Abstract
Methamphetamine use is associated with HIV infection, especially among gay and bisexual men. Methamphetamine use contributes to disease progression both directly, by increasing viral load and damaging the immune system, and indirectly, by decreasing medication adherence. Research examining the association of methamphetamine use and non-adherence has traditionally compared groups of users and nonusers on adherence, compared methamphetamine use between participants above or below some threshold level of adherence (e.g. >90% dose adherence), or examined aggregate relationships. Using Timeline Follow-back procedures, the present study examined aggregate, threshold, and day-level associations of methamphetamine use with non-adherence in 210 HIV-positive gay and bisexual methamphetamine-using men. Methamphetamine use was not associated with adherence behavior at the aggregate-level, but methamphetamine use on a given day was associated with 2.3 times the odds of non-adherence on that day. Threshold results were equivocal. These data suggest that the methamphetamine and non-adherence relationship is complicated: non-adherence is more likely to occur on days in which methamphetamine is used, but participants reported more non-adherence days in which methamphetamine was not used. This seeming paradox generates questions about the selection of analytical techniques and has important implications for behavioral interventions targeting substance use and adherence among HIV-positive individuals.
Keywords: ADHERENCE, METHAMPHETAMINE, METHODS, DAY-LEVEL
INTRODUCTION
The availability of antiretroviral therapy, or ART, has led to the suppression of HIV, significantly improving the health of those living with HIV (1). Recent research suggests that ART not only prolongs the lives of HIV+ persons, but also helps prevent the spread of HIV by reducing viral load and therefore decreasing infectiousness (2–6). These benefits are contingent upon rates of ART adherence being sufficient to provide continuous prevention against HIV replication. Among the many barriers that exist to proper ART adherence is the abuse of substances (i.e., alcohol and drugs). A number of theories have been articulated regarding the association between substance use and poor adherence, including the lifestyle associated with substance use (7, 8), cognitive impairment (7–12) and the exacerbation of psychiatric issues (8, 11). However, in order to best evaluate the evidence for these theories, it is important to contextualize research findings within the methods most commonly used to examine the association between substance use and non-adherence.
Traditionally, the association between substance use and adherence has been examined in one of three ways: 1) by exploring differences in adherence between substance use groups; 2) by associating a specified threshold adherence level (e.g., less than 90% adherence) with substance use; or 3) by examining the association between aggregate substance use and non-adherence behavior over a certain period. Each of these methods has made an important contribution to the literature, but has different implications for interpretation. Research that examines differences in adherence behavior by substance use groups – using t-tests, ANOVA, or logistic regression, for example – provides information about the qualitative differences between substance users and non-users, or between substance dependent individuals and those who do not meet criteria for dependence. These studies general conclude that substance users are more likely to miss medication than non-users (8, 12–16), and help identify variables that might drive or moderate differences between groups (12). However, such analyses do not address the question of whether the amount of drug use makes a difference in non-adherence, and do not usually allow researchers to distinguish between substance use patterns within a particular class of individuals.
Research that compares individuals who meet a particular adherence threshold (e.g., 90% adherence) to those who do not have concluded that greater levels of drug use, and methamphetamine use in specific, is associated with poorer adherence (17–23). These data offer important information for intervention development; however, questions remain about the choice of appropriate thresholds and the durability of proven associations as thresholds shift. At one time, 95% adherence was considered a gold standard for all patients, though results of newer studies suggest that adherence levels as low as 80% (24–27), or even 55% (24, 28) may be sufficient for viral suppression with certain medication regimens. Indeed, studies that utilize the threshold approach vary widely in their chosen cut-offs, with some using 100% (14, 18, 21), 95% (17, 22), 90% (19, 29), or 80% adherence (23, 30). It may be difficult to understand intervention implications in situations in which amount of substance use predicts non-adherence at a particular threshold, but not another.
Investigations that examine linear associations of aggregate substance use and adherence behavior data are perhaps the most straightforward and easiest to interpret; they provide direct information about whether more substance use is correlated with less medication adherence. However, recent review articles reveal that these types of associations are rarely reported (11, 31, 32). One meta-analysis chose to remove any studies that did not stratify participants into discrete categories of substance use or adherence, thus eliminating opportunities to examine the full range of variability (33). One study that reported a linear association used degree of substance dependence, rather than amount of substance use, and found a significant association for one measure of adherence, but not another (34). The lack of consistent reports of linear associations between amounts of use and adherence rates presents difficulty for those seeking support for the claim that substance use causes missed medications.
Although each of the above methods provides important information about the role of substance use in adherence behavior, none provides information regarding within-person temporal associations (i.e., how does substance use on a given day impact non-adherence on that same day?). A strong association between substance use and non-adherence at the day level suggests different types of intervention strategies than those designed simply to reduce drug use. A few studies have examined day-level associations between alcohol use and adherence, indicating that as the number of drinks on a given day increases, the likelihood of missing medication increases both on that day (35–37) and on the day after drinking (36). However, despite the growing body of literature on multilevel modeling of event-level and day-level data for the association of substance use and other HIV risk behaviors (38), little if any published work has examined the day-level effects of other substances (i.e., drugs) on adherence.
The present study compares three methods of examining the association between substance use and adherence – aggregate/linear, day-level, and threshold – in a sample of HIV-positive methamphetamine users in New York City. In past research, methamphetamine users and abusers demonstrate high levels of HIV medication non-adherence (8, 14, 19, 39), frequent treatment interruptions (40), a lower likelihood of achieving optimal adherence (41), and higher viral loads (42). In qualitative and ethnographic research, methamphetamine users cite their use as a direct cause of ART non-adherence, above and beyond other barriers to adherence such as side effects and complex regimens (7, 43). Methamphetamine use is a particular problem for gay and bisexual men, with rates being 10 to 20 times higher in this group compared to the general population (44, 45). In addition, methamphetamine is consistently shown to be a risk factor for contracting HIV, especially among gay and bisexual men (46–48), due to its association with increased sexual risk behavior (16, 41, 44, 49–53). As recent research underscores the importance of HIV treatment adherence for both secondary and primary HIV prevention, a better understanding of the true association between methamphetamine use and HIV adherence among HIV-positive gay and bisexual men is paramount. In comparing different methods of association, this study is designed to illuminate nuances in the role of methamphetamine use in non-adherence behavior and guide future research.
METHODS
Participants and Procedures
This article presents baseline data collected from gay and bisexual men recruited in the New York City metropolitan area for Project ACE, a randomized clinical trial of a behavioral intervention focused on crystal methamphetamine use and HIV medication adherence. Between August 2008 and December 2011, 210 participants completed a baseline quantitative survey and a face-to-face interview. Participants were recruited through a multi-method approach implemented in diverse geographic areas of New York City using techniques previously effective in the recruitment and enrollment of substance-using gay and bisexual men (54, 55). Eligible participants were biologically male, at least 18 years of age, HIV-positive (confirmed with documentation), currently prescribed an ART medication regimen, and English speaking. Additionally, screening criteria required that participants report at least 3 days of methamphetamine use during the previous 90 days and 1 day in the last 30 days, and a minimum of 3 days of HIV medication non-adherence within the last 30 days. All study participants gave written informed consent and all study procedures were approved by the Institutional Review Board of the City University of New York.
Measures
Participants reported their age, year of HIV diagnosis, race and ethnicity, sexual identity, income, and education level using audio-assisted computer administered self-interviews (ACASI). Drug use and HIV medication adherence data were collected using a 14-day Timeline Follow-back (TLFB) interview (56). A period of 14 days was used to collect two weeks of both weekday and weekend activity so as to capture consistent and inconsistent patterns that may be missed with a shorter assessment period. The TLFB has previously demonstrated good test-retest reliability, convergent validity, and agreement with collateral reports for drug abuse (57) and has been previously utilized with substance-using gay and bisexual men (55, 58–60). In addition, the TLFB has been previously used to measure HIV medication adherence in substance-using populations (61, 62), and adherence data collected with TLFB interviews correlates well with HIV biological markers (10). Interviewers received extensive training in the administration of the TLFB, and demonstrated skills (as evidenced by ongoing quality assurance of audiotapes of the TLFB interviews and supervision) in the development of rapport with participants and remaining non-judgmental in order to facilitate honest self-reports and to respect the values and behaviors of all participants.
An interviewer asked participants to reflect back on the past 14 days, mark memorable events (e.g., vacations, birthdays, paycheck days, parties) on the calendar as anchor points, and then recall day by day whether or not they had used methamphetamine, as well as other drug use, and whether or not they had missed any doses of their HIV medications. This method of data collection allowed us to analyze data at the aggregate level (i.e., number of methamphetamine use days, number of missed medication days) as well as at the day level (for each day, whether methamphetamine was used and whether any doses of HIV medications were not taken).
In addition to the training interviewers received in remaining non-judgmental throughout the data collection process, urine tests of drug use were also obtained to create an environment where participants have less incentive to lie, and to validate the TLFB. In fact, percent concordance between methamphetamine self report and qualitative urine toxicology was 90.1%. This concordance indicates valid self-report as it is as good, or greater, than what has been found for other substances of abuse (63) or stimulants in specific (64).
Data Analysis
Data analysis took place in four steps. First, we examined whether there were differences in the number of days of HIV medication non-adherence by demographic factors, including age, race/ethnicity, sexual identity, income, education level, and years since HIV diagnosis. No significant differences were identified; therefore, no covariates were entered into subsequent models. Second, we examined the aggregate-level association between methamphetamine use and adherence (i.e., the association between participants’ total number of methamphetamine use days and total number of non-adherence days in the past two weeks). Because the number of days is a positively skewed count variable, we examined this association using a negative binomial regression model. Negative binomial models are capable of modeling skewed count data, which often result in over-dispersion, and provides more accurate estimates of parameters in such cases than the identity or poisson link functions (65, 66).
Third, we examined the day-level relationship between methamphetamine use and adherence (i.e., the extent to which using methamphetamine on a given day was associated with increased odds of non-adherence on that day). This day-level association was tested using General Estimating Procedures (GEE), which is an extension of Generalized Linear Modeling (GLM). GEE is used to model the average response (e.g., non-adherence across days for any given individual) when multiple observations within the units of analysis are correlated (as they are when the data consist of multiple days for each participant). GEE produces robust estimates of regression coefficients and their variances which can be refined by specifying more accurate estimates of the models variance/covariance structure. Using GEE we were able to test whether using methamphetamine on a given day decreases the odds of taking all HIV medications (non-adherence) on that day compared to a day on which methamphetamine was not used.
Finally, we examined the association between number of methamphetamine use days and two commonly used adherence “thresholds”: 90% dose adherence and 80% dose adherence in the past 14 days. These data were analyzed using logistic regression, with total number of meth days in the same period (past 14 days) used as a predictor.
RESULTS
Demographics of the sample and descriptive statistics for HIV adherence and substance use variables are listed in Table 1. Participants were, on average, 41 years old, and had been positive for 13.9 years. Most participants identified as gay, were non-White, made less than $20,000 in the past year, and did not have a college education. Participants averaged 4.1 (Median = 2) days of non-adherence, and 2.7 (Median = 2) days of methamphetamine use.
Table 1.
Sample demographics, HIV bio-markers and risk events baseline participants.
| n | % | |
|---|---|---|
| Ethnicity/Race | ||
| Black | 70 | 33.7 |
| White | 70 | 33.7 |
| Latino | 54 | 26.0 |
| Other | 14 | 6.7 |
| Sexual Identity | ||
| Gay | 185 | 88.1 |
| Bi-sexual | 23 | 11.0 |
| Education | ||
| HS Grad or Less | 51 | .25 |
| Some College | 73 | .35 |
| College Grad Plus | 85 | .40 |
| Income | ||
| Lt $20K | 148 | 71.5 |
| GE $20K | 59 | 28.1 |
| Viral Load Failure | ||
| GE 200+ | 78 | 37.1 |
| GE 50+ | 104 | 49.5 |
| Mean | SD | Median | Range | |
|---|---|---|---|---|
| Age at Baseline | 40.8 | 8.8 | 41 | 24–63 |
| Years HIV Positive | 13.9 | 7.6 | 11 | 0–31 |
| HIV RNA(log10) | 2.60 | 1.4 | 1.67 | 1.29–6.12 |
| CD4 Count (Absolute) | 460 | 261 | 451 | 19–1144 |
| Meth Use Last 14-days | 2.7 | 2.0 | 2.0 | 0–14 |
| Missed ART Last 14-days | 4.1 | 4.2 | 2.0 | 0–14 |
| Missed ART/Used Meth 14-days | 1.2 | 1.5 | 1 | 0–9 |
In aggregate analyses using negative binomial regression, number of methamphetamine use days was not significantly associated with the number of days of medication non-adherence, β = .02, p = .42 (Table 2). In contrast, GEE analyses showed a strong day-level association between methamphetamine use and non-adherence behavior. On a day in which a participant used methamphetamine, his odds of non-adherence on that day increased by 2.30 times, compared to a day in which methamphetamine was not used, p < .001 (Table 2). In other words, there is no significant aggregate-level association between total number of methamphetamine use days and total number of missed medication days in this sample; however, using methamphetamine use on any given day increases the odds of missing medication on that day by more than 200%.
Table 2.
Differences in Aggregate versus Day Level Analysis of the relationship between Methamphetamine Use and HIV Medication Non-Adherence (n = 210)
| Negative Binomial Regression Approach (Aggregate level analysis. Number of methamphetamine use days predicts number of days of medication non-adherence)
| ||||
|---|---|---|---|---|
| β | Exp. β | 95% CI | p | |
|
|
||||
| Intercept | 1.34 | 3.82 | 3.08–4.74 | <.001 |
| Number methamphetamine days | 0.02 | 1.02 | 0.97–1.08 | .42 |
| Likelihood Ratio χ2 (1, n = 210) = 0.67, p = .42 | ||||
| Generalized Estimated Equation Approach (Day level analysis. Predicts odds of HIV medication non-adherence on a day methamphetamine was used)
| ||||
|---|---|---|---|---|
| β | Exp. β | 95% CI | p | |
|
|
||||
| Intercept | −1.07 | 0.34 | 0.31–0.38 | <.001 |
| Methamphetamine day | 0.83 | 2.30 | 1.90–2.78 | <.001 |
| Non-methamphetamine day | 1 | 0 | ||
Logistic regression analyses of the association between methamphetamine use and adherence threshold groups are presented in Table 3. There was a significant association between days of methamphetamine use and 90% dose adherence, such that for every additional day of methamphetamine use, the odds of being less than 90% adherent increased by 20% (OR = 1.20, p < .01). However, there was no significant association between days of methamphetamine use and the 80% dose adherence threshold
Table 3.
Analyses predicting 14 Day ART Dose Adherence Threshold Groups with Total Meth 14 Day Use
| β | OR | CI | p | |
|---|---|---|---|---|
| <90 Percent Dose Adherence | ||||
| Constant | 0.11 | 0.90 | .608 | |
| Total Meth 14 Days | 0.18 | 0.83 | 1.06–1.36 | .005 |
| <80 Percent Dose Adherence | ||||
| Constant | −0.45 | 1.56 | .021 | |
| Total Meth 14 Days | 0.07 | 0.94 | 0.97–1.18 | .183 |
Note: 113 participants (63.3%) reported less than 90% dose adherence; 91 participants (43.3%) reported less than 80% dose adherence.
In order to better understand the differences between aggregate, day-level, and threshold associations, Figure 1 displays methamphetamine use and adherence data for five study participants. The total number of methamphetamine use days for each participant ranges from 1 (participants #1 and #2) to 5 (participant #5), and the total number of non-adherence days ranges from 2 (participants #1 and #3) to 13 (participant #2). If there were an aggregate-level association between number of methamphetamine use days and non-adherence days, we would expect to see an association between these two numbers for each participant, i.e., as the number of methamphetamine use days increased across participants, we would expect a similar increase in number of non-adherence days. In contrast, among the two participants who reported only one day of methamphetamine use (participant s #1 and #2), one reported the lowest number of missed medication days (2 days), while the other reported the highest number of days (13 days). Similarly, the participant with the highest number of methamphetamine days (participant #5, 5 days), reported fewer non-adherence days (8 days) than participant #2, who reported only one methamphetamine use day, but 13 non-adherence days.
Figure 1.
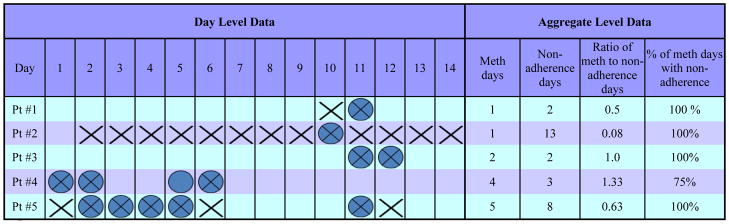
Comparing Day-Level and Aggregate-Level Data
 = Methamphetamine Use day
= Methamphetamine Use day
 = HIV Medication Non-Adherence day
= HIV Medication Non-Adherence day
 = Co-occurence of Methamphetamine Use and Non-Adherence day
= Co-occurence of Methamphetamine Use and Non-Adherence day
However, if we examine the percentage of methamphetamine use days in which non-adherence co-occurred, the data tell a different story. For each participant, the percentage of methamphetamine use days that were also non-adherence days ranges from 75%-100%, with four out of five participants missing medication on 100% of their methamphetamine-use days. As such, the difference in the aggregate versus day-level associations between methamphetamine use and non-adherence can be accounted for by the differential pattern of covariation among these behaviors. Because participants miss their medication on both days in which they use methamphetamine and days in which they do not, the aggregate association between these two behaviors is reduced. However, on days in which participants do use methamphetamine, they are much more likely to miss their HIV medication. Therefore, the day-level association between methamphetamine and non-adherence remains strong. In other words, methamphetamine use means non-adherence, but non-adherence does not necessarily mean methamphetamine use.
DISCUSSION
The present study is the first, to our knowledge, to compare aggregate, day-level, and threshold associations between methamphetamine use and HIV medication non-adherence. Although no aggregate relationship was found, a strong day-level association was identified – participants had more than twice the odds of reporting non-adherence to their HIV medications on a day in which they had used methamphetamine. While this finding does not necessarily imply a causal relationship between these behaviors, it suggests that methamphetamine use plays a critical role in adherence behavior for HIV-positive gay and bisexual men at the day-level. In other words, the mechanism of association between methamphetamine use and non-adherence appears to operate in the co-occurrence of these behaviors, rather than in a general atmosphere or life context that makes adherence difficult. These findings have significant implications for both methodological approaches to understanding the intersection between adherence and other problematic health behaviors (in this case, methamphetamine use), as well as for intervention approaches aimed at HIV-positive gay and bisexual men.
Aggregate level data suggest that, among methamphetamine users, it is not necessarily the frequency with which methamphetamine is used that leads to problems with non-adherence. The day-level analyses, however, provide clear evidence of the connection between the two behaviors – methamphetamine use on a given day significantly increases the odds of medication non-adherence on that particular day. Threshold analyses suggest that the number of days of methamphetamine use may distinguish between patients who are 90% adherent and those who are not, but it does not distinguish between patients who meet lower thresholds. The three sets of analyses suggest that the methamphetamine and non-adherence relationship is complicated: non-adherence is more likely to occur on days in which methamphetamine is used, but participants actually reported more non-adherence days in which methamphetamine was not used. As a result, simplistic measures of the frequency of methamphetamine use and the frequency of medication non-adherence are likely to significantly underestimate the nature of the relationship between these two behaviors. Day-level measurement, be it retrospective using calendar-based assessments, or prospective using diary-based or real time measurement tools, may help researchers to more fully understand the relationships across behaviors. That is, understanding the co-occurrence of behaviors requires analysis of their co-occurrence and not simply their association at the aggregate level.
Methodologically, these findings also underscore the importance of examining behavior within specific risk populations. Certainly, studies have shown that methamphetamine users have higher rates of HIV medication non-adherence compared to non-users (8, 14, 19, 40, 41), but these findings suggest that the impact of methamphetamine may occur at two levels. At the day-level, methamphetamine use itself is associated with non-adherence. But reported non-adherence by methamphetamine users even on days in which the drug is not used suggests other mechanisms of association. In other words, all participants in this study had in common the impact of methamphetamine use generally on their adherence, so the differences in adherence between users are likely driven by other non-methamphetamine factors related to non-adherence. For example, there is evidence that methamphetamine damages limbic regions of the brain in a manner that makes methamphetamine users more susceptible to mood disorders (67). In turn, negative affect has been shown to be associated with more frequent stimulant use and lower adherence in HIV-positive stimulant users (68, 69).
Day level analyses could be used to examine the individual differences in reasons for ART non-adherence behavior. The relative impact of other behaviors could be examined relative to the effect of methamphetamine use. Day level data can be combined with person level factors to examine the relative impact and interaction of cognitive performance or mental health. With data collected at the day level it would also be possible to categorize participants based on the percentage of missed medication that co-occurs with methamphetamine use (or any substance of abuse). An examination of a non-adherent drug using population that has a low percentage of missed medication that co-occurs with drug use could illuminate behaviors that adherence interventions could target in addition to drug use to yield a more effective outcome, especially for those who’s problems with adherence extend beyond the substance use-missed medication connection.
The fact that HIV medication non-adherence among methamphetamine users is so much more likely to occur on a day in which methamphetamine was used has clear intervention implications. Decreasing the frequency of days in which methamphetamine is used may have limited impact - with the need for high levels (e.g., 90% or better) of adherence to HIV medications (70–73), even a few days of methamphetamine use, and the corresponding strong effect on non-adherence, could result in viral replication and the development of resistance (73, 74). Pharmacologic approaches to treat methamphetamine have generally been found to lack efficacy (32), although one recent study suggests some success with mirtazapine (75). The only published behavioral intervention that has been evaluated with HIV-positive gay and bisexual men, consisting of eight 90-minute sessions of individual therapy, found significant effects on reducing unprotected sex, but not reducing methamphetamine use (76). Similarly, contingency management interventions to treat methamphetamine abuse have shown mixed results, with two trials focused on gay and bisexual men showing significant effects (77, 78), but one recent trial showing none (79). This trial has been called into question due to twice weekly urine testing, allowing a window for those engaged in the treatment condition to possibly use and still receive contingencies. Better results have been identified when contingency management is combined with cognitive behavioral therapy (80, 81), particularly when the therapy is culturally tailored for gay and bisexual men (82, 83). It is important to note that all of these intervention trials involved rather intensive courses of treatment – it is unlikely methamphetamine abuse will be responsive to briefer forms of counseling. For HIV-positive gay and bisexual men in particular, in which multiple behaviors – methamphetamine use, HIV medication adherence, and risky sexual practices – intersect, it is likely that interventions will need to address both motivation and skills-development in order to have the potential for effect.
The current study is not without limitations. Both behaviors of focus - methamphetamine use and HIV medication non-adherence - were self-reported by participants. However, significant training efforts went into creating a non-judgmental environment in which participants were urged to be as honest as possible. In addition, biological markers (urine testing for methamphetamine and viral load and CD4 counts as proxy indicators of adherence) were collected, which could result in participants being more honest in their self-reports. Indeed, the concordance between TLFB self-report and methamphetamine urine toxicology suggests that this sample’s self-report data are valid for methamphetamine, at the very least. These data were also collected retrospectively, using the TLFB. It is possible that drug use events create an anchor such that participants are more likely to recall other behaviors on that day. This is in contrast to assessing these behaviors prospectively, using diary-like measures. This method, however, comes with unique problems, foremost being missing data when the participants forget to complete the diary. The sample was entirely self-selected and comprised of HIV-positive gay and bisexual men interested in an intervention trial designed to improve medication adherence and/or reduce methamphetamine use. An Australian study found only 10% of regular methamphetamine users seek treatment (84), so it is not clear to what degree these results would be generalizable to men not presenting for an intervention study. Further, the sample was comprised entirely of men living in a large urban area - although our sample was quite diverse with regard to race and ethnicity, age, education, and years living with HIV.
The study is also limited in that it is not feasible to examine viral load or CD4 count on a day level, thus it is not possible to know if the day level associations are associated with changes in day level biological markers. It is also important to note that an association between a behavior or a psychological state and the likelihood of non-adherence does not ensure high viral loads or drug resistance. Just as one should not suggest that a medical provider withhold prescribing ART to those who are depressed or have some neurocognitive impairment, one should not take the present findings as a reason to withhold medication from a methamphetamine user. In fact, the lack of a more general association between methamphetamine use and adherence, while a strong day level association exists, suggests that the causes of non-adherence are far more varied than simply methamphetamine use, even for methamphetamine users. There is benefit to prescribing ART to methamphetamine users. Indeed, studies have shown that the small negative impact of the cumulative exposure to stimulants on biological HIV outcomes is dwarfed by the large positive effect of the cumulative exposure to ART (85).
Future analyses and studies should consider the duration of the effect of a methamphetamine use day on medication non-adherence. That is, it is possible that methamphetamine use on Saturday not only negatively affects adherence on Saturday, but may also negatively affect adherence on Sunday. In addition, the day-level predictors of non-adherence on non-methamphetamine use days, as well as predictors of adherence on methamphetamine use days should be examined. As such, this study represents the first step in a more comprehensive understanding of the relationship between methamphetamine use and HIV medication non-adherence. In addition, because of the previously identified connections between methamphetamine use and risky sexual behaviors (16, 41, 44, 49–53), and the fact that non-adherence to HIV medications can result in the transmission of drug resistant strains (73, 74, 86, 87), future studies should examine the day-level associations between methamphetamine use, non-adherence, and sexual risk behavior.
Acknowledgments
The ACE Project was supported by a grant from the National Institute on Drug Abuse (NIDA) (R01 DA023395, Jeffrey T. Parsons, Principal Investigator). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors acknowledge the contributions of the ACE Project Team—Michael Adams, Kristi Gamarel, Chris Hietikko, Zak Hill-Whilton, Catherine Holder, John Pachankis, Gregory Payton, Jonathan Rendina, Kevin Robin, Tyrel Starks, and the CHEST recruitment team. We also gratefully acknowledge Shoshana Kahana for her support of the project.
References
- 1.Nath A, Schiess N, Venkatesan A, Rumbaugh J, Sacktor N, McArthur J. Evolution of HIV dementia with HIV infection. International Review of Psychiatry. 2008 Feb;20(1):25–31. doi: 10.1080/09540260701861930. [DOI] [PubMed] [Google Scholar]
- 2.HPTN-052. HIV Prevention Trials Network. 2011. Initiation of Antiretroviral Treatment Protects Uninfected Sexual Partners from HIV Infection (HPTN Study 052) [Google Scholar]
- 3.Carey CL, Woods SP, Rippeth JD, Gonzalez R, Heaton RK, Grant I. Additive deleterious effects of methamphetamine dependence and immunosuppression on neuropsychological functioning in HIV infection. AIDS Behav. 2006;10(2):185–90. doi: 10.1007/s10461-005-9056-4. [DOI] [PubMed] [Google Scholar]
- 4.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009 Jul 17;23(11):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 5.Montaner JS. Treatment as prevention--a double hat-trick. Lancet. 2011 Jul 16;378(9787):208–9. doi: 10.1016/S0140-6736(11)60821-0. [DOI] [PubMed] [Google Scholar]
- 6.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003 Dec;15(6):775–85. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- 8.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007 Mar;11(2):185–94. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rendell PG, Mazur M, Henry JD. Prospective memory impairment in former users of methamphetamine. Psychopharmacology. 2009 Apr;203(3):609–16. doi: 10.1007/s00213-008-1408-0. [DOI] [PubMed] [Google Scholar]
- 10.Parsons JT, Rosof E, Mustanski B. Medication adherence mediates the relationship between adherence self-efficacy and biological assessments of HIV health among those with alcohol use disorders. AIDS Behav. 2008 Jan;12(1):95–103. doi: 10.1007/s10461-007-9241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez A, Barinas J, O’Cleirigh C. Substance use: impact on adherence and HIV medical treatment. Current HIV/AIDS reports. 2011 Dec;8(4):223–34. doi: 10.1007/s11904-011-0093-5. [DOI] [PubMed] [Google Scholar]
- 12.Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. Journal of Behavioral Medicine. 2010 Sep 21; doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moore DJ, Blackstone K, Woods SP, Ellis RJ, Atkinson JH, Heaton RK, et al. Methamphetamine use and neuropsychiatric factors are associated with antiretroviral non-adherence. AIDS Care. 2012 Apr 24; doi: 10.1080/09540121.2012.672718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tucker JS, Burnam MA, Sherbourne CD, Kung F-Y, Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114(7):573–80. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 15.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, et al. Medication adherence in HIV-infected adults: effect of patient age, cognitive status, and substance abuse. AIDS. 2004 Jan 1;18( Suppl 1):S19–25. doi: 10.1097/00002030-200418001-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009 May;21(5):575–82. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- 17.McGowan CC, Weinstein DD, Samenow CP, Stinnette SE, Barkanic G, Rebeiro PF, et al. Drug use and receipt of highly active antiretroviral therapy among HIV-infected persons in two U.S. clinic cohorts. PLoS One. 2011;6(4):e18462. doi: 10.1371/journal.pone.0018462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharpe TT, Lee LM, Nakashima AK, Elam-Evans LD, Fleming PL. Crack cocaine use and adherence to antiretroviral treatment among HIV-infected black women. J Community Health. 2004 Apr;29(2):117–27. doi: 10.1023/b:johe.0000016716.99847.9b. [DOI] [PubMed] [Google Scholar]
- 19.Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. 2007 Nov;69(8):785–92. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- 20.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, et al. Medication adherence among HIV+ adults: effects of cognitive dysfunction and regimen complexity. Neurology. 2002 Dec 24;59(12):1944–50. doi: 10.1212/01.wnl.0000038347.48137.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Jong BC, Prentiss D, McFarland W, Machekano R, Israelski DM. Marijuana use and its association with adherence to antiretroviral therapy among HIV-infected persons with moderate to severe nausea. Journal of Acquired Immune Deficiency Syndromes. 2005 Jan 1;38(1):43–6. doi: 10.1097/00126334-200501010-00008. [DOI] [PubMed] [Google Scholar]
- 22.Ingersoll K. The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004 Feb;16(2):199–211. doi: 10.1080/09540120410001641048. [DOI] [PubMed] [Google Scholar]
- 23.Avants SK, Margolin A, Warburton LA, Hawkins KA, Shi J. Predictors of nonadherence to HIV-related medication regimens during methadone stabilization. Am J Addict. 2001 Winter;10(1):69–78. doi: 10.1080/105504901750160501. [DOI] [PubMed] [Google Scholar]
- 24.Maggiolo F, Airoldi M, Kleinloog HD, Callegaro A, Ravasio V, Arici C, et al. Effect of adherence to HAART on virologic outcome and on the selection of resistance-conferring mutations in NNRTI- or PI-treated patients. HIV Clinical Trials. 2007 Sep-Oct;8(5):282–92. doi: 10.1310/hct0805-282. [DOI] [PubMed] [Google Scholar]
- 25.Martin M, Del Cacho E, Codina C, Tuset M, De Lazzari E, Mallolas J, et al. Relationship between adherence level, type of the antiretroviral regimen, and plasma HIV type 1 RNA viral load: a prospective cohort study. AIDS Research and Human Retroviruses. 2008 Oct;24(10):1263–8. doi: 10.1089/aid.2008.0141. [DOI] [PubMed] [Google Scholar]
- 26.Parienti JJ, Ragland K, Lucht F, de la Blanchardiere A, Dargere S, Yazdanpanah Y, et al. Average adherence to boosted protease inhibitor therapy, rather than the pattern of missed doses, as a predictor of HIV RNA replication. Clinical Infectious Diseases. 2010 Apr 15;50(8):1192–7. doi: 10.1086/651419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shuter J, Sarlo JA, Kanmaz TJ, Rode RA, Zingman BS. HIV-infected patients receiving lopinavir/ritonavir-based antiretroviral therapy achieve high rates of virologic suppression despite adherence rates less than 95% Journal of Acquired Immune Deficiency Syndromes. 2007 May 1;45(1):4–8. doi: 10.1097/QAI.0b013e318050d8c2. [DOI] [PubMed] [Google Scholar]
- 28.Bangsberg DR. Less than 95% adherence to nonnucleoside reverse-transcriptase inhibitor therapy can lead to viral suppression. Clinical Infectious Diseases. 2006 Oct 1;43(7):939–41. doi: 10.1086/507526. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Longshore D, Williams JK, Rivkin I, Loeb T, Warda US, et al. Substance abuse and medication adherence among HIV-positive women with histories of child sexual abuse. AIDS And Behavior. 2006 May;10(3):279–86. doi: 10.1007/s10461-005-9041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bouhnik AD, Preau M, Vincent E, Carrieri MP, Gallais H, Lepeu G, et al. Depression and clinical progression in HIV-infected drug users treated with highly active antiretroviral therapy. Antivir Ther. 2005;10(1):53–61. [PubMed] [Google Scholar]
- 31.Hendershot CS, Stoner SA, Pantalone DW, Simoni JM. Alcohol use and antiretroviral adherence: review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2009 Oct 1;52(2):180–202. doi: 10.1097/QAI.0b013e3181b18b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajasingham R, Mimiaga MJ, White JM, Pinkston MM, Baden RP, Mitty JA. A systematic review of behavioral and treatment outcome studies among HIV-infected men who have sex with men who abuse crystal methamphetamine. AIDS Patient Care STDS. 2012 Jan;26(1):36–52. doi: 10.1089/apc.2011.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malta M, Magnanini MM, Strathdee SA, Bastos FI. Adherence to antiretroviral therapy among HIV-infected drug users: a meta-analysis. AIDS And Behavior. 2010 Aug;14(4):731–47. doi: 10.1007/s10461-008-9489-7. [DOI] [PubMed] [Google Scholar]
- 34.Applebaum AJ, Reilly LC, Gonzalez JS, Richardson MA, Leveroni CL, Safren SA. The impact of neuropsychological functioning on adherence to HAART in HIV-infected substance abuse patients. AIDS Patient Care STDS. 2009 Jun;23(6):455–62. doi: 10.1089/apc.2008.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braithwaite RS, Conigliaro J, McGinnis KA, Maisto SA, Bryant K, Justice AC. Adjusting alcohol quantity for mean consumption and intoxication threshold improves prediction of nonadherence in HIV patients and HIV-negative controls. Alcoholism, clinical and experimental research. 2008 Sep;32(9):1645–51. doi: 10.1111/j.1530-0277.2008.00732.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braithwaite RS, McGinnis KA, Conigliaro J, Maisto SA, Crystal S, Day N, et al. A temporal and dose-response association between alcohol consumption and medication adherence among veterans in care. Alcoholism, clinical and experimental research. 2005 Jul;29(7):1190–7. doi: 10.1097/01.alc.0000171937.87731.28. [DOI] [PubMed] [Google Scholar]
- 37.Parsons JT, Rosof E, Mustanski B. The temporal relationship between alcohol consumption and HIV-medication adherence: a multilevel model of direct and moderating effects. Health Psychology. 2008 Sep;27(5):628–37. doi: 10.1037/a0012664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vosburgh HW, Mansergh G, Sullivan PS, Purcell DW. A review of the literature on event-level substance use and sexual risk behavior among men who have sex with men. AIDS Behav. 2012 Aug;16(6):1394–410. doi: 10.1007/s10461-011-0131-8. [DOI] [PubMed] [Google Scholar]
- 39.Das M, Santos D, Matheson T, Santos GM, Chu P, Vittinghoff E, et al. Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high-risk men who have sex with men. AIDS. 2010;24(7):991. doi: 10.1097/qad.0b013e328336e98b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrico AW, Riley ED, Johnson MO, Charlebois ED, Neilands TB, Remien RH, et al. Psychiatric risk factors for HIV disease progression: the role of inconsistent patterns of antiretroviral therapy utilization. J Acquir Immune Defic Syndr. 2011;56(2):146–50. doi: 10.1097/QAI.0b013e318201df63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halkitis PN, Parsons JT. Methamphetamine use and sexual risk taking among gay/bisexual men in New York City. Paper presented at: 5th International AIDS Impact Conference; Brighton. 2001. [Google Scholar]
- 42.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, Grant I. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003 Dec 15;188(12):1820–6. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 43.Carroll RT. Daily living, functional health, and adherence to anti-viral drug regimens in young adult, HIV+ methamphetamine users. Paper presented at: AIDS 2006 - XVI International AIDS Conference; Toronto. 2006. [Google Scholar]
- 44.Grov C, Parsons JT, Bimbi DS. In the shadows of a prevention campaign: Sexual risk behavior in the absence of crystal methamphetamine. AIDS Educ Prev. 2008;20(1):42–55. doi: 10.1521/aeap.2008.20.1.42. [DOI] [PubMed] [Google Scholar]
- 45.Mimiaga MJ, Fair AD, Mayer KH, Koenen K, Gortmaker S, Tetu AM, et al. Experiences and sexual behaviors of HIV-infected MSM who acquired HIV in the context of crystal methamphetamine use. AIDS Educ Prev. 2008;20(1):30–41. doi: 10.1521/aeap.2008.20.1.30. [DOI] [PubMed] [Google Scholar]
- 46.Plankey MW, Ostrow DG, Stall R, Cox C, Li X, Peck JA, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45(1):85. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, et al. Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS. 2005;19(13):1423–4. doi: 10.1097/01.aids.0000180794.27896.fb. [DOI] [PubMed] [Google Scholar]
- 48.Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–9. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 49.Bousman C, Cherner M, Ake C, Letendre S, Atkinson J, Patterson T, et al. Negative mood and sexual behavior among non-monogamous men who have sex with men in the context of methamphetamine and HIV. J Affect Disord. 2009;119(1–3):84–91. doi: 10.1016/j.jad.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Colfax G, Shoptaw S. The methamphetamine epidemic: Implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005 Nov;2(4):194–9. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- 51.Forrest DW, Metsch LR, LaLota M, Cardenas G, Beck DW, Jeanty Y. Crystal methamphetamine use and sexual risk behaviors among HIV-positive and HIV-negative men who have sex with men in South Florida. J Urban Health. 2010;87(3):480–5. doi: 10.1007/s11524-009-9422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorbach PM, Weiss RE, Jeffries R, Javanbakht M, Drumright LN, Daar ES, et al. Behaviors of recently HIV-infected men who have sex with men in the year postdiagnosis: effects of drug use and partner types. J Acquir Immune Defic Syndr. 2011;56(2):176. doi: 10.1097/QAI.0b013e3181ff9750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halkitis PN, Mukherjee PP, Palamar JJ. Longitudinal modeling of methamphetamine use and sexual risk behaviors in gay and bisexual men. AIDS Behav. 2009;13(4):783–91. doi: 10.1007/s10461-008-9432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grov C, Bux D, Parsons JT, Morgenstern J. Recruiting Hard-to-Reach Drug-Using Men Who Have SexWith Men into an Intervention Study: Lessons Learned and Implications for Applied Research. Subst Use Misuse. 2009;44(13):1855–71. doi: 10.3109/10826080802501570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morgenstern J, Bux DA, Jr, Parsons J, Hagman BT, Wainberg M, Irwin T. Randomized trial to reduce club drug use and HIV risk behaviors among men who have sex with men. J Consult Clin Psychol. 2009;77(4):645–56. doi: 10.1037/a0015588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sobell M, Sobell L. Problem drinkers: Guided self-change treatment. New York, NY: Guilford Press; 1993. [Google Scholar]
- 57.Fals-Stewart W, O’Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000 Feb;68(1):134–44. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 58.Irwin TW, Morgenstern J, Parsons JT, Wainberg M, Labouvie E. Alcohol and sexual HIV risk behavior among problem drinking men who have sex with men: An event level analysis of timeline followback data. AIDS Behav. 2006 May;10(3):299–307. doi: 10.1007/s10461-005-9045-7. [DOI] [PubMed] [Google Scholar]
- 59.Velasquez MM, von Sternberg K, Johnson DH, Green C, Carbonari JP, Parsons JT. Reducing sexual risk behaviors and alcohol use among HIV-positive men who have sex with men: a randomized clinical trial. J Consult Clin Psychol. 2009 Aug;77(4):657–67. doi: 10.1037/a0015519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golub SA, Starks TJ, Payton G, Parsons JT. The Critical Role of Intimacy in the Sexual Risk Behaviors of Gay and Bisexual Men. AIDS Behav. 2011 Jun 1; doi: 10.1007/s10461-011-9972-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ingersoll K, Farrell-Carnahan L, Cohen-Filipic J, Heckman CJ, Ceperich SD, Hettema J, et al. A pilot randomized clinical trial of two medication adherence and drug use interventions for HIV+ crack cocaine users. Drug Alcohol Depend. 2011 doi: 10.1016/j.drugalcdep.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parsons JT, Rosof E, Mustanski B. Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. J Health Psychol. 2007 Mar;12(2):357–70. doi: 10.1177/1359105307074298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison L. The validity of self-reported drug use in survey research: an overview and critique of research methods. NIDA Res Monogr. 1997;167:17–36. [PubMed] [Google Scholar]
- 64.Reinhard MJ, Hinkin CH, Barclay TR, Levine AJ, Marion S, Castellon SA, et al. Discrepancies between self-report and objective measures for stimulant drug use in HIV: cognitive, medication adherence and psychological correlates. Addictive Behaviors. 2007 Dec;32(12):2727–36. doi: 10.1016/j.addbeh.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coxe S. The Analysis of Count Data: A Gentle Introduction to Poisson Regression and Its Alternatives. J Pers Assess. 2009;91(2):121–36. doi: 10.1080/00223890802634175. [DOI] [PubMed] [Google Scholar]
- 66.Long JS. Regression models for categorical and limited dependent variables. Thousand Oaks, CA: Sage, Inc; 1997. [Google Scholar]
- 67.London ED, Simon SL, Berman SM, Mandelkern MA, Lichtman AM, Bramen J, et al. Mood disturbances and regional cerebral metabolic abnormalities in recently abstinent methamphetamine abusers. Arch Gen Psychiatry. 2004 Jan;61(1):73–84. doi: 10.1001/archpsyc.61.1.73. [DOI] [PubMed] [Google Scholar]
- 68.Carrico AW, Johnson MO, Colfax GN, Moskowitz JT. Affective correlates of stimulant use and adherence to anti-retroviral therapy among HIV-positive methamphetamine users. AIDS Behav. 2010 Aug;14(4):769–77. doi: 10.1007/s10461-008-9513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, Charlebois ED, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosomatic Medicine. 2007 Nov;69(8):785–92. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- 70.Kitahata MM, Reed SD, Dillingham PW, Van Rompaey SE, Young AA, Harrington RD, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. Int J STD AIDS. 2004;15(12):803–10. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- 71.Palombi L, Marazzi MC, Guidotti G, Germano P, Buonomo E, Scarcella P, et al. Incidence and predictors of death, retention, and switch to second-line regimens in antiretroviral- treated patients in sub-Saharan African Sites with comprehensive monitoring availability. Clin Infect Dis. 2009;48(1):115–22. doi: 10.1086/593312. [DOI] [PubMed] [Google Scholar]
- 72.Paterson DL, Swindells S, Mohr J, Brester M, Vergis EN, Squier C, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133(1):21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 73.Read T, Mijch A, Fairley CK. Adherence to antiretroviral therapy: are we doing enough? Intern Med J. 2003 May-Jun;33(5–6):254–6. doi: 10.1046/j.1445-5994.2003.00333.x. [DOI] [PubMed] [Google Scholar]
- 74.Racey CS, Zhang W, Brandson EK, Fernandes KA, Tzemis D, Harrigan PR, et al. HIV antiviral drug resistance: patient comprehension. AIDS Care. 2010;22(7):816–26. doi: 10.1080/09540120903431355. [DOI] [PubMed] [Google Scholar]
- 75.Colfax GN, Santos G-M, Das M, Santos DM, Matheson T, Gasper J, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168–75. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mausbach BT, Semple SJ, Strathdee SA, Zians J, Patterson TL. Efficacy of a behavioral intervention for increasing safer sex behaviors in HIV-positive MSM methamphetamine users: results from the EDGE study. Drug Alcohol Depend. 2007;87(2–3):249–57. doi: 10.1016/j.drugalcdep.2006.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shoptaw S, Klausner JD, Reback CJ, Tierney S, Stansell J, Hare CB, et al. A public health response to the methamphetamine epidemic: the implementation of contingency management to treat methamphetamine dependence. BMC Public Health. 2006;6:214. doi: 10.1186/1471-2458-6-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reback CJ, Peck JA, Dierst-Davies R, Nuno M, Kamien JB, Amass L. Contingency management among homeless, out-of-treatment men who have sex with men. J Subst Abuse Treat. 2010;39(3):255–63. doi: 10.1016/j.jsat.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Menza TW, Jameson DR, Hughes JP, Colfax GN, Shoptaw S, Golden MR. Contingency management to reduce methamphetamine use and sexual risk among men who have sex with men: a randomized controlled trial. BMC Public Health. 2010;10:774. doi: 10.1186/1471-2458-10-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, et al. Contingency management for the treatment of methamphetamine use disorders. Am J Psychiatry. 2006;163(11):1993–9. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- 81.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, et al. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction. 2006;101(2):267–74. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 82.Shoptaw S, Reback CJ, Peck JA, Yang X, Rotheram-Fuller E, Larkins S, et al. Behavioral treatment approaches for methamphetamine dependence and HIV-related sexual risk behaviors among urban gay and bisexual men. Drug Alcohol Depend. 2005;78(2):125–34. doi: 10.1016/j.drugalcdep.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 83.Jaffe A, Shoptaw S, Stein JA, Reback CJ, Rotheram-Fuller E. Depression ratings, reported sexual risk behaviors, and methamphetamine use: Latent growth curve models of positive change among gay and bisexual men in an outpatient treatment program. Exp Clin Psychopharmacol. 2007;15(3):301–7. doi: 10.1037/1064-1297.15.3.301. [DOI] [PubMed] [Google Scholar]
- 84.Kelly E, McKetin R, McLaren J. Health service utilisation among regular methamphetamine users: National Drug and Alcohol Research Centre. 2005. Report No.: 233. [Google Scholar]
- 85.Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, et al. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. International Journal Of STD & AIDS. 2012 Aug;23(8):576–80. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colfax GN, Vittinghoff E, Grant R, Lum P, Spotts G, Hecht F. Frequent methanphetamine use is associated with primary non-nucleoside reverse transcriptase inhibitor resistance. AIDS. 2007;21(2):239–41. doi: 10.1097/QAD.0b013e3280114a29. [DOI] [PubMed] [Google Scholar]
- 87.Gorbach PM, Drumright LN, Javanbakht M, Pond SL, Woelk CH, Daar ES, et al. Antiretroviral drug resistance and risk behavior among recently HIV-infected men who have sex with men. J Acquir Immune Defic Syndr. 2008;47(5):639–43. doi: 10.1097/QAI.0b013e3181684c3d. [DOI] [PMC free article] [PubMed] [Google Scholar]


