Abstract
Objective
In 2009 the U.S. Food and Drug Administration issued a warning regarding suicidality and antiepileptic drugs based on meta-analyses of 199 randomized trials (over 43,000 subjects with different illnesses) of 11 antiepileptics. The present study examines the hypothesis that the three antiepileptics approved for bipolar disorder (carbamazepine, lamotrigine, and valproate) are associated with an elevated risk of suicide attempts and suicides.
Method
A prospective observational study was conducted at five U.S. academic medical centers from 1978 to 2009. Analyses included 199 participants with bipolar disorder for whom 1,077 time intervals were classified as either exposed to an antiepileptic (carbamazepine, lamotrigine, or valproate) or not exposed to an antiepileptic, an antidepressant, or lithium during 30 years of follow-up.
Results
Participants who had more severe manic symptoms were more likely to receive antiepileptic drugs. Mixed-effects grouped-time survival models revealed no elevation in risk of suicide attempt or suicide during periods when participants were receiving antiepileptics relative to periods when they were not (hazard ratio= 0.93, 95% CI=0.45–1.92), controlling for demographic and clinical variables through propensity score matching.
Conclusions
In this longitudinal observational study, the risk of suicide attempts or suicides was not associated with the antiepileptics approved for bipolar disorder.
Antiepileptic drugs are approved for the treatment of epilepsy, bipolar disorder, and neuropathic pain. Each of these conditions is associated with an elevated risk of suicide (1–5). On January 31, 2008, the U.S. Food and Drug Administration (FDA) issued an alert to warn prescribers of an elevated risk of suicidality (suicidal behavior or ideation) associated with antiepileptic drugs relative to risk with placebo (6).
This warning was based on an FDA examination of data from 199 randomized clinical trials of 11 antiepileptic medications (carbamazepine, divalproex, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, pregabalin, tiagabine, topiramate, and zonisamide). The trials included 43,892 participants with epilepsy, psychiatric disorders, or other conditions (20 indications in total) randomly assigned to receive antiepileptic drugs or placebo (7). The risk of suicidal behavior or ideation was significantly elevated in patients who received an antiepileptic compared with those who received placebo (0.37% compared with 0.24%; odds ratio=1.87, 95% confidence interval [CI]=1.24–2.66; number needed to harm=769) when no adjustments were made for trial differences. The increased risk was observed as early as 1 week after starting the antiepileptic drug and continued through 24 weeks. Results were generally consistent among the 11 drugs. Additional analyses showed that participants treated with antiepileptics for epilepsy, psychiatric disorders, or other conditions were each at greater risk compared with those who received placebo. Two-thirds (67.6%) of the observed suicidality was suicidal ideation. Analyses that focused on suicidal behavior found that patients in the antiepileptic arm (132.8/100,000) had a significantly elevated risk, nearly three times that of patients in the placebo arm (56.1/100,000; odds ratio=2.92, 95% CI=1.44–6.47; number needed to harm=1,304). There were four suicide deaths, all in participants in active treatment arms.
Based on these results, the antiepileptic drug class warning issued by the FDA states, “All patients who are currently taking or starting on any antiepileptic drug for any indication should be monitored for notable changes in behavior that could indicate the emergence or worsening of suicidal thoughts or behavior or depression” (8). In contrast to FDAactions on antidepressants, this was not a boxed warning (9, 10).
Our objective in this study was to examine the risk, in patients with bipolar disorder, of suicide attempts or suicide deaths associated with antiepileptics that have FDA approval for the treatment of bipolar disorder. The study participants were part of the National Institute of Mental Health Collaborative Program on the Psychobiology of Depression–Clinical Studies (Collaborative Depression Study), a longitudinal observational study that began collecting prospective follow-up data in 1978 (11). The methodological strengths of this study include frequent, direct participant interviews with standardized instruments and up to 30 years of follow-up. The study sample included patients who would typically be excluded from randomized controlled trials, such as asymptomatic and mildly symptomatic patients and suicidal or psychotic patients, and thus the study findings may better generalize to the majority of individuals with bipolar disorder than do findings from randomized controlled trials. In aggregate, the Collaborative Depression Study offers a rare opportunity to examine the risk associated with antiepileptics in the treatment of bipolar disorder. Based on the FDA findings, we hypothesized that suicide attempts and suicides would be elevated during periods when participants received an antiepileptic drug approved for bipolar disorder compared with periods when they did not.
Method
Participants
The Collaborative Depression Study was conducted at five academic medical centers (in Boston, Chicago, Iowa City, New York, and St. Louis). It recruited patients who were treated for depressive disorders, bipolar disorder, or schizoaffective disorder from 1978 through 1981 (11). All participants provided written informed consent. At intake, participants had to be at least 17 years of age, English speaking, and white (genetic hypotheses were tested). The study protocol was approved by the institutional review boards of all sites.
No antiepileptic drugs were used in the Collaborative Depression Study until week 105. Therefore, the analyses described here exclude data from the first 2 years of follow-up. The resulting study sample involved 199 participants who, at intake or during follow-up, met Research Diagnostic Criteria (RDC) (12) for bipolar I disorder or schizoaffective mania, mainly affective subtype. The RDC for schizoaffective mania, mainly affective subtype, are similar to DSM-IV criteria for bipolar I disorder. The inclusion of patients with schizoaffective disorder in this study is consistent with other longitudinal studies of bipolar I disorder (13–15).
Assessments
RDC diagnoses were based on Schedule for Affective Disorders and Schizophrenia interviews (16) and medical records. The date, method, and medical severity of suicide attempts and deaths were systematically recorded. Information about antiepileptic treatment was collected from Longitudinal Interval Follow-Up Evaluation interviews (17) and medical records. These interviews also recorded the dose and duration of somatic treatment, level of functional impairment, and level of psychopathology. The severity of symptoms was recorded using psychiatric status ratings, which for major affective disorders range from 1 (not present) to 6 (definite criteria, severe symptoms). For minor depression and hypomania, ratings range from 1 (no symptoms) to 3 (definite criteria). Raters assigned psychiatric status ratings for each week since the previous interview. To assist the participant in recalling when significant clinical improvement or deterioration took place, raters identified chronological anchor points (e.g., holidays). After each interview, raters also wrote a narrative description. Available clinical records and informants were used to corroborate ratings.
The Longitudinal Interval Follow-Up Evaluation was administered by supervised, trained, and certified raters semiannually during the first 5 years of follow-up and annually thereafter. The interrater reliability for this instrument is very good, with intraclass correlation coefficients of 0.92 for rating changes in symptoms, 0.88 for reappearance of symptoms, and 0.95 for recovery from mood episodes (17).
Classification of Antiepileptic Exposure
Each week of follow-up was classified as either “exposed to an antiepileptic” or “unexposed” based on the medications the patient received that week. If the participant received carbamazepine, lamotrigine, or valproate (all of which have demonstrated efficacy and are FDA approved for bipolar disorder) and did not receive an antidepressant or lithium, that week was classified as exposed; patients receiving the latter agents were excluded because of the boxed warning for suicidality for antidepressants (10) and the protective effect of lithium (18, 19). Weeks were classified as unexposed if the participant did not receive any of the three antiepileptic drugs approved for bipolar disorder, any of four antiepileptic drugs that have not been demonstrated as efficacious in bipolar disorder (gabapentin, nimodipine, verapamil, or topiramate), lithium, or any antidepressant. Neither the dosage of antiepileptic drug nor use of any other medications had any bearing on classification of weekly exposure.
In this study, the unit of analysis is not the participant but the “antiepileptic exposure interval,” defined as a period of consecutive weeks during which antiepileptic exposure classification remained unchanged. Any interval that did not meet criteria for exposed or unexposed intervals was excluded from all analyses. A switch from any of the three antiepileptics to another did not initiate a new exposure interval, but instead constituted a continuation of the current interval. Exposure intervals were examined in survival analyses of time to suicidal behavior (suicide attempt or suicide death). Each antiepileptic exposure interval ended in one of three ways: a suicide attempt or suicide death; a change in antiepileptic exposure status (exposure versus no exposure); or end of follow-up. New exposure intervals began in the week following a suicide attempt or in the week when antiepileptic exposure status changed. Over the course of 30 years of follow-up, most participants had several periods of antiepileptic exposure and other periods of no exposure.
Statistical Analysis
Analyses were conducted in two stages: 1) a model of propensity for antiepileptic exposure and 2) a model of treatment safety, focusing exclusively on suicidal behavior, which compared the rates during antiepileptic-exposed intervals and unexposed intervals. The unit of analysis in both the propensity and safety models was the antiepileptic exposure interval. The longitudinal approach to analyses accounted for the variability in the duration of treatment and multiple correlated within-participant exposure intervals, and it allowed for within-participant variation in propensity scores and exposure status over time. A two-tailed alpha level of 0.05 was used for each statistical test.
Primary analyses: safety models
A mixed-effects grouped-time survival model (20) with a complementary log-log link examined the number of weeks from the start of an antiepileptic exposure interval until suicidal behavior. Time until suicidal behavior is the survival time, which represents the number of consecutive weeks during which treatment remained at the initial status (either exposure or no exposure to an antiepileptic drug). A new antiepileptic exposure interval (i.e., survival interval) began with each change in antiepileptic exposure status. To correspond with each new period of risk, a new exposure interval commenced the week after each suicide attempt. Survival intervals were classified as censored if they terminated either with a change in antiepileptic exposure status or with the end of follow-up. The survival model assumed the conditional independence of the censoring mechanism and suicidal behavior, given other variables included in the model (propensity score, prior treatment exposure status, and prior observations of suicidality).
The safety analyses were conducted in such a way that exposed and unexposed intervals were matched based on the propensity score (21). Full matching, as opposed to pairwise matching, was used such that each matched set included at least one exposed and one unexposed interval, but the number need not be identical. Furthermore, an “optimal” matching procedure (as opposed to “greedy matching”) was used to minimize the total propensity score difference within each matched set (22–24). (Our previous implementation of the propensity adjustment involved propensity score quintile stratification [25–27]. Here, because of sparse strata, matching was used.) Our criteria required that, within a matched set, propensity scores could differ by no more than 0.10 propensity score standard deviation units. This was implemented with the OptMatch package, version 0.7–1 (28, 29) for R, version 2.12.2. This distance criterion is referred to as a caliper. Sensitivity analyses examined results with a caliper of 0.40. The safety model, conducted using Proc GLIMMIX in SAS, version 9.2 (SAS Institute, Cary, N.C.), included antiepileptic exposure as a binary fixed effect and two crossed random effects—the participant-specific and matched-set intercepts. It is this latter term that accounted for the variables comprising the propensity score, as described below. By virtue of including random intercepts, but not a random slope, compound symmetry was assumed (30).
Propensity for antiepileptic exposure
Given that treatment assignment was not determined by randomization but rather by self-selection and clinician decision, it is conceivable that antiepileptics were initiated for the more symptomatic participants. As a consequence, an unadjusted comparison of suicidal behavior in the exposed and unexposed intervals could be influenced by pretreatment confounding variables. For that reason, the propensity score was used as an adjustment for comparisons of exposure intervals. The propensity score represents the conditional probability of exposure to antiepileptics, given the covariates included in the score (21).
The analyses of propensity for antiepileptic exposure model involved a mixed-effects logistic regression model that examined the association of the binary dependent variable (receiving carbamazepine, lamotrigine, or valproate) with clinical and demographic characteristics. The model included a participant-specific intercept as a random effect. Fixed effects included variables that, based on our earlier studies (25–27), we hypothesized to be associated with receiving treatment. These included both time-invariant variables—gender, marital status, socioeconomic status, and study site—and time-varying variables, each assessed prior to the corresponding exposure interval—age at start of the exposure interval, suicide attempt from study intake to the start of the exposure interval, severity of manic or schizoaffective manic symptoms (mean psychiatric status ratings in the previous 8 weeks), severity of hypomanic symptoms (mean psychiatric status ratings in the previous 8 weeks), use of antipsychotics prior to exposure interval, and cumulative morbidity (percent of prior Collaborative Depression Study follow-up time in episode).
The propensity score, derived as a linear combination of variables, represents the probability of antiepileptic exposure, ranging from zero to unity. A propensity score close to zero represents an exposure interval with features not associated with exposure, whereas a score close to 1 denotes an interval with characteristics associated with antiepileptic exposure. A participant’s propensity for antiepileptics could vary over the follow-up period because the propensity score algorithm included several time-varying variables. The application of the propensity adjustment with repeated survival data has been shown to reduce bias with observational data (27). SuperMix was used to analyze the propensity models (31).
Results
Study Sample
Of the 199 study participants, 122 (61.3%) were women. The mean age at intake into the Collaborative Depression Study was 36.7 years (SD=12.9). Additional demographic and clinical characteristics of the sample are presented in Table 1. Follow-up time ranged from 3 years to 30 years, with a median of 24 years (mean=21.0, SD=8.2).
Table 1.
Demographic and Clinical Characteristics of Patients with Bipolar Disorder at Intake into the Collaborative Depression Study (N=199)
| Characteristic | N | % |
|---|---|---|
| Gender | ||
| Female | 122 | 61.3 |
| Male | 77 | 38.7 |
| Marital status | ||
| Never married | 70 | 35.2 |
| Married | 77 | 38.7 |
| Divorced, separated, or widowed | 52 | 26.1 |
| Hollingshead socioeconomic status scalea | ||
| I | 4 | 2.0 |
| II | 28 | 14.1 |
| III | 68 | 34.2 |
| IV | 56 | 28.1 |
| V | 43 | 21.6 |
| Intake site | ||
| New York | 31 | 15.6 |
| St. Louis | 41 | 20.6 |
| Boston | 29 | 14.6 |
| Iowa City | 56 | 28.1 |
| Chicago | 42 | 21.1 |
| Intake status | ||
| Inpatient | 178 | 89.4 |
| Outpatient | 21 | 10.6 |
| Major depressive episodes before study intake | ||
| 0 | 43 | 21.6 |
| 1 | 32 | 16.1 |
| 2 | 30 | 15.1 |
| 3 | 19 | 9.5 |
| 4 | 17 | 8.5 |
| 5 or more | 58 | 29.1 |
| Manic episodes prior to study intake | ||
| 0 | 93 | 46.7 |
| 1 | 30 | 15.1 |
| 2 | 21 | 10.6 |
| 3 or more | 55 | 27.6 |
| Mean | SD | |
| Global Assessment Scale score | 32.2 | 11.1 |
| Hamilton Depression Rating Scaleb | 25.5 | 8.2 |
| Age (years) | 36.7 | 12.9 |
| Duration of follow-up (years) | 21.0 | 8.2 |
The scale ranges from 1 (higher status) to V (lower status).
17-item version, extracted from the Schedule for Affective Disorders and Schizophrenia; see ref #32
Antiepileptic Exposure
The analyses included 199 participants with 1,077 exposure intervals over the course of follow-up. It is likely that many of the intervals would not have met criteria for inclusion in the acute randomized clinical trials reviewed by the FDA. For example, 8.9% of participants made a suicide attempt in the 3 months preceding the exposure interval. Additionally, based on the psychiatric status ratings at exposure interval commencement, 33.1% were at most mildly symptomatic and 15% had psychosis or extreme functional impairment.
Most of the intervals did not involve antiepileptic drug exposure, as defined for our analyses; 216 were exposed (20.1%) and 861 were unexposed (79.9%). On average, exposed participants spent 35.6% of their follow-up time on antiepileptics (median=24.3; SD=22.5; range=0.2–92.1). The participants’ mania severity at the commencement of exposed intervals was significantly higher than for the unexposed intervals (psychiatric status ratings for exposed intervals, mean=2.6, SD=1.12; for unexposed intervals, mean=1.9, SD=1.08; z=5.51, p<0.001). Severity of hypomania did not differ across groups (psychiatric status ratings for exposed intervals, mean=1.1, SD=0.46; for unexposed intervals, mean=1.1, SD=0.37; z=1.78, p=0.075). Overall, 60.6% of the exposed intervals began when the participant was in a mood episode, compared with 47.2% of the unexposed intervals (z=2.85, p=0.004).
Of the 216 exposed intervals, 113 involved carbamazepine (53.3%), 24 involved lamotrigine (11.1%), and 101 involved valproate (46.8%). (The sum of exposed intervals for each medication exceeds the total number of exposed intervals because there was concomitant use of these antiepileptics.)
Propensity for Antiepileptic Exposure
The propensity model (Table 2) shows that participants with more severe manic symptoms (odds ratio=3.83, 95% CI=2.39–6.14; z=5.59, p<0.001) or hypomanic symptoms (odds ratio=5.93, 95% CI=2.09–16.86; z=3.34, p<0.001) were significantly more likely to receive antiepileptics. Those who were treated with antipsychotics were significantly less likely to receive antiepileptics (odds ratio=0.50, 95% CI=0.32–0.79; z=−2.97, p=0.003). There were also significant omnibus effects of age, social class, and site, but not marital status.
Table 2.
Model of Propensity for Exposure to Any of the Three Antieplileptic Drugs for Patients with Bipolar Disorder in the Collaborative Depression Studya
| Variable | Odds Ratio | 95% CI | z | p |
|---|---|---|---|---|
| Hollingshead socioeconomic status scaleb | ||||
| I or II | 1.00 | |||
| III | 1.52 | 0.62–3.75 | 0.91 | 0.364 |
| IV | 0.64 | 0.23–1.79 | −0.85 | 0.394 |
| V | 0.36 | 0.13–1.02 | −1.92 | 0.055 |
| Marital status | ||||
| Married | 1.00 | |||
| Never married | 2.25 | 1.04–4.84 | 2.07 | 0.038 |
| Divorced, separated, or widowed | 0.97 | 0.44–2.14 | −0.08 | 0.934 |
| Site | ||||
| New York | 1.00 | |||
| St. Louis | 2.94 | 0.94–9.16 | 1.86 | 0.063 |
| Boston | 1.67 | 0.43–6.53 | 0.74 | 0.459 |
| Iowa City | 5.93 | 1.85–18.95 | 3.00 | 0.003 |
| Chicago | 3.28 | 1.08–9.97 | 2.10 | 0.036 |
| Gender | ||||
| Female | 1.00 | |||
| Male | 1.72 | 0.87–3.40 | 1.55 | 0.121 |
| Severity of mania | 3.83 | 2.39–6.14 | 5.59 | <0.001 |
| Severity of hypomania | 5.93 | 2.09–16.86 | 3.34 | 0.001 |
| Antipsychotics in week prior to exposure interval | 0.50 | 0.32–0.79 | −2.97 | 0.003 |
| Suicide attempt between intake and start of exposure interval | 1.66 | 0.93–2.95 | 1.71 | 0.086 |
| Age | 1.06 | 1.03–1.08 | 4.68 | <0.001 |
| Cumulative morbidity | 1.01 | 1.00–1.02 | 1.61 | 0.108 |
The antiepileptics included in the analysis were carbamazepine, lamotrigine, and valproate. Analyses included 199 participants with 1,077 exposure intervals.
The scale ranges from I (higher status) to V (lower status).
Primary Results: Safety Analyses
Unadjusted rates of suicidal behavior were 6.3% among 847 unexposed intervals (52 attempts [6.1%] and one suicide [0.1%]) and 5.1% among 216 exposed intervals (nine attempts [4.2%] and two suicides [0.9%]), as depicted in Figure 1. The unadjusted number needed to harm was 86, favoring antiepileptic drugs. The drug-specific rates of suicidal behavior were 3.5% for carbamazepine (4/113), 12.5% for lamotrigine (3/24), and 5.0% for valproate (4/101).
FIGURE 1. Rates of Suicide Attempts and Suicides for Anti-epileptic Drug-Exposed and Unexposed Intervalsa.
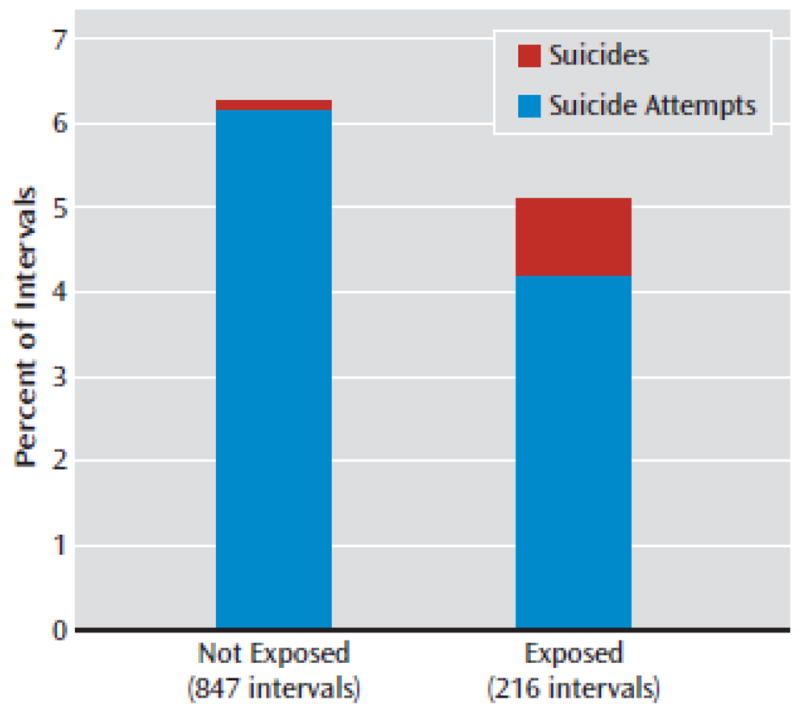
aThe antiepileptics included in the analysis were carbamazepine, lamotrigine, and valproate. Propensity-adjusted hazard ratio=0.93 (95% Cl=0.45–1.92).
Of the 1,077 exposed and unexposed intervals, 852 (79.1%) were matched based on the caliper of 0.10 propensity score standard deviation units and thus were included in the safety model. The rates of suicidal behavior in this matched set (6.4% unexposed, 5.2% exposed) are similar to those in the larger study sample. The risk of suicidal behavior was not significantly elevated among participants exposed to antiepileptics (hazard ratio=0.93, 95% CI=0.45–1.92; z=−0.20, p=0.814), controlling for variables in the propensity score through matching. Sensitivity analyses were conducted to examine results with a caliper of 0.40, in which 1,063 of 1,077 (98.7%) intervals were matched. Once again there was a nonsignificant association (hazard ratio=0.87, 95% CI=0.42–1.79; z=−0.38, p=0.707).
In 600 (70.8%) of the 847 unexposed intervals (again, defined as an interval with no antiepileptic, no antidepressant, and no lithium), the participants had no contemporaneous exposure to other mood-stabilizing treatments (such as antipsychotics or ECT). For intervals classified as unexposed, higher rates of suicidal behavior were observed among participants receiving other mood stabilizers (7.7%) compared with those not receiving mood stabilizers (5.7%), but this difference was nonsignificant (odds ratio=1.38, 95% CI=0.66–2.87; z=0.851, p=0.395).
Discussion
In this study we hypothesized that there would be an elevated risk of suicide attempts and suicide deaths among participants with bipolar disorder when they received an antiepileptic drug compared with periods when they did not. Our hypothesis was based on meta-analyses conducted by the FDA with data from 199 placebo-controlled clinical trials of antiepileptic drugs for epilepsy, pain, bipolar disorder, and a variety of other neurological and psychiatric indications. We found no significant elevation in risk of suicide attempts or suicides associated with the use of three antiepileptic drugs in a longitudinal observational study of participants with bipolar disorder. We emphasize that we conducted analyses consistent with a superiority design and therefore do not purport to conclude that there is equivalence or noninferiority in risk of suicidality for antiepileptic drugs relative to no antiepileptic treatment (33).
Although it may appear so on the surface, our findings do not directly conflict with those supporting the FDA warning, which were based on trials for 20 different indications, of which bipolar disorder was only one. Our study was limited to participants with bipolar disorder. The comparator was not placebo but intervals during which antiepileptics, lithium, and antidepressants were not used. Furthermore, the majority of the suicidality in the FDA analyses was based on suicidal ideation, whereas we focused entirely on suicide attempts and suicides. It has been shown that only a small percentage of patients who report suicidal ideation eventually commit suicide (34). Finally, the FDA data came from relatively short-term clinical trials, whereas our analyses included data from up to 30 years of follow-up. Any of these factors may account for the difference in conclusions.
Our results are generally consistent with large observational pharmacoepidemiological studies. Studies in which samples were limited to bipolar disorder or in which relevant subgroup analyses were performed for bipolar disorder have not demonstrated a greater risk of suicide attempts or completions when antiepileptic medications are used compared with when they are not used (35–37). Studies using patients taking lithium as a comparison group have demonstrated greater risk with exposure to antiepileptic medications, although not consistently (18, 19, 35). With some data supporting a benefit of lithium on suicide risk, lithium may not be a relevant comparison for the question of whether antiepileptic medications convey risk. Pharmacoepidemiological studies using claims data may underreport suicide attempts. Ascertainment of suicide attempts in our prospective cohort did not rely on the documentation of suicide attempts from billing codes but instead was made in direct, routine assessments. Confounding by indication can be a challenge in observational studies. Our use of a propensity score that incorporates the severity of affective symptoms at the time antiepileptics were initiated adds to the existing literature, which primarily captures severity based on comorbidity. The convergent findings of our analysis with the existing pharmacoepidemiological literature are also supported by the results of a recent meta-analysis that found no elevation in the risk of suicidal ideation or behavior associated with divalproex sodium in 14 controlled clinical studies (37).
There are several limitations to the observational study design we used. First, participants were not randomly assigned to treatment, and therefore baseline imbalance might account, in part, for the findings. For that reason, our analyses used a matching procedure based on the propensity score to account for differences among exposure intervals. The propensity score included a wide array of possible confounding demographic and clinical variables. The analyses estimated safety risk after controlling for influences of observed covariates. We acknowledge that the propensity adjustment assumes that all confounding variables are included in the analyses, and propensity model misspecification could result in biased estimates (38, 39). Second, the exposure intervals varied widely in length. For that reason, we used survival analyses of the time until suicidal behavior. As noted, we assumed conditional independence of censoring mechanism and suicidal behavior, given other variables included in the model. Third, we did not attempt to distinguish the risk among the three antiepileptics. Instead we examined a class risk, attempting to mirror the strategy used in the FDA meta-analyses.
Conclusions
Our analyses lend no empirical support to the conjecture that antiepileptic drugs elevate the risk of suicidal behavior in patients with bipolar disorder. Nevertheless, patients treated with anticonvulsants should be regularly monitored by their clinician, as with any bipolar patient in treatment, with emphasis on mood episode symptoms. Therapeutic options should be discussed with the patient and family members, and suicidal ideation and behavior must be carefully monitored before and after the commencement of treatment.
References
- 1.Christensen J, Vestergaard M, Mortensen PB, Sidenius P, Agerbo E. Epilepsy and risk of suicide: a population-based case-control study. Lancet Neurol. 2007;6:693–698. doi: 10.1016/S1474-4422(07)70175-8. [DOI] [PubMed] [Google Scholar]
- 2.Nilsson L, Ahlbom A, Farahmand BY, Asberg M, Tomson T. Risk factors in suicide in epilepsy: a case-control study. Epilepsia. 2002;43:644–651. doi: 10.1046/j.1528-1157.2002.40001.x. [DOI] [PubMed] [Google Scholar]
- 3.Ratcliffe GE, Enns MW, Belik SL, Sareen J. Chronic pain conditions and suicidal ideation and suicide attempts: an epidemiologic perspective. Clin J Pain. 2008;24:204–210. doi: 10.1097/AJP.0b013e31815ca2a3. [DOI] [PubMed] [Google Scholar]
- 4.Tsai SY, Kuo CJ, Chen CC, Lee HC. Risk factors for completed suicide in bipolar disorder. J Clin Psychiatry. 2002;63:469–476. doi: 10.4088/jcp.v63n0602. [DOI] [PubMed] [Google Scholar]
- 5.Simon GE, Hunkeler E, Fireman B, Lee JY, Savarino J. Risk of suicide attempt and suicide death in patients treated for bipolar disorder. Bipolar Disord. 2007;9:526–530. doi: 10.1111/j.1399-5618.2007.00408.x. [DOI] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration. FDA Alerts Health Care Providers to Risk of Suicidal Thoughts and Behavior With Antiepileptic Medications. 2008 Jan 31; http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsand-Providers/ucm100200.htm.
- 7.US Food and Drug Administration. Statistical Review and Evaluation: Antiepileptic Drugs and Suicidality. 2008 May 23; http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4372b1-01-FDA.pdf.
- 8.US Food and Drug Administration. Antiepileptic Drugs and Suicidality. 2009 May 5; http://www.fda.gov/CDER/Drug/infopage/antiepileptics/default.htm.
- 9.US Food and Drug Administration. Labeling Change Request Letter for Antidepressant Medications. 2004 http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/ucm096352.htm.
- 10.US Food and Drug Administration. Labeling Template for Antidepressant Medications. 2005 http://www.fda.gov/cder/drug/antidepressants/default.htm.
- 11.Katz MM, Klerman GL. Introduction: overview of the clinical studies program (NIMH Clinical Research Branch Collaborative Study on Psychobiology of Depression) Am J Psychiatry. 1979;136:49–51. doi: 10.1176/ajp.136.1.49. [DOI] [PubMed] [Google Scholar]
- 12.Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria: rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 13.Angst J, Gamma A, Sellaro R, Lavori PW, Zhang H. Recurrence of bipolar disorders and major depression: a life-long perspective. Eur Arch Psychiatry Clin Neurosci. 2003;253:236–240. doi: 10.1007/s00406-003-0437-2. [DOI] [PubMed] [Google Scholar]
- 14.Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, Lavori P, Lebowitz B, Rudorfer M, Frank E, Nierenberg AA, Fava M, Bowden C, Ketter T, Marangell L, Calabrese J, Kupfer D, Rosenbaum JF. Rationale, design, and methods of the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Biol Psychiatry. 2003;53:1028–1042. doi: 10.1016/s0006-3223(03)00165-3. [DOI] [PubMed] [Google Scholar]
- 15.Nolen WA, Luckenbaugh DA, Altshuler LL, Suppes T, McElroy SL, Frye MA, Kupka RW, Keck PE, Jr, Leverich GS, Post RM. Correlates of 1-year prospective outcome in bipolar disorder: results from the Stanley Foundation Bipolar Network. Am J Psychiatry. 2004;161:1447–1454. doi: 10.1176/appi.ajp.161.8.1447. [DOI] [PubMed] [Google Scholar]
- 16.Endicott J, Spitzer RL. A diagnostic interview: the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 17.Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald- Scott P, Andreasen NC. The Longitudinal Interval Follow-Up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44:540–548. doi: 10.1001/archpsyc.1987.01800180050009. [DOI] [PubMed] [Google Scholar]
- 18.Goodwin FK, Fireman B, Simon GE, Hunkeler EM, Lee J, Revicki D. Suicide risk in bipolar disorder during treatment with lithium and divalproex. JAMA. 2003;290:1467–1473. doi: 10.1001/jama.290.11.1467. [DOI] [PubMed] [Google Scholar]
- 19.Collins JC, McFarland BH. Divalproex, lithium, and suicide among Medicaid patients with bipolar disorder. J Affect Disord. 2008;107:23–28. doi: 10.1016/j.jad.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 20.Hedeker D, Siddiqui O, Hu FB. Random-effects regression analysis of correlated grouped-time survival data. Stat Methods Med Res. 2000;9:161–179. doi: 10.1177/096228020000900206. [DOI] [PubMed] [Google Scholar]
- 21.Rosenbaum P, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 22.Gu X, Rosenbaum PR. Comparison of multivariate matching methods: structures, distances, and algorithms. J Comput Graph Stat. 1993;2:405–420. [Google Scholar]
- 23.Hansen BB. Full matching in an observational study of coaching for the SAT. J Am Stat Assoc. 2004;99:609–618. [Google Scholar]
- 24.Hansen BB, Klopfer SO. Technical Report 416. Department of Statistics, University of Michigan; Ann Arbor: 2005. Optimal Full Matching and Related Designs Via Network Flows. [Google Scholar]
- 25.Leon AC, Solomon DA, Mueller TI, Endicott J, Rice JP, Maser JD, Coryell W, Keller MB. A 20-year longitudinal observational study of somatic antidepressant treatment effectiveness. Am J Psychiatry. 2003;160:727–733. doi: 10.1176/appi.ajp.160.4.727. [DOI] [PubMed] [Google Scholar]
- 26.Leon AC, Solomon DA, Li C, Fiedorowicz JG, Coryell WH, Endicott J, Keller MB. Antidepressants and risks of suicide and suicide attempts: a 27-year observational study. J Clin Psychiatry. 2011;72:580–586. doi: 10.4088/JCP.10m06552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leon AC, Hedeker D, Teres JJ. Bias reduction in effectiveness analyses of longitudinal ordinal doses with a mixed-effects propensity adjustment. Stat Med. 2007;26:110–123. doi: 10.1002/sim.2458. [DOI] [PubMed] [Google Scholar]
- 28.Kleyman Y, Hansen B. Basic Uses of the OptMatch Package. 2005 http://www.stat.lsa.umich.edu/~bbh/optmatch/doc/optmatch.pdf.
- 29.Hansen BB, Fredrickson M. OptMatch package, version 0.7–1. http://cran.r-project.org/web/packages/optmatch/index.html.
- 30.Hedeker D, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: John Wiley & Sons; 2006. [Google Scholar]
- 31.Hedeker D, Gibbons RD, Du Toit SHC, Patterson D. SuperMix: A Program for Mixed-Effects Regression Models. Chicago: Scientific Software International; 2008. [Google Scholar]
- 32.Endicott J, Cohen J, Nee J, Fleiss J, Sarantakos S. Hamilton Depression Rating Scale: extracted from regular and change versions of the Schedule for Affective Disorders and Schizophrenia. Arch Gen Psychiatry. 1981;38:98–103. doi: 10.1001/archpsyc.1981.01780260100011. [DOI] [PubMed] [Google Scholar]
- 33.Leon AC. Comparative effectiveness clinical trials in psychiatry: superiority, noninferiority, and the role of active comparators. J Clin Psychiatry. 2011;72:1344–1349. doi: 10.4088/JCP.10m06089whi. [DOI] [PubMed] [Google Scholar]
- 34.Wenzel A, Berchick ER, Tenhave T, Halberstadt S, Brown GK, Beck AT. Predictors of suicide relative to other deaths in patients with suicide attempts and suicide ideation: a 30-year prospective study. J Affect Disord. 2011;132:375–382. doi: 10.1016/j.jad.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Gibbons RD, Hur K, Brown CH, Mann JJ. Relationship between antiepileptic drugs and suicide attempts in patients with bipolar disorder. Arch Gen Psychiatry. 2009;66:1354–1360. doi: 10.1001/archgenpsychiatry.2009.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arana A, Wentworth CE, Ayuso-Mateos JL, Arellano FM. Suiciderelated events in patients treated with antiepileptic drugs. N Engl J Med. 2010;363:542–551. doi: 10.1056/NEJMoa0909801. [DOI] [PubMed] [Google Scholar]
- 37.Redden L, Pritchett Y, Robieson W, Kovacs X, Garofalo M, Tracy K, Saltarelli M. Suicidality and divalproex sodium: analysis of controlled studies in multiple indications. Ann Gen Psychiatry. 2011;10:1. doi: 10.1186/1744-859X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drake C. Effects of misspecification of the propensity score on estimators of the treatment effect. Biometrics. 1993;49:1231–1236. [Google Scholar]
- 39.Leon AC, Hedeker D. Quintile stratification based on a misspecified propensity score in longitudinal treatment effectiveness analyses of ordinal doses. Comput Stat Data Anal. 2007;51:6114–6122. doi: 10.1016/j.csda.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


