Abstract
Huntington’s disease (HD) is a devastating genetic neurodegenerative disease caused by CAG trinucleotide expansion in the exon-1 region of the huntingtin gene. Currently, no cure is available. It is becoming increasingly apparent that mutant HTT impairs metabolic homeostasis and causes transcriptional dysregulation. The peroxisome proliferator-activated receptor gamma (PPAR-γ) is a transcriptional factor that plays a key role in regulating genes involved in energy metabolism; recent studies demonstrated that PPAR-γ activation prevented mitochondrial depolarization in cells expressing mutant HTT and attenuated neurodegeneration in various models of neurodegenerative diseases. PPAR-γ-coactivator 1α (PGC-1 α) transcription activity is also impaired by mutant HTT. We now report that the PPAR-γ agonist, rosiglitazone (RSG), significantly attenuated mutant HTT-induced toxicity in striatal cells and that the protective effect of RSG is mediated by activation of PPAR-γ. Moreover, chronic administration of RSG (10 mg/kg/d, i.p) significantly improved motor function and attenuated hyperglycemia in N171-82Q HD mice. RSG administration rescued BDNF deficiency in the cerebral cortex, and prevented loss of orexin-A-immunopositive neurons in the hypothalamus of N171-82Q HD mice. RSG also prevented PGC-1α reduction and increased Sirt6 protein levels in HD mouse brain. Our results suggest that modifying the PPAR-γ pathway plays a beneficial role in rescuing motor function as well as glucose metabolic abnormalities in HD.
Keywords: PPAR-γ, huntingtin, glucose metabolism, PGC-1α, Sirt6, BDNF
Introduction
Huntington disease (HD) is an autosomal dominant neurodegenerative disease characterized primarily by progressive motor dysfunction, weight loss, cognitive decline and psychiatric symptoms. The prevalence of HD is 7–10/100,000 in the Western world (Hoppitt et al. 2010). The trinucleotide expansion in exon 1 of the Huntingtin (HTT) gene is the cause of clinical manifestations in HD patients (1993). Transcriptional dysregulation, mitochondrial dysfunction, increased oxidative stress, excitotoxicity, and neurotrophic factor deficiency have been implicated in HD pathogenesis (Cui et al. 2006, Panov et al. 2002, Rosenstock et al. 2010, Bithell et al. 2009, Giralt et al. 2009, Ross & Tabrizi 2011, Xie et al. 2010, Zuccato et al. 2011). Abnormal bioenergetic deficits such as body weight loss, reduced glucose uptake in the brain, and increased incidence of diabetes have also been observed during the progression of this disease (Djousse et al. 2002). Currently there is no treatment to delay onset or slow progression of HD.
Peroxisome proliferator-activated receptors (PPARs) are members of the nuclear hormone receptor family of ligand-activated transcription factors (Rosen & Spiegelman 2001). There are three mammalian subtypes of PPARs termed PPAR-α, PPAR-β, and PPAR-γ. PPAR-γ agonists have been used as an anti-type II diabetes drug. Recent studies suggest that treatment with PPARγ agonists has beneficial effects in models of Alzheimer’s disease (Watson et al. 2005), Parkinson’s disease (Randy & Guoying 2007, Schintu et al. 2009), and amyotrophic lateral sclerosis (Kiaei 2008, Kiaei et al. 2005, Schutz et al. 2005), as well as Huntington’s disease (Napolitano et al. 2011, Johri et al. 2012, Jin et al. 2012). Activation of PPAR-γ upregulates Bcl-2, enhances its cell survival pathway, and prevents neuronal degeneration, with a concomitant increase in mitochondrial viability (Fuenzalida et al. 2007, Quintanilla et al. 2008, Chiang et al. 2011, Hunter et al. 2007, Quintanilla & Johnson 2009). In addition, PPAR-γ coactivator 1α (PGC-1α), a key transcription factor regulating mitochondrial biogenesis and metabolism, is compromised by mutant HTT (Cui et al. 2006). PGC-1α knockout mice display neurodegeneration in the striatum and abnormal metabolism as seen in HD (Lin et al. 2004). In both human caudate nucleus and N171-82Q HD mouse striatum, reduced levels of PGC-1α mRNA were detected (Weydt et al. 2006). Recent studies show that administration of PPAR agonist increases expression of PGC-1α, mitochondrial DNA, and ATP (Wenz et al. 2008).
The PPAR-γ agonist rosiglitazone (RSG) is an FDA-approved drug that has been used for clinical treatment of diabetes. It has been shown that RSG prevents mitochondrial dysfunction in cells expressing mutant huntingtin (Quintanilla et al. 2008); RSG is able to cross the blood-brain barrier and induce mitochondrial biogenesis in mouse brain (Strum et al. 2007). In the present study we examined whether RSG would prevent toxicity in a cell model and improve motor function and metabolic abnormalities in the N171-82Q HD mouse model. We further determined the molecular mechanisms mediated by RSG in HD mouse brains and cells expressing mutant HTT.
Materials and Methods
Materials
RSG was purchased from Cayman Chemical (Michigan, USA). For cell culture experiments, RSG was dissolved in DMSO to the concentration of 40 mM stocking solution and stored at −20°C. Just before the experiment, it was diluted to 5 mM and added to the culture medium at 1:1000 dilution. For in vivo experiments, RSG was prepared fresh daily with water to the concentration of 1 mg/ml and used within 1 h. RSG was given to mice at 10 mg/kg, this dose was chosen based on previous studies showing that this dose of rosiglitazone had neuroprotective effects in mice (Carta et al. 2011, Fatehi-Hassanabad & Tasker 2011). PPARγ antagonist GW9662, and PPAR-α antagonist GW6471 were purchased from Sigma and were prepared in 100 mM stocking solution with DMSO and kept at 4°C. SsoFast EvaGreenR Supermix was purchased from Bio-Rad; protein assay BCA kits were purchased from Thermo Scientific. Immunostaining ABC kits and DAB kits were purchased from Vector Laboratories.
Animals
N171-82Q HD mice express a human N-terminal truncated HTT with 82 polyQ repeats driven by a mouse prion protein promoter. Male N171-82Q HD were mated to hybrid (C3H/HEJ×C57 BL/6J F1) female mice, and the mice were maintained on the hybrid background. Genomic DNA was extracted from mouse tail and genotyping was conducted by using a three-way PCR analysis: two primers were complementary to the prion protein genomic DNA sequence (PrP-sense 5′-CCTCTTGTGACTATGTGGACTGATGTCGG-3′ and PrP-antisense 5′-GTGGATACCCCCTCCCCCAGCCTAGACC-3′). The amplified product of this reaction is 700 bp in length. The antisense primer is also complementary to the 3′-untranslated portion of the PrP vector and, in combination with a third sense primer to the HD sequence (HD-591-5′: 5′-GAACTTTCAGCTACCAAGAAAGACCGTGT-3′), generated a transgene-specific product that is 350 bp in length.
Because we found a sex-dependent difference between males and females, as we reported previously1,2, only male mice were used in the current study. The mice were housed in groups with ad libitum access to food and water and a reversed 12-h light/dark cycle. Mice were divided randomly into vehicle or RSG treatment groups. Mice were treated with rosiglitazone (RSG) or vehicle (water) starting from 8 weeks of age. RSG (10 mg/kg body weight) or vehicle was administered between 10:00–11:00AM daily by oral gavage until 32 weeks of age. All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by Johns Hopkins University Animal Care Committees. Body weight and survival were monitored routinely. At 14 weeks, mice were anesthetized and brain tissues were dissected and immediately frozen in −80C° for mRNA and protein extraction. At 32 weeks of age, mice were anesthetized and perfused transcardially with phosphorylated saline followed by 4% paraformadehyde. After perfusion, the brains were post-fixed overnight, and transferred into 30% sucrose solution for 2 days before immunohistochemistry.
Immortalized striatal cells
Immortalized striatal precursor cells with normal HTT (SThdhQ7/Q7) or mutant HTT (SThdhQ111/Q111) were derived from Hdh knock-in mice, and were kindly provided by Dr. Marcy McDonald 3. These cells were maintained at 33°C in high glucose DMEM medium (Invitrogen) with 10% fetal bovine serum (FBS, GIBCO), 1% Penicillin-Streptomycin (Invitrogen), 1% L-glutamate (Invitrogen), and 400 μg/ml of G418 (Mediatech), in a humidified atmosphere of 95% air: 5% CO2.
Statistical Analysis
Data are expressed as means ± S.E. from at least three independent experiments in cell cultures studies or with more than three mice in each group in animal studies. Statistical significance was determined by using one-way-ANOVA with Fisher’s posthoc analysis and a level of p <0.05 was accepted as significant.
RESULTS
Rosiglitazone protected cells against mutant HTT-induced toxicity in SThdhQ111/Q111 cells by activation of PPAR-γ
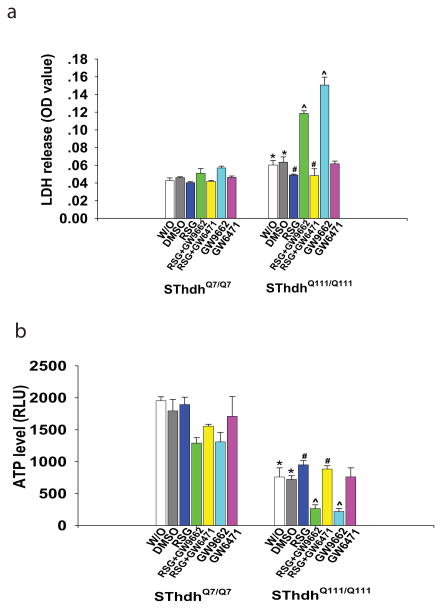
Striatal cells expressing mutant HTT (SThdhQ111/Q111) were used to evaluate the effect of PPAR-γ agonist on mutant HTT-induced cell toxicity, and cells expressing normal HTT (SThdhQ7/Q7) were used as controls. The cells expressing mutant HTT were more susceptible to serum withdrawal compared to the cells expressing normal HTT, as indicated by enhanced LDH release (Fig. 1a) and decreased ATP levels (Fig. 1b) after serum withdrawal (W/O) in mutant HTT cells. PPAR-γ agonist rosiglitazone (RSG, 5 μM) significantly reduced the cell toxicity induced by mutant HTT in striatal cells, indicated by attenuated LDH release (Fig. 1a) and preserved ATP levels (Fig. 1b) in RSG-treated SThdhQ111/Q111 cells compared to vehicle-treated SThdhQ111/Q111 cells.
Fig. 1. Rosiglitazone (RSG) protected cells against mutant huntingtin-induced toxicity in STHdhQ111/Q111 cells through activating PPAR-γ.
Cells were plated and maintained at 33°C in high glucose DMEM medium until 80% confluence, then switched to serum-free medium (W/O) for 24 h; treatment with vehicle DMSO or RSG was started when the medium was switched. RSG treatment (5 μM) decreased LDH release (a) and preserved ATP levels (b). The protective effects of RSG were abolished by PPAR-γ antagonist GW9662 (10 μM), not by PPAR-α antagonist GW6471 (10 μM) in STHdhQ111/Q111 cells. n=3 independent experiments. *p<0.05 vs the values of STHdh Q7/Q7 cells with corresponding treatment. #p<0.05 vs the values of DMSO-treated STHdh Q111/Q111 cells. ^ p<0.05 vs the values of RSG-treated STHdh Q111/Q111 cells by one-way-ANOVA with Fisher’s posthoc analysis.
In order to investigate whether the neuroprotective effect of RSG is mediated by the PPAR-γ pathway, we pretreated cells with the PPAR-γ antagonist GW 9662 (10 μM) or PPAR-α antagonist GW 6471(10 μM). The neuroprotective effect of RSG was abolished by the PPAR-γ antagonist, GW9662, whereas the PPAR-α antagonist, GW6471did not abolish the protective effect of RSG on mutant HTT- induced toxicity (Fig. 1a,b). These results suggest that RSG protected cells from mutant HTT toxicity by activating PPAR-γ.
Rosiglitazone preserved mRNA levels of PGC-1α in brains of N171-82Q HD mice
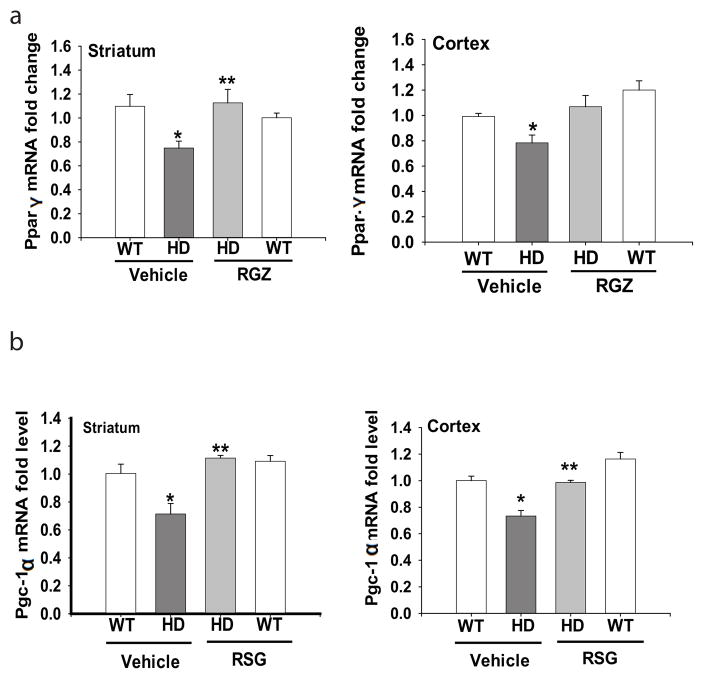
PPAR-γ and its coactivator PCG-1α play an important role in regulating genes involved in metabolism and mitochondrial function. It has been shown that the PPAR-γ signaling pathway was impaired in cells expressing mutant HTT (Quintanilla et al. 2008). We found that the mRNA levels of PPAR-γ (Fig 2a), as well as PGC-1α (Fig. 2b) were decreased in the striatum and cerebral cortex of N171-82Q HD mice, further supporting the view that PPAR-γ signaling pathway is impaired in HD. Chronic RSG administration significantly restored the mRNA levels of PGC-1α and PPAR-γ in brains of N171-82Q mice (Fig. 2a &b).
Fig. 2. Rosiglitazone (RSG) restored mRNA levels of PGC -1α and PPAR-γ levels in the brains of N171-82Q HD mice.
(a) PPAR-γ mRNA levels in the striatum and cortex of N171-82Q HD mice. (b) PGC-1α mRNA levels in the striatum and cortex of N171-82Q HD mice. *p<0.05 vs the WT-Vehicle group; **p<0.05 vs the HD-vehicle group by one-way-ANOVA with Fisher’s posthoc analysis.
Rosiglitazone improved motor impairment and prevented BDNF depletion in N171-82Q HD mice
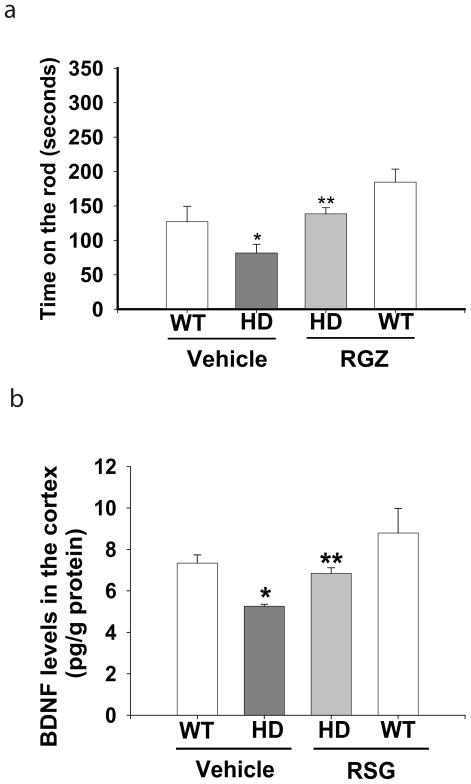
The motor function of mice was assessed by accelerating rotarod tests. N171-82Q HD mice exhibited significant motor impairment, indicated by reduction of the time staying on the rod compared to that in age-matched wild type littermate controls. Chronic treatment with RSG significantly improved the motor function, indicated by extended time on the rotarod (Fig. 3a). In contrast, RSG administration had no effect on the survival and body weight loss in N171-82Q HD mice (data not shown).
Fig. 3. Rosiglitazone (RSG) improved motor performance and attenuated BDNF depletion in the brains of N171-82Q HD mice.
(a) Motor function was assessed with an accelerating rotarod apparatus, n=8. (b) The protein levels of BDNF were measured by using an ELISA in the cerebral cortex of N171-82Q HD mice. n=4. *p<0.05 vs WT-Vehicle group; **p<0.05 vs HD-Vehicle group by one-way-ANOVA with Fisher’s posthoc analysis.
BDNF deficiency occurs commonly in HD patients as well as in HD mouse models, including N171-82Q HD mice (Duan et al. 2003). Because striatal medium spiny neurons do not produce BDNF, and BDNF levels in the striatum rely on transport from cortical projection neurons, we therefore measured BDNF levels in the cortex. We examined whether the protective effect of RSG on motor function results from restoring BDNF protein levels in HD mouse brain. The levels of BDNF were decreased significantly in the N171-82Q HD mouse cortex compared to those in wild type controls as we reported previously, and chronic administration of RSG restored the BDNF levels in the cortex of N171-82Q HD mice (Fig. 3b).
Rosiglitazone attenuated hyperglycemia and preserved orexin-A-immunopositive neurons in the hypothalamus of N171-82Q HD mice
We previously reported that N171-82Q mice exhibited hyperglycemia (Duan et al. 2003). Blood glucose was measured in 20-week-old mice, that is, 13 weeks after chronic administration of RSG. HD mice had significantly higher blood glucose levels compared to age-matched littermate control mice. Chronic treatment with RSG maintained the blood glucose levels in the normal range in N171-82Q HD mice (Fig. 4a).
Fig. 4. Rosiglitazone (RSG) attenuated hyperglycemia and rescued the loss of orexin-A-immunoreactive neurons in the hypothalamus of N171-82Q HD mice.
(a) Blood glucose levels were measured in 20-week-old mice following overnight fasting. n=8–10. (b) Top panel, representative pictures of orexin-A immunostaining in the hypothalamus in indicated groups. Scale bars = 100 μm. Bottom panel, stereology counting of orexin-A-immunoreactive neurons in the ventromedial hypothalamus. n=4. *p<0.05 vs WT-Vehicle group; **p<0.05 vs HD-Vehicle group by one-way-ANOVA with Fisher’s posthoc analysis.
Orexin-A plays a key role in regulating glucose homeostasis. In HD mouse hypothalamus, immunohistological examination indicated that the numbers of orexin-A-positive neurons were significantly decreased in HD mice, which could be caused by either loss of orexin neurons or reduction of orexin expression. Chronic treatment with RSG preserved the numbers of orexin-A-positive neurons (Fig. 4b), indicating that orexin-A-positive neurons may contribute to the control of the glucose levels in HD mice.
Rosiglitazone upregulated Sirt6 levels in HD models
In order to further understand the molecular mechanism mediated by RSG in HD mice, we measured the levels of Sirt6, a member of the sirtuin family; Sirt6 is localized in the nucleus and is involved in metabolic homeostasis. Sirt6 deficiency leads to metabolic abnormalities. Interestingly, the expression of Sirt6 in the cortex of N171-82Q HD mouse brain is lower than that in control mice, and chronic administration of RSG preserved the Sirt6 levels in both cerebral cortex (Fig. 5a) and striatum (Fig. 5b). Consistently, Sirt6 levels were decreased in cells expressing mutant HTT. As already mentioned, cells expressing mutant HTT (STHdh Q111/Q111 ) rapidly undergo cell death after serum withdrawal, and we found that Sirt6 levels were dramatically reduced after serum withdrawal, but treatment with RSG resulted in significantly recovery of Sirt6 levels in cells expressing mutant HTT (Fig. 5c).
Fig. 5. Rosiglitazone (RSG) prevented Sirt6 reduction in both mouse model and cell model of HD.
(a) Chronic administration of RSG prevented reduction of Sirt6 mRNA and protein levels in the cerebral cortex of N171-82Q HD mice. *p<0.05 vs WT-Vehicle group; **p<0.05 vs HD-Vehicle group by one-way-ANOVA with Fisher’s posthoc analysis. (b) Chronic administration of RSG prevented reduction of Sirt6 mRNA and protein levels in the striatum of N171-82Q HD mice. *p<0.05 vs WT-Vehicle group; **p<0.05 vs HD-Vehicle group by one-way-ANOVA with Fisher’s posthoc analysis. (c) RSG treatment (5 μM) attenuated reduced Sirt6 protein levels in cells expressing mutant Htt (SThdhQ111/Q111 cells). *p<0.05 vs the values of DMSO-treated STHdh Q7/Q7 cells. #p<0.05 vs the values of DMSO-treated STHdh Q111/Q111 cells by one-way-ANOVA with Fisher’s posthoc analysis.
DISCUSSION
It has been reported that rosiglitazone (RSG) attenuates mitochondrial dysfunction in cells expressing mutant HTT (Quintanilla et al. 2008). In the present study, we further confirmed that RSG protects cells against mutant HTT-induced cell toxicity, and that the protective effect is mediated by activation of PPAR-γ. Furthermore, we demonstrated that PPAR-γ mRNA and its coactivator PGC-1α levels were reduced in the cerebral cortex and striatum of N171-82Q HD mice. RSG restored the levels of PGC-1α and rescued BDNF, thereby improving motor function in HD mice.
PPAR-γ is a key transcription factor involved in energy metabolism (Jones & Hughes 2011, Diano et al. 2011, Etgen et al. 2002); mutant HTT disrupts PPAR-γ transcription and consequently leads to metabolic abnormalities. Indeed, it has been shown that PPAR-γ agonists thiazolidinedione (Chiang et al. 2010), piglitazone (Napolitano et al. 2011), and pan-PPAR agonist bezafibrate (Johri et al. 2012), exhibited neuroprotective effects in different HD mouse models. PPAR-γ coactivator PGC-1α interacts with a number of transcriptional factors, and regulates genes involved in mitochondrial respiration (Rasbach & Schnellmann 2007, Valle et al. 2005, McGill & Beal 2006, Zheng et al. 2010, Liang & Ward 2006). Many nuclear encoded mitochondrial genes are modulated by PGC-1α (Martin et al. 2011, Jin & Johnson 2010, Turner & Schapira 2010). Repression of PGC-1α leads to mitochondrial dysfunction, and mutant HTT interferes with PGC-1α, disrupts its transcriptional activity in HD (Cui et al. 2006, Weydt et al. 2006), and represses genes targeted by PGC-1α in HD patients, as well as in HD mouse models (Chaturvedi et al. 2010). Overexpression of PGC-1α protects neurons from mutant HTT-induced cell death, while PGC-1α knockout mice exhibited impaired mitochondrial dysfunction, movement disorders, and striatal degeneration (Chiang et al. 2010). We found that reduced mRNA levels of PGC-1α and PPAR-γ are rescued by chronic administration of RSG in N171-82Q mouse cerebral cortex and striatum. However, protein levels of these molecules were not measured and therefore it is possible that the protein levels may or may not be restored as were mRNA levels by RSG in HD mice. RSG exhibits neuroprotective effects similar to compounds activating PGC-1α in HD models (Canto & Auwerx 2009, Chaturvedi et al. 2009).
BDNF deficiency is a major contributor to striatal degeneration and many phenotypes in HD (Strand et al. 2007, Baquet et al. 2004, Diekmann et al. 2009). Conditional release of BDNF improved pathology and delayed neuronal dysfunction in HD mice (Giralt et al. 2011), and overexpression of BDNF in the striatum or administration of compounds increasing BDNF levels delayed the onset of motor dysfunction in these mice (Simmons et al. 2011, Xie et al. 2010). Most notably, RSG treatment significantly preserved BDNF levels and improved motor function in N171-82Q HD mice, suggesting that activation of PPAR-γ preserved the neurotrophic factor, and protected neuronal function and thereby improved motor function in these mice.
Orexin-A is a neuropeptide, selectively expressed in the hypothalamus, that controls metabolism including glucose homeostasis; it has been shown that orexin levels are decreased in HD mouse models (Petersen et al. 2005, Williams et al. 2011, Gabery et al. 2012). Orexin-A-positive neurons send axonal projections to a wide variety of brain regions and influence a broad range of functions, such as sleep architecture, state-dependent behavior stabilization, and modulation of food intake, and thus respond to metabolic status (Ebrahim et al. 2002). Selectively expressing mutant HTT in the hypothalamus is sufficient to produce the abnormal metabolic symptoms in mice, such as increased food intake and obesity on a normal diet, and these abnormalities could be prevented by selectively inactivating mutant HTT expression in the hypothalamus (Hult et al. 2011). In R6/2 HD mice, although there was no significant overall neuronal loss, orexin-A-positive neurons were decreased dramatically in the late stage of disease (Petersen et al. 2005). We found a similar loss of orexin-A-positive neurons in N171-82Q mouse hypothalamus, indicating that decreased orexin expression or loss of orexin neurons are common pathologies in HD. RSG treatment preserved orexin-A-positive neurons, and maintained glucose homeostasis in these mice.
Disrupted metabolic homeostasis is a hallmark of HD. Sirt6, a member of the sirtuin families, appears to have particular significance in regulating metabolism and life span (Zhong et al. 2010, Zhong & Mostoslavsky 2010, Lombard et al. 2008). Mice deficient in Sirt6 develop a variety of degenerative conditions, including complete loss of subcutaneous fat, lymphopenia, osteopenia, and lordokyphosis (Xiao et al. 2010). In a study of the effect of RSG on hepatic steatosis, RSG treatment ameliorated accumulation of hepatic lipids and increased the expression of Sirt6 in rat liver. Sirt6 knockdown abolished the effects of RSG, suggesting that Sirt6 is involved in RSG-mediated metabolic regulation (Yang et al. 2011). We therefore examined the Sirt6 levels in brains of HD mice as well as RSG-treated mice. Interestingly, we first found that the levels of Sirt6 were significantly low in both HD mouse brain and mutant HTT-expressing cells. RSG treatment restored the Sirt6 levels in brains of HD mice, and increased the Sirt6 level in mutant HTT-expressing cells. These results implicate RSG in regulation of Sirt6, thereby attenuating the metabolic abnormality in HD mice.
It is noteworthy that that RSG treatments had no effect on body weight or survival in N171-82Q HD mice. In this regard, the effects of RSG treatment are similar to results obtained from several other treatment regimens or genetic manipulation, in which significant improvements in motor performance and glucose homeostasis are noted despite lack of effect on body weight or life span (Chopra et al. 2007, Chou et al. 2005, Li et al. 2010, Jiang et al. 2012). The mechanism of body weight changes in HD model is not fully understood; although it has been shown that there is correlation between body weight gain and huntingtin levels in YAC HD mice (Pouladi et al. 2010). But this phenomenon seems not specific to mutant huntingtin-induced changes, as HD patients often lose body weight, and other fragment HD mouse models, such as N171-82Q and R6/2, and full-length Hdh knock-in mouse, including HdhQ150, HdhQ140 models, display body weight loss. These results suggest that the body weight changes in HD mouse models may be related to the mouse background strain and/or non-specific effects from mutant huntingtin expression. It is most likely that the weight loss and life-span are not responsive to RSG-driven increases in neurotrophin signaling, PGC-1α, or Sirt6 levels in brain regions controlling movement and glucose metabolism. Interestingly, weight loss does not correlate with various motor scores in HD patients.
It is noteworthy that the role of PPAR-γ in HD has been explored by other groups in cell models(Quintanilla et al. 2008, Jin et al. 2012), chemically induced HD models(Napolitano et al. 2011), and R6/2 transgenic HD mouse models(Chiang et al. 2010, Chiang et al. 2012); the consistent conclusion is that PPARγ is involved in the pathomechanism of mutant huntingtin-induced mitochondrial dysfunction. To our knowledge, the current study is the first report showing the beneficial effects of the PPAR-γ agonist rosiglitazone in the N171-82Q transgenic mouse model; the novelty of the present results is that we found that activation of PPARγ normalizes the levels of the Sirt6 in HD models. Sirt6 appears to have particular significance in regulating metabolism and life-span(Zhong & Mostoslavsky 2010, Zhong et al. 2010, Lombard et al. 2008). Mice deficient in Sirt6 develop a variety of degenerative conditions, including complete loss of subcutaneous fat, lymphopenia, osteopenia, and lordokyphosis (Xiao et al. 2010). Thus our results shed lights into the new possible mechanism on mutant huntingtin-induced energy deficiency.
In conclusion, our results indicate that chronic administration of the PPAR-γ agonist RSG is sufficient to restore the markedly reduced HD-related BDNF deficiency and preserve levels of PGC-1α and Sirt6 in HD mouse brain, and that these changes are accompanied by equally pronounced improvements in a striatum-dependent motor task, as well as maintenance of glucose homeostasis. The treatments did not affect body weight loss or survival, characteristic of N171-82Q mice. Future studies will extend the analysis of RSG effects to other measures of HD pathology (e.g., changes in neuropeptide expression) and test if more potent PPAR-γ agonists can further alleviate motor impairments in mouse models expressing full-length mutant huntingtin, in which the pathology develops more slowly. The promising protective effects of RSG in HD mice suggest that targeting the PPAR-γ signaling pathway should be considered in developing HD therapy.
Supplementary Material
Acknowledgments
This study was supported by NIH NS072344 (to WD). We acknowledge Dr. Pamela Talalay for dedicated editorial advice. Dr. Marcy Macdonald provided striatal cells.
Footnotes
All authors have no conflict of interest to declare.
References
- The Huntington’s Disease Collaborative Research Group. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Baquet ZC, Gorski JA, Jones KR. Early striatal dendrite deficits followed by neuron loss with advanced age in the absence of anterograde cortical brain-derived neurotrophic factor. J Neurosci. 2004;24:4250–4258. doi: 10.1523/JNEUROSCI.3920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bithell A, Johnson R, Buckley NJ. Transcriptional dysregulation of coding and non-coding genes in cellular models of Huntington’s disease. Biochem Soc Trans. 2009;37:1270–1275. doi: 10.1042/BST0371270. [DOI] [PubMed] [Google Scholar]
- Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Frau L, Pisanu A, Wardas J, Spiga S, Carboni E. Rosiglitazone decreases peroxisome proliferator receptor-gamma levels in microglia and inhibits TNF-alpha production: new evidences on neuroprotection in a progressive Parkinson’s disease model. Neuroscience. 2011;194:250–261. doi: 10.1016/j.neuroscience.2011.07.046. [DOI] [PubMed] [Google Scholar]
- Chaturvedi RK, Adhihetty P, Shukla S, et al. Impaired PGC-1alpha function in muscle in Huntington’s disease. Hum Mol Genet. 2009;18:3048–3065. doi: 10.1093/hmg/ddp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, Calingasan NY, Yang L, Hennessey T, Johri A, Beal MF. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington’s disease following chronic energy deprivation. Hum Mol Genet. 2010;19:3190–3205. doi: 10.1093/hmg/ddq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang MC, Chen CM, Lee MR, et al. Modulation of energy deficiency in Huntington’s disease via activation of the peroxisome proliferator-activated receptor gamma. Hum Mol Genet. 2010;19:4043–4058. doi: 10.1093/hmg/ddq322. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Chern Y, Huang RN. PPARgamma rescue of the mitochondrial dysfunction in Huntington’s disease. Neurobiol Dis. 2012;45:322–328. doi: 10.1016/j.nbd.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Chiang MC, Chern Y, Juo CG. The dysfunction of hepatic transcriptional factors in mice with Huntington’s Disease. Biochim Biophys Acta. 2011;1812:1111–1120. doi: 10.1016/j.bbadis.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Chopra V, Fox JH, Lieberman G, et al. A small-molecule therapeutic lead for Huntington’s disease: preclinical pharmacology and efficacy of C2-8 in the R6/2 transgenic mouse. Proc Natl Acad Sci U S A. 2007;104:16685–16689. doi: 10.1073/pnas.0707842104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SY, Lee YC, Chen HM, et al. CGS21680 attenuates symptoms of Huntington’s disease in a transgenic mouse model. J Neurochem. 2005;93:310–320. doi: 10.1111/j.1471-4159.2005.03029.x. [DOI] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Diano S, Liu ZW, Jeong JK, et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat Med. 2011;17:1121–1127. doi: 10.1038/nm.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekmann H, Anichtchik O, Fleming A, Futter M, Goldsmith P, Roach A, Rubinsztein DC. Decreased BDNF levels are a major contributor to the embryonic phenotype of huntingtin knockdown zebrafish. J Neurosci. 2009;29:1343–1349. doi: 10.1523/JNEUROSCI.6039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djousse L, Knowlton B, Cupples LA, Marder K, Shoulson I, Myers RH. Weight loss in early stage of Huntington’s disease. Neurology. 2002;59:1325–1330. doi: 10.1212/01.wnl.0000031791.10922.cf. [DOI] [PubMed] [Google Scholar]
- Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci U S A. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahim IO, Howard RS, Kopelman MD, Sharief MK, Williams AJ. The hypocretin/orexin system. J R Soc Med. 2002;95:227–230. doi: 10.1258/jrsm.95.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etgen GJ, Oldham BA, Johnson WT, et al. A tailored therapy for the metabolic syndrome: the dual peroxisome proliferator-activated receptor-alpha/gamma agonist LY465608 ameliorates insulin resistance and diabetic hyperglycemia while improving cardiovascular risk factors in preclinical models. Diabetes. 2002;51:1083–1087. doi: 10.2337/diabetes.51.4.1083. [DOI] [PubMed] [Google Scholar]
- Fatehi-Hassanabad Z, Tasker RA. Peroxisome proliferator-activated receptor-gamma (PPAR-gamma) activation confers functional neuroprotection in global ischemia. Neurotox Res. 2011;19:462–471. doi: 10.1007/s12640-010-9201-3. [DOI] [PubMed] [Google Scholar]
- Fuenzalida K, Quintanilla R, Ramos P, Piderit D, Fuentealba RA, Martinez G, Inestrosa NC, Bronfman M. Peroxisome proliferator-activated receptor gamma up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J Biol Chem. 2007;282:37006–37015. doi: 10.1074/jbc.M700447200. [DOI] [PubMed] [Google Scholar]
- Gabery S, Sajjad MU, Hult S, Soylu R, Kirik D, Petersen A. Characterization of a rat model of Huntington’s disease based on targeted expression of mutant huntingtin in the forebrain using adeno-associated viral vectors. Eur J Neurosci. 2012 doi: 10.1111/j.1460-9568.2012.08193.x. [DOI] [PubMed] [Google Scholar]
- Giralt A, Carreton O, Lao-Peregrin C, Martin ED, Alberch J. Conditional BDNF release under pathological conditions improves Huntington’s disease pathology by delaying neuronal dysfunction. Mol Neurodegener. 2011;6:71. doi: 10.1186/1750-1326-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Rodrigo T, Martin ED, Gonzalez JR, Mila M, Cena V, Dierssen M, Canals JM, Alberch J. Brain-derived neurotrophic factor modulates the severity of cognitive alterations induced by mutant huntingtin: involvement of phospholipaseCgamma activity and glutamate receptor expression. Neuroscience. 2009;158:1234–1250. doi: 10.1016/j.neuroscience.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Hoppitt T, Calvert M, Pall H, Rickards H, Sackley C. Huntington’s disease. Lancet. 2010;376:1463–1464. doi: 10.1016/S0140-6736(10)61989-7. [DOI] [PubMed] [Google Scholar]
- Hult S, Soylu R, Bjorklund T, Belgardt BF, Mauer J, Bruning JC, Kirik D, Petersen A. Mutant huntingtin causes metabolic imbalance by disruption of hypothalamic neurocircuits. Cell Metab. 2011;13:428–439. doi: 10.1016/j.cmet.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hunter JM, Lesort M, Johnson GV. Ubiquitin-proteasome system alterations in a striatal cell model of Huntington’s disease. J Neurosci Res. 2007;85:1774–1788. doi: 10.1002/jnr.21287. [DOI] [PubMed] [Google Scholar]
- Jiang M, Wang J, Fu J, et al. Neuroprotective role of Sirt1 in mammalian models of Huntington’s disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YN, Hwang WY, Jo C, Johnson GV. Metabolic state determines sensitivity to cellular stress in Huntington disease: normalization by activation of PPARgamma. PLoS One. 2012;7:e30406. doi: 10.1371/journal.pone.0030406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YN, Johnson GV. The interrelationship between mitochondrial dysfunction and transcriptional dysregulation in Huntington disease. J Bioenerg Biomembr. 2010;42:199–205. doi: 10.1007/s10863-010-9286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johri A, Calingasan NY, Hennessey TM, Sharma A, Yang L, Wille E, Chandra A, Beal MF. Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Hum Mol Genet. 2012;21:1124–1137. doi: 10.1093/hmg/ddr541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Hughes A. Pathogenic mechanisms in Huntington’s disease. Int Rev Neurobiol. 2011;98:373–418. doi: 10.1016/B978-0-12-381328-2.00015-8. [DOI] [PubMed] [Google Scholar]
- Kiaei M. Peroxisome Proliferator-Activated Receptor-gamma in Amyotrophic Lateral Sclerosis and Huntington’s Disease. PPAR Res. 2008;2008:418765. doi: 10.1155/2008/418765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiaei M, Kipiani K, Chen J, Calingasan NY, Beal MF. Peroxisome proliferator-activated receptor-gamma agonist extends survival in transgenic mouse model of amyotrophic lateral sclerosis. Exp Neurol. 2005;191:331–336. doi: 10.1016/j.expneurol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Li XJ, Orr AL, Li S. Impaired mitochondrial trafficking in Huntington’s disease. Biochim Biophys Acta. 2010;1802:62–65. doi: 10.1016/j.bbadis.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Ward WF. PGC-1alpha: a key regulator of energy metabolism. Adv Physiol Educ. 2006;30:145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119:121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Schwer B, Alt FW, Mostoslavsky R. SIRT6 in DNA repair, metabolism and ageing. J Intern Med. 2008;263:128–141. doi: 10.1111/j.1365-2796.2007.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Betuing S, Pages C, Cambon K, Auregan G, Deglon N, Roze E, Caboche J. Mitogen- and stress-activated protein kinase 1-induced neuroprotection in Huntington’s disease: role on chromatin remodeling at the PGC-1-alpha promoter. Hum Mol Genet. 2011;20:2422–2434. doi: 10.1093/hmg/ddr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill JK, Beal MF. PGC-1alpha, a new therapeutic target in Huntington’s disease? Cell. 2006;127:465–468. doi: 10.1016/j.cell.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Napolitano M, Costa L, Palermo R, Giovenco A, Vacca A, Gulino A. Protective effect of pioglitazone, a PPARgamma ligand, in a 3 nitropropionic acid model of Huntington’s disease. Brain Res Bull. 2011;85:231–237. doi: 10.1016/j.brainresbull.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Panov AV, Gutekunst CA, Leavitt BR, Hayden MR, Burke JR, Strittmatter WJ, Greenamyre JT. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- Petersen A, Gil J, Maat-Schieman ML, et al. Orexin loss in Huntington’s disease. Hum Mol Genet. 2005;14:39–47. doi: 10.1093/hmg/ddi004. [DOI] [PubMed] [Google Scholar]
- Pouladi MA, Xie Y, Skotte NH, et al. Full-length huntingtin levels modulate body weight by influencing insulin-like growth factor 1 expression. Hum Mol Genet. 2010;19:1528–1538. doi: 10.1093/hmg/ddq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, Jin YN, Fuenzalida K, Bronfman M, Johnson GV. Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. J Biol Chem. 2008;283:25628–25637. doi: 10.1074/jbc.M804291200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla RA, Johnson GV. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res Bull. 2009;80:242–247. doi: 10.1016/j.brainresbull.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randy LH, Guoying B. Agonism of Peroxisome Proliferator Receptor-Gamma may have Therapeutic Potential for Neuroinflammation and Parkinson’s Disease. Curr Neuropharmacol. 2007;5:35–46. doi: 10.2174/157015907780077123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasbach KA, Schnellmann RG. PGC-1alpha over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. PPARgamma : a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Rosenstock TR, Duarte AI, Rego AC. Mitochondrial-Associated Metabolic Changes and Neurodegeneration in Huntington’s Disease - From Clinical Features to the Bench. Curr Drug Targets. 2010 doi: 10.2174/1389450111007011218. [DOI] [PubMed] [Google Scholar]
- Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- Schintu N, Frau L, Ibba M, Caboni P, Garau A, Carboni E, Carta AR. PPAR-gamma-mediated neuroprotection in a chronic mouse model of Parkinson’s disease. Eur J Neurosci. 2009;29:954–963. doi: 10.1111/j.1460-9568.2009.06657.x. [DOI] [PubMed] [Google Scholar]
- Schutz B, Reimann J, Dumitrescu-Ozimek L, Kappes-Horn K, Landreth GE, Schurmann B, Zimmer A, Heneka MT. The oral antidiabetic pioglitazone protects from neurodegeneration and amyotrophic lateral sclerosis-like symptoms in superoxide dismutase-G93A transgenic mice. J Neurosci. 2005;25:7805–7812. doi: 10.1523/JNEUROSCI.2038-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DA, Mehta RA, Lauterborn JC, Gall CM, Lynch G. Brief ampakine treatments slow the progression of Huntington’s disease phenotypes in R6/2 mice. Neurobiol Dis. 2011;41:436–444. doi: 10.1016/j.nbd.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand AD, Baquet ZC, Aragaki AK, et al. Expression profiling of Huntington’s disease models suggests that brain-derived neurotrophic factor depletion plays a major role in striatal degeneration. J Neurosci. 2007;27:11758–11768. doi: 10.1523/JNEUROSCI.2461-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strum JC, Shehee R, Virley D, et al. Rosiglitazone induces mitochondrial biogenesis in mouse brain. J Alzheimers Dis. 2007;11:45–51. doi: 10.3233/jad-2007-11108. [DOI] [PubMed] [Google Scholar]
- Turner C, Schapira AH. Mitochondrial matters of the brain: the role in Huntington’s disease. J Bioenerg Biomembr. 2010;42:193–198. doi: 10.1007/s10863-010-9290-y. [DOI] [PubMed] [Google Scholar]
- Valle I, Alvarez-Barrientos A, Arza E, Lamas S, Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry. 2005;13:950–958. doi: 10.1176/appi.ajgp.13.11.950. [DOI] [PubMed] [Google Scholar]
- Wenz T, Diaz F, Spiegelman BM, Moraes CT. Activation of the PPAR/PGC-1alpha pathway prevents a bioenergetic deficit and effectively improves a mitochondrial myopathy phenotype. Cell Metab. 2008;8:249–256. doi: 10.1016/j.cmet.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Weydt P, Pineda VV, Torrence AE, et al. Thermoregulatory and metabolic defects in Huntington’s disease transgenic mice implicate PGC-1alpha in Huntington’s disease neurodegeneration. Cell Metab. 2006;4:349–362. doi: 10.1016/j.cmet.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Williams RH, Morton AJ, Burdakov D. Paradoxical function of orexin/hypocretin circuits in a mouse model of Huntington’s disease. Neurobiol Dis. 2011;42:438–445. doi: 10.1016/j.nbd.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Kim HS, Lahusen T, Wang RH, Xu X, Gavrilova O, Jou W, Gius D, Deng CX. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285:36776–36784. doi: 10.1074/jbc.M110.168039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Hayden MR, Xu B. BDNF overexpression in the forebrain rescues Huntington’s disease phenotypes in YAC128 mice. J Neurosci. 2010;30:14708–14718. doi: 10.1523/JNEUROSCI.1637-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SJ, Choi JM, Chae SW, et al. Activation of peroxisome proliferator-activated receptor gamma by rosiglitazone increases sirt6 expression and ameliorates hepatic steatosis in rats. PLoS One. 2011;6:e17057. doi: 10.1371/journal.pone.0017057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, et al. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D’Urso A, Toiber D, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–293. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, Mostoslavsky R. SIRT6: a master epigenetic gatekeeper of glucose metabolism. Transcription. 2010;1:17–21. doi: 10.4161/trns.1.1.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuccato C, Marullo M, Vitali B, et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS One. 2011;6:e22966. doi: 10.1371/journal.pone.0022966. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.