs, has also been reported to play a role in defense against RNA and DNA viruses in vitro and in vivo (Berlanga et al, 2006; Won et al, 2012). Similarly, the type-I interferon (IFN)-inducible PKR exerts an antiviral activity through its activation by double-stranded RNA (Williams, 2001; Dabo and Meurs, 2012), but is also regulated by cellular cofactors such as p58IPK, ribosomal protein L18, the TAR RNA-binding protein (TRBP) and the PKR activator (PACT) (Daher et al, 2009).
Figure 2.
Schematic description of eIF2a phosphorylation pathway. Upon stress sensing, four known different eIF2α kinases, PKR, PERK, GCN2 and HRI, act via phosphorylation of serine 51 of eIF2α to limit global protein synthesis. GADD34 (PPP1R15a) and CReP (PPP1R15b) are regulatory subunits of PP1 that promote eIF2α dephosphorylation and counteract eIF2α kinases activity. Under normal conditions, ATF4, GADD34 and CHOP mRNA translation is repressed by competition for translation initiation of several short open reading frames (decoy ORFs) located upstream and frame shifted from the true translation initiation site. Upon phosphorylation of eIF2α, translation can now initiate at the AUG of the downstream coding regions allowing synthesis of these molecules during stress-induced protein synthesis inhibition.
Thus, viral infection or accumulation of misfolded proteins can result in a sustained eIF2α phosphorylation by PKR, GCN2 or PERK, which can become lethal if prolonged (Srivastava et al, 1998). Cells must therefore tightly regulate the level of phosphorylated eIF2α in order to survive and carry on with their function (Tabas and Ron, 2011). GADD34 (PPP1R15a) and the constitutive repressor of eIF2α phosphorylation CReP (PPP1R15b) are regulatory subunits of protein phosphatase 1 (PP1) that promote the dephosphorylation of eIF2α (Connor et al, 2001; Novoa et al, 2001, 2003; Jousse et al, 2004). CReP contributes to a basal level of eIF2α dephosphorylation (Jousse et al, 2004), while GADD34 negatively controls eIF2α phosphorylation during the UPR and other stress, including viral infection (Clavarino et al, 2012a). GADD34 expression is mostly dependent on ATF4 (Brush et al, 2003; Ma and Hendershot, 2003; Novoa et al, 2003), which binds to a conserved consensus site in the promoter region of the GADD34 gene and induces its transcription (Ma and Hendershot, 2003). GADD34 transcription is also known to involve other transcription factors, such as the pro-apoptotic CHOP, ATF3 (Jiang et al, 2004; Marciniak et al, 2004) and potentially ATF6, whose proteolytic activation contributes to the UPR. GADD34 expression and its eIF2α phosphatase activity are therefore critical to determine cellular fate following various forms of stress (Harding et al, 2003) and to define a biochemical response commensurate to stress intensity and duration.
During stress, eIF2α phosphorylation and its inhibitory impact on translation initiation are essential to promote ATF4 synthesis (Figure 2). Under normal conditions, ATF4 mRNA translation is repressed by competition for translation initiation of several short open reading frames (uORFs) located upstream and frame shifted from the true translation initiation site. These uORFs are translated by ribosomes, which generally initiate translation on the first available AUG codon placed in the right neighbouring nucleotide sequence context. As a consequence, the last downstream ORF that encodes ATF4 is only translated at low levels, if at all. During stress conditions, phosphorylation of eIF2α and the accompanying reduction in the levels of eIF2α-GTP increase the time required for the scanning ribosomes to become competent to initiate translation. This delay allows the ribosomes to scan through the uORFs and initiate at the AUG of the downstream ATF4-coding region allowing full translation of this transcription factor (Holcik and Sonenberg, 2005). This in turn promotes the transcription of the downstream target genes CHOP and GADD34 (Lee et al, 2009; Palam et al, 2011) that share a similar upstream uORF-competition translation mechanism (Lee et al, 2009; Palam et al, 2011) (Figure 2).
Importantly, most of our current knowledge on ATF4 and GADD34 was obtained by investigating their role during artificial induction of the UPR. Recent work from our laboratory, however, indicates that ATF4 and GADD34 induction are also important components of antimicrobial responses, although the modalities of their expression upon infection are clearly distinct from the ‘classical’ UPR.
Phosphorylation of eIF2α and viral detection
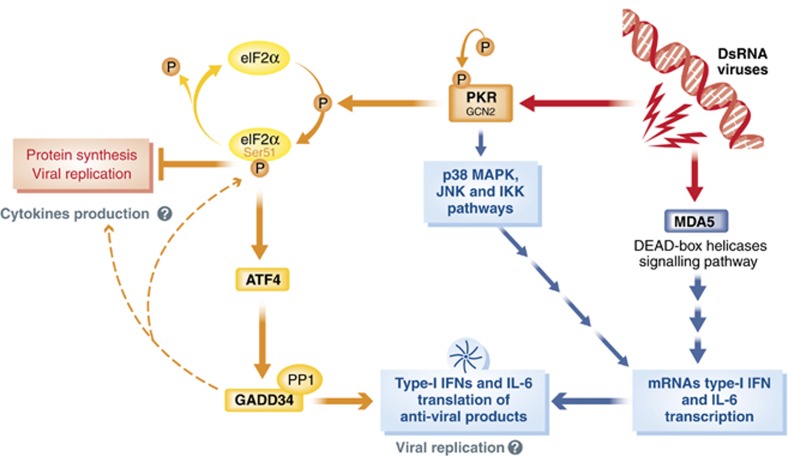
In addition to ER stress, virus infection and dsRNA also induce eIF2α phosphorylation via PKR to inhibit cellular protein synthesis and viral replication (Dabo and Meurs, 2012). During their replication, RNA and DNA viruses generate RNA intermediates that elicit antiviral responses mostly through type-I IFN production (Kawai and Akira, 2006; Pichlmair and Reis e Sousa, 2007). In addition to PKR, several families of proteins are known to sense dsRNA and trigger IFN release, including endocytic TLR3 (Alexopoulou et al, 2001) and several cytosolic DEAD-box RNA helicases, such as MDA5 (Yoneyama and Fujita, 2007; Loo and Gale, 2011) (Figure 3). Type-1 IFN binding to cell surface receptors leads to activation of the Janus tyrosine kinase pathway, which induces the expression of a wide spectrum of IFN-stimulated genes, including PKR itself, which participates in the cellular defense against viral infection (Williams, 1999). PKR mediates phosphorylation of eIF2α, leading to inhibition of translation and triggering of apoptosis (Williams, 1999, 2001; Dabo and Meurs, 2012). Initial analysis revealed that the PKR−/− mice (129terSv × C57/BL6) are healthy and presented normal antiviral responses after intravascular inoculation of EMCV or Vaccinia virus (Yang et al, 1995; Abraham et al, 1999). However, differences in genetic backgrounds that may compensate for the PKR deficiency were later observed, and PKR-deficient mice in the 129terSv × BALB/c background died due to intranasal infection with Vesicular Stomatitis Virus (VSV). The PKR−/− mice also showed increased susceptibility to influenza virus infection (Balachandran et al, 2000).
Figure 3.
Schematic description of dsRNA and viral sensing in the cytosol. During their replication, viruses generate RNA intermediates, which are sensed by several cytosolic DEAD-box RNA helicases, such as MDA5, which signal to promote type-I IFN production, through a cascade of adaptors leading to IRF3 phosphorylation and nuclear translocation. Concomitantly upon dsRNA sensing, PKR or GCN2 autophosphorylate and mediates phosphorylation of eIF2α, leading to inhibition of translation, while activating other signalling pathways promoting cytokines expression (e.g., p38 and JNK). The large production of inflammatory cytokines and antiviral factors, despite this rapid and efficient concomitant shut down of cellular protein synthesis, implies the existence of specific regulatory mechanisms allowing the translation of host antiviral mRNAs during eIF2α phosphorylation. ATF4 and GADD34 are necessary to allow cytokine translation.
Independently of PKR detection, some viruses use the ER as a site of replication, which can lead to the activation of ER stress and PERK (Cheng et al, 2005). The GCN2 and PERK eIF2α-kinases can thus phosphorylate eIF2α upon viral detection (Won et al, 2012), and like PKR, limit infection by preventing viral replication and inducing apoptosis in contaminated cells. These three eIF2α kinases can therefore contribute to a powerful antiviral pathway (Langland et al, 2006; Domingo-Gil et al, 2011). Consequently, many viruses have evolved strategies to ensure completion of their infection cycle and efficient spreading, by escaping host translation shut-down, for example through antagonizing PKR (Schneider and Mohr, 2003). Some viruses have thus acquired factors homologous to GADD34 containing PP1-activating motives to promote eIF2α dephosphorylation (Cruz et al, 2011). The protein ICP34.5 of herpes simplex virus 1 (HSV-1) enables HSV-1 to escape the inhibitory effect of both PKR and PERK activation (He et al, 1997, 1998; Cheng et al, 2005), via its C-terminal domain, which mimics the C-terminal region of GADD34. Functional homology was established by domain-swapping experiments demonstrating that the infectivity of an ICP34.5-deleted mutant of HSV-1 could be rescued with the PP1-interacting domain of GADD34 (Chou and Roizman, 1990, 1994; Zhan et al, 1994). The current list of identified GADD34 viral homologues include DP71L from African swine fever virus (ASFV) (Zsak et al, 1996), Gene 7 of the transmissible gastroenteritis virus (TGEV) (Malathi et al, 2007; Cruz et al, 2011) and by the human papillomavirus (HPV) type 18 E6 oncoprotein (Kazemi et al, 2004).
Unresolved issues concerning innate immunity and eIF2α phosphorylation
Although the antiviral role of mammalian eIF2α kinases is well established, several ambiguities have to be resolved to fully understand the integration of antiviral host cell responses within the more complex systemic immune responses. First, the large production of inflammatory cytokines and antiviral factors, including type-I IFN, despite a rapid and efficient shut down of cellular protein synthesis in infected cells, implies the existence of specific regulatory mechanisms allowing the translation of host antiviral mRNAs during PKR-dependent eIF2α phosphorylation. Conversely, if one applies to viral infection, the biochemical model built using the observations drawn from PERK activation and ATF4 production during the UPR, phosphorylation of eIF2α in response to direct dsRNA-dependent activation of PKR, should lead to a rapid ATF4 and GADD34 induction in infected cells (Figure 3). GADD34 will, in turn, promote eIF2α dephosphorylation, which in the particular context of viral infection should antagonize PKR and promote viral replication, in a similar fashion to what is observed for ICP34.5 and other GADD34 viral homologues. Thus, based on the UPR model, eIF2α phosphorylation-mediated GADD34 induction in infected cells would be counter productive for the host. Dendritic cells (DCs) and macrophages, which are key for the initiation of the immune response, are equipped with a broad array of these microbial PRRs (Kawai and Akira, 2011) and are able to detect and control a large variety of pathogens. In these cells, bacterial LPS detection by TLR4 (Hsu et al, 2004; Nakamura et al, 2010) and exposure to inflammasome agonists induce PKR autophosphorylation (Figure 4) and activation (Lu et al, 2012). PKR deficiency in these stimulated cells significantly inhibits the expression of cytokines, like type-I IFN (Diebold et al, 2003), and prevents the secretion of IL-1β, IL-18 and HMGB1 (Lu et al, 2012). However, despite PKR activation, protein synthesis is enhanced and not inhibited in TLR-stimulated cells (Hsu et al, 2004; Lelouard et al, 2007; Clavarino et al, 2012b), indicating that differently from the UPR, microbial activation of eIF2α kinases is not always associated with translational arrest and increased phosphorylation of eIF2α (Goldfinger et al, 2011). These observations can be also extended to plasma cells stimulated with LPS, although in these cells, a precise molecular dissection of the pathways involved is made more complex by the exhibition of a chronic ER stress due to their massive immunoglobulin secretory activity (Cenci et al, 2006; Goldfinger et al, 2011). Some clues to these yet unresolved issues could be given by the surprising observation that upon dsRNA sensing and viral infection, GADD34 is necessary to ensure efficient inflammatory cytokines production both at the transcriptional and translational level (Clavarino et al, 2012a, 2012b), thereby unravelling novel and important features of this molecule during infection and the initiation of innate immunity.
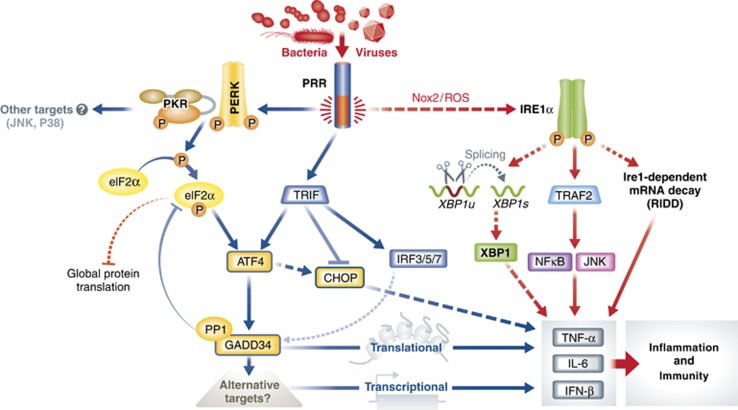
Figure 4.
Schematic description of the MSR. During the MSR, microbes- and virus-associated molecular patterns (e.g., LPS) are sensed directly or indirectly by different receptors, such as Toll-like-receptors (TLRs), Rig I-like receptors (RLRs) or the dsRNA-sensing kinase (PKR), which through complex signalling cascades, involving the TRIF adaptor and different TRAF ubiquitin ligase, leads to the nuclear translocation of NFkB or IRF-3, and subsequent IFN-I and inflammatory cytokines transcription. Microbial detection leads also to GADD34 expression, which, however, in this context has little effect on controlling global translation, while participates in the regulation of cytokine production both at the translational and transcriptional level. During the MSR, XBP1 splicing levels varies greatly according to cell models and microbe stimulus used; however, a striking distinctive feature of this pathway is the translational inhibition of CHOP synthesis, together with enhanced level of eIF2α de-phosphorylation, GADD34 and expression. GADD34, ATF4 and XBP1 are likely to favour the expression of cytokines through the targeting of yet undefined partners at the translational, signal transduction and transcriptional level. Cross-talks between the UPR and MSR clearly exist, and the direct activation of the TRAF2 or RIDD pathway by IRE1 and subsequent inflammatory cytokines transcription could be an example of those commonalities.
GADD34, a novel player in the cellular antiviral response
In cells not expressing TLR3, GADD34 is strongly induced upon cytosolic delivery and detection of the synthetic dsRNA analogue polyriboinosinic:polyribocytidylic acid (poly I:C) (Figure 3). GADD34 expression obeys the UPR/PERK paradigm during which phosphorylation of eIF2α by its cognate kinase induces translation inhibition while favouring ATF4 synthesis and subsequent GADD34 expression (Clavarino et al, 2012a). As expected, GADD34 induction in response to cytosolic poly I:C is PKR- and ATF4-dependent, and triggers the negative control loop of eIF2α dephosphorylation, despite the continuous presence of the dsRNA stimulus and steadily increased PKR activation. Conversely, in GADD34-deficient cells, eIF2α phosphorylation is strongly increased in response to poly I:C, demonstrating the functionality of the PP1 cofactor in this system. Apparently, this biochemical cascade is closely related to what is observed during the UPR. However, conversely to the drug-induced UPR during which translation is only profoundly inhibited for few hours prior full recovery, cytosolic poly I:C induces a near to complete and irreversible protein synthesis extinction within 8 h of cytosolic delivery, despite a rapid and concomitant induction of GADD34 and eIF2α dephosphorylation (Clavarino et al, 2012a). Although eIF2α phosphorylation and PKR are required for the initiation of protein synthesis inhibition, this process becomes rapidly eIF2α-independent and, surprisingly, GADD34 inactivation has no impact on neither the intensity nor the speed of translation loss. This observation contrasts with the UPR, during which functional GADD34 is absolutely required to prevent total and rapid protein synthesis inhibition in response to the PERK-activating drug thapsigargin. These observations clearly show that although eIF2α phosphorylation and GADD34 expression represent common consequences of PERK and PKR activation, their impact on the cell physiology are absolutely not equivalent. The functional importance of GADD34 induction in response to dsRNA remained unclear, until it was demonstrated that GADD34-deficient cells were unable to produce type-I IFN and IL-6 proteins in response to poly I:C or Chikungunya Virus (ChikV) infection, despite close to normal mRNA induction (Clavarino et al, 2012a).
At the mechanistic level, these observations point directly to a role of GADD34 in controlling the translation of specific mRNAs upon PKR-dependent eIF2α phosphorylation and global translation repression, which is prolonged by yet other undefined factors than eIF2α phosphorylation itself. Interestingly, the synthesis of PKR does not seem inhibited in any of these situations, suggesting that some mRNAs are insensitive to the translation repression exerted by poly I:C detection and do not absolutely depend on GADD34 for their synthesis. However, apart from IFN-β and IL-6, the identity of the mRNAs dependent on GADD34 for their translation is still ill defined. This list could encompass messengers translated specifically by ER-associated polysomes, such as those coding for secreted and membrane-associated proteins expressed after microbial detection. This possibility infers to the existence of a compartment-specific regulation of mRNA translation, enabling controlled synthesis of selected proteins possibly at specific cellular locations in a globally repressed environment. The existence of such segregation has been proposed for ER-associated translation during the UPR by Nicchita and collaborators (Stephens et al, 2005). This compartmentalization of protein synthesis might possibly be extremely important during PKR-dependent responses, and GADD34 could have a qualitative role on the selection of mRNAs being translated during viral infections. The activity of GADD34 viral homologues, such as ICP34.5, might therefore be more subtle, than merely counteracting PKR, and could influence the translation of specific messengers, leaving others untouched. Consequently, the importance of GADD34 for cytokines production makes this molecule a novel actor in the antiviral arsenal, with a role particularly obvious in neonates mice, that are exquisitely sensitive to ChikV infection and die of myocarditis in the absence of functional GADD34 (Clavarino et al, 2012a).
Specificity of the innate immunity-sensing pathways
Independently of direct cellular infection by pathogens, activation of innate cells by microbial products is a key event in the initiation of a productive immune response. Interestingly, TLR stimulations by LPS or other microbial agonists, such as soluble poly I:C, result in the activation of multiple signalling cascades resulting in inflammatory cytokines and type-I IFN production. In macrophages, PKR has been shown to be activated downstream of TLR4 and TLR3 in a TIR-domain-containing adaptor-inducing IFN-β (TRIF)-dependent manner (Hsu et al, 2004) and to participate to the induction of type-1 IFN in macrophages and DCs (Diebold et al, 2003; Hsu et al, 2004). Interestingly, prior to PKR activation, non-activated DCs display extremely high level of eIF2α phosphorylation both in vitro and in vivo (Clavarino et al, 2012b). This unconventional eIF2α phosphorylation pattern may reflect special needs of DCs for translational regulation to fully exert their function (Lelouard et al, 2007; Ceppi et al, 2009). PKR activation in response to TLR triggering was found not to increase further the levels of phosphorylated eIF2α (P-eIF2α), which are even decreased rapidly upon microbial products detection (Hsu et al, 2004; Lelouard et al, 2007; Ceppi et al, 2009; Goldfinger et al, 2011; Clavarino et al, 2012b). In DCs, eIF2α dephosphorylation was shown to be mediated by GADD34 induction, which occurs together with ATF4 synthesis during TLR-dependent LPS or poly I:C detection (Clavarino et al, 2012b). This type of transcriptional response has been also observed in macrophages infected with L. monocytogenes or Mycobacterium tuberculosis during which transcription of Chop, Gadd34 and activating transcription factor 3 (Atf3) are also strongly induced (Leber et al, 2008; Seimon et al, 2010). Importantly ATF3, in addition to PAMPs detection, is induced by many forms of stress, including tunicamycin and tapsigargin treatments (Mungrue et al, 2009). Atf3−/− animals exhibit no obvious developmental phenotypes, only when the mice are challenged with LPS do they display significantly elevated IL-6 and IL-12 serum levels compared to wild-type controls, suggesting that ATF3 negatively regulates pro-inflammatory cytokine production (Thompson et al, 2009).
Gadd34 together with Atf3 and Atf4 should therefore be considered as TLR-induced genes, and the signal transduction pathways leading to their expression seem different from what is classically observed during the UPR. Interestingly, recent work on the signalling cascades induced in DCs by West Nile virus infection suggests that GADD34 represents a key signature gene associated to the triggering of RLRs and of mitochondria-associated adapter molecule (MAVS, also called IPS-1, VISA or CARDIF), and could depend on IRF5 translocation for its expression (Lazear et al, 2013). This view is confirmed by the direct comparison of the mRNA transcription signatures obtained from cells exposed to tunicamycin treatment or poly I:C exposure, which are clearly different, and contain only few co-regulated genes, including ATF3, ATF4 and GADD34 (Clavarino et al, 2012b) (Table I). Expression of ATF4 and several of its downstream targets, including GADD34, normally requires increased eIF2α phosphorylation to allow their translation at the right initiation codon (Harding et al, 2000a) (Figure 2). At an early stage of DC activation, the level of eIF2α phosphorylation could be high enough to permit the synthesis of ATF4 and GADD34 immediately upon transcriptional induction. It is, however, striking that ATF4 is not synthetized in non-stimulated cells, which display massive eIF2α phosphorylation, and that it solely accumulates in activated DCs, mostly concomitant with GADD34-dependent eIF2α dephosphorylation (Clavarino et al, 2012b). Moreover, GADD34 and ATF4 expressions are occurring normally in PKR-deficient DCs, which display, upon activation, much lower levels of eIF2α phosphorylation than their normal counterparts. Thus, ATF4 and GADD34 expression are part of a specific response to pathogens and their function seems to have other purposes than solely participating in ER homeostasis and global translation regulation during stress (Figure 4). In fact, it has been recently proposed that IFN regulatory factor (IRF) 7, a master regulator of type-I IFN gene expression, upregulates ATF4 activity and expression, whereas ATF4 in return inhibits IRF7 activation, suggesting a cross-regulation between the IFN response and a participation of ATF4 in a negative-feedback loop of the IFN antiviral response (Liang et al, 2011). Interestingly, TLR stimulation seems to induce cellular resistance to eIF2α phosphorylation-dependent inhibition of translation, and this independently of the presence of functional GADD34 explaining how TLR-activated GADD34-deficient DCs are able to synthesize cytokines.
In GADD34-deficient DCs, the transcription levels of IFN-β and IL-6 in response to lipofected dsRNA were found significantly decreased, indicating that during microbial induction, GADD34 probably impacts other signalling cascades important for the transcriptional regulation of cytokines. In the absence of inflammatory stimuli, NF-κB remains in an inactive state via its binding to inhibitor of NF-κB (IκB), which is constitutively expressed. Amplification of NF-κB signalling and inflammatory cytokines production through a P–eIF2α-mediated attenuation of translation has been proposed on the basis that the half-life of IκB is much shorter than that of NF-κB (Schröder and Kaufman, 2005). Translation attenuation should increase the ratio of free NF-κB to IκB, thereby reducing NF-κB quenching by neo-synthesized IκB and prolonging NF-ϰb-dependent transcription events in response to ER stress. This phenomenon is, however, clearly not at work during DC responses to lipofected dsRNA, since protein synthesis is not attenuated and GADD34 inactivation was found to decrease cytokines expression. Thus, contrary to a bona fide UPR, GADD34 activity on eIF2α phosphorylation and protein synthesis might become secondary to its other regulatory functions during TLR stimulation.
ER stress Response versus Microbial Stress Response
Interestingly, although functional ATF4 and GADD34 are clearly detected in activated DCs, microbial stimulation alleviates the synthesis of the pro-apoptotic transcription factor CHOP (Marciniak et al, 2004; Nakayama et al, 2010), despite an upregulation of its transcripts. Transient CHOP expression has been suggested to be beneficial during ER stress, possibly by avoiding the action of pro-apoptotic regulator Bax (Sok et al, 1999). However, when the stress is permanent, expression of CHOP is prolonged and cell death induced (Boyce and Yuan, 2006; Rutkowski et al, 2006). This observation was confirmed by work in macrophages demonstrating that CHOP expression in response to UPR-inducing agent is inhibited by TLR stimulation (Figure 4) (Woo et al, 2009; Nakayama et al, 2010; Woo et al, 2012). This inhibition is potentially favouring cell survival during microbial detection, since LPS-induced apoptosis is suppressed in different CHOP-deficient cell types (Endo et al, 2005, 2006). However, these experiments (Woo et al, 2009, 2012) were carried out in the presence of UPR-inducing drugs. Loss of CHOP expression in response to TLR activation was attributed to a deficit in ATF4 synthesis and nuclear translocation, therefore limiting the level of ATF4-dependent CHOP mRNA transcription in stressed macrophages (Woo et al, 2009, 2012). Contrasting with these studies, experiments performed in LPS-activated human monocyte-derived DCs have demonstrated that CHOP participates in the enhanced production of IL-23 p19 (Goodall et al, 2010), suggesting that CHOP expression is not always inhibited by LPS sensing and associated with increased apoptosis. In activated bone-marrow-derived DCs, ATF4 is synthetized and translocated, while GADD34 and CHOP mRNAs are induced, suggesting that, in these cells, the control of a potentially detrimental CHOP synthesis is occuring at the translational level. This extinction of CHOP synthesis, while maintaining the translation of ATF4 and GADD34 active, again, clearly singularizes the microbial-induced stress genes transcriptional response from a classical ER stress response.
When the IRE-1α and XBP-1 pathways (Figure 1) were explored during macrophage stimulation with different microbial products, it was found that although XBP-1 mRNA splicing could be detected, the transcriptional consequences of this unusual IRE1α activation were qualitatively different from the traditional UPR response (Martinon et al, 2010). The NADPH oxidase NOX2 plays an important role in the signalling downstream of the TLRs, through the production of reactive oxygen species (ROS) and their damaging activities on cell structures (Matsuzawa et al, 2005). NOX2-dependent activation of IRE1 was shown to be required for XBP1 mRNA maturation, yet this process was found independent of ER stress as downstream targets of XBP1 normally activated in the context the UPR were not induced in response to LPS (Table I) (Martinon et al, 2010). Indeed, transcription of the XBP1 canonical UPR targets, such as BiP or ERdj4, was not observed. Like for Chop transcription, when LPS was administered together with tunicamycin, the induction of several UPR-related mRNAs was suppressed. Conversely, during a chemically induced UPR, XBP1 synthesis did not promote the transcription of inflammatory cytokines such as IL-6 or TNF-α, whereas in the context of microbial activation, XBP1 clearly enhanced the transcription of these different cytokines. Independently of XBP1 mRNA splicing, Ire1-dependent decay (RIDD), a recently described pathway specialized in the degradation of different mRNAs by IRE-1 homologous to the type-I-inducible antiviral RNAse L, could also participate in the inflammatory response through host mRNAs or viral mRNA degradation during infection (Malathi et al, 2007; Hollien et al, 2009). Interestingly, XBP-1 mRNA splicing is not very efficient in DCs activated with LPS or poly I:C (A Dalet, personal communication). The lower microbicidal activities of DCs compared to macrophages could explain this difference, as also suggested by the fact that activated Nox2-deficient DCs display normal GADD34 expression levels (Clavarino et al, 2012b). In that case, GADD34 expression was found to be TRIF-dependent, further suggesting that ATF4 and GADD34 inductions are a direct consequence of TLR triggering and not part of an indirect response to ER stress, linked to protein overload or misfolding in microbe-stimulated innate cells.
Perspective
The induction or activation of several molecules linked to the ER stress response pathway by microbial stimuli have created the impression that innate sensing is always associated with an UPR. The detection of transcriptional programme linked to the UPR in numerous human diseases, including atherosclerosis, cancer, diabetes and neurodegenerative disorders, have naturally led a wealth of data describing the importance of the UPR in the generation and maintenance of inflammation by immune cells, including macrophages and DCs. However, we would like to underline that the characterization of non-canonical roles of ATF4/GADD34 and IRE-1/XBP-1 deeply linked to the immune context suggests the existence of a specific microbial stress response (MSR) distinct from the now ‘classical’ UPR. Like the UPR during ER stress, the MSR could allow individual cells to cope with the considerable and deleterious impact of microbe detection on their physiology, while they are expected to participate actively in systemic response to infection. The coexistence and synergy of the two responses are obviously not excluded, as suggested by plasma cell biology or several viral infection situations, but we suspect that the MSR could be dominant in some respect, since TLR stimulation has been shown to counteract most of the conserved features of the UPR (Martinon et al, 2010), such as Bip or ERdj4 transcription (Martinon et al, 2010), CHOP synthesis (Woo et al, 2009, 2012), eIF2α phosphorylation and translation inhibition (Goldfinger et al, 2011; Clavarino et al, 2012b). There is emerging evidence that immune responses can be negatively affected by abnormalities in the UPR and that this could contribute to the development of autoimmunity and metabolic diseases (Todd et al, 2008; Hotamisligil, 2010). Artificial induction of the UPR, together with microbial stimulation, has become a standard to study UPR contribution to inflammation (Woo et al, 2009; Goodall et al, 2010; Hotamisligil, 2010; Woo et al, 2012). However, XBP-1, ATF4 and ATF3 synthesis and their nuclear translocations are directly induced by TLR triggering or type-I IFN stimulation (Litvak et al, 2009; Martinon et al, 2010; Clavarino et al, 2012b), with poor experimental evidences for concomitant protein misfolding and true ER stress induction, independent of XBP-1 or ATF4 expressions themselves.
GADD34 induction during TLR activation might have an additional purpose than mediating eIF2α dephosphorylation. GADD34 can form stable interactions with the Tuberous sclerosis complex (TSC1/2) and inhibits mTOR signalling (Uddin et al, 2011). A cross-talk between stress-inducible GADD34 and the mTOR-signalling pathway might therefore exist (Goldfinger et al, 2011) and play an important role in innate signalling, as well as autophagy regulation (Hyrskyluoto et al, 2012). In parallel, GADD34 has been reported to interact with CUE domain-containing 2 (CUEDC2) and form together a stable complex with PP1. This complex has been proposed to be part of a negative-feedback loop that specifically dephosphorylates IKKα and IKKβ after their TLR-dependent activation, thereby decreasing the levels of activated transcription factor NF-κB (Li et al, 2008). Thus, CUEDC2 and, by association, GADD34 were proposed to function as an anti-inflammatory complex promoting NF-κB inactivation. These observations contrast, however, with the result obtained in GADD34-inactivated DCs, in which inflammatory cytokines production is decreased suggesting that GADD34 expression can be pro-inflammatory (Clavarino et al, 2012b).
Thus, we would like to propose that microbial recognition induces a specific stress response (MSR) with some molecular determinants in common with the UPR, but with different levels of regulation and, more importantly, with a different functional outcome (Table I). Consequently, as transduction cascades downstream of TLRs could be seen as parallel and complementary signalling modules, the MSR should be considered as a novel signal transduction and transcription module involved in the coordination of inflammatory cytokines induction (e.g., IFN-β/IRFs and IL6/NFkB), metabolism activation (e.g., mTOR) and cellular antimicrobial pathways (e.g., PKR and eIF2α) during the detection of microbial products and pathogens.
Acknowledgments
We thank all our colleagues at CIML for lively discussions and help with the manuscript, in particular Jonathan Ewbank, Lena Alexopoulou and Philippe Naquet. This work is supported by grants from La Ligue Nationale Contre le Cancer, the ANR 07-MIME-005 ‘DC-TRANS’, ANR Blanc SVSE 2-2012 ‘Stressor’ and the ANRS. NC is supported by FCT (SFRH/BD/40112/2007), Portugal.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abraham N, Stojdl DF, Duncan PI, Methot N, Ishii T, Dube M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, Koromilas AE, Brown EG, Sonenberg N, Bell JC (1999) Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J Biol Chem 274: 5953–5962 [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA (2001) Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413: 732–738 [DOI] [PubMed] [Google Scholar]
- Balachandran S, Roberts PC, Brown LE, Truong H, Pattnaik AK, Archer DR, Barber GN (2000) Essential role for the dsRNA-dependent protein kinase PKR in innate immunity to viral infection. Immunity 13: 129–141 [DOI] [PubMed] [Google Scholar]
- Berlanga JJ, Santoyo J, De Haro C (1999) Characterization of a mammalian homolog of the GCN2 eukaryotic initiation factor 2alpha kinase. Eur J Biochem 265: 754–762 [DOI] [PubMed] [Google Scholar]
- Berlanga JJ, Ventoso I, Harding HP, Deng J, Ron D, Sonenberg N, Carrasco L, de Haro C (2006) Antiviral effect of the mammalian translation initiation factor 2alpha kinase GCN2 against RNA viruses. EMBO J 25: 1730–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce M, Yuan J (2006) Cellular response to endoplasmic reticulum stress: a matter of life or death. Cell Death Differ 13: 363–373 [DOI] [PubMed] [Google Scholar]
- Brush MH, Weiser DC, Shenolikar S (2003) Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 23: 1292–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci S, Mezghrani A, Cascio P, Bianchi G, Cerruti F, Fra A, Lelouard H, Masciarelli S, Mattioli L, Oliva L, Orsi A, Pasqualetto E, Pierre P, Ruffato E, Tagliavacca L, Sitia R (2006) Progressively impaired proteasomal capacity during terminal plasma cell differentiation. EMBO J 25: 1104–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceppi M, Clavarino G, Gatti E, Schmidt EK, de Gassart A, Blankenship D, Ogola G, Banchereau J, Chaussabel D, Pierre P (2009) Ribosomal protein mRNAs are translationally-regulated during human dendritic cells activation by LPS. Immunome Res 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Shen J, Prywes R (2002) The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 277: 13045–13052 [DOI] [PubMed] [Google Scholar]
- Cheng G, Feng Z, He B (2005) Herpes Simplex Virus 1 infection activates the endoplasmic reticulum resident kinase PERK and mediates eIF2α dephosphorylation by the γ134.5 protein. J Virol 79: 1379–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Roizman B (1990) The herpes-simplex virus-1 gene for icp34.5, which maps in inverted repeats, is conserved in several limited-passage isolates but not in strain 17syn+. J Virol 64: 1014–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Roizman B (1994) Herpes-simplex virus-1 gamma(1)34.5-gene function, which blocks the host response to infection, maps in the homologous domain of the genes expressed during growth arrest and dna-damage. Proc Natl Acad Sci USA 91: 5247–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavarino G, Cláudio N, Couderc T, Dalet A, Judith D, Camosseto V, Schmidt EK, Wenger T, Lecuit M, Gatti E, Pierre P (2012a) Induction of GADD34 is necessary for dsRNA-dependent interferon-β production and participates in the control of Chikungunya Virus infection. PLoS Pathog 8: e1002708–e1002708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clavarino G, Cláudio N, Dalet A, Terawaki S, Couderc T, Chasson L, Ceppi M, Schmidt EK, Wenger T, Lecuit M, Gatti E, Pierre P (2012b) Protein phosphatase 1 subunit Ppp1r15a/GADD34 regulates cytokine production in polyinosinic:polycytidylic acid-stimulated dendritic cells. Proc Natl Acad Sci USA 109: 3006–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor JH, Weiser DC, Li S, Hallenbeck JM, Shenolikar S (2001) Growth arrest and DNA damage-inducible protein GADD34 assembles a novel signaling complex containing protein phosphatase 1 and inhibitor 1. Mol Cell Biol 21: 6841–6850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz JLG, Sola I, Becares M, Alberca B, Plana J, Enjuanes L, Zuñiga S (2011) Coronavirus gene 7 counteracts host defenses and modulates virus virulence. PLoS Pathog 7: e1002090–e1002090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabo S, Meurs EF (2012) DsRNA-dependent protein kinase PKR and its role in stress, signaling and HCV infection. Viruses 4: 2598–2635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daher A, Laraki G, Singh M, Melendez-Pena CE, Bannwarth S, Peters AH, Meurs EF, Braun RE, Patel RC, Gatignol A (2009) TRBP control of PACT-induced phosphorylation of protein kinase R is reversed by stress. Mol Cell Biol 29: 254–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J, Harding HP, Raught B, Gingras A-C, Berlanga JJ, Scheuner D, Kaufman RJ, Ron D, Sonenberg N (2002) Activation of GCN2 in UV-irradiated cells inhibits translation. Curr Biol 12: 1279–1286 [DOI] [PubMed] [Google Scholar]
- Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D (2004) Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 24: 10161–10168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, Haswell LE, Al-Shamkhani A, Flavell R, Borrow P, Reis e Sousa C (2003) Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424: 324–328 [DOI] [PubMed] [Google Scholar]
- Domingo-Gil E, Toribio R, Najera JL, Esteban M, Ventoso I (2011) Diversity in viral anti-PKR mechanisms: a remarkable case of evolutionary convergence. PloS One 6: e16711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Mori M, Akira S, Gotoh T (2006) C/EBP homologous protein (CHOP) is crucial for the induction of caspase-11 and the pathogenesis of lipopolysaccharide-induced inflammation. J Immunol 176: 6245–6253 [DOI] [PubMed] [Google Scholar]
- Endo M, Oyadomari S, Suga M, Mori M, Gotoh T (2005) The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem 138: 501–507 [DOI] [PubMed] [Google Scholar]
- Goldfinger M, Shmuel M, Benhamron S, Tirosh B (2011) Protein synthesis in plasma cells is regulated by crosstalk between endoplasmic reticulum stress and mTOR signaling. Eur J Immunol 41: 491–502 [DOI] [PubMed] [Google Scholar]
- Goodall JC, Wu C, Zhang Y, McNeill L, Ellis L, Saudek V, Gaston JS (2010) Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc Natl Acad Sci USA 107: 17698–17703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han AP, Yu C, Lu L, Fujiwara Y, Browne C, Chin G, Fleming M, Leboulch P, Orkin SH, Chen JJ (2001) Heme-regulated eIF2alpha kinase (HRI) is required for translational regulation and survival of erythroid precursors in iron deficiency. EMBO J 20: 6909–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D (2000a) Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000b) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633 [DOI] [PubMed] [Google Scholar]
- Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10: 3787–3799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B (1997) The gamma(1)34.5 protein of herpes simplex virus 1 complexes with protein phosphatase 1alpha to dephosphorylate the alpha subunit of the eukaryotic translation initiation factor 2 and preclude the shutoff of protein synthesis by double-stranded RNA-activa. Proc Natl Acad Sci USA 94: 843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Gross M, Roizman B (1998) The gamma134.5 protein of herpes simplex virus 1 has the structural and functional attributes of a protein phosphatase 1 regulatory subunit and is present in a high molecular weight complex with the enzyme in infected cells. J Biol Chem 273: 20737–20743 [DOI] [PubMed] [Google Scholar]
- Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13: 89–102 [DOI] [PubMed] [Google Scholar]
- Holcik M, Sonenberg N (2005) Translational control in stress and apoptosis. Nat Rev Mol Cell Biol 6: 318–327 [DOI] [PubMed] [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS (2010) Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140: 900–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, Eckmann L, Guiney DG, Karin M (2004) The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature 428: 341–345 [DOI] [PubMed] [Google Scholar]
- Hyrskyluoto A, Reijonen S, Kivinen J, Lindholm D, Korhonen L (2012) GADD34 mediates cytoprotective autophagy in mutant huntingtin expressing cells via the mTOR pathway. Exp Cell Res 318: 33–42 [DOI] [PubMed] [Google Scholar]
- Jiang H-Y, Wek SA, McGrath BC, Lu D, Hai T, Harding HP, Wang X, Ron D, Cavener DR, Wek RC (2004) Activating transcription factor 3 is integral to the eukaryotic initiation factor 2 kinase stress response. Mol Cell Biol 24: 1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jousse C, Averous J, Bruhat A, Carraro V, Mordier S, Fafournoux P (2004) Amino acids as regulators of gene expression: molecular mechanisms. Biochem Biophys Res Commun 313: 447–452 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S (2006) Innate immune recognition of viral infection. Nat Immunol 7: 131–137 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384 [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34: 637–650 [DOI] [PubMed] [Google Scholar]
- Kazemi S, Papadopoulou S, Li S, Su Q, Wang S, Yoshimura A, Matlashewski G, Dever TE, Koromilas AE (2004) Control of alpha subunit of eukaryotic translation initiation factor 2 (eIF2 alpha) phosphorylation by the human papillomavirus type 18 E6 oncoprotein: implications for eIF2 alpha-dependent gene expression and cell death. Mol Cell Biol 24: 3415–3429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langland JO, Cameron JM, Heck MC, Jancovich JK, Jacobs BL (2006) Inhibition of PKR by RNA and DNA viruses. Virus Res 119: 100–110 [DOI] [PubMed] [Google Scholar]
- Lazear HM, Lancaster A, Wilkins C, Suthar MS, Huang A, Vick SC, Clepper L, Thackray L, Brassil MM, Virgin HW, Nikolich-Zugich J, Moses AV, Gale M Jr, Früh K, Diamond MS (2013) IRF-3, IRF-5, and IRF-7 coordinately regulate the type I IFN response in myeloid dendritic cells downstream of MAVS signaling. PLoS Pathog 9: e1003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber JH, Crimmins GT, Raghavan S, Meyer-Morse NP, Cox JS, Portnoy DA (2008) Distinct TLR- and NLR-mediated transcriptional responses to an intracellular pathogen. PLoS Pathog 4: e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 7448–7459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Scapa EF, Cohen DE, Glimcher LH (2008) Regulation of hepatic lipogenesis by the transcription factor XBP1. Science 320: 1492–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y-Y, Cevallos RC, Jan E (2009) An upstream open reading frame regulates translation of GADD34 during cellular stresses that induce eIF2alpha phosphorylation. J Biol Chem 284: 6661–6673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelouard H, Schmidt EK, Camosseto V, Clavarino G, Ceppi M, Hsu HT, Pierre P (2007) Regulation of translation is required for dendritic cell function and survival during activation. J Cell Biol 179: 1427–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Scull C, Ozcan L, Tabas I (2010) NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J Cell Biol 191: 1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Liu H, Wang CH, Zhang JY, Man JH, Gao YF, Zhang PJ, Li WH, Zhao J, Pan X, Zhou T, Gong WL, Li AL, Zhang XM (2008) Deactivation of the kinase IKK by CUEDC2 through recruitment of the phosphatase PP1. Nat Immunol 9: 533–541 [DOI] [PubMed] [Google Scholar]
- Liang Q, Deng H, Sun CW, Townes TM, Zhu F (2011) Negative regulation of IRF7 activation by activating transcription factor 4 suggests a cross-regulation between the IFN responses and the cellular integrated stress responses. J Immunol 186: 1001–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A (2009) Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol 10: 437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo YM, Gale M Jr. (2011) Immune signaling by RIG-I-like receptors. Immunity 34: 680–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, Valdes-Ferrer SI, Olofsson PS, Kalb T, Roth J, Zou Y, Erlandsson-Harris H, Yang H, Ting JP, Wang H, Andersson U, Antoine DJ, Chavan SS, Hotamisligil GS, Tracey KJ (2012) Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488: 670–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM (2003) Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J Biol Chem 278: 34864–34873 [DOI] [PubMed] [Google Scholar]
- Malathi K, Dong B, Gale M Jr, Silverman RH (2007) Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448: 816–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18: 3066–3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Chen X, Lee AH, Glimcher LH (2010) TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol 11: 411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A, Saegusa K, Noguchi T, Sadamitsu C, Nishitoh H, Nagai S, Koyasu S, Matsumoto K, Takeda K, Ichijo H (2005) ROS-dependent activation of the TRAF6-ASK1-p38 pathway is selectively required for TLR4-mediated innate immunity. Nat Immunol 6: 587–592 [DOI] [PubMed] [Google Scholar]
- Mungrue IN, Pagnon J, Kohannim O, Gargalovic PS, Lusis AJ (2009) CHAC1/MGC4504 is a novel proapoptotic component of the unfolded protein response, downstream of the ATF4-ATF3-CHOP cascade. Journal of immunology 182: 466–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS (2010) Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140: 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama Y, Endo M, Tsukano H, Mori M, Oike Y, Gotoh T (2010) Molecular mechanisms of the LPS-induced non-apoptotic ER stress-CHOP pathway. J Biochem 147: 471–483 [DOI] [PubMed] [Google Scholar]
- Novoa I, Zeng H, Harding HP, Ron D (2001) Feedback inhibition of the unfolded protein response by GADD34-mediated dephosphorylation of eIF2alpha. J Cell Biol 153: 1011–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D (2003) Stress-induced gene expression requires programmed recovery from translational repression. EMBO J 22: 1180–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palam LR, Baird TD, Wek RC (2011) Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem 286: 10939–10949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Reis e Sousa C (2007) Innate recognition of viruses. Immunity 27: 370–383 [DOI] [PubMed] [Google Scholar]
- Proud CG (2005) eIF2 and the control of cell physiology. Semin Cell Dev Biol 16: 3–12 [DOI] [PubMed] [Google Scholar]
- Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4: e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7: 1165–1176 [DOI] [PubMed] [Google Scholar]
- Schneider RJ, Mohr I (2003) Translation initiation and viral tricks. Trends Biochem Sci 28: 130–136 [DOI] [PubMed] [Google Scholar]
- Schröder M, Kaufman RJ (2005) The mammalian unfolded protein response. Ann Rev Biochem 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Seimon TA, Kim MJ, Blumenthal A, Koo J, Ehrt S, Wainwright H, Bekker LG, Kaplan G, Nathan C, Tabas I, Russell DG (2010) Induction of ER stress in macrophages of tuberculosis granulomas. PloS One 5: e12772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Chen X, Hendershot L, Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3: 99–111 [DOI] [PubMed] [Google Scholar]
- Sok J, Wang XZ, Batchvarova N, Kuroda M, Harding H, Ron D (1999) CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol 19: 495–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava SPP, Kumar KUU, Kaufman RJJ (1998) Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J Biol Chem 273: 2416–2416 [DOI] [PubMed] [Google Scholar]
- Stephens SB, Dodd RD, Brewer JW, Lager PJ, Keene JD, Nicchitta CV (2005) Stable ribosome binding to the endoplasmic reticulum enables compartment-specific regulation of mRNA translation. Mol Biol Cell 16: 5819–5831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13: 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MR, Xu D, Williams BR (2009) ATF3 transcription factor and its emerging roles in immunity and cancer. J Mol Med 87: 1053–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd DJ, Lee AH, Glimcher LH (2008) The endoplasmic reticulum stress response in immunity and autoimmunity. Nat Rev Immunol 8: 663–674 [DOI] [PubMed] [Google Scholar]
- Trinh MA, Kaphzan H, Wek RC, Pierre P, Cavener DR, Klann E (2012) Brain-specific disruption of the eIF2alpha kinase PERK decreases ATF4 expression and impairs behavioral flexibility. Cell Rep 1: 676–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin MN, Ito S, Nishio N, Suganya T, Isobe K (2011) Gadd34 induces autophagy through the suppression of the mTOR pathway during starvation. Biochem Biophys Res Commun 407: 692–698 [DOI] [PubMed] [Google Scholar]
- Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287: 664–666 [DOI] [PubMed] [Google Scholar]
- Wang Y, Shen J, Arenzana N, Tirasophon W, Kaufman RJ, Prywes R (2000) Activation of ATF6 and an ATF6 DNA binding site by the endoplasmic reticulum stress response. J Biol Chem 275: 27013–27020 [DOI] [PubMed] [Google Scholar]
- Wek RC, Jiang HY, Anthony TG (2006) Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans 34: 7–11 [DOI] [PubMed] [Google Scholar]
- Williams BR (1999) PKR; a sentinel kinase for cellular stress. Oncogene 18: 6112–6120 [DOI] [PubMed] [Google Scholar]
- Williams BR (2001) Signal integration via PKR. Sci STKE 2001: re2. [DOI] [PubMed] [Google Scholar]
- Won S, Eidenschenk C, Arnold CN, Siggs OM, Sun L, Brandl K, Mullen T-M, Nemerow GR, Moresco EMY, Beutler B (2012) Increased susceptibility to DNA virus infection in mice with a GCN2 mutation. J Virol 86: 1802–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Cui D, Arellano J, Dorweiler B, Harding H, Fitzgerald KA, Ron D, Tabas I (2009) Adaptive suppression of the ATF4-CHOP branch of the unfolded protein response by toll-like receptor signalling. Nat Cell Biol 11: 1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo CW, Kutzler L, Kimball SR, Tabas I (2012) Toll-like receptor activation suppresses ER stress factor CHOP and translation inhibition through activation of eIF2B. Nat Cell Biol 14: 192–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YL, Reis LF, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams BR, Aguet M, Weissmann C (1995) Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J 14: 6095–6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama M, Fujita T (2007) Function of RIG-I-like receptors in antiviral innate immunity. J Biol Chem 282: 15315–15318 [DOI] [PubMed] [Google Scholar]
- Yoshida H (2007) ER stress and diseases. FEBS J 274: 630–658 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891 [DOI] [PubMed] [Google Scholar]
- Zhan QM, Lord KA, Alamo I, Hollander MC, Carrier F, Ron D, Kohn KW, Hoffman B, Liebermann DA, Fornace AJ (1994) The gadd and myd genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell-growth. Mol Cell Biol 14: 2361–2371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Kaufman RJ (2008) From endoplasmic-reticulum stress to the inflammatory response. Nature 454: 455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsak L, Lu Z, Kutish GF, Neilan JG, Rock DL (1996) An African swine fever virus virulence-associated gene NL-S with similarity to the herpes simplex virus ICP34.5 gene. J Virol 70: 8865–8871 [DOI] [PMC free article] [PubMed] [Google Scholar]