Abstract
The cytotoxic farnesyl transferase inhibitor BMS-214662 has been shown to potently induce mitochondrial apoptosis in primitive CD34+ chronic myeloid leukaemia (CML) stem/progenitor cells. Here, to enhance the BMS-214662 apoptotic effect, we further targeted the RAS-MEK-ERK pathway, downstream of BCR-ABL, by treating CD34+ CML stem/progenitor cells with a highly selective ATP non-competitive MEK inhibitor, PD184352. PD184352 increased the apoptotic effect of BMS-214662 in a CML blast crisis cell line, K562, and in primary chronic phase CD34+ CML cells. Compared to BMS-214662, after combination treatment we observed inhibition of ERK phosphorylation, increased Annexin-V levels, caspase-3, 8 and 9 activation and potentiated mitochondrial damage, associated with decreased levels of anti-apoptotic BCL-2 family protein MCL-1. Inhibition of K-RAS function by a dominant negative mutant resulted in CML cell death and this process was further enhanced by the addition of BMS-214662 and PD184352. Together, these findings suggest that the addition of a MEK inhibitor improves the ability of BMS-214662 to selectively target CML stem/progenitor cells, notoriously insensitive to tyrosine kinase inhibitor treatment and presumed to be responsible for the persistence and relapse of the disease.
Keywords: CML, FTI, BMS-214662, MEK inhibitor, PD184352, HSC, TKI, CD34+
Introduction
Chronic myeloid leukaemia (CML) is a clonal disorder of haemopoietic stem cells (HSC) characterised by the enhanced production of myeloid cells as a consequence of the reciprocal translocation t(9;22) (1). As a result of the translocation, the BCR-ABL oncogene encodes for a constitutively active tyrosine kinase (TK) and imatinib mesylate (IM) is a TK inhibitor (TKI) used as conventional treatment for CML (2, 3). Although IM treatment results in achievement of complete cytogenetic response only a minority of patients achieve complete molecular response (4, 5). Persistence of BCR-ABL+ stem cells with repopulating capacity in CML patients in prolonged complete cytogenetic response after 5 years of IM treatment, indicates that patients remain at risk of relapse on drug discontinuation or through acquisition of IM resistance (6). The second generation TKIs, nilotinib and dasatinib, are significantly more potent ABL inhibitors than IM, but they are still not able to target the most primitive CML stem cells (7–11). Recently, the “non-randomised Stop IM” (STIM) trial has shown that 61% of CML patients that discontinued IM after achieving complete molecular remission relapsed (12).
Recently, new therapeutic targets are actively being sought, mainly among the BCR-ABL downstream pathway proteins. Amongst them the RAS signalling pathway plays a pivotal role. Localisation of RAS to the cell membrane, where it is capable of activating downstream signalling events, requires modification by intracellular farnesyl transferases (FT). BMS-214662 is a cytotoxic FT inhibitor (FTI) designed to inhibit farnesylation of RAS and able to induce apoptosis in both proliferating and quiescent CML stem/progenitor cells in chronic phase (CP) samples (13, 14). We have shown that BMS-214662, in addition to its activity as a FTI, is able to induce apoptosis through a different mechanism of action (15). Recent studies have demonstrated that BMS-214662 has a significantly higher potency to reduce quiescent CML stem cells through the induction of apoptosis, as compared to other FTIs, such as BMS-225975 or lonafarnib (14–16). Additional targeting of MEK has been shown to further increase the activity of IM and dasatinib in BCR-ABL-positive cell lines (17, 18). The first MEK inhibitor to enter clinical trials is PD184352. It is a highly potent and selective ATP non-competitive inhibitor of both MEK isoforms (MEK1 and MEK2) (19, 20). In this study we used a combination of the MEK inhibitor PD184352 with BMS-214662 to increase the effect of BMS-214662 in a CML blast crisis (BC) cell line, K562 and in primary CP CML stem/progenitor cells (CD34+, CD34+38−).
Material and methods
Reagents
BMS-214662 was obtained from Bristol-Myers Squibb (Princeton, USA). PD184352 (CI-1040) was chemically synthesized in-house based on the published structure of the drug. U0126 was from Calbiochem (Chemicals Ltd., Nottingham, UK). All reagent grade chemicals were purchased from Sigma-Aldrich Company Ltd. (Poole, UK), unless otherwise stated.
Isolation and culture of CD34+ cells and culture of K562 cell line
Fresh leukapheresis or peripheral blood samples were obtained with written informed consent from patients with CP CML at diagnosis prior treatment or non-CML donors. Samples were enriched for CD34+ cells using CliniMACS (Miltenyi Biotec Inc., Auburn, CA, USA) according to the manufacturer’s instructions. Samples were cultured as previously described (21). CD34+38− population was isolated as previously described (15). After treatment for 24 hrs as indicated, CD34+ cells were set up in Long-Term Culture-Initiating Cell (LTC-IC) assays as previously described (14). The Ph+ BC K562 cell line was maintained in 10% FCS/RPMI 1640 medium (Invitrogen, Paisley, UK).
Flow cytometry (FACS) and synergy analysis
Cells were stained with Annexin-V FITC or Annexin-V APC and 7-Amino-actinomycin D (7-AAD; BD Via-Probe) in Annexin-V binding buffer (both from Becton Dickinson, Oxford, UK). The level of active caspase-3 was assessed by intracellular staining. Cells were re-suspended in ‘Fix and Perm’ (Merck, Whitehouse Station, USA). The primary anti-caspase-3-PE antibody (Becton Dickinson) was incubated for 1 hr. 10 μM FMK-ZVAD (Bachem, St. Helens, UK) was added to the culture 2 hrs prior to treatment with BMS-214662 and PD184352. Decrease in mitochondrial membrane potential was detected using 50 nM TMRE (Cell Technology, CA, USA) for 20 mins at 37°C. FACS analysis was performed using the FACSCanto Flow Cytometer (Becton Dickinson). Data analysis was performed with FACSDiva (Becton Dickinson) or FlowJo (Tree Star, Ashland, USA) software. Drug synergy for PD184352 and BMS-214662 was determined after 24 hrs culture using K562 viable cell gate in Annexin-V and 7-AAD measurement and the median-effect method of Chou and Talalay (22) and analyzed using CalcuSyn software (Biosoft, Cambridge, UK).
K562 transfection and lentiviral infection
The K-RAS-N17 dominant negative mutant plasmid was obtained from Dr D Matallanas-Gomez (23). K562 cells were transfected using Amaxa nucleofection with cell-line Nucleofector® Kit V (Lonza Group Ltd, Cologne, Germany) as per manufacturer’s instructions. 5 μg of DNA from K-RAS N17 plasmid was transfected. RAS activity was detected using a RAS activation assay (Millipore, Billerica, MA, USA) according to the manufacturer’s instructions. Efficiency of the pull down assay was shown by using positive and negative controls (GTPγS and GDP, respectively). DN-MEK1 plasmid was obtained from Prof Chris Marshall (24). For retroviral infection, virus was produced by transfection of Phoenix cells with DN-MEK1 and control empty plasmids by the calcium phosphate method. Virus containing supernatant was collected 48 and 72 hrs following transfection, filtered through a 0.45-μm filter and added to K562 cells. Polybrene was added to a final concentration of 8 mg/ml. Infected K562 cells were selected in 5 μg/ml puromycin.
Western blotting
Western blotting was performed as previously described (15). Incubation at 1:500 (p-ERK, p-STAT5, STAT5 and MCL-1) or 1:1000 (caspase-8, caspase-9, PARP, HDJ2, ERK and GAPDH) dilution of primary antibodies was carried out at 4°C overnight, and with secondary antibody (1:5000 anti-rabbit HRP) for 1 hr at room temperature and then the blots were developed with the ECL kit (Immun-Star WesternC; Bio-Rad, Hemel Hempstead, UK) according to the manufacturer’s instructions. All the above antibodies were purchased from New England Biolabs (Hitchin, UK). Anti-RAS (RAS10, Millipore) was used to show RAS pull down.
Statistical analysis
Statistical analyses were performed using the Student’s T-test. A level of p≤0.05 was taken to be statistically significant. A level of p≤0.005 was taken to be highly statistically significant.
Results
Co-treatment with PD184352 and BMS-214662 synergistically enhances apoptosis in K562 cell line
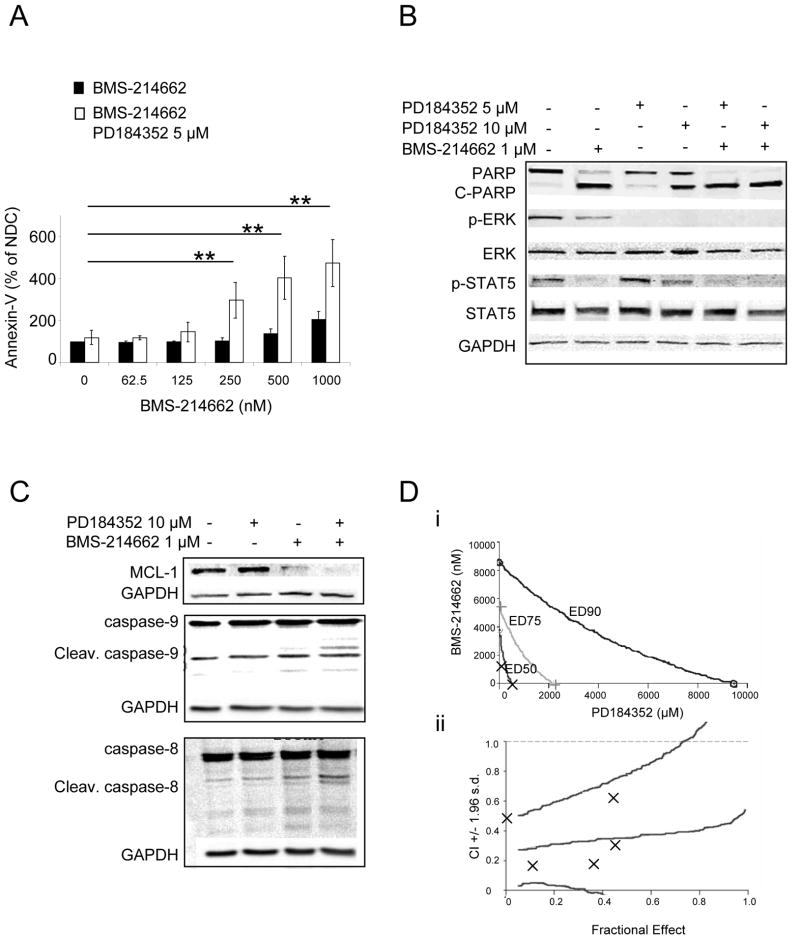
BCR-ABL-positive K562 cells are sensitive to combination treatment with dasatinib and MEK inhibitors (17). Therefore, we investigated the activity of BMS-214662 in combination with PD184352 in K562 cells and whether there are synergistic or additive effects between these two drugs. Treatment of K562 cells with increasing concentrations of BMS-214662 for 24 hrs resulted in only a modest increase in early apoptosis. However, co-administration of 5 μM PD184352 with BMS-214662 resulted in significantly enhanced early apoptosis (p=0.008) (Figure 1A). Whereas an incomplete cleavage of PARP (the main target of the pro-apoptotic factor caspase-3) was still noticeable in the sample treated with BMS-214662 alone, the un-cleaved band completely disappeared after co-treatment with PD184352 (Figure 1B). 5 μM PD184352 showed almost no effect on PARP cleavage, whereas it did have an effect at 10 μM, although not to the same extent as with the combination. To prove that PD184352 blocks the MAPK pathway, phosphorylation of ERK, the unique substrate of MEK, was measured (Figure 1B). Addition of PD184352 inhibited phosphorylation. Only a mild decrease in p-ERK was detected using BMS-214662 alone, which likely affected MAPK signalling through inhibition of RAS farnesylation. These data support the findings that BMS-214662 target CML cells not entirely through its FTI activity as previously shown (15). The STAT family proteins are among the downstream proteins highly activated by BCR-ABL (25). Interestingly, strong inhibition of p-STAT5 on tyrosine 694 - the major phosphorylation site by SRC kinases in K562 cells (26) - was observed after treatment with BMS-214662 (Figure 1B). Little decrease was observed when 5 μM PD184352 was added, whereas it was more evident with 10 μM PD184352. The combination with PD184352 and BMS-214662 showed a complete inhibition of STAT5 phosphorylation, presumably due mostly to BMS-214662 activity. We have recently shown that BMS-214662 induces mitochondrial apoptosis in CML stem/progenitor cells (15). The mitochondrial anti-apoptotic protein MCL-1 is known to be constitutively activated by BCR-ABL, through activation of the RAS-RAF-MAPK pathway (27). Down-regulation of MCL-1 was seen as a result of treatment with BMS-214662, which was increased in combination with PD184352 (Figure 1C). PD184352 alone had no effect on MCL-1 levels. To further investigate the effect of the treatment on the apoptotic pathways we investigated activation of caspase-8 and caspase-9. We observed cleavage of caspase-9 after combination treatment with BMS-214662 and PD184352. Neither of these drugs alone caused cleavage of caspase-9 (Figure 1C). Analysis of caspase-8 also revealed cleavage after combination treatment (Figure 1C).
Figure 1. Efficiency of combined treatment with PD184352 and BMS-214662 against K562.
(A) Cells as indicated for 24 hrs and subjected to measurement of Annexin-V and 7AAD. (B–C) Cells were exposed to BMS-214662 and PD184352 for 24 hrs and lysed analysed by Western blotting (NDC, no drug control). (D) A conservative isobologram for K562 cells indicated synergism between BMS-214662 and PD184352 (n=3) (i). Combination of BMS-214662 plus PD184352 indicated synergistic activity (CI values < 1). CI values are represented by points below the dotted line (n=3) (ii).
Finally, we investigated synergism between BMS-214662 and PD184352 on K562 cells using the CalcuSyn software program. Combination Index (CI) at IC50 was 0.357 for doses ranging from 125 to 2000 nM of BMS-214662 and from 2.5 to 40 μM of PD184352, mixed in a constant ratio of 20:1 (Figure 1D), indicating that this combination was synergistic.
Taken together, these data indicate that exposure of K562 to a combination of BMS-214662 and PD184352 caused a demonstrable enhancement in apoptosis. This is presumably as a result of the apoptotic effect of BMS-214662, which is potentiated by the abrogation of MAPK activation.
MEK inhibition with K-RAS N17 and DN-MEK enhances BMS-214662/PD184352- induced apoptosis in K562 cells
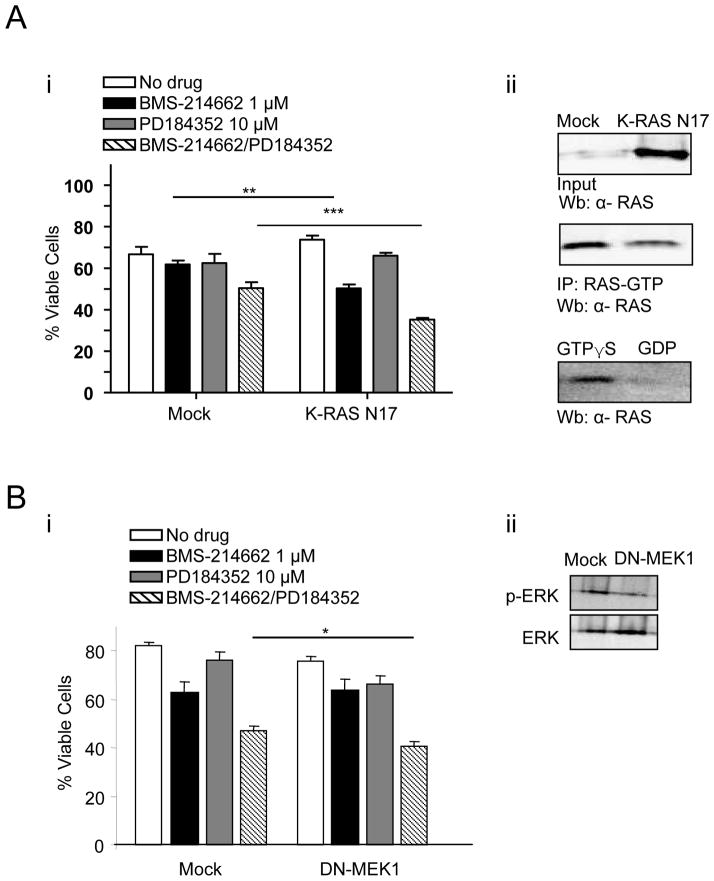
To confirm the involvement of the RAS pathway in BMS-214662-induced apoptosis, we blocked the function of the K-RAS protein by transfecting a K-RAS N17 dominant negative mutant into K562 cells (Figure 2A). This particular RAS mutant, with a substitution of serine 17 by asparagine (RAS N17), competes with the wild-type form of RAS (23). BMS-214662 treatment alone resulted in a reduction in cell viability in the K-RAS N17 mutant compared to the mock treated cells (Fig. 2Ai). Furthermore, the combination of BMS-214662 and PD184352, used at sub-lethal concentrations, resulted in significantly greater reduction of viable cells in the K-RAS N17 mutant compared to the mock treated cells. This confirms the importance of RAS inhibition to the combination effect of BMS-214662 with MAPK inhibition. RAS pull down assay was used to demonstrate the reduction of active RAS in K-RAS N17 transfected cells in comparison to the mock treated control (Fig. 2Aii). Finally K562 cells were retrovirally infected with the dominant negative form of MEK1 (DN-MEK1) (24) and treated with BMS-214662 or PD184352 or the combination (Figure 2B). The combination of BMS-214662 and PD184352 resulted in significant reduction of viable cells in the DN-MEK1 cells compared to the mock infected cells (Figure 2Bi). Western blot analysis showed a decrease in p-ERK after infection with DN-MEK1 (Figure 2Bii).
Figure 2. Inhibition of RAS and MEK increased BMS-214662/PD184352-induced cell death.
(A) K562 were transfected with K-RAS N17. After 24 hrs of treatment, viable cells were measured (n=3) (**p<0.005; ***p<0.0005) (i). Protein lysates from K562 transfected with K-RAS N17 and the empty vector (Mock) were analysed for active RAS using a RAS-GTP pulldown assay. GTPγS (positive) and GDP (negative) treated lysates act as controls for the assay (ii). (B) K562 were infected with DN-MEK1. After 24 hrs of treatment, viable cells were measured (n=3) (*p<0.05) (i). Protein lysates from K562 infected with DN-MEK1 and the empty vector control (Mock) were analysed for p-ERK (ii).
BMS-214662-induced apoptosis in CD34+ CML stem/progenitor cells is further enhanced by PD184352 through inhibition of MEK
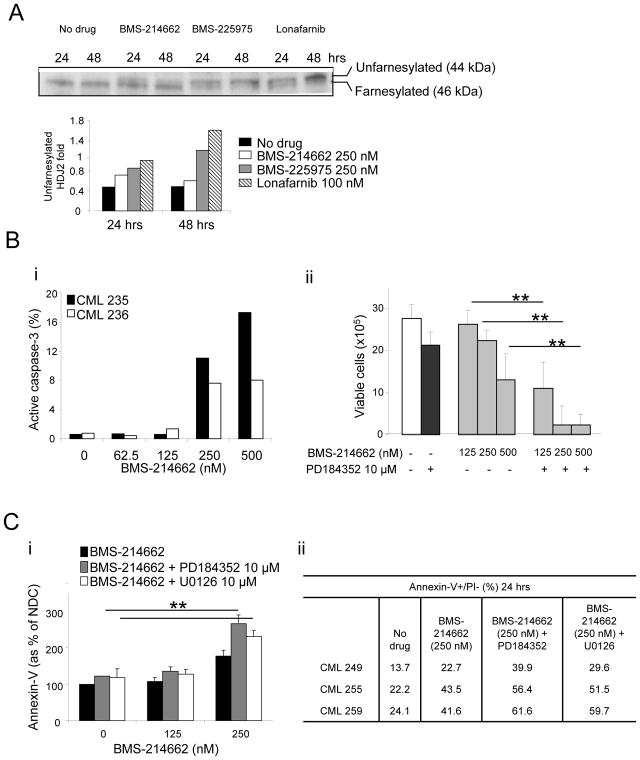
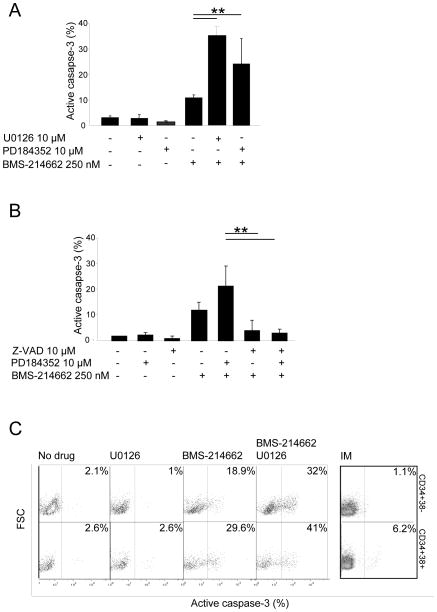
FTI activity of BMS-214662 in CD34+ primary CML cells was compared to that of other FTIs, BMS-225975 and lonafarnib, by measuring farnesylation of HDJ2 chaperone protein. The upper band indicated farnesylated protein, whereas the lower band indicated unfarnesylated protein. Compared to no drug control, BMS-214662 showed a mild increase in unfarnesylated HDJ2 after 24 hrs of treatment (Figure 3A). BMS-225975 and lonafarnib showed a stronger upper band, suggesting stronger activity than BMS-214662 in terms of FTI activity. CD34+ CML cells were treated with a range of concentrations of BMS-214662 (Figure 3Bi). BMS-214662 alone was able to induce activation of caspase-3 in these cells at the concentration of 250 nM. These data confirm previous observations in which primary CML CD34+ cells showed higher sensitivity to BMS-214662 compared to cell lines, which required high concentrations of the drug to enter apoptosis (IC50 for primary CML: 62.5 nM; IC50 for K562 cells: 2 μM) (14, 28). CML CD34+ cells were then treated with different concentrations of BMS-214662 alone or in combination with of PD184352 (n=3) (Figure 3Bii). Treatment combination with the MEK inhibitor significantly decreased the viable cell count compared to BMS-214662 alone, particularly when high concentrations of BMS-214662 were added (p=0.01, p=0.009, p=0.0006). 10 μM PD184352 alone had a minor effect on CD34+ cells. The enhanced effect of PD184352 on BMS-214662-induced apoptosis was then examined in CD34+ CML cells from patients in CP (n=3) (Figure 3Ci). Co-administration of 10 μM PD184352 with 250 nM BMS-214662 for 24 hrs resulted in a significant increase in early apoptosis (p=0.002). Addition of 10 μM of the equally effective MEK inhibitor U0126 enhanced the apoptotic effect of BMS-214662 in a similar manner (p=0.007), proving that inhibition of the MAPK pathway, in particular of MEK, leads to enhancement of BMS-214662-induced apoptosis (Figure 3Ci–ii). Western blotting was performed to analyze cleavage of the apoptotic marker PARP after treatment of CD34+ CML cells (Figure 4A). Although BMS-214662 alone caused cleavage of PARP after 24 hrs, a further increase in PARP cleavage was observed in the combination treatment with PD184352. We then investigated the phosphorylation of ERK and found it was blocked after 24 hrs of treatment with PD184352. As we observed in K562, a milder effect was detected after treatment with BMS-214662, supporting the previous indication that BMS-214662 has weak FTI activity in CML cells (Figure 3A). The decrease of p-ERK observed with the combination therapy was likely to be caused mainly by the addition of PD184352.
Figure 3. Combination treatment with PD184352 and BMS-214662 targets primary CD34+ cells.
(A) The effects on HDJ2 farnesylation with FTIs was assessed by analyzing the mobility gel shift, comparing 44 to 46 kDa. The percentage of unfarnesylated HDJ2 was determined for each time point by performing densitometry and ratio between the upper and lower band. (B) Percentage of active caspase-3 in CD34+ CML cells (n = 2) was measured after 24 hrs of treatment with BMS-214662 at the concentrations indicated (i). Total viable cells were counted after 24 hrs culture in the different treatment arms (n = 3) (ii). (C) CD34+ cells were treated for 24 hrs as indicated and Annexin-V was measured to assess level of early apoptosis (n=3) (i). CML samples used and percentage of Annexin-V positive cells after each treatment are indicated in the table (ii) (*p<0.05; **p<0.005).
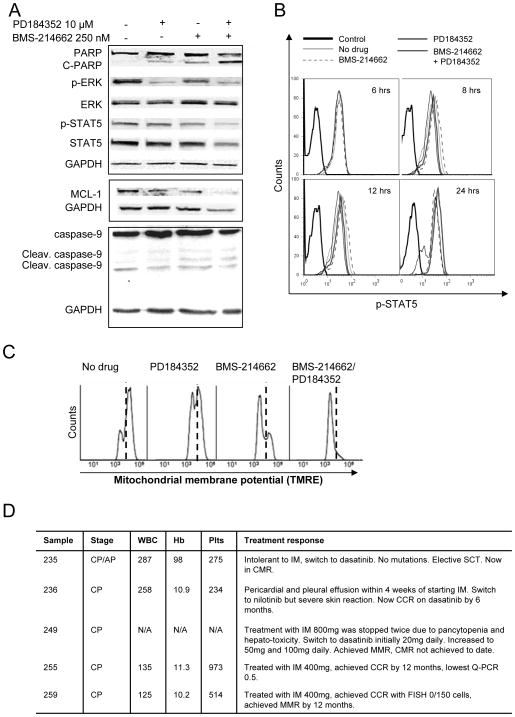
Figure 4. Treatment with PD184352 and BMS-214662 induces mitochondrial apoptosis.
(A) Cells were exposed to 250 nM of BMS-214662 and 10 μM of PD184352 either alone or in combination and lysed analysed by Western blotting. (B) Cells were treated as above and time course analysis and p-STAT5 measured. (C) CD34+ CML cells were treated with 250 nM BMS-214662 alone or in combination with 10 μM PD184352 and mitochondrial membrane potential was measured. (D) Detailed information of CML patients/samples used for this study. White blood count (WBC), haemoglobin (Hb), platelets (Plts) at diagnosis are reported. Complete cytogenetic response (CCR), Stem cell transplant (SCT), major molecular response (MMR).
In K562 we detected a decrease in p-STAT5 after treatment with BMS-214662, which was further enhanced with the addition of PD184352. Densitometry analysis relative to GAPDH loading control (not shown) showed that in CD34+ cells both the levels of total STAT5 and p-STAT5 decreased after combination treatment with BMS-214662 and PD184352, likely due to cell death. A similar effect was observed when a time course treatment with the drugs alone or in combination was performed. After 6, 8 and 12 hrs no effect was detected, but after 24 hrs there was a clear decrease in p-STAT5 in the combination treatment (Figure 4B). Finally, we analysed the level of MCL-1 and the cleavage of caspase-9 in CML CD34+ cells. We found that a decrease in MCL-1 levels was detected after 24 hrs treatment with BMS-214662 and PD184352 (Figure 4A), although because of reduction in GAPDH at this time point the change was not dramatic. Increase in the level of cleaved caspase-9 was detected after treatment with BMS-214662 alone and no further increase was observed in the drug combination.
To additionally characterise the apoptotic effects of the combination of BMS-214662 and PD184352, we examined changes in mitochondrial membrane potential. We have previously shown that BMS-214662 alone causes a decrease in mitochondrial membrane potential (15). Similar results were obtained in this study (Figure 4C). When PD184352 was added, the mitochondrial membrane potential further decreased, suggesting that the combination treatment caused a more effective activation of the mitochondrial apoptotic pathway. All the samples used for this study were from patients at diagnosis and before therapy and detailed information of CML patients/samples used for this study is included (Figure 4D).
Combination treatment with BMS-214662 and MEK inhibitors leads to enhanced activation of caspase-3 in CD34+ and CD34+38− populations
In CD34+ CML cells caspase-3 activation was detected with 250 nM BMS-214662, but the co-addition of either PD184352 or U0126 greatly increased the effect (p<0.005). PD184352 or U0126 alone did not induce activation of caspase-3 (Figure 5A). Parallel studies were performed in CD34+ CML cells treated with BMS-214662 and PD184352 exposed to the pan-caspase inhibitor FMK-ZVAD (Figure 5B). We observed that by inhibiting caspases the apoptosis measured by caspase-3 activation was rescued, both with BMS-214662 alone and in combination with PD184352. CD34+38− cells are the most primitive fraction within bulk CD34+ cells and have previously been shown to be predominantly quiescent (29). Standard TKIs are ineffective in eradicating CML, probably due to their incapacity to target this sub-population. Here CD34+38+ cells were separated from CD34+38− (<5% of total CD34+ cells) CML cells by FACS. We treated the CD34+38+ and CD34+38− CML cells with 250 nM of BMS-214662, 10 μM U0126, or the combination (Figure 5C). As expected, after 24 hrs an increase in activation of caspase-3 was detected in both populations after treatment with BMS-214662 alone, although the more mature cells were more sensitive (18.9 and 29.6%, respectively). U0126 alone did not have any effect on the cells (1 and 2.6% respectively). A dramatic increase in apoptosis was detected when BMS-214662 was combined with U0126, both in CD34+38+ and CD34+38− CML cells (32 and 41%, respectively) (n=1, due to limitation of sample availability). IM treatment only caused a mild increase in apoptosis in CD34+38+ but not in CD34+38− (6.2 and 1.1% respectively). Taken together these experiments confirmed that apoptosis induced by a combination of BMS-214662 and PD184352 leads to a strong activation of the caspase-mediated apoptotic pathway.
Figure 5. Treatment with MEK inhibitors and BMS-214662 induces activation of caspase-3.
(A) Percentage of active caspase-3 in CD34+ CML cells (n = 3) was measured after 24 hrs of treatment with BMS-214662, PD184352, U0126 or the combination at the concentrations indicated. (B) Co-treatment of the CD34+ CML cells with FMK- ZVAD pan-caspase inhibitor rescued apoptosis caused both by BMS-214662 alone and in combination with 10 μM PD184352 after 24 hrs. (C) Treatment of CD34+38− and CD34+38+ with BMS-214662 and U0126, but not with IM, induced activation of caspase-3 in both populations after 24 hrs (n=1).
Combination treatment with BMS-214662 and MEK inhibitors is relatively selective for CML versus normal stem/progenitor cells
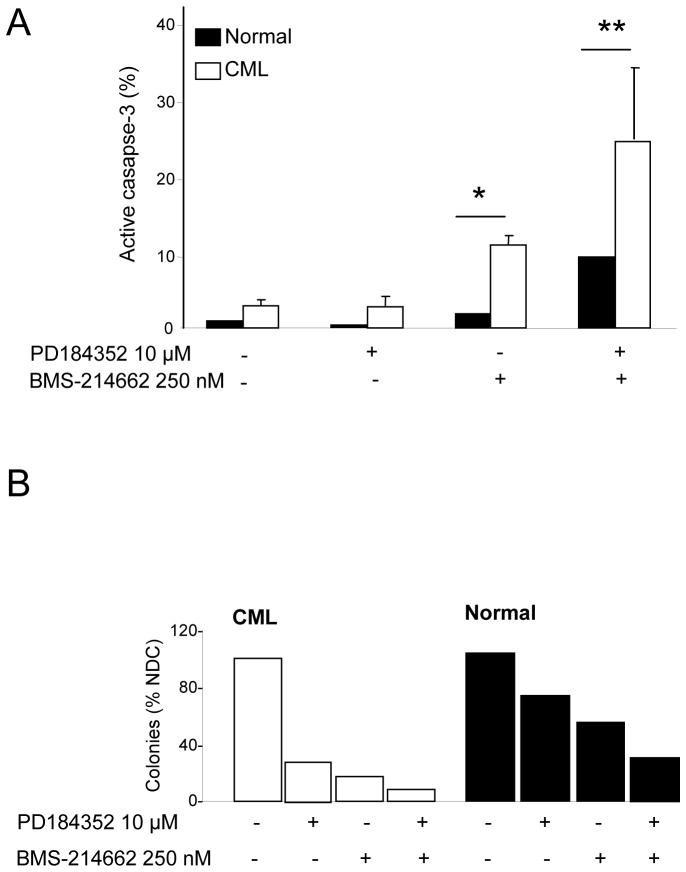
Activation of caspase-3 was compared between CML and normal CD34+ cells. BMS-214662 was selective in inducing apoptosis in CML CD34+ cells after 24 hrs of treatment (Figure 6A). Combination of PD184352 and BMS-214662 caused a far greater increase in caspase-3 activation in CML (24.1%) than in normal cells.
Figure 6. Combination treatment with PD184352 and BMS-214662 is more selective for CML versus normal cells.
(A) Percentage of active caspase-3 in CD34+ CML (n=3) and normal cells (n = 1) was measured after 24 hrs of treatment with BMS-214662 and PD184352 or with the combination of both drugs (*=p<0.05; **p<0.005). (B) (Left panel) Results of LTC-IC assay in CML stem cells (n=1). (Right panel) Results of LTC-IC assay in normal stem cells (n=1). Colony counts are normalised according to the cell counts after 5 weeks cultivation on stromal layer.
To measure the effect of combination treatment on the most primitive CML cells, we performed LTC-IC assays with CML and normal CD34+ cells after 24 hrs of treatment with PD184352, BMS-214662 or BMS-214662 plus PD184352 (Figure 6B). Surprisingly, in CML CD34+ cells, the addition of PD184352 alone reduced LTC-IC compared to the no drug control. Similarly, BMS-214662 reduced the number of colonies compared to PD184352 alone, but the strongest effect on LTC-IC was observed after the treatment with the combination of PD184352 and BMS-214662. In normal CD34+ cells we observed a similar but weaker trend and the decrease in the number of colonies was not as marked, suggesting that the combination of PD184352 and BMS-214662 is relatively selective for CML versus normal stem cells (n=1, due to limitation of sample availability).
Discussion
In this study, we investigated the activity of BMS-214662 and PD184352 in targeting the CML BC cell line, K562, and primary CD34+ CML stem/progenitor cells. CML is one of the most extensively studied malignancies and serves as a paradigm for targeting molecular pathogenic events. A single genetic abnormality allows attention to be focused on deregulated BCR-ABL signal transduction and the pivotal role of BCR-ABL downstream MAPK proteins in intracellular signalling makes them attractive therapeutic targets.
We have previously shown that the FTI BMS-214662 selectively targets quiescent CML stem cells, by a mechanism that is not solely through inhibition of farnesylation of RAS, which BMS-214662 was specifically designed to inhibit (15). The unique ability of BMS-214662 to target quiescent CML stem/progenitor cells is partially explained by aberrant activation of CDK2 and an up-regulation of PKCβ, in addition to FT inhibition. These changes result in activation of the intrinsic apoptotic pathway (15). Here, we have focused on targeting the MAPK pathway in CML cells, by using both a specific MEK inhibitor and the FTI BMS-214662. BMS-214662, compared to FTIs BMS-225975 and lonafarnib, did not show as marked FTI activity, despite its ability to target primitive stem/progenitor cells. We hypothesised that combining BMS-214662 with an additional inhibitor of the MAPK pathway may improve its activity, either as a FTI or through another mechanism. Previous studies have shown that PD184352 interacts synergistically with TKIs to induce apoptosis in BCR-ABL-expressing cells (17, 18). Therefore, we used PD184352 in combination with BMS-214662. The data presented suggest that the combination of BMS-214662 with the MEK inhibitors PD184352 or U0126 induces a greater level of apoptosis in K562 and CD34+ CML cells than BMS-214662 alone. We confirmed enhanced apoptosis by 24 hrs of combination treatment using various methods, including Annexin-V in combination with 7-AAD, measurement of active caspase-3, caspase-8, caspase-9 and PARP. In K562 we also detected a strong decrease in the expression of the anti-apoptotic protein MCL-1, which has previously been implicated in the pathogenesis of CML and cleavage of caspase-8 (30, 31). Taken together, these data suggest, that combination treatment affects the mitochondrial pathway of apoptosis in CML cells. It is still not clear whether cleavage of caspase-8 was a downstream effect of active caspase-3 or caused by activation of a parallel non-mitochondrial apoptotic pathway.
In K562 we found a decrease in p-STAT5 on Tyrosine 694 after treatment with BMS-214662. Interestingly, p-STAT5 down-regulation was seen also in CD34+ CML cells, but together with decreased level in total STAT-5. Similarly for p-ERK, the decrease in phosphorylation detected was likely to be caused solely by PD184352, since no additional effect was seen after the combination of the two drugs or BMS-214662 alone. These results suggest the possibility of a difference in drug responses between K562 and primary CML CP cells. In fact, our previous studies suggested that CD34+ CML cells are more sensitive to BMS-214662 than K562 cells (14, 28). This difference may be due to the fact that K562 is a BC cell line, whereas the CD34+ cells used here were CP. In addition K562 cells have aberrant copy numbers of BCR-ABL, which is likely to provide stronger survival signalling. We have previously shown that, although inducing mitochondrial apoptosis, BMS-214662 does not affect the protein level of the BCL-2 family members, including BCL-2, MCL-1, BIM and BCLXL. Here we have shown that the addition of PD184352 to BMS-214662 blocks the expression of the anti-apoptotic protein MCL-1 in the CML cell line (only modestly in primary CML cells), whereas the two drugs alone do not have a significant effect. It is unlikely that increased potency of BMS-214662 in inducing apoptosis in primary CML cells after the addition of PD184352 is caused solely by the mild inhibition of MCL-1. CML cells express MCL-1 in a constitutive manner and dominant-negative RAS mutant, RAS-N17, reversed BCR-ABL-dependent MCL-1 activity in Ba/F3 cells (27). The role that MCL-1 plays in the survival and accumulation of leukaemic cells in CML patients is still unclear.
Finally, inhibition of the MAPK pathway by using DN forms for RAS and MEK together with the combination of the drugs, further decreased cell viability. Interestingly, when either DN form was used with BMS-214662 this did not mirror the effect of PD184352 when combined with BMS-214662, suggesting that full inhibition of the pathway, likely given only by PD184352, is necessary to see an enhanced effect on cell death.
Results of this work suggest that inhibition of the RAS-MEK-ERK pathway by MEK inhibitors increases the apoptotic effect of BMS-214662 in CD34+ CML stem/progenitor cells. BMS-214662 does not appear to have a strong FTI activity in CML primary cells and it is possible that the addition of a second agent targeting the RAS pathway potentiates its ability to inhibit this signalling cascade, leading to the inhibition of the protective factor MCL-1 and consequently causing enhanced apoptosis. It is also plausible that the addition of a MEK inhibitor may potentiate the off target effects of BMS-214662, independently from its FTI activity. However, until the exact mechanism of action of BMS-214662 is understood this remains speculative. This study suggests an important role for potent MEK inhibitors in the treatment of CML.
Acknowledgments
We thank Dr R. Weinmann and Dr F. Lee (Bristol-Myers Squibb, Princeton) for providing BMS-214662 and BMS-225975, Prof C. Marshall for the DN-MEK1 plasmid and Dr D Matallanas-Gomez for the K-RAS N17 plasmid. FP received funding from Bristol-Myers Squibb, the Rockefeller Foundation, Chief Scientific Office and Glasgow Royal Infirmary Endowments. PS received funding from the Ministry of Education of Czech Republic MSM-0021622430 and NPVII (2B06052). GVH and AS received funding from Biotechnology and Biological Sciences Research Council and Medical Research Council. SG received funding from the NCI (CA 100866-01, CA93738-05) and the Leukemia and Lymphoma Society of America (LSA-6181-10). We would like to thank all UK CML patients and normal donors, haematologists and non-medical staff who have contributed to our biobank.
Footnotes
Authorship
Contributions: FP designed the research. FP, PS, AS and GVH performed the experiments and analysed the data. TLH and MC provided suggestions in experimental design. FP, PS and TLH co-wrote the manuscript. TLH supervised the project. SG provided PD184352. All authors commented on the manuscript.
Conflict of interest disclosure
TLH receives per year approximately $10 000 combined from consultancy, speaking fees, honoraria, and service on advisory boards from Bristol-Myers Squibb and Novartis. TLH also received research funding from Bristol-Myers Squibb. MC has undertaken consultancy work for Bristol-Myers Squibb and is on its Advisory Board and has received honoraria from both Bristol-Myers Squibb and Novartis within the last 2 years. The remaining authors declare no competing financial interests
References
- 1.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 2.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 3.Druker BJ. Translation of the Philadelphia chromosome into therapy for CML. Blood. 2008;112:4808–4817. doi: 10.1182/blood-2008-07-077958. [DOI] [PubMed] [Google Scholar]
- 4.Hughes T, Branford S. Molecular monitoring of chronic myeloid leukemia. Semin Hematol. 2003;40:62–68. doi: 10.1053/shem.2003.50044. [DOI] [PubMed] [Google Scholar]
- 5.Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 6.Chu S, Allen L, Tinisha M, David S, Stephen F, Ravi B. Persistence of Leukemia Stem Cells in Chronic Myelogenous Leukemia Patients in Complete Cytogenetic Remission on Imatinib Treatment for 5 Years. Blood. 2008;112:194. (ASH Annual Meeting Abstracts) [Google Scholar]
- 7.Weisberg E, Manley PW, Breitenstein W, Broggen J, Cowan-Jacob SW, Ray A, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 9.Nicaise C, Wang X, Roy A, Pfister M, Chen T-T, Bleickardt E, et al. Dasatinib pharmacokinetics and exposure-response: relationships to efficacy and safety in patients with chronic myelogenous leukemia in chronic phase (CML-CP) Haematologica. 93 (EHA Annual Meeting Abstracts) [Google Scholar]
- 10.Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 11.Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109:4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- 12.Mahon FX, Rea D, Guilhot J, Guilhot F, Huguet F, Nicolini F, et al. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 13.Gajate C, Mollinedo F. The antitumor ether lipid ET-18-OCH(3) induces apoptosis through translocation and capping of Fas/CD95 into membrane rafts in human leukemic cells. Blood. 2001;98:3860–3863. doi: 10.1182/blood.v98.13.3860. [DOI] [PubMed] [Google Scholar]
- 14.Copland M, Pellicano F, Richmond L, Allan EK, Hamilton A, Lee FY, et al. BMS-214662 potently induces apoptosis of chronic myeloid leukemia stem and progenitor cells and synergizes with tyrosine kinase inhibitors. Blood. 2008;111:2843–2853. doi: 10.1182/blood-2007-09-112573. [DOI] [PubMed] [Google Scholar]
- 15.Pellicano F, Copland M, Jorgensen HG, Mountford J, Leber B, Holyoake TL. BMS-214662 induces mitochondrial apoptosis in chronic myeloid leukemia (CML) stem/progenitor cells, including CD34+38− cells, through activation of protein kinase Cbeta. Blood. 2009;114:4186–4196. doi: 10.1182/blood-2009-05-219550. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen HG, Allan EK, Graham SM, Godden JL, Richmond L, Elliott MA, et al. Lonafarnib reduces the resistance of primitive quiescent CML cells to imatinib mesylate in vitro. Leukemia. 2005;19:1184–1191. doi: 10.1038/sj.leu.2403785. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TK, Rahmani M, Harada H, Dent P, Grant S. MEK1/2 inhibitors sensitize Bcr/Abl+ human leukemia cells to the dual Abl/Src inhibitor BMS-354/825. Blood. 2007;109:4006–4015. doi: 10.1182/blood-2006-09-045039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yu C, Krystal G, Varticovksi L, McKinstry R, Rahmani M, Dent P, et al. Pharmacologic mitogen-activated protein/extracellular signal-regulated kinase kinase/mitogen-activated protein kinase inhibitors interact synergistically with STI571 to induce apoptosis in Bcr/Abl-expressing human leukemia cells. Cancer Res. 2002;62:188–199. [PubMed] [Google Scholar]
- 19.Lorusso PM, Adjei AA, Varterasian M, Gadgeel S, Reid J, Mitchell DY, et al. Phase I and pharmacodynamic study of the oral MEK inhibitor CI-1040 in patients with advanced malignancies. J Clin Oncol. 2005;23:5281–5293. doi: 10.1200/JCO.2005.14.415. [DOI] [PubMed] [Google Scholar]
- 20.Barrett SD, Bridges AJ, Dudley DT, Saltiel AR, Fergus JH, Flamme CM, et al. The discovery of the benzhydroxamate MEK inhibitors CI-1040 and PD 0325901. Bioorg Med Chem Lett. 2008;18:6501–6504. doi: 10.1016/j.bmcl.2008.10.054. [DOI] [PubMed] [Google Scholar]
- 21.Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 22.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 23.Matallanas D, Arozarena I, Berciano MT, Aaronson DS, Pellicer A, Lafarga M, et al. Differences on the inhibitory specificities of H-Ras, K-Ras, and N-Ras (N17) dominant negative mutants are related to their membrane microlocalization. J Biol Chem. 2003;278:4572–4581. doi: 10.1074/jbc.M209807200. [DOI] [PubMed] [Google Scholar]
- 24.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 25.Coffer PJ, Koenderman L, de Groot RP. The role of STATs in myeloid differentiation and leukemia. Oncogene. 2000;19:2511–2522. doi: 10.1038/sj.onc.1203479. [DOI] [PubMed] [Google Scholar]
- 26.Okutani Y, Kitanaka A, Tanaka T, Kamano H, Ohnishi H, Kubota Y, et al. Src directly tyrosine-phosphorylates STAT5 on its activation site and is involved in erythropoietin-induced signaling pathway. Oncogene. 2001;20:6643–6650. doi: 10.1038/sj.onc.1204807. [DOI] [PubMed] [Google Scholar]
- 27.Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H, Florian S, Sonneck K, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 28.Copland M, Hamilton A, Allan EK, Brunton V, Holyoake TL. BMS-214662 Targets Quiescent Chronic Myeloid Leukaemia Stem Cells and Enhances the Activity of Both Imatinib and Dasatinib (BMS-354825) Blood. 2005;106:693. (ASH Annual Meeting Abstracts) [Google Scholar]
- 29.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 30.Fukuchi Y, Kizaki M, Yamato K, Kawamura C, Umezawa A, Hata J, et al. Mcl-1, an early-induction molecule, modulates activin A-induced apoptosis and differentiation of CML cells. Oncogene. 2001;20:704–713. doi: 10.1038/sj.onc.1204142. [DOI] [PubMed] [Google Scholar]
- 31.Yu C, Rahmani M, Almenara J, Subler M, Krystal G, Conrad D, et al. Histone deacetylase inhibitors promote STI571-mediated apoptosis in STI571-sensitive and -resistant Bcr/Abl+ human myeloid leukemia cells. Cancer Res. 2003;63:2118–2126. [PubMed] [Google Scholar]