Abstract
Background: Limited evidence suggests that calcium intake before puberty influences adolescent height growth and the timing of puberty. Such an effect might be particularly marked in populations in whom low calcium intake, stunting, and delayed puberty are common.
Objective: The objective was to test whether 12 mo of calcium supplementation at age 8–12 y to increase intakes toward international recommendations had long-term effects on adolescent growth and pubertal development in rural Gambian children.
Design: This was a longitudinal study of 160 Gambian boys (n = 80) and girls (n = 80) who had participated in a 12-mo, randomized, double-blind, placebo-controlled, calcium carbonate supplementation trial (1000 mg Ca/d, 5 d/wk) at age 8–12 y. Anthropometric measures were made every 1–2 y until age 21–25 y; pubertal status and menarche data were collected. Repeated-measures ANCOVA and Superimposition by Translation and Rotation Method (SITAR) growth models were used to assess the effects of treatment.
Results: In boys, midadolescent height growth was advanced in the calcium group, which resulted in greater stature at a mean age of 15.5 y (mean ± SEM: 2.0 ± 0.8 cm; P = 0.01) and an earlier age of peak height velocity by 7.4 ± 2.9 mo. Subsequently, the calcium group stopped growing earlier (P = 0.02) and was 3.5 ± 1.1 cm shorter (P = 0.002) at a mean age of 23.5 y. Weight and midupper arm circumference paralleled height. No significant effects were observed in girls, but a sex-by-supplement interaction on height growth could not be confirmed.
Conclusion: Calcium supplementation of boys in late childhood advanced the age of peak height velocity and resulted in shorter adult stature in a population in whom low calcium intakes and delayed puberty are common. This trial was registered at isrctn.org as ISRCTN28836000.
INTRODUCTION
Calcium is an essential bone-forming mineral and, as such, must be consumed in adequate amounts during childhood and adolescence for healthy skeletal growth and development (1, 2). The calcium intakes of young people in many populations are considerably below those recommended for optimal skeletal growth and mineral accretion and are often close to the theoretical biological accretion rate (3). In consequence, very low calcium intakes in childhood and adolescence, particularly at times of high requirement, such as the pubertal growth spurt, have been implicated in linear growth retardation, low bone mineral accretion, and increased risk of osteoporotic fracture in adolescence and old age (1–3).
Several researchers, including ourselves, have investigated whether an increased calcium intake in childhood and adolescence improves growth and bone mineral accretion through controlled intervention studies in pre-, peri-, and postpubertal boys and girls. A variety of approaches have been used, including supplementation, food fortification, and increased supply of calcium-rich foods—most commonly cow milk. The results of such studies suggest a positive effect of calcium supplementation on bone mineral density, although the observed increases are largely transient except, possibly, in the upper limbs (2, 4).
The effects on height growth and the timing of the pubertal growth spurt have been less studied. However, limited evidence suggests that calcium intake in late childhood and adolescence may influence the pubertal growth trajectory. In controlled intervention studies, a calcium carbonate supplement for 12 mo increased the stature of British boys aged 16–18 y (5), and a milk calcium-phosphate salt consumed for 1 y by Swiss girls at age 6.6–9.4 y advanced the age of menarche (6). In observational studies in the United States, milk and dairy consumption at age 9–12 y was associated with greater female height growth (7) and earlier menarche (8).
It is possible, therefore, that calcium intake in childhood may influence skeletal growth and pubertal development in adolescence. If so, such an effect is likely to be particularly evident in populations in whom a low calcium intake, stunting, and delayed puberty are common. Children in rural areas of The Gambia characteristically have very low calcium intakes, poor childhood growth, and delayed puberty (9). We report here the results of a longitudinal study of the growth and pubertal development of rural Gambian boys and girls who had participated at age 8.3–11.9 y in a randomized, placebo-controlled calcium carbonate supplementation study for 12 mo. The calcium supplement, which tripled the mean calcium intake from 340 to 1060 mg/d, increased forearm bone mineral density and altered calciotropic hormone concentrations—effects that were still evident 12–24 mo after supplementation ceased—but had no significant effect on height growth or pubertal development at that time (9–11). Since then, the participants have been followed up at regular intervals throughout puberty and into early adulthood to see whether the calcium supplement had any long-term effects on adolescent growth.
SUBJECTS AND METHODS
Participants and study design
Full details of the study design, subject characteristics, outcome measures, and short-term results of the calcium supplementation have been reported elsewhere (9–11). The primary outcome of the intervention was bone mineral accretion in the radius at the end of 1 y, and the study was powered (at α = 5% and 1-β = 80%) to detect a 5% difference in bone mineral density between the supplement groups for each sex separately or 4% combined.
In summary, children aged 8.0–11.99 y in 1995–1996 and resident in the rural village of Keneba, The Gambia, were eligible for the study. Families were approached with the aim of recruiting 80 boys and 80 girls. Baseline (year 1) measurements were conducted in 2 boys and 2 girls each week, until the target number was reached, starting with the oldest child first. The youngest child enrolled was aged 8.3 y. Participants were randomized, double-blind, in permuted blocks of 4 to receive either a calcium carbonate supplement that provided 1000 mg elemental Ca on 5 d/wk (an average of 714 mg/d) or a matching placebo. The calcium supplement was 2 tablets of orange-flavored Calcichew, and the placebo was matching tablets of microcrystalline cellulose and lactose of similar texture and taste (Shire Pharmaceuticals Ltd and Nycomed Pharma AS). The supplements were provided for ∼12 mo (mean ± SD: 385 ± 41 d), starting the week after the baseline measurements for each set of 4 children. The tablets were dispensed to each participant individually and consumed in the early evening at a centrally situated “youth club” run by the study team to encourage compliance. Tablets missed because of illness or absence from the village were consumed on weekends. The supplements were well accepted; all participants consumed all the tablets assigned to them over the course of the 12 mo.
Serial measurements were made at ∼1-y or 2-y intervals; the time points are identified in this report by year relative to the first set of measurements at baseline (year 1). Repeat measurements were performed at the end of the intervention year (year 2) for each set of 4 participants, before stopping the tablets, and at successive follow-up sessions scheduled to be as close as possible to the anniversary of the baseline measurements. For boys, the interval was initially 1 y (2 sessions, year 3 and year 4) and then approximately every 2 y (5 sessions) to the age of 22.1–25.6 y (year 14). The intervals were the same for girls until year 10, after which yearly measurements were made to year 14. For those girls who had started a family, the sessions were arranged at specific times during and after each lactation (2 wk, 13 wk, 52 wk postpartum, and 13 wk after weaning). No measurements were made during pregnancy. Boys and girls who had moved out of the district—generally for schooling, employment, or marriage—were traced and invited back for measurements. The reasons for declining further participation were not documented.
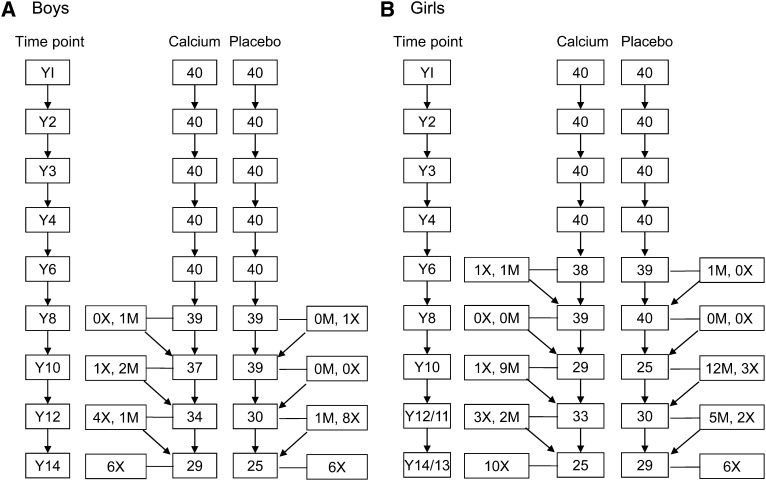
In total, 1431 sets of growth measurements were obtained (boys: n = 672; girls: n = 759). The numbers of participants measured at each time point, and an indication of the numbers of permanent dropouts and those who missed a session, are given in Figure 1. For simplicity, the data for girls presented in the figure and used in the analysis of growth by measurement year were those obtained at time points most closely mirroring those of the boys. For older girls who had attended more than one measurement session during a calendar year because of lactation, the session latest in the year was selected to minimize the use of measurements obtained at 2 wk postpartum. In addition, when year 12 data were not available for an individual girl, year 11 data were used (year 12/11); similarly when year 14 data were not available, year 13 data were used (year 14/13).
FIGURE 1.
Flowchart of participants measured in the study at each time point by sex and supplement group. M, missed this time point but returned for a subsequent measurement; X, permanent dropout or not available for Y14 measurement; Y, year of measurement relative to baseline (Y1).
Ethical approval for the supplementation study, successive follow-up sessions, and the additional measurements on the girls after year 10 were obtained from the Medical Research Council/Gambian Government Joint Ethics Committee. Written informed consent was obtained from a parent or guardian at enrollment for the supplementation study and at each successive follow-up session. No measurements were performed without the full assent of the child. After the age of 18 y, the participants themselves gave written informed consent for the further follow-up sessions. The participants and the investigators involved in the data collection remained blinded to the tablet assignments throughout.
Anthropometric measures
Height was measured to the nearest 0.1 cm with a stadiometer by trained members of the study team by using standardized protocols and checked before each session for accuracy by using a calibrated pole. Measurements were made in the early to mid-morning while the subjects were shoeless and not wearing a headdress and were positioned to ensure a horizontal Frankfort Plane. Weight was measured to the nearest 0.1 kg while the subjects were wearing light clothing and no shoes by using scales that were checked regularly. BMI was calculated as weight divided by the square of height (kg/m2). Midupper arm circumference and triceps skinfold thickness were measured at the midpoint of the upper left arm with a nonstretchable tape measure and a skinfold caliper (Holtain Ltd), respectively.
Age and pubertal status assessments
The people of Keneba have participated for generations in demographic data-gathering by the Medical Research Council (12). All births in the village are recorded during the first days postpartum and individuals entered into the register. The dates of birth of the participants in this study were obtained from this register, enabling accurate calculation of age at each measurement.
Pubertal assessments were made on the basis of the 5 Tanner stages of classification by physical examination, as described elsewhere (11, 13). The pubertal status of the boys was based on genital and pubic hair development and that of the girls by breast and pubic hair development. Tanner stage assessments were made in years 1–6 for the boys and years 1–8 for the girls. They were discontinued because of decreasing acceptability of the assessments among the participants. Information on age at menarche was obtained at each session until menarche had been achieved by asking the girls whether their first menstruation had occurred since the last visit, and, if yes, how many months previously.
Statistical analyses
Descriptive statistics used to compare the calcium and placebo groups are presented as mean differences ± SEMs and the characteristics of the cohort as a whole as means ± SDs. Multiple regression analysis (DataDesk 6.2.1; Data Description Inc) was performed to consider the effect of group on achieved growth at each time point with adjustment of each variable for baseline value and age. Repeated-measures ANCOVA, using the Linear Model software in DataDesk, was used to test for an interaction between supplement group and time point in hierarchical data sets with adjustment for age at each time point and with subject ID nested by supplement group. Logistic regression was used to test for group differences in the prevalence of notable height growth cessation, defined as a height increment of <0.4 cm from year 12 to year 14. The sexes were analyzed separately for most analyses but were combined to test for group-by-sex interactions.
Longitudinal growth in height, weight, and BMI was modeled for boys and girls separately by using the Superimposition by Translation and Rotation Method (SITAR) model, described in detail elsewhere (14), fitted by using the statistical program R (version 2.14.0; http://www.R-project.org/). This is a shape invariant model with a single-fitted cubic spline curve common to all individuals and 3 parameters per individual, estimated as random effects, that determine how the common curve is modified to obtain the best-fitting curve for each individual. The parameters summarize each individual's pattern of growth during adolescence and represent modifications in 1) size (taller/shorter or heavier/lighter overall), 2) tempo (earlier or later pubertal growth spurt), and 3) velocity (higher or lower peak velocity). The 3 parameters can be thought of geometrically as follows: size is an up or down shift on the height axis for each individual growth curve; tempo is a corresponding left or right shift of the curve on the age axis, and velocity is a shrinking or stretching of the age axis, which affects the mean slope (ie, velocity) of the curve. Estimates of mean age at peak velocity were obtained by differentiating the mean growth curve and identifying the age when this velocity curve peaked; its SEM was assumed to be the same as that of the mean tempo random effect. Mean differences in the 3 parameters between the calcium and placebo groups were estimated by including the trial allocation as fixed effects in the model. The 3 SITAR parameters were also fitted as fixed effects. Interactions between the allocation and SITAR parameters were not fitted. To improve the fit by minimizing the Schwarz Bayesian Criterion, the height and weight models in boys used age transformed to natural logs, and boys’ weight was also log transformed (with the Jacobian of the transformation included in the Bayesian Information Criterion). The effect of log transformation on the size and tempo effects was adjusted for by multiplying the effects and their SEMs by the geometric means of weight and age, respectively, giving the size effects in measurement units and the tempo effects in years. The velocity effects were multiplied by 100 to give percentage units. A natural spline curve with 7 df provided the best fit for the boys’ data, whereas 6 df were optimal for the girls’ data, based on the Schwarz Bayesian Criterion.
For the boys, all available measurements were included in the summary tables, regression analyses, and SITAR height analyses. For the girls, the summary statistics and regression analyses were based on the condensed data set described in Figure 1. This made no material difference to the results (data not shown). In addition, the SITAR analysis highlighted 6 outlying heights (2 boys, 4 girls) and 2 outlying weights (1 boy, 1 girl); these measurements were excluded; however, their inclusion did not alter the conclusions drawn.
The Gambia has 2 distinct seasons, wet and dry, which are known to influence child growth and adult body habitus (15). The possibility of confounding by season in the analysis of supplement effects was minimized by the study design, whereby supplement randomization was in permuted blocks of 4, with 4 children measured each week, and the early follow-up sessions were timed at annual intervals. However, by the later sessions, especially in the girls, sessions were not timed according to the week of initial measurements. Tests for the influence of current season were therefore performed, but none were significant and have not been included in the results presented. In addition, a series of analyses was performed with the full data sets restricted to those participants who remained in the study at year 14 for boys and year 13/14 for girls, to see whether bias had been introduced by attrition. The results were not materially different from those obtained with the full models and are not presented.
RESULTS
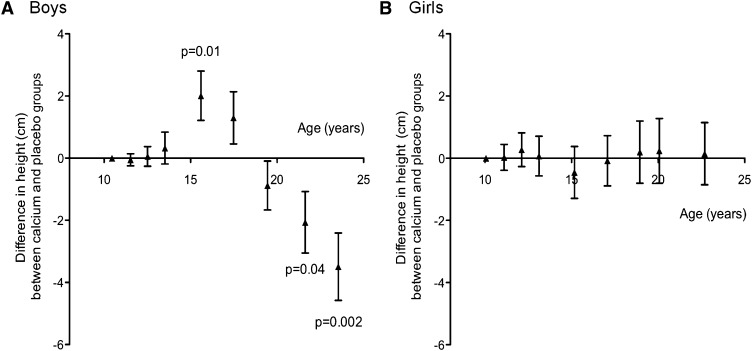
The anthropometric measures are presented by time point and supplement group in Table 1, and the group differences in height by time point are shown in Figure 2. The supplement had no significant effect on growth during the supplementation phase or early follow-up. However, the calcium group was taller in midadolescence (mean ± SEM difference between calcium and placebo groups adjusted for initial height and age: year 6, +2.0 ± 0.8 cm; P = 0.01) and shorter in early adulthood (year 12: −2.1 ± 1.0 cm, P = 0.04; year 14: −3.5 ± 1.1 cm, P = 0.002). The supplement group–by–time point interaction was significant (P = 0.002).
TABLE 1.
Anthropometric measures of Gambian boys in the calcium-supplementation and placebo groups1
| Age |
Height |
Weight |
Midupper arm circumference |
Triceps skinfold thickness |
||||||
| Time point | Calcium | Placebo | Calcium | Placebo | Calcium | Placebo | Calcium | Placebo | Calcium | Placebo |
| y | cm | kg | cm | mm | ||||||
| Y1 | 10.4 ± 0.1 | 10.5 ± 0.1 | 132.5 ± 0.9 | 132.5 ± 1.2 | 25.7 ± 0.5 | 25.4 ± 0.6 | 18.0 ± 0.2 | 17.8 ± 0.2 | 7.2 ± 0.2 | 7.0 ± 0.2 |
| Y2 | 11.5 ± 0.1 | 11.6 ± 0.1 | 137.2 ± 0.8 | 137.2 ± 1.2 | 27.4 ± 0.5 | 26.9 ± 0.7 | 19.0 ± 0.2 | 18.7 ± 0.2 | 7.6 ± 0.3 | 7.4 ± 0.3 |
| Y3 | 12.5 ± 0.1 | 12.6 ± 0.1 | 141.8 ± 0.9 | 141.7 ± 1.3 | 30.0 ± 0.7 | 29.7 ± 0.8 | 19.7 ± 0.2 | 19.5 ± 0.3 | 7.3 ± 0.3 | 7.0 ± 0.2 |
| Y4 | 13.5 ± 0.1 | 13.6 ± 0.1 | 146.2 ± 1.1 | 145.9 ± 1.4 | 33.0 ± 0.8 | 32.3 ± 0.9 | 20.3 ± 0.3 | 20.1 ± 0.3 | 7.2 ± 0.3 | 7.1 ± 0.3 |
| Y6 | 15.5 ± 0.1 | 15.6 ± 0.1 | 158.1 ± 0.8 | 156.2 ± 1.62 | 41.4 ± 1.0 | 40.0 ± 1.3 | 20.2 ± 0.3 | 19.9 ± 0.3 | 6.0 ± 0.2 | 6.3 ± 0.3 |
| Y8 | 17.5 ± 0.1 | 17.5 ± 0.1 | 166.8 ± 0.7 | 165.1 ± 1.4 | 49.6 ± 1.0 | 47.2 ± 1.4 | 22.4 ± 0.3 | 21.9 ± 0.3 | 6.2 ± 0.2 | 6.2 ± 0.2 |
| Y10 | 19.4 ± 0.1 | 19.5 ± 0.1 | 171.1 ± 0.8 | 172.0 ± 1.1 | 54.7 ± 0.9 | 54.0 ± 1.1 | 24.0 ± 0.3 | 24.0 ± 0.3 | 5.7 ± 0.2 | 6.0 ± 0.3 |
| Y12 | 21.7 ± 0.2 | 21.6 ± 0.2 | 173.3 ± 0.8 | 174.7 ± 1.23 | 59.1 ± 1.2 | 57.8 ± 1.2 | 25.6 ± 0.3 | 25.4 ± 0.3 | 7.0 ± 0.3 | 6.6 ± 0.2 |
| Y14 | 23.5 ± 0.2 | 23.6 ± 0.2 | 173.7 ± 0.8 | 176.3 ± 1.44 | 58.9 ± 1.4 | 59.8 ± 1.52 | 25.1 ± 0.5 | 25.7 ± 0.43 | 6.0 ± 0.3 | 6.3 ± 0.3 |
All values are means ± SEMs. The numbers of boys in the calcium and placebo groups, respectively, were as follows: 40 and 40 at Y1–6, 39 and 39 at Y8, 37 and 39 at Y10, 34 and 30 at Y12, and 29 and 25 at Y14. Significance of group differences by multiple regression analysis after adjustment for value and age at Y1 (all else P > 0.1): 2P ≤ 0.01, 3P ≤ 0.05, 4P = 0.002. Y1, baseline; Y2–14, the year of follow-up relative to Y1.
FIGURE 2.
Mean (±SEM) differences in height in boys (A) and girls (B) between the calcium and placebo groups by age at each measurement time point. The numbers of boys in the calcium and placebo groups, respectively, were as follows: 40 and 40 at Y1–6, 39 and 39 at Y8, 37 and 39 at Y10, 34 and 30 at Y12, and 29 and 25 at Y14. The numbers of girls in the calcium and placebo groups, respectively, were as follows: 40 and 40 at Y1–4, 38 and 39 at Y6, 39 and 40 at Y8, 29 and 25 at Y10, 33 and 30 at Y12/11, and 25 and 29 at Y14/13. The data are the differences between supplement groups at each time point, obtained as the group coefficient (calcium = 1, placebo = 0) from regression models adjusted for height and age at baseline. Significant differences are shown; all other differences were not significant (P > 0.1). Y, year of measurement relative to baseline (Y1).
A similar pattern was observed for boys’ weight, although the differences were significant only at year 14 (−3.6 ± 1.4 kg; P = 0.01), and the supplement group–by–time point interaction was marginally significant (P = 0.06). Consequently, no significant effect of supplement group on BMI at any time point and no supplement group–by–time point interaction (P = 0.2) was found. The calcium group had a smaller mean midupper arm circumference than the placebo group at year 14 (−1.4 ± 0.6 cm; P = 0.02), but there were no significant group differences in triceps skinfold thickness at any age.
The girls’ anthropometric measures are presented by time point and supplement group in Table 2. No significant group differences were found for any variable at any time. With the sexes combined, the boys and girls differed in the effects of the calcium supplement on height growth and attained adult stature. At year 6, a significant sex-by-group interaction adjusted for height and age at year 1 was found (P = 0.05); the calcium group was taller than the placebo group for boys (+1.9 ± 0.9 cm, P = 0.04) but not for girls (−0.7 ± 0.9 cm; P = 0.4). Similarly at year 14, a significant sex-by-group interaction was found (P = 0.01); the calcium group grew less since year 1 than did the placebo group for boys (−3.3 ± 1.1 cm; P = 0.004) but not girls (+0.6 ± 1.1 cm; P = 0.6).
TABLE 2.
Anthropometric measures of Gambian girls in the calcium-supplementation and placebo groups1
| Age |
Height |
Weight |
Midupper arm circumference |
Triceps skinfold thickness |
||||||
| Time point | Calcium | Placebo | Calcium | Placebo | Calcium | Placebo | Calcium | Placebo | Calcium | Placebo |
| y | cm | kg | cm | mm | ||||||
| Y1 | 10.1 ± 0.2 | 10.0 ± 0.2 | 132.6 ± 1.3 | 130.7 ± 1.2 | 25.4 ± 0.7 | 24.5 ± 0.7 | 18.5 ± 0.3 | 18.2 ± 0.3 | 8.8 ± 0.3 | 8.7 ± 0.4 |
| Y2 | 11.2 ± 0.2 | 11.1 ± 0.2 | 138.1 ± 1.6 | 136.4 ± 1.2 | 28.1 ± 0.9 | 26.7 ± 0.8 | 19.6 ± 0.3 | 19.1 ± 0.3 | 9.6 ± 0.4 | 9.8 ± 0.5 |
| Y3 | 12.1 ± 0.2 | 12.1 ± 0.2 | 143.6 ± 1.4 | 141.6 ± 1.4 | 31.7 ± 1.2 | 30.1 ± 1.0 | 20.5 ± 0.4 | 20.0 ± 0.3 | 9.4 ± 0.5 | 9.6 ± 0.5 |
| Y4 | 13.2 ± 0.2 | 13.1 ± 0.2 | 148.5 ± 1.4 | 146.9 ± 1.4 | 36.2 ± 1.4 | 33.9 ± 1.2 | 22.2 ± 0.5 | 21.1 ± 0.4 | 10.3 ± 0.6 | 9.5 ± 0.5 |
| Y6 | 15.2 ± 0.2 | 15.1 ± 0.2 | 157.0 ± 1.1 | 155.7 ± 1.1 | 44.6 ± 1.4 | 43.2 ± 1.3 | 21.9 ± 0.5 | 21.5 ± 0.4 | 10.5 ± 0.7 | 10.1 ± 0.5 |
| Y8 | 17.1 ± 0.2 | 17.0 ± 0.2 | 160.5 ± 0.8 | 159.3 ± 0.9 | 50.7 ± 1.2 | 49.8 ± 1.0 | 24.1 ± 0.5 | 23.6 ± 0.4 | 12.8 ± 0.7 | 12.0 ± 0.7 |
| Y10 | 19.0 ± 0.2 | 18.9 ± 0.2 | 161.5 ± 0.8 | 160.5 ± 1.2 | 53.6 ± 1.3 | 52.5 ± 1.3 | 25.3 ± 0.6 | 24.6 ± 0.5 | 14.1 ± 1.1 | 13.6 ± 1.2 |
| Y12/11 | 20.6 ± 0.2 | 20.5 ± 0.3 | 162.8 ± 0.8 | 161.1 ± 1.1 | 53.5 ± 1.2 | 53.9 ± 1.7 | 24.8 ± 0.8 | 25.4 ± 0.5 | 13.6 ± 0.8 | 14.2 ± 1.0 |
| Y14/13 | 22.9 ± 0.2 | 23.0 ± 0.3 | 163.0 ± 0.9 | 162.0 ± 1.1 | 54.6 ± 1.5 | 52.9 ± 1.2 | 25.9 ± 0.6 | 25.3 ± 0.5 | 13.4 ± 1.0 | 12.5 ± 1.1 |
All values are means ± SEMs. The numbers of girls in the calcium and placebo groups, respectively, were as follows: 40 and 40 at Y1–4, 38 and 39 at Y6, 39 and 40 at Y8, 29 and 25 at Y10, 33 and 30 at Y12/11, and 25 and 29 at Y14/13. There were no significant group differences by multiple regression analysis after adjustment for value and age at Y1, all P > 0.1. Y1, baseline; Y2–14, the year of follow-up relative to Y1.
Some of the boys continued to grow in height from year 12 to year 14, but less so in the calcium than in the placebo group (height increment >0.4/<0.4 cm: calcium group, 8/20; placebo group, 15/9; P = 0.02). This was independent of mean age or time interval between measurements. Despite this, the mean increment from year 12 to year 14 was not significantly different between the 2 groups (calcium group: 0.5 ± 0.2 cm; placebo group, 0.8 ± 0.2 cm; P = 0.1). In the girls, there were no such group differences in growth cessation (calcium group, 8/16; placebo group, 7/16; P = 0.8) or mean height increment (both groups: 0.3 ± 0.1 cm).
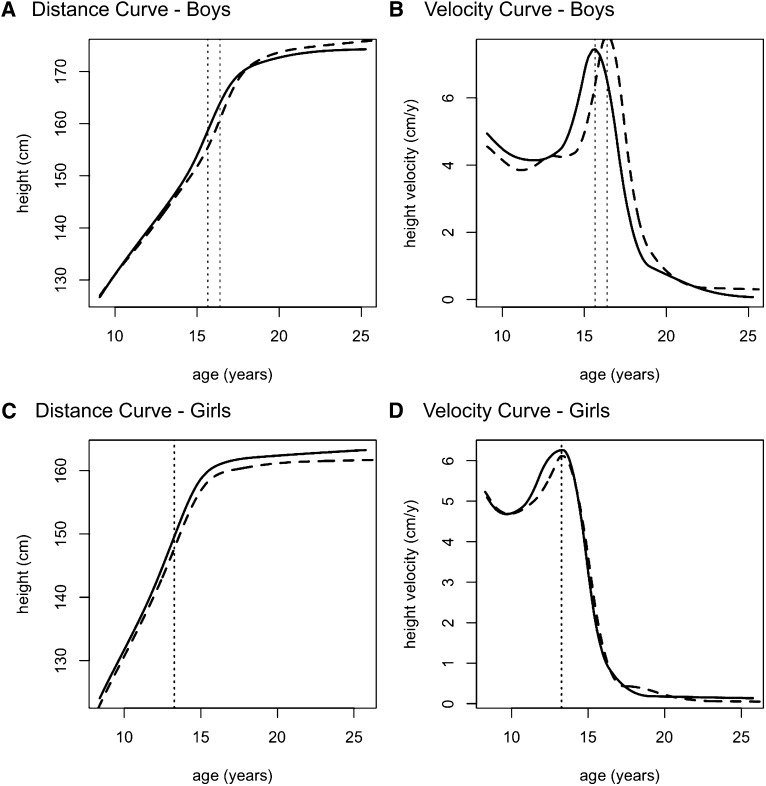
The SITAR height distance and velocity curves for boys and girls are depicted in Figure 3, and the group differences in the estimated mean SITAR parameters for height, weight, and BMI are shown in Table 3; the mean ages at peak velocity are given in Table 4. The summary growth curves confirm that boys in the calcium group were taller in midadolescence but shorter in early adulthood (Figure 3A) and that some boys were still growing at year 14, particularly in the placebo group. The mean ages at peak height velocity for the 2 groups were 15.7 and 16.4 y, respectively, in the calcium group 0.7 y (8 mo) earlier. For the groups combined, the mean age at peak height velocity was 16.1 y, and the SITAR tempo fixed effect showed this age to be significantly earlier in the calcium group by 0.62 ± 0.24 y (P = 0.01) or 7.4 mo (Table 3, Figure 3B). The other SITAR parameters, size and velocity, were not significantly different between the calcium and placebo groups.
FIGURE 3.
SITAR (14) plots of height compared with age in the calcium group (solid lines) and the placebo group (dashed curves). The diagrams illustrate the distance curve for boys (A), the velocity curve for boys (B), the distance curve for girls (C), and the velocity curve for girls (D) in each group. Vertical dotted lines denote age at peak height velocity in each sex-supplement group. n = 40 boys and 40 girls in each supplement group in the longitudinal study. The total numbers of data points in the SITAR models were as follows: 670 for the boys and 755 for the girls. SITAR, Superimposition by Translation and Rotation Method.
TABLE 3.
Height, weight, and BMI: mean differences in SITAR parameters between the calcium and placebo groups, by sex1
| Boys |
Girls |
|||
| SITAR parameter | Difference | P | Difference | P |
| Height | ||||
| Size (cm) | −1.6 ± 1.3 | 0.2 | 1.3 ± 1.1 | 0.2 |
| Tempo (y) | −0.62 ± 0.24 | 0.01 | −0.15 ± 0.28 | 0.6 |
| Velocity (%) | −0.7 ± 2.9 | 0.8 | 3.4 ± 3.1 | 0.3 |
| Weight | ||||
| Size (kg) | 0.12 ± 0.96 | 0.9 | 0.64 ± 1.31 | 0.6 |
| Tempo (y) | −0.39 ± 0.27 | 0.15 | −0.14 ± 0.26 | 0.6 |
| Velocity (%) | 1.0 ± 3.2 | 0.7 | 2.8 ± 6.2 | 0.7 |
| BMI | ||||
| Size (kg/m2) | 0.47 ± 0.36 | 0.2 | −0.03 ± 0.46 | 0.9 |
| Tempo (y) | 0.14 ± 0.48 | 0.8 | −0.50 ± 0.42 | 0.2 |
| Velocity (%) | 13.6 ± 10.6 | 0.2 | −3.1 ± 13.0 | 0.8 |
All values are means ± SEMs of parameters obtained from SITAR models (14). n = 40 boys and 40 girls in each supplement group in the longitudinal study. The total numbers of data points in the SITAR models were as follows: 670 for boys and 755 for girls. SITAR, Superimposition by Translation and Rotation Method.
TABLE 4.
Height, weight, and BMI: mean age of peak velocity in boys and girls1
| Age at peak velocity |
||
| Boys | Girls | |
| y | ||
| Height | 16.1 ± 0.12 | 13.1 ± 0.14 |
| Weight | 16.0 ± 0.12 | 14.3 ± 0.10 |
| BMI | 16.6 ± 0.21 | 14.8 ± 0.12 |
All values are means ± SEMs; derived from SITAR models (14). n = 80 boys and 80 girls in the longitudinal study. The total numbers of data points in the SITAR models were as follows: 670 for boys and 753 for girls. SITAR, Superimposition by Translation and Rotation Method.
In the girls, the mean ages at peak height velocity were 13.3 and 13.2 y for the calcium and placebo groups, respectively. The calcium supplement effect on the SITAR tempo parameter of −0.15 ± 0.28 y was far from significant (P = 0.6); similar results were found for the SITAR size and velocity parameters (Table 3, Figure 3). The sex-by-group interaction for tempo was not significant (P = 0.2).
For weight in the boys, the age at peak velocity was 16.0 y overall, and the effect of supplementation on tempo, −0.39 ± 0.27 y, was two-thirds that for height and not significant (P = 0.15). For BMI in boys, and for weight and BMI in girls, the supplement had no effect on growth (Table 4). A comparison of the individual random effects for tempo in height and weight among the boys, by supplement group, is shown in Figure 4. The correlation of 0.86 between the two was large, which indicates that the timings of peak velocity in height and weight are closely linked in individuals. In contrast, the correlation was only 0.56 in the girls. The random effects for size in height and weight were less strongly correlated (0.67 in boys, 0.25 in girls), and for velocity the correlations were very small (0.08 and 0.02).
FIGURE 4.
Random effects for height tempo and weight tempo in boys in the calcium group (•) and the placebo group (○) obtained from SITAR models (14). The point of intersection and the length of the solid vertical and horizontal lines indicate the group mean (±2 SEM) difference in weight and height tempo, respectively. n = 40 in each supplement group in the longitudinal study. The total number of data points in the SITAR models was 670. SITAR, Superimposition by Translation and Rotation Method.
Also shown in Figure 4 are the tempo effects for boys in the calcium group (shifted toward the lower left of the plot) and for those in the placebo group (toward the upper right), and the group mean differences in height and weight tempo are indicated (±2 SEM). This emphasizes the effect of the calcium supplement on growth tempo.
The pubertal status of boys assessed at years 1–6 with respect to genital and pubic hair development is given in Table 5. By year 6, relatively few boys had reached stage 5 (genital stage 5 = 11%; pubic hair stage 5 = 7%), and several were still in stage 1 (genital stage 1 = 11%; pubic hair stage 1 = 26%). No discernible effect of the calcium supplement on the pattern of Tanner stage was found at each time point. Similarly in the girls at years 1–8, no significant group effects were found in breast or pubic hair stage (Table 5) or in mean age of menarche (P = 0.9). The age of menarche when a direct assessment had been possible (n = 78/80) was as follows: mean ± SD: 15.1 ± 1.1 y; median (IQR): 14.9 (14.3–15.8) y; range: 12.7–18.6 y.
TABLE 5.
Pubertal status of Gambian boys and girls by study year and supplement group1
| Genital or breast stage(calcium group, placebo group)2 |
Pubic hair stage(calcium group, placebo group)2 |
||||||||||
| Time point | n(calcium group, placebo group ) | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| % of participants | % of participants | ||||||||||
| Boys | |||||||||||
| Y1 | 40, 40 | 90, 80 | 10, 20 | 0, 0 | 0, 0 | 0, 0 | 100, 98 | 0, 3 | 0, 0 | 0, 0 | 0, 0 |
| Y2 | 40, 40 | 70, 68 | 25, 23 | 5, 10 | 0, 0 | 0, 0 | 95, 95 | 5, 1 | 5, 1 | 0, 0 | 0, 0 |
| Y3 | 40, 40 | 53, 58 | 35, 23 | 10, 18 | 3, 3 | 0, 0 | 83, 85 | 15, 13 | 0, 0 | 0, 0 | 0, 0 |
| Y4 | 40, 40 | 53, 60 | 25, 25 | 13, 8 | 10, 8 | 0, 0 | 60, 65 | 30, 23 | 5, 5 | 8, 8 | 0, 0 |
| Y6 | 36, 37 | 8, 14 | 31, 32 | 33, 22 | 19, 19 | 8, 14 | 22, 30 | 25, 24 | 19, 19 | 28, 19 | 7, 8 |
| Girls | |||||||||||
| Y1 | 40, 40 | 80, 93 | 15, 8 | 5, 0 | 0, 0 | 0, 0 | 80, 85 | 15, 13 | 5, 3 | 0, 0 | 0, 0 |
| Y2 | 40, 39 | 73, 85 | 8, 10 | 15, 3 | 5, 3 | 0, 0 | 70, 82 | 13, 13 | 13, 3 | 5, 3 | 0, 0 |
| Y3 | 40, 40 | 48, 65 | 25, 15 | 13, 18 | 15, 3 | 0, 0 | 63, 75 | 15, 10 | 13, 10 | 8, 5 | 3, 0 |
| Y4 | 38, 40 | 21, 25 | 37, 38 | 16, 18 | 13, 15 | 13, 5 | 49, 47 | 22, 32 | 16, 13 | 14, 8 | 0, 0 |
| Y6 | 37, 38 | 6, 0 | 6, 5 | 8, 16 | 53, 50 | 28, 29 | 14, 0 | 6, 16 | 28, 41 | 36, 30 | 17, 14 |
| Y8 | 38, 33 | 0, 0 | 0, 0 | 11, 6 | 29, 24 | 58, 70 | 0, 0 | 6, 0 | 6, 12 | 30, 35 | 52, 54 |
Y1, baseline; Y2–8, the year of follow-up relative to Y1.
The data are the percentage of participants assessed in the calcium and placebo groups in each Tanner stage.
DISCUSSION
This study has shown that 12 mo of calcium carbonate supplementation before puberty had a long-term effect on the pattern of adolescent height growth in Gambian boys, which suggests an earlier pubertal growth spurt and resultant greater achieved height in midadolescence, followed by an earlier cessation of growth and shorter adult stature. The calcium supplement had advanced the age of peak height velocity of boys by 7.4 mo, despite no apparent differences in the passage through the Tanner stages of genital and pubic hair development. Height velocity itself was also not affected, which indicated that the supplementation effects were attributable to a shortened period of prepubertal growth coupled with earlier growth cessation rather than to a more rapid and accentuated pubertal transit. These data are in line with the general observation that children with earlier puberty are taller during childhood but shorter as adults, which reflects the earlier cessation of growth (16, 17). The results of this Gambian study in boys, therefore, lend support to previous studies that have suggested an influence of childhood calcium intake on pubertal maturation and adolescent height growth (5, 6).
In the girls, no effects of the calcium supplement were found on growth and maturation, in contrast with studies in other populations that have suggested advancements in the timing of menarche and in adult stature associated with childhood calcium consumption (6–8). In addition, significant sex-by-supplement interactions on height growth were noted at year 6 and year 14 with the sexes combined, which suggests that the effect was specific to the boys, although the difference between the sexes in growth tempo was not significant in the SITAR analysis. However, it is possible that the lack of an effect in the girls reflected that they were closer than the boys to their pubertal growth spurt when supplementation started, because inclusion in the study was based on chronologic age. Thus, the study leaves open the question of whether boys and girls differ in their long-term response to prepubertal calcium supplementation.
There was some evidence that the calcium supplement had also altered the pattern of adolescent growth in weight in the boys but not in BMI in either sex. An earlier pubertal growth spurt is a risk factor for central adiposity and obesity in young adulthood (17–19), whereas lower obesity rates have been associated with higher calcium intakes (20). However, the results of this study suggest that there was no long-term effect of the calcium supplement on adiposity and that the weight gain of the boys in both the calcium and placebo groups was in proportion to their height growth. Certainly, there was a close association between height and weight tempo, and to a lesser extent between height and weight size, in boys. These associations were weaker in girls, but this may have reflected the effect of pregnancy and lactation on their weight growth curves after menarche.
We reported previously that calcium carbonate supplementation in these Gambian children was associated with increased forearm bone mineral density and altered plasma calciotropic hormone concentrations that persisted for ≥12–24 mo after supplementation was withdrawn (9–11). The effects of the calcium supplement on their growth, as described here, were delayed, appearing only some years after supplementation stopped. This suggests that the intervention had stimulated the activation of the hypothalamic-pituitary-gonadal axis, which occurs several years before the appearance of visible pubertal signs and the growth spurt, the latter being driven by insulin-like growth factor I (21, 22). This possibility is supported by a calcium supplementation study of prepubertal girls in which delayed effects on the timing of menarche were noted (6) and by reports of elevated plasma concentrations of insulin-like growth factor I concentrations in boys and girls supplemented with calcium carbonate (23) and milk (24, 25).
At the start of supplementation, the children in this cohort were small for their age relative to Western references in terms of both weight and height (11). Also, as expected from previous studies in this population (26, 27), the children experienced puberty later than children in Western countries. The mean age of peak height velocity was 16.4 y for boys and 13.1 y for girls, which contrasts with estimates, for example, in the United States (boys/girls: 13.6/11.5 y) (28), Canada (boys/girls: 13.4/11.8 y) (29), and Switzerland (boys/girls: 14.5/12.5 y) (30). In addition, the mean age of menarche of the girls in this Gambian cohort was 15 y, compared with ∼12.5 y in the United States and Britain (31, 32). It is possible, therefore, that the effect of the calcium supplement seen in this study was a reflection of the poor nutritional status and delayed puberty of the Gambian children and may not be evident in well-nourished populations.
In conclusion, these data for Gambian children with low calcium intakes, poor childhood growth, and delayed puberty suggest that calcium supplementation in late childhood had influenced the subsequent pattern of height growth in adolescence and final adult stature, with proportional effects on weight. The effects of the calcium carbonate supplement were observed only in boys, but a sex-by-supplement interaction could not be confirmed. The study involved only a small group of individuals and the findings could have arisen by chance. The results therefore need to be replicated in larger cohorts. In addition, there may have been effects of the calcium supplement on skeletal mineral content, shape, and architecture that were not addressed in this study. However, the observed effects on age of peak height velocity (7.4 mo earlier) and adult height (3.5 cm shorter) were of sufficient magnitude to raise concerns that calcium supplementation of Gambian children may have had unintended consequences by shortening the period of prepubertal growth and reducing adult height. International dietary recommendations are currently set to ensure that growth and bone mineral accretion are not limited by a lack of calcium and are based on data derived from populations with higher habitual intakes and different genetic, dietary, lifestyle, and environmental backgrounds than the children described here. In common with our recent findings in Gambian mothers and infants of unexpected outcomes of calcium supplementation in pregnancy, namely greater maternal bone loss in the subsequent lactation and no apparent benefit for bone mineral accretion of the infant (33, 34), this study cautions against the application of calcium dietary recommendations between populations without supporting evidence.
Acknowledgments
We thank the participants in the study, their families, and the staff of Medical Research Council (MRC) Keneba and MRC Human Nutrition Research who contributed over the years to this intensive study. We particularly thank Landing Jarjou, Mustapha Ceesay, Michael Mendy, Mariama Jammeh, Fatou Manneh, Lamin Jammeh, and Buba Sise (members of the research staff at MRC Keneba), who coordinated and performed the measurements, and Gail Goldberg, Jing Yin, Sheila Levitt, Jennifer Thompson, and Duangporn Harpanich (MRC Human Nutrition Research) for data entry, collation, and checking.
The authors’ responsibilities were as follows—AP: conceived and designed the study, supervised BD, conducted the data analysis, drafted the manuscript, and had responsibility for the decision to submit the manuscript for publication; BD (Principal Investigator for the intervention and follow-up studies to 2002, and who conducted the work as part of his PhD and postdoctoral program): was responsible for the design and data collection in The Gambia; YS (local lead in The Gambia): was responsible for conducting the follow-up study data collection from 2002; and TJC: was responsible for expert statistical input on the study design and analysis, conducted the SITAR analyses, and critically reviewed the manuscript. All authors had full access to the data. None of the authors reported a financial or personal conflict of interest. The sources of funding and donation had no role in the study design, collection, analysis, and interpretation of the data or the decision to publish. This work was supported by the UK Medical Research Council under program numbers U105960371, U123261351, and G0700961.
REFERENCES
- 1.Department of Health Nutrition and bone health: with particular reference to calcium and vitamin D. London, United Kingdom: The Stationery Office, 1998 [Google Scholar]
- 2.Institute of Medicine Food and Nutrition Board Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011 [PubMed] [Google Scholar]
- 3.Prentice A, Bates CJ. An appraisal of the adequacy of dietary mineral intakes in developing countries for bone growth and development in children. Nutr Res Rev 1993;6:51–69 [DOI] [PubMed] [Google Scholar]
- 4.Winzenberg TM, Shaw K, Fryer J, Jones G. Calcium supplementation for improving bone mineral density in children. Cochrane Database Syst Rev 2006 Apr. 19;(2):CD005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentice A, Ginty F, Stear SJ, Jones SC, Laskey MA, Cole TJ. Calcium supplementation increases stature and bone mineral mass of 16- to 18-year old boys. J Clin Endocrinol Metab 2005;90:3153–61 [DOI] [PubMed] [Google Scholar]
- 6.Chevalley T, Rizzoli R, Hans D, Ferrari S, Bonjour J-P. Interaction between calcium intake and menarcheal age on bone mass gain: an eight-year follow-up study from prepuberty to postmenarche. J Clin Endocrinol Metab 2005;90:44–51 [DOI] [PubMed] [Google Scholar]
- 7.Berkey CS, Colditz GA, Rockett HRH, Frazier AL, Willett WC. Dairy consumption and female height growth: prospective cohort study. Cancer Epidemiol Biomarkers Prev 2009;18:1881–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiley AS. Milk intake and total dairy consumption: associations with early menarche in NHANES 1999-2004. PLoS ONE 2011;6:e14685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentice A. Studies of Gambian and UK children and adolescents: insights into calcium requirements and adaptation to a low calcium intake. Int Congress Series 2007;1297:15–24 [Google Scholar]
- 10.Dibba B, Prentice A, Ceesay M, Mendy M, Darboe S, Stirling DM, Cole TJ, Poskitt EME. Bone mineral content and plasma osteocalcin concentration of Gambian children 12 and 24 months after the withdrawal of a calcium supplement. Am J Clin Nutr 2002;76:681–6 [DOI] [PubMed] [Google Scholar]
- 11.Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EME. Effect of calcium supplementation on bone mineral accretion in Gambian children accustomed to a low calcium diet. Am J Clin Nutr 2000;71:544–9 [DOI] [PubMed] [Google Scholar]
- 12.Rayco-Solon P, Moore SE, Fulford AJ, Prentice AM. Fifty-year mortality trends in three rural African villages. Trop Med Int Health 2004;9:1151–60 [DOI] [PubMed] [Google Scholar]
- 13.Tanner JM. Growth at adolescence. 2nd ed. Oxford, United Kingdom: Blackwell Scientific, 1962 [Google Scholar]
- 14.Cole TJ, Donaldson MDC, Ben-Shlomo Y. SITAR–a useful instrument for growth curve analysis. Int J Epidemiol 2010;39:1558–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayco-Solon P, Fulford AJ, Prentice AM. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr 2005;81:134–9 [DOI] [PubMed] [Google Scholar]
- 16.McIntyre MH. Adult stature, body proportions and age and menarche in the United States National Health and Nutrition Survey (NHANES) III. Ann Hum Biol 2011;38:716–20 [DOI] [PubMed] [Google Scholar]
- 17.Golub MS, Collman GW, Foster PM, Kimmel CA, Rajpert-De Meyts E, Reiter EO, Sharpe RM, Skakkebaek NE, Toppari J. Public health implications of altered puberty timing. Pediatrics 2008;121:S218–30 [DOI] [PubMed] [Google Scholar]
- 18.Kindblom JM, Lorentzon M, Norjavaara E, Lonn L, Brandberg J, Angelhed JE, Hellqvist A, Nilsson S, Ohlsson C. Pubertal timing is an independent predictor of cetral adiposity in young adult males: the Gothenburg osteoporosis and obesity determinants study. Diabetes 2006;55:3047–52 [DOI] [PubMed] [Google Scholar]
- 19.Johnson W, Stovitz SD, Choh AC, Czerwinski SA, Towne B, Demerath EW. Patterns of linear growth and skeletal maturation from birth to 18 years of age in overweight young adults. Int J Obes (Lond) 2012;36:535–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zemel MB, Shi H, Greer B, Dirienzo D, Zemel PC. Regulation of adiposity by dietary calcium. FASEB J 2000;14:1132–8 [PubMed] [Google Scholar]
- 21.Palmert MR, Dunkel L. Delayed puberty. N Engl J Med 2012;366:443–53 [DOI] [PubMed] [Google Scholar]
- 22.Cannata D, Vijayakumar A, Fierz Y, LeRoith D. The GH/IGF-1 axis in growth and development: new insights derived from animal models. Adv Pediatr 2010;57:331–51 [DOI] [PubMed] [Google Scholar]
- 23.Ginty F, Prentice A, Laidlaw A, McKenna L, Jones SC, Stear SJ, Cole TJ. Calcium carbonate supplementation is associated with higher plasma IGF-1 in 16-18 year old boys and girls. In: Burckhardt P, Dawson-Hughes B, Heaney RP, eds. Nutritional aspects of osteoporosis. 2nd ed. Elsevier Science (USA), 2004:45–57.
- 24.Cadogan J, Eastell R, Jones N, Barker ME. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ 1997;315:1255–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-1 and IGF-BP3 in eight year old boys. Eur J Clin Nutr 2004;58:1211–6 [DOI] [PubMed] [Google Scholar]
- 26.Lo CW, Jarjou LMA, Poppitt S, Cole TJ, Neer R, Prentice A. Delayed development of bone mass in West African adolescents In: Christiansen C, Overgaard H, eds. Osteoporosis 1990. Aalborg, Denmark: Handelstrykveriat, 1991:73–7.
- 27.Prentice S, Fulford AJ, Jarjou LMA, Goldberg GR, Prentice A. Evidence for a downward secular trend in age of menarche in a rural Gambian population. Ann Hum Biol 2010;37:717–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkey CS, Wang X, Dockery DW, Ferris BG. Adolescent height growth of US children. Ann Hum Biol 1994;21:435–42 [DOI] [PubMed] [Google Scholar]
- 29.Bailey DA, Martin AD, McKay H, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty. J Bone Miner Res 2000;15:2245–50 [DOI] [PubMed] [Google Scholar]
- 30.Fournier P-E, Rizzoli R, Slosman D-O, Theintz G, Bonjour J-P. Asynchrony between rates of standing height gain and bone mass accumulation during puberty. Osteoporos Int 1997;7:525–32 [DOI] [PubMed] [Google Scholar]
- 31.McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the National Health and Nutrition Examination Survey (NHANES) 1999-2004. J Adolesc Health 2007;40:227–31 [DOI] [PubMed] [Google Scholar]
- 32.Christensen KY, Maisonet M, Rubin C, Holmes A, Flanders WD, Heron J, Ness A, Drews-Botsch C, Dominguez C, McGeehin MA, et al. Progression through puberty in girls enrolled in a contemporary British cohort. J Adolesc Health 2010;47:282–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jarjou L, Prentice A, Sawo Y, Laskey MA, Bennett J, Goldberg GR, Cole TJ. Randomized, placebo-controlled calcium supplementation study of pregnant Gambian women: effects on breast-milk calcium concentration and infant birth weight, growth and bone mineral accretion in the first year of life. Am J Clin Nutr 2006;83:657–66 [DOI] [PubMed] [Google Scholar]
- 34.Jarjou LM, Laskey MA, Sawo Y, Goldberg GR, Cole TJ, Prentice A. Effect of calcium supplementation in pregnancy on maternal bone outcomes in women with a low calcium intake. Am J Clin Nutr 2010;92:450–7 [DOI] [PMC free article] [PubMed] [Google Scholar]