Abstract
AIM: To investigate the prevalence of chronic dyspnea and its relationship to respiratory muscle function in end-stage liver disease.
METHODS: Sixty-eight consecutive, ambulatory, Caucasian patients with end-stage liver disease, candidates for liver transplantation, were referred for preoperative respiratory function assessment. Forty of these (29 men) were included in this preliminary study after applying strict inclusion and exclusion criteria. Seventeen of 40 patients (42%) had ascites, but none of them was cachectic. Fifteen of 40 patients (38%) had a history of hepatic encephalopathy, though none of them was symptomatic at study time. All patients with a known history and/or presence of co-morbidities were excluded. Chronic dyspnea was rated according to the modified medical research council (mMRC) 6-point scale. Liver disease severity was assessed according to the Model for end-stage liver disease (MELD). Routine lung function tests, maximum static expiratory (Pemax) and inspiratory (Pimax) mouth pressures were measured. Respiratory muscle strength (RMS) was calculated from Pimax and Pemax values. In addition, arterial blood gases and pattern of breathing (VE: minute ventilation; VT: tidal volume; VT/TI: mean inspiratory flow; TI: duration of inspiration) were measured.
RESULTS: Thirty-five (88%) of 40 patients aged (mean ± SD) 52 ± 10 years reported various degrees of chronic dyspnea (mMRC), ranging from 0 to 4, with a mean value of 2.0 ± 1.2. MELD score was 14 ± 6. Pemax, percent of predicted (%pred) was 105 ± 35, Pimax, %pred was 90 ± 29, and RMS, %pred was 97 ± 30. These pressures were below the normal limits in 12 (30%), 15 (38%), and 14 (35%) patients, respectively. Furthermore, comparing the subgroups of ascites to non-ascites patients, all respiratory muscle indices measured were found significantly decreased in ascites patients. Patients with ascites also had a significantly worse MELD score compared to non-ascites ones (P = 0.006). Significant correlations were found between chronic dyspnea and respiratory muscle function indices in all patients. Specifically, mMRC score was significantly correlated with Pemax, Pimax, and RMS (r = -0.53, P < 0.001; r = -0.42, P < 0.01; r = -0.51, P < 0.001, respectively). These correlations were substantially closer in the non-ascites subgroup (r = -0.82, P < 0.0001; r = -0.61, P < 0.01; r = -0.79, P < 0.0001, respectively) compared to all patients. Similar results were found for the relationship between mMRC vs MELD score, and MELD score vs respiratory muscle strength indices. In all patients the sole predictor of mMRC score was RMS (r = -0.51, P < 0.001). In the subgroup of patients without ascites this relationship becomes closer (r = -0.79, P < 0.001), whilst this relationship breaks down in the subgroup of patients with ascites. The disappearance of such a correlation may be due to the fact that ascites acts as a “confounding” factor. PaCO2 (4.4 ± 0.5 kPa) was increased, whereas pH (7.49 ± 0.04) was decreased in 26 (65%) and 34 (85%) patients, respectively. PaO2 (12.3 ± 0.04 kPa) was within normal limits. VE (11.5 ± 3.5 L/min), VT (0.735 ± 0.287 L), and VT/TI (0.449±0.129 L/s) were increased signifying hyperventilation in both subgroups of patients. VT/TI was significantly higher in patients with ascites than without ascites. Significant correlations, albeit weak, were found for PaCO2 with VE and VT/TI (r = -0.44, P < 0.01; r = -0.41, P < 0.01, respectively).
CONCLUSION: The prevalence of chronic dyspnea is 88% in end-stage liver disease. The mMRC score closely correlates with respiratory muscle strength.
Keywords: Liver transplantation, Lung function testing, Maximum static mouth pressures, Pattern of breathing, Respiratory muscle strength
INTRODUCTION
Liver transplantation has become the therapeutic option for patients with end-stage liver disease[1]. These patients are usually characterized by tiring easily, chronic fatigue, protein wasting and muscle mass loss[2]. The loss of muscle mass may affect both peripheral and respiratory muscles[3]. However, there are only a few reports on respiratory muscle function[4-8] or pattern of breathing in patients awaiting liver transplantation. These patients frequently report chronic dyspnea, however, its exact prevalence and severity remains unknown. To the best of our knowledge, no report can be found in the literature relating chronic dyspnea to respiratory muscle function in these patients. Therefore, we wondered whether chronic dyspnea is related to respiratory muscle strength and/or any other lung function index. We conducted this preliminary study to determine the prevalence of chronic dyspnea and the interrelationships among chronic dyspnea, measured with the modified Medical Research Council (mMRC) scale, respiratory muscle strength, and lung function in patients with end-stage liver disease[9-13].
MATERIAL AND METHODS
Subjects
Sixty-eight consecutive, ambulatory, Caucasian patients with end-stage liver disease, candidates for liver transplantation, were referred for preoperative respiratory function assessment. Twenty-eight patients were excluded applying the imposed (see below) strict inclusion and exclusion criteria [i.e., chronic obstructive pulmonary disease (COPD) n = 11; bronchiectasis n = 2; previous abdominal and thoracic surgical operation n = 3; pleural effusion n = 5; unsatisfactory lung function testing performed n = 7]. Forty of them (29 men) were included in this preliminary study. The causes of end-stage liver disease among the patients finally included in the study were cirrhosis from hepatitis B or C (n = 23; 58%; 4 of them additionally had hepatocellular carcinoma), alcoholic liver disease (n = 9; 22%), cryptogenic cirrhosis (n = 2; 5%), primary biliary cirrhosis (n = 2; 5%), and other causes (n = 4; 10%; 1 of them additionally had hepatocellular carcinoma). Their anthropometric characteristics and lung function data are shown in Table 1.
Table 1.
Anthropometric characteristics and routine respiratory function data of 40 end-stage liver disease patients, stratified in two groups according to the presence or absence of ascites
| All patients (n = 40) | Ascites (n = 17) | Non-ascites (n = 23) | P value | |
| Gender, M/F | 29/11 | 15/2 | 14/9 | 0.079 |
| Age, yr | 52 ± 10 | 51 ± 10 | 52 ± 9 | 0.752 |
| Ht, m | 1.69 ± 0.90 | 1.73 ± 0.90 | 1.66 ± 0.90 | 0.015 |
| BMI, kg ∙ m-2 | 28 ± 4 | 26 ± 4 | 29 ± 5 | 0.144 |
| Pack- years, yr | 19 ± 22 | 27 ± 25 | 13 ± 18 | 0.0871 |
| FVC | 104 ± 20 | 91 ± 17 | 114 ± 16 | < 0.001 |
| FEV1 | 100 ± 18 | 89 ± 15 | 108 ± 15 | < 0.001 |
| FEV1/FVC, % | 78 ± 3 | 79 ± 3 | 78 ± 3 | 0.083 |
| PEF | 95 ± 19 | 84 ± 18 | 103 ± 17 | 0.002 |
| TLC | 95 ± 20 | 86 ± 16 | 102 ± 21 | 0.011 |
| FRC | 97 ± 28 | 87 ± 24 | 101 ± 29 | 0.136 |
| RV | 92 ± 28 | 91 ± 25 | 93 ± 30 | 0.840 |
| DLCO | 78 ± 18 | 70 ± 16 | 84 ± 18 | 0.013 |
Mann-Whitney rank sum test was used. Values are mean ± SD obtained in a sitting position. Unless otherwise specified, values are expressed as % of predicted. Statistical significance tested with Student’s t test between ascites and non-ascites groups. M: Male; F: Female; Ht: Height; BMI: Body mass index; FVC: Forced vital capacity; FEV1: Forced expiratory volume in one second; PEF: Peak expiratory flow; TLC: Total lung capacity; FRC: Functional residual capacity; RV: Residual volume; DLCO: Diffusing capacity for carbon monoxide.
Inclusion criteria were: (1) age 18 years and older; (2) the ability to perform a full lung function testing satisfactorily; and (3) stable clinical and functional state for at least four weeks before testing. Exclusion criteria were presence of any kind of: (1) cardiovascular disorders diagnosed by a cardiologist; (2) known lung disease caused by conditions other than liver, such as asthma or COPD requiring regular bronchodilator treatment; (3) pleural effusion; (4) previous abdominal and thoracic surgical operation; and (5) neuromuscular disorders. None of the patients studied was cachectic (body mass index < 18). Nineteen out of 40 patients had never been smokers. Fifteen of 40 patients had a history of hepatic encephalopathy, but none of them was symptomatic at study time. Seventeen of 40 patients presented with ascites, and 21 out of 40 patients were on b-blockers at study time.
The study was approved by the local ethics committee. All subjects gave informed consent.
Liver disease severity, dyspnea, and respiratory function
Liver disease severity was assessed according to the model for end-stage liver disease (MELD, United Network for Organ Sharing modification)[14]. The United Network for Organ Sharing and Euro Transplant currently use MELD score for prioritizing patients’ allocation for liver transplantation instead of the older Child-Pugh score[15]. Serum laboratory data (used for MELD score calculation) were measured maximum ± 7 d the day of respiratory testing. The MELD score used was not corrected for the patients with hepatocellular carcinoma.
Chronic dyspnea was rated according to the modified mMRC 6-point scale[9]. The mMRC breathlessness scale comprises six statements that describe almost the entire range of respiratory disability from none (Grade 0) to almost complete incapacity (Grade 5). The score is the number that best fits the patient’s level of activity. Simple spirometry was measured with the Vmax apparatus (Vmax Encore 22: Sensor Medics, Yorba Linda, CA, United States) using the “fast inspiratory maneuver”[16]. Static Lung Volumes were determined by the multiple nitrogen washout technique[17] (Vmax Encore 22 apparatus). The diffusing capacity for carbon monoxide (DLCO) single breath technique was also determined (Vmax Encore 22 apparatus)[18]. Predicted values for spirometry, static lung volumes, and DLCO were from the European Community for Coal and Steel[19]. The arterial pH, PaO2, and PaCO2 were measured with the Stat Profile Critical Care Xpress apparatus (Nova Biomedical, Waltham, MA, United States).
Maximum static expiratory (Pemax) and inspiratory (Pimax) mouth pressures were measured with a plastic semi-rigid flanged mouthpiece fitted to a metallic stem incorporating a 3-way tap, manufactured according to the design of Ringqvist[10,11]. The dimensions of the metallic stem were length 27 cm and internal diameter 2.6 cm. A leak tube of length 3.7 cm and 2 mm internal diameter was incorporated into the stem 3 cm from the point of attachment to the plastic mouthpiece. A differential pressure transducer (range ± 340 cm H2O, Validyne MP45-36-871, Validyne Co, Northridge, CA) connected to the 3-way tap with a 70 cm fine polyethylene catheter. The pressure transducer was calibrated before each study using a U-tube water manometer. Pressures were displayed on a computer screen. All studies were performed with a nose-clip on and with the subjects seated comfortably in a high-backed chair at 90º where they could view a computer screen. The flanged mouthpiece was held in the mouth behind the lips and gripped firmly by the teeth, the operator holding the stem. The subjects used their hands to hold the lips firmly onto the mouthpiece if a leak was noticed. Prior to a Pemax or Pimax effort, the operator closed the 3-way tap with the subject at total lung capacity or residual volume, respectively. All subjects were given verbal encouragement and received visual feedback from the computer screen. A period of learning the procedure preceded the definitive measurements [10]. All measurements followed the criteria of Ringqvist[11] such that: (1) no extra leakage occurred; (2) the three highest pressures were similar (within 5%) and later attempts did not yield higher results; and (3) the subjects felt that they had given a maximum effort. At least 1 min rest was allowed between efforts. Pressures maintained for less than 1 s were disregarded. The highest pressure generated by an individual was used for analysis. Predicted values for Pemax and Pimax standardized for age, height, and gender were obtained from Wilson et al[12]. Respiratory muscle strength (RMS) was also calculated as the arithmetic mean of Pemax and Pimax[13].
Pattern of breathing (VE: minute ventilation; VT: tidal volume; TE: duration of expiration; TI: duration of inspiration; TTOT: total cycle duration; RR: respiratory rate; VT/TI: mean inspiratory flow; TI/TTOT: duty cycle) was also assessed during resting breathing. Subjects with a noseclip on breathed through a heated pneumotachograph (Screenmate-Box; Erich Jaeger GmbH and Co, Germany) while they were seated comfortably in a high-backed chair at 90º in a quiet room. In order to minimize the effects of anxiety, all indices were measured after the patient had become familiar with the procedure and actual measurements were made only when ventilation had remained constant for at least 10 min[20]. Normal values for the pattern of breathing were obtained from Tobin et al[21].
Statistical analysis
Data are expressed as mean ± SD, unless otherwise stated. For comparisons between groups Student’s t test, Mann-Whitney rank sum test, and Fisher’s exact test were used. Pearson and Spearman correlation coefficients, linear and backward stepwise regressions analyses were used where appropriate. A P ≤ 0.05 value was considered as significant. Statistical analysis was performed using SigmaStat V3.5 and SigmaPlot V10.0 statistical software (Jandel Scientific, CA, United States).
RESULTS
Table 1 provides anthropometric and routine respiratory function data from the 40 end-stage liver disease patients stratified according to the presence or absence of ascites (17 with ascites; 42%). Non-ascites patients had better routine lung function compared to ascites ones. DLCO was abnormal in 24 out of 40 patients, 13 of whom had ascites.
Serum laboratory data are shown in Table 2. Patients with ascites had abnormal values in the majority of the serum laboratory tests, whereas non-ascites patients had values almost within normal range.
Table 2.
Serum laboratory data of the 40 patients with end-stage liver disease stratified according to the presence or absence of ascites
| All patients (n = 40) | Ascites (n = 17) | Non-ascites (n = 23) | P value | |
| INR | 1.40 ± 0.31 | 1.53 ± 0.34 | 1.30 ± 0.25 | 0.0171 |
| Haemoglobin, g/L | 121.00 ± 23.80 | 108.60 ± 24.00 | 130.10 ± 19.20 | 0.003 |
| Creatinine, mg/dL | 1.19 ± 1.02 | 1.43 ± 1.49 | 1.01 ± 0.39 | 0.3281 |
| Total protein, g/L | 72.20 ± 8.70 | 68.80 ± 8.80 | 74.70 ± 7.90 | 0.032 |
| Albumin, g/L | 34.80 ± 6.70 | 32.00 ± 6.90 | 37.00 ± 5.90 | 0.019 |
| Total bilirubin, mg/dL | 4.67 ± 9.61 | 6.68 ± 11.41 | 3.19 ± 7.97 | 0.0041 |
| Sodium, mmol/L | 137.00 ± 5.00 | 134.00 ± 5.00 | 139.00 ± 4.00 | < 0.001 |
Values obtained maximum ± 7 d of respiratory tests day.
Mann-Whitney rank sum test was used. Statistical significance tested with Student’s t test between ascites and non-ascites groups. INR: International normalised ratio for prothrombin time.
Thirty-five (88%) of 40 patients reported various degrees of chronic dyspnea, ranging from 0 to 4 (Table 3). Pemax, Pimax, and RMS were below the normal limits[12,13] in 12 (30%), 15 (38%), and 14 (35%) patients, respectively. Furthermore, comparing the subgroups of ascites to non-ascites patients, all respiratory muscle indices measured were significantly decreased in ascites patients. Patients with ascites had significantly worse MELD score compared to non-ascites ones.
Table 3.
Comparison of model for end-stage liver disease score, modified medical research council dyspnea score, and respiratory muscle strength indices between ascites and non-ascites liver disease patients
| All patients (n = 40) | Ascites (n = 17) | Non-ascites (n = 23) | P value | |
| MELD | 14 ± 6 | 17 ± 7 | 12 ± 5 | 0.0061 |
| mMRC | 2.0 ± 1.2 | 2.2 ± 1.1 | 1.9 ± 1.2 | 0.4441 |
| Pemax, %pred | 105 ± 35 | 87 ± 30 | 118 ± 33 | 0.004 |
| Pimax, %pred | 90 ± 29 | 72 ± 20 | 103 ± 28 | < 0.001 |
| RMS, %pred | 97 ± 30 | 79 ± 23 | 110 ± 28 | < 0.001 |
Values are mean ± SD obtained in a sitting position. Statistical significance tested with Student’s t test between ascites and non-ascites groups.
For MELD and mMRC Mann-Whitney rank sum test was used. MELD: Model for end-stage liver disease; mMRC: Modified medical research council; Pemax: Maximum static expiratory mouth pressure; %pred: Percent of predicted; Pimax: Maximum static inspiratory mouth pressure; RMS: Respiratory muscle strength.
Arterial blood gases and pattern of breathing data are shown in Table 4. PaCO2 was increased (< 4.7 kPa) and pH was decreased (> 7.45) in 26 (65%) and 34 (85%) patients, respectively. PaO2 was within normal limits. VE, VT, and VT/TI were increased indicating hyperventilation in both subgroups of patients. VT/TI was significantly higher in patients with ascites than without ascites. Significant correlations, albeit weak, were found for PaCO2 with VE and VT/TI (r = -0.44, P < 0.01; r = -0.41, P < 0.01, respectively).
Table 4.
Arterial blood gases and pattern of breathing of 40 end-stage liver disease patients, according to presence or absence of ascites
| All patients (n = 40) | Ascites (n = 17) | Non-ascites (n = 23) | P value | |
| pH | 7.49 ± 0.04 | 7.50 ± 0.04 | 7.48 ± 0.04 | 0.216 |
| PaO2, kPa | 12.3 ± 1.3 | 12.0 ± 1.5 | 12.3 ± 1.3 | 0.508 |
| PaCO2, kPa | 4.4 ± 0.5 | 4.3 ± 0.5 | 4.5 ± 0.4 | 0.261 |
| VE, L/min | 11.5 ± 3.5 | 12.7 ± 3.2 | 10.6 ± 3.5 | 0.065 |
| VT, L | 0.735 ± 0.287 | 0.800 ± 0.255 | 0.686 ± 0.305 | 0.218 |
| TE, s | 2.26 ± 0.70 | 2.25 ± 0.68 | 2.26 ± 0.74 | 0.953 |
| TI, s | 1.64 ± 0.40 | 1.65 ± 0.40 | 1.64 ± 0.42 | 0.941 |
| RR, bpm | 16 ± 4 | 17 ± 5 | 16 ± 4 | 0.938 |
| VT/TI, L/s | 0.449 ± 0.129 | 0.496 ± 0.130 | 0.414 ± 0.119 | 0.044 |
| TI/TTOT | 0.43 ± 0.05 | 0.43 ± 0.06 | 0.42 ± 0.05 | 0.928 |
Values are obtained in a sitting position. Statistical significance tested with Student’s t test between ascites and non-ascites groups. PaO2: Arterial oxygen tension; PaCO2: Arterial carbon dioxide tension; VE: Minute ventilation; VT: Tidal volume; TE: Duration of expiration; TI: Duration of inspiration; RR: Respiratory rate; bpm: Breaths per minute; TTOT: Total cycle duration; VT/TI: Mean inspiratory flow; TI/TTOT: Duty cycle.
Significant correlations were found between chronic dyspnea and respiratory muscle strength indices. Specifically, mMRC score was significantly correlated with Pemax, Pimax, and RMS (r = -0.53, P < 0.001; r = -0.42, P < 0.01; r = -0.51, P < 0.001, respectively). These correlations were substantially closer in the non-ascites subgroup (r = -0.82, P < 0.0001; r = -0.61, P < 0.01; r = -0.79, P < 0.0001, respectively). Backward Stepwise Regression analysis showed that the sole predictor of mMRC score was RMS (r = -0.51, P < 0.001) (all parameters in Table 2 being eliminated) in all patients. In the subgroup of patients without ascites the predictive power of RMS was higher (r = -0.79, P < 0.001) (Figure 1). This prediction breaks down in the ascites subgroup. The correlation between mMRC score and MELD score was r = 0.43 (P < 0.01), whilst it was higher in the subgroup of end-stage liver disease without ascites (r = 0.51, P = 0.01). No correlation was found between mMRC score and MELD score in the ascites subgroup.
Figure 1.
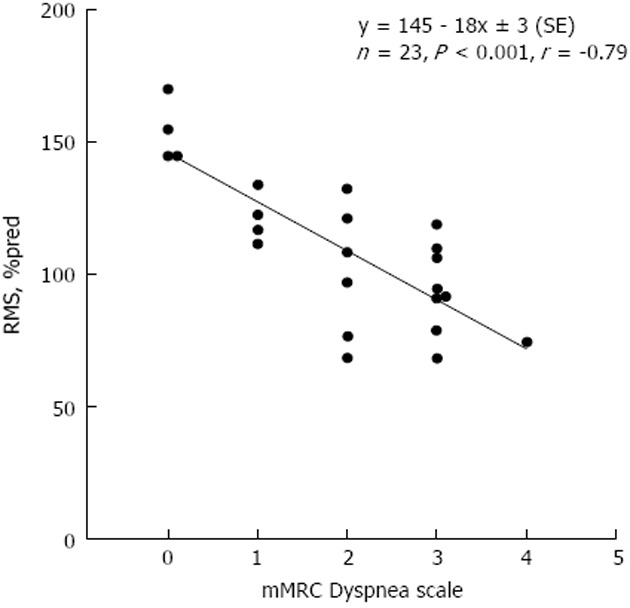
Relationship of respiratory muscle strength (percent of predicted) and modified medical research council dyspnea score in end-stage liver patients without ascites. Solid line: Linear regression. Linear regression equation and corresponding Pearson’s correlation coefficient are shown. The slope of the line indicates that the dyspnea score increases, on average, by one modified medical research council score unit per approximately 20% decrease in respiratory muscle strength, percent of predicted (%pred). RMS: respiratory muscle strength; mMRC: modified medical research council.
MELD score was correlated with Pemax, Pimax, and RMS (r = -0.48, P < 0.01; r = -0.48, P < 0.01; r = -0.48, P < 0.01, respectively). The latter correlations were higher in the subgroup of non-ascites patients (r = -0.64, P < 0.01; r = -0.47, P = 0.02; r = -0.61, P < 0.01, respectively) (Figure 2). MELD score was not correlated with respiratory muscle strength indices in the ascites subgroup.
Figure 2.
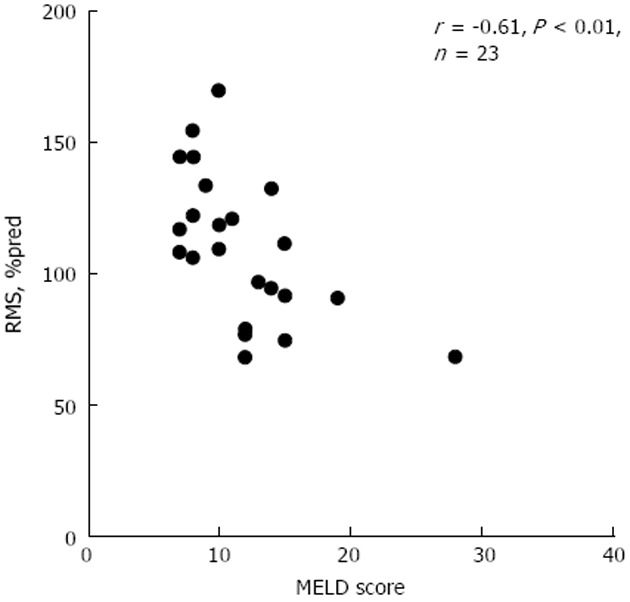
Relationship of respiratory muscle strength (percent of predicted) and end-stage liver disease score in end-stage liver patients without ascites. RMS: respiratory muscle strength; MELD: model for end-stage liver disease; %pred: percent of predicted.
DISCUSSION
The main findings of the present preliminary investigation in patients awaiting liver transplantation are: (1) most patients (88%) report a mild to severe degree of chronic dyspnea; (2) the degree of chronic dyspnea correlates well with respiratory muscle strength; and (3) respiratory muscle strength indices are weakly related to MELD score.
Most liver disease patients studied reported mild to severe chronic dyspnea (range: 0-4) (Table 3). Although chronic dyspnea is the predominant respiratory symptom in patients with liver disease[22], there are scarce reports on its prevalence and measurement. These reports show contradictory data regarding the prevalence of chronic dyspnea, ranging from 0% to 95%[3,23-26]. Nevertheless, none had assessed the severity of chronic dyspnea using such a widely accepted tool as the mMRC chronic dyspnea scale (Table 3). Thus, the prevalence and severity of chronic dyspnea may have been underestimated in previous reports.
There are also scarce data on respiratory muscle strength. Pemax was abnormally low in 30%, Pimax in 38%, and RMS in 35% of our patients. These findings are in agreement with Hourani et al[3]. We have also found that the mean value for Pemax is higher than the mean value of Pimax, consistent with the findings of previous reports[3-5,7]. We also compared Pemax and Pimax in our patients, stratified according to the presence or absence of ascites (Table 3). Our findings are consistent with a few studies comparing these respiratory muscle indices before and after the removal of the ascitic fluid[8,27]. A possible explanation for the lower values of respiratory muscle strength indices in ascites compared to non-ascites group is that the former group of patients had more severe liver disease and a variable degree of mechanical compromise due to ascites per se. In fact, in patients undergoing continuous ambulatory peritoneal dialysis (a situation similar to ascites), Siafakas et al[28] and Prezant et al[29] reported that Pimax, measured in a sitting position, was low during this procedure and increased after the drainage of the fluid. In contrast, Duranti et al[8] found that large volume drainage in patients with tense cirrhotic ascites showed a lack of effect on Pimax, indicating that the cause is not solely mechanical.
Respiratory alkalosis, due to hyperventilation, is the commonest acid-base disorder in patients with chronic liver disease[30,31]. Indeed, PaCO2 was decreased (< 4.7 kPa) in 85% of patients, whilst pH was increased (> 7.45) in 65% of patients in our study (Table 4). This respiratory alkalosis was due to increased VE, VT, and VT/TI (whilst TI/TTOT was normal) indicating increased inspiratory drive intensity (Table 4). Significantly, correlations were found for PaCO2 with VE and VT/TI, providing corroborative evidence. Furthermore, VT/TI was significantly higher in patients with ascites compared to those without ascites (Table 4). This result indicates that the presence of ascites and/or the progression of disease could lead to a further increase in central respiratory drive intensity. What triggers hyperventilation is yet unknown[32]. However, hyperventilation can explain in part the close correlation between chronic dyspnea and RMS in end-stage liver disease patients without ascites. Hyperventilation causes excessive use of the respiratory muscles, which may be mildly impaired. This results in neuromechanical dissociation between increased respiratory drive and respiratory muscle strength probably leading to an increased dyspnea sensation. Therefore, the combination of mild impairment of respiratory muscles and their increased use can cause dyspnea in patients with liver disease.
Correlations were found between chronic dyspnea severity and respiratory muscle strength indices (Pemax, Pimax, and RMS). In the subgroup of patients without ascites, Pemax was abnormally low in 13%, Pimax in 22%, and RMS in 22%. In this subgroup the correlations between respiratory muscle indices and mMRC score were very close, as a result of the absence of ascites (Figure 1). Our data show that patients studied could experience chronic dyspnea, which depends on RMS and not vice versa. Because it is difficult to suggest that chronic dyspnea, known to occur in liver disease patients, causes a decrease of RMS due to disuse atrophy, as increased intensity of respiratory drive tends to exercise, and hence strengthen, the respiratory muscles. Treatment with b-blockers in a subgroup of patients (n = 21; 52%) did not have any significant impact on dyspnea, respiratory muscle strength, and spirometric indices. In contrast, the presence of ascites acts as a strong confounding factor, hence the correlation between chronic dyspnea and respiratory muscle strength breaks down.
A weak correlation was found between mMRC score and MELD score. This correlation was relatively higher in the subgroup of end-stage liver disease without ascites compared to the subgroup of patients with ascites, possibly due to the above-mentioned confounding factor.
A good correlation was detected between MELD score and respiratory muscle indices only in the non-ascites subgroup (Figure 2). Only one study, by Galant et al[4], has shown a correlation between Pimax and MELD score. Few papers discuss the relationship between severity of liver disease as measured by Child-Pugh score and respiratory muscles strength indices[5,6].
In our study, statistical analysis showed that chronic dyspnea does not correlate with hypoxemia, ascites, anemia, and fluid retention.
Low DLCO is the commonest lung function abnormality in end-stage liver disease. In our study, DLCO was abnormally low in 60% of patients. This is in agreement with previous reports showing low DLCO in 55% of liver transplantation candidates[3,33].
As Pimax is a predictor of weaning success from mechanical ventilation and Pemax is a predictor of effective cough[34], further investigations are needed to show whether preoperative measurement of the maximum static respiratory pressures (Pimax and Pemax) could be helpful in liver transplantation candidates.
In conclusion, a mild to severe degree of chronic dyspnea is quite common (88%) in patients with end-stage liver disease. Chronic dyspnea correlates with respiratory muscle strength in all patients. This correlation is close in the subgroup of patients without ascites. This correlation breaks down in the ascites subgroup despite the fact that these patients had weaker respiratory muscle strength. This indicates that the presence of ascites acts as a confounding factor. Respiratory muscle strength indices are weakly related to MELD score. Further studies with increased number of patients are needed to elucidate these findings.
ACKNOWLEDGMENTS
We thank technician Mr Stelios Vechlidis for his valued technical assistance.
COMMENTS
Background
End-stage liver disease may cause mild reduction of respiratory muscle strength and increase ventilation. The combination of both can induce various degrees of dyspnea. The cause of this increased ventilation still remains unknown.
Research frontiers
The exact prevalence and severity of chronic dyspnea in end-stage liver disease remains unknown. Additionally, no report can be found in the literature relating chronic dyspnea to respiratory muscle function in end-stage liver disease patients.
Innovations and breakthroughs
Although chronic dyspnea is the predominant respiratory symptom in patients with liver disease, there are few reports on its prevalence and measurement. These reports show contradictory data regarding the prevalence of chronic dyspnea, ranging from 0% to 95%. Nevertheless, none had assessed the severity of chronic dyspnea using such a widely accepted tool as the modified medical research council chronic dyspnea scale. Thus, the prevalence and severity of chronic dyspnea may have been underestimated in previous reports. There are also scarce data on respiratory muscles strength in end-stage liver disease patients. Measurement of Pemax and Pimax are not fully standardized. No reports in the literature describe in detail the assessment of Pemax and Pimax in liver disease patients. Furthermore, the correlation between chronic dyspnea and respiratory muscle function has not been previously reported in end-stage liver disease patients.
Applications
Further investigations are needed to show whether preoperative measurement of the maximum static respiratory pressures (Pimax and Pemax) could be helpful in the management of liver transplantation candidates.
Terminology
Maximum static expiratory (Pemax) and inspiratory (Pimax) mouth pressures are tests in which patients generate as low inspiratory and as high expiratory pressure as possible against a closed airway. These are tests of respiratory muscle function. Respiratory muscle strength is the arithmetic mean of Pemax and Pimax.
Peer review
The authors studied pulmonary functions of 40 patients with end -stage liver disease referred to a pretransplant clinic. They found that dyspnea was frequently reported by patients and was associated with respiratory muscle strength only in the patients without ascites. The paper is overall well written and provides useful information.
Footnotes
P- Reviewer Xia VW S- Editor Song XX L- Editor Hughes D E- Editor Yan JL
References
- 1.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement: liver transplantation--June 20-23, 1983. Hepatology. 1984;4:107S–110S. [PubMed] [Google Scholar]
- 2.Tessari P. Protein metabolism in liver cirrhosis: from albumin to muscle myofibrils. Curr Opin Clin Nutr Metab Care. 2003;6:79–85. doi: 10.1097/00075197-200301000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Hourani JM, Bellamy PE, Tashkin DP, Batra P, Simmons MS. Pulmonary dysfunction in advanced liver disease: frequent occurrence of an abnormal diffusing capacity. Am J Med. 1991;90:693–700. [PubMed] [Google Scholar]
- 4.Galant LH, Ferrari R, Forgiarini LA, Monteiro MB, Marroni CA, Dias AS. Relationship between MELD severity score and the distance walked and respiratory muscle strength in candidates for liver transplantation. Transplant Proc. 2010;42:1729–1730. doi: 10.1016/j.transproceed.2010.02.087. [DOI] [PubMed] [Google Scholar]
- 5.Augusto VS, Castro E Silva O, Souza ME, Sankarankutty AK. Evaluation of the respiratory muscle strength of cirrhotic patients: relationship with Child-Turcotte-Pugh scoring system. Transplant Proc. 2008;40:774–776. doi: 10.1016/j.transproceed.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Peng S, Plank LD, McCall JL, Gillanders LK, McIlroy K, Gane EJ. Body composition, muscle function, and energy expenditure in patients with liver cirrhosis: a comprehensive study. Am J Clin Nutr. 2007;85:1257–1266. doi: 10.1093/ajcn/85.5.1257. [DOI] [PubMed] [Google Scholar]
- 7.Carvalho EM, Isern MRM, Lima PA, Machado CS, Biagini AP, Massarollo PCB. Força muscular e mortalidade na lista de espera de transplante de fígado. Rev Bras Fisioter. 2008;12:235–240. [Google Scholar]
- 8.Duranti R, Laffi G, Misuri G, Riccardi D, Gorini M, Foschi M, Iandelli I, Mazzanti R, Mancini M, Scano G, et al. Respiratory mechanics in patients with tense cirrhotic ascites. Eur Respir J. 1997;10:1622–1630. doi: 10.1183/09031936.97.10071622. [DOI] [PubMed] [Google Scholar]
- 9.Jones PW. Measurement of breathlessness. In: Hughes JMB, Pride NB, eds , editors. Lung Function Tests: Physiological Principles and Clinical Applications. 1st ed. London: Saunders; 1999. pp. 121–131. [Google Scholar]
- 10.Koulouris N, Mulvey DA, Laroche CM, Green M, Moxham J. Comparison of two different mouthpieces for the measurement of Pimax and Pemax in normal and weak subjects. Eur Respir J. 1988;1:863–867. [PubMed] [Google Scholar]
- 11.Ringqvist T. The ventilatory capacity in healthy subjects. An analysis of causal factors with special reference to the respiratory forces. Scand J Clin Lab Invest Suppl. 1966;88:5–179. [PubMed] [Google Scholar]
- 12.Wilson SH, Cooke NT, Edwards RH, Spiro SG. Predicted normal values for maximal respiratory pressures in caucasian adults and children. Thorax. 1984;39:535–538. doi: 10.1136/thx.39.7.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braun NM, Arora NS, Rochester DF. Respiratory muscle and pulmonary function in polymyositis and other proximal myopathies. Thorax. 1983;38:616–623. doi: 10.1136/thx.38.8.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 15.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 16.D’Angelo E, Prandi E, Marazzini L, Milic-Emili J. Dependence of maximal flow-volume curves on time course of preceding inspiration in patients with chronic obstruction pulmonary disease. Am J Respir Crit Care Med. 1994;150:1581–1586. doi: 10.1164/ajrccm.150.6.7952618. [DOI] [PubMed] [Google Scholar]
- 17.Darling RC, Cournand A, Richards DW. Studies On The Intrapulmonary Mixture Of Gases. III. An Open Circuit Method For Measuring Residual Air. J Clin Invest. 1940;19:609–618. doi: 10.1172/JCI101163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 19.Quanjer PhH. Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J. 1993;Suppl 16:1–100. [PubMed] [Google Scholar]
- 20.Milic-Emili J. Recent Advances in Clinical Assessment of Control of Breathing. Lung. 1982;160:1–17. [Google Scholar]
- 21.Tobin MJ, Chadha TS, Jenouri G, Birch SJ, Gazeroglu HB, Sackner MA. Breathing patterns. 1. Normal subjects. Chest. 1983;84:202–205. doi: 10.1378/chest.84.2.202. [DOI] [PubMed] [Google Scholar]
- 22.Rodríguez-Roisin R, Krowka MJ. Hepatopulmonary syndrome--a liver-induced lung vascular disorder. N Engl J Med. 2008;358:2378–2387. doi: 10.1056/NEJMra0707185. [DOI] [PubMed] [Google Scholar]
- 23.Fallon MB, Abrams GA. Pulmonary dysfunction in chronic liver disease. Hepatology. 2000;32:859–865. doi: 10.1053/jhep.2000.7519. [DOI] [PubMed] [Google Scholar]
- 24.Mohammad Alizadeh AH, Fatemi SR, Mirzaee V, Khoshbaten M, Talebipour B, Sharifian A, Khoram Z, Haj-sheikh-oleslami F, Gholamreza-shirazi M, Zali MR. Clinical features of hepatopulmonary syndrome in cirrhotic patients. World J Gastroenterol. 2006;12:1954–1956. doi: 10.3748/wjg.v12.i12.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Charalabopoulos K, Peschos D, Zoganas L, Bablekos G, Golias C, Charalabopoulos A, Stagikas D, Karakosta A, Papathanasopoulos A, Karachalios G, et al. Alterations in arterial blood parameters in patients with liver cirrhosis and ascites. Int J Med Sci. 2007;4:94–97. doi: 10.7150/ijms.4.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata T, Matsumoto A, Uehara Y, Tanaka G, Oonuma H, Hara K, Igarashi K, Hazama H, Hirata Y, Shiratori Y, et al. Oxygenation abnormalities in normoxemic patients with mild liver cirrhosis. Intern Med. 2002;41:435–440. doi: 10.2169/internalmedicine.41.435. [DOI] [PubMed] [Google Scholar]
- 27.Ordiales Fernández JJ, Fernández Moya A, Nistal de Paz F, Linares Rodríguez A, Colubi Colubi L, Alvarez Asensio E, Rodrigo Sáez L. [Influence of liver cirrhosis with and without ascites on ventilatory mechanics] Rev Esp Enferm Dig. 1995;87:853–857. [PubMed] [Google Scholar]
- 28.Siafakas NM, Argyrakopoulos T, Andreopoulos K, Tsoukalas G, Tzanakis N, Bouros D. Respiratory muscle strength during continuous ambulatory peritoneal dialysis (CAPD) Eur Respir J. 1995;8:109–113. doi: 10.1183/09031936.95.08010109. [DOI] [PubMed] [Google Scholar]
- 29.Prezant DJ, Aldrich TK, Karpel JP, Lynn RI. Adaptations in the diaphragm’s in vitro force-length relationship in patients on continuous ambulatory peritoneal dialysis. Am Rev Respir Dis. 1990;141:1342–1349. doi: 10.1164/ajrccm/141.5_Pt_1.1342. [DOI] [PubMed] [Google Scholar]
- 30.Heinemann HO, Emirgil C, Mijnssen JP. Hyperventilation and arterial hypoxemia in cirrhosis of the liver. Am J Med. 1960;28:239–246. doi: 10.1016/0002-9343(60)90187-x. [DOI] [PubMed] [Google Scholar]
- 31.Snell RE, Luchsinger PC. The relation of arterial hypoxemia to the hyperventilation of chronic liver disease. Am J Med Sci. 1963;245:289–292. [PubMed] [Google Scholar]
- 32.Lustik SJ, Chhibber AK, Kolano JW, Hilmi IA, Henson LC, Morris MC, Bronsther O. The hyperventilation of cirrhosis: progesterone and estradiol effects. Hepatology. 1997;25:55–58. doi: 10.1002/hep.510250110. [DOI] [PubMed] [Google Scholar]
- 33.Krowka MJ, Dickson ER, Wiesner RH, Krom RA, Atkinson B, Cortese DA. A prospective study of pulmonary function and gas exchange following liver transplantation. Chest. 1992;102:1161–1166. doi: 10.1378/chest.102.4.1161. [DOI] [PubMed] [Google Scholar]
- 34.Enright PL, Kronmal RA, Manolio TA, Schenker MB, Hyatt RE. Respiratory muscle strength in the elderly. Correlates and reference values. Cardiovascular Health Study Research Group. Am J Respir Crit Care Med. 1994;149:430–438. doi: 10.1164/ajrccm.149.2.8306041. [DOI] [PubMed] [Google Scholar]


