Summary
There are little data on the impact of antiretroviral therapy (ART) regimen factors on adherence in ART naïve HIV patients on contemporary once or twice daily regimens. Ninety-nine newly diagnosed patients in a prospective observational cohort study completed a visual analogue scale (VAS) to assess their ART adherence. Adherence by type of ART and dosing frequency were compared by Brown-Mood median tests. Participants taking once daily regimens had higher adherence (n=70, 99.5%) compared to participants taking twice daily regimens (n=29, 94%; p=0.01). Adherence of participants taking fixed dose combination efavirenz-emtricitabine-tenofovir (n=34, 100%) compared to those taking once daily, >1-pill regimens was no different (n=36, 99.3%; p=0.34). Among a cohort of newly diagnosed, ART naïve patients, once daily dosing of ART resulted in higher adherence than twice daily dosing. Pill burden among once daily regimens did not predict adherence, suggesting that factors other than pill burden should drive regimen selection.
Keywords: HIV, HAART, North America
Introduction
Highly active antiretroviral therapy (HAART) provides immunologic and virologic benefits to patients with HIV. However, such benefits cannot be achieved without medication adherence. In the early years of the HAART era, HAART consisted of complicated regimens with high pill burden and frequent dosing. Newer regimens consist of fixed-dose combination pills. In October 2004, the Department of Health and Human Services treatment guidelines recommended use of efavirenz (EFV) with emtricitabine (FTC) and tenofovir (TDF) in HIV-treatment naïve patients as one of the preferred regimens.1 Thereafter, a fixed dose combination one-pill, once-daily regimen of EFV/FTC/TDF was developed.
Several studies have shown that characteristics of antiretroviral regimens can affect patients’ medication adherence. A recent meta-analysis of 11 randomized, controlled trials of different HAART regimens found that adherence was higher with once-daily regimens (+2.9%; 95% confidence interval 1.0%–4.8%; p<0.003) than twice-daily regimens, with a more profound effect seen at the time of treatment initiation.2 Fixed dose combination EFV/FTC/TDF was not studied in comparison to other once-daily regimens in that meta-analysis. Participants in a prospective cohort study had higher adherence on HAART with low dosing frequencies (the lowest being twice daily) but not with low pill burden.3 A 5-month observational cohort study of participants who had been on HAART for more than four months found that a lower number of pills (p= 0.02) and fewer daily doses (p=0.02) were associated with higher adherence as measured by the Adult AIDS Clinical Trial Group (AACTG) 4-day self-report instrument.4 In addition, a prospective observational study in Italy found higher AACTG 4-day self-report adherence for participants with a lower number of pills in their prescribed HAART regimen (p=0.02) and a lower number of required daily doses (p=0.04).5 Fixed dose combination EFV/FTC/TDF was not available in the latter three studies. Bangsberg et al. recently conducted a prospective observational cohort study in primarily HAART-experienced homeless participants for six months and found that adherence by unannounced pill count was higher in participants who took fixed dose combination EFV/FTC/TDF compared to participants taking any other regimen (p<0.01).6 Airoldi et al. found that patients who were first treated with FTC + TDF + EFV or lamivudine (3TC) + TDF + EFV and had a HIV-RNA <50 copies/mL and were then switched to fixed-dose combination EFV/FTC/TDF had better adherence after the switch (93.8% vs. 96.1%, respectively, p<0.01).7 These studies, including the studies in the meta-analysis, did not assess adherence to contemporary HAART regimens (including fixed dose combination EFV/FTC/TDF) in newly diagnosed, HAART-naïve patients. Whether a one-pill once-daily regimen promotes higher adherence than other once daily regimens in HAART-naive patients is unknown. We report the adherence to contemporary HAART regimens of newly diagnosed, HAART-naïve participants in an 18-month prospective cohort study.
Patients and Methods
Study Design, Participants & Setting
We conducted a prospective, observational cohort study of patients newly diagnosed with HIV infection in Houston, TX. Details of screening and enrollment into the Attitudes and Beliefs and the Steps of HIV Care study (the Steps Study) are outlined by Bhatia, et al.8 Participants completed an interviewer-administered questionnaire at baseline and every three months for up to 18 months. These questionnaires were generally completed outside of the clinical setting. The questionnaires included items on demographics, HIV risk factors, and incarceration history, as well as a visual analogue scale (VAS) adherence measure for each of their HIV medications if they had been prescribed them.9 Participants did not undergo standardized adherence counseling as part of the research protocol. Most patients starting HAART in the clinic receive adherence counseling as part of routine care from their provider and either a nurse adherence counselor or a clinical pharmacist. Laboratory data from routine care were retrieved, and medical records were reviewed to verify participants’ HAART regimens.
Outcome Measures and Data Analysis
For 30-day VAS adherence, the location of the ‘X’ that the participant wrote on the response scale was converted to a percent. For example, if they placed an ‘X’ at the 50% mark on the scale for a medication, their adherence for that medication would be recorded as 50%.9 The mean adherence for all of the HIV medications in a participant’s regimen was calculated at each study time point. The median adherence was then computed for all of the adherence assessments a participant completed over time. Adherence was censored at a participant’s first change in HAART regimen or last VAS measurement, whichever came first, as no further data points for adherence were available. Because the adherence data were not normally distributed, median rather than mean adherence values were used. The Brown-Mood statistical test was used, as it is more robust against outliers in data compared to the Kruskal-Wallis test.10 We used generalized estimating equations to conduct adjusted analyses to account for the repeated measures design of the study. The outcome variable was dichotomized at 99–100% or <99%, and analyses were adjusted for gender, race, age, education level, income, CD4 count at baseline, type of insurance, employment status, and place of diagnosis.
At the time of our study, HIV viral load assays with a lower limit of detection of 400 and 50 c/mL were both in use at our clinic. To avoid misclassifying participants, we dichotomized viral load results as either <400 c/mL or ≥400 c/mL. We used the latest HIV viral load that was at least 90 days after a participant started HAART but was not 30 days or more after a change in HAART. Rates of achieving an undetectable VL for different dosing frequencies and pill burden were compared using Fisher’s exact tests.
The study was approved by the institutional review boards of Baylor College of Medicine and The University of Texas Health Science Center at Houston. All participants provided written informed consent.
Results
Ninety-nine out of 184 Steps study participants contributed adherence data to the present analysis while seventy-six contributed viral load data. The remaining participants were not prescribed HAART during the study, died, or were lost to follow-up before contributing adherence data. Characteristics of the Steps study participants in the present analyses are shown in Table 1. The majority of the participants were male, <50 years old, African-American or Hispanic, had no high school degree, and had relatively low incomes. The median (25th, 75th percentile) baseline HIV viral load was 5.32 log10 c/mL (4.90, 5.73), and the median (25th, 75th percentile) baseline CD4 cell count was 135 K/mm3 (36, 271).
Table 1.
Characteristics of 99 Steps study participants with adherence data
| Characteristic | N |
|---|---|
| Gender | |
| Male | 72 |
| Female | 27 |
| Age | |
| <30 years old | 22 |
| 30–39 years old | 35 |
| 40–49 years old | 24 |
| 50 and above | 18 |
| Race/Ethnicity | |
| African American, non-Hispanic | 44 |
| White, non-Hispanic | 11 |
| Hispanic | 44 |
| Degree Attained | |
| Never finished high school | 45 |
| High school diploma or GED | 26 |
| Some college | 28 |
| Yearly Income | |
| 0–$24,999 | 81 |
| $25,000 and above | 16 |
| Insurance | |
| Private, Medicare and/or Medicaid | 17 |
| Uninsured | 82 |
| HIV Risk Factors | |
| Any IDU | 6 |
| MSM | 36 |
| Heterosexual/Other | 57 |
| Place of HIV diagnosis | |
| Inpatient | 49 |
| Outpatient | 51 |
| HIV Viral Load before HAART, median (IQR) | 5.32 log10 c/mL (4.90, 5.73) |
| CD4 cell count before HAART, median (IQR) | 135 K/mm3 (36, 271) |
| HAART Type | |
| NNRTI | 47 |
| Boosted PI | 50 |
| Unboosted PI | 2 |
| Dosing Frequency | |
| Once daily | 70 |
| Twice daily | 29 |
| Pills/day in HAART regimen | |
| 1 | 34 |
| >1 | 65 |
| Number of pills/day if >1* | |
| 2 | 7 |
| 3 | 16 |
| 4 | 15 |
| 5 | 17 |
| 6 | 5 |
| 7 | 3 |
Exact pills/day were not available for two participants who were on a boosted PI regimen.
GED, general equivalency diploma; IDU, intravenous drug use; MSM, men who have sex with men.
Forty-seven (47%) participants were on an NNRTI and 50 (51%) were on a boosted PI. Seventy participants (71%) were on a once-daily regimen, including 34 (34%) who were taking the fixed dose combination EFV/FTC/TDF and 36 (36%) who were taking a once-daily regimen that included more than one pill. Twenty-nine participants (29%) were on a twice-daily regimen. Accounting for gender, age, race/ethnicity, education, income, type of insurance, and place of HIV diagnosis, no differences were found between participants who were prescribed an NNRTI and a PI, a once daily regimen and a twice daily regimen, or a one-pill once daily regimen and a >1-pill once daily regimen. Of the participants taking more than one pill a day, seven participants were taking two pills a day, 16 were taking three pills a day, 15 were taking four pills per day, and 17 were taking five pills per day (Table 1). The median number of days between when participants started HAART and when they changed HAART or completed their last VAS was 371 days (IQR 182, 488).
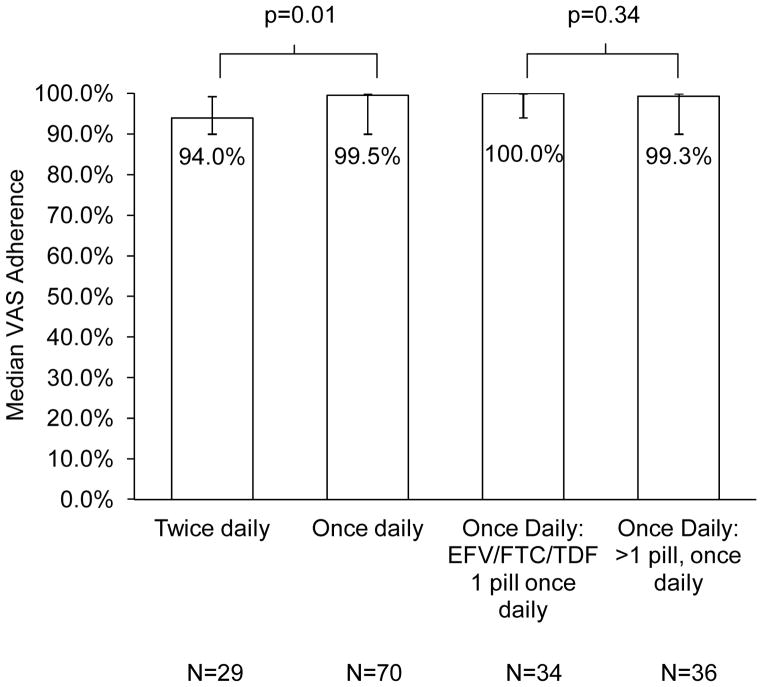
The median number of visual analogue scales completed per participant was three. The median VAS adherence for participants taking once-daily regimens was 99.5% (25th, 75th percentile 90.0, 100.0), which was significantly higher than the adherence of participants taking twice-daily regimens (94.0%; 25th, 75th percentile 90.0, 99.3; p=0.01; Figure 1). Median VAS adherence for participants taking fixed dose combination EFV/FTC/TDF was 100.0% (25th, 75th percentile 93.0, 100.0); for participants taking once daily regimens that were more than one pill, it was 99.3% (25th, 75th percentile 90.0, 100.0). There was no significant difference in VAS adherence between participants taking fixed dose combination EFV/FTC/TDF and participants taking more than one pill, once daily regimens (p=0.34). Adherence was lower in the twice daily group compared to the one pill once daily and more than one pill once daily groups after controlling for gender, race, age, education level, income, CD4 count at baseline, type of insurance, employment status, and place of diagnosis (Table 2, p=0.01). We dichotomized adherence as 99–100% and <99% given that the overall median adherence of our study population was 97.5% (25th, 75th percentile 90.0, 100.0).
Figure 1. Median VAS adherence by HAART regimen characteristics.
The four groups above are not mutually exclusive: the fixed dose combination EFV/FTC/TDF 1 pill once daily and the >1 pill, once daily groups are part of the once daily group
Table 2.
Generalized estimating equation predictors of 99–100% adherence in 99 Steps study participants
| OR (95% CI) | z | p | |
|---|---|---|---|
| ART Characteristics (Referent: One-pill once daily) | |||
| Twice daily | 0.35 (0.15, 0.79) | −2.53 | 0.01 |
| >1 pill once daily | 0.75 (0.34, 1.67) | −0.97 | 0.33 |
| Male sex (Referent: Female sex) | 0.47 (0.20, 1.09) | −1.90 | 0.06 |
| Race (Referent: Non-Hispanic White) | |||
| Hispanic | 1.34 (0.43, 4.15) | 0.70 | 0.48 |
| African-American | 1.02 (0.32, 3.27) | 0.16 | 0.87 |
| Age (Referent: Age <30) | |||
| 50 and above | 1.72 (0.57, 5.21) | 1.22 | 0.22 |
| 40–49 | 1.96 (0.74, 5.19) | 1.47 | 0.14 |
| 30–39 | 1.42 (0.61, 3.30) | 0.98 | 0.32 |
| Education (Referent: < High school) | |||
| At least some college | 1.42 (0.51, 3.96) | 0.59 | 0.56 |
| High school diploma or GED | 1.70 (0.70, 4.13) | 1.10 | 0.27 |
| Annual Income (Referent: <$25,000) | |||
| $25,000 and above | 1.20 (0.49, 2.99) | 0.51 | 0.61 |
| CD4 cell count before HAART (Referent: CD4 < 200 K/mm3) | |||
| >200K/mm3 | 0.83 (0.41, 1.69) | −0.54 | 0.59 |
| Insurance (Referent: No insurance) | |||
| Private, Medicare and/or Medicaid | 0.87 (0.32, 2.39) | −0.19 | 0.85 |
| Employed (Referent: Unemployed) | 0.81 (0.39, 1.69) | −0.49 | 0.62 |
| Inpatient HIV diagnosis (Referent: Outpatient HIV diagnosis) | 1.02 (0.52, 2.00) | 0.09 | 0.92 |
GED, general equivalency diploma
The median number of days between when participants started HAART and their outcome viral load measurement was 352 days (IQR 179, 505). Participants who had 99–100% adherence by VAS were slightly more likely to have virologic suppression (n=38, 84% suppressed) compared to participants with less than 99% adherence (n=36, 64% suppressed, p=0.06). There was no statistically significant difference in viral suppression between participants taking once daily regimens (n=42, 79.3% suppressed) and participants taking twice daily regimens (n=14; 60.9% suppressed; p=0.15). There was also no statistically significant difference in viral suppression between participants taking fixed dose combination EFV/FTC/TDF (n=23, 82.1% suppressed) and participants taking more-than-one-pill once daily (n=19; 76.0% suppressed; p=0.74), and between participants taking one-pill EFV/FTC/TDF and participants taking all other regimens (n=33; 68.6% suppressed; p=0.28).
Discussion
In this study of participants recently diagnosed with HIV infection, once daily dosing of HAART resulted in higher adherence than twice daily dosing, as measured by a VAS. In contrast, among participants on once daily regimens, adherence to a one-pill regimen was no different than adherence to regimens with more than one pill. These results suggest that pill burden is not an important factor in determining adherence to contemporary once daily HAART regimens in HAART naïve patients.
Several studies have shown that participants exhibit higher adherence with once daily dosing of antiretrovirals compared to twice daily dosing.2,4,5,11 Some studies have also shown that a lower pill burden results in higher adherence.4–6 Specifically, Airoldi et al. found that patients who had a HIV-RNA <50 copies/mL on a more-than-one-pill once daily regimen and were then switched to fixed-dose combination EFV/FTC/TDF had better adherence after the switch (93.8% vs. 96.1%, respectively, p<0.01).7 To our knowledge, our study is the first to compare adherence between one-pill once daily regimens and more-than-one-pill once daily regimens in HAART-naïve patients. We expected that therapy with a single tablet once daily would result in higher adherence. Somewhat surprisingly, we found no differences in adherence between the once daily regimens. Our data support once daily dosing, but do not support the hypothesis that a one-pill once daily dose is associated with higher adherence than other once daily regimens.
Although our study participants who took HAART once daily had higher rates of virologic suppression compared to participants taking HAART twice daily, the difference was not statistically significant (p=0.15), though this result is likely due to our small sample size. Parienti et al. also found no significant difference in the proportion of subjects who achieved HIV RNA levels <50 copies/mL (p=0.21) between participants taking once daily versus twice daily regimens.2 Bangsberg et al. found that viral suppression was higher in participants taking fixed dose combination EFV/FTC/TDF (69%) compared to participants taking any other regimen (46%, p=0.02) but did not compare once daily versus twice daily regimens exclusively.6
We also did not find a statistically significant difference in achievement of virologic suppression based on pill burden, likely due to our small sample size. Bartlett et al. in 2001 found that a higher percentage of patients had an HIV RNA level <50 copies/mL at 48 weeks with lower pill burdens 12. In 2006, Bartlett et al. found no difference in viral suppression based on pill count, likely due to the fact that their 2006 systematic overview included more potent HAART regimens compared to their overview in 2001.13 Bangsberg et al. found that viral suppression was higher in participants taking fixed dose combination EFV/FTC/TDF (69%) compared to participants taking any other regimen with more than pill (46%, p=0.02).6 Together, these results and the results of the present study suggest that pill burden for contemporary regimens is low enough that, among once-daily regimens, it is not a major factor in determining adherence or viral suppression as long as it is below some as yet undefined threshold.
This study has several limitations. Adherence was measured by self-report rather than more objective measures like pharmacy refill data or Medication Event Monitoring System (MEMS) caps. These data were available for a subset of participants, but not enough to produce reliable estimates for the questions addressed in the present set of analyses. As noted earlier, only 99 participants out of the entire Steps cohort of 184 contributed to the analysis, and only 76 out of 99 participants were included in the viral suppression analyses, thus decreasing our ability to detect small differences in adherence and virologic suppression. Some participants did not have a viral load in their medical record after starting HAART because they dropped out of or transferred care. The generalizibility of our results may be impacted by the fact that participants who were not prescribed HAART during the study, died, or were lost to follow-up are not included in these analyses, Also, adherence in participants was reported to be very high, which may not have been the case for the excluded participants. The high reported adherence is likely partly due to patients overstating their adherence, possibly due to social desirability. Finally, these data are observational, and participants should have been prescribed regimens that fit their lifestyle and clinical needs best. Patients thought to be less likely to adhere may have been placed on less demanding regimens from the start, thus confounding later adherence results. In that sense these data represent what is achievable in routine HIV care with contemporary HAART regimens.
AIDS Drug Assistance Programs (ADAPs) are state-run programs that provide HIV medications for low income, uninsured, and underinsured individuals in the United States. As ADAP client enrollment continues to increase, cost-cutting measures have had to be implemented. Each ADAP has adopted its own formulary, and 24 ADAPs do not cover all FDA-approved antiretrovirals in all drug classes.14 High drug costs are a concern in other developed and developing countries as well.15 Our study results suggest that fixed-dose combination pills are not as critical as dosing schedule in promoting excellent adherence. Cost containment strategies that rely on generic drugs not available in fixed-dose combination pills will, however, need careful evaluation for their clinical impact.
This is the first study to our knowledge to examine the effect of regimen factors on adherence in exclusively newly diagnosed, HAART-naïve participants on contemporary HAART regimens that include fixed dose combination EFV/FTC/TDF. We found that once daily dosing was associated with greater adherence than twice daily dosing. In contrast, the number of pills did not predict adherence among participants on a once daily regimen. When possible, once daily dosing may be the recommended dosing schedule, compared to twice daily dosing. Among once daily regimens, factors other than pill burden (e.g. side effects, drug interactions, daily schedule, and patient preference) should drive regimen selection.
Acknowledgments
This work was supported by National Institute of Mental Health [grant number R34MH074360], Agency for Healthcare Research and Quality [grant number U18HS016093], the Baylor/UT Houston Center for AIDS Research [grant number P30AI036211], and the National Institutes of Allergy and Infectious Diseases [grant number T32AI07456]. This work was also supported by the facilities and resources of the Harris County Hospital District and the Michael E. DeBakey VA Medical Center. Dr. Giordano is a researcher at the Michael E. DeBakey VA Medical Center Health Services Research and Development Center of Excellence, Houston, TX. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Declarations: None
References
- 1.Department of Health and Human Services. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2011 Jan 10;:1–166. http://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL.pdf. 4-15-2011.
- 2.Parienti JJ, Bangsberg DR, Verdon R, Gardner EM. Better adherence with once-daily antiretroviral regimens: a meta-analysis. Clin Infect Dis. 2009;48:484–488. doi: 10.1086/596482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golin CE, Liu H, Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maggiolo F, Ripamonti D, Arici C, et al. Simpler regimens may enhance adherence to antiretrovirals in HIV-infected patients. HIV Clin Trials. 2002;3:371–378. doi: 10.1310/98b3-pwg8-pmyw-w5bp. [DOI] [PubMed] [Google Scholar]
- 5.Maggiolo F, Ravasio L, Ripamonti D, et al. Similar adherence rates favor different virologic outcomes for patients treated with nonnucleoside analogues or protease inhibitors. Clin Infect Dis. 2005;40:158–163. doi: 10.1086/426595. [DOI] [PubMed] [Google Scholar]
- 6.Bangsberg DR, Ragland K, Monk A, Deeks SG. A single tablet regimen is associated with higher adherence and viral suppression than multiple tablet regimens in HIV+ homeless and marginally housed people. AIDS. 2010;24:2835–2840. doi: 10.1097/QAD.0b013e328340a209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Airoldi M, Zaccarelli M, Bisi L, et al. On-pill once-a-day HAART: a simplification strategy that improves adherence and quality of life of HIV-infected subjects. Patient Preference and Adherence. 2010;4:115–125. doi: 10.2147/ppa.s10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatia R, Hartman C, Kallen MA, Graham J, Giordano TP. Persons Newly Diagnosed with HIV Infection are at High Risk for Depression and Poor Linkage to Care: Results from the Steps Study. AIDS Behav. 2011;15:1161–1170. doi: 10.1007/s10461-010-9778-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self-report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS. 2002;16:269–277. doi: 10.1097/00002030-200201250-00017. [DOI] [PubMed] [Google Scholar]
- 10.Thakur AK. Tests of homogeneity and trend with medians. Environ Mutagen. 1985;7 (Suppl 4):23–30. doi: 10.1002/em.2860070806. [DOI] [PubMed] [Google Scholar]
- 11.Flexner C, Tierney C, Gross R, et al. Comparison of once-daily versus twice-daily combination antiretroviral therapy in treatment-naive patients: results of AIDS clinical trials group (ACTG) A5073, a 48-week randomized controlled trial. Clin Infect Dis. 2010;50:1041–1052. doi: 10.1086/651118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett JA, Demasi R, Quinn J, Moxham C, Rousseau F. Overview of the effectiveness of triple combination therapy in antiretroviral-naive HIV-1 infected adults. AIDS. 2001;15:1369–1377. doi: 10.1097/00002030-200107270-00006. [DOI] [PubMed] [Google Scholar]
- 13.Bartlett JA, Fath MJ, Demasi R, et al. An updated systematic overview of triple combination therapy in antiretroviral-naive HIV-infected adults. AIDS. 2006;20:2051–2064. doi: 10.1097/01.aids.0000247578.08449.ff. [DOI] [PubMed] [Google Scholar]
- 14.National Alliance of State and Territorial AIDS Directors. National ADAP Monitoring Project Annual Report. 2010. 201053_2010 National ADAP Monitoring Project Annual Report.pdf. 9-20-2011. [Google Scholar]
- 15.Jones R, Gazzard B. The cost of antiretroviral drugs and influence on prescribing policies. Int J STD AIDS. 2006;17:499–506. doi: 10.1258/095646206778145587. [DOI] [PubMed] [Google Scholar]