Abstract
Polybrominated diphenyl ethers (PBDEs) and their oxidative metabolites (hydroxylated PBDEs; OH-BDEs) are known endocrine disrupting contaminants that have been shown to disrupt thyroid hormone regulation both in mammals and in fish. The purpose of this study was to determine the precise organ and tissue locations that express genes critical to thyroid hormone regulation in developing zebrafish (Danio rerio), and to determine the effects of an OH-BDE on their expression. While RT-PCR can provide quantitative data on gene expression, it lacks spatial sensitivity to examine localized gene expression; and, isolation of organs from zebrafish embryos is technically difficult, if not impossible. For this reason, the present study used whole mount in situ hybridization to simultaneously localize and quantify gene expression in vivo. While PBDEs and OH-BDEs have been shown to inhibit the activity and expression of deiodionases, a family of enzymes that regulate thyroid hormone concentrations intracellularly, it is unclear whether or not they can affect regional expression of the different isoforms during early development. In this study we investigated deiodinase 1 (Dio1), deiodinase 2 (Dio2), and deiodinase 3 (Dio3) mRNA expression at the following life stages (2, 8, and 1k-cells; 50%-epiboly, 6 and 18-somites, 22, 24, 48, 72 hpf and/or 10 dpf) in zebrafish and found life stage specific expression of these genes that were highly localized. To demonstrate the use of this technique for investigating potential endocrine disrupting effects, zebrafish embryos were exposed to 1, 10 and 100 nM 6-OH-BDE-47. Significant increases in mean intensity of Dio1 and Dio3 expression in the periventricular zone of brain and pronephric duct, respectively (quantified by measuring intensity of coloration using ImageJ analysis software) were observed, suggesting localized response at the HPT axis with the possibility of impacting neurodevelopment. Our results demonstrate effects of OH-BDEs on thyroid regulating gene expression and provide more insight into potential sites of injury during early life stages.
Keywords: Zebrafish, Gene Expression, Endocrine Disruptors, Flame Retardants, Whole Mount In Situ Hybridization
1. Introduction
In humans, the thyroid hormones thyroxine (T4) and 3, 5, 3′-triiodothyronine (T3) play an important role in regulating development and growth in target tissues (Chan et al., 2003; Johnson and Lema, 2011). This is especially important in regards to the development of the central nervous system (CNS), which begins in utero (Calvo et al., 2002; Fisher, 1997; Kilby et al., 2000). Mild maternal hypothyroidism in early pregnancy has been associated with adverse neuropsychological outcomes, demonstrating that changes in maternal thyroid status affect fetal CNS development (Pop et al., 2003).
In most vertebrates (particularly mammals, fish and amphibians), T4 is considered a pro-hormone, and must first be metabolized to T3 in peripheral tissues by the selenocysteine containing enzymes deiodinase 1 (Dio1) or deiodinase 2 (Dio2) in order to bind to thyroid hormone nuclear receptors (Kohrle, 2000). Dio1 catalyzes both outer ring deiodination (ORD) and inner ring deiodination (IRD), while Dio2 only catalyzes ORD. In contrast, deiodinase 3 (Dio3) only catalyzes IRD and metabolizes T4 into the biologically inactive reverse triiodothyronine (rT3) (Darras and Van Herck, 2012). From the mammalian literature, Dio1 is primarily distributed in kidney, liver and thyroid (Brtko et al., 2002; Richard et al., 1998; Schoenmakers et al., 1992; Visser et al., 1988); Dio2 is distributed in brain, anterior pituitary, placenta, thyroid, skeletal muscle and heart (Bernal, 2002; Bianco et al., 2002; Croteau et al., 1996; Hosoi et al., 1999; Murakami et al., 2001; Salvatore et al., 1996a; Salvatore et al., 1996b); and Dio3 is found in the brain, uterus, and placenta (Darras et al., 1999; Galton et al., 1999; Richard et al., 1998; Salvatore et al., 1995). To date, studies investigating the presence and expression of Dio1, Dio2 and Dio3 have been mostly conducted in juvenile and adult rats.
In contrast to mammals, teleost Dio1 has been detected only in kidney and liver (Johnson and Lema, 2011(; Klaren et al., 2012), Dio2 has been detected in brain, liver, kidney and gonads but not in heart or skeletal muscle (Sambroni et al., 2001); and Dio3 expression was found in gill, brain, eyes and skin (Orozco and Valverde, 2005; Picard-Aitken et al., 2007; Sanders et al., 1999). However, it is worth noting that these studies were restricted to adults. This is due, in part, to the difficulty of characterizing gene expression in embryonic or larval fish that are small in size and undergo rapid differentiation and development (Teraoka et al., 2009).
Zebrafish embryos/larvae has been proposed as a good alternative model for human disease and developmental biology research, particularly for their use in identifying target genes responsive to endocrine disrupting chemicals in the environmental (Hoshijima and Hirose, 2007; Segner, 2009). For example, Walpita et al (2007) reported Dio mRNA expression in early life stages of zebrafish at 8, 12, 23, 30, 36, 48 and 75 hours post fertilization (hpf) using quantitative Real time polymerase chain reactions (qRT-PCR). From 8–48 hpf, Dio2 expression remained at low levels but increased dramatically at 75 hpf. Moreover, Walpita et al knocked down Dio2 expression and found that gene knockdown increased Dio1 mRNA expression, while knocking down Dio1 did not lead to significant change in Dio2 nor Dio3 expression (Walpita et al., 2010; Walpita et al., 2009).
Polybrominated diphenyl ethers (PBDEs) are a class of flame retardant chemicals that are ubiquitous contaminants and have known impacts on thyroid hormone regulation (Birnbaum and Staskal, 2004; Dingemans et al., 2011). They have been added to myriad products, including building materials, furniture,, textiles, plastics, electronic equipment and motor vehicles to decrease risks from fire (Schreiber et al., 2010). However, because of the large volume use of PBDEs, and their widespread contamination and accumulation in human tissues, there has been an increased concern about their potential to elicit human health effects. Maternal transfer (either in utero or through lactation) of PBDEs is one of the major routes of exposure to developing infants, which may be more vulnerable to their effects (de Wit, 2002). Studies suggest that early development may be particularly sensitive to PBDE exposures and toxicity. For example, rodents exposed to PBDEs in utero were found to develop problems with learning, memory function and behavior later in life(Johansson et al., 2008; Viberg et al., 2002, 2003, 2004, 2005, 2007; Viberg et al., 2006). Deficits in neurodevelopment in children have also been associated with PBDE exposure at early life stages (Eskenazi et al., 2013; Herbstman et al., 2010). PBDEs are structurally similar to thyroid hormones, and become even more similar in structure when they are hydroxylated through oxidative metabolism to form OH-BDEs (Qiu et al., 2007; Stapleton et al., 2009). Several studies have demonstrated that PBDE exposure is associated with changes in thyroid hormone concentrations, both in animal exposure studies and with the human population(Chevrier et al., 2010; Fernie et al., 2005; Stapleton et al., 2011; Tomy et al., 2004; Turyk et al., 2008; Zhou et al., 2001). In addition, in vitro studies have demonstrated that the OH-BDEs inhibit thyroid deiodinase activity and likely have greater potency than PBDEs in disrupting thyroid hormone regulation (Butt et al., 2011; Meerts et al., 2001).
Recently, Dio1, Dio2 and Dio3 transcripts were used as biomarkers of endocrine disruption in rats and in other organisms including fish (Schmutzler et al., 2007). Several studies indicated Dio1, Dio2 and Dio3 gene transcript levels were significantly affected by exposure to environmentally realistic concentrations of PBDEs (Egloff et al., 2011; Lema et al., 2008; Schmutzler et al., 2007). For example, after zebrafish embryos were exposed to 0.625 ppm 2,2,4,4′-tetrabromodiphenyl ether (BDE-47) from 24 to 28 hpf, Dio1 and Dio2 mRNA were up-regulated (Usenko et al., 2012). Exposure to the commercial deca-BDE flame retardant (BDE-209) for 14 or 21 days in zebrafish (Li et al., 2011) and Chinese rare minnow (Chen et al., 2012b) significantly up-regulated Dio1 and/or Dio2 mRNA expression. In addition, both BDE-99 and BDE-209 exposure resulted in up-regulation of Dio1 mRNA expression in human hepatocytes (Stapleton et al., 2009). In vivo studies in fish have also demonstrated that exposure to BDE-209 can lead to significant inhibition of Dio activity (Noyes et al., 2011). Furthermore, OH-BDEs have been shown to significantly inhibit activity of Di1 enzyme in vitro in human liver tissues (Butt et al., 2011).
All the above studies measured mRNA expression using qRT-PCR. In the studies with zebrafish and the Chinese rare minnow, results were derived from pools of whole animals and do not provide any information as to the organ/tissues in which these changes occurred, which would be crucial for understanding mechanisms of toxicity. We are then left to wonder whether changes restricted to specific sites would have been determined when diluted by non-responsive organ/tissues of the whole animal. While one could possibly isolate organs/tissues of interest, such tedious procedures are technically difficult in small developing organisms, such as zebrafish embryos. Whole mount in situ hybridization (WISH) has the potential to provide a better platform to localize and quantify gene expression in vivo (Dong et al., 2002). Given these considerations, the aims of this study were to develop a WISH method to examine localized gene expression of Dio1, Dio2 and Dio3 in early zebrafish embryos, and to use this method to investigate the effects of 6-OH-BDE-47, a common PBDE metabolite found in human tissues, and with known potential for Dio inhibition, on mRNA expression of the three deiodinase isoforms.
2. Materials and Methods
2.1. Fish care
Adult zebrafish (Danio rerio) were maintained in a recirculating AHAB system (Aquatic Habitats, Apopka, FL, USA) at 28 °C under a 14:10 light:dark cycle. Adult fish were fed brine shrimp and Zeigler’s Adult Zebrafish Complete Diet (Aquatic Habitats). Embryos were collected after natural spawning of adult zebrafish and were maintained in 30% Danieau solution in petri dishes in an incubator under the same conditions as the adults. From 5 day post-fertilization (dpf), larvae were transferred to 1 L beaker at a density of ~150 larvae/L. Between 6–16 dpf, larvae were fed with dry food LARVAL AP 100 (larval food supplement, Zeigler Bros Inc. Gardeners. PA. USA) 2 times daily. After 16 dpf, larvae were fed with brine shrimp nauplii daily. All larvae were maintained at same conditions as the adults. All care and reproductive techniques were non-invasive and approved by the Duke University Institutional Animal Care & Use Committee (A053-10-03).
2.2. Dosing
OH-BDE standards were purchased neat (>99.5% purity) from Accustandard (New Haven, CT). Stock solutions of 1mM concentration were prepared by dissolving an appropriate amount of the neat standard in dimethylsulfoxide (DMSO; Sigma, St. Louis, MO, USA). Exposure solutions were prepared via serial dilution from stock solution with fish culture system water with concentration of DMSO <0.4%. Zebrafish embryos were exposed to three concentrations of 6-OH-BDE-47 (1, 10 and 100 nM) from 4 hours post fertilization (hpf) until 22 hpf. Around 24 hpf pigmentation of the zebrafish embryo begins to occur. Therefore, exposures ceased at 22 hpf to prevent any confounding of the WISH method from pigmentation in the embryo.
2.3. Whole mount in situ hybridization
Upon reaching desired life stages, zebrafish embryos were fixed in 4% (w/v) paraformaldehyde in phosphate saline solution (pH 7.4) overnight, dechorionated by watchmaker’s forceps, and then stored at −20°C. Whole mount in situ hybridization was carried out according to (Dong et al., 2002). Embryos were hybridized with an antisense probe of 987 base pairs for zebrafish Deiodinase 1 (Dio1, BC_076008.1), an antisense probe of 774 base pairs for zebrafish Deiodinase 2 (Dio2, NM_212789.3), or an antisense probe of 809 base pairs for zebrafish Deiodinase 3 (Dio3, NM_001177935.2), respectively. Dio1, Dio2 and Dio3 probe were cloned with the following primers: Dio1 forward primer, 5′-TGCACTCGCAAATCTCTCACGATG-3′; Dio1 reverse primer, 5′-GCTACTGATGCTACCATTAC-3′; Dio2 forward primer, 5′-ATGGGCTTGCTTAGTGTG GACCTC-3′; Dio2 reverse primer, 5′-TCACTTTCCGTAGCACTTCTCCAG-3′; Dio3 forward primer, 5′-TAGACGTGCAGCACCGCGGA -3′; Dio2 reverse primer, 5′-CGCTCCAGCCAGTCTCTGAG-3′. Following hybridization overnight at 64°C, embryos were washed with 2× SSC (300 mM NaCl, 30 mM sodium citrate, pH 7.0) and 0.2× SSC twice for 30 min, respectively. Next the embryos were blocked with 2% blocking reagent (Roche, Mannheim, Germany), then incubated overnight with 3000× diluted anti-DIG antibody conjugated with alkaline phosphatase (Roche) at 4°C. Lastly, the color reaction was carried out by incubation with BM-purple substrate (Roche).
2.4. Expression localization and quantification
Dio1, Dio2 and Dio3 mRNA expression were recorded with a Nikon Eclipse E600 light microscope, a Nikon DXM 1200 digital camera, and EclipseNet imaging software (Nikon, Melville, NY). To best identify sites of Dio expression indicated by WISH, locations of these sites were compared and referenced with histologic sections of zebrafish of appropriate ages using the online Zfish atlas (http://zfatlas.psu.edu/progress.php).
For quantifying the intensity of Dio1, Dio2 and Dio3 expression, the following procedure was used. First the area, or tissue, of greatest staining intensity was outlined by ImageJ (National Institutes of Health, Bethesda, MD) Analysis Software and the sum of intensity (reaction product plus background) was determined in this region of interest (ROI). Next, the outline was moved to an adjacent region/tissue of the embryo that showed no reaction product and the background intensity was determined. This was then subtracted from the ROI. Using the sum intensity and dividing by the ROI pixel area, the mean intensity was calculated. A minimum of 5 images were examined per developmental stage per treatment.
2.5. Statistical analyses
Expression data are expressed as mean ± SD. GraphPad Prism 4 software was used for statistical analysis. Differences between means were analyzed using one-way ANOVA followed by Dunn’s test. A p value of <0.05 was considered statistically significant.
3. Result
3.1. Localization of Dio1, Dio2 and Dio3 mRNA by WISH
In this experiment, we used WISH to determine the embryonic tissue/organ distribution of Dio1, Dio2 and Dio3 mRNA in various early life stages of zebrafish embryos. Consistent results were found in at least two repeated experiments using 5 embryos or larvae in each developmental stage in each experiment.
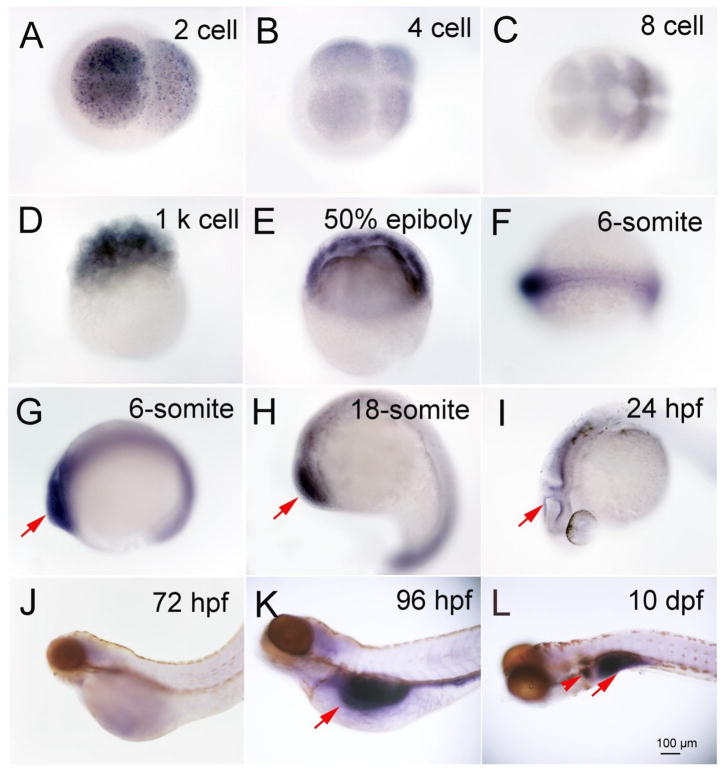
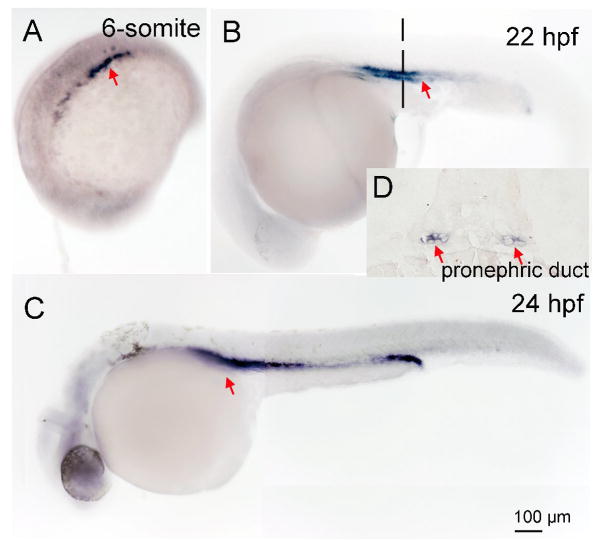
A summary of tissue specific mRNA localization and intensity of the three deiodinases is show in Table 1. Dio1 and Dio2 mRNA expression was detected starting at the two cell stage (0.75 hour, Fig. 1A), and in all subsequent stages [four - (1 h, Fig. 1B), 8- (1.25 h, Fig. 1C), and 1k-cell (3 h, Fig. 1D), 50% epiboly (5.3 h, Fig. 1E), 6- (12 h Fig. 1F and G), and 18 somite (18 h, Fig. 1H), 24- (Fig. 1I), 72 -, and 96 hours post fertilization (hpf) and 10 days post fertilization (dpf) (Fig. 1J, K and L)]. Dio1 and Dio2 expression were co-localized from the two cell stage through the 18 somite stage (Table 1). Between two cell - through 1 k-cell stages, Dio1 and Dio2 mRNA was expressed ubiquitously in cells with variable intensity, and the highest expression was found to be at the 50% epiboly stage. From the 6--through the 18 somite stage, Dio1 and Dio2 mRNA expression was more concentrated at the rostral and caudal regions. Expression was also found in the periventricular zone of brain at 22 - and 24 hpf (Fig. 1I, and Fig. 4A and B), approximate to the cerebral cortex. After 24 hpf, Dio1 and Dio2 mRNA expression differed as to localization. There was only weak Dio1 mRNA expression in the mid-brain region at 48 and 72 hpf, and no other regions displayed any significant expression (Fig. 1J). At 96 hpf, Dio1 was strongly expressed in liver, kidney, and proximal intestine (Fig. 1K). From 96 hpf until 10 day post fertilization (10 dpf), Dio1 mRNA expression was found in the proximal intestine and in the internal tissue (adrenal gland equivalent tissue in zebrafish) (Fig. 1L). In contrast, Dio2 mRNA expression was found in adenohypophysis at 48 hpf (Fig 2. A, B and C), in the swim bladder at 96 hpf, and in the proximal intestine at 10 dpf (Fig. 2E and F). Dio3 mRNA expression was first found in pronephric duct from the 6-somite stage (Fig. 3A) and with increasing area and intensity through 22 - (Fig. 3B) and 24 hpf (Fig. 3C). There was also weak expression in the mid-brain region at 24 hpf (Fig. 3C).
Table 1.
A summary of tissue mRNA localization of three deiodinase.
| Stage/tissues/Gene | Dio1 | Dio2 | Dio3 |
|---|---|---|---|
| 2cell | + | + | − |
| 4cell | + | + | − |
| 8cell | + | + | − |
| 1k cell | +++ | +++ | − |
| 50% epiboly | +++ | +++ | − |
| 6 somite | +++ head |
+++ head |
++ pronephric duct |
| 18 somite | ++ head |
++ head |
++ pronephric duct |
| 24 hpf | ++ periventricular zone of brain |
++ periventricular zone of brain |
+++ pronephric duct |
| 48 hpf | − | ++ Pituitary, spinal cord |
|
| 72 hpf | + head |
++ head |
|
| 96 hpf | +++ Liver and swimming bladder |
+ swimming bladder |
|
| 10 dpf | +++ Kidney and liver |
+ liver |
−, no signal; +, positive singal; moderate signal; +++, strong signal.
Fig. 1.
Expression of Deiodinase 1 in different developmental stages of zebrafish embryo and larva as shown by dark coloration using in situ hybridization. A, 2 cell; B, 4 cell; C, 8 cell; D,1 k cell; E, 6-somite; F and G, 6-somite; H, 18-somite; I, 24 hpf; J, 72 hpf; K, 96 hpf; L, 10 dpf. Red arrows indicate the location of expression.
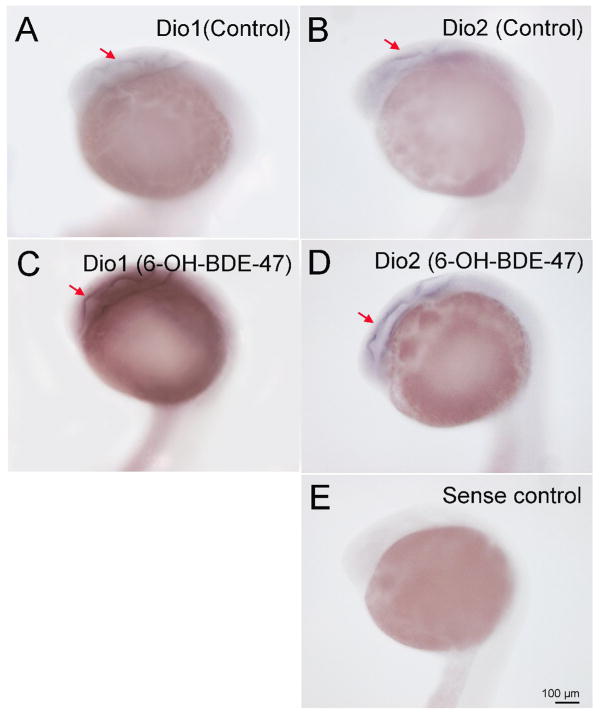
Fig. 4.
Representative images 6-OH-BDE 47 induced Dio1 and Dio2 mRNA expression as detected by whole mount in situ hybridization. A: DMSO control for Dio1; B, DMSO control for Dio2; C 6-OH-BDE 47 exposed for Dio1; D: 6-OH-BDE 47 for Dio2; E: sense control. Red arrows indicated locations of mRNA expression.
Fig. 2.
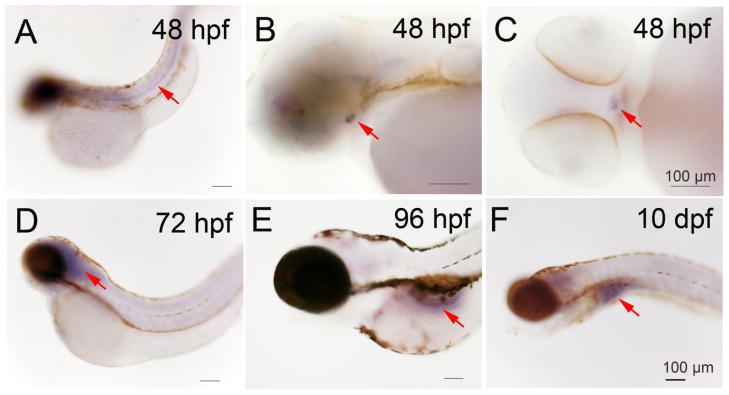
Expression of Deiodinase 2 in zebrafish larva at different time points as shown by dark coloration using in situ hybridization. A, B and C, 48 hpf; D, 72 hpf; E, 96 hpf; F, 10 dpf.
Fig. 3.
Expression of Deiodinase 3 in different developmental stages of zebrafish embryo as shown by dark coloration using in situ hybridization. A, 6-Sommite; B, 22 hpf; C 24 hpf; D, a section of B. Red arrows indicate location of expression.
3.2. Effects of 6-OH-BDE 47 on Dio1, Dio2 and Dio3 mRNA expression visualized by WISH
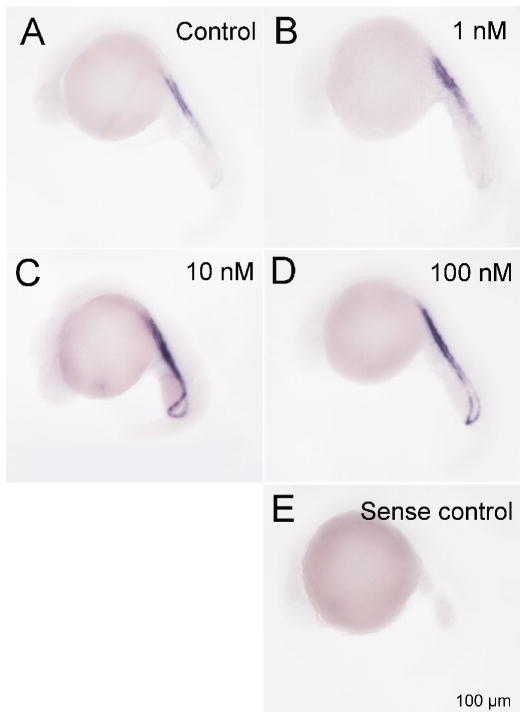
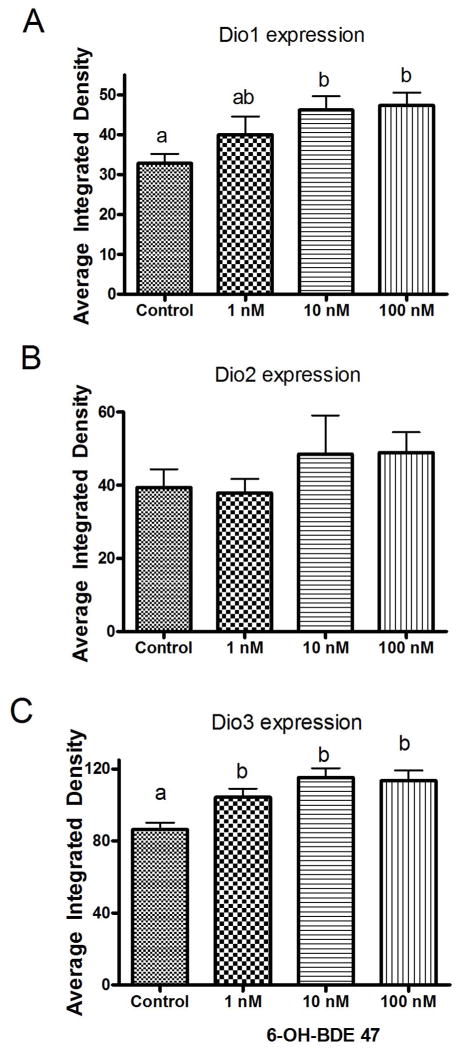
At 22 hpf, Dio1 and Dio2 mRNA expression was found in periventricular zone (Fig. 4), and Dio3 mRNA expression in the pronephric duct respectively (Fig. 3 and 6). Therefore, we selected 22 hpf to conduct the exposure assessments with 6-OH-BDE 47 and examined impacts on Dio1, Dio2 and Dio3 mRNA expression by WISH. We found that exposure to 1-, 10- and 100 nM 6-OH BDE47 increased Dio1 mRNA expression by 21.7% (p-value =0.603), 41% (p <0.05) and 44.2% (p <0.05) in the periventricular zone at 22 hpf relative to control embryos, respectively (Fig. 4A, C and Fig. 5A). Similarly, Dio3 mRNA was significantly increased by 21 %, 33.8% and 31.8% (all p <0.05) by 1 -, 10 -and 100 nM 6-OH BDE 47 concentrations, respectively (Fig. 6 and Fig. 5C). On the contrary, Dio2 mRNA expression was slightly, but not significantly, increased by exposure at 10 - or 100 nM of 6-OH BDE 47 (by 23 % and 24.1% respectively) (p-value =0.297), when compared to the control group (Fig. 4B, C and Fig. 5B).
Fig. 6.
Representative images of 6-OH-BDE-47 induced Dio3 mRNA expression detected using whole mount in situ hybridization. A, Control; B 1nM 6-OH-BDE-47; C, 10 nM 6-OH-BDE-47 and D 100 nM 6-OH-BDE-47; E: sense control. Red arrows indicate locations of Dio3 mRNA expression.
Fig. 5.
Gene expression levels as indicated by intensity of coloration in in situ hybridization between control and 6-OH-BDE-47 exposed groups. Embryos were exposed from 4 hpf to 22 hpf to 1, 10 and 100 nM 6-OH-BDE 47. A. Dio1 B. Dio2 C. Dio3. Different letters indicate significant differences (p<0.05). n=10–15.
4. Discussion
4.1. Differential expression and role of Dio1, Dio2 and Dio3 during early zebrafish development
The results of this study demonstrate that in situ hybridization can provide an indispensable tool for examining regional gene expression in early developmental life stages such as embryos, cells and tissues (Schad et al., 2003; Wang et al., 2012). WISH analysis yielded sufficient sensitivity and resolution to determine tissue-specific gene expression of deiodinases at different developmental stages within the intact organism. We demonstrated that Dio1, Dio2 and Dio3 are expressed in a spatially and temporally specific manners during zebrafish early development. Dio1 and Dio2 expression appeared to decrease steadily as zebrafish embryos developed from 2 - through 8 cell stages. Such findings may indicate that the initial Dio1 and Dio2 were from a maternal source and were distributed amongst daughter cells as the embryos underwent subsequent divisions. Such, a process would result in a steady decrease in expression. From 1k cell stage onward, Dio1 and Dio2 expression levels increased and were highly expressed in the 50% epiboly and 6-somite stages, when neuromere development and primary organogenesis take place in the zebrafish (Kimmel et al., 1995).
Notably, at 22 hpf, Dio1 and Dio2 mRNA expression were concentrated at the periventricular zone of the brain (Fig. 4). In contrast, Dio3 mRNA was found in the pronephric duct from 6-somite stage to 24 hpf. This suggests that embryos have specialized tissues/organs concentrating at de-activating the pro-hormone T4. To our knowledge, this is the first study showing locations where Dio1 Dio2 and Dio3 mRNA expression changes in different embryonic stages (from 2 cell and/or 6-somite stage) of zebrafish. There were no other reports of Dio1 and Dio2 expression in early embryonic stage for fish, but some data were available in amphibians, birds and mammals to support the presence of deiodinase expression in embryos. In most cases, significant Dio1 Dio2 and Dio3 expression was found in the developing brain, which is highly sensitive to thyroid hormones (Horn and Heuer, 2010; Johnson and Lema, 2011; Kaplan and Yaskoski, 1981; Tu et al., 1999; Van Herck et al., 2012). Dio1 and Dio2 are known to play an important role in fish embryonic development (Eales et al., 1993; Kohrle, 2000). Walpita et al (Walpita et al., 2010; Walpita et al., 2009) investigated the biological functions of Dio1 and Dio2 using antisense oligonucleotides and combined knockdown of both deiodinases. Their results demonstrated that Dio2 is essential to otic vesicle and head trunk development, and pigmentation. Remarkably, pigmentation inhibition and development delay due to knockdown of Dio2 was rescued by addition of 50 nM T3 treatment to embryos at 31 hpf (Schmutzler et al., 2007). Given these results, it is not surprising that we found Dio2 mRNA expression in periventricular zone of the brain at 22 and 24 hpf (pharyngula period) in zebrafish embryos. During this period (22–24 hpf) zebrafish embryos show early pigmentation in both the retina and skin (Kimmel et al., 1995). Our observations suggest that zebrafish embryos express Dio2 to regulate T3 in these tissues at 22–24 hpf.
Although T3 concentrations were reported to be very low throughout the morphogenesis period in zebrafish (Walpita et al., 2007), the Dio1 or Dio2 mRNA levels detected in this study suggest there may be a significant capacity for T3 synthesis via deiodination of T4 in these early life stages. Dio1 and Dio2 expression in the periventricular zone at 22 hpf also suggests a role for thyroid hormone signaling in very early neurogenesis. Thus, these results support the role of deiodinases in early embryonic organogenesis of the central nervous system and neuron development. Similar expression patterns were also found in early Xenopus embryos (Morvan Dubois et al., 2006), where deiodinase mRNA (Dio1, Dio2 and Dio3) expression was detected in the head region and axial structures of early stages (NF30 and NF35). This expression pattern has been reported in rats as well (Meerts et al., 2004). This conservation of deiodinase expression pattern between different taxa supports zebrafish as a suitable alternative model for studying thyroid biology and toxicology.
Earlier reports on Dio1 and Dio2 mRNA expression in early stages of zebrafish using qRT PCR indicated that Dio1 mRNA gradually increased during development while Dio2 levels were stable until hatching (Walpita et al., 2007). Our results here are a little different. After hatching and organ differentiation, we only found very weak Dio1 and Dio2 mRNA expression in the brain area at 72 hpf. Similar to a study by Thisse (Thisse et al., 2003), we detected strong Dio1 mRNA expression in the liver, kidney and intestinal bulb at 96 hpf (Fig. 1K) and weak Dio2 mRNA expression in intestinal bulb at 96 hpf (Fig. 2E). This is in agreement with previous data of T3 concentrations peaking at the “metamorphic climax”, i.e., that time when progressive filling of melanophores in the lateral stripe, and swim bladder occur. Also, inflation of the swim bladder occurs at this time (Kimmel et al., 1995). A high level of Dio1 mRNA expression in liver may, in addition, suggest that T4 is being converted to T3 and circulated to support pigmentation. Up through 10 dpf, strong Dio1 expression was detected in the inter-renal gland (adrenal equivalent) and intestine. Interestingly, no Dio2 mRNA was found in inter-renal gland, and was only detected weakly in the swim bladder. In summary both Dio1 and Dio2 appeared to play important roles for maintaining larval development and differentiation, and Dio1 may have more influence than Dio2 over this duration.
Dio3 expression is known to affect cell proliferation (Huang, 2009), and Dio3 has been identified as a thyroid hormone target gene involved in metamorphosis in Xenopus (Becker et al., 1997; Kawahara et al., 1999; Marsh-Armstrong et al., 2004). In our study, zebrafish Dio3 mRNA expression was only detected in the pronephric duct from the 6-somite cell stage to 24 hpf. Nakajima et al (Nakajima et al., 2012) reported that Dio3 expression was the highest at metamorphic climax (stage 60) as measured by qRT-PCR in amphibians. Given that the biological function of Dio3 is likely conserved between amphibians and fish, we hypothesize that it has the same influence on cell proliferation in zebrafish embryos. Our results then indicate that Dio3 was likely involved in kidney development in zebrafish, and this result was similar to Xenopus tropicalis, where Dio3 was detected in pro-nephros at stage 28 early tailbud embryos (Tindall et al., 2007). A similar finding of high expression level of Dio3 transcripts in bovine kidney has also been reported (Connor et al., 2005).
4.2. WISH for detection of Dio1, Dio2 and Dio3 mRNA expression after exposure 6-OH BDE 47
In this study we provided evidence demonstrating that WISH analysis provides information on Dio tissue-specific gene expression at different developmental stages in intact organisms. Given the potential thyroid disrupting effects of some PBDE flame retardants, and their metabolites, we therefore sought to examine the impacts of one specific metabolite, 6-OH BDE 47, on Dio1, Dio2 and Dio3 gene expression in zebrafish. According to our results, 6-OH-BDE-47 (10 and 100 nM) significantly upregulated Dio1 mRNA expression, but no significant induction of Dio2 mRNA at 22 hpf was observed (Fig. 5). In fish, T4 is the predominant form of thyroid hormone synthesized but T4 is not biologically active. Dio1 and Dio2 covert T4 into the biologically active T3 (Orozco and Valverde, 2005). T3 is crucial for growth and differentiation in early stages of zebrafish. Dio3, on the other hand, converts T4 into biologically inactive rT3. Chen et al reported that BDE208 induced T3 concentration in zebrafish lavae and also induced Dio1 and Dio2 mRNA expression in same period. Increased Dio1 can lead to increased conversion of T4 into T3, thus increasing T3 concentrations (Chen et al., 2012a). The decrease of Dio3 may further decrease the inactivation of T3 into T2, thus add to the effect of increasing T3 concentrations.
PBDE congeners (BDE 47, 100 and 209) and OH-BDE metabolites (6-OH-BE 47, 4′-OH-BDE 49) have been detected in high concentrations in human serum, human breast milk and in animals (Birnbaum and Staskal, 2004; Koh et al., 2010; Schecter et al., 2010; Stapleton et al., 2011). 6-OH-BDE 47 is a known metabolite of BDE 47, which is a common PBDE in human tissues. It is worth nothing that 6-OH-BDE 47 is also synthesized naturally in marine sponges and red algae, and thus is a natural product found in the marine environment, and likely, seafood consumed (Hamers et al., 2008; Malmvarn et al., 2005). Therefore not all 6-OH-BDE-47 in humans may be attributable to flame retardant exposure. 6-OH-BDE-47 has also been identified as a ubiquitous compound of toxicological concern in human serum (Zota et al., 2011). Comparisons of several PBDEs and their metabolites revealed that 6-OH-BDE-47 is more toxic to zebrafish embryos than 5-OH-BDE-47, 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47), DE-71 (a commercial PBDE mixture), 2,2′,4,4′,5-pentabromodiphenyl ether (BDE-99), BDE-100, 2,2′,4,4′,5,5′-hexabromodiphneyl ether (BDE-153) and 2,2′,4,4′,5,5′6,6′-deca-bromodiphenyl ether (BDE-209). It leads to induced apoptotic cell death, developmental arrest, and disrupted gene regulations in zebrafish (Nyholm et al., 2009; Usenko et al., 2012). Dio1 and Dio2 mRNA expression in zebrafish adult liver was increased by 18 and 7 fold respectively at 168 hpf by qRT-PCR after exposure to 1.243 μM (0.625 ppm) 6-OH-BDE-47 from 24 hpf to 48 hpf (Usenko et al., 2012). Amongst other PBDEs, BDE-209 and DE-71 have also shown to significantly induce Dio1 and Dio2 mRNA expression at 14 dpf in zebrafish larva (Chen et al., 2012b; Gao et al., 2010). Unsurprisingly BDE-99 and BDE-209 were reported to significantly up-regulate Dio1 mRNA expression in human hepatocytes, but Dio2 mRNA expression was not measured in the study (Stapleton et al., 2009). Conversely, Szabo et al reported both the Dio1 enzyme activity and mRNA expression were decreased in liver of rat male pups at postnatal day 4 (PND4) and PND 21 after maternal exposure to DE-71 from gestational day 6 to PND21. However, DE-71 is mixture of PBDEs including BDE-47, BDE-99, BDE-100, BDE-153 and BDE-154, and low concentrations of brominated dioxin and dibenzofuran contaminants (Hanari et al., 2006; Szabo et al., 2009). Therefore, exposure to DE-71 may result in multiple mechanisms and lead to complex results.
6-OH-BDE-47 also significantly induced Dio3 in a dose-dependent manner, but in the pronephric duct at 6-somite, 22 hpf and 24 hpf zebrafish embryos (Fig. 3). This compares with the amplitude of Dio3 induction by T3 in goldfish (Nelson and Habibi, 2008). In rodents, Dio3 is also expressed in placental tissues and is important in maintaining low thyroid hormone concentrations in the developing fetus (Huang et al., 2003). Up-regulation of Dio3 may lead to lower than normal T3 concentrations in the developing fetus (Chan et al., 2005).
In conclusion, we have used WISH to monitor Dio1, Dio2, and Dio3 tissue specific mRNA expression in very early embryonic stages and to show how such patterns differ temporally. Dio1 and Dio2 expression were present from 2 cell stage onwards but the mRNA may be from maternal source from the 2 to the 1k cell stage. Dio3 was not detected until the 6-somite cell stage. After exposure to a ubiquitous contaminant, 6-OH-BDE 47, Dio1 was increased substantially while Dio2 and Dio3 were only weakly increased. There was also no change in the locations/tissues where the respective Dio1, Dio2 and Dio3 were expressed. Our results suggests that WISH may provide a more detailed account of toxic mechanism(s) and the resultant injury caused by exposure to PBDE and OH-BDEs in early life stages.
Highlights.
Whole mount In situ hybridization (WISH) identifies regional and developmental deiodinase mRNA expression in zebrafish
Deiodinase 1 and 2 were localized in periventricular zone of the brain in zebrafish embryos
Deiodinase 3 was localized in the pronephric duct of zebrafish embryos
6-OH-BDE-47 exposure increases Deiodinase 1 and 3 mRNA expression in developing zebrafish
Acknowledgments
This study was primarily funded by a grant from the National Institute of Environmental Health Sciences (NIEHS), P42 ES010356-10A2. Drs. Dong and Stapleton were also partially supported by a grant from NIEHS, R01ESO16099. We are grateful to Alain Lescure for Deiodinase 1 and Deiodinase 2 plasmid.
Footnotes
The authors declare no competing financial interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Becker KB, Stephens KC, Davey JC, Schneider MJ, Galton VA. The type 2 and type 3 iodothyronine deiodinases play important roles in coordinating development in Rana catesbeiana tadpoles. Endocrinology. 1997;138:2989–2997. doi: 10.1210/endo.138.7.5272. [DOI] [PubMed] [Google Scholar]
- Bernal J. Action of thyroid hormone in brain. J Endocrinol Invest. 2002;25:268–288. doi: 10.1007/BF03344003. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Staskal DF. Brominated flame retardants: cause for concern? Environmental health perspectives. 2004;112:9–17. doi: 10.1289/ehp.6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brtko J, Bobalova J, Podoba J, Schmutzler C, Kohrle J. Thyroid hormone receptors and type I iodothyronine 5′-deiodinase activity of human thyroid toxic adenomas and benign cold nodules. Exp Clin Endocrinol Diabetes. 2002;110:166–170. doi: 10.1055/s-2002-32147. [DOI] [PubMed] [Google Scholar]
- Butt CM, Wang D, Stapleton HM. Halogenated phenolic contaminants inhibit the in vitro activity of the thyroid-regulating deiodinases in human liver. Toxicological sciences : an official journal of the Society of Toxicology. 2011;124:339–347. doi: 10.1093/toxsci/kfr117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo RM, Jauniaux E, Gulbis B, Asuncion M, Gervy C, Contempre B, Morreale de Escobar G. Fetal tissues are exposed to biologically relevant free thyroxine concentrations during early phases of development. J Clin Endocrinol Metab. 2002;87:1768–1777. doi: 10.1210/jcem.87.4.8434. [DOI] [PubMed] [Google Scholar]
- Chan S, Kachilele S, Hobbs E, Bulmer JN, Boelaert K, McCabe CJ, Driver PM, Bradwell AR, Kester M, Visser TJ, Franklyn JA, Kilby MD. Placental iodothyronine deiodinase expression in normal and growth-restricted human pregnancies. J Clin Endocrinol Metab. 2003;88:4488–4495. doi: 10.1210/jc.2003-030228. [DOI] [PubMed] [Google Scholar]
- Chan SY, Andrews MH, Lingas R, McCabe CJ, Franklyn JA, Kilby MD, Matthews SG. Maternal nutrient deprivation induces sex-specific changes in thyroid hormone receptor and deiodinase expression in the fetal guinea pig brain. J Physiol. 2005;566:467–480. doi: 10.1113/jphysiol.2005.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Jia H, Tian Q, Du L, Gao Y, Miao X, Liu Y. Protecting effect of phosphorylation on oxidative damage of D1 protein by down-regulating the production of superoxide anion in photosystem II membranes under high light. Photosynth Res. 2012a;112:141–148. doi: 10.1007/s11120-012-9750-9. [DOI] [PubMed] [Google Scholar]
- Chen Q, Yu L, Yang L, Zhou B. Bioconcentration and metabolism of decabromodiphenyl ether (BDE-209) result in thyroid endocrine disruption in zebrafish larvae. Aquat Toxicol. 2012b;110–111:141–148. doi: 10.1016/j.aquatox.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Chevrier J, Harley KG, Bradman A, Gharbi M, Sjodin A, Eskenazi B. Polybrominated diphenyl ether (PBDE) flame retardants and thyroid hormone during pregnancy. Environmental health perspectives. 2010;118:1444–1449. doi: 10.1289/ehp.1001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor EE, Laiakis EC, Fernandes VM, Williams JL, Capuco AV. Molecular cloning, expression and radiation hybrid mapping of the bovine deiodinase type II (DIO2) and deiodinase type III (DIO3) genes. Anim Genet. 2005;36:240–243. doi: 10.1111/j.1365-2052.2005.01282.x. [DOI] [PubMed] [Google Scholar]
- Croteau W, Davey JC, Galton VA, St Germain DL. Cloning of the mammalian type II iodothyronine deiodinase. A selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest. 1996;98:405–417. doi: 10.1172/JCI118806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darras VM, Hume R, Visser TJ. Regulation of thyroid hormone metabolism during fetal development. Mol Cell Endocrinol. 1999;151:37–47. doi: 10.1016/s0303-7207(99)00088-x. [DOI] [PubMed] [Google Scholar]
- Darras VM, Van Herck SL. Iodothyronine deiodinase structure and function: from ascidians to humans. The Journal of endocrinology. 2012 doi: 10.1530/JOE-12-0204. [DOI] [PubMed] [Google Scholar]
- de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46:583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- Dingemans MM, van den Berg M, Westerink RH. Neurotoxicity of brominated flame retardants: (in)direct effects of parent and hydroxylated polybrominated diphenyl ethers on the (developing) nervous system. Environmental health perspectives. 2011;119:900–907. doi: 10.1289/ehp.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Teraoka H, Yamazaki K, Tsukiyama S, Imani S, Imagawa T, Stegeman JJ, Peterson RE, Hiraga T. 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in the zebrafish embryo: local circulation failure in the dorsal midbrain is associated with increased apoptosis. Toxicological sciences : an official journal of the Society of Toxicology. 2002;69:191–201. doi: 10.1093/toxsci/69.1.191. [DOI] [PubMed] [Google Scholar]
- Eales JG, Morin PP, Tsang P, Hara TJ. Thyroid hormone deiodination in brain, liver, gill, heart and muscle of Atlantic salmon (Salmo salar) during photoperiodically-induced parr-smolt transformation. II. Outer- and inner-ring 3,5,3′-triiodo-L-thyronine and 3,3′,5′-triiodo-L-thyronine (reverse T3) deiodination. Gen Comp Endocrinol. 1993;90:157–167. doi: 10.1006/gcen.1993.1070. [DOI] [PubMed] [Google Scholar]
- Egloff C, Crump D, Chiu S, Manning G, McLaren KK, Cassone CG, Letcher RJ, Gauthier LT, Kennedy SW. In vitro and in ovo effects of four brominated flame retardants on toxicity and hepatic mRNA expression in chicken embryos. Toxicol Lett. 2011;207:25–33. doi: 10.1016/j.toxlet.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rauch SA, Kogut K, Harley KG, Johnson C, Trujillo C, Sjodin A, Bradman A. In Utero and Childhood Polybrominated Diphenyl Ether (PBDE) Exposures and Neurodevelopment in the CHAMACOS Study. Environmental health perspectives. 2013;121:257–262. doi: 10.1289/ehp.1205597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernie KJ, Shutt JL, Mayne G, Hoffman D, Letcher RJ, Drouillard KG, Ritchie IJ. Exposure to polybrominated diphenyl ethers (PBDEs): changes in thyroid, vitamin A, glutathione homeostasis, and oxidative stress in American kestrels (Falco sparverius) Toxicological sciences : an official journal of the Society of Toxicology. 2005;88:375–383. doi: 10.1093/toxsci/kfi295. [DOI] [PubMed] [Google Scholar]
- Fisher DA. Thyroid function in very low birthweight infants. Clin Endocrinol (Oxf) 1997;47:419–421. doi: 10.1046/j.1365-2265.1997.3021106.x. [DOI] [PubMed] [Google Scholar]
- Galton VA, Martinez E, Hernandez A, St Germain EA, Bates JM, St Germain DL. Pregnant rat uterus expresses high levels of the type 3 iodothyronine deiodinase. J Clin Invest. 1999;103:979–987. doi: 10.1172/JCI6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Dong W, Hu M, Yu M, Guo L, Qian L, Guo N, Song L. GADD45alpha mediates arsenite-induced cell apoptotic effect in human hepatoma cells via JNKs/AP-1-dependent pathway. J Cell Biochem. 2010;109:1264–1273. doi: 10.1002/jcb.22509. [DOI] [PubMed] [Google Scholar]
- Hamers T, Kamstra JH, Sonneveld E, Murk AJ, Visser TJ, Van Velzen MJ, Brouwer A, Bergman A. Biotransformation of brominated flame retardants into potentially endocrine-disrupting metabolites, with special attention to 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) Molecular nutrition & food research. 2008;52:284–298. doi: 10.1002/mnfr.200700104. [DOI] [PubMed] [Google Scholar]
- Hanari N, Kannan K, Miyake Y, Okazawa T, Kodavanti PR, Aldous KM, Yamashita N. Occurrence of polybrominated biphenyls, polybrominated dibenzo-p-dioxins, and polybrominated dibenzofurans as impurities in commercial polybrominated diphenyl ether mixtures. Environmental science & technology. 2006;40:4400–4405. doi: 10.1021/es060559k. [DOI] [PubMed] [Google Scholar]
- Herbstman JB, Sjodin A, Kurzon M, Lederman SA, Jones RS, Rauh V, Needham LL, Tang D, Niedzwiecki M, Wang RY, Perera F. Prenatal exposure to PBDEs and neurodevelopment. Environmental health perspectives. 2010;118:712–719. doi: 10.1289/ehp.0901340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S, Heuer H. Thyroid hormone action during brain development: more questions than answers. Mol Cell Endocrinol. 2010;315:19–26. doi: 10.1016/j.mce.2009.09.008. [DOI] [PubMed] [Google Scholar]
- Hoshijima K, Hirose S. Expression of endocrine genes in zebrafish larvae in response to environmental salinity. The Journal of endocrinology. 2007;193:481–491. doi: 10.1677/JOE-07-0003. [DOI] [PubMed] [Google Scholar]
- Hosoi Y, Murakami M, Mizuma H, Ogiwara T, Imamura M, Mori M. Expression and regulation of type II iodothyronine deiodinase in cultured human skeletal muscle cells. J Clin Endocrinol Metab. 1999;84:3293–3300. doi: 10.1210/jcem.84.9.5969. [DOI] [PubMed] [Google Scholar]
- Huang SA. Deiodination and cellular proliferation: parallels between development, differentiation, tumorigenesis, and now regeneration. Endocrinology. 2009;150:3–4. doi: 10.1210/en.2008-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SA, Dorfman DM, Genest DR, Salvatore D, Larsen PR. Type 3 iodothyronine deiodinase is highly expressed in the human uteroplacental unit and in fetal epithelium. J Clin Endocrinol Metab. 2003;88:1384–1388. doi: 10.1210/jc.2002-021291. [DOI] [PubMed] [Google Scholar]
- Johansson N, Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to deca-brominated diphenyl ether (PBDE 209) causes dose-response changes in spontaneous behaviour and cholinergic susceptibility in adult mice. Neurotoxicology. 2008;29:911–919. doi: 10.1016/j.neuro.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Lema SC. Tissue-specific thyroid hormone regulation of gene transcripts encoding iodothyronine deiodinases and thyroid hormone receptors in striped parrotfish (Scarus iseri) Gen Comp Endocrinol. 2011;172:505–517. doi: 10.1016/j.ygcen.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Kaplan MM, Yaskoski KA. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest. 1981;67:1208–1214. doi: 10.1172/JCI110136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara A, Gohda Y, Hikosaka A. Role of type III iodothyronine 5-deiodinase gene expression in temporal regulation of Xenopus metamorphosis. Dev Growth Differ. 1999;41:365–373. doi: 10.1046/j.1440-169x.1999.413431.x. [DOI] [PubMed] [Google Scholar]
- Kilby MD, Gittoes N, McCabe C, Verhaeg J, Franklyn JA. Expression of thyroid receptor isoforms in the human fetal central nervous system and the effects of intrauterine growth restriction. Clin Endocrinol (Oxf) 2000;53:469–477. doi: 10.1046/j.1365-2265.2000.01074.x. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Klaren PH, Geven EJ, Nagelkerke A, Flik G. Kinetics and thiol requirements of iodothyronine 5′-deiodination are tissue-specific in common carp (Cyprinus carpio L.) Comp Biochem Physiol B Biochem Mol Biol. 2012;161:275–282. doi: 10.1016/j.cbpb.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Koh TW, Chih-Cheng Chen S, Chang-Chien GP, Lin DY, Chen FA, Chao HR. Breast-milk levels of polybrominated diphenyl ether flame retardants in relation to women’s age and pre-pregnant body mass index. Int J Hyg Environ Health. 2010;213:59–65. doi: 10.1016/j.ijheh.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Kohrle J. The deiodinase family: selenoenzymes regulating thyroid hormone availability and action. Cell Mol Life Sci. 2000;57:1853–1863. doi: 10.1007/PL00000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema SC, Dickey JT, Schultz IR, Swanson P. Dietary exposure to 2,2′,4,4′-tetrabromodiphenyl ether (PBDE-47) alters thyroid status and thyroid hormone-regulated gene transcription in the pituitary and brain. Environmental health perspectives. 2008;116:1694–1699. doi: 10.1289/ehp.11570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Zhu L, Zha J, Wang Z. Effects of decabromodiphenyl ether (BDE-209) on mRNA transcription of thyroid hormone pathway and spermatogenesis associated genes in Chinese rare minnow (Gobiocypris rarus) Environ Toxicol. 2011 doi: 10.1002/tox.20767. [DOI] [PubMed] [Google Scholar]
- Malmvarn A, Marsh G, Kautsky L, Athanasiadou M, Bergman A, Asplund L. Hydroxylated and methoxylated brominated diphenyl ethers in the red algae Ceramium tenuicorne and blue mussels from the Baltic Sea. Environmental science & technology. 2005;39:2990–2997. doi: 10.1021/es0482886. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, Cai L, Brown DD. Thyroid hormone controls the development of connections between the spinal cord and limbs during Xenopus laevis metamorphosis. Proc Natl Acad Sci U S A. 2004;101:165–170. doi: 10.1073/pnas.2136755100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerts IA, Hoving S, van den Berg JH, Weijers BM, Swarts HJ, van der Beek EM, Bergman A, Koeman JH, Brouwer A. Effects of in utero exposure to 4-hydroxy-2,3,3′,4′,5-pentachlorobiphenyl (4-OH-CB107) on developmental landmarks, steroid hormone levels, and female estrous cyclicity in rats. Toxicological sciences : an official journal of the Society of Toxicology. 2004;82:259–267. doi: 10.1093/toxsci/kfh251. [DOI] [PubMed] [Google Scholar]
- Meerts IA, Letcher RJ, Hoving S, Marsh G, Bergman A, Lemmen JG, van der Burg B, Brouwer A. In vitro estrogenicity of polybrominated diphenyl ethers, hydroxylated PDBEs, and polybrominated bisphenol A compounds. Environmental health perspectives. 2001;109:399–407. doi: 10.1289/ehp.01109399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morvan Dubois G, Sebillot A, Kuiper GG, Verhoelst CH, Darras VM, Visser TJ, Demeneix BA. Deiodinase activity is present in Xenopus laevis during early embryogenesis. Endocrinology. 2006;147:4941–4949. doi: 10.1210/en.2006-0609. [DOI] [PubMed] [Google Scholar]
- Murakami M, Araki O, Hosoi Y, Kamiya Y, Morimura T, Ogiwara T, Mizuma H, Mori M. Expression and regulation of type II iodothyronine deiodinase in human thyroid gland. Endocrinology. 2001;142:2961–2967. doi: 10.1210/endo.142.7.8280. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Fujimoto K, Yaoita Y. Regulation of thyroid hormone sensitivity by differential expression of the thyroid hormone receptor during Xenopus metamorphosis. Genes Cells. 2012;17:645–659. doi: 10.1111/j.1365-2443.2012.01614.x. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Habibi HR. Functional significance of a truncated thyroid receptor subtype lacking a hormone-binding domain in goldfish. Endocrinology. 2008;149:4702–4709. doi: 10.1210/en.2008-0107. [DOI] [PubMed] [Google Scholar]
- Noyes PD, Hinton DE, Stapleton HM. Accumulation and debromination of decabromodiphenyl ether (BDE-209) in juvenile fathead minnows (Pimephales promelas) induces thyroid disruption and liver alterations. Toxicological sciences : an official journal of the Society of Toxicology. 2011;122:265–274. doi: 10.1093/toxsci/kfr105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm JR, Norman A, Norrgren L, Haglund P, Andersson PL. Uptake and biotransformation of structurally diverse brominated flame retardants in zebrafish (Danio rerio) after dietary exposure. Environ Toxicol Chem. 2009;28:1035–1042. doi: 10.1897/08-302.1. [DOI] [PubMed] [Google Scholar]
- Orozco A, Valverde RC. Thyroid hormone deiodination in fish. Thyroid. 2005;15:799–813. doi: 10.1089/thy.2005.15.799. [DOI] [PubMed] [Google Scholar]
- Picard-Aitken M, Fournier H, Pariseau R, Marcogliese DJ, Cyr DG. Thyroid disruption in walleye (Sander vitreus) exposed to environmental contaminants: cloning and use of iodothyronine deiodinases as molecular biomarkers. Aquat Toxicol. 2007;83:200–211. doi: 10.1016/j.aquatox.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59:282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- Qiu X, Mercado-Feliciano M, Bigsby RM, Hites RA. Measurement of polybrominated diphenyl ethers and metabolites in mouse plasma after exposure to a commercial pentabromodiphenyl ether mixture. Environmental health perspectives. 2007;115:1052–1058. doi: 10.1289/ehp.10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard K, Hume R, Kaptein E, Sanders JP, van Toor H, De Herder WW, den Hollander JC, Krenning EP, Visser TJ. Ontogeny of iodothyronine deiodinases in human liver. J Clin Endocrinol Metab. 1998;83:2868–2874. doi: 10.1210/jcem.83.8.5032. [DOI] [PubMed] [Google Scholar]
- Salvatore D, Bartha T, Harney JW, Larsen PR. Molecular biological and biochemical characterization of the human type 2 selenodeiodinase. Endocrinology. 1996a;137:3308–3315. doi: 10.1210/endo.137.8.8754756. [DOI] [PubMed] [Google Scholar]
- Salvatore D, Low SC, Berry M, Maia AL, Harney JW, Croteau W, St Germain DL, Larsen PR. Type 3 lodothyronine deiodinase: cloning, in vitro expression, and functional analysis of the placental selenoenzyme. J Clin Invest. 1995;96:2421–2430. doi: 10.1172/JCI118299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore D, Tu H, Harney JW, Larsen PR. Type 2 iodothyronine deiodinase is highly expressed in human thyroid. J Clin Invest. 1996b;98:962–968. doi: 10.1172/JCI118880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambroni E, Gutieres S, Cauty C, Guiguen Y, Breton B, Lareyre JJ. Type II iodothyronine deiodinase is preferentially expressed in rainbow trout (Oncorhynchus mykiss) liver and gonads. Molecular reproduction and development. 2001;60:338–350. doi: 10.1002/mrd.1096. [DOI] [PubMed] [Google Scholar]
- Sanders JP, Van der Geyten S, Kaptein E, Darras VM, Kuhn ER, Leonard JL, Visser TJ. Cloning and characterization of type III iodothyronine deiodinase from the fish Oreochromis niloticus. Endocrinology. 1999;140:3666–3673. doi: 10.1210/endo.140.8.6902. [DOI] [PubMed] [Google Scholar]
- Schad A, Fahimi HD, Volkl A, Baumgart E. Expression of catalase mRNA and protein in adult rat brain: detection by nonradioactive in situ hybridization with signal amplification by catalyzed reporter deposition (ISH-CARD) and immunohistochemistry (IHC)/immunofluorescence (IF) J Histochem Cytochem. 2003;51:751–760. doi: 10.1177/002215540305100606. [DOI] [PubMed] [Google Scholar]
- Schecter A, Colacino J, Sjodin A, Needham L, Birnbaum L. Partitioning of polybrominated diphenyl ethers (PBDEs) in serum and milk from the same mothers. Chemosphere. 2010;78:1279–1284. doi: 10.1016/j.chemosphere.2009.12.016. [DOI] [PubMed] [Google Scholar]
- Schmutzler C, Gotthardt I, Hofmann PJ, Radovic B, Kovacs G, Stemmler L, Nobis I, Bacinski A, Mentrup B, Ambrugger P, Gruters A, Malendowicz LK, Christoffel J, Jarry H, Seidlova-Wuttke D, Wuttke W, Kohrle J. Endocrine disruptors and the thyroid gland--a combined in vitro and in vivo analysis of potential new biomarkers. Environmental health perspectives. 2007;115(Suppl 1):77–83. doi: 10.1289/ehp.9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenmakers CH, Pigmans IG, Visser TJ. Species differences in liver type I iodothyronine deiodinase. Biochim Biophys Acta. 1992;1121:160–166. doi: 10.1016/0167-4838(92)90349-i. [DOI] [PubMed] [Google Scholar]
- Schreiber T, Gassmann K, Gotz C, Hubenthal U, Moors M, Krause G, Merk HF, Nguyen NH, Scanlan TS, Abel J, Rose CR, Fritsche E. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: evidence for endocrine disruption. Environmental health perspectives. 2010;118:572–578. doi: 10.1289/ehp.0901435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segner H. Zebrafish (Danio rerio) as a model organism for investigating endocrine disruption. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2009;149:187–195. doi: 10.1016/j.cbpc.2008.10.099. [DOI] [PubMed] [Google Scholar]
- Solis LM, Behrens C, Dong W, Suraokar M, Ozburn NC, Moran CA, Corvalan AH, Biswal S, Swisher SG, Bekele BN, Minna JD, Stewart DJ, Wistuba II. Nrf2 and Keap1 abnormalities in non-small cell lung carcinoma and association with clinicopathologic features. Clin Cancer Res. 2010;16:3743–3753. doi: 10.1158/1078-0432.CCR-09-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Eagle S, Anthopolos R, Wolkin A, Miranda ML. Associations between polybrominated diphenyl ether (PBDE) flame retardants, phenolic metabolites, and thyroid hormones during pregnancy. Environmental health perspectives. 2011;119:1454–1459. doi: 10.1289/ehp.1003235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton HM, Kelly SM, Pei R, Letcher RJ, Gunsch C. Metabolism of polybrominated diphenyl ethers (PBDEs) by human hepatocytes in vitro. Environmental health perspectives. 2009;117:197–202. doi: 10.1289/ehp.11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo DT, Richardson VM, Ross DG, Diliberto JJ, Kodavanti PR, Birnbaum LS. Effects of perinatal PBDE exposure on hepatic phase I, phase II, phase III, and deiodinase 1 gene expression involved in thyroid hormone metabolism in male rat pups. Toxicological sciences : an official journal of the Society of Toxicology. 2009;107:27–39. doi: 10.1093/toxsci/kfn230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teraoka H, Kubota A, Dong W, Kawai Y, Yamazaki K, Mori C, Harada Y, Peterson RE, Hiraga T. Role of the cyclooxygenase 2-thromboxane pathway in 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced decrease in mesencephalic vein blood flow in the zebrafish embryo. Toxicol Appl Pharmacol. 2009;234:33–40. doi: 10.1016/j.taap.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Thisse C, Degrave A, Kryukov GV, Gladyshev VN, Obrecht-Pflumio S, Krol A, Thisse B, Lescure A. Spatial and temporal expression patterns of selenoprotein genes during embryogenesis in zebrafish. Gene Expr Patterns. 2003;3:525–532. doi: 10.1016/s1567-133x(03)00054-1. [DOI] [PubMed] [Google Scholar]
- Tindall AJ, Morris ID, Pownall ME, Isaacs HV. Expression of enzymes involved in thyroid hormone metabolism during the early development of Xenopus tropicalis. Biol Cell. 2007;99:151–163. doi: 10.1042/BC20060074. [DOI] [PubMed] [Google Scholar]
- Tomy GT, Palace VP, Halldorson T, Braekevelt E, Danell R, Wautier K, Evans B, Brinkworth L, Fisk AT. Bioaccumulation, biotransformation, and biochemical effects of brominated diphenyl ethers in juvenile lake trout (Salvelinus namaycush) Environmental science & technology. 2004;38:1496–1504. doi: 10.1021/es035070v. [DOI] [PubMed] [Google Scholar]
- Tu HM, Legradi G, Bartha T, Salvatore D, Lechan RM, Larsen PR. Regional expression of the type 3 iodothyronine deiodinase messenger ribonucleic acid in the rat central nervous system and its regulation by thyroid hormone. Endocrinology. 1999;140:784–790. doi: 10.1210/endo.140.2.6486. [DOI] [PubMed] [Google Scholar]
- Turyk ME, Persky VW, Imm P, Knobeloch L, Chatterton R, Anderson HA. Hormone disruption by PBDEs in adult male sport fish consumers. Environmental health perspectives. 2008;116:1635–1641. doi: 10.1289/ehp.11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usenko CY, Hopkins DC, Trumble SJ, Bruce ED. Hydroxylated PBDEs induce developmental arrest in zebrafish. Toxicol Appl Pharmacol. 2012;262:43–51. doi: 10.1016/j.taap.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Van Herck SL, Geysens S, Delbaere J, Tylzanowski P, Darras VM. Expression profile and thyroid hormone responsiveness of transporters and deiodinases in early embryonic chicken brain development. Mol Cell Endocrinol. 2012;349:289–297. doi: 10.1016/j.mce.2011.11.012. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2′,4,4′,5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicological sciences : an official journal of the Society of Toxicology. 2002;67:104–107. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to polybrominated diphenyl ether (PBDE 153) disrupts spontaneous behaviour, impairs learning and memory, and decreases hippocampal cholinergic receptors in adult mice. Toxicol Appl Pharmacol. 2003;192:95–106. doi: 10.1016/s0041-008x(03)00217-5. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame-retardant, 2,2′,4,4′,5-pentabromodiphenyl ether, decreases cholinergic nicotinic receptors in hippocampus and affects spontaneous behaviour in the adult mouse. Environmental toxicology and pharmacology. 2004;17:61–65. doi: 10.1016/j.etap.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Deranged spontaneous behaviour and decrease in cholinergic muscarinic receptors in hippocampus in the adult rat, after neonatal exposure to the brominated flame-retardant, 2,2′,4,4′,5-pentabromodiphenyl ether (PBDE 99) Environmental toxicology and pharmacology. 2005;20:283–288. doi: 10.1016/j.etap.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Changes in spontaneous behaviour and altered response to nicotine in the adult rat, after neonatal exposure to the brominated flame retardant, decabrominated diphenyl ether (PBDE 209) Neurotoxicology. 2007;28:136–142. doi: 10.1016/j.neuro.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Viberg H, Johansson N, Fredriksson A, Eriksson J, Marsh G, Eriksson P. Neonatal exposure to higher brominated diphenyl ethers, hepta-, octa-, or nonabromodiphenyl ether, impairs spontaneous behavior and learning and memory functions of adult mice. Toxicological sciences : an official journal of the Society of Toxicology. 2006;92:211–218. doi: 10.1093/toxsci/kfj196. [DOI] [PubMed] [Google Scholar]
- Visser TJ, Kaptein E, Terpstra OT, Krenning EP. Deiodination of thyroid hormone by human liver. J Clin Endocrinol Metab. 1988;67:17–24. doi: 10.1210/jcem-67-1-17. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Darras VM. Combined antisense knockdown of type 1 and type 2 iodothyronine deiodinases disrupts embryonic development in zebrafish (Danio rerio) Gen Comp Endocrinol. 2010;166:134–141. doi: 10.1016/j.ygcen.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Crawford AD, Janssens ED, Van der Geyten S, Darras VM. Type 2 iodothyronine deiodinase is essential for thyroid hormone-dependent embryonic development and pigmentation in zebrafish. Endocrinology. 2009;150:530–539. doi: 10.1210/en.2008-0457. [DOI] [PubMed] [Google Scholar]
- Walpita CN, Van der Geyten S, Rurangwa E, Darras VM. The effect of 3,5,3′-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen Comp Endocrinol. 2007;152:206–214. doi: 10.1016/j.ygcen.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Wang DO, Matsuno H, Ikeda S, Nakamura A, Yanagisawa H, Hayashi Y, Okamoto A. A quick and simple FISH protocol with hybridization-sensitive fluorescent linear oligodeoxynucleotide probes. RNA. 2012;18:166–175. doi: 10.1261/rna.028431.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou T, Ross DG, DeVito MJ, Crofton KM. Effects of short-term in vivo exposure to polybrominated diphenyl ethers on thyroid hormones and hepatic enzyme activities in weanling rats. Toxicological sciences : an official journal of the Society of Toxicology. 2001;61:76–82. doi: 10.1093/toxsci/61.1.76. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol. 2011;45:7896–7905. doi: 10.1021/es200422b. [DOI] [PMC free article] [PubMed] [Google Scholar]