Abstract
Cortical development involves complex interactions between neurons and non-neuronal elements including precursor cells, blood vessels, meninges and associated extracellular matrix. Because they provide a suitable organotypic environment, cortical slice explants are often used to investigate those interactions that control neuronal differentiation and development. Although beneficial, the slice explant model can suffer from drawbacks including aberrant cellular lamination and migration. Here we report a whole cerebral hemisphere explant system for studies of early cortical development that is easier to prepare than cortical slices and shows consistent organotypic migration and lamination. In this model system, early lamination and migration patterns proceed normally for a period of two days in vitro, including the period of preplate splitting, during which prospective cortical layer six forms. We then developed an ex utero electroporation (EUEP) approach that achieves ~80% success in targeting GFP expression to neurons developing in the dorsal medial cortex.
The whole hemisphere explant model makes early cortical development accessible for electroporation, pharmacological intervention and live imaging approaches. This method avoids the survival surgery required of in utero electroporation (IUEP) approaches while improving both transfection and areal targeting consistency. This method will facilitate experimental studies of neuronal proliferation, migration and differentiation.
Keywords: Neuroscience, Issue 74, Genetics, Neurobiology, Developmental Biology, Anatomy, Physiology, Molecular Biology, Cellular Biology, Bioengineering, Tissue Engineering, preplate splitting, in vitro preparation, dendritogenesis, gene function assay, in utero electroporation, GFP, hemisphere explants, gene expression, plasmid, explant, tissue, cell culture, tissue culture, animal model
Introduction
The mammalian cerebral cortex forms through the concerted proliferation, migration and differentiation of successively generated neurons. Each neuron is born in the ventricular zone (VZ) and migrates from the VZ into the intermediate zone (IZ), forming the cortical plate (CP) 1. As they pass through different cortical domains, the migrating neurons display multiple modes of migration 2,3 that depend on the extracellular environment and other cellular elements (e.g. radial glia) within the developing tissue. Cortical neurons then arrest migration at the top of the forming cortical plate in the coincident processes of neuronal migration arrest and dendritogenesis 4.
Cortical development is initiated between embryonic days 11-13 (E11-13) 5 through establishment of the primordial plexiform layer 6 or preplate (PP), a layer of pioneer neurons that overlies the VZ. Prospective layer 6 cortical neurons (i.e. the first cortical neurons born in the VZ) then orient their somata in a stereotypical pattern and coalesce into a distinct layer within the PP 7. These events split the preplate into a superficial marginal (future cortical layer 1) and a deep zone, the latter composed of subplate cells (transient cortical layer 7). This process, termed preplate splitting, is a foundational event in the future growth of the cerebral cortex 8.
Many genetic mutations have been identified that disrupt various aspects of cortical development 9. Cortical development can also be negatively impacted by exposure to ingested toxins such as cocaine 10 and alcohol 11. Because cortical malformations that arise during development are likely contributors to neurological disorders (e.g. autism, schizophrenia), empirical investigations of perturbations to cortical development are inherently important. It is therefore of considerable importance to establish approaches to study cortical development that allow rapid assays of genetic or toxin effects but that also preserve the possible interactions between differentiating neurons, other cell types and extracellular matrix (ECM) during this early period of brain development 12.
Slice explants 13 have provided such a system and have been widely used to assay cortical neuron development 14-16. However, slice assays can suffer from the drawback that neuronal migration and lamination can be abnormal 17 possibly due to damage to the meningeal cells that surround the developing brain and anchor the radial glial scaffold. As radial glial fibers are an important substrate for cortical neuron migration 18 disruption of the basal lamina by slicing may locally disrupt radial glial architecture and lead to altered cortical migration. In addition the sliced surfaces of explants provides a region of dead cells that may alter the normal composition of the ECM in these areas.
More recent approaches have focused their analysis on cells located deep in the slice that are surrounded by appropriate healthy cell types and ECM. However, in some cases these newer approaches can require that the original thick cultured slice be cryo-sectioned or paraffin-sectioned after fixation so that the relatively normal interior of the slice is made available for analysis 19-21. Both the original vibratome sectioning to prepare the live slices for culture as well as the subsequent cryosectioning of fixed slices for analysis require care and effort for these assays to work.
To provide a simple, complementary approach for studies of early cortical development, we have modified an existing slice approaches 13 to facilitate studies of early cortical development. We have developed a whole hemisphere explant model similar to an existing E14 whole hemisphere model that involved shaking cultures at 65 rotations per minute and permitted organotypic growth for 16-18 hr 22,23 . In our approach, whole hemisphere explants are placed on semi-permeable membrane 13 with in a high oxygen culture atmosphere 21,24 to extend organotypic cortical growth for 48 hr. This approach also allows for consistent electroporation of developing cortical neurons. The embryos are removed from the uterus and electroporated to introduce plasmid DNA and the telencephalon is then dissected. Each hemisphere is isolated and placed medial side down on a collagen-coated filter. The explant is then cultured for a period of 48 hr, a period that encompasses preplate splitting 8. During the culture period, L6 neurons develop from precursor to differentiated neuron 25, correctly positioned within the cortical plate. Throughout this period the developing neuron is surrounded by the appropriate ECM and cell types that would confront the corresponding cell in vivo. This system has already proved valuable in deciphering the cellular events that underlie ethanol toxicity 26, layer 6 formation and preplate splitting 7,25 .
Protocol
1. Ex utero Electroporations with Green Fluorescent Protein Expression Plasmid
Plasmid DNA injection solutions are prepared with CAG-eGFP DNA 27 diluted to the final working concentration of 0.33 mg/ml in ddH2O. Qiagen Endo-Free Maxi-Preps are used to purify the plasmid from transformed bacteria. Fast green dye at ~0.02% (w/v final) is added to the DNA solution as an injection tracer.
To prepare the surgical area, spray down bench top and dissecting microscope stage with a 70% ethanol solution and wipe dry. Spray surgical scissors and forceps with 70% ethanol prior to dissection and wipe dry.
Timed Pregnant Swiss/Webster dams are sacrificed on E13 by transfer into a chamber filled with CO2 from a pressurized cylinder and the dam is monitored until all motion has stopped for at least 1 min. After sacrifice by CO2 inhalation the embryos are removed from the uterus and placed in a 10 cm Petri dish containing ice cold Hanks balanced saline solution (HBSS).
Carefully dissect each embryo from the surrounding extraembryonic membranes and keep in ice-cold HBSS.
Transfer each embryo individually to a second 10 cm Petri dish containing cold HBSS. Use a Hamilton syringe to inject 2-3 μl of the DNA fast green mixture into the ventricle of the left cerebral hemisphere, taking care to inject in a cortical area spatially separate from the cortical area for future analysis.
To electroporate, hold the embryo gently with forceps and lightly place the positive paddle of the tweezer electrode on the dorsal midline of the head and lightly place the negative paddle underneath the embryo chin. Electroporations are achieved with a BTX830 electroporator programmed to deliver five 30 V pulses of 50 msec duration, separated by 950 msec intervals. These settings are for the BTX830 model. Other systems can be used according to manufacturer's instructions.
2. Whole Hemisphere Explant Preparation
After electroporation the embryos are placed back into ice-cold HBSS. The brain is removed using two #5 jeweler forceps to remove the skin and cartilaginous skull from the embryo head. A forcep is then slid underneath the brain to remove the intact brain from the skull. The electroporated hemisphere (left) is then dissected away from the brain and attached mid-brain tissue is removed. Throughout the dissection procedure care is taken to not damage the meninges overlying the left cortical hemisphere.
Once dissected each embryo is transferred in HBSS using a pipettor with a cut 1 ml pipette tip. The hemisphere is gently expelled onto a collagen coated culture insert. Up to six hemisphere explants are arranged medial side down on each 24 mm insert. Once arranged, excess HBSS is removed from the insert and the insert is then placed into one 35 mm well of a 6 well dish. The well contains exactly 2.7 ml of media: DMEM-F12 media containing Glutamax and supplemented with 2% B-27, 1% G5 and 1% Penicillin-Strep. A few drops of the media from the well can be pipetted on top of each explant, but do not add media in excess of the original 2.7 ml per well.
Once the explants have been placed onto Collagen filters the 6 well dish containing the explants is placed into a Billups Rothenberg (BR) chamber (that also contains a humidifying dish of water). A 95%/5% Oxygen/CO2 gas mixture is infused into the chamber for at least 1 min before sealing the chamber shut and disconnecting the 95%/5% Oxygen/CO2 gas supply tube. The BR chamber is then placed into a 37 °C tissue culture incubator for the remainder of the culture period, 1-2 DIV.
3. Fixation of Explant Tissue for Histology
In a fume hood prepare the Pagano fixation solution by first solubilizing 8 g of paraformaldehyde in 100 ml of preheated (~80 °C) ddH2O. Add 1-3 drops concentrated NaOH to promote solubilization and gently stir. Once solubilized mix the 8% paraformaldehyde solution 1:1 with a solution that is 500 mM sucrose, 100 mM Hepes, pH 7.4, 50 mM MgCl2, 5 mM KCl for a final concentration of 4% paraformaldehyde in 250 mM sucrose 50 mM Hepes, 25 mM MgCl2, 2.5 mM KCl, pH 7.4.
To fix the explants warm the Pagano fix solution to 37 °C. Quickly remove most of the DMEM/F12 media from each culture well and add 5 ml of Pagano Fix to each well of explants. Make sure to cover each explant completely in fix. Fix for 1 hr at RT. Do not remove explants from filter prior to 1 hr of fixation.
After fixation remove the Pagano fix solution and replace with Pagano solution without fix (250 mM sucrose 50 mM Hepes, 25 mM MgCl2, 2.5 mM KCl, pH 7.4). Add a few drops of 10% sodium azide and store at 4 °C until embedding.
4. Embedding and Sectioning for Histology
Prepare a 10% calf skin gelatin solution by solubilizing 10 g of calf skin gelatin in 100 ml of warm water (55-60 °C). Place gelatin solution on a hot plate set to 60 °C and swirl periodically until solubilized.
Pour approximately 10 ml of the 10% gelatin solution into the bottom of a 10 cm Petri dish and allow 30 min to solidify. This will form a "pad" for embedding the explants. These pads can be prepared days in advance and stored at 4 °C. Use a ruler to draw a grid on the bottom of the Petri dish and transfer individual explants into each box of the grid. Remove residual Pagano solution and using a pipettor, cover each explant with warm 10% gelatin solution and allow to solidify. Resume slow addition of gelatin solution until each explant is completely surrounded by gelatin, taking care not to melt the pad by the addition of too much warm gelatin at once. Allow for the gelatin to harden for ~1 hr. Once hardened the individual explants can be cut out in small blocks of gelatin. The blocks are then post-fixed in Pagano fix for 24-48 hr prior to sectioning with a vibratome.
Explants in gelatin blocks are positioned olfactory bulb up and sectioned in the coronal plane at 100 μm thickness. The sections are collected and stored in PBS + 0.1% sodium azide at 4 °C until immunohistochemical processing.
Immunohistochemical detection is performed by transferring sections into a 24 well plate (2-3 sections per well). First block non-specific antibody binding by adding 0.5 ml PBST+B (PBS + 0.5% Triton X 100 and 2% BSA) to each well, incubate 1 hr with gentle shaking. The appropriate primary antibody is then diluted in PBST+B and 0.4 ml per well is added for overnight incubation at RT. The primary is washed out with 3X PBS washes for at least 10 min each. The secondary antibody and 2 μg/ml Hoechst 33342 nuclear stain are then diluted in PBST+B and the samples are incubated for 2 hr at RT with gentle shaking. Three PBS washes (10 min each) are performed and then the sections are mounted on slides using a 90% glycerol 50 mM Tris, pH7.4, mounting media.
Representative Results
The embryonic rodent cortex exhibits a transverse neurogenetic gradient throughout the developmental period, such that lateral neocortex is approximately 1 day more mature than dorsal medial neocortex 28. The bulk of layer 6 neurons are thus generated (i.e. exhibit their final S-phase) on E12 in lateral cortex (also called Field 405) and at E13 in the dorsal medial cortex (also called Field 15). Preplate splitting begins approximately 1 day after Layer 6 neuron generation and thus is initiated in the lateral cortex on E13 and the dorsal cortex on E14. To study preplate splitting and the initiation of cortical plate development, we modified a slice explant assay 21,24 such that entire cerebral hemispheres were cultured typically from E13 to E15 (Figure 1).
We tested the viability of the cortical explant technique by examining the growth of whole hemisphere explants derived from Tg(Eomes::eGFP)Gsat mice (Figure 2). This mouse strain contains a transgene that reports immature neurons of the excitatory cortical lineage 7,29,30 . Whole hemisphere cortical explants were prepared at E13, at a time point prior to preplate splitting in the dorsal neocortex (Figure 2A). Cortical explants from the same litter were drop fixed sequentially over a period of 2 DIV and processed for subsequent histological analysis. At the initial analysis time point (DIV 0), preplate splitting has not yet occurred in Field 1 (Dorsal neocortex; Figures 2A, 2E). By 1 DIV the CP is apparent (Figures 2B, 2C, 2F, 2G), and it continues to grow up to 2 DIV (Figures 2D, 2H). Thus the whole hemisphere explants cultured initially at E13 support for two days the maturation of the cerebral cortex and captures preplate splitting in the dorsal neocortex.
To confirm normal histological development in the dorsal neocortex, we immunostained 2 DIV explants for chondroitin sulfate proteoglycans (CSPGs), an extracellular matrix component that is also an established marker of the PP and its derivatives the MZ and SP 8,31,32 . Immunostaining for CSPGs reveals two bands of CSPG immunoreactivity in the dorsal cortex indicating appropriate splitting of the PP into MZ and SP during the in vitro culture period (Figures 3A-3C). The PP is split by L6 cortical neurons that are known to express the transcription factors Ctip2 and Tbr1 7,33,34 . The immunostaining pattern of these markers (Figures 3D-3F, 3G-I, respectively) confirms the appropriate expression of these transcription factors in the newly formed CP. Together these analyses confirm organotypic early cortical development in the whole hemisphere explants.
In utero electroporations have been used extensively to assay for cell autonomous gene function in the developing cortex 35. We therefore tested whether electroporation could successfully transfect neurons in the whole hemisphere explant model. The left ventricle of intact E13 embryos were injected with 0.33 mg/ml CAG-GFP plasmid and electroporated (see Methods) to introduce the plasmid DNA into the neuronal precursors that line the lateral ventricle (Figure 1A). After 2 DIV the explants were dropped fixed and sectioned for histological analysis. At this time point, GFP expressing cells were observed in all cortical zones across the cerebral wall (Figures 4A-4C). GFP expression could be observed from neuronal precursors in the VZ while intense GFP expressing cells were observed in the IZ and CP. Importantly, numerous GFP+ neurons were observed at the top of the forming CP with emerging dendrites and axons. The cells have formed a discrete layer at the interface between the CP and MZ indicating appropriate migration arrest and lamination (Figures 4A-4C, arrows). The dendrites extended into the MZ while corticofugal axons extend below the CP and into the IZ (Figures 4A-4C and 5, See also Supplemental Movie S1). Below the differentiating cells are GFP+ cells in varying states of maturity, including neurons with a bipolar morphology, juxtaposed to radial glial fibers and below them, multipolar neurons in the IZ (Figures 4D-4F). In addition, the dendrites are observed extending into the MZ where the ECM protein Reelin is appropriately immunolocalized (Figures 4G-4I). The electroporation and explant conditions thus permit normal development of cortical neurons through the stages of precursor to migrating neuron to immature differentiating neuron.
At the time of electroporation (E13) 100% of the neurons produced by the dorsal neocortical VZ are L6 neurons, the neurons that split the PP 5. Successful electroporation requires that constructs be introduced into neurons during the 6 hr immediately prior to, or during, M-phase of the cell cycle 36. It is thus expected that the earliest postmitotic, GFP+ neurons following E13 electroporation in the dorsal cortex (Field 1) should populate the forming CP. Such neurons were observed (Figure 4) indicating that the protocol can reliably target L6 cortical neurons for studies of their migration and maturation.
To estimate the "success rate" of the electroporation/explant procedure we analyzed an ongoing investigation from our lab. In the course of that study, 21 litters of explants have been prepared, a total of 248 embryos were electroporated (as described above) with CAG-GFP, and a single hemisphere explant from each embryo was prepared. Of the original 248 explants, 195 (79%) were judged suitable for imaging and analysis. About half of the 53 explants that could not be analyzed failed to express GFP or had mis-targeted GFP expression. The remaining losses were explained by dysmorphic growth due to explant detachment from the culture filter, or problems associated with histological processing. This success rate of roughly 80% (Figure 5) has rendered the explant system suitable for pharmacological studies 26, as well as the analysis of recessive mutations 7 that adversely affect early cortical development.
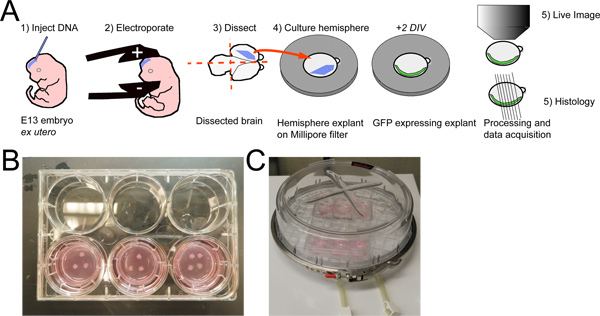 Figure 1. Whole hemisphere explant procedure with ex utero electroporation. A) E13 mouse embryos are injected with a plasmid DNA solution and electroporated. The brains are dissected and transected sagitally. The electroporated hemisphere are then placed medial side down on a collagen coated filter and cultured for 2 DIV. The hemispheres can then be imaged live or fixed for histology and subsequent imaging analysis. B) A photo of 10 explants placed on three filters in a six well tissue culture dish. C) The six well dish with explants is placed in a high oxygen environment (95% O2 / 5% CO2) within a Billups-Rothenberg Incubator chamber, which is then placed in a standard 37 °C tissue culture incubator.
Figure 1. Whole hemisphere explant procedure with ex utero electroporation. A) E13 mouse embryos are injected with a plasmid DNA solution and electroporated. The brains are dissected and transected sagitally. The electroporated hemisphere are then placed medial side down on a collagen coated filter and cultured for 2 DIV. The hemispheres can then be imaged live or fixed for histology and subsequent imaging analysis. B) A photo of 10 explants placed on three filters in a six well tissue culture dish. C) The six well dish with explants is placed in a high oxygen environment (95% O2 / 5% CO2) within a Billups-Rothenberg Incubator chamber, which is then placed in a standard 37 °C tissue culture incubator.
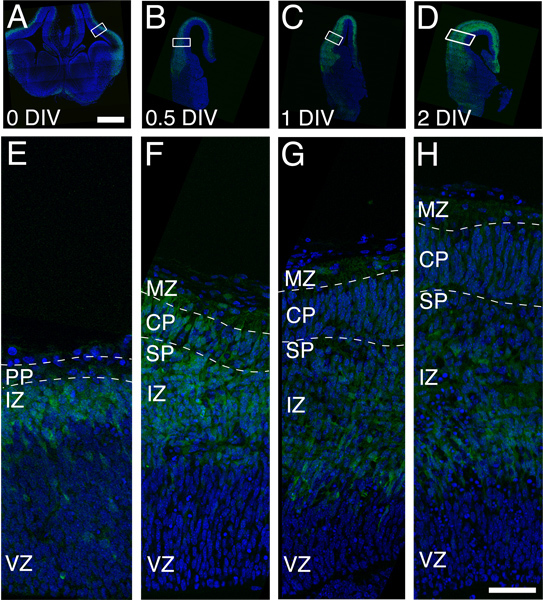 Figure 2. Organotypic growth of cortical plate in whole hemisphere explants derived from a single litter of Eomes::eGFP embryos. A, E) A coronal section from a drop-fixed brain at the beginning of the culture period (O DIV). Note that the PP is present but that CP has yet to form. The green signal is produced by GFP expression in immature excitatory neurons and the blue is Hoechst dye that labels the nucleus of all cells. B, F). CP growth, denoted by dashed lines, is observed by 0.5 DIV and increases by 1 DIV (C and G) and 2 DIV (panels D and H). Note the increased thickness of the GFP expressing cell domain in panels A-D, indicating the proliferation and differentiation of the excitatory neuron lineage in the explant. Scale bars are 500 μm (panel A) and 50 μm (panel H). Abbreviations: CP, Cortical Plate; IZ, Intermediate Zone; MZ, Marginal Zone; SP, Subplate; VZ, Ventricular Zone.
Figure 2. Organotypic growth of cortical plate in whole hemisphere explants derived from a single litter of Eomes::eGFP embryos. A, E) A coronal section from a drop-fixed brain at the beginning of the culture period (O DIV). Note that the PP is present but that CP has yet to form. The green signal is produced by GFP expression in immature excitatory neurons and the blue is Hoechst dye that labels the nucleus of all cells. B, F). CP growth, denoted by dashed lines, is observed by 0.5 DIV and increases by 1 DIV (C and G) and 2 DIV (panels D and H). Note the increased thickness of the GFP expressing cell domain in panels A-D, indicating the proliferation and differentiation of the excitatory neuron lineage in the explant. Scale bars are 500 μm (panel A) and 50 μm (panel H). Abbreviations: CP, Cortical Plate; IZ, Intermediate Zone; MZ, Marginal Zone; SP, Subplate; VZ, Ventricular Zone.
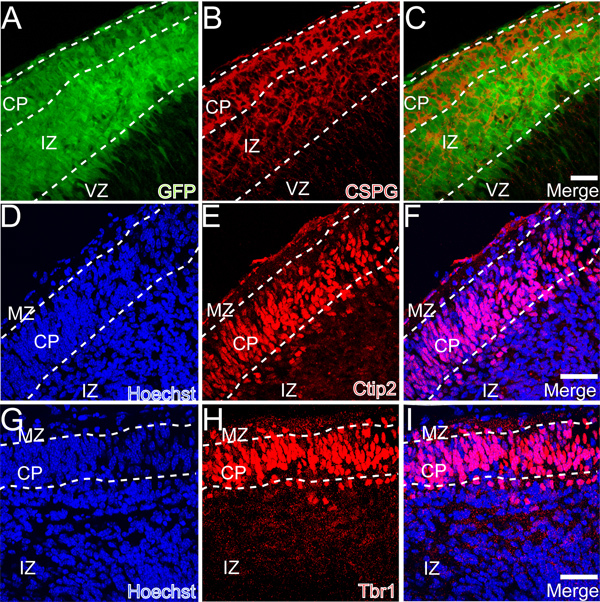 Figure 3. Preplate splitting in whole hemisphere explants. A-C) Chondroitin sulfate proteoglycan (CSPG) immunostaining illustrates the division (splitting) of the PP into the MZ and SP. D-F) Expression of the transcription factor Ctip2 in the newly formed CP. G-I) Expression of the transcription factor Tbr1 in the newly formed CP. Scale bars are 50 μm in panels C, F, I.
Figure 3. Preplate splitting in whole hemisphere explants. A-C) Chondroitin sulfate proteoglycan (CSPG) immunostaining illustrates the division (splitting) of the PP into the MZ and SP. D-F) Expression of the transcription factor Ctip2 in the newly formed CP. G-I) Expression of the transcription factor Tbr1 in the newly formed CP. Scale bars are 50 μm in panels C, F, I.
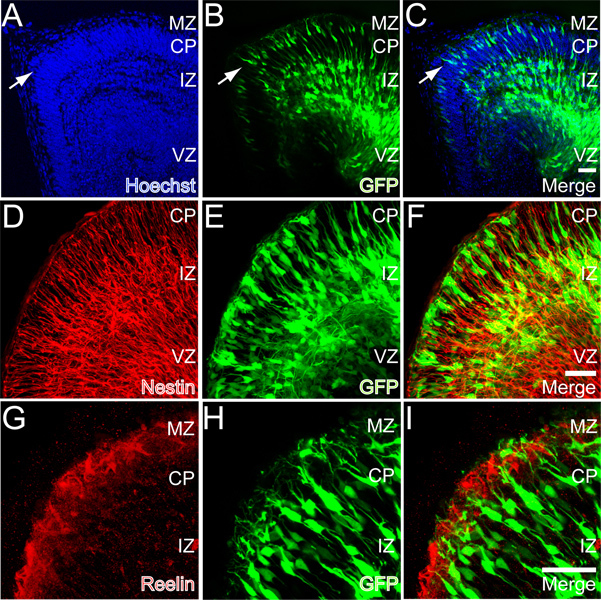 Figure 4. GFP expression in whole hemisphere explants after E13 ex utero electroporation. A-C) GFP expression after 2 DIV. Note the layer of cortical neurons forming at the top of the CP (arrows). D-F) Nestin (red) immunoreactivity in a section from a whole hemisphere explant. G-H) Reelin (red) immunoreactivity in a section derived from a whole hemisphere explant. Scale bars in panels C, F, and I are 50 μm.
Figure 4. GFP expression in whole hemisphere explants after E13 ex utero electroporation. A-C) GFP expression after 2 DIV. Note the layer of cortical neurons forming at the top of the CP (arrows). D-F) Nestin (red) immunoreactivity in a section from a whole hemisphere explant. G-H) Reelin (red) immunoreactivity in a section derived from a whole hemisphere explant. Scale bars in panels C, F, and I are 50 μm.
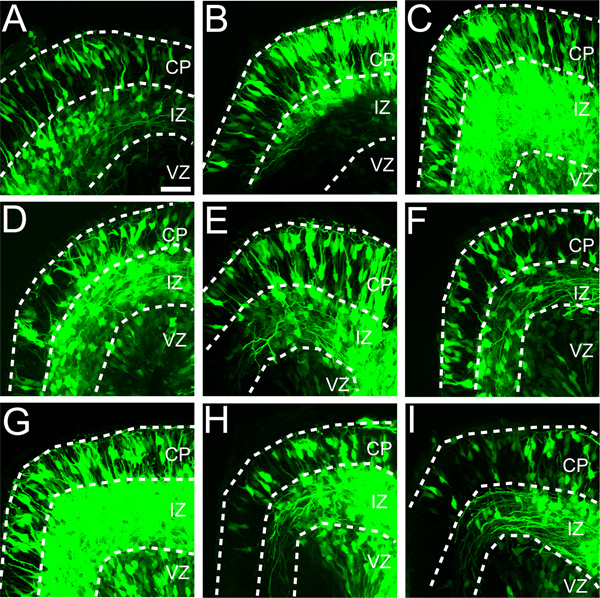 Figure 5. Variability in targeting ex utero electroporations to dorsal medial cortex (Field 1). Eleven embryos from a single litter were electroporated with the CAG-GFP expression construct (0.33 mg/ml) and cultured as whole hemisphere explants. A-I) Nine of eleven explants showed expression of GFP in dorsal medial cortex. Although GFP expression varies between explants, in each explant GFP is detected in all three cortical zones (i.e. VZ, IZ and CP). Scale bar is 50 μm in panel A.
Figure 5. Variability in targeting ex utero electroporations to dorsal medial cortex (Field 1). Eleven embryos from a single litter were electroporated with the CAG-GFP expression construct (0.33 mg/ml) and cultured as whole hemisphere explants. A-I) Nine of eleven explants showed expression of GFP in dorsal medial cortex. Although GFP expression varies between explants, in each explant GFP is detected in all three cortical zones (i.e. VZ, IZ and CP). Scale bar is 50 μm in panel A.
Discussion
We have improved and evaluated an experimental model - whole hemisphere explants - for the study of early cortical development (E13-E15). The model has proven useful for the analysis of migration and differentiation of the excitatory neuron lineage that constitutes layer 6 of the cerebral cortex 25,37 . The principle advantages of the system are 1) organotypic growth for 2 DIV, 2) simplicity of preparation, and 3) experimental access to the neurons for electroporation, pharmacological manipulation and imaging during this early, critical period of brain development. We have used the system to document subtle differences in the morphology, orientation, and dendritic growth of layer 6 cortical neurons 7,38 during the period of preplate splitting.
The excitatory neurons that populate layer 6 are largely composed of corticothalamic projection neurons that provide reciprocal input to the dorsal thalamus 39,40 and are hypothesized to participate functionally in the synchronization of neural function in the cortex 41. Although these neurons are physiologically specialized, the generation, migration and maturation process of layer 6 neurons shares basic features with all excitatory neurons in layers 2-6 of the cortex. Specifically, all cortical excitatory neurons are generated in the dorsal telencephalic VZ, migrate through the IZ as multipolar neurons, and differentiate within the CP 7. The maturation of excitatory cortical neurons is driven by a core set of sequentially expressed transcription factors: Pax6, Tbr2, and Tbr1 29. This sequence is shared by all excitatory neurons in layers 2-6 42 and we confirmed the appropriate expression of Tbr1 in the newly formed CP (Figures 3G-3I). Utilizing this approach to investigate the development of layer 6 cortical neurons should thus provide essential insights into the development of neurons that constitute all cortical layers.
Whole hemisphere explants complement existing approaches for studies of cortical neuron development. Prior studies have employed an E14 whole hemisphere explant approach to study cortical development for 1 DIV 22,23 . We have coupled whole hemisphere explants with ex utero electroporation to investigate 2 days of cortical development during the period of L6 formation and preplate splitting. In contrast to in utero electroporations at E13, the success rate of ex utero electroporations followed by whole hemisphere explant is higher: in our hands we achieve ~80% success in targeting electroporation to the dorsal telencephalon. Additionally, in utero electroporations require a survival surgery and take considerably more technical effort than the whole hemisphere ex utero approach. Finally, in utero studies are not readily amenable to precise pharmacological manipulations and imaging approaches. Precise temporal control in the delivery or activation of drugs and molecular agents (expression vectors, silencing constructs, etc) at defined concentrations is provided with the whole hemisphere explants, but is more difficult to achieve in utero. Live optical monitoring of the resultant effects of such manipulations, with spatial resolution in the micron range, is readily available with EUEP, but not IUEP. Such precision is likely to substantially enhance the interpretive power of data collected from experiments involving cortical development and function. Thus for studies of early cortical development whole hemisphere explants possess distinct experimental advantages over the in utero approach. At this time, however, for studies of upper layer cortical neurons and experiments requiring data collection > 48 hr, slice explants and in utero approaches remain preferable.
There are several critical requirements for success of the whole hemisphere explant technique. First, growth of the explant is highly sensitive to the mechanical integrity of the meninges. Physical damage to the meninges (i.e. punctures or tears) will at a minimum disrupt local development of the underlying cortex. Secondly, explant growth is sensitive to the total volume of media within the culture well: with too much media the explants will disengage from the filter and develop distorted CP architecture; with too little media and the explants are likely to dehydrate and experience excessive cell death. Lastly, with the current technique explants we have documented cortical growth for 2 DIV. When starting at E13 this developmental time window, although admitting many valuable investigations, restricts analyses to a period prior to synaptogenesis and canonical neuronal communication. Although the cortical neurons at E14.5 express many mRNAs encoding synaptic proteins (www.genepaint.org), synaptogenesis does not begin until ~E15 in the lateral cortex, and this synaptogenesis is confined to preplate cells rather than layer 6 neurons 43. Some critical questions concerning cortical synapse formation and function are therefore not currently addressable with the whole hemisphere explant system.
In conclusion the whole hemisphere explant preparation can be used reliably and consistently to investigate early cortical development in mouse models of heritable brain malformations. The technique is readily adaptable to RNAi, shRNA, and other molecular and pharmacological manipulations. The whole hemisphere explant is also suitable for studies involving applied recombinant protein, drugs and environmental toxins.
Disclosures
The authors declare that they have no competing financial interests.
Acknowledgments
This work was supported grants from NINDS (NS066071) and the NIAAA. (P50AA017823) to ECO. The authors thank Dr. Robert Quinn and the staff in the Department of Laboratory Animal Resources for animal care. We thank Judson Belmont for technical support, Nicole Belletier for assistance as a Summer Undergraduate Research Fellow (SURF). We also thank Dr. David Cameron for comments and edits on an earlier version of the manuscript.
References
- Rakic P. Principles of neural cell migration. Experientia. 1990;46:882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J. Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat. Neurosci. 2004. [DOI] [PubMed]
- Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J. Neurosci. 2006;26:1767–1775. doi: 10.1523/JNEUROSCI.3000-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Goto T, Miyama S, Nowakowski RS, Caviness VS. Sequence of neuron origin and neocortical laminar fate: relation to cell cycle of origin in the developing murine cerebral wall. J. Neurosci. 1999;19:10357–10371. doi: 10.1523/JNEUROSCI.19-23-10357.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Padilla M. Early prenatal ontogenesis of the cerebral cortex (neocortex) of the cat (Felis domestica). A Golgi study. I. The primordial neocortical organization. Z. Anat. Entwicklungsgesch. 1971;134:117–145. doi: 10.1007/BF00519296. [DOI] [PubMed] [Google Scholar]
- Nichols AJ, Olson EC. Reelin promotes neuronal orientation and dendritogenesis during preplate splitting. Cerebral Cortex. 2010;20:2213–2223. doi: 10.1093/cercor/bhp303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard AM, Pearlman AL. Abnormal reorganization of preplate neurons and their associated extracellular matrix: an early manifestation of altered neocortical development in the reeler mutant mouse. J. Comp. Neurol. 1997;378:173–179. doi: 10.1002/(sici)1096-9861(19970210)378:2<173::aid-cne2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Manzini MC, Walsh CA. What disorders of cortical development tell us about the cortex: one plus one does not always make two. Current Opinion in Genetics & Development. 2011;21:333–339. doi: 10.1016/j.gde.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones L, Fischer I, Levitt P. Nonuniform alteration of dendritic development in the cerebral cortex following prenatal cocaine exposure. Cereb. Cortex. 1996;6:431–445. doi: 10.1093/cercor/6.3.431. [DOI] [PubMed] [Google Scholar]
- Olney JW. Fetal alcohol syndrome at the cellular level. Addict. Biol. 2004;9:137–149. doi: 10.1080/13556210410001717006. [DOI] [PubMed] [Google Scholar]
- Levitt P, Reinoso B, Jones L. The critical impact of early cellular environment on neuronal development. Prev. Med. 1998;27:180–183. doi: 10.1006/pmed.1998.0273. [DOI] [PubMed] [Google Scholar]
- Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Dailey ME, Smith SJ, McConnell SK. Diverse migratory pathways in the developing cerebral cortex. Science. 1992;258:299–302. doi: 10.1126/science.1411527. [DOI] [PubMed] [Google Scholar]
- Elias LA, Wang DD, Kriegstein AR. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Muller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz M, Bolz J. Formation and preservation of cortical layers in slice cultures. J. Neurobiol. 1992;23:783–802. doi: 10.1002/neu.480230702. [DOI] [PubMed] [Google Scholar]
- Cameron RS, Rakic P. Identification of membrane proteins that comprise the plasmalemmal junction between migrating neurons and radial glial cells. J Neurosci. 1994;14:3139–3155. doi: 10.1523/JNEUROSCI.14-05-03139.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizarraga SB, Coser KR, Sabbagh M, Morrow EM. Methods for Study of Neuronal Morphogenesis: Ex vivo RNAi Electroporation in Embryonic Murine Cerebral Cortex. J. Vis. Exp. 2012. p. e3621. [DOI] [PMC free article] [PubMed]
- Jossin Y, Goffinet AM. Reelin signals through phosphatidylinositol 3-kinase and Akt to control cortical development and through mTor to regulate dendritic growth. Mol. Cell Biol. 2007;27:7113–7124. doi: 10.1128/MCB.00928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Ogawa M, Metin C, Tissir F, Goffinet AM. Inhibition of SRC family kinases and non-classical protein kinases C induce a reeler-like malformation of cortical plate development. J. Neurosci. 2003;23:9953–9959. doi: 10.1523/JNEUROSCI.23-30-09953.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, Rehen SK, Contos JJ, Higgins CM, Chun J. Non-proliferative effects of lysophosphatidic acid enhance cortical growth and folding. Nat. Neurosci. 2003;6:1292–1299. doi: 10.1038/nn1157. [DOI] [PubMed] [Google Scholar]
- Rehen SK, et al. A new method of embryonic culture for assessing global changes in brain organization. J. Neurosci. Methods. 2006;158:100–108. doi: 10.1016/j.jneumeth.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Jossin Y, et al. The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development. J. Neurosci. 2004;24:514–521. doi: 10.1523/JNEUROSCI.3408-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dell RS, et al. Layer 6 cortical neurons require Reelin-Dab1 signaling for cellular orientation, Golgi deployment, and directed neurite growth into the marginal zone. Neural. Dev. 2012;7:25. doi: 10.1186/1749-8104-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrozek TA, Olson EC. Ethanol-induced disruption of Golgi apparatus morphology, primary neurite number and cellular orientation in developing cortical neurons. Alcohol. 2012. [DOI] [PMC free article] [PubMed]
- Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc. Natl. Acad. Sci. U.S.A. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suter B, Nowakowski RS, Bhide PG, Caviness VS. Navigating neocortical neurogenesis and neuronal specification: a positional information system encoded by neurogenetic gradients. The Journal of Neuroscience : The Official Journal of The Society for Neuroscience. 2007;27:10777–10784. doi: 10.1523/JNEUROSCI.3091-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, et al. and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon GS, Hadjantonakis AK. Eomes::GFP-a tool for live imaging cells of the trophoblast, primitive streak, and telencephalon in the mouse embryo. Genesis. 2007;45:208–217. doi: 10.1002/dvg.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman AL, Sheppard AM. Extracellular matrix in early cortical development. Prog. Brain Res. 1996;108:117–134. [PubMed] [Google Scholar]
- Milev P, et al. Differential regulation of expression of hyaluronan-binding proteoglycans in developing brain: aggrecan, versican, neurocan, and brevican. Biochemical and Biophysical Research Communications. 1998;247:207–212. doi: 10.1006/bbrc.1998.8759. [DOI] [PubMed] [Google Scholar]
- Kwan KY, et al. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna WL, et al. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2011;31:549–564. doi: 10.1523/JNEUROSCI.4131-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF. Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J. Neurosci. 2010;30:7028–7036. doi: 10.1523/JNEUROSCI.6131-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powrozek TA, Zhou FC. Effects of prenatal alcohol exposure on the development of the vibrissal somatosensory cortical barrel network. Brain Res. Dev. Brain Res. 2005;155:135–146. doi: 10.1016/j.devbrainres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- O'Dell R, et al. Society for Neuroscience. Washington D.C: 2011. [Google Scholar]
- Tombol T, Hajdu F, Somogyi G. Identification of the Golgi picture of the layer VI cortic-geniculate projection neurons. Experimental Brain Research. Experimentelle Hirnforschung. Experimentation Cerebrale. 1975;24:107–110. doi: 10.1007/BF00236022. [DOI] [PubMed] [Google Scholar]
- Brumberg JC, Hamzei-Sichani F, Yuste R. Morphological and physiological characterization of layer VI corticofugal neurons of mouse primary visual cortex. Journal of Neurophysiology. 2003;89:2854–2867. doi: 10.1152/jn.01051.2002. [DOI] [PubMed] [Google Scholar]
- Jones EG. Synchrony in the interconnected circuitry of the thalamus and cerebral cortex. Annals of the New York Academy of Sciences. 2009;1157:10–23. doi: 10.1111/j.1749-6632.2009.04534.x. [DOI] [PubMed] [Google Scholar]
- Kowalczyk T, et al. Intermediate Neuronal Progenitors (Basal Progenitors) Produce Pyramidal-Projection Neurons for All Layers of Cerebral Cortex. Cereb. Cortex. 2009. [DOI] [PMC free article] [PubMed]
- Konig N, Roch G, Marty R. The onset of synaptogenesis in rat temporal cortex. Anatomy and Embryology. 1975;148:73–87. doi: 10.1007/BF00315564. [DOI] [PubMed] [Google Scholar]


