Abstract
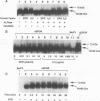
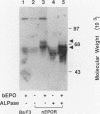
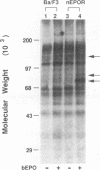
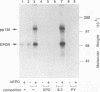
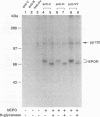
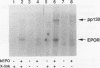
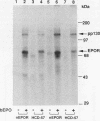
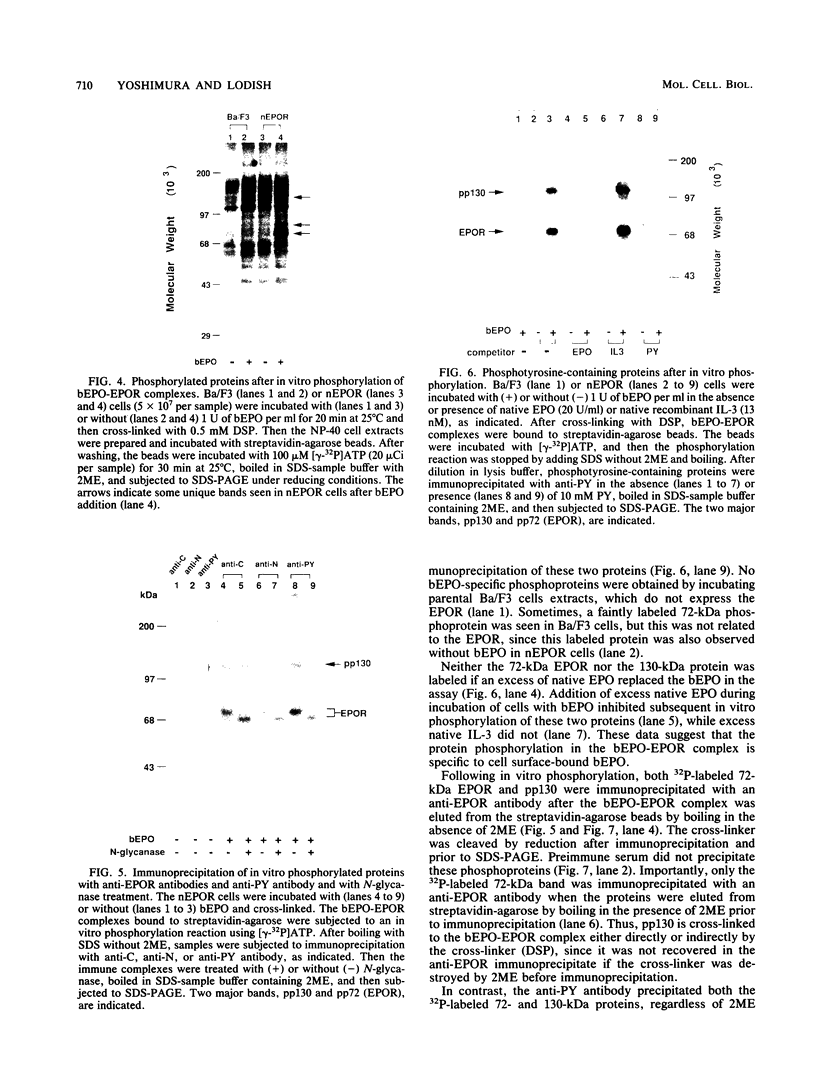
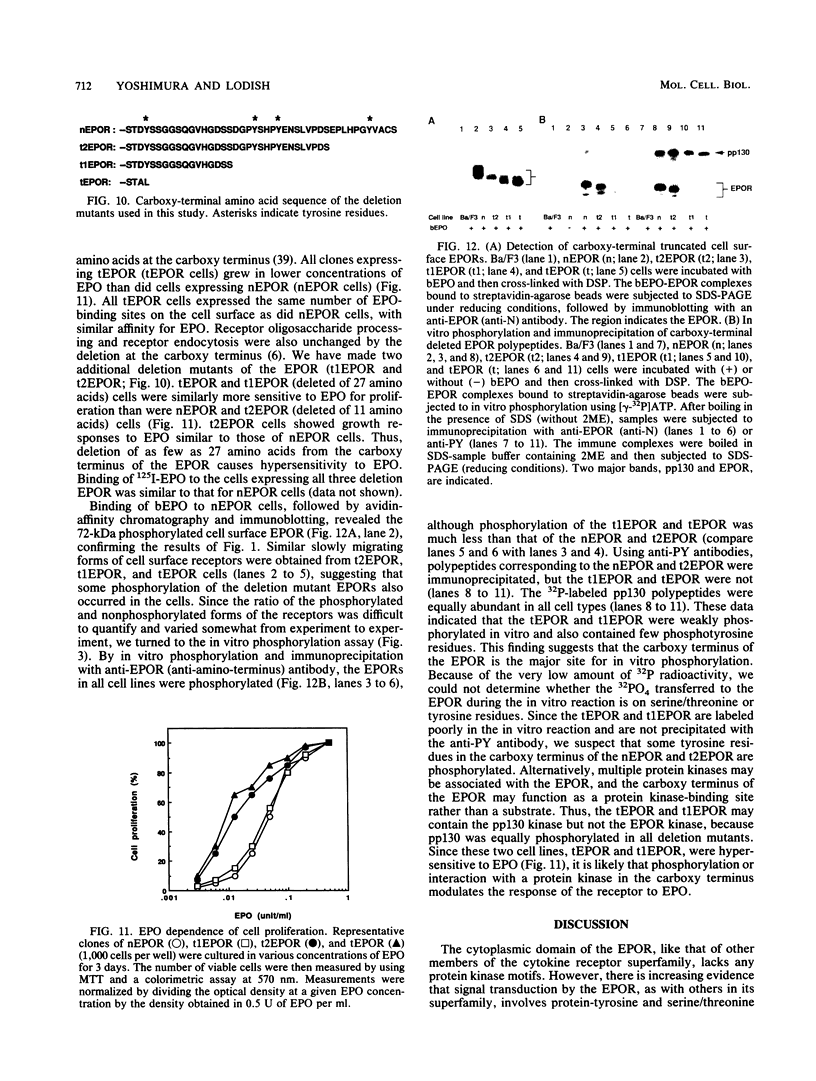
The cytoplasmic domain of the cloned erythropoietin (EPO) receptor (EPOR) contains no protein kinase motif, yet addition of EPO to EPO-responsive cells causes an increase in protein-tyrosine phosphorylation. Here we show that addition of EPO or interleukin-3 (IL-3) to an IL-3-dependent cell line expressing the wild-type EPOR causes a small fraction (less than 5%) of total cellular EPOR to shift in gel mobility from 66 to 72 kDa, due at least in part to phosphorylation. Using biotinylated EPO as an affinity reagent, we show that the 72-kDa species is greatly enriched on the cell surface. To demonstrate that a protein kinase activity associates with cell surface EPOR, cells were incubated with biotinylated EPO and then cross-linked with a thiol-cleavable chemical cross-linker. The avidin-agarose-selected complexes were incubated with [gamma-32P]ATP. After in vitro phosphorylation and denaturation without reducing agent, both antiphosphotyrosine and anti-EPOR antibodies immunoprecipitated labeled 72-kDa EPOR and an unidentified 130-kDa phosphoprotein (pp130), indicating that a protein kinase is associated with cell surface EPOR and that a fraction of the EPOR was phosphorylated on tyrosine residues either in the cells or during the cell-free phosphorylation reaction. Under reducing conditions, the 72-kDa phosphorylated EPOR but not pp130 was immunoprecipitated with an anti-EPOR antibody, suggesting that the pp130 is bound to the EPOR by the thiol-cleavable chemical cross-linker. Previously, we showed that deletion of the 42 carboxy-terminal amino acids of the EPOR allows cells to grow in 1/10 the normal EPO concentration, without affecting receptor number or affinity. Two carboxy-terminal truncated EPO receptors that are hyperresponsive to EPO were poorly phosphorylated during the in vitro reaction, suggesting that the carboxy-terminal region of the EPOR contains a site for phosphorylation or a site for interaction with a protein kinase. Our data suggests that phosphorylation or interaction with a protein kinase in the carboxy-terminal region may down-modulate the proliferative action of the EPOR.
Full text
PDF


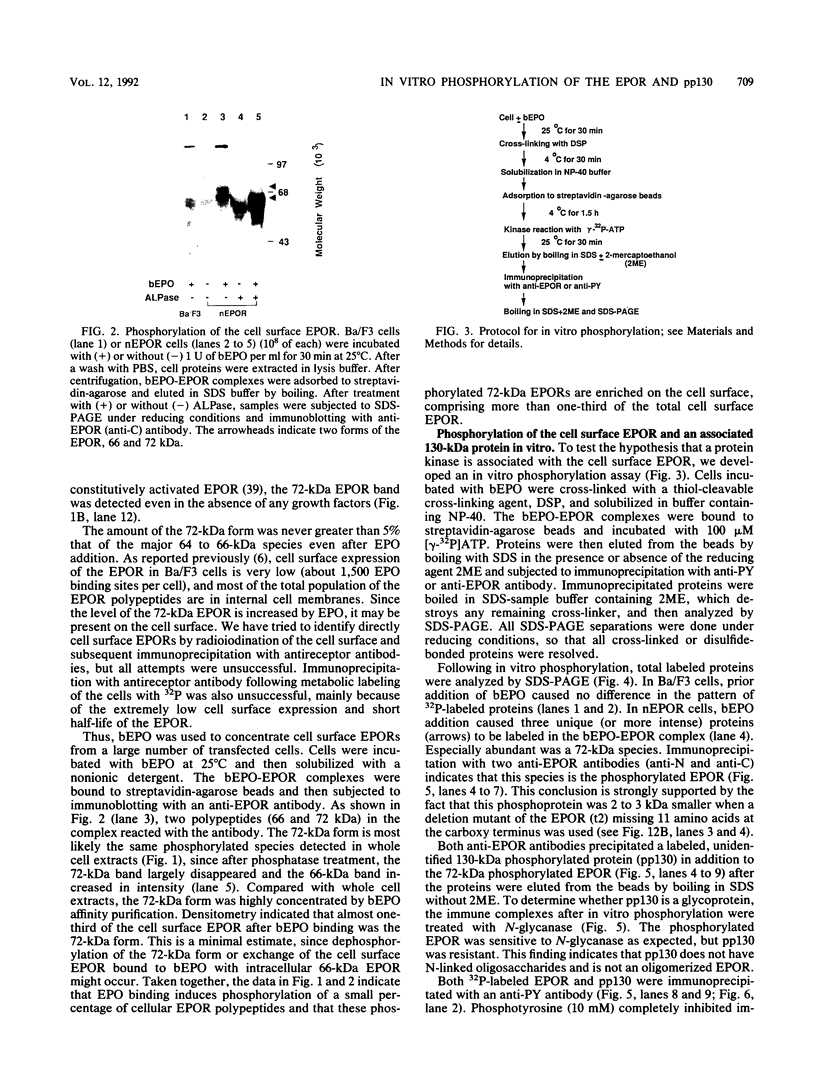




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Klinken S. P., Hankins W. D. A murine recombinant retrovirus containing the src oncogene transforms erythroid precursor cells in vitro. Mol Cell Biol. 1985 Dec;5(12):3369–3375. doi: 10.1128/mcb.5.12.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll M. P., Clark-Lewis I., Rapp U. R., May W. S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [PubMed] [Google Scholar]
- Carroll M. P., Spivak J. L., McMahon M., Weich N., Rapp U. R., May W. S. Erythropoietin induces Raf-1 activation and Raf-1 is required for erythropoietin-mediated proliferation. J Biol Chem. 1991 Aug 15;266(23):14964–14969. [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Lodish H. F., Wong G. G. Expression cloning of the murine erythropoietin receptor. Cell. 1989 Apr 21;57(2):277–285. doi: 10.1016/0092-8674(89)90965-3. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Yoshimura A., Youssoufian H., Zon L. I., Koo J. W., Lodish H. F. The cytoplasmic region of the erythropoietin receptor contains nonoverlapping positive and negative growth-regulatory domains. Mol Cell Biol. 1991 Apr;11(4):1980–1987. doi: 10.1128/mcb.11.4.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung M. R., Scearce R. M., Hoffman J. A., Peffer N. J., Hammes S. R., Hosking J. B., Schmandt R., Kuziel W. A., Haynes B. F., Mills G. B. A tyrosine kinase physically associates with the beta-subunit of the human IL-2 receptor. J Immunol. 1991 Aug 15;147(4):1253–1260. [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Honjo T. Shared partners in receptors. Curr Biol. 1991 Aug;1(4):201–203. doi: 10.1016/0960-9822(91)90055-2. [DOI] [PubMed] [Google Scholar]
- Horak I. D., Gress R. E., Lucas P. J., Horak E. M., Waldmann T. A., Bolen J. B. T-lymphocyte interleukin 2-dependent tyrosine protein kinase signal transduction involves the activation of p56lck. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1996–2000. doi: 10.1073/pnas.88.5.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Stevens D., May W. S., Ihle J. N. Interleukin 3 binds to a 140-kDa phosphotyrosine-containing cell surface protein. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7982–7986. doi: 10.1073/pnas.85.21.7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Margolis B., Li N., Koch A., Mohammadi M., Hurwitz D. R., Zilberstein A., Ullrich A., Pawson T., Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 1990 Dec;9(13):4375–4380. doi: 10.1002/j.1460-2075.1990.tb07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux P., Billat C., Jacquot R. The erythropoietin receptor of rat erythroid progenitor lens. Characterization and affinity cross-linkage. J Biol Chem. 1987 Oct 15;262(29):13985–13990. [PubMed] [Google Scholar]
- Merida I., Gaulton G. N. Protein tyrosine phosphorylation associated with activation of the interleukin 2 receptor. J Biol Chem. 1990 Apr 5;265(10):5690–5694. [PubMed] [Google Scholar]
- Mills G. B., May C., McGill M., Fung M., Baker M., Sutherland R., Greene W. C. Interleukin 2-induced tyrosine phosphorylation. Interleukin 2 receptor beta is tyrosine phosphorylated. J Biol Chem. 1990 Feb 25;265(6):3561–3567. [PubMed] [Google Scholar]
- Miura O., D'Andrea A., Kabat D., Ihle J. N. Induction of tyrosine phosphorylation by the erythropoietin receptor correlates with mitogenesis. Mol Cell Biol. 1991 Oct;11(10):4895–4902. doi: 10.1128/mcb.11.10.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morla A. O., Schreurs J., Miyajima A., Wang J. Y. Hematopoietic growth factors activate the tyrosine phosphorylation of distinct sets of proteins in interleukin-3-dependent murine cell lines. Mol Cell Biol. 1988 May;8(5):2214–2218. doi: 10.1128/mcb.8.5.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D. Subunit promiscuity among hemopoietic growth factor receptors. Cell. 1991 Oct 4;67(1):1–4. doi: 10.1016/0092-8674(91)90564-f. [DOI] [PubMed] [Google Scholar]
- Noguchi T., Fukumoto H., Mishina Y., Obinata M. Differentiation of erythroid progenitor (CFU-E) cells from mouse fetal liver cells and murine erythroleukemia (TSA8) cells without proliferation. Mol Cell Biol. 1988 Jun;8(6):2604–2609. doi: 10.1128/mcb.8.6.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patschinsky T., Hunter T., Esch F. S., Cooper J. A., Sefton B. M. Analysis of the sequence of amino acids surrounding sites of tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):973–977. doi: 10.1073/pnas.79.4.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle D. E., Wojchowski D. M. Localized cytosolic domains of the erythropoietin receptor regulate growth signaling and down-modulate responsiveness to granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4801–4805. doi: 10.1073/pnas.88.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle F. W., Wojchowski D. M. Proliferative action of erythropoietin is associated with rapid protein tyrosine phosphorylation in responsive B6SUt.EP cells. J Biol Chem. 1991 Jan 5;266(1):609–614. [PubMed] [Google Scholar]
- Ruscetti S. K., Janesch N. J., Chakraborti A., Sawyer S. T., Hankins W. D. Friend spleen focus-forming virus induces factor independence in an erythropoietin-dependent erythroleukemia cell line. J Virol. 1990 Mar;64(3):1057–1062. doi: 10.1128/jvi.64.3.1057-1062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Shackelford D. A., Trowbridge I. S. Ligand-stimulated tyrosine phosphorylation of the IL-2 receptor beta chain and receptor-associated proteins. Cell Regul. 1991 Jan;2(1):73–85. doi: 10.1091/mbc.2.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen P., Mui A. L., Krystal G. Interleukin-3 stimulates the tyrosine phosphorylation of the 140-kilodalton interleukin-3 receptor. J Biol Chem. 1989 Nov 15;264(32):19253–19258. [PubMed] [Google Scholar]
- Spangler R., Bailey S. C., Sytkowski A. J. Erythropoietin increases c-myc mRNA by a protein kinase C-dependent pathway. J Biol Chem. 1991 Jan 15;266(2):681–684. [PubMed] [Google Scholar]
- Turner B., Rapp U., App H., Greene M., Dobashi K., Reed J. Interleukin 2 induces tyrosine phosphorylation and activation of p72-74 Raf-1 kinase in a T-cell line. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1227–1231. doi: 10.1073/pnas.88.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Shiraishi T., Sasaki H., Oishi M. Inhibitors for protein-tyrosine kinases, ST638 and genistein: induce differentiation of mouse erythroleukemia cells in a synergistic manner. Exp Cell Res. 1989 Aug;183(2):335–342. doi: 10.1016/0014-4827(89)90394-7. [DOI] [PubMed] [Google Scholar]
- Wognum A. W., Lansdorp P. M., Humphries R. K., Krystal G. Detection and isolation of the erythropoietin receptor using biotinylated erythropoietin. Blood. 1990 Aug 15;76(4):697–705. [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Longmore G., Lodish H. F. Point mutation in the exoplasmic domain of the erythropoietin receptor resulting in hormone-independent activation and tumorigenicity. Nature. 1990 Dec 13;348(6302):647–649. doi: 10.1038/348647a0. [DOI] [PubMed] [Google Scholar]