Abstract
Background
Beside autoimmunity, coagulation pathway is also involved in the pathogenesis of chronic urticaria (CU). Previous studies showed that plasma D-dimer levels paralleled the severity of the disease. To date, there are no data concerning D-dimer level in Thai patients with CU.
Objective
This study aimed to find the relationship between plasma D-dimer levels and the disease severity of Thai CU patients. The secondary objective is to analyze plasma D-dimer level in each group of patients who performed autologous plasma skin testing (APST) and autologous serum skin testing (ASST).
Methods
We retrospectively reviewed case record forms of chronic idiopathic urticaria (CIU) patients aged at least 18 years in Skin Allergy Clinic, Siriraj Hospital Mahidol University, Bangkok, during June 2008 to June 2011.
Results
Of 120 patients, plasma D-dimer level was abnormal in 58 patients (48.3%). The study showed statistically significant positive correlation between disease severity and plasma D-dimer level (p < 0.05, r = 0.537). There was no statistically significant difference in plasma D-dimer level between APST positive and negative groups, and also between ASST positive and negative groups. In APST negative group, plasma D-dimer level was elevated in 29 patients (47.5%) and correlated with disease severity.
Conclusion
This study showed elevated plasma D-dimer levels in nearly half of Thai patients with CIU. There was a positive correlation between plasma D-dimer levels and the severity of disease activity. Investigation for plasma D-dimer level may be an alternative way to evaluate disease severity in patients with CIU.
Keywords: Chronic urticaria, D-dimer, Severity of the disease, Coagulation pathway
INTRODUCTION
Chronic urticaria (CU) is characterized by recurrent eruption of itchy short-lived wheals and flares for longer than six weeks duration [1]. Without identifiable causes after full investigation, these patients are classified as chronic idiopathic urticaria (CIU) [2]. One fourth to half of CIU patients, autoimmunity was involved in the pathogenesis as proven by the detection of autoantibodies (IgG) to IgE or to high affinity IgE receptors (FcεRI) on mast cell surfaces [3-8].
Besides autoimmunity, some parts of the pathogenesis of CIU remain unclear. Fagiolo et al. [9] showed that IgG depleted serums from CIU patients retain ability to induce a wheal-and-flare reaction upon intradermal injection. These observations suggest that there are some other mechanisms involving in the pathogenesis of CIU. Another indirect evidence showed that the percentage of positive autologus plasma skin testing (APST) is higher than that of positive autologus serum skin testing (ASST) (80% vs. 50%). This difference suggested that coagulation cascade is possibly involved in the pathogenesis of CIU [10].
Asero et al. [10] showed significant elevated plasma levels of prothrombin fragment (F1+2) in the circulation in CIU patients and the level correlated with the severity of the disease [11]. Normally, thrombin is involved in hemostasis including vessel wound healing [12]. For the pathophysiology of urticaria, thrombin increases vascular permeability [13], stimulates mast cell degranulation [14] and may activate protease activated receptor1 (PAR1) on mast cells [15]. Subsequent study reveals that activated Factor VII, D-dimer level and F1+2 are elevated in CU patient but Factor XII level is normal, leading to the conclusion of activated extrinsic pathway involvement in CIU [11]. Recently, Takeda et al. [16] demonstrated hypercoagulable pattern in activated partial thromboplastin time (APTT) clot waveform analysis in patients with CIU, suggesting a high potential involvement of intrinsic pathway in the pathogenesis. It is probable that pathogenesis of CIU involves both intrinsic and extrinsic pathway.
To date, there is no data concerning D-dimer level in Thai patients with CIU. We aim to study the correlation between D-dimer level and the severity of disease activity of Thai patients with CIU.
MATERIALS AND METHODS
This study was approved by Siriraj Institutional Review Board. CIU was diagnosed on basis of recurrent eruption of wheals and flare with or without angioedema for at least 6 weeks without specific causes after appropriate evaluation [2].
We retrospectively reviewed case record forms of CIU patients aged at least 18 years who attended Skin Allergy Clinic, Siriraj Hospital Mahidol University, Bangkok, during June 2008 to June 2011. Inclusion criteria was CIU patients who had performed plasma D-dimer level testing. Exclusion criterias were CIU patients with underlying diseases or taking any medicine which would affect D-dimer level and patients with other causes of urticaria (such as physical induced urticaria, drug-induced urticaria and etc.).
The demographic data, personal history, family history of atopy and underlying disease of patients were recorded. Other available data from patients' records such as ASST, APST and other laboratory investigations (complete blood count [CBC], erythrocyte sedimentation rate [ESR], anti-HBsAg, anti-HCV, anti-nuclear antibodies [ANA], anti-thyroglobulin and anti-microsomal antibodies) were also reviewed.
Assessment of disease activity of CU
At the time of blood sampling, APST, ASST and disease activity assessment, all patients discontinued short acting antihistamines for at least three days, long acting antihistamines and systemic corticosteroids for at least seven days. Disease activity was assessed by EAACI/GA2LEN/EDF activity score [17] which is composed of wheals score; none = 0, mild (< 20 lesions in 24 hours) = 1, moderate (20-50 lesions in 24 hours) = 2, intense (> 50 lesions in 24 hours) = 3 and the pruritus score for which none = 0, mild (presence but not annoying or troublesome) = 1, moderate (troublesome but does not interfere with normal activity or sleeping) = 2, and intense (severe pruritus, which is sufficiently troublesome to interfere with normal daily activity or sleep) = 3. The disease activity is scored 0 to 6. We graded activity of disease as follows; patients with 0 score considered as none, score 1-2 as mild, score 3-4 as moderate and score 5-6 as severe disease activity.
D-dimer measurement
Measurement of plasma D-dimer level was done at the same time as ASST, APST and clinical assessment. D-dimer plasma level was measured by enzyme-linked fluorescence assay (ELFA) kits (VIDAS® D-Dimer Exclusion II) from bioMérieux, France. The cut-off level is 500 ng/mL.
Autologous serum skin test and autologous plasma skin test technique
Five mL of venous blood was placed in sterile plastic tubes containing a buf fered sodium citrate 3.2% (0.109 mol/L), centrifuged at 500xg for 15 minutes and kept in aliquots for use in APST. Another 5 mL of venous blood was allowed to clot at room t emperature for 30 minutes and then centrifuged at 500xg for 15 minutes and kept in aliquots for use in ASST. Sterile serum, plasma and 0.9% normal saline (negative control), 0.05 mL each were injected intradermally into patient's volar side of forearm with 27 G needles at least 3 cm apart by the same well-trained doctor. The result was observed in 30 minutes. Wheal and flare at the site of injection with the serum and/or plasma that was 3 mm larger than saline control was interpreted as a positive result. Skin prick testing with histamine (10 µg/µL) was used as a positive control and was interpreted at 15 minutes.
Statistical methods
Descriptive statistics, e.g. mean, median, maximum, minimum and percentages were used to describe demographic data, plasma D-dimer level and positivity of ASST and APST. The prevalence of positive ASST and ASPT results was recorded as a number and percentage. Correlation between D-dimer level and disease severity score (0-6) was calculated by Spearmen's rank correlation. Difference in proportion of abnormal D-dimer level among the disease severity was tested using Pearson's chi-square test; Mann-Whitney U test and Kruskal-Wallis test were employed to test the difference in D-dimer level between two groups (e.g., APST positive vs. negative) and three groups (i.e., disease severity) respectively. All statistical data analyses were performed using SPSS for Windows version 17.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
One hundred and twenty patients, 30 male (25%) and 90 female (75%); with a mean age of 38.8 years (range 16-73 years) were enrolled in the study. Of 120 patients, at the time of D-dimer level tested, 63 (52.5%), 37 (30.8%) and 20 (16.7%) were categorized in the mild, moderate and severe disease activity group, respectively. Median duration of the disease was 12 months (min,max; 2,360 months). Six patients (5%) also had angioedema. Thirty-six patients (30%) had personal history of atopy (allergic rhinitis: 24; 20%, asthma: 8; 6.7%, allergic conjunctivitis: 4; 3.3%). Twenty-five patients (20.8%) had family history of atopy (allergic rhinitis: 18; 15%, asthma: 6; 5%, atopic dermatitis: 1; 0.8%). There were no associations between demographic data and plasma D-dimer level or disease severity.
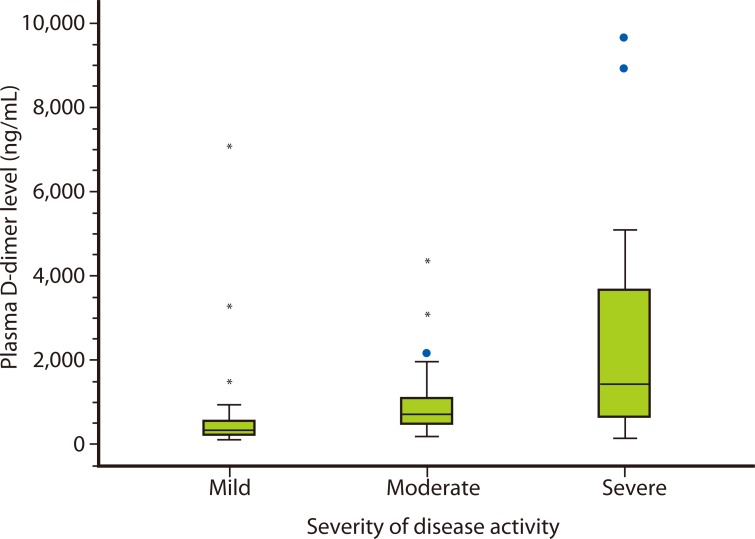
There was a statistically significant difference in D-dimer levels among the three groups with different disease activities (p value < 0.05; Fig. 1 and Table 1). The study showed statistically significant positive correlation between disease severity and plasma D-dimer level (p < 0.05, r = 0.537). D-dimer levels were abnormal (> 500 ng/mL) in 58 patients (48.3%). The proportion of abnormal plasma D-dimer level was higher in the more severe disease activity group (Table 1).
Fig. 1.
Plasma D-dimer levels of chronic idiopathic urticaria patients with mild, moderate and severe disease activity
Table 1.
Plasma D-dimer levels and proportion of abnormal plasma D-dimer levels in CIU patients with various disease activity scores

CIU, chronic idiopathic urticaria.
ASST and APST were both performed in 96 patients. ASST was positive in 33 out of 96 patients (34.4%). APST was positive in 35 out of 96 patients (36.5%). Plasma D-dimer level was also elevated in APST negative group (Table 2). There was no statistically significant difference in plasma D-dimer level between APST positive and negative groups, nor between ASST positive and negative groups.
Table 2.
Plasma D-dimer levels in CIU patients who received APST and ASST

CIU, chronic idiopathic urticaria; APST, autologous plasma skin testing; ASST, autologous serum skin testing.
Three patients in our study had repeated plasma D-dimer testing during the follow up period. All had decreased plasma D-dimer levels together with decrease of the disease severity scores, and their diseases became easily controlled by using lower amounts of oral antihistamines, without using systemic corticosteroids, or receiving anticoagulant treatment.
Thyroid autoantibodies were positive in 11 of 70 patients (15.7%). Anti-nuclear antibodies were positive in 14 of 78 patients (17.9%). Complete blood counts were normal in all patients (n = 104). ESR levels were elevated in 19 of 56 patients (33.9%)
DISCUSSION
Apart from the autoimmunity that explains pathogenesis of CIU, coagulation cascade was also involved in the disease from the many evidences proposed [18]. Previous data showed increase D-dimer level in 2 of 21 patients (10%) [11], 14 of 68 patients (20%) [10] and 27 of 82 patients (35%) [19]. In our study, the plasma D-dimer levels were elevated in 58 out of 120 patients (48.3%), a percentage which was higher than previous reports [10, 11, 19]. The percentage difference is due perhaps to the differences of the disease severity in each study. Our study also showed the positive correlation between plasma D-dimer level and disease severity, which is similar to previous studies performed in Europe [10, 11, 20]. Previous studies showed plasma D-dimer and F1+2 levels were normal after disease remission or during treatment [10, 16, 20]. Our three patients who had repeated D-dimer levels also had decreased plasma D-dimer levels with decreased disease severity scores.
Asero et al. [10] showed that ASST was positive in 53% (51 of 96 patients) whereas APST was positive in 86% (61 of 71). In their study, 21 of 30 patients (70%) with negative ASST had positive APST results. However, our study showed that ASST was positive in 28.1% of the patients (33 of 96), APST was positive in 34.4% (35 of 96) and 8 out of 96 patients (8.3%) with ASST negative had positive APST.
Recent study also showed evidences of coagulation pathway activation in patients with negative APST. This suggests that in CU, the coagulation system is activated irrespective of the subset of the disease. D-dimer and F1+2 levels were elevated in 7 of 16 patients with negative APST (43.7%) and correlated with the disease activities. These levels were higher than the control group but lower than the APST positive group [21]. Similarly, we also detected that plasma D-dimer levels were elevated in APST negative group (29 of 61 patients, 47.5%) and had a positive correlation with the severity of disease (p < 0.05, r = 0.649). Perhaps plasma D-dimer is more sensitive than APST. Further studies are required. It should be noted here that plasma D-dimer levels in the APST positive and APST negative groups were not statistically different.
Beside activation of the extrinsic pathway, recent study also showed activation of intrinsic pathway in CIU patients. However, D-dimer level is a plasmatic marker for fibrinolysis in coagulation pathway; we could not determine whether the extrinsic or intrinsic or both pathways were activated.
In our study, thyroid autoantibodies, anti-nuclear antibodies and ESR level were also recorded. There were no differences between plasma D-dimer levels and the results of APST/ASST in patients with normal and abnormal results in those laboratory tests.
In conclusion, this study showed elevated plasma D-dimer levels in nearly half of Thai patients with CIU. There was a positive correlation between plasma D-dimer levels and the severity of disease activity. Investigation for plasma D-dimer level may be an alternative way to evaluate disease severity in patients with CIU.
ACKNOWLEDGEMENTS
We would like to express our gratitude towards Associate Professor Dr. Chulaluk Komoltri for her kind support in statistical analysis.
References
- 1.Greaves M. Chronic urticaria. J Allergy Clin Immunol. 2000;105:664–672. doi: 10.1067/mai.2000.105706. [DOI] [PubMed] [Google Scholar]
- 2.Grattan CE, Sabroe RA, Greaves MW. Chronic urticaria. J Am Acad Dermatol. 2002;46:645–657. doi: 10.1067/mjd.2002.122759. [DOI] [PubMed] [Google Scholar]
- 3.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–217. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 4.Grattan CE, Francis DM, Hide M, Greaves MW. Detection of circulating histamine releasing autoantibodies with functional properties of anti-IgE in chronic urticaria. Clin Exp Allergy. 1991;21:695–704. doi: 10.1111/j.1365-2222.1991.tb03198.x. [DOI] [PubMed] [Google Scholar]
- 5.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–1604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 6.Fiebiger E, Maurer D, Holub H, Reininger B, Hartmann G, Woisetschläger M, Kinet JP, Stingl G. Serum IgG autoantibodies directed against the alpha chain of Fc epsilon RI: a selective marker and pathogenetic factor for a distinct subset of chronic urticaria patients? J Clin Invest. 1995;96:2606–2612. doi: 10.1172/JCI118325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrer M, Kinét JP, Kaplan AP. Comparative studies of functional and binding assays for IgG anti-Fc(epsilon)RIalpha (alpha-subunit) in chronic urticaria. J Allergy Clin Immunol. 1998;101:672–676. doi: 10.1016/s0091-6749(98)70176-9. [DOI] [PubMed] [Google Scholar]
- 8.Greaves MW, O'Donnell BF. Not all chronic urticaria is "idiopathic"! Exp Dermatol. 1998;7:11–13. doi: 10.1111/j.1600-0625.1998.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 9.Fagiolo U, Kricek F, Ruf C, Peserico A, Amadori A, Cancian M. Effects of complement inactivation and IgG depletion on skin reactivity to autologous serum in chronic idiopathic urticaria. J Allergy Clin Immunol. 2000;106:567–572. doi: 10.1067/mai.2000.108913. [DOI] [PubMed] [Google Scholar]
- 10.Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113–1117. doi: 10.1016/j.jaci.2005.12.1343. [DOI] [PubMed] [Google Scholar]
- 11.Asero R, Tedeschi A, Coppola R, Griffini S, Paparella P, Riboldi P, Marzano AV, Fanoni D, Cugno M. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119:705–710. doi: 10.1016/j.jaci.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 12.Goldsack NR, Chambers RC, Dabbagh K, Laurent GJ. Thrombin. Int J Biochem Cell Biol. 1998;30:641–646. doi: 10.1016/s1357-2725(98)00011-9. [DOI] [PubMed] [Google Scholar]
- 13.Cirino G, Cicala C, Bucci MR, Sorrentino L, Maraganore JM, Stone SR. Thrombin functions as an inflammatory mediator through activation of its receptor. J Exp Med. 1996;183:821–827. doi: 10.1084/jem.183.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Razin E, Marx G. Thrombin-induced degranulation of cultured bone marrow-derived mast cells. J Immunol. 1984;133:3282–3285. [PubMed] [Google Scholar]
- 15.Vliagoftis H. Thrombin induces mast cell adhesion to fibronectin: evidence for involvement of protease-activated receptor-1. J Immunol. 2002;169:4551–4558. doi: 10.4049/jimmunol.169.8.4551. [DOI] [PubMed] [Google Scholar]
- 16.Takeda T, Sakurai Y, Takahagi S, Kato J, Yoshida K, Yoshioka A, Hide M, Shima M. Increase of coagulation potential in chronic spontaneous urticaria. Allergy. 2011;66:428–433. doi: 10.1111/j.1398-9995.2010.02506.x. [DOI] [PubMed] [Google Scholar]
- 17.Zuberbier T, Asero R, Bindslev-Jensen C, Walter Canonica G, Church MK, Giménez-Arnau AM, Grattan CE, Kapp A, Maurer M, Merk HF, Rogala B, Saini S, Sánchez-Borges M, Schmid-Grendelmeier P, Schünemann H, Staubach P, Vena GA, Wedi B Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization. EAACI/GA(2)LEN/EDF/WAO guideline: management of urticaria. Allergy. 2009;64:1427–1443. doi: 10.1111/j.1398-9995.2009.02178.x. [DOI] [PubMed] [Google Scholar]
- 18.Asero R, Riboldi P, Tedeschi A, Cugno M, Meroni P. Chronic urticaria: a disease at a crossroad between autoimmunity and coagulation. Autoimmun Rev. 2007;7:71–76. doi: 10.1016/j.autrev.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Takahagi S, Mihara S, Iwamoto K, Morioke S, Okabe T, Kameyoshi Y, Hide M. Coagulation/fibrinolysis and inflammation markers are associated with disease activity in patients with chronic urticaria. Allergy. 2010;65:649–656. doi: 10.1111/j.1398-9995.2009.02222.x. [DOI] [PubMed] [Google Scholar]
- 20.Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M. Severe chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy. 2008;63:176–180. doi: 10.1111/j.1398-9995.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- 21.Asero R, Cugno M, Tedeschi A. Activation of blood coagulation in plasma from chronic urticaria patients with negative autologous plasma skin test. J Eur Acad Dermatol Venereol. 2011;25:201–205. doi: 10.1111/j.1468-3083.2010.03752.x. [DOI] [PubMed] [Google Scholar]