Abstract
Background: Cervical intraepithelial neoplasia (CIN) is a premalignant lesion capable of progressing to cervical cancer. Despite the existing well-defined criteria, the histomorphologic diagnosis is subject to high rates of discordance among pathologists. The aim of this study was to evaluate Ki-67 (MIB-1), CK17 and p16 INK4a (p16) markers by immunohistochemical methods in differentiating CIN from benign cervical lesions.
Methods: The present study reviewed and re-classified 77 cervical biopsies, originally diagnosed as 31 non-CIN, and 46 CIN, as 54 non-CIN, and 23 CIN based on at least two similar diagnoses. Immunostaining by Ki67, p16 and CK17 markers was performed on all cases and the results were compared with pervious and consensus diagnosis.
Results: The overall agreement between pervious and consensus diagnosis was 67.5% (Kappa=0.39, P<0.001). The sensitivity and specificity of Ki67 immunostaining were 95.6% and 85.1% respectively, while for p16 the corresponding values were 91.3% and 98.1%. The overall agreement, for both p16 and Ki67, with consensus diagnosis were significant (P<0.001). The sensitivity and specificity of CK17 negative staining in CIN detection were 39.1% and 40.7% respectively.
Conclusion: Ki67 and p16 markers are recommended as complementary tests for differentiating between dysplastic and non-dysplastic lesions. CK17 does not discriminate between immature metaplasia with and without dysplasia.
Key Words: CIN, Ki67 (MIB-1), p16 INK4a
Introduction
Almost all of the invasive cervical cancers are preceded by cervical intraepithelial neoplasia (CIN).1,2 Persistent infections with high risk human papilloma virus (hr-HPV) types lead to CIN and invasive cancer.3 Despite well-defined criteria, the histopathologic diagnosis is subject to high rates of discrepancy among pathologists.4-6
Supplementary methods using objective biomarkers are needed to achieve more accurate diagnosis. Ki-67 is a well-known cell proliferation marker, useful for confirmation of the diagnosis in ambiguous cases and CIN grading.2,7 p16 INK4a is a specific biomarker used for identification of dysplastic cervical epithelium with tendency to invasive cervical cancer.8,9
The diagnosis of atypical immature metaplasia (AIM) has poor intra- and inter-observer reproducibility on routine hematoxylin and eosin (H&E) stained sections because of its resemblance to CIN 3.10 Ki-67 immunostaining of AIM revealed variable results, with a wide range of reactivity and marked overlap between HPV-negative and HPV-positive cases. Ki-67 and p16 are complementary alternative biomarkers for HPV-related cervical neoplasia.7 Cytokeratin (CK) 17 is a marker for endocervical reserve stem cells which gives rise to metaplasia and expression of CK17 that decreases and disappeares as the metaplastic epithelium matures. Antibody to CK17 is used to differentiate between immature squamous metaplasia (ISM) and high grade CIN (CIN3). 11 AIM may be re-classified into metaplasia and CIN3 based on p16 and CK17 immuoreactivity and mmunohistochemistry.10 Recent studies have shown that Ki67 and p16 could be used as progression markers in cervical lesions.12
The aim of this study was to evaluate and compare staining pattern for Ki67, p16 and CK17, as adjunct tests, in differentiating CIN from benign lesions to increase the diagnostic accuracy in equivocal cases.
Materials and Methods
Case Selection and Immunohistochemical Staining
A total of 77 formalin-fixed paraffin embedded cervical specimens were selected from the pathology archives of hospitals affiliated with Shiraz University of Medical Sciences Shiraz, Iran during 2004 to 2009. All the samples were cervical (punch) biopsy or endocervical curettage specimens. The patients aged from 20 to 80 years (mean 39.8 years).
Initial diagnosis comprised 10 negative dysplasia (NEG), 21 ISM with or without reactive atypia, and 46 CIN, (18 CIN1, 11 CIN2, and 17 CIN3). All H&E stained sections were first reviewed by 2 independent pathologists blinded to the initial diagnosis. The consensus diagnosis was a gold standard, and defined as diagnostic agreement between the pathologists concerned. For patients with diagnostic disagreement, 12 of 77 cases, a third review was obtained from a gynecopathologist. All the specimens were immunostained for Ki-67, p16 and CK17 antigens.
Immunohistochemical (IHC) Staining
IHC staining for Ki-67, p16 and CK-17 antigens was performed on 5 µm sections obtained from formalin-fixed, paraffin embedded blocks, using avidin-biotin peroxidase complex method. The primary antibodies were monoclonal mouse anti Ki-67 antigen, clone MIB-1 (Dako, code: N1633, Denmark; diluted 1:2); mouse monoclonal anti p16INK4a, (Santa cruz, (JC8) SC-56330, USA; diluted 1:50) and monoclonal mouse anti-CK17, clone E3 (Dako, code: M7046, Denmark; diluted 1:30). Secondary antibodies included goat anti-mouse and anti-rabbit immunoglobulines (Dako, code: K4061, Denmark; Ready to use) and DAB (3,3’ Diaminobenzidine; chromogen (Dako).
Immunohistochemical Scoring
The sections stained by IHC were examined alongside H&E stained specimens, to identify the precise locations of the lesions. Ki-67 (MIB-1) staining was interpreted positive when a cluster of at least 2 strongly stained epithelial nuclei were present in the upper two thirds of the epithelial thickness anywhere within the lesion. Presence of the para-basal cells staining was used as an internal positive control.4,7 The p16 was considered positive when it showed nuclear, as well as continuous diffuse cytoplasmic staining of the cells in the basal and para-basal cell layers of the squamous epithelium, variably reaching intermediate and superficial cell layer characterized by diffuse staining pattern. p16 was considered negative when it was completely unstained, or showing focal or sporadic epithelial staining, particularly not of the basal and para-basal cells (focal staining pattern). Scoring of IHC results was evaluated on the basis of distribution of immunoreactive cells. However, staining intensity was not graded to avoid subjective interpretation.1 CIN3 specimens were used as positive controls. CK17 staining was considered positive, when cytoplasmic staining involved all squmous cell layers. Focal staining or completely unstained cell layers was considered as negative.13
Statistical Analysis
Histologic diagnoses were categorized as (NEG), CIN1 (LG-SIL), CIN2 & CIN3 (HG-SIL), and ISM. The concordance between each of the initial and consensus diagnosis with Ki67, p16 and CK17 reactivity was determined by kappa statistical analysis using SPSS software package, version 15. Kappa values (K) of (1.0-0.75), (0.5-0.75), (0.25-0.5) and less than 0.25 were considered as thresholds for excellent, good, moderate, and poor concordance respectively.
Results
In review of 77 patients with previous diagnoses, the cases were reclassified as 24 negative for dysplasia (NEG); 4 as CIN1; 5 as CIN2; 14 as CIN3; and 30 ISM (figures 1A, 2A, 3A). The sensitivity and specificity of the previous diagnoses were 95.6% and 55.5% with 47.8% and 96.8% PPV and NP respectively. The overall agreement between previous and consensus diagnosis was 67.5%, (Kappa=0.39, P<0.001).
Figure 1.
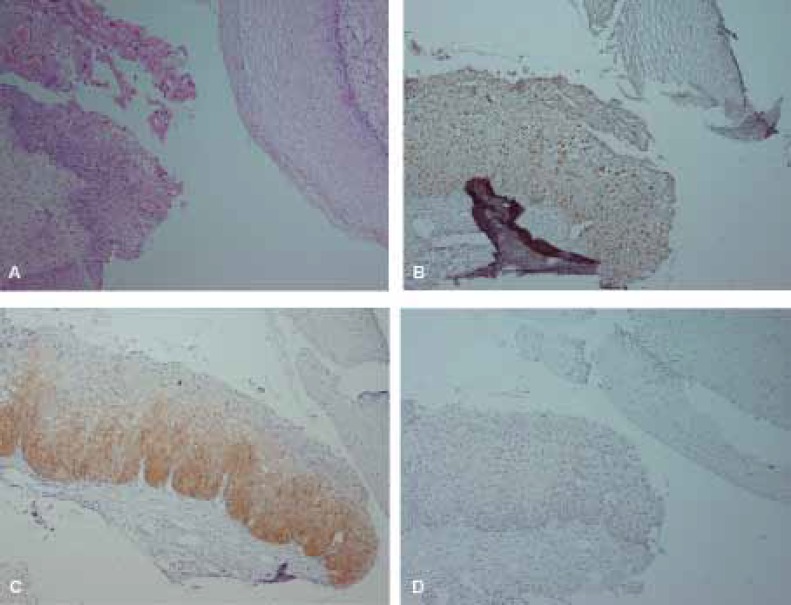
Hematoxylin and Eosin (H&E) and immunohistochemical staining of Ki67, p16 and CK17 in CIN1. A, H&E staining. B, scattered Ki67 immunostaining in CIN1 and negative in normal epithelium. C, diffuse (one-third) p16 immunostaining in CIN1 and negative staining in normal epithelium. D, negative staining for CK17 in both normal tissue and CIN1. (×100)
Ki67 Immunostaining
The patterns of positive Ki67 staining were regarded as scattered (5.2%) or/and diffuse (97.3%); (figures 1B, 2B, 3B). All cases of HG-SIL were positive for Ki67.
Ki67 was positive in 26.6% of ISM cases. One ISM specimen showed a pattern of staining identical to HG-SIL. Other 7 cases of ISM were only positive for Ki67 with scattered patterns (figure 2B). Of 54 non-CIN cases, Ki67 was negative in 46 cases. The sensitivity and specificity of Ki67 staining are 95.6% and 85.1% respectively with 73.3 positive predictive value (PPV) and 97.8% negative predictive value (NPV). The overall agreement regarding Ki67 with consensus diagnosis was 88.3% (Kappa=0.74, P<0.001).
Figure 2.
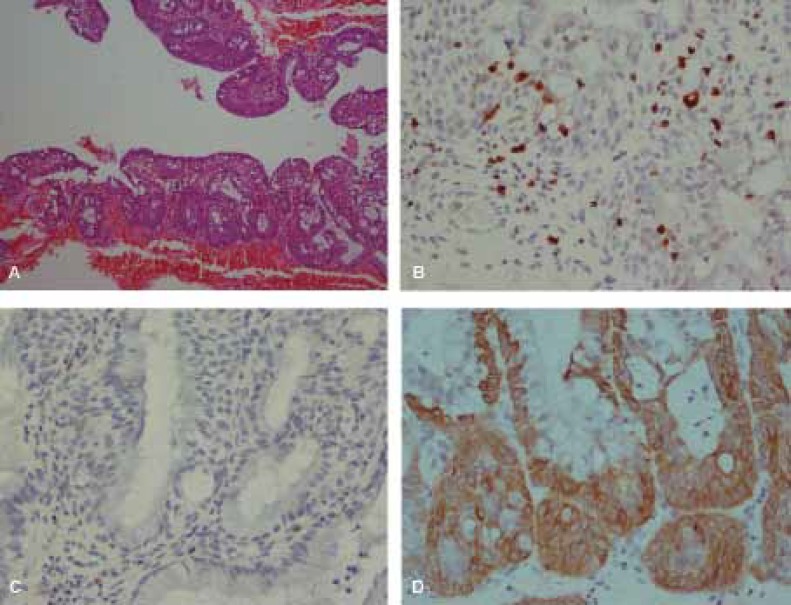
H&E and immunohistochemical staining of Ki67, p16 and CK17 in Immature Squamous Metaplasia (ISM). A, H&E staining. B, Scattered Ki67 immunostaining. C, negative p16. D, positive cyrokeratin 17. (A: ×100, B,C,D: ×400)
p16 Immunostaining
All cases of HG-SIL were positive with strongly diffuse staining. All NEG specimens were negative for both p16 and Ki67. The staining was both nuclear and cytoplasmic, and mostly involved full-thickness of the epithelium (figure 3C). Also p16 was positive in 2 of 4 CIN1 patients, of which one was diffuse basal and the other diffuse one-third thickness (figure 1C). Of ISM cases, 73.3% were negative for both p16 and Ki67. Additionally, p16 staining was entirely negative for NEG and ISM cases (figure 2C).
Figure 3.
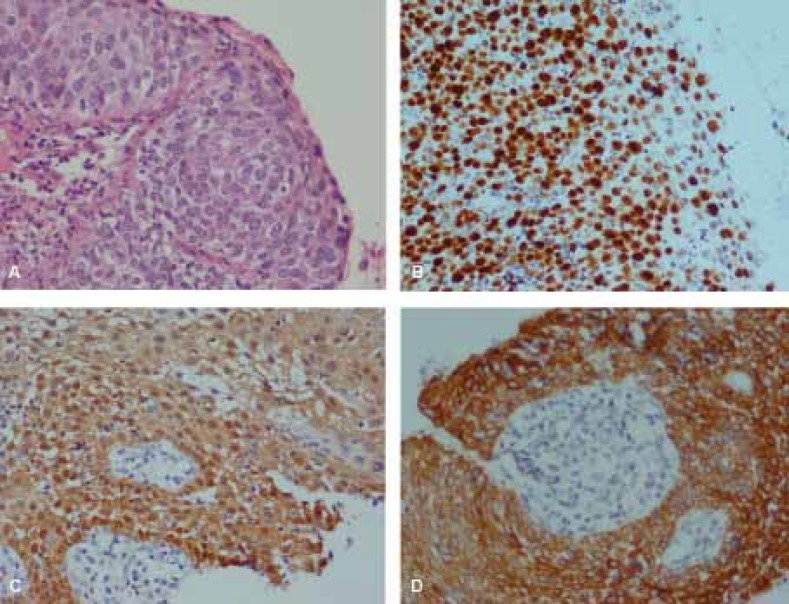
H&E and Immunohistochemical staining of Ki67, p16 and CK17 in CIN3. A, H&E staining. B, diffuse full thickness Ki67 staining. C, diffuse full thickness p16 staining. D, CK17 positive staining. (A,B,C,D: ×400)
The sensitivity and specificity of p16 staining were 91.3% and 98.1%, with 95.4% PPV and 96.3% NPV, respectively. The overall agreement between p16 and consensus diagnosis was 96.1% (Kappa=0.90, P<0.001), which were higher than those of Ki67 and consensus diagnosis. All of the p16 positive cases were also positive for Ki67, but none of the p16 positive cases were negative for Ki67 (table 1). The agreement between Ki67 and p16 was higher than Ki67 and consensus diagnosis, but lower than p16 and consensus diagnosis.
Table 1.
Correlation between p16 and Ki67 immunostaining
| Results |
Ki67
|
||
|---|---|---|---|
| Negative | Positive | Total | |
| p16 Negative | 47 | 8 | 55 |
| p16 Positive | 0 | 22 | 22 |
| Total | 47 | 30 | 77 |
CK17 Immunostaining
CK17 was positive in 3 of 24 NEG, 3 of 4 CIN1, 1 of 5 CIN2, 9 of 14 CIN3, and 30 of 30 ISM cases (figures 1D, 2D, 3D). The sensitivity and specificity of CK17 negativity for CIN detection were 39.1% and 40.7% with 21.9% PPV and 61.1% NPV, respectively. The overall agreement between CK17 with consensus diagnosis was 46.7% (Kappa=-0.015, P=0.89). There was poor negative correlation between CK17 negative staining and consensus diagnosis in CIN detection.
Discussion
The evaluation of CIN is subjective in relation to intra- and inter-observer variability regarding interpretation of histomorphologic features.14 Variability in diagnosis of CIN by assessment of H&E staining in the current and some other studies are presented in table 2.
Table 2.
Interobserver variability for the assessment of H&E stained sections
| Author | Non dysplastic tissue * | CIN1 | CIN2 | CIN3 | Cervical carcinoma |
|---|---|---|---|---|---|
| Klaes et al.5 (2002) | 71%** | 52% | 35% | 72% | 94% |
| Bergeron et al.14 (2010) | 76% | 42.3% | 36% | 76.6% | - |
| Galgano et al.6 (2010) | 86.5% | 61.9% | 47.6% | 75% | 83.3% |
| Present study | 96.8% | 0% | 18.2% | 70.6% | - |
*Nondysplastic tissue includes normal, inflammation, mature and immature metaplasia, atypical cells. **71% in normal and 3% in the other nondysplastic tissues
Diagnosis of CIN1 on the basis of H&E staining alone is subject to a high level of intra-observer variability.15 Many studies show that IHC staining for Ki67 and p16 is a very useful adjunctive aid in the diagnosis of equivocal cervical biopsies.4,6,7 In the previous studies, Ki67 expression has been found to be associated with the grade of dysplasia, indicating that IHC for Ki67 is a useful adjunctive test in the evaluation of low-grade lesions of the cervix. The advantage of MIB-1 staining over HPV testing is its higher specificity, since the staining is negative in subclinical HPV infections. Other advantages of this marker are simplicity, availability, reproducibility, and low-cost laboratory techniques.3 Although presence of MIB-1 positive nuclei in the upper two thirds of epithelial thickness is outstanding criteria for MIB-1 positivity, there are few false positive interpretations of the staining, such as tangential sectioning with the presence of positive nuclei in the superficial layers of the epithelium, MIB-1 positive lymphocytes throughout the epithelial thickness in the cervicitis, MIB-1 positive cells in the upper layers of epithelium in the ISM and areas of repair.4,5 Any Ki67 positivity in an atrophic epithelium, especially when diffuse, is consistent with SIL, since atrophic epithelium has virtually no staining.4 Two atrophic lesions in our study reported as HSIL were negative for Ki67. Another study showed sensitivity of 71.4%, 94.7%, and 7.7% for Ki67 in LSIL, HSIL and non-dysplastic lesions, respectively.4 In the present study, the respective sensitivity and specificity of Ki67 were 95.6% and 85.1%. In problematic cases, Ki67 alone cannot differentiate between dysplasia and ISM.
IHC staining for p16 yields greater accuracy of CIN grading with less variability and helps to avoid unnecessary diagnostic and surgical procedures related to pregnancy-associated morbidity and psychological distress.16 Several studies show that p16 is a sensitive and specific marker with a better predictive value than HPV-DNA testing for high grade SIL. This staining decreased inter-observer variation in the histopathologic examinations.2,9,15,17,18 The results of our study is concordant with the results of previous studies (table 3).
Table 3.
Comparison of p16 staining in the previous reports and the present study
| Author | Nondysplastic* | CIN1 | CIN2 | CIN3 |
|---|---|---|---|---|
| Klaes et al.5 (2002) | 7.58 (12%) | 15.17 (88%) | 10.10 (100%) | 43.43 (100%) |
| Benevolo et al.16 (2006) | 0.17 (0%) | 17.54 (31%) | 9.10 (90%) | 11.11 (100%) |
| Ishikawa et al.20 (2006) | 0.7 (0%) | 13.53 (25%) | 32.40 (80%) | 45.48 (94%) |
| Focchi et al.21 (2007) | 0.114 (0%) | 80.88 (91%) | 33.33 (100%) | 32.32 (100%) |
| Ikeda et al.13 (2008) | - | 29.39 (74.4%) | 29.31 (93.5%) | 42.43 (97.7%) |
| Reuschenbach et al.3 (2010) | 0.21 (0%) | 12.21 (57.1%) | 17.23 (73.9%) | 27.27 (100%) |
| Present study | 1.54 (1.8%) | 2.4 (50%) | 5.5 (100%) | 14.14 (100%) |
*Nondysplastic tissue includes normal, reactive, inflammation, mature and immature metaplasia, and atypical cells
CIN1 lesions show a more variable reactivity, with percentages of diffuse positive staining ranging from 20-50%.12,18,19 p16 staining is considered as a useful and reliable diagnostic adjunct for distinguishing biopsies with and without CIN2 or is more severe but not so useful for discriminating between CIN1 and non-CIN. Ki67 staining was inferior to p16 and its inclusion with p16 shows no marked improvement in clinical performance over p16 per se.6 In our study, sensitivity and specificity for p16 were 91.3% and 98.1% respectively.
One study reveals that unlike non-progressive cases with negative CIN1, all CIN1 biopsies from patients who progressed to CIN 2-3 were positive for p16, 16 In this context, CIN1 lesions with positive p16 showed a markedly higher tendency to progress to CIN2-3, indicating that p16 may have a significant role in the evaluation of CIN1 lesions, excluding about half of the cases from an invasive clinical follow up.
Supplementary use of p16 staining significantly improves the accuracy of grading CIN lesions by a single pathologist, equivalent to an expert consensus diagnosis.17 Some authors re-classified AIM lesions in consensus diagnosis based on Ki67 and p16 IHC and HPV tests. Almost two-thirds of AIM cases could be re-classified as benign based on negative p16 staining. Another one-third could be re-classified as HSIL regarding positive Ki67 and p16 staining. Another study showed a strong uniform cytoplasmic CK17 positivity of the proliferating cells together with p16 negativity in ISM lesions. The lesions featuring both metaplastic changes and atypia with staining of both p16 and CK17 are classified as high-grade dysplasia.12 Another study shows variable positivity with CK17 staining in CIN.13 The current study revealed that CK17 can be positive in immature squamous metaplasia, and in some CIN lesions. For differentiation between metaplastic lesions with or without dysplasia, it would be helpful to consider another marker such as p16.
Based on H&E stained sections, consensus diagnosis was reached in one of the cases with ISM. However, IHC study showed Ki67 and p16 positivity, which was compatible with CIN2. In another case, consensus diagnosis was CIN1 but IHC staining for Ki67, and p16 were negative, a finding consistent with non-dysplastic lesion. The limitation of this study was the number of CIN cases collected from the files hospitals concerned. It should be considered that CIN cases in this region are not as common as those in western countries.
Conclusions
We recommend using Ki67 and p16 markers as complementary tests for differentiation between dysplastic and non-dysplastic lesions. Ki67 can be positive in some immature squamous metaplastic lesions, thus p16 is useful to rule out dysplasia. CK17 can also be positive in ISM cases with dysplastic change. Testing for p16 is proposed to rule out dysplasia which is positive in almost all HSIL cases. However, it may be positive or negative in LSIL. A complementary study including more cases and follow up examinations is warranted for better evaluation and definitive prognostic significance of these biomarkers.
Acknowledgment
The authors would like to thank Dr. Nasrin Shokrpour at Center for Development of Clinical Research of Nemazee Hospital for editorial assistance.
Conflict of interest: None declared
References
- 1.Rosai J. Female reproductive system. In: Houston M, editor. Rosai and Ackerman’s surgical pathology. 9th ed. New York: Mosby; 2004. pp. 1523–68. [Google Scholar]
- 2.Nam EJ, Kim JW, Hong JW, Jang HS, Lee SY, Jang SY, et al. Expression of the p16 and Ki-67 in relation to the grade of cervical intraepithelial neoplasia and high-risk human papillomavirus infection. J Gynecol Oncol. 2008;19:162–8. doi: 10.3802/jgo.2008.19.3.162. doi: 10.3802/jgo.2008.19.3.162. PubMed PMID: 19471565; PubMed Central PMCID: PMC2676462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reuschenbach M, Seiz M, von KnebelDoeberitzC, Vinokurova S, Duwe A, Ridder R, et al. Evaluation of cervical cone biopsies for coexpression of p16INK4a and Ki-67 in epithelial cells. Int J Cancer. 2012;130:388–94. doi: 10.1002/ijc.26017. doi: 10.1002/ijc.26017. PubMed PMID: 21387293. [DOI] [PubMed] [Google Scholar]
- 4.Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–91. doi: 10.1097/00000478-200107000-00006. PubMed PMID: 11420459. [DOI] [PubMed] [Google Scholar]
- 5.Klaes R, Benner A, Friedrich T, Ridder R, Herrington S, Jenkins D, et al. p16INK4a immunohistochemistry improves interobserver agreement in the diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2002;26:1389–99. doi: 10.1097/00000478-200211000-00001. doi: 10.1097/00000478-200211000-00001. PubMed PMID: 12409715. [DOI] [PubMed] [Google Scholar]
- 6.Galgano MT, Castle PE, Atkins KA, Brix WK, Nassau SR, Stoler MH. Using biomarkers as objective standards in the diagnosis of cervical biopsies. Am J Surg Pathol. 2010;34:1077–87. doi: 10.1097/PAS.0b013e3181e8b2c4. doi: 10.1097/PAS.0b013e3181e8b2c4. PubMed PMID: 20661011; PubMed Central PMCID: PMC2921638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iaconis L, Hyjek E, Ellenson LH, Pirog EC. p16 and Ki-67 immunostaining in atypical immature squamous metaplasia of the uterine cervix: correlation with human papillomavirus detection. Arch Pathol Lab Med. 2007;131:1343–9. doi: 10.5858/2007-131-1343-PAKIIA. PubMed PMID: 17824788. [DOI] [PubMed] [Google Scholar]
- 8.Wang JL, Zheng BY, Li XD, Nokelainen K, Angström T, Lindström MS, et al. p16INK4A and p14ARF expression pattern by immunohistochemistry in human papillomavirus-related cervical neoplasia. Mod Pathol. 2005;18:629–37. doi: 10.1038/modpathol.3800308. 10.1038/modpathol.3800308.PubMed PMID: 15502810. [DOI] [PubMed] [Google Scholar]
- 9.Wang SS, Sherman ME, Robboy Js. Robboy’s pathology of the Female Reproductive Tract. 2nd ed. London: Churchil livingstone; 2009. Cervix: epidemiology of squamous metaplasia; pp. 173–88. [Google Scholar]
- 10.Wentzensen N, von KnebelDoeberitzM. Biomarkers in cervical cancer screening. Dis Markers. 2007;23:315–30. doi: 10.1155/2007/678793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malpica A, Robboy SJ. Cervical benign and non-neoplastic conditions. In: Robboy Js, Mutter GL, Prat J, Bentley RC, Russell P, Malcolm C., editors. Robboy’s pathology of the Female Reproductive Tract. 2nd ed. London: Churchil livingstone; 2009. pp. 141–72. [Google Scholar]
- 12.Regauer S, Reich O. CK17 and p16 expression patterns distinguish (atypical) immature squamous metaplasia from high-grade cervical intraepithelial neoplasia (CIN III. Histopathology. 2007;50:629–35. doi: 10.1111/j.1365-2559.2007.02652.x. doi: 10.1111/j.1365-2559.2007.02652.x. PubMed PMID: 17394499; PubMed Central PMCID: PMC1890920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda K, Tate G, Suzuki T, Mitsuya T. Coordinate expression of cytokeratin 8 and cytokeratin 17 immunohistochemical staining in cervical intraepithelial neoplasia and cervical squamous cell carcinoma: an immunohistochemical analysis and review of the literature. Gynecol Oncol. 2008;108:598–602. doi: 10.1016/j.ygyno.2007.11.042. doi: 10.1016/j.ygyno.2007.11.042. PubMed PMID: 18191996. [DOI] [PubMed] [Google Scholar]
- 14.Bergeron C, Ordi J, Schmidt D, Trunk MJ, Keller T, Ridder R. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol. 2010;133:395–406. doi: 10.1309/AJCPXSVCDZ3D5MZM. doi: 10.1309/AJCPXSVCDZ3D5MZM.PubMed PMID: 20154278. [DOI] [PubMed] [Google Scholar]
- 15.Horn LC, Reichert A, Oster A, Arndal SF, Trunk MJ, Ridder R, et al. Immunostaining for p16INK4a used as a conjunctive tool improves interobserver agreement of the histologic diagnosis of cervical intraepithelial neoplasia. Am J Surg Pathol. 2008;32:502–12. doi: 10.1097/PAS.0b013e31815ac420. doi: 10.1097/PAS.0b013e31815ac420. PubMed PMID: 18223479. [DOI] [PubMed] [Google Scholar]
- 16.Benevolo M, Mottolese M, Marandino F, Vocaturo G, Sindico R, Piperno G, et al. Immunohistochemical expression of p16(INK4a) is predictive of HR-HPV infection in cervical low-grade lesions. Mod Pathol. 2006;19:384–91. doi: 10.1038/modpathol.3800551. PubMed PMID: 16415792. [DOI] [PubMed] [Google Scholar]
- 17.Dijkstra MG, Heideman DA, de RoySC, Rozendaal L, Berkhof J, van KrimpenK, et al. p16(INK4a) immunostaining as an alternative to histology review for reliable grading of cervical intraepithelial lesions. J Clin Pathol. 2010;63:972–7. doi: 10.1136/jcp.2010.078634. doi: 10.1136/jcp.2010.078634. PubMed PMID: 20924035. [DOI] [PubMed] [Google Scholar]
- 18.del PinoM, Garcia S, Fusté V, Alonso I, Fusté P, Torné A, et al. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. Am J Obstet Gynecol. 2009;201:488. doi: 10.1016/j.ajog.2009.05.046. doi: 10.1016/j.ajog.2009.05.046. PubMed PMID: 19683687. [DOI] [PubMed] [Google Scholar]
- 19.Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev. 2008;17:2536–45. doi: 10.1158/1055-9965.EPI-08-0306. doi: 10.1158/1055-9965.EPI-08-0306. PubMed PMID: 18842994; PubMed Central PMCID: PMC2900792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ishikawa M, Fujii T, Saito M, Nindl I, Ono A, Kubushiro K, et al. Overexpression of p16 INK4a as an indicator for human papillomavirus oncogenic activity in cervical squamous neoplasia. Int J Gynecol Cancer. 2006;16:347–53. doi: 10.1111/j.1525-1438.2006.00355.x. PubMed PMID: 16445657. [DOI] [PubMed] [Google Scholar]
- 21.Focchi GR, Silva ID, Nogueira-de-Souza NC, Dobo C, Oshima CT, Stavale JN. Immunohistochemical expression of p16(INK4A) in normal uterine cervix, nonneoplastic epithelial lesions, and low-grade squamous intraepithelial lesions. J Low Genit Tract Dis. 2007;11:98–104. doi: 10.1097/01.lgt.0000245042.29847.dd. doi: 10.1097/01.lgt.0000245042.29847.dd. PubMed PMID: 17415114. [DOI] [PubMed] [Google Scholar]