Abstract
Bisphenol-A (BPA), a polymer used in plastics manufacturing, and methochychlor (MXC) a pesticide, are endocrine disrupting compounds with estrogenic and anti-androgenic properties. Prenatal BPA or MXC treatment induces reproductive defects in sheep with BPA causing prepubertal luteinizing hormone (LH) hypersecretion and dampening of periovulatory LH surges and MXC lengthening follicular phase and delaying the LH surge. In this study, we addressed the underlying neuroendocrine defects by testing the following hypotheses: 1) prenatal BPA but not MXC reduces sensitivity to estradiol and progesterone negative feedback, 2) prenatal BPA but not MXC increases pituitary responsiveness to gonadotropin releasing hormone (GnRH), and 3) prenatal BPA dampens LH surge response to estradiol positive feedback challenge while prenatal MXC delays the timing of the LH surge. Pregnant sheep were treated with either 1) 5 mg/kg/day BPA (produces approximately twice the level found in human circulation, n=8), 2) 5 mg/kg/day MXC (lowest observed effect level stated in the EPA National Toxicology Program’s Report; n=6), or 3) vehicle (cotton seed oil: C: n=6) from days 30 to 90 of gestation. Female offspring of these ewes were ovariectomized at 21 months of age and tested for progesterone negative, estradiol negative, estradiol positive feedback sensitivities and pituitary responsiveness to GnRH. Results revealed that sensitivity to all 3 feedbacks as well as pituitary responsiveness to GnRH were not altered by either of the prenatal treatments. These findings suggest that the postpubertal reproductive defects seen in these animals may have stemmed from ovarian defects and the steroidal signals emanating from them.
Keywords: Endocrine disrupting chemicals, Luteinizing Hormone, Reproductive neuroendocrine, Estradiol positive feedback, Estradiol negative feedback, Progesterone negative feedback
Introduction
Endocrine disrupting compounds (EDCs) are substances that can mimic, modulate, or block the normal function of physiological systems (Diamanti-Kandarakis et al., 2009). In the last 50 years, the number of EDCs that humans are exposed to has increased dramatically (T, Colborn 2011; http://www.endocrinedisruption.com/endocrine.TEDXList.overview.php) posing a real threat to their wellbeing. Bisphenol-A (BPA) and metoxychlor (MXC) are two such EDCs widely studied. Annually, more than eight billion pounds of BPA are produced with more than 100 tons being diffused into the atmosphere (Vandenberg et al., 2012). It is used in the manufacture of plastics and has both estrogenic and anti-androgenic properties. BPA is released into the food chain from polycarbonate containers (baby food bottles and reusable food containers), epoxy resins used in inner coating of metallic food cans, and dental sealants (Vandenberg et al., 2012). BPA is also released from paints and lining of drinking water pipes (vom Saal and Hughes, 2005). A recent NHANES study reported that over 90% of subjects studied from different age categories have detectable levels of BPA in their urine (Calafat et al., 2008). Measurable levels of BPA have been reported in human maternal circulation (Padmanabhan et al., 2008; Schönfelder et al., 2002), cord blood (Kuroda et al., 2003), colostrum (Kuruto-Niwa et al., 2007), breast milk (Ye et al., 2008), and the placenta (Schönfelder et al., 2002).
Methoxychlor (MXC), which has anti-androgenic and estrogenic properties (Staub et al., 2002), is an organochlorine pesticide applied to fruits, vegetables, and animal feed (Reynolds et al., 1976, Agency for Toxic Substances and Disease Registry 2002 http://www.atsdr.cdc.gov/toxguides/toxguide-47.pdf). It has been found in the circulation of men and women (Bottela et al., 2004; Carreño et al., 2007) and adipose tissue of women (Botella et al., 2004). MXC was banned in the European Union in 2002 (http://ec.europa.eu/sanco_pesticides/public/index.cfm?event=activesubstance.selection&a=1) and U.S.A. in 2003 (http://www.epa.gov/oppsrrd1/REDs/methoxychlor_red.htm). It is a persistent chemical (Howard, 1991) and is still found in the environment (Bempah and Donkor, 2011).
Studies in rats and mice have provided evidence that EDCs can alter reproductive function in both males (reviewed in: Wong and Cheng, 2011) and females (mice, rabbits, and cattle; reviewed in Fowler et al., 2012). There is growing interest in EDCs with steroidogenic potential due to their ability to induce cancers (breast [Fenton, 2006], prostate [Muir, 2005], testicular [Garner et al., 2008]), endometriosis (Missmer et al., 2004), and genital abnormalities in boys (Paulozzi et al., 1997). They have been implicated in lower sperm quality in human (Dallinga et al., 2002) as well as pubertal advancement in girls (Roy et al., 2009). A growing body of evidence suggests that perinatal exposure to EDCs leads to adult reproductive dysfunction (reviewed in: Crain et al., 2008). For example, prenatal exposure to BPA was found to advance puberty in mouse (Howdeshell et al., 1999) and trigger sex reversal in crocodilian reptile (Stoker et al., 2003). Similarly, perinatal MXC exposure was found to negatively impact reproductive function in rats (Suzuki et al., 2004).
In female sheep, prenatal exposure to BPA and MXC induces reproductive defects (Savabieasfahani et al., 2006) during postpubertal life. While prenatal BPA treatment was found to induce LH excess early in life and disruption of the periovulatory LH surge manifested as absent or reduced LH surge amplitude (Savabieasfahani et al., 2006), prenatal MXC treatment delayed onset of LH surge without having an effect on the LH surge amplitude. LH excess in prenatal BPA-treated females may be the result of reduced estradiol/progesterone input from the ovary thus providing reduced negative feedback signal, reduced sensitivity of the hypothalamo-pituitary axis to steroid negative feedback, and/or increased pituitary responsiveness to GnRH. The reduced magnitude of LH surge or delay in timing of LH surge on the other hand may result from deficits in estradiol positive feedback mechanisms. In previous studies with prenatal testosterone-treated females, deficits in neuroendocrine feedback systems and increased pituitary sensitivity to GnRH were found to underlie LH excess and LH surge defects (Padmanabhan et al., 2010).
In this study, we addressed if prenatal BPA and MXC treatment has differential effects on neuroendocrine feedback systems (estradiol negative and positive, progesterone negative) and pituitary responsiveness to GnRH. Specifically, we tested the following hypotheses: 1) prenatal BPA, but not MXC treatment would reduce sensitivity to estradiol and progesterone negative feedback, 2) prenatal BPA, but not MXC would increase pituitary responsiveness to GnRH, and 3) prenatal BPA treatment would dampen the LH surge response to estradiol positive feedback challenge while prenatal MXC treatment would delay the timing of LH surge response to estradiol positive feedback challenge.
Material and Methods
Animal breeding and prenatal treatment
All experimental procedures involving animals used in this study were approved by the Institutional Animal Care and Use Committee of the University of Michigan and are consistent with National Research Council’s Guide for the Care and Use of Laboratory Animals. Adult Suffolk ewes of proven fertility were obtained from a local farmer and maintained at the University of Michigan Sheep research facility (Ann Arbor, MI, USA; 42° 18′ N). Beginning two to three weeks prior to breeding, ewes were group fed a daily ration of 0.5 kg shelled corn and 1.0–1.5 kg alfalfa hay to enhance energy intake. Ewes were mated with fertility-proven raddled Suffolk male. Day of mating was determined by visual confirmation of paint marks left on the rumps of ewes by the raddled rams.. Once ewes were bred, they were all kept in the same pasture under natural photoperiod and group-fed a daily ration of 1.25 kg alfalfa/brome mix hay per ewe.
Pregnant ewes (average weight 87.2±2.3 kg) were administrated BPA (purity 99% catalog no. 239658–250G; Sigma-Aldrich) or MXC (purity 95%, catalog number M-1501; Sigma-Aldrich, St. Louis, MO, USA) at 5 mg/kg per day in cotton seed oil (s.c. injections) from day 30 to 90 of gestation (term: 147 days). The doses of prenatal BPA and MXC were selected on the basis of the lowest-observed-adverse-effect-level (LOAEL) stated in the EPA National Toxicology Program’s Report of the Endocrine Disruptors (EPA/630/R-96/012, 1997, http://www.epa.gov/raf/publications/pdfs/ENDOCRINE.PDF). Controls received equal volume of cottonseed oil for the same duration. Levels of BPA achieved in maternal plasma following BPA treatment averaged approximately 40 ng/ml (Savabieasfahani et al., 2006), which is two to three times greater than the highest level reported in pregnant women (Schönfelder et al., 2002; Padmanabhan et al., 2008). Levels of MXC averaged 209 μg/g in fat samples of MXC-treated pregnant ewes and their fetuses and 3.9 μg/g fat. in controls (Savabieasfahani et al., 2006). One study in human reported MXC levels of 0.160 μg/g in fat and 0. 39 ng/ml in serum (Bottela et al., 2004). The levels of MXC residues varied between 10–30 mg/kg of pawpaw fruit obtained from different local markets in Ghana (Bempah and Donkor, 2011). As such MXC residues has the potential to reach the bloodstream through ingestion and accumulate in fat (Bottela et al., 2004).
Experimental design
Control (C, n=6), prenatal BPA-treated (n=8) and prenatal MXC-treated (MXC, n=6) females were utilized in studies described below. These animals are a subset of the same cohort used in our earlier study (Savabieasfahani et al., 2006). Studies described in Savabieasfahani et al., 2006 were carried out during the prepubertal period and at 10 months of age (postpubertal). Only one female offspring from each dam was chosen when twin female births were involved. To enable study of steroid feedback mechanisms, all animals were ovariectomized at 21 months of age. Ovaries were excised via midline abdominal incision under anesthesia (18 mg/kg Sodium Pentobarbitol i.v. [Butler Co., Columbus, OH], 1.5% halothane gas).
Mechanisms underlying differential impact of BPA and MXC on LH excess
Previous studies found prenatal BPA but not MXC treatment induced LH hypersecretion during early life (Savabieasfahani et al., 2006). The following studies were undertaken to determine if alterations in negative feedback mechanisms namely reduced sensitivity to estradiol/progesterone negative feedback at the hypothalamo-pituitary level or increased pituitary responsiveness to GnRH stimulation contributed to the LH excess seen in prenatal BPA- but not MXC-treated females (Savabieasfahani et al., 2006). Figure one illustrates the temporal sequence of the neuroendocrine tests conducted.
Figure 1.
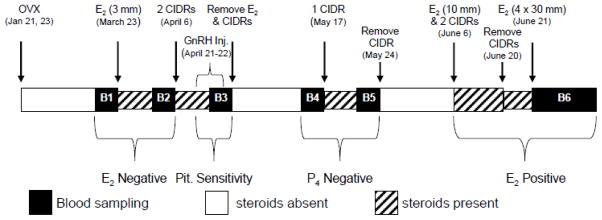
Schematic showing the sequence of neuroendocrine feedback (estradiol and progesterone negative feedback, and estradiol positive feedback) tests and tests for pituitary responsiveness to GnRH. B1 and B2 represent the 6 h estradiol negative feedback bleeds (before and after estradiol, respectively), B3 represents the 9 h pituitary responsiveness bleed, B4 and B5 represent the 6 h progesterone negative feedback test (before and after CIDR, respectively) and B6 represents the 96- h estradiol positive feedback test.
Estradiol negative feedback
The estradiol negative feedback test was carried out 2 months after ovariectomy. Blood samples were obtained at 10 min intervals from all animals to establish circulating patterns LH in the absence of ovarian feedback. Thereafter, a 10 mm SILASTIC capsule (inner diameter, 3.35 mm and outer diameter, 4.65mm; Dow Corning Corp., Midland, MI) filled with 3 mm length of 17β estradiol (Sigma-Aldrich, St. Louis, MO, USA), was inserted subcutaneously into each animal. This size implant produces ≤ 1 pg/ml of estradiol in circulation, levels similar to that found during the early follicular phase of the ovine estrous cycle (Legan et al., 1985). Estradiol levels achieved after insertion of various size implants have been previously reported (Foster, 1984). Eleven days after insertion of estradiol implant, blood samples were collected for a second time at 10 min intervals for 6 h.
Progesterone negative feedback
Approximately 4 months after ovariectomy, all animals were subjected to a progesterone negative feedback test. This involved procurement of blood samples for 6 h at 10 min intervals prior to insertion of one progesterone-releasing device (control internal drug release device: CIDR; DEC International NZ/Ltd, Hamilton, New Zealand). Previous studies have documented that one CIDR produces circulating progesterone levels of 3 to 7 ng/ml (Robinson et al., 2000), levels comparable to that found during the midluteal phase of the ovine estrous cycle (Hauger et al., 1977). One week after CIDR insertion, blood samples were again collected at 10 min intervals for 6 h.
Testing pituitary responsiveness to GnRH
This test was conducted 2 weeks after completion of estradiol negative feedback test and approximately one month prior to the progesterone negative feedback test (three months after ovariectomy). In addition to the 3 mm estradiol implant that was left in place after the estradiol negative feedback test, two CIDRa were inserted into all animals to ablate endogenous GnRH and consequently LH release. Fifteen days after CIDR insertion, a jugular catheter was placed and blood samples were procured for 6 h at 24 min intervals to assess baseline levels. On the following day, all animals were primed by administering 2 ng/kg of GnRH (L7134, Sigma-Aldrich) i.v. at 90 min intervals for a total of 17 injections (Manikkam et al., 2008). GnRH administration of 2 ng/kg has been shown to produce peak GnRH levels of ~ 6 pg/min/mL (Sharma et al., 2012). Peak levels of GnRH achieved during the luteal phase ranges between 0.7 to 18.3 pg/min (Moenter et al., 1991). Blood samples were collected at 10 min intervals during the last 9 h spanning the last 6 GnRH injections. All implants were then removed to maintain a steroid-free environment.
Mechanisms underlying LH surge defects
Defects in timing or magnitude of LH surge may stem from inadequate positive feedback signal from the ovary or disrupted estradiol positive feedback mechanisms at the neuroendocrine level. This study tested the contribution of estradiol positive feedback in inducing LH surge defects in prenatal BPA- and MXC-treated animals; dampened LH surge in BPA- and delayed in MXC-treated females (Savabieasfahani et al., 2006).
Estradiol positive feedback test was undertaken five months after ovariectomy. All animals received a 10 mm estradiol implant and two CIDRs to create an artificial luteal phase. Previous studies have shown that this replacement approach produces plasma concentrations of progesterone and estradiol approximating 6 ng/ml and 0.6 pg/ml, respectively (Van Cleeff et al., 1998). CIDRS were removed 14 days later. Sixteen hours after removal of CIDRs, all animals received four 30 mm estradiol implants subcutaneously (inner diameter, 3.35 mm and outer diameter, 4.65mm; Dow Corning Corp., Midland, MI), which has been shown to produce circulating levels of 3.7 to 11 pg/ml estradiol in adult ovariectomized sheep (Goodman et al., 1981). Periovulatory increases in estradiol range between 7 and 11 pg/ml (Karsch et al., 1979). Blood samples were collected at 2 h intervals beginning 2 h before insertion of estradiol implants and lasting 96 h.
Circulating concentrations of LH was determined in all samples from all tests. Circulating levels of FSH were determined only in samples collected during the test for assessing pituitary responsiveness to GnRH.
Hormone Measurements
Plasma LH and FSH were measured in duplicate using well validated radiommunoassays (LH: Niswender et al., 1969; FSH: Padmanabhan et al., 1997) and expressed in terms of NIH-LH-S12 and NIDDK-ovine FSH-1. The detection limit of LH assay was 0.7±0.1 ng/ml (n=29 assays; mean±SEM). The mean intraassay coefficients of variation calculated based on four quality control pools measuring 2.2±0.1, 15.7±0.4, 13.7±0.5, and 23.8±0.4 ng/ml were 6.8, 5.2, 4.7, and 5%, respectively. The interassay coefficients of variation for the same four quality control pools were 11.9, 13.6, 7.8, and 10.2%, respectively. The detection limit of the FSH assay was 0.7±0.3 ng/ml (n=6). The intra assay coefficients of variation based on FSH quality control pools averaging 4.9±2.2, 5.4±2.2, 21.2±8.6, and 22.9±9.3 ng/ml were 7, 4.1, 2.8, and 2.3%, respectively. The interassay coefficients of variation for the same quality control pools were 7.3, 2.3, 3.3, and 3.3 %, respectively.
Statistical analysis
Values that were below detectable limit (assay sensitivity) were assigned assay sensitivity. Pulse detection from data obtained during estradiol and progesterone negative feedbacks and pituitary GnRH responsiveness tests was by Cluster analysis (Veldhuis and Johnson, 1986). For cluster analysis, the minimum number of data points to identify either a peak (highest concentration reached during a pulse) or a nadir (basal level) was set at two. The Student’s t statistic values used to identify significant increases from preceding nadirs and decreases to following nadirs were set at 2.0.
The variables compared for estradiol and progesterone negative feedbacks are number of pulses, pulse amplitude (difference between nadir and peak), and total LH released per pulse (total of the increment of concentration of LH measured during a detected pulse). For the pituitary GnRH responsiveness test, LH pulse amplitude, total LH and total FSH released in response to GnRH were analyzed.
For the estradiol positive feedback, the variables analyzed were onset, duration, peak (maximum concentration reached), and total LH released. For characterizing LH surge variables, the onset of LH surge was defined as an increase circulating LH above the baseline by two times assay sensitivity lasting 8 h. The end of the surge was defined as the time when LH levels decreased below the threshold for LH surge onset. The LH peak was defined as maximum concentration reached during the LH surge. The interval between onset and end of the LH surge defined its duration.
All variables were tested for homogeneity of variance. Data from LH pulse amplitude and total LH released from the estradiol negative feedback test, LH pulse amplitude and total FSH released during the pituitary responsiveness to GnRH test, and timing of LH surge onset during estradiol positive feedback test were log transformed prior to analyses. To analyze differences in the number of LH pulses a general linear model was used. For all other variables a mixed model was used to test the effects of treatment, time, and interaction between both factors. For multiple comparisons Dunnett adjustment (Field, 2005) was incorporated in both models. P value less than 0.05 was considered significant.
Results
Estradiol negative feedback
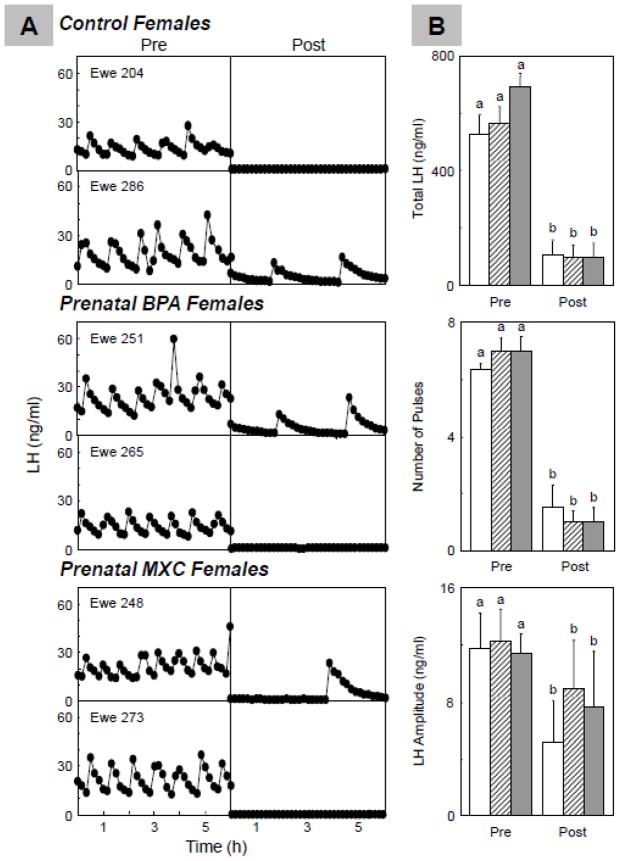
Figure 2 summarizes representative circulating patterns of LH (Panel A) and summary statistics (Panel B) during the estradiol negative feedback test. Prior to the estradiol treatment, number of pulses (7.0±0.5), LH pulse amplitude (12.1±2.3 ng/ml), and total LH released (566.5±54.7 ng/ml) in prenatal BPA-treated females were similar to the controls (number of pulses: 6.3±0.2; LH pulse amplitude: 11.7±2.2 ng/ml; and total LH released: 527.2±61.1 ng/ml) (P=0.147, P=0.996, P=0.908 for the three variables respectively). Number of pulses: (7.0±0.4 total), LH pulse amplitude (11.3±1.3 ng/ml), and total LH released (692.5±41.9 ng/ml) in MXC-treated animals also were similar to controls (control vs. MXC P=0.128, P=0.984, P=0.658, respectively for the 3 variables).
Figure 2.
Results of the estradiol negative feedback. Panel A: Representative LH profiles from control (top) and prenatal BPA- (middle) and MXC-treated (bottom) groups before and after estradiol; Panel B: Mean±SEM of total LH released (top), number of LH pulses (middle), and LH pulse amplitude (bottom) before and after the estradiol challenge in control (n=6; open bars), prenatal BPA- (n=8; hatched bars) and MXC-treated (n=6; closed bars) sheep. For each treatment group (C, BPA and MXC), significant differences between pre and post periods are indicated by differing superscripts (a vs.b; P<0.01).
Estradiol treatment was efficacious in suppressing LH pulse frequency (pre vs. post estradiol, P<0.001), amplitude (pre vs. post estradiol, P<0.01) and total LH released (pre vs. post estradiol, P<0.001) for all 3 groups. Number of LH pulses post estradiol treatment in prenatal BPA-treated (1.0±0.4), and prenatal MXC-treated sheep (1.0±0.4) did not differ from controls (1.5±0.7; BPA vs. C: P=0.492, MXC vs. control P=0.498). LH pulse amplitude in prenatal BPA-treated animals (8.8±3.4 ng/ml) and prenatal MXC-treated animals (7.6±3.5 ng/ml) also did not differ from controls (5.1±2.5 ng/ml; BPA vs. C: P=0.650, MXC vs. P=0.761). Similarly, total LH released in prenatal BPA-treated animals (97.5±38.9 ng/ml) and prenatal MXC-treated animals (98.2±42.1 ng/ml) also did not differ from total LH released in controls (103.4.2±47.8 ng/ml; BPA vs. Control: P=0.832, MXC vs. control: P=0.896).
Progesterone negative feedback
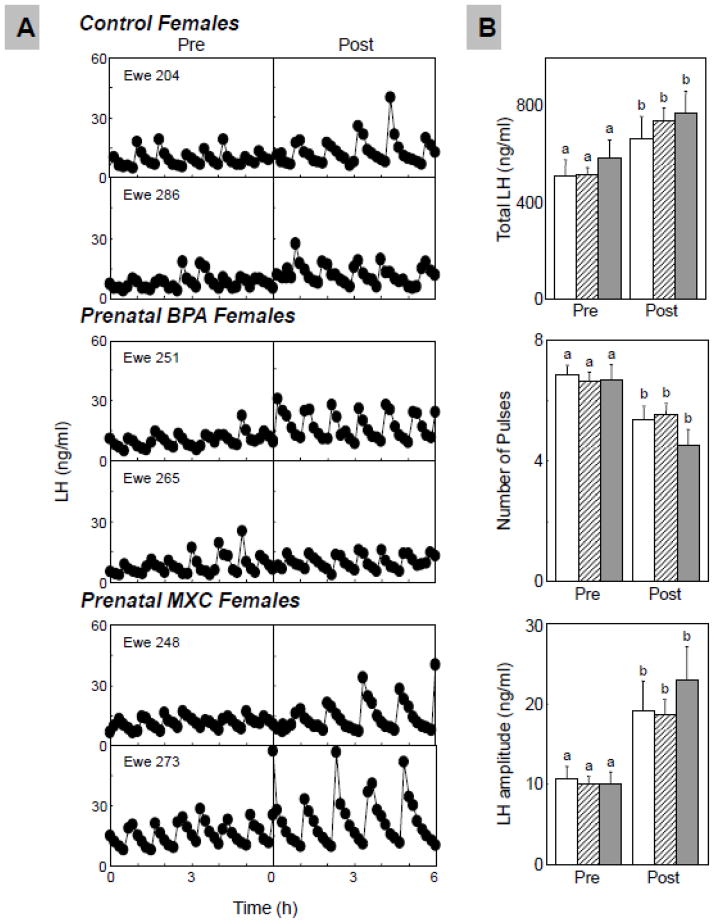
Figure 3 summarizes circulating patterns of LH (Panel A) and summary statistics (Panel B) from the progesterone negative feedback test. Prior to progesterone treatment, variables of LH dynamics in prenatal BPA-treated (number of pulses: 6.6±0.3, P=0.576; LH pulse amplitude: 9.9±0.9 ng/ml, P=0.810; total LH released: 509.5±32.8 ng/ml, P=0.994), or prenatal MXC-treated (number of pulses: 6.7±0.5, P=0.755; LH pulse amplitude: 10.1±1.5 ng/ml, P=0.851; total LH released: 578.2±78.9 ng/ml, P=0.461) females did not differ from the control animals (number of LH pulses: 6.8±0.3; LH pulse amplitude: 10.7±1.4 ng/ml; total LH released: 502.9±63.7 ng/ml).
Figure 3.
Results of the progesterone negative feedback. Panel A: Representative LH profiles of control (top) and prenatal BPA- (middle) and MXC-treated (bottom) group before and after CIDR administration; Panel B: Mean±SEM of total LH released (top), number of LH pulses (middle), and LH pulse amplitude (bottom) before and after CIDR insertion in control (n=6; open bars), prenatal BPA- (n=8; hatched bars) and MXC-treated (n=6; closed bars) sheep. For each treatment group (C, BPA and MXC), significant differences between pre and post periods are indicated by differing superscripts (a vs.b; P<0.01).
Progesterone treatment was efficacious in suppressing the number of LH pulses in all 3 treatment groups (pre vs. post progesterone P<0.001 for all 3 groups). After progesterone treatment, number of LH pulses of prenatal BPA-treated animals (5.5±0.3), and prenatal MXC-treated animals (4.5±0.5) did not differ from number of pulses in control animals (5.3±0.4, BPA vs. control: P=0.736, MXC vs. control: P=0.172). Progesterone treatment significantly increased LH pulse amplitude (P<0.01 for all 3 treatment groups) and total LH released (P<0.001 for all 3 treatment groups), relative to the pretreatment period. During the progesterone treatment period, LH pulse amplitude of prenatal BPA-treated animals (18.6±1.6 ng/ml) and MXC-treated group (23.1±4.2 ng/ml) did not differ from LH amplitude of controls (19.1±3.8 ng/ml; BPA vs. control: P=0.916, MXC vs. control: P=0.287). Similarly, total LH released in the BPA-treated animals (734.4±53.4 ng/ml) and MXC-treated animals (767.2±91.7 ng/ml) also did not differ from the control animals (661.1±78.9 ng/ml; BPA vs. control: P=0.443, MXC vs. control: P=0.303).
Pituitary responsiveness to GnRH test
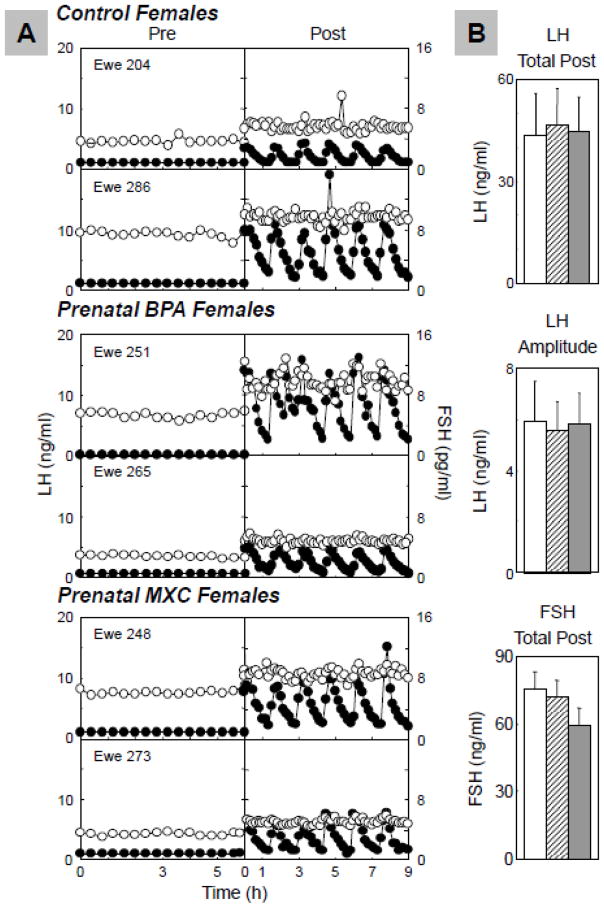
Figure 4 summarizes representative circulating patterns of LH and FSH during the GnRH test (Panel A) and the summary statistics (Panel B). All animals responded to pulsatile administration of GnRH. Total LH released per pulse of prenatal BPA-treated animals (47.0±4.0 ng/ml) and prenatal MXC-treated animals (44.7±3.8) did not differ from controls (43.9±4.2 ng/ml; BPA vs. control: P= 0.833, MXC vs. control: P=0.955). LH pulse amplitude of prenatal BPA-treated animals (5.7±0.6 ng/ml) and prenatal MXC-treated animals (5.8±0.6 ng/ml) also did not differ from controls (5.9±0.7 ng/ml; BPA vs. control: P=0.908, MXC vs. control: P=0.906). Similarly, total FSH released per pulse of prenatal BPA-treated animals (69.5±2.4 ng/ml) and prenatal MXC-treated animals (60.7±2.9 ng/ml) did not differ from controls (74.3±2.6 ng/ml; BPA vs. control: P= 0.317, MXC vs. control: P=0.150).
Figure 4.
Results of pituitary sensitivity to GnRH. Panel A: Representative profiles of LH (solid circles) and FSH (open circles) responses to GnRH of control (top) and prenatal BPA- (middle) and MXC-treated (bottom) sheep; Panel B: Mean±SEM of total LH released/GnRH bolus (top), LH pulse amplitude (middle), and total FSH released (bottom) in control (n=6; open bars), prenatal BPA- (n=8; hatched bars) and MXC-treated (n=6; closed bars) sheep.
Estradiol positive feedback
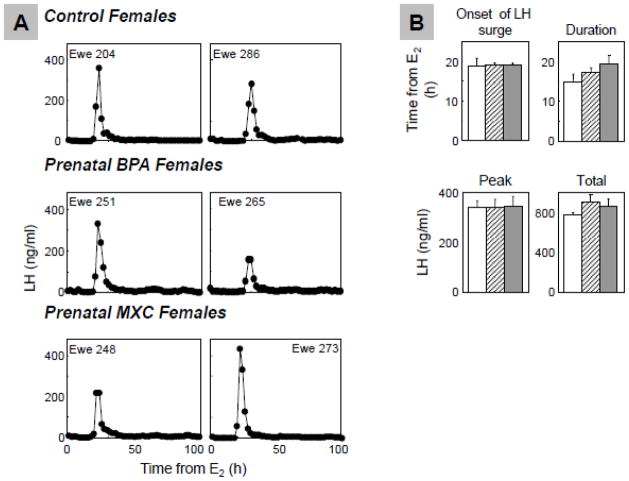
Figure 5 shows the LH surge profiles (Panel A) and summary statistics (Panel B) resulting from estradiol positive feedback tests. All ewes in all treatment groups exhibited an LH surge in response to the estradiol positive feedback test. LH surge onset in prenatal BPA-treated animals (19.1±0.5 h) and prenatal MXC-treated (19.1±0.4 h) did not differ from controls (18.7±1.9 h; BPA vs. control: P=0.826, MXC vs. control: P=0.842). The duration of the LH surge in prenatal BPA-treated females (17.2±1.2 h) and MXC-treated animals (19.3±2.4 h) did not differ from controls (15.0±1.8 h; BPA vs. control: P=0.574, MXC vs. control: P=0.207). Furthermore, peak of LH surge of prenatal BPA-treated animals (336.8±32.3 ng/ml) and MXC-treated animals (342.6±38.7 ng/ml) did not differ from controls (338.3±26.1 ng/ml; BPA vs. control: P=0.999, MXC vs. control: P=0.994). Similarly, total LH released from prenatal BPA-treated (900.3±79.5 ng/m) and prenatal MXC-treated sheep (853.9±76.8 ng/ml) did not differ from controls (767.8±24.7 ng/ml; BPA vs. control: P=0.311, MXC vs. control: P=0.622).
Figure 5.
Results of the estradiol positive feedback. Panel A; Representative patterns of LH surges in control (top), prenatal BPA- (middle) and MXC-treated (bottom) groups; Panel B: Mean±SEM of onset of the LH surge (top left), LH surge duration (top right), LH surge peak (bottom left), total LH releases in surge (bottom right) of control (n=6; open bars), prenatal BPA- (n=8; hatched bars) and MXC-treated (n=6; closed bars) groups.
Discussion
The findings from this study fail to support the hypothesis that the LH excess seen in prenatal BPA-treated sheep is a function of altered sensitivity of neuroendocrine systems to steroid feedback and/or increased pituitary responsiveness to GnRH. Similarly, the LH surge defects seen in prenatal BPA- and MXC-treated females do not appear to stem from estradiol positive feedback disruptions. These findings are discussed in detail below.
The finding that prenatal BPA treatment fails to reduce sensitivity to estradiol negative feedback suggests that the LH excess found early during life (Savabieafsahani et al., 2006) may be a transient phenomenon; the LH excess was evident in prepubertal lambs (first two months of age; Savabieafsahani et al., 2006) while the estradiol feedback test described in this study was conducted during adult life (21 months of age), using a subset of the original cohort of animals. Considering that estrogen receptor 1 (ESR1) is the primary mediator of estradiol negative feedback (Stefanovic et al., 2000; Hardy et al., 2003), the elevation in hypothalamic ESR1 expression seen in prenatal BPA-treated sheep during late follicular phase (Mahoney and Padmanabhan, 2010) is inconsistent with lack of BPA effects on the estradiol negative feedback. One possibility is that the increase in ESR1 mRNA expression in the earlier sheep studies was not reflected at the protein level. While no studies have investigated the impact of the developmental exposure to BPA on estradiol negative feedback in rodents, neonatal exposure to BPA was found not to impact circulating levels of LH in female rats (Fernández et al., 2009).
In contrast, the fact that prenatal MXC treatment did not reduce the sensitivity of the hypothalamo-pituitary axis to estradiol negative feedback is consistent with findings of lack of LH excess in our previous study (Savabieasfahani et al., 2006) and lack of increase in hypothalamic ESR1 expression (Mahoney and Padmanabhan, 2010). Consistent with a lack of effect on the estradiol negative feedback, rodent studies have also found that perinatal MXC treatment had no effect on the hypothalamic ESR1 mRNA expression (Takagi et al., 2005) and number of LH positive cells in the pituitary (Masutomi et al., 2003). Lack of effect of BPA and MXC on the estradiol negative feedback suggests that estrogen is not a mediator of this regulation. Our earlier comparative studies with prenatal testosterone (aromatizable androgen) and DHT (non-aromatizable androgen)-treated sheep (Sarma et al., 2005; Veiga-Lopez et al., 2009), found that estradiol negative feedback deficits were programmed by androgenic not estrogenic actions of testosterone. BPA and MXC have estrogenic and anti-androgenic properties (Staub et al., 2002).
The finding of lack of difference in LH pulse frequency between prenatal BPA- and MXC-treated sheep relative to controls during the progesterone negative feedback test is consistent with our earlier findings of absence of LH pulse frequency changes during the luteal phase of BPA and MXC-treated animals (Savabieasfahani et al., 2006). It is well documented that progesterone negative feedback is exerted at the level of LH pulse frequency (Karsch, 1987). The increase in LH pulse amplitude during the luteal phase of prenatal BPA-treated sheep (Savabieasfahani et al., 2006) was however not evident during the progesterone negative feedback test with the ovariectomized animals (this study). Similar studies have not been carried out in rodent models. Increase in circulating LH concentrations following developmental (between 19 and 22 days post coitum and postnatally between day 0 and 7) exposure to MXC appear to relate to reduced circulating progesterone levels stemming from reduced number of corpora lutea in rats (Armenti et al., 2008).
The lack of effect of prenatal BPA treatment on pituitary responsiveness to GnRH also fail to support altered pituitary sensitivity as a contributor to the LH increase seen in BPA-treated females during early life (Savabiefsahani et al., 2006). On the other hand, the finding of the lack of effects of prenatal MXC treatment on pituitary responsiveness to GnRH is consistent with lack of LH increase. Lack of effect of BPA, the estrogen mimic, is however consistent with our earlier comparative studies in prenatal testosterone (estradiol precursor) and DHT (non aromatizable steroid)-treated sheep that support androgenic mediation of increased pituitary responsiveness to GnRH in sheep (Manikkam et al., 2008). Confirmation of this premise requires further studies targeting blockade of androgen action with anti-androgen. In contrast to lack of effect of prenatal BPA or MXC treatment on LH responsiveness to GnRH in sheep, neonatal treatment with BPA at a higher dose ranging from 25 to 62.5 mg/kg/day diminished pituitary sensitivity to GnRH in female rats (Fernández et al., 2009). Other studies with mice found female offspring exposed in utero to BPA had increased pituitary proliferation and increased gonadotrope number (Brannick et al., 2012). A possibility that cannot be ignored is that enhanced pituitary sensitivity to GnRH was a feature of prepubertal animals thus accounting for the LH increase seen during early life in our earlier study (Savabieasfahani et al., 2006). Differences between studies may relate to species differences, timing of treatment or multiple effects of BPA.
Lack of effect of prenatal BPA- and MXC-treated sheep on estradiol positive feedback at 21 months of age (this study) relative to the reduced magnitude of the LH surge in prenatal BPA-treated and the delayed onset of the LH surge in prenatal MXC-treated sheep evident at 10 months of age (Savabieafsahani et al., 2006) indicates that altered estradiol positive feedback may be a mediator prior to puberty and pubertal hormonal changes may have had an impact in overcoming this deficit. Considering that BPA is an estrogen mimic, the findings from the present study are also at odds with our earlier findings from prenatal testosterone, prenatal DHT, and testosterone and androgen antagonist-treated sheep (Veiga-Lopez et al., 2009), which indicate that estradiol positive feedback is influenced by estrogenic programming,. Lack of impact on estrogen positive feedback in the ovariectomized prenatal BPA and MXC-treated animals, where similar levels of estradiol are delivered via the 4 implants, suggests that compromised LH surge in the ovary-intact prenatal BPA and MXC-treated females may be the result of compromised ovarian estradiol output. While insufficient estradiol signal can fail to elicit an LH surge (Foster 1984), excessive estradiol can downregulate the neuroendocrine system from responding, as appears to be the case with prenatal testosterone-treated sheep (Veiga-Lopez et al., 2009).
The impact of prenatal BPA and MXC treatment on circulating estradiol levels has not been studied in adult sheep. Neonatal BPA was found to increase the number of antral follicles in rats (Adewale et al., 2009), the primary source of estradiol. On the other hand, neonatal MXC treatment decreased the number of antral follicles in rats suggestive of reduced estradiol signal (Uzumcu et al., 2006). Alternatively, changes in nonsteroidal ovarian paracrine modulators (activin/inhibin) may have contributed to the diminution of LH surge in the ovary-intact prenatal BPA-treated sheep reported earlier. For example, in the prenatal testosterone sheep model compromised LH surge dynamics were accompanied by an increase in activin/inhibin ratio prior to LH surge (Veiga-Lopez et al., 2008). Earlier studies in sheep have shown that activin suppresses LH secretion and inhibin increases it (Ghosh et al., 1996).
In summary, changes in neuroendocrine feedback mechanisms or pituitary responsiveness to GnRH fail to explain the LH excess seen in prenatal BPA-treated animals or compromised LH surge dynamics in BPA- and MXC-treated sheep. Instead, they point to mediation via changes in estradiol signal emanating from the ovary that likely reflects changes in the follicular dynamics and estradiol production by the antral follicle.
Highlights.
Prenatal BPA or MXC treatment had no impact on reproductive neuroendocrine steroid feedbacks.
Prenatal BPA or MXC treatment failed to alter pituitary sensitivity to GnRH.
LH hypersecretion in prenatal BPA treated sheep may reduced feedback signals from the ovary.
Acknowledgments
Funding
This work was supported by NIEHS grant ES016541 to VP and training grant support to BAS (T32DK071212).
We are indebted to Doug Doop and Gary McCalla for help with breeding and lambing and excellent animal care; Olga Astapova, David Han, Danielle Djoumbi, Jonathan Flak, Esther Aizenberg, Mozhgan Savabieasfahani and Mohan Manikkam for help with prenatal treatments, participation in feedback tests, and/or performance of LH/FSH assays; Dr. Almudena Veiga-Lopez for her helpful edits and comments.
Footnotes
Declaration of interest
All authors have no conflict of interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81:690–699. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–296. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bempah CK, Donkor AK. Pesticide residues in fruits at the market level in Accra Metropolis, Ghana, a preliminary study. Environ Monit Assess. 2011;175:551–561. doi: 10.1007/s10661-010-1550-0. [DOI] [PubMed] [Google Scholar]
- Botella B, Crespo J, Rivas A, Cerrillo I, Olea-Serrano MF, Olea N. Exposure of women to organochlorine pesticides in Southern Spain. Environ Res. 2004;96:34–40. doi: 10.1016/j.envres.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT. Prenatal Exposure to Low Doses of Bisphenol A Increases Pituitary Proliferation and Gonadotroph Number in Female Mice Offspring at Birth. Biol Reprod. 2012 doi: 10.1095/biolreprod.112.100636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the US population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreño J, Rivas A, Granada A, Jose Lopez-Espinosa M, Mariscal M, Olea N, Olea-Serrano F. Exposure of young men to organochlorine pesticides in Southern Spain. Environ Res. 2007;103:55–61. doi: 10.1016/j.envres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–940. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallinga JW, Moonen EJ, Dumoulin JC, Evers JL, Geraedts JP, Kleinjans JC. Decreased human semen quality and organochlorine compounds in blood. Hum Reprod. 2002;17:1973–1979. doi: 10.1093/humrep/17.8.1973. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147:S18–24. doi: 10.1210/en.2005-1131. [DOI] [PubMed] [Google Scholar]
- Fernández M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117:757–762. doi: 10.1289/ehp.0800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. Discovering Statistics Using:SPSS. 2. Sage Publications; London, Thousand Oaks, New Delhi: 2005. Comparing several means: ANOVA (GLM1) pp. 309–362. [Google Scholar]
- Foster DL. Preovulatory gonadotropin surge system of prepubertal female sheep is exquisitely sensitive to the stimulatory feedback action of estradiol. Endocrinology. 1984;115:1186–1189. doi: 10.1210/endo-115-3-1186. [DOI] [PubMed] [Google Scholar]
- Fowler PA, Bellingham M, Sinclair KD, Evans NP, Pocar P, Fischer B, Schaedlich K, Schmidt JS, Amezaga MR, Bhattacharya S, Rhind SM, O’Shaughnessy PJ. Impact of endocrine-disrupting compounds (EDCs) on female reproductive health. Mol Cell Endocrinol. 2012;355:231–239. doi: 10.1016/j.mce.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Garner M, Turner MC, Ghadirian P, Krewski D, Wade M. Testicular cancer and hormonally active agents. J Toxicol Environ Health B Crit Rev. 2008;11:260–275. doi: 10.1080/10937400701873696. [DOI] [PubMed] [Google Scholar]
- Ghosh BR, Wu JC, Strahl BD, Childs GV, Miller WL. Inhibin and estradiol alter gonadotropes differentially in ovine pituitary cultures: changing gonadotrope numbers and calcium responses to gonadotropin releasing hormone. Endocrinology. 1996;137:5144–5154. doi: 10.1210/endo.137.11.8895389. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol. 1981;89:229–240. doi: 10.1677/joe.0.0890229. [DOI] [PubMed] [Google Scholar]
- Hardy SL, Anderson GM, Valent M, Connors JM, Goodman RL. Evidence that estrogen receptor alpha, but not beta, mediates seasonal changes in the response of the ovine retrochiasmatic area to estradiol. Biol Reprod. 2003;68:846–852. doi: 10.1095/biolreprod.102.010215. [DOI] [PubMed] [Google Scholar]
- Hauger RL, Karsch FJ, Foster DL. A new concept for control of the estrous cycle of the ewe based on the temporal relationships between luteinizing hormone, estradiol and progesterone in peripheral serum and evidence that progesterone inhibits tonic LH secretion. Endocrinology. 1977;101:807–817. doi: 10.1210/endo-101-3-807. [DOI] [PubMed] [Google Scholar]
- Howard PH. Handbook of environmental fate and exposure data for organic chemicals. Vol. 3. Lewis Publishers Inc; Chelsea, Michigan: 1991. pp. 502–504. [Google Scholar]
- Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–764. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- Karsch FJ, Foster DL, Legan SJ, Ryan KD, Peter GK. Control of the preovulatory endocrine events in the ewe: interrelationship of estradiol, progesterone, and luteinizing hormone. Endocrinology. 1979;105:421–426. doi: 10.1210/endo-105-2-421. [DOI] [PubMed] [Google Scholar]
- Karsch FJ. Central actions of ovarian steroids in the feedback regulation of pulsatile secretion of luteinizing hormone. Annu Rev Physiol. 1987;49:356–382. doi: 10.1146/annurev.ph.49.030187.002053. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Kinoshita Y, Sun Y, Wada M, Kishikawa N, Nakashima K, Makino T, Nakazawa H. Measurement of bisphenol A levels in human blood serum and ascetic fluid by HPLC using a fluorescent labeling reagent. J Pharm Biomed Anal. 2003;30:1743–1749. doi: 10.1016/s0731-7085(02)00516-2. [DOI] [PubMed] [Google Scholar]
- Kuruto-Niwa R, Tateoka Y, Usuki Y, Nozawa R. Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160–1164. doi: 10.1016/j.chemosphere.2006.06.073. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Goodman RL, Ryan KD, Foster DL, Karsch FJ. Can the transition into anoestrus in the ewe be accounted for solely by insufficient tonic LH secretion? J Endocrinol. 1985;106:55–60. doi: 10.1677/joe.0.1060055. [DOI] [PubMed] [Google Scholar]
- Mahoney MM, Padmanabhan P. Developmental programming: Impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus. Toxicol Appl Pharmacol. 2010;247:98–104. doi: 10.1016/j.taap.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manikkam M, Thompson RC, Herkimer C, Welch KB, Flak J, Karsch FJ, Padmanabhan V. Developmental programming: impact of prenatal testosterone excess on pre- and postnatal gonadotropin regulation in sheep. Biol Reprod. 2008;78:648–660. doi: 10.1095/biolreprod.107.063347. [DOI] [PubMed] [Google Scholar]
- Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–170. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;6:1501–1508. doi: 10.1016/j.fertnstert.2004.04.065. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129:1175–1182. doi: 10.1210/endo-129-3-1175. [DOI] [PubMed] [Google Scholar]
- Muir KR. Endocrine-disrupting pesticides and selected hormonally dependent cancers. Scand J Work Environ Health. 2005;31:55–61. [PubMed] [Google Scholar]
- Niswender GD, Reichert LE, Midgley AR, Nalbandov AV. Radioimmunoassay for bovine and ovine luteinizing hormone. Endocrinology. 1969;84:1166–1173. doi: 10.1210/endo-84-5-1166. [DOI] [PubMed] [Google Scholar]
- Padmanabhan V, Siefert K, Ransom S, Johnson T, Pinkerton J, Anderson L, Tao L, Kannan K. Maternal bisphenol-A levels at delivery: a looming problem? J Perinatol. 2008;28:258–263. doi: 10.1038/sj.jp.7211913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, Sarma HN, Savabieasfahani M, Steckler TL, Veiga-Lopez A. Developmental reprogramming of reproductive and metabolic dysfunction in sheep: native steroids vs environmental steroid receptor modulators. Int J Androl. 2010;33:394–404. doi: 10.1111/j.1365-2605.2009.01024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan V, McFadden K, Mauger DT, Karsch FJ, Midgley AR., Jr Neuroendocrine control of follicle-stimulating hormone (FSH) secretion: I.Direct evidence for separate episodic and basal components of FSH secretion. Endocrinology. 1997;138:424–432. doi: 10.1210/endo.138.1.4892. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ, Erickson JD, Jackson RJ. Hypospadias trends in two US surveillance systems. Pediatrics. 1997;100:831–834. doi: 10.1542/peds.100.5.831. [DOI] [PubMed] [Google Scholar]
- Reynolds PJ, Lindahl IL, Cecil HC, Bitman J. A comparison of DDT and methoxychlor accumulation and depletion in sheep. Bull Environ Contam Toxicol. 1976;16:240–247. doi: 10.1007/BF01685234. [DOI] [PubMed] [Google Scholar]
- Robinson JE, Healey AE, Harris TG, Messent EA, Skinner DC, Taylor JA, Evans NP. The negative feedback action of progesterone on luteinizing hormone release is not associated with changes in GnRH mRNA expression in the ewe. J Neuroendocrinol. 2000;12:121–129. doi: 10.1046/j.1365-2826.2000.00426.x. [DOI] [PubMed] [Google Scholar]
- Roy JR, Chakraborty S, Chakraborty TR. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Med Sci Monit. 2009;15:RA137–145. [PubMed] [Google Scholar]
- Sarma HN, Manikkam M, Herkimer C, Dell’Orco J, Welch KB, Foster DL, Padmanabhan V. Fetal programming: excess prenatal testosterone reduces postnatal luteinizing hormone, but not follicle-stimulating hormone responsiveness to estradiol negative feedback in the female. Endocrinology. 2005;146:4281–4291. doi: 10.1210/en.2005-0322. [DOI] [PubMed] [Google Scholar]
- Savabieasfahani M, Kannan K, Astapova O, Evans NP, Padmanabhan V. Developmental programming: differential effects of prenatal exposure to bisphenol-A or methoxychlor on reproductive function. Endocrinology. 2006;147:5956–5966. doi: 10.1210/en.2006-0805. [DOI] [PubMed] [Google Scholar]
- Schönfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ Health Perspect. 2002;110:A703–707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Nett TM, Karsch FJ, Phillips DJ, Lee JS, Herkimer C, Padmanabhan V. Neuroendocrine control of FSH secretion: IV. Hypothalamic control of pituitary FSH-regulatory proteins and their relationship to changes in FSH synthesis and secretion. Biol Reprod. 2012;86:1–9. doi: 10.1095/biolreprod.111.098442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub C, Hardy VB, Chapin RE, Harris MW, Johnson L. The hidden effect of estrogenic/antiandrogenic methoxychlor on spermatogenesis. Toxicol Appl Pharmacol. 2002;180:129–135. doi: 10.1006/taap.2002.9369. [DOI] [PubMed] [Google Scholar]
- Stefanovic I, Adrian B, Jansen HT, Lehman MN, Goodman RL. The ability of estradiol to induce Fos expression in a subset of estrogen receptor-alpha-containing neurons in the preoptic area of the ewe depends on reproductive status. Endocrinology. 2000;141:190–196. doi: 10.1210/endo.141.1.7286. [DOI] [PubMed] [Google Scholar]
- Stoker C, Rey F, Rodriguez H, Ramos JG, Sirosky P, Larriera A, Luque EH, Munoz-de-Toro M. Sex reversal effects on Caiman latirostris exposed to environmentally relevant doses of the xenoestrogen bisphenol A. Gen Comp Endocrinol. 2003;133:287–296. doi: 10.1016/s0016-6480(03)00199-0. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Lee HC, Chiba S, Yonezawa T, Nishihara M. Effects of methoxychlor exposure during perinatal period on reproductive function after maturation in rats. J Reprod Dev. 2004;50:455–461. doi: 10.1262/jrd.50.455. [DOI] [PubMed] [Google Scholar]
- Takagi H, Shibutani M, Lee KY, Masutomi N, Fujita H, Inoue K, Mitsumori K, Hirose M. Impact of maternal dietary exposure to endocrine-acting chemicals on progesterone receptor expression in microdissected hypothalamic medial preoptic areas of rat offspring. Toxicol Appl Pharmacol. 2005;15:127–136. doi: 10.1016/j.taap.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol. 2006;191:549–558. doi: 10.1677/joe.1.06592. [DOI] [PubMed] [Google Scholar]
- Van Cleeff J, Karsch FJ, Padmanabhan V. Characterization of endocrine events during the periestrous period in sheep after estrous synchronization with controlled internal drug release (CIDR) device. Domest Anim Endocrinol. 1998;15:23–34. doi: 10.1016/s0739-7240(97)00059-3. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schönfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet. 2012;17:407–434. doi: 10.1590/s1413-81232012000200015. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Astapova OI, Aizenberg EF, Lee JS, Padmanabhan V. Developmental programming: contribution of prenatal androgen and estrogen to estradiol feedback systems and periovulatory hormonal dynamics in sheep. Biol Reprod. 2009;80:718–725. doi: 10.1095/biolreprod.108.074781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga-Lopez A, Ye W, Phillips DJ, Herkimer C, Knight PG, Padmanabhan V. Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol Reprod. 2008;78:636–647. doi: 10.1095/biolreprod.107.065904. [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Johnson ML. Cluster analysis: a simple versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250:E486–493. doi: 10.1152/ajpendo.1986.250.4.E486. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong EW, Cheng CY. Impacts of environmental toxicants on male reproductive dysfunction. Trends Pharmacol Sci. 2011;32:290–299. doi: 10.1016/j.tips.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Bishop AM, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for measuring parabens, triclosan, and other environmental phenols in human milk. Anal Chim Acta. 2008;622:150–156. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]