Abstract
Background
The purpose of this study was to evaluate the safety, tolerability, and efficacy of milnacipran following a direct switch from duloxetine in fibromyalgia patients experiencing inadequate clinical response to duloxetine after receiving treatment for 6 weeks or longer.
Methods
This exploratory study included 107 patients with fibromyalgia who had been treated with duloxetine 60 mg/day for at least 4 weeks prior to enrollment. Following a 2-week open-label period on duloxetine, patients who had visual analog scale pain scores ≥ 40 and were dissatisfied with current treatment were randomized 4:1 to milnacipran 100 mg/day (n = 86) or placebo (n = 21) for 10 weeks of double-blind treatment. The small placebo group was included solely to blind the study and minimize expectation bias among patients and investigators, and there was no preplanned statistical comparison between treatment groups. The primary efficacy parameter was the percentage of patients rating themselves as “much improved” or “very much improved” on the Patient Global Impression of Change (PGIC) at the final visit. Other efficacy parameters included changes in one-week recall visual analog scale pain, Fibromyalgia Impact Questionnaire Revised (FIQR), and Multiple Ability Self-Report Questionnaire (MASQ).
Results
Of patients switched to milnacipran, 32.9% were classified as PGIC responders, and they also demonstrated improvement in visual analog scale pain, FIQR total, and MASQ total scores (mean changes from baseline were −12.3, −7.77, and −2.39, respectively). Nausea and dizziness were the most common treatment-emergent adverse events in patients switched to milnacipran, reported in 21% and 15%, respectively, of patients in this group.
Conclusion
Results from this exploratory study suggest that switching from duloxetine to milnacipran may be beneficial in some patients with fibromyalgia who have an inadequate response to duloxetine. Further research investigating the efficacy and safety of switching fibromyalgia therapies is warranted.
Keywords: fibromyalgia, milnacipran, duloxetine, switch
Introduction
Fibromyalgia, a disorder characterized by chronic widespread pain and tenderness, is estimated to affect 2%–4% of the population and occurs more often in women than men.1 The diagnostic criteria for fibromyalgia define a heterogeneous group of patients with challenging symptoms and comorbidities.2,3 In addition to widespread pain, most patients experience fatigue, sleep disturbances, cognitive dysfunction, stiffness, and a higher lifetime history of anxiety or depression.4–6 Fibromyalgia patients may also suffer from comorbid conditions, such as primary sleep disorders, migraine headaches, interstitial cystitis, irritable bowel syndrome, and restless leg syndrome.7,8 These multiple symptoms and comorbidities contribute to reduced quality of life in patients9–11 and present a considerable economic burden, including increased health care costs, disability costs, missed work, and unemployment.7,12,13
Because of the heterogeneity of this population, it is recommended that treatment be individualized to each patient based on specific symptoms, comorbidities, and disease severity.14 Although a number of medications have demonstrated efficacy in fibromyalgia patients, no single medication results in full symptom resolution in all patients.15 Identifying appropriate fibromyalgia treatments is essential because untreated symptoms may contribute to decreased functioning and diminished quality of life.5,6 Also, tolerability is an important issue since drugs have different side effects. In addition, given that fibromyalgia patients may need multiple medications to manage symptoms and/or comorbid conditions,7,8 choosing medications with low potential for drug-drug interactions should be a priority.16 Changing and adjusting fibromyalgia medications may help to achieve greater efficacy, reduce undesirable side effects, or avoid potential interactions with other concomitant medications.
While switching medications may be common clinical practice, no clinical studies to our knowledge have been conducted to investigate this treatment strategy in fibromyalgia patients. When the current study was designed, not enough information was available to estimate the sample size required to conduct a randomized, placebo-controlled, trial. In order to assess the feasibility of a direct switch between two fibromyalgia drugs in the same class, this pilot study was designed to emulate clinical practice so that patients who were already receiving duloxetine, but were dissatisfied with treatment, could switch to milnacipran without down-tapering of duloxetine or uptitration of milnacipran. Both of these medications are serotonin and norepinephrine reuptake inhibitors (SRNIs) approved for the management of fibromyalgia in the United States.17,18 Studies of SNRIs and selective serotonin reuptake inhibitors for depression have shown that patients failing to respond to one treatment can benefit from a switch to medications with a similar mechanism of action,19–21 but such studies have not been conducted in patients with fibromyalgia. Therefore, two of the main outcomes expected from this study were as follows: whether patients experience any improvements in pain or other symptoms after switching medications; and whether patients could switch directly without having to decrease duloxetine dosage before initiating treatment with milnacipran in order to avoid a gap in SNRI therapy. The current report presents preliminary findings regarding the safety, tolerability, and potential clinical benefits of switching between drugs in the same class when treatment response to one drug is inadequate.
Materials and methods
This was an exploratory, multicenter, randomized, double-blind, placebo-controlled Phase IV trial that evaluated the safety, tolerability, and efficacy of milnacipran in patients with an inadequate response to duloxetine for the treatment of fibromyalgia (Clinical Trial Registration ID NCT01077375). The trial was conducted at 25 study centers in the United States from March to December 2010. An institutional review board approved the protocol, which was developed in accordance with the ethical guidelines of Good Clinical Practice and the Declaration of Helsinki. All patients provided written consent after the study was explained and their questions answered and before study procedures were initiated.
Study design
The study included female and male outpatients, aged 18–70 years, with a diagnosis of fibromyalgia who had been receiving the recommended dosage of duloxetine 60 mg/day (ie, the maximum dosage recommended by the US Food and Drug Administration)17 at stable doses for at least 4 weeks prior to screening. Duloxetine prescriptions had to be for the management of fibromyalgia and not for the treatment of depression or another pain syndrome. Patients with a one-week visual analog scale (VAS) pain recall score ≥40 mm to ≤90 mm at screening were entered into the open-label, run-in period of this study and continued receiving duloxetine 60 mg/day for an additional 2 weeks in order to confirm that they were not having an adequate response to duloxetine under study conditions. After this 2-week run-in period, patients who continued to have a VAS pain score ≥40 mm and who still expressed dissatisfaction with treatment were eligible for randomization. A deliberately general question was used to evaluate treatment satisfaction (ie, “Are you satisfied with duloxetine treatment?”) in order to allow for any potential dissatisfaction (eg, unsatisfactory improvement in pain or non-pain symptoms, poor tolerability, simple desire to try a new medication), as might occur in clinical practice.
In order to minimize expectation bias that would occur with an open-label switch to milnacipran, patients were randomized (4:1) to milnacipran or placebo in a double-blind fashion for 10 weeks of treatment, followed by a one-week, double-blind, down-taper period. The placebo arm was included to lessen the potential for bias in patients who might otherwise expect improvement from being switched to another therapy, rather than to provide a comparison group, because the placebo arm was too small to allow direct comparison.
Patients randomized to milnacipran were switched directly without down-tapering of duloxetine or uptitration of milnacipran. During the first 2 weeks of the double-blind treatment period, milnacipran could be lowered to 50 or 75 mg/day if patients had initial difficulty tolerating the minimum recommended dosage (100 mg/day).18 However, patients unable to tolerate milnacipran 100 mg/day by the end of week 2 were discontinued from the study. After week 2, the milnacipran dosage could be escalated to 200 mg/day (ie, the highest dosage recommended by the US Food and Drug Administration)18 as needed to treat symptoms effectively, and the dosage could also be decreased to 50 or 75 mg/day for tolerability. Patients randomized to the placebo group continued receiving duloxetine 30 mg/day for the first week of double-blind treatment during a duloxetine down-taper, then continued on placebo for the remainder of the study. In addition, all patients who withdrew from the study for any reason were down-tapered from active medication (duloxetine or milnacipran) in accordance with prescribing recommendations.17,18
Patients meeting any of the following criteria were excluded from the study: history or current diagnosis of serious psychiatric disorder; substantial alcohol use or abuse; behavior that would, in the investigator’s judgment, prohibit participation in the study; serious suicide risk; Beck Depression Inventory22 >25; pregnancy or breastfeeding; unacceptable contraception in those of childbearing potential; untreated hypertension; cardiovascular disease, including myocardial infarction or stroke within the past 6 months; sitting mean systolic blood pressure >160 mmHg or diastolic blood pressure >100 mmHg; active or unstable medical illness; evidence of active liver disease; prostate enlargement or other genitourinary disorders; renal impairment (creatinine clearance <30 mL per minute); uncontrolled narrow-angle glaucoma; body mass index ≥45 kg/m2.
Excluded concomitant medications included drugs with central nervous system activity, such as antidepressants, anorectics, antiepileptic agents, opiates, and related analgesics (eg, oxycodone, codeine, tramadol, narcotic patches), dopamine agonists, stimulants, and sodium oxybate. Permitted analgesic medications were acetaminophen, aspirin, and nonsteroidal anti-inflammatory agents. Patients requiring short-term pain rescue medication were allowed opioid analgesics, but opioids were not permitted within 7 days of scheduled study visits. Triptans were permitted for acute migraine treatment. Nonbenzodiazepine hypnotics were also allowed for patients requiring treatment of insomnia.
Outcomes
The primary efficacy parameter was responder status based on Patient Global Impression of Change (PGIC) score, defined as the proportion of patients rating their overall improvement from baseline to week 10 of the double-blind period as “very much improved” (score = 1) or “much improved” (score = 2) based on a 7-point scale ranging from 1 to 7 (“very much worse”). As with the question used to evaluate patient satisfaction with duloxetine, the general wording of the PGIC (ie, “Since I started investigational product at visit 2 [randomization], overall my fibromyalgia is …”) allowed patients to consider any reason for improvement, including better efficacy or tolerability.
The secondary efficacy parameter was change from randomization to week 10 of the double-blind treatment period in one-week recall VAS pain score. A post hoc analysis was also conducted to identify the percentages of patients with ≥30%, ≥40%, and ≥50% improvement from baseline in pain severity. Additional efficacy assessments included the Multiple Ability Self-Report Questionnaire (MASQ),23 the European Quality of Life-5-Dimensions (EQ-5D),24 the Fibromyalgia Impact Questionnaire-Revised (FIQR),25 and the Arizona Sexual Experiences Scale (ASEX).26 Safety was evaluated at all study visits. Assessments included adverse event recording, vital sign parameters, and Beck Depression Inventory scores.
Statistical analyses
Since this was designed as an exploratory study rather than a hypothesis-testing study, all analyses were descriptive with no statistical comparisons between treatment groups. Missing values were imputed using a last observation carried forward approach. For analyses of mean changes, baseline was defined as the randomization visit. Safety analyses were based on the double-blind safety population, defined as randomized patients who received at least one dose of study treatment. All efficacy analyses were conducted in the intent-to-treat population, defined as all patients in the double-blind safety population who had at least one post-baseline assessment of the primary efficacy parameter.
Results
Patients
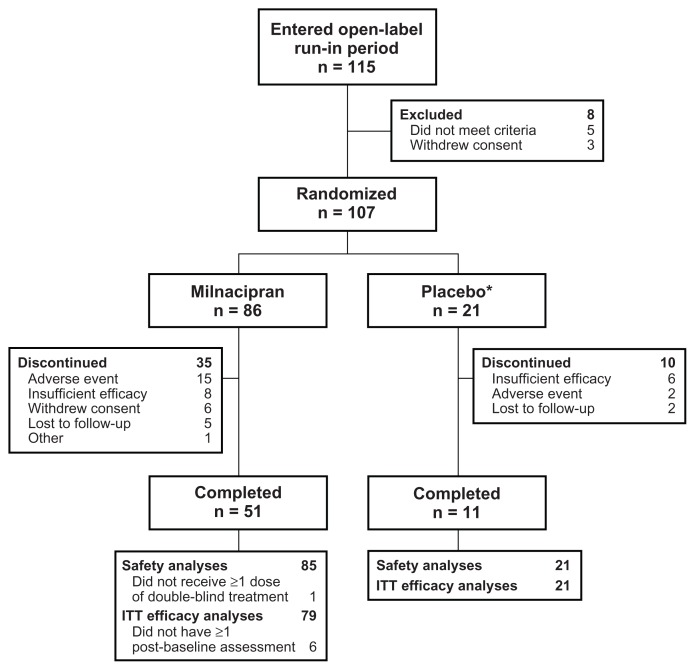
Of the 115 patients who received at least one dose of open-label duloxetine during the 2-week run-in period, 107 met the criteria for inadequate response to duloxetine and were randomized to double-blind treatment (Figure 1). In the group switched to milnacipran, 51/86 (59.3%) patients completed the study. The most common reasons for discontinuation were adverse events for milnacipran (15/86 [17.4%]) and insufficient therapeutic response for placebo (6/21 [28.6%]). In the milnacipran group, one patient who did not receive double-blind treatment was excluded from safety and intent-to-treat efficacy analyses; five patients without post-baseline efficacy assessments were excluded from the intent-to-treat population. All randomized patients in the placebo group were included in the safety and efficacy analyses.
Figure 1.
Study flow.
Note: *Placebo group included for blinding purposes only.
Abbreviation: ITT, intent to treat.
Demographics and baseline characteristics are presented in Table 1. Most patients (>90%) were female; mean age for all intent-to-treat patients was 48.6 years. Patients in this study were generally overweight or obese, as indicated by mean body mass index (>30 kg/m2).27 Mean baseline pain scores (>60 mm) suggest moderate-to-severe pain intensity in both groups.25 In addition, mean baseline EQ-5D index scores were lower than the mean US general population score of 0.87,24 indicating diminished quality of life in these patients.
Table 1.
Demographics and baseline characteristics, intent-to-treat population
| Milnacipran (n = 79) | Placebo (n = 21) | |
|---|---|---|
| Mean age, years (SD) | 48.6 (10.2) | 48.5 (11.3) |
| Female, n (%) | 73 (92.4) | 19 (90.5) |
| Race, n (%) | ||
| White | 71 (89.9) | 20 (95.2) |
| Black/African-American | 7 (8.9) | 0 (0.0) |
| Other | 1 (1.3) | 1 (4.8) |
| Mean BMI, kg/m2 (SD) | 31.8 (6.8) | 30.5 (6.3) |
| Mean scores (SD) at baseline | ||
| VAS pain, 1-week recall, 0–100 | 65.4 (13.2) | 62.2 (12.2) |
| MASQ total, 38–190 | 91.5 (21.0) | 92.8 (20.4) |
| EQ-5D index, −0.11–1 | 0.68 (0.17) | 0.70 (0.18) |
| EQ-5D VAS, 0–100 | 57.4 (18.6) | 48.4 (16.5) |
| FIQR total, 0–100 | 54.7 (16.2) | 53.1 (11.2) |
| ASEX total, 5–30 | 18.7 (5.5) | 20.6 (3.5) |
| Mean BDI total score (SD) at screening, 0–63 | 11.1 (7.0) | 11.1 (7.8) |
Abbreviations: ASEX, Arizona Sexual Experience Scale; BDI, Beck Depression Inventory; BMI, body mass index; EQ-5D, EuroQOL-5D; MASQ, Multiple Ability Self-Report Questionnaire; FIQR, Fibromyalgia Impact Questionnaire-Revised; SD, standard deviation; VAS, visual analog scale.
Efficacy
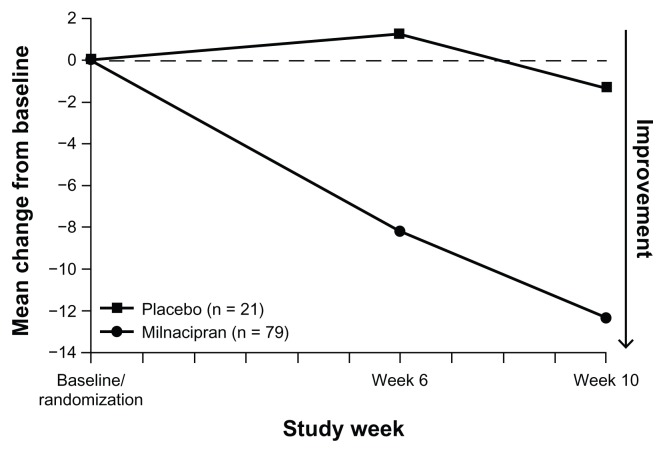
Clinically significant improvements in global status (ie, PGIC score of 1 or 2) were found in 26/79 (32.9%) patients who were switched to milnacipran. In the small group of patients switched to placebo, 5/21 (23.8%) were improved (Table 2); however, as planned, sample sizes were not powered to evaluate between-group differences. At the week 6 and week 10 study visits, mean improvements in VAS pain score were found with milnacipran (−8.2 and −12.3, respectively, Figure 2); mean changes from baseline with placebo were minimal (+1.3 and −1.3 at weeks 6 and 10, respectively). Clinically meaningful improvements in pain (ie, ≥30% decrease from randomization in VAS pain score) were found in 27/29 (34.2%) of milnacipran-treated patients; 20/79 (25.3%) patients reported ≥50% pain improvement. In patients switched to placebo, 6/21 (28.6%) and 3/21 (14.3%) had ≥30% and ≥50% improvements in pain, respectively.
Table 2.
Efficacy outcomes at end of week 10
| Milnacipran (n = 79) | Placebo (n = 21) | |
|---|---|---|
| Responders, n (%) | ||
| PGIC, score ≤ 2 | 26 (32.9) | 5 (23.8) |
| VAS pain, ≥30% improvement | 27 (34.2) | 6 (28.6) |
| VAS pain, ≥40% improvement | 23 (29.1) | 4 (19.0) |
| VAS pain, ≥50% improvement | 20 (25.3) | 3 (14.3) |
| Change from randomization, mean (SEM)a | ||
| VAS pain, 1-week recall | −12.3 (3.07) | −1.3 (4.91) |
| MASQ total | −2.39 (1.66) | 3.23 (2.32) |
| MASQ language ability | −0.02 (0.06) | 0.11 (0.08) |
| MASQ visual-perceptual ability | −0.11 (0.06) | 0.05 (0.10) |
| MASQ verbal memory | −0.09 (0.06) | 0.15 (0.10) |
| MASQ visual memory | −0.07 (0.05) | 0.03 (0.06) |
| MASQ attention | −0.04 (0.06) | 0.08 (0.08) |
| EQ-5D index | 0.01 (0.03) | 0.01 (0.04) |
| EQ-5D VAS | 0.75 (2.57) | 3.05 (6.25) |
| FIQR total | −7.77 (2.35) | −1.38 (3.5) |
| FIQR function | −2.15 (0.77) | −1.43 (1.01) |
| FIQR overall impact | −2.16 (0.69) | −0.52 (1.10) |
| FIQR symptom domain | −3.46 (1.16) | 0.57 (2.00) |
| ASEX total | −0.45 (0.62) | −1.16 (1.00) |
Note:
Negative values indicate improvement for all scales except EQ-5D.
Abbreviations: ASEX, Arizona Sexual Experience Scale; EQ-5D, EuroQOL-5D; MASQ, Multiple Ability Self-Report Questionnaire; FIQR, Fibromyalgia Impact Questionnaire-Revised; PGIC, Patient Global Impression of Change; SEM, standard error of the mean; VAS, visual analog scale.
Figure 2.
Mean change from baseline in VAS pain scores (LOCF).
Note: No statistical comparisons were performed.
Abbreviations: LOCF, last observation carried forward; VAS, visual analog scale.
Mean changes from randomization to week 10 in MASQ, EQ-5D, FIQR, and ASEX suggested improvements in multiple symptom domains among patients switched to milnacipran (Table 2).
Tolerability and safety
Switching from duloxetine to milnacipran was generally well tolerated in patients with fibromyalgia. The most commonly reported treatment-emergent adverse events were nausea, dizziness, headache, and insomnia (Table 3). In patients receiving placebo, the most common treatment-emergent adverse events were nausea and diarrhea. Discontinuations due to adverse events occurred in 15 (17.6%) milnacipran-treated patients; nine of these patients discontinued during the first week after switching from duloxetine to milnacipran. Dizziness (3.5%) was the most common adverse event leading to discontinuation in this group. Two patients switched to placebo discontinued due to an adverse event (one with nausea, one with contact dermatitis). No deaths occurred in the study. Serious adverse events were reported in two patients switched to milnacipran (one with hypersensitivity, one with suicidal ideation), both of whom were discontinued from the study. No serious adverse events were reported in any patient who switched to placebo.
Table 3.
Incidence of treatment-emergent adverse events
| Milnacipran (n = 85) | Placebo (n = 21) | |
|---|---|---|
| Patients with ≥1 TEAE, n (%) | 63 (74.1) | 16 (76.2) |
| Nausea | 18 (21.2) | 6 (28.6) |
| Dizziness | 13 (15.3) | 1 (4.8) |
| Headache | 10 (11.8) | 2 (9.5) |
| Insomnia | 9 (10.6) | 2 (9.5) |
| Hot flushes | 7 (8.2) | 1 (4.8) |
| Irritability | 6 (7.1) | 1 (4.8) |
| Nasopharyngitis | 6 (7.1) | 0 (0.0) |
| Blood pressure increased | 5 (5.9) | 0 (0.0) |
| Anxiety | 5 (5.9) | 0 (0.0) |
| Hyperhidrosis | 5 (5.9) | 0 (0.0) |
| Diarrhea | 4 (4.7) | 3 (14.3) |
| Fatigue | 3 (3.5) | 2 (9.5) |
| Migraine | 1 (1.2) | 2 (9.5) |
| Paresthesia | 1 (1.2) | 2 (9.5) |
| Muscle spasms | 0 (0.0) | 2 (9.5) |
Note: Reported in ≥5% of patients in either treatment group.
Abbreviation: TEAE, treatment-emergent adverse event.
In patients switched to milnacipran, mean changes in vital signs from randomization to end of study, defined as the last available assessment in the double-blind period, were as follows: systolic blood pressure, 1.13 mmHg (placebo 0.39 mmHg); diastolic blood pressure, 2.84 mmHg (placebo −0.08 mmHg); heart rate, 7.42 beats per minute (placebo −2.00 beats per minute); body weight, 0.04 kg (placebo 0.37 kg). Potentially clinically significant changes in these measures were found in three milnacipran-treated patients, including two who had diastolic blood pressure ≥110 mmHg with a ≥10 mmHg increase from baseline and one who had a ≥7% decrease in body weight from baseline.
Mean change from randomization to end of study in Beck Depression Inventory total score was 1.0 (placebo 3.4), indicating no significant worsening of depressive symptoms following the switch from duloxetine to milnacipran.
Discussion
The results from this pilot study suggest that switching between medications within the same drug class may be a useful treatment strategy in some patients with fibromyalgia. This study was conducted in patients who had already been prescribed duloxetine for fibromyalgia by their physicians and had received the recommended dosage of 60 mg/day for 6 weeks or longer prior to randomization (ie, ≥4 weeks before screening and 2 weeks during the open-label, run-in period of this study). Because switching medications is not usually necessary when individuals respond well to treatment, this study was limited to patients who were not experiencing an adequate response to duloxetine, as indicated by moderate or severe pain at randomization (ie, VAS pain score ≥40) and confirmation of patient dissatisfaction (ie, response of “no” to the question “Are you satisfied with duloxetine treatment?”). In the patients who met these criteria, switching to milnacipran appeared to have some benefits. After 10 weeks of milnacipran treatment, approximately one third of patients had clinically meaningful improvements in global status (32.9% with PGIC score ≤2) or pain (34.2% with ≥30% reduction in VAS pain score). In the 21 patients who were randomized to placebo, five patients (23.8%) had improvement in PGIC and six patients (28.6%) had improvement in pain. However, no conclusive statements can be made regarding treatment effects because the small placebo group was designed for blinding purposes only and statistical analyses of between-group differences were neither planned nor conducted.
An important issue raised by this study is whether patients can safely switch from duloxetine to milnacipran without a washout period or down-tapering of duloxetine. The results suggest that a majority of fibromyalgia patients may tolerate a direct switch from duloxetine to milnacipran. Of the 85 milnacipran-treated patients in the safety population, 15 (17.6%) discontinued due to an adverse event, and nine (10.6%) left the study within one week after switching from duloxetine. It is unclear whether these withdrawals were related to stopping duloxetine or starting milnacipran, and it is possible that dose titration of milnacipran and/or tapering duloxetine, rather than a direct switch between medications, may help to alleviate side effects in this subset of patients. No unexpected treatment-emergent adverse events were found, and nausea was the most commonly reported adverse event in both groups (milnacipran, 21.2%; placebo, 28.6%). Nausea, headache, and constipation, which were the three most common adverse events in the pivotal milnacipran studies,18 occurred less frequently in this study than in those previous trials. Moreover, nausea occurred less frequently in patients switched to milnacipran than in those switched to placebo. Although the reasons for these results are unclear, it is possible that prior exposure to duloxetine mitigated some of the nausea and other SNRI-related adverse events in patients switched to milnacipran; it is also possible that withdrawal of duloxetine induced nausea in the placebo group.17 Mean increases in blood pressure and heart rate were detected in patients switching from duloxetine to milnacipran. These changes were similar to results seen when starting milnacipran in other, placebo-controlled studies.18
Although duloxetine and milnacipran are in the same class of drugs, there are a few differences that may affect how patients respond to these medications. While both compounds inhibit serotonin and norepinephrine reuptake, duloxetine is more selective for serotonin reuptake inhibition while milnacipran is more selective for norepinephrine.28 In addition, these drugs have slightly different adverse event profiles.17,18 Although no definitive statements can be made based on these pharmacologic differences, it is possible that they account for the milnacipran treatment response observed in this study.
Although this study provides preliminary information about the potential efficacy and safety of switching medications in patients with fibromyalgia, the findings are limited by the absence of a true placebo control and formal between-group analyses. The small placebo group was designed to reduce the risk of expectation bias rather than to provide for statistical comparisons between milnacipran and placebo, and no hypothesis testing was planned for this study. Nonetheless, the results of this study suggest that switching medications might be a useful treatment strategy in patients with fibromyalgia and that larger and more rigorous clinical studies are warranted.
Conclusion
The results of this exploratory study suggest that fibromyalgia patients with an inadequate response to duloxetine may benefit from switching to milnacipran, although further studies are needed to confirm these findings. Because only three drugs have been approved for the management of fibromyalgia, it is important for clinicians to know that some patients may benefit from this switch and that the approach is safe and tolerable.
Footnotes
Disclosure
LB has received research support and speaker fees from Forest Laboratories, Inc. and Forest Research Institute, Inc. RHP, JMT, and YL are full-time employees of Forest Research Institute, Inc., a wholly owned subsidiary of Forest Laboratories, Inc., and hold stock in the parent company. This study was supported by Forest Laboratories, Inc. The authors thank Allan Spera at Forest Research Institute, Inc. for his contributions to the study and development of this paper. The authors also thank Mildred Bahn at Prescott Medical Communications Group (Chicago, IL, USA) for medical writing assistance supported by Forest Research Institute, Inc.
References
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Wolfe F, Clauw DJ, Fitzcharles MA, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 4.Bennett RM. Clinical manifestations and diagnosis of fibromyalgia. Rheum Dis Clin North Am. 2009;35(2):215–232. doi: 10.1016/j.rdc.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Arnold LM, Crofford LJ, Mease PJ, et al. Patient perspectives on the impact of fibromyalgia. Patient Educ Couns. 2008;73(1):114–120. doi: 10.1016/j.pec.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choy E, Perrot S, Leon T, et al. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv Res. 2010;10:102. doi: 10.1186/1472-6963-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachaine J, Beauchemin C, Landry PA. Clinical and economic characteristics of patients with fibromyalgia syndrome. Clin J Pain. 2010;26(4):284–290. doi: 10.1097/AJP.0b013e3181cf599f. [DOI] [PubMed] [Google Scholar]
- 8.McNett M, Goldenberg D, Schaefer C, et al. Treatment patterns among physician specialties in the management of fibromyalgia: results of a cross-sectional study in the United States. Curr Med Res Opin. 2011;27(3):673–683. doi: 10.1185/03007995.2011.553214. [DOI] [PubMed] [Google Scholar]
- 9.Salaffi F, Sarzi-Puttini P, Girolimetti R, Atzeni F, Gasparini S, Grassi W. Health-related quality of life in fibromyalgia patients: a comparison with rheumatoid arthritis patients and the general population using the SF-36 health survey. Clin Exp Rheumatol. 2009;27(5 Suppl 56):S67–S74. [PubMed] [Google Scholar]
- 10.Ovayolu N, Ovayolu O, Karadag G. Health-related quality of life in ankylosing spondylitis, fibromyalgia syndrome, and rheumatoid arthritis: a comparison with a selected sample of healthy individuals. Clin Rheumatol. 2011;30(5):655–664. doi: 10.1007/s10067-010-1604-2. [DOI] [PubMed] [Google Scholar]
- 11.Campos RP, Vazquez Rodriguez MI. Health-related quality of life in women with fibromyalgia: clinical and psychological factors associated. Clin Rheumatol. 2012;31(2):347–355. doi: 10.1007/s10067-011-1870-7. [DOI] [PubMed] [Google Scholar]
- 12.Winkelmann A, Perrot S, Schaefer C, et al. Impact of fibromyalgia severity on health economic costs: results from a European cross-sectional study. Appl Health Econ Health Policy. 2011;9(2):125–136. doi: 10.2165/11535250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 13.Chandran A, Schaefer C, Ryan K, Baik R, McNett M, Zlateva G. The comparative economic burden of mild, moderate, and severe fibromyalgia: results from a retrospective chart review and cross-sectional survey of working-age US adults. J Manag Care Pharm. 2012;18(6):415–426. doi: 10.18553/jmcp.2012.18.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arnold LM, Clauw DJ, Dunegan LJ, Turk DC. A framework for fibromyalgia management for primary care providers. Mayo Clin Proc. 2012;87(5):488–496. doi: 10.1016/j.mayocp.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clauw DJ. Pain management: Fibromyalgia drugs are ‘as good as it gets’ in chronic pain. Nat Rev Rheumatol. 2010;6(8):439–440. doi: 10.1038/nrrheum.2010.120. [DOI] [PubMed] [Google Scholar]
- 16.Preskorn SH, Lacey RL. Polypharmacy: when is it rational? J Psychiatr Pract. 2007;13(2):97–105. doi: 10.1097/01.pra.0000265766.25495.3b. [DOI] [PubMed] [Google Scholar]
- 17.Cymbalta (duloxetine), prescribing information. Indianapolis, IN: Eli Lilly and Company; Nov, 2012. [Google Scholar]
- 18.Savella (milnacipran), prescribing information. St Louis MO: Forest Laboratories Inc; Oct, 2012. [Google Scholar]
- 19.Thase ME, Rush AJ, Howland RH, et al. Double-blind switch study of imipramine or sertraline treatment of antidepressant-resistant chronic depression. Arch Gen Psychiatry. 2002;59(3):233–239. doi: 10.1001/archpsyc.59.3.233. [DOI] [PubMed] [Google Scholar]
- 20.Denys D, van Megen HJ, van der Wee N, Westenberg HG. A double-blind switch study of paroxetine and venlafaxine in obsessive-compulsive disorder. J Clin Psychiatry. 2004;65(1):37–43. doi: 10.4088/jcp.v65n0106. [DOI] [PubMed] [Google Scholar]
- 21.Perahia DG, Quail D, Desaiah D, Corruble E, Fava M. Switching to duloxetine from selective serotonin reuptake inhibitor antidepressants: a multicenter trial comparing 2 switching techniques. J Clin Psychiatry. 2008;69(1):95–105. doi: 10.4088/jcp.v69n0113. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 23.Seidenberg M, Haltiner A, Taylor MA, Hermann BB, Wyler A. Development and validation of a Multiple Ability Self-Report Questionnaire. J Clin Exp Neuropsychol. 1994;16(1):93–104. doi: 10.1080/01688639408402620. [DOI] [PubMed] [Google Scholar]
- 24.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult US population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43(11):1078–1086. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 25.Bennett RM, Schein J, Kosinski MR, Hewitt DJ, Jordan DM, Rosenthal NR. Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum. 2005;53(4):519–527. doi: 10.1002/art.21319. [DOI] [PubMed] [Google Scholar]
- 26.McGahuey CA, Gelenberg AJ, Laukes CA, et al. The Arizona Sexual Experience Scale (ASEX): reliability and validity. J Sex Marital Ther. 2000;26(1):25–40. doi: 10.1080/009262300278623. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. Obesity and overweight. Fact sheet No 311. [Accessed March 15, 2013]. updated Mar 2011. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- 28.Vaishnavi SN, Nemeroff CB, Plott SJ, Rao SG, Kranzler J, Owens MJ. Milnacipran: a comparative analysis of human monoamine uptake and transporter binding affinity. Biol Psychiatry. 2004;55(3):320–322. doi: 10.1016/j.biopsych.2003.07.006. [DOI] [PubMed] [Google Scholar]