Abstract
Background
Recent literature suggests that Staphylococcal enterotoxin specific IgE may be a risk factor for asthma.
Objective
To investigate the associations between Staphylococcal enterotoxin sensitization and asthma.
Methods
A systematic review and meta-analysis was performed for relevant case-control or population-based studies, published in the peer-reviewed journals until February 2013. Data were extracted on study designs, subjects, definitions and the prevalence of Staphylococcal enterotoxin sensitization.
Results
A total of 683 studies were initially identified, of which 7 studies finally met the inclusion criteria (5 case-control and 2 population-based studies). All the included studies reported higher prevalence of the sensitization in asthmatics than in controls, despite clinical and methodological heterogeneity. In a meta-analysis, the pooled odds ratio of the sensitization for asthma was 2.95 (95% confidence intervals 2.28-3.82).
Conclusion
Staphylococcal enterotoxin sensitization was significantly associated with asthma. The mechanisms of associations warrant further elucidation.
Keywords: Asthma, Staphylococcus, Meta-analysis
INTRODUCTION
Now it is accepted that asthma is a heterogeneous disorder [1]. In the past, asthma was mainly associated with inhalant allergen sensitization; however, recently it has been found that various risk factors are involved in the complex pathophysiology of asthma.
Current literature suggests the microbial exposure to play various regulatory roles for asthma [2, 3]. Particularly, Staphylococcus aureus (S. aureus) is a frequent colonizer in upper airways and skin among healthy individuals [4]. It has unique immune modulatory properties by secreting enterotoxins [5]. In this regard, previous studies have elucidated the significant associations between Staphylococcal enterotoxins and allergic diseases such as atopic dermatitis [6] or chronic rhinosinusitis with nasal polyps [7].
However, several recent observations [8, 9] and meta-analyses [10] suggested that Staphylococcal enterotoxin (SE) specific IgE (sIgE) is a potential risk factor for asthma also, but there is still scarce data to address the issue. We present here a systematic review and meta-analysis of currently available literatures to summarize the associations between SE sensitization and asthma.
MATERIALS AND METHODS
Literature search
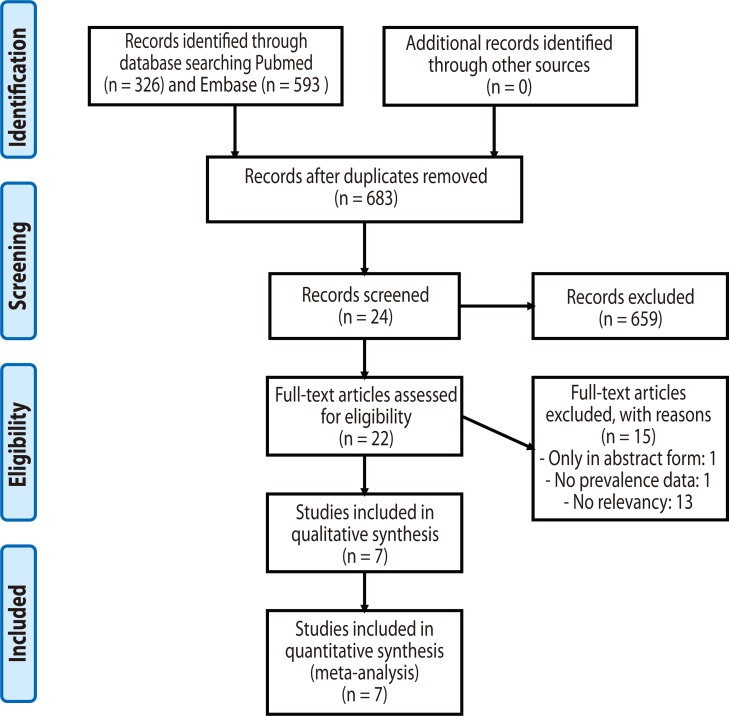
A systematic literature review was performed on Pubmed and Embase databases to identify peer-reviewed articles reporting the prevalence of SE sensitization in asthmatics and controls, published from January 1960 until February 2013, without language restriction. The search utilized the keywords 'asthma OR wheeze OR wheezing' AND 'Staphylococcal OR Staphylococcus'. Additional articles were manually sought through the reference lists of the retrieved articles. The review process followed the recommendations of the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [11], as presented in Fig. 1. Inclusion criteria were 1) the population-based or case-control studies which compared the prevalence of SE sensitization in asthmatics with non-asthmatic controls. Exclusion criteria were 1) the articles without peer-reviewed full-text (i.e., conference abstracts) and 2) the studies which did not determine the positivity of SE sensitization in a standardized manner.
Fig. 1.
Flowchart for the identification of relevant studies.
The literature search and review process was performed by two researchers. In cases of disagreement during the selection of relevant studies, it was resolved by discussion within all the authors. The outcome data extracted were: study design, subjects, region/population, and the definition and prevalence of SE sIgE positivity. If prevalence of SE sIgE positivity was not described, corresponding authors were contacted to obtain the data.
Statistical analyses
A pooled estimate of risk for asthma by SE sIgE positivity was calculated by using the fixed-effect models with Mantel-Haenszel methods. The results were expressed as odds ratio (OR) with 95% confidence intervals (CI). Homogeneity testing was performed using the I2 test. The analysis was performed using the "metan" command in STATA package (release 12.0; StataCorp., College Station, TX, USA).
RESULTS
Pooled analyses
Of 683 potentially relevant publications identified through the literature search, 659 papers were excluded after reading the abstract and title. Further 17 papers were excluded after reading the full text. Finally, 5 case-control studies and 2 population-based studies from peer-reviewed journals were included (Fig. 1). The summary of seven included studies is described in Tables 1 and 2.
Table 1.
Summary of hospital-based case-control studies on the association between Staphylococcal enterotoxin sensitization and asthma
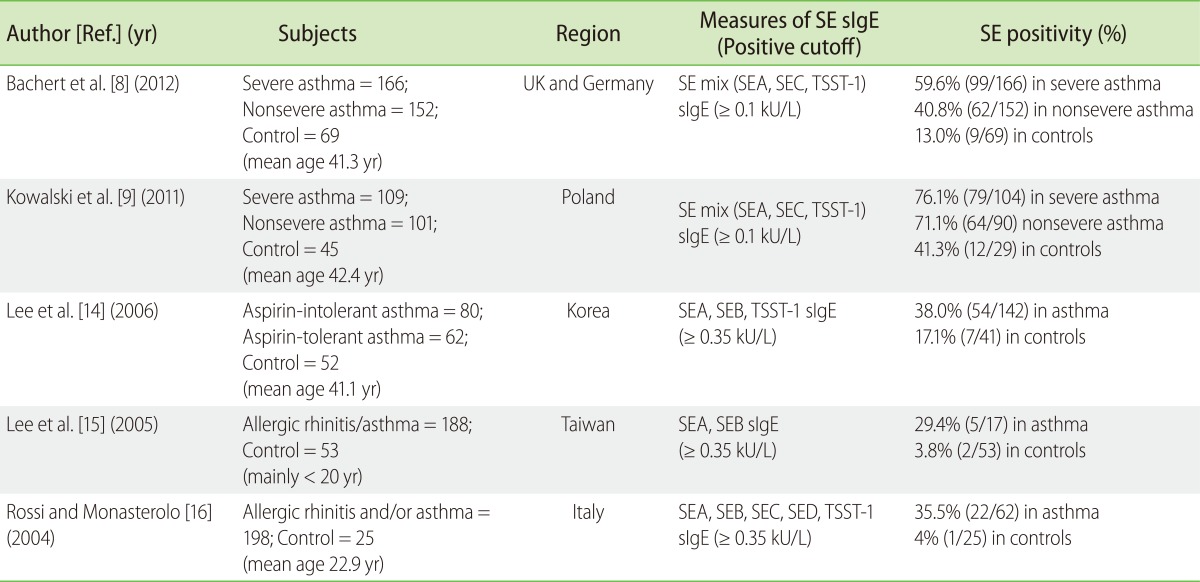
SE, Staphylococcus aureus enterotoxin; SEA-D, Staphylococcal enterotoxin A-D; TSST-1, toxic shock syndrome toxin-1; sIgE, specific IgE.
Table 2.
Summary of population-based studies on the association between Staphylococcal enterotoxin sensitization and asthma
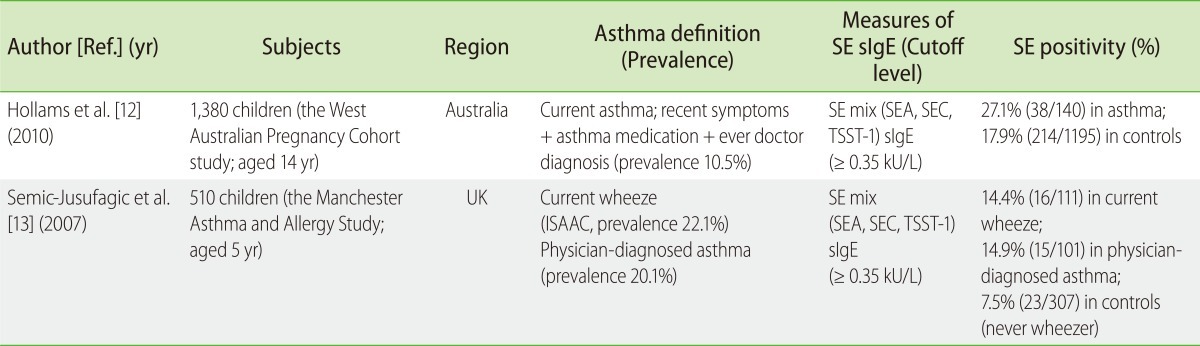
SE, Staphylococcus aureus enterotoxin; SEA-C, Staphylococcal enterotoxin A-C; TSST-1, toxic shock syndrome toxin-1; sIgE, specific IgE; ISAAC, the International Study of Asthma and Allergies in Childhood questionnaire.
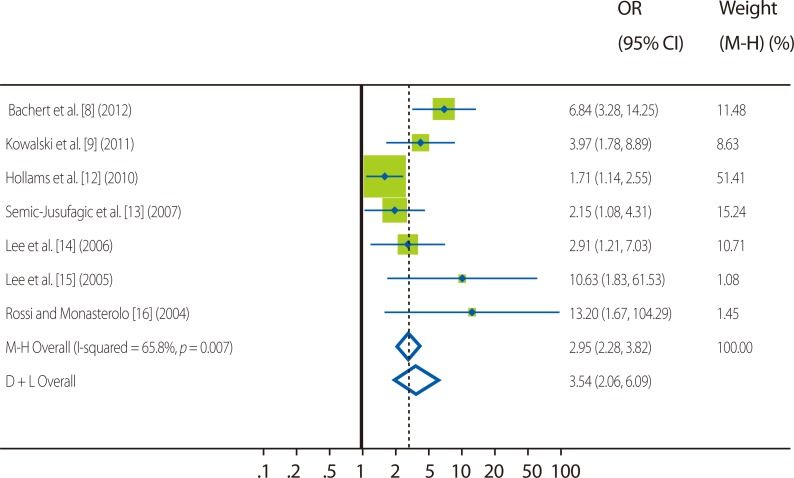
The prevalence of SE sIgE positivity among asthmatics widely varied with study populations, ranging from 14.9% to 79.1%; however, the rates showed trends to increase in older subjects and in more severe asthmatics. In non-asthmatic controls, the rate of SE sensitization also ranged widely, from 3.8% to 41.3%. Collectively, all the included articles consistently showed that SE sensitization is more frequent among asthmatics than controls, irrespective of study populations. In the meta-analysis, the pooled odds ratio (OR) of SE sensitization for asthma was 2.95 (95% confidence interval [CI] 2.28-3.82, Fig. 2).
Fig. 2.
Forest plots of studies comparing the frequency of Staphylococcal enterotoxin sensitization in asthmatics and controls. Green squares represent individual studies, and the size of squares is proportional to the number of subjects in the study. Horizontal lines indicate 95% confidence interval ranges. Vertical dotted lines and diamond shapes represent pooled summary estimates for the analysis (the width of the diamond represents the 95% CI).
OR, odds ratio; 95% CI, 95% confidence interval; M-H, Mantel-Haenzel test; D+L, DerSimonian and Laird method.
However, it should be noted that the definition of SE positivity was considerably heterogeneous. To specify, 2 case-control studies (Bachert et al. [8] and Kowalski et al. [9]) and 2 population studies [12, 13] utilized the SE mix (Staphylococcal enterotoxin A [SEA], Staphylococcal enterotoxin C [SEC], toxic shock syndrome toxin-1 [TSST-1]) antigen kits; but three other case-control studies [14-16] used each enterotoxin sIgE tests (Table 1). Moreover, the cut-off value for positivity also varied. Two recent case-control studies (Bachert et al. [8] and Kowalski et al. [9]) adapted ≥ 0.1 kU/L, whereas other 5 previous case-control or population-based studies used ≥ 0.35 kU/L as the cutoff levels.
Considering the methodological heterogeneity, the pooled OR was calculated respectively for similarly designed study collection. For two case-control studies (Bachert et al. [8] and Kowalski et al. [9]) utilizing the cutoff of 0.1 kU/L for SE mix, the OR was 5.61 (95% CI 3.27-9.63); in three case-control studies (Lee et al. [14], Lee et al. [15] and Rossi and Monasterolo [16]) with different definition (as any of tested SE IgE ≥ 0.35 kU/L), the OR was 4.67 (95% CI 2.25-9.68). In two population-based studies (Hollams et al. [12] and Semic-Jusufagic et al. [13]), the pooled OR was relatively lower (OR 1.81, 95% CI 1.28-2.56) than case-control studies but was also significant. The OR for each study was presented in Fig. 2.
Specific findings
The studies by Rossi and Monasterolo [16] were conducted in allergic rhinitis and/or asthma patients with house dust mite sensitization. Particularly, they reported the correlation between SE sensitization and serum eosinophil cationic protein levels, indicating that SE sIgE is a potential marker for clinical severity of allergic diseases.
The studies by Lee et al. [15] in Taiwanese children/adolescents reported that the association with SE is more related to asthma or airway hyper-responsiveness (AHR) than allergic rhinitis alone. Lee et al. [14] also found significantly high prevalence of SE sensitization among Korean asthmatic adults. Additionally, they revealed that SE sensitization is related to the degree of AHR (methacholine PC20).
Two later case-control studies extend the previous findings to severe asthma. In the studies by Kowalski et al. [9], they found significantly higher serum levels of SE sIgE among severe asthmatics than nonsevere counterparts (1.39 ± 0.30 vs. 0.38 ± 0.07 kU/L; p = 0.01), despite similar rates of SE sIgE positivity (76.1% vs. 71.1%). They also found that the presence of SE sensitization significantly correlated with various lung function parameters, when adjusted for age. In recent studies by Bachert et al. [8], the association between SE sensitization and severe asthma was clearly demonstrated by utilizing various sophisticated statistical models. Particularly, they found that SE sIgE was more closely related to asthma severity than house dust mite or grass pollen sIgE was.
Two population-based studies were available for children/adolescents; however, their associations with asthma were significant but less strong than the case-control studies. In the studies by Semic-Jusufagic et al. [13] on UK children aged 5 years, SE sensitization significantly correlated with current wheeze, wheeze frequency and persistence, and dry air bronchial reactivity. Later studies by Hollams et al. [12] on Australian children aged 14 years found dose-dependent relationships of SE-sIgE for asthma (in univariate analyses), and particularly for AHR (also in multivariate analyses).
Although not included in the present analyses, the studies by Tee and Pepys [17] were the first to compare sIgE to bacterial antigens (S. aureus, Streptococcus pneumoniae and Haemophilus influenzae) in subjects with various allergic diseases. The reasons for exclusion were that they did not define the positivity of SE sensitization in a standardized manner and did not report the prevalence of SE sensitization and thus were not directly comparable to other included studies. Methodologically, they utilized the radio-allergosorbent tests (RAST) and the RAST score ratio (= patient's specific RAST score/cord blood specific RAST score) for comparisons. In their reports, IgE RAST scores for S. aureus were slightly higher in asthmatics (n = 20, mean 1.2) than controls (n = 20, mean 1.0), but without statistical significance.
DISCUSSION
The present systematic review demonstrated that SE sensitization has significant associations with asthma. Particularly, it was suggested to have relationships with the clinical reactivity and severity of asthma by individual studies. The relationships have been consistently observed, despite methodological heterogeneity.
In the literature, the history of studies on the role of bacterial antigens for asthma go back to about 100 years ago [18]. Since then, numerous researchers have long been interested in the roles of bacteria, particularly S. aureus, in the pathogenesis of asthma but with controversy [17, 19-25]. It is just until recently that their significant associations have come into the spotlight.
In a sense, it is not surprising that S. aureus may contribute to the pathogenesis of asthma, as it has already been consistently associated with other allergic disorders like atopic dermatitis [6] or chronic rhinosinusitis with nasal polyp [7]. S. aureus has a peculiar characteristic to produce enterotoxins which act as superantigens, and thus exerts potent immunologic stimulatory effects on various immune cells [5]. Of note, Staphylococcal enterotoxin B (SEB) has been well demonstrated to have pro-allergic actions. In vitro experiments have revealed that SEB induces the corticosteroid insensitivity in human peripheral blood mononuclear cells [25], modulates dendritic cells to drive Th2 polarization [26], and influences nasal epithelium to secrete granulocyte migration and survival factors [27]. In vivo experiments have demonstrated that nasal SEB administration can promote allergen sensitization and airway inflammation in ovalbumin-induced murine asthma [28], or induce non-allergic eosinophilic asthma by itself [29]. In another animal model using epicutaneous SEB exposure, it enhanced ovalbumin-induced experimental 'atopic march' from dermatitis to asthma, supporting its pathophysiological plausibility [30].
Nevertheless, the direct role of S. aureus for asthma has been questioned, as it is a colonizer mostly in the upper airways and skins, but not in the lower airways [4]. Lower airways have long been considered to be sterile, and the invasion of pathogenic bacteria into the lower tracts may cause pneumonia not asthma. However, recent advances in metagenomics technologies have uncovered that lower airways, particularly in the subjects with asthma, are not sterile as previously thought, but rather have high burden of colonized bacteria [31, 32].
Still we do not have direct evidence that S. aureus causes asthma without causing pneumonia. However, we postulate the hypothesis that S. aureus has a mechanism to survive within the bronchial epithelium at small numbers and may secrete enterotoxins to promote various immunologic modulation. Theoretically, the survival could be more favorable in allergic subjects; as M2 macrophages are more frequent in allergic micro-environments, resulting in the decreased phagocytotic activity and the increased intracellular survival of microbes [33]. To prove this, it may be needed to directly compare the presence of S. aureus within the bronchial biopsy samples obtained from asthmatics and controls, with utilizing techniques such as peptide nucleic acid-fluorescence in situ hybridization (PNA-FISH). In a recent study using the epithelium from nasal polyps and PNA-FISH, S. aureus was found to be able to invade into the nasal epithelium and survive in patients with nasal polyposis [34, 35].
Another possible link between S. aureus and asthma could be the effects of chronic repeated spillover of low dose Staphylococcal exotoxins from the upper airways. About a quarter of individuals are known to have S. aureus colonization in their upper airways [4]. In subsets of subjects with genetically susceptible T-cell receptor β-variable region 8, the exposure to Staphylococcal enterotoxins may stimulate responsive immune cells and diseases [36]. Another genetic factor may be the IL-5 promoter polymorphism [37].
Then, which asthma subtype would be more related to SE sensitization? To answer this, it needs to be examined in large scale unbiased population samples. Particularly, studies are still lacking on adult community populations. Based on the findings from the case-control studies [8, 9], older age is presumably a clinical factor to link asthma and SE sIgE. In older adult asthma, eosinophilic airway inflammation is frequently observed while no serum sIgE is detectable for common inhalant allergens. We speculate that Th2 responses to inhaled bacterial antigens may contribute to non-atopic eosinophilic asthma in older adults. Severe asthma is another subtype which is related to SE sIgE, as suggested by two recent case-control studies [8, 9].
There still remains possibility that SE sensitization is a 'surrogate marker' just to reflect the effects of still 'undiscovered risk factors'. In fact, S. aureus and various bacteria may co-exist in indoor dust [38], and its sensitization could be correlated with exposure to indoor dust, toxins or other pathogens. High indoor levels of bacteria and mold spores have already been associated with asthma severity [39]. Moreover, serum antibody levels do not directly represent the local inflammation in the airways. Further studies would be necessary to delineate whether it is a surrogate marker or the causative factor.
A limitation of this study should be considered in the interpretation of findings. All the included studies reported the positive associations between asthma and SE sensitization, which might be indicative of publication bias. Small numbers of included studies (n = 7) and substantial heterogeneity (I2 = 65.8%) warrants careful interpretation of the results. Particularly in adults, large-scale community population-based studies are necessary to confirm the findings.
In conclusions, the systematic review and meta-analyses of current literatures demonstrated that Staphylococcal enterotoxin sensitization was significantly associated with asthma. These findings warrant further studies for elucidating mechanisms, and for confirming their relationships in large-scale populations.
ACKNOWLEDGEMENTS
We thank Professor Hae-Sim Park (Ajou University College of Medicine, Korea), Professor Jae-Young Lee (Hallym University College of Medicine, Korea), Professor Patrick Holt (University of Western Australia, Australia), and Professor Claus Bachert (Ghent University, Belgium) for data provision and kind advice. This study was supported by grants of Seoul National University Bundang Hospital and the Korean Health 21 R&D Project, Ministry for Health, Welfare and Family Affairs, R.O.K. (A030001).
References
- 1.Anderson GP. Endotyping asthma: new insights into key pathogenic mechanisms in a complex, heterogeneous disease. Lancet. 2008;372:1107–1119. doi: 10.1016/S0140-6736(08)61452-X. [DOI] [PubMed] [Google Scholar]
- 2.Heederik D, von Mutius E. Does diversity of environmental microbial exposure matter for the occurrence of allergy and asthma? J Allergy Clin Immunol. 2012;130:44–50. doi: 10.1016/j.jaci.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 3.Brooks C, Pearce N, Douwes J. The hygiene hypothesis in allergy and asthma: an update. Curr Opin Allergy Clin Immunol. 2013;13:70–77. doi: 10.1097/ACI.0b013e32835ad0d2. [DOI] [PubMed] [Google Scholar]
- 4.Wilson M. Bacteriology of humans: an ecological perspective. Malden, MA: Wiley-Blackwell; 2008. [Google Scholar]
- 5.Bachert C, Gevaert P, van Cauwenberge P. Staphylococcus aureus enterotoxins: a key in airway disease? Allergy. 2002;57:480–487. doi: 10.1034/j.1398-9995.2002.02156.x. [DOI] [PubMed] [Google Scholar]
- 6.Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125:4–13. doi: 10.1016/j.jaci.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bachert C, Zhang N, Patou J, van Zele T, Gevaert P. Role of staphylococcal superantigens in upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:34–38. doi: 10.1097/ACI.0b013e3282f4178f. [DOI] [PubMed] [Google Scholar]
- 8.Bachert C, van Steen K, Zhang N, Holtappels G, Cattaert T, Maus B, Buhl R, Taube C, Korn S, Kowalski M, Bousquet J, Howarth P. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130:376–381.e8. doi: 10.1016/j.jaci.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Kowalski ML, Cieślak M, Pérez-Novo CA, Makowska JS, Bachert C. Clinical and immunological determinants of severe/refractory asthma (SRA): association with staphylococcal superantigen-specific IgE antibodies. Allergy. 2011;66:32–38. doi: 10.1111/j.1398-9995.2010.02379.x. [DOI] [PubMed] [Google Scholar]
- 10.Pastacaldi C, Lewis P, Howarth P. Staphylococci and staphylococcal superantigens in asthma and rhinitis: a systematic review and meta-analysis. Allergy. 2011;66:549–555. doi: 10.1111/j.1398-9995.2010.02502.x. [DOI] [PubMed] [Google Scholar]
- 11.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hollams EM, Hales BJ, Bachert C, Huvenne W, Parsons F, de Klerk NH, Serralha M, Holt BJ, Ahlstedt S, Thomas WR, Sly PD, Holt PG. Th2-associated immunity to bacteria in teenagers and susceptibility to asthma. Eur Respir J. 2010;36:509–516. doi: 10.1183/09031936.00184109. [DOI] [PubMed] [Google Scholar]
- 13.Semic-Jusufagic A, Bachert C, Gevaert P, Holtappels G, Lowe L, Woodcock A, Simpson A, Custovic A. Staphylococcus aureus sensitization and allergic disease in early childhood: population-based birth cohort study. J Allergy Clin Immunol. 2007;119:930–936. doi: 10.1016/j.jaci.2006.12.639. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Kim HM, Ye YM, Bahn JW, Suh CH, Nahm D, Lee HR, Park HS. Role of staphylococcal superantigen-specific IgE antibodies in aspirin-intolerant asthma. Allergy Asthma Proc. 2006;27:341–346. doi: 10.2500/aap.2006.27.2908. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Lin YT, Yang YH, Wang LC, Chiang BL. Increased levels of serum-specific immunoglobulin e to staphylococcal enterotoxin a and B in patients with allergic rhinitis and bronchial asthma. Int Arch Allergy Immunol. 2005;138:305–311. doi: 10.1159/000088868. [DOI] [PubMed] [Google Scholar]
- 16.Rossi RE, Monasterolo G. Prevalence of serum IgE antibodies to the Staphylococcus aureus enterotoxins (SAE, SEB, SEC, SED, TSST-1) in patients with persistent allergic rhinitis. Int Arch Allergy Immunol. 2004;133:261–266. doi: 10.1159/000076833. [DOI] [PubMed] [Google Scholar]
- 17.Tee RD, Pepys J. Specific serum IgE antibodies to bacterial antigens in allergic lung disease. Clin Allergy. 1982;12:439–450. doi: 10.1111/j.1365-2222.1982.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 18.Walker IC, Adkinson J. Study XIII: the relationship between the Cutaneous Reaction, Serum Agglutination Tests and Bacteriological Examination of the Sputum and Nasal Secretions in determining the part Staphylococcus Pyogenes Aureus and Albus may play in the cause of Bronchial Asthma. J Med Res. 1917;36:295–308. [PMC free article] [PubMed] [Google Scholar]
- 19.Virtue CM, Wittig HJ, Cook TJ. Lymphocyte transformation with bacterial antigens in intrinsic asthma. J Allergy Clin Immunol. 1971;48:321–330. doi: 10.1016/0091-6749(71)90078-9. [DOI] [PubMed] [Google Scholar]
- 20.Bacigaluppi JE, Negroni R, de Severino HM. Bacterial allergy in allergic rhinitis and bronchial asthma. Ann Allergy. 1979;42:95–98. [PubMed] [Google Scholar]
- 21.Alam R, Kuna P, Rozniecki J, Kuzminska B. Bacterial antigens stimulate the production of histamine releasing factor (HRF) by lymphocytes from intrinsic asthmatic patients. Clin Exp Immunol. 1986;63:241–248. [PMC free article] [PubMed] [Google Scholar]
- 22.Beklemishev ND, Belyaev NN, Sukhodoeva GS, Bulvakhter YL. The mechanisms of bronchospasm in experimental microbial sensitization. I. Immunological stage. Allergol Immunopathol (Madr) 1984;12:129–134. [PubMed] [Google Scholar]
- 23.del Real Sánchez JH. Controversy in asthma. I. Staphylococcus aureus as the main factor responsible for bronchospasm. Rev Alerg Mex. 1987;34:89–97. [PubMed] [Google Scholar]
- 24.Brarda OA, Vanella LM, Boudet RV. Anti-Staphylococcus aureus, anti-Streptococcus pneumoniae and anti-Moraxella catarrhalis specific IgE in asthmatic children. J Investig Allergol Clin Immunol. 1996;6:266–269. [PubMed] [Google Scholar]
- 25.Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000;105:782–787. doi: 10.1067/mai.2000.105807. [DOI] [PubMed] [Google Scholar]
- 26.Mandron M, Ariès MF, Brehm RD, Tranter HS, Acharya KR, Charveron M, Davrinche C. Human dendritic cells conditioned with Staphylococcus aureus enterotoxin B promote TH2 cell polarization. J Allergy Clin Immunol. 2006;117:1141–1147. doi: 10.1016/j.jaci.2005.12.1360. [DOI] [PubMed] [Google Scholar]
- 27.Huvenne W, Callebaut I, Reekmans K, Hens G, Bobic S, Jorissen M, Bullens DM, Ceuppens JL, Bachert C, Hellings PW. Staphylococcus aureus enterotoxin B augments granulocyte migration and survival via airway epithelial cell activation. Allergy. 2010;65:1013–1020. doi: 10.1111/j.1398-9995.2009.02313.x. [DOI] [PubMed] [Google Scholar]
- 28.Huvenne W, Callebaut I, Plantinga M, Vanoirbeek JA, Krysko O, Bullens DM, Gevaert P, Van Cauwenberge P, Lambrecht BN, Ceuppens JL, Bachert C, Hellings PW. Staphylococcus aureus enterotoxin B facilitates allergic sensitization in experimental asthma. Clin Exp Allergy. 2010;40:1079–1090. doi: 10.1111/j.1365-2222.2010.03464.x. [DOI] [PubMed] [Google Scholar]
- 29.Herz U, Rückert R, Wollenhaupt K, Tschernig T, Neuhaus-Steinmetz U, Pabst R, Renz H. Airway exposure to bacterial superantigen (SEB) induces lymphocyte-dependent airway inflammation associated with increased airway responsiveness---a model for non-allergic asthma. Eur J Immunol. 1999;29:1021–1031. doi: 10.1002/(SICI)1521-4141(199903)29:03<1021::AID-IMMU1021>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 30.Yu J, Oh MH, Park JU, Myers AC, Dong C, Zhu Z, Zheng T. Epicutaneous exposure to staphylococcal superantigen enterotoxin B enhances allergic lung inflammation via an IL-17A dependent mechanism. PLoS One. 2012;7:e39032. doi: 10.1371/journal.pone.0039032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, Davies J, Ervine A, Poulter L, Pachter L, Moffatt MF, Cookson WO. Disordered microbial communities in asthmatic airways. PLoS One. 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, Woyke T, Allgaier M, Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M, Denlinger LC, Dimango E, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, Lynch SV National Heart, Lung, and Blood Institute's Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372–381.e1-3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 34.Sachse F, Becker K, von Eiff C, Metze D, Rudack C. Staphylococcus aureus invades the epithelium in nasal polyposis and induces IL-6 in nasal epithelial cells in vitro. Allergy. 2010;65:1430–1437. doi: 10.1111/j.1398-9995.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 35.Corriveau MN, Zhang N, Holtappels G, Van Roy N, Bachert C. Detection of Staphylococcus aureus in nasal tissue with peptide nucleic acid-fluorescence in situ hybridization. Am J Rhinol Allergy. 2009;23:461–465. doi: 10.2500/ajra.2009.23.3367. [DOI] [PubMed] [Google Scholar]
- 36.Hauk PJ, Wenzel SE, Trumble AE, Szefler SJ, Leung DY. Increased T-cell receptor vbeta8+ T cells in bronchoalveolar lavage fluid of subjects with poorly controlled asthma: a potential role for microbial superantigens. J Allergy Clin Immunol. 1999;104:37–45. doi: 10.1016/s0091-6749(99)70111-9. [DOI] [PubMed] [Google Scholar]
- 37.Losol P, Kim SH, Hwang EK, Shin YS, Park HS. IL-5 Promoter polymorphism enhances IgE responses to staphylococcal superantigens in adult asthmatics. Allergy Asthma Immunol Res. 2013;5:106–109. doi: 10.4168/aair.2013.5.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MR, Hong SW, Choi EB, Lee WH, Kim YS, Jeon SG, Jang MH, Gho YS, Kim YK. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy. 2012;67:1271–1281. doi: 10.1111/all.12001. [DOI] [PubMed] [Google Scholar]
- 39.Ross MA, Curtis L, Scheff PA, Hryhorczuk DO, Ramakrishnan V, Wadden RA, Persky VW. Association of asthma symptoms and severity with indoor bioaerosols. Allergy. 2000;55:705–711. doi: 10.1034/j.1398-9995.2000.00551.x. [DOI] [PubMed] [Google Scholar]