Abstract
Background
Oxidative stress is postulated to contribute to the initiation, promotion, and progression of non-small cell lung cancer (NSCLC). We investigated the effects of supervised, moderate-intensity aerobic training on urinary markers of oxidative status in patients with postsurgical NSCLC.
Patients and Methods
Sixteen patients with histologically confirmed stage I-IIIB NSCLC were recruited. Exercise training consisted of aerobic cycle ergometry sessions at 60 to ≥70% of baseline peak workload 20–45 min·d−1, 3 d·wk−1for 14 weeks. Oxidative status was assessed via four urinary F2-isoprostanes isomers: iPF (2-alpha)-III, 2,3-dinor-iPF(2 alpha)-III, iPF (2-alpha)-VI, and 8,12-iso-iPF(2 alpha)-VI using liquid chromatography with tandem mass spectrometry detection. Peak oxygen consumption (VO2peak) was assessed using a maximal, incremental, cardiopulmonary exercise test with expired gas analysis.
Results
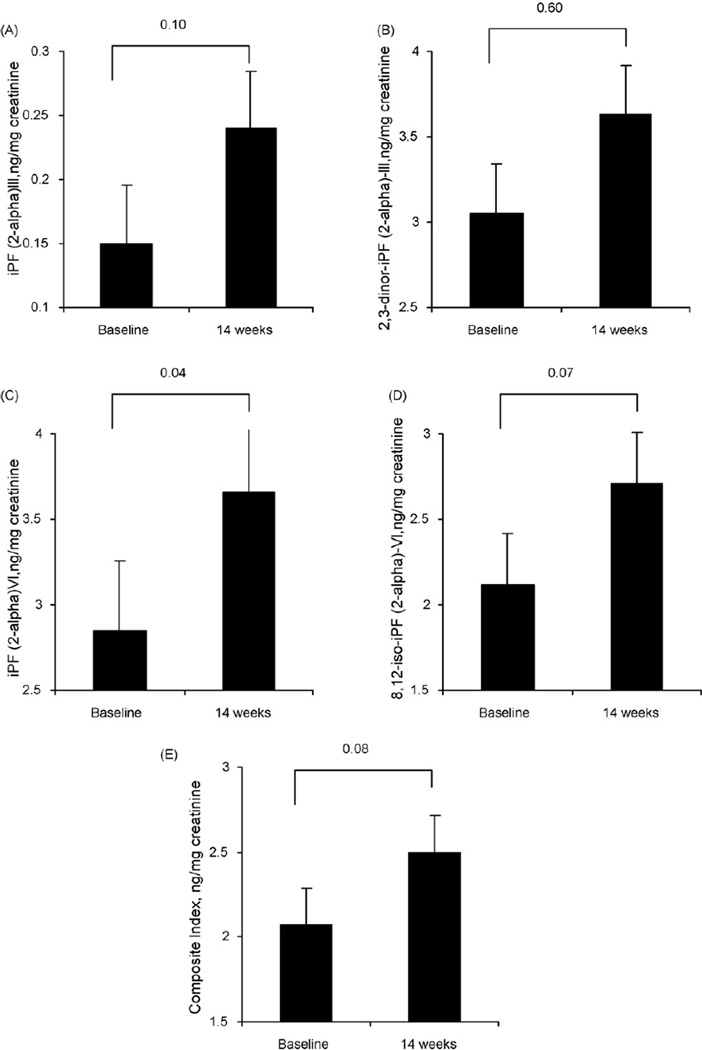
A composite index of all four F2-isoprostanes isomers increased from baseline to post-intervention by 32% (p = 0.08). Concerning individual isomers, iPF (2-alpha)-III increased by 0.09 (+55%; p = .010), iPF (2-alpha)-VI by 0.81 (+29%; p = 0.04), and 8,12-iso-iPF(2 alpha)-VI by 0.59 (+28%; p = 0.07) from baseline to postintervention. There was no change in 2,3-dinor-iPF(2 alpha)-III levels. VO2peak increased 1.1 mL.kg.−1min−1 (p = 0.14) and peak workload increased 10 Watts (p < .001). Change in VO2peak was not associated with change in markers of oxidative status.
Conclusions
Aerobic training was associated with significant increases in urinary measures of oxidative status in postsurgical NSCLC. The clinical implications of these findings are currently unknown. Further studies are required to elucidate the complex relationship between aerobic training, oxidative stress, tumor biology, and response to cytotoxic agents in mouse and human models of cancer.
Keywords: Exercise, Lung Cancer, Cardiorespiratory Fitness, Oxidative Stress
1. INTRODUCTION
The past decade has witnessed significant increase in research and clinical interest in the efficacy of exercise training as an adjunct therapy for patients diagnosed with non-small cell lung cancer (NSCLC) both before and following pulmonary resection.[1] Overall, the existent literature provides ‘proof of principle’ that exercise training is safe and feasible for presurgical and postsurgical NSCLC patients that may also be associated with significant improvements in cardiorespiratory fitness as well as patient-reported outcomes, including overall quality of life, lung cancer-specific quality of life, and fatigue.[2–9] Our group also found that peak oxygen consumption (VO2peak) was an independent predictor of mortality when controlling for performance status, gender, and age in 398 presur- gical NSCLC patients.[10] Together, these findings suggest that improvements in VO2peak via exercise training may be an attractive therapeutic target to improve prognosis in NSCLC.
Elucidation of the biologic mechanisms underpinning the potential relationship between exercise training and disease outcome in NSCLC is required to ensure mechanistically-driven trials that optimize the efficacy of exercise training.[11] Several pathways have been postulated, including modulation of metabolic and sex-steroid hormone levels, improved immune surveillance, and reduced systemic inflammation, as well as lowering oxidative damage via induction of antioxidant response to exercise-induced transient oxidative stress, although scant evidence exists to support these pathways.[11,12] Of the postulated biologic pathways, modulation of oxidative damage is a particularly relevant pathway that may mediate the exercise–NSCLC prognosis relationship.
In living cells, reactive oxygen species (ROS) are formed continuously, with mitochondria being the most significant source of ROS as an inevitable consequence of oxidative metabolism.[13,14] Endogenous antioxidant defense systems provide protection from noxious effects of ROS. The two opposing processes–ROS production and antioxidant defense–set constitutive levels of ROS within the tissues and at the systemic level, defining the internal oxidative environment or oxidative status of an individual.[13,14] Variability in the pro-oxidant–antioxidant balance may contribute to individual differences in susceptibility to chronic diseases including the initiation and progression of lung cancer.[15,16]
Exercise training transiently increases ROS production due to increased skeletal muscle oxidative metabolism. However, transient increases in ROS levels during chronic repeated bouts of aerobic training induces concomitant expression of endogenous antioxidant enzyme machinery that, in turn, should reduce the systemic ROS levels as well as provide enhanced protection against subsequent oxidative insult; a process known as hormesis.[13,14,17] Evidence supporting the role of oxidative damage in promotion and aggressiveness of NSCLC carcinogenesis together with the antioxidant properties of exercise training provides one plausible mechanism to explain the exercise–NSCLC prognosis relationship.
Against this background, we explored the effects of supervised aerobic training on systemic markers of oxidative status in postsurgical NSCLC patients. Oxidative status was assessed via urinary F2–isoprostanes, the most accurate assessment of systemic oxidative status in humans.[18] Isoprostanes are prostaglandin-like compounds formed via a non-enzymatic mechanism involving the free radical-initiated peroxidation of arachidonic acid.[18,19] Specifically, we measured iPF(2 alpha)-III, iPF(2 alpha)-VI, 8,12-iso-iPF(2 alpha)-VI along with the prostaglandin 2,3-dinor-iPF(2 alpha)-III, ametabolite of iPF(2 alpha)-III. All four biomarkers have been validated in a clinical model of oxidative stress.[20]
2. METHODS
2.1. Setting and Patients
Full details regarding the study sample, recruitment, and procedures have been reported elsewhere.[5] In brief, patients with histologically confirmed stage I–IIIB NSCLC being treated for curative or palliative intent at Duke University Medical Center (DUMC) between January 2006 and December 2007. The DUMC institutional review board approved the study and written informed consent was obtained from all participants prior to initiation of any study procedures. Using a prospective, single-group design, potential participants were identified and screened for eligibility via medical record review of patients scheduled for their new patient consultation. Following the successful completion of the baseline assessments, all participants were scheduled for supervised exercise training. After 14 weeks all baseline assessments were repeated. Urine samples were collected after an 8-hour, water only fast at both timepoints, and a minimum of 48 hrs following the completion of the last chemotherapy and aerobic training session at the postintervention timepoint.
2.2. Exercise Training Intervention
Exercise training consisted of three aerobic cycle ergometry (Lifestyle Fitness 9500HR; Life Fitness, Franklin Park, IL) sessions per week on nonconsecutive days for 14 weeks. In week 1, exercise intensity was initially set at 60% of baseline peak workload for a duration of 15 to 20 minutes. Duration and/or intensity were then subsequently increased throughout weeks 2 to 4 up to 30minutes at 65% peak workload. In weeks 5 and 6, exercise intensity varied between 60%–65% of peak workload for a duration of 30 to 45 minutes for 2 sessions; in the remaining session patients cycled for 20–25 minutes at ventilatory threshold determined by a systematic increase in the VE/VO2 ratio, while VE/VCO2 remained constant. From the 7th week onwards, patients performed 2 sessions at 60% to 70% peak workload with one threshold workout for 20–30 minutes. Finally, in weeks 10 to 14, patients performed 2 sessions at 60% to 70% peak workload with one interval session. Interval workouts consisted of 30 s at peak workload followed by 60 s of active recovery for 10–15 intervals. All exercise sessions included a 5-min warm-up and 5-min cool down. Exercise training intensity and safety was monitored continuously via heart rate and oxyhemoglobin saturation (SpO2) while blood pressure was monitored every two minutes.
2.3. Measurements of Urinary F2-isoprostanes
Four isomers of F2-isoprostanes–iPF(2 alpha)-III, 2,3-dinoriPF(2 alpha)-III, iPF(2 alpha)-VI, and 8,12-iso-iPF(2 alpha)-VI–were quantified by liquid chromatography-tandem mass spectrometry (LC-MS/MS) on Shimadzu 20A series LC and Applied Biosystems API 4000 QTrap MS/MS instruments.[21,22] Prior to isoprostane analysis, creatinine levels were determined by LC-MS/MS (1/1000 diluted urine with addition of creatinine-d3 as internal standard). Based on creatinine measurements, urine samples were diluted to 0.65mg/mL creatinine and samples with creatinine levels equal or below this value were analyzed without dilution. Sample preparation included: (1) addition of internal standards (iPF(2 alpha)-III-d4, 8,12-iso-iPF(2 alpha)-VI-d11, iPF(2 alpha)-VI-d4) and 10µL 1M HCl; (2) washing of sample (500µL) with 1mL hexane; (3) extraction of the analytes by ethyl acetate/hexane mixture (3/1, v/v); (4) evaporation of the liquid and re-suspension of the residue in 150µL of mixture containing 70% mobile phase A (0.1% formic acid in water) and 30% methanol. The samples (100µL) then were injected into LC-MS/MS system. Two solid core C18 columns (Phenomenex Kinetex C18, 150 × 4.6mm)in series were used to achieve chromatographic separation of the F2-isoprostane isomers. Mass spectrometer was operated in negative mode with the following MRM transitions (m/z): 353/193 [iPF(2 alpha)-III], 357/197 [iPF(2 alpha)-III-d4], 325/237 [2,3-dinor-iPF(2 alpha)-III], 353/115 [iPF(2 alpha)-VI and 8,12-iso-iPF(2 alpha)-VI], 364/115 [iPF(2 alpha)-VId11], 357/115 [8,12-iso-iPF(2 alpha)-VI-d4]. Calibration samples covering expected range of concentrations were prepared by addition of pure material into pooled human urine and were injected before and after the patient samples. Lower limits of quantification (LLOQ, >80% accuracy) were 0.007, 0.34, 0.25, and 0.12 ng/mL for iPF(2 alpha)-III, 2,3-dinor-iPF(2 alpha)-III, iPF(2 alpha)-VI, and 8,12-iso-iPF(2 alpha)-VI, respectively.
2.4. Measures of Cardiorespiratory Fitness and Pulmonary Function
Cardiorespiratory fitness was determined using an incremental, cardiopulmonary exercise test on an electronically-braked cycle ergometer (Ergoline, Ergoselect 100, Bitz, Germany) with breath-by-breath expired gas analysis (ParvoMedics TrueOne® 2400, Sandy, UT) as per recommendations for assessing peak oxygen consumption (VO2peak) in cancer patients.[23] Routine spirometry and single-breath diffusion capacity for carbon monoxide (DLCO) was measured in the sitting position according to American Thoracic Society guidelines.[24] Lung volumes were determined using a body plethysmograph (6200 Autobox; SensorMedics, Yorba Linda CA).
2.5. Statistical Analysis
Values equal to half quantification limit adjusted for creatinine were assigned to three samples with values below quantification limit. Agreement between all four biomarkers was evaluated using Chronbach’s alpha. To examine overall changes in oxidative status, a composite index using all four measurements was calculated as follows: each value was standardized (divided by the standard deviation for a corresponding biomarker at the corresponding time point), mean standardized value for four markers was calculated for each participant at baseline and post-intervention.
Analyses included all study participants (n = 16) regardless of adherence to the intervention. The paired t-test was used to test whether the mean values in the measurements of F2-isoprostanes differed significantly between at baseline and after intervention controlling for smoking status (i.e., ex-smoker vs. current smoker). To examine correlations between oxidative status markers and subjects’ characteristics, Pearson correlation coefficient was used for continuous and Wilcoxon test for categorical variables. A 2-sided alpha of 0.05 was used for all tests. No adjustment was made for multiple comparisons.
3. RESULTS
Details regarding response rates and profiles of the participants have been reported previously.[5] Briefly, 149 patients attended a new patient consultation at DUMC during the study period and 40 (40/149 = 27%) met inclusion criteria. Of these, 20 (20/40 = 50%) agreed to participate and 18 (18/20 = 90%) provided urine samples at baseline and post-intervention. Samples from two patients exhibited low LC-MS/MS signal and were excluded due to poor biomarker quantification for a total subsample of 16 (16/20 = 80%). Participant characteristics of this sub-sample are presented in Table 1. In brief, mean age was 63±10 years, 50% were male and mean body mass index was 26±8 kg/m2. Seventy five percent underwent a lobectomy, 39% were receiving adjuvant chemotherapy. The overall adherence rate was 86±19% (planned sessions/sessions attended). At baseline, there were no associations between any demographic and medical or cardiorespiratory fitness endpoints and any F2-isoprostanes (p > 0.05).
Table 1.
Characteristics of the Participants (n = 16)
| Variable | No. | % | |
|---|---|---|---|
| Mean ± SD age, yrs | 64 ± 10 | ||
| Male, % | 8 | 50 | |
| Mean ± SD weight, kg | 74 ± 16 | ||
| Mean ± SD BMI, kg/m2 | 24 ± 4 | ||
| Smoking History | |||
| Current | 2 | 12.5 | |
| Former | 14 | 87.5 | |
| Histologic features-no. (%) | |||
| Adenocarcinoma | 12 | 75 | |
| Squamous | 4 | 25 | |
| Undifferentiated | − | − | |
| Stage-no. (%) | |||
| IA | 8 | 50 | |
| IB | 2 | 12.5 | |
| IIA | 1 | 6.3 | |
| IIB | 1 | 6.3 | |
| IIIA | − | − | |
| IIIB | 4 | 25.0 | |
| Extent of Resection-no. (%) | |||
| Lobectomy | 10 | 71.4 | |
| Pneumonectomy | 1 | 7.1 | |
| Bilobectomy | 1 | 7.1 | |
| Wedge | 1 | 7.1 | |
| VATS | 1 | 7.1 | |
| Bronchoscopy | 1 | 7.1 | |
| Adjuvant therapy | |||
| Received Chemotherapy-no. (%) | 5 | 31.3 | |
| Received Radiotherapy-no. (%) | 1 | 6.3 | |
| Concomitant comorbidities-no. (%) | 13 | 81 | |
| Cardiopulmonary Function Data | |||
| VO2peak, mL·kg−1·min−1, predicted (%) | 15.7 ± 3.4 (63%) | ||
| VO2peak, mL·min−1 | 1.13 ± 0.21 | ||
| Workload, Watts | |||
| 72 ± 20 |
Abbreviations: BMI, body mass index; VO2peak, peak oxygen consumption.
3.1. Changes in Markers of Oxidative Status and Cardiorespiratory Fitness
Changes in F2-isoprostanes are shown in Table 2. Urinary levels of all F2-isoprostanes except 2,3-dinor-iPF(2 alpha)-III were higher following the completion of aerobic training, with statistical significant increases in iPF (2-alpha)-VI and 8,12-iso-iPF(2 alpha)-VI levels (p < 0.10); increase in iPF (2-alpha)-III was borderline statistically significant (p = 0.10).
Table 2.
Effects of Aerobic Training on Changes in Markers of Oxidative Status (n= 16)
| Variable | Baseline Mean±SD | Post-intervention Mean±SD |
Mean Difference [95% CI] |
Range of Mean Difference |
% change | P-values |
|---|---|---|---|---|---|---|
| F2-isoprostanes, ng/mg creatinine | ||||||
| iPF(2-alpha)-III | 0.15 ± 0.13 | 0.24 ± 0.22 | +0.09 [−0.02 to 0.19] | −0.140 to 0.77 | +55% | 0.10 |
| 2,3-dinor-iPF(2 alpha)-III | 3.05 ± 2.67 | 3.63 ± 4.02 | +0.59 [−1.96 to3.13] | −5.49 to 16.85 | +19% | 0.60 |
| iPF (2-alpha)-VI | 2.85 ± 1.33 | 3.66 ± 2.12 | +0.81 [0.06 to 1.57] | −1.79 to 3.07 | +29% | 0.04 |
| 8,12-iso-iPF(2alpha)-VI | 2.12 ± 1.25 | 2.71 ± 1.84 | +0.59 [−0.06 to 1.23] | −0.86 to 2.62 | +28% | 0.07 |
| Composite Index* | 1.61 ± 0.90 | 2.12 ± 1.33 | +0.51 [−0.08 to 1.10] | −0.81 to 3.49 | +32% | 0.08 |
Composite index was calculated as each value was standardized (divided by the standard deviation for a corresponding biomarker at the corresponding time point), mean standardized value for four markers was calculated for each participant at baseline and post-intervention.
Based on excellent agreement between the four biomarkers at each time point (p-values for Chronbach’s alpha were 0.90), the overall change in oxidative status was assessed using a composite index calculated for each participant at baseline and post-intervention. Statistical significant increases in the composite index were observed from baseline to postintervention (Table 2). Change in VO2peak nor total exercise volume did not correlate with change in individual markers of oxidative status or the composite index (p < 0.05).
3.2. Changes in Oxidative Status and Cardiorespiratory Fitness by Treatment Status
Changes in F2-isoprostanes by chemotherapy (received chemotherapy vs. no chemotherapy) are presented in Table 3. Exercise adherence was 93% and 75% for patients not receiving and those receiving chemotherapy, respectively. Changes in markers of oxidative status were similar in these subgroups. In terms of cardiorespiratory fitness, only patients not receiving chemotherapy showed increase in absolute VO2peak (p = 0.007) and relative VO2peak (1.7 mL.kg−1.min−1, p = 0.008), and peak workload (p < .001)(data not presented).
Table 3.
Effects of Aerobic Training on Changes in Markers of Oxidative Status by Adjuvant Therapy (chemotherapy vs. no chemotherapy) (n= 16)
| Variable | Baseline Mean ±SD | Post-intervention Mean±SD |
Mean Difference [95% CI] |
% change | P |
|---|---|---|---|---|---|
| iPF(2-alpha)-III | |||||
| Chemotherapy (n = 5) | 0.12 ± 0.10 | 0.18 ± 0.15 | +0.06 [−0.05 to 0.16] | +50% | 0.21 |
| No chemotherapy (n = 11) | 0.17 ± 0.14 | 0.27 ± 0.25 | +0.10 [−0.07 to 0.26] | +59% | 0.22 |
| 2,3dinor-iPF(2 alpha)-III | |||||
| Chemotherapy(n = 5) | 2.03 ± 1.12 | 2.24 ± 1.73 | +0.21 [−2.39 to 2.81] | + 10% | 0.81 |
| No chemotherapy(n = 11) | 3.51 ± 3.07 | 4.27 ± 4.65 | +0.76 [−3.06 to 4.58] | +22% | 0.70 |
| iPF (2-alpha)-VI | |||||
| Chemotherapy(n = 5) | 2.37 ± 1.13 | 3.27 ± 2.38 | +0.90 [−0.69 to 2.48] | +38% | 0.23 |
| No chemotherapy(n = 11) | 3.06 ± 1.40 | 3.84 ± 2.09 | +0.78 [−0.26 to 1.81] | +25% | 0.18 |
| 8,12-iso-iPF(2alpha)-VI | |||||
| Chemotherapy (n = 5) | 1.78 ± 1.06 | 2.44 ± 2.37 | +0.66 [−1.14 to 2.47] | +37% | 0.49 |
| No chemotherapy(n = 11) | 2.28 ± 1.35 | 2.84 ± 1.67 | +0.56 [−0.22 to 1.34] | +25% | 0.15 |
| Composite Index | |||||
| Chemotherapy (n = 5) | 1.28 ± 0.62 | 1.73 ± 1.41 | +0.46 [−0.59 to 1.50] | +35% | 0.23 |
| No chemotherapy(n= 11) | 1.76 ± 0.99 | 2.30 ± 1.33 | +0.54 [−0.30 to 1.38] | +31% | 0.22 |
4. DISCUSSION
The major finding of this exploratory study was that chronic supervised aerobic training was associated with statistical significant increases in urinary F2-isoprostanes, nonenzymatic products of arachidonic acid peroxidation which are considered sensitive biomarkers of oxidative status in humans.[18] Secondary findings were that change in VO2peak was not associated with change in F2-isoprostanes, and treatment with adjuvant chemotherapy did not modify this relationship. To our knowledge, this is one of the first studies to investigate the effects of aerobic training on markers of oxidative status in patients with cancer.
Prior work investigating the effects of exercise on oxidative status in humans exploiting urinary F2-isoprostanes is equivocal, with studies reporting both decreases as well as increases in these endpoints. Campbell et al. [25] reported that 12 months of moderate-intensity aerobic training (60–75% heart ratemax, ≥45 min d−1, 5d wk−1) was associated with statistical non-significant reductions in urinary F2-isoprostane (as measured by its major urinary metabolite, 15-F2t-isoprostane, 2,3-dinor-8-iso-prostaglandin F2α) in 173 sedentary and overweight/obese but otherwise healthy women relative to a sedentary control group. Specifically, F2-isoprostanes decreased 6.2% and increased 3.3% in the exercise and control groups, respectively. Change in VO2peak was significantly inversely associated with change in F2-isoprostane. Similarly, Roberts et al. [26] found that the combination of a low-fat, high-fiber diet with a 3-week moderate-intensity (70% to 85% of heart rate maximum) aerobic training was associated with a statistically significant reduction in urinary F2-isoprostane (8-isoprostaglandin F2α) among11menwith hypercholesterolemia or hypertension. Interestingly, in the only other study in patients with cancer, Allgayer et al. [27] reported that low-intensity aerobic training (30%–40% maximal workload) was associated with a 29% reduction in urinary excretion of 8-oxo- 2′-deoxyguanosine (8-oxo-dG), a specific marker of DNA damage. However, moderate-intensity training (50%–60% maximal workload) was associated with a 42% increase over a 2-week period in patients with colorectal cancer.
The precise explanations for the contrasting findings are not known but likely reflect differences in exercise prescription (i.e., duration, frequency, intensity, and program length) and study population. In terms of exercise prescription, our study tested the effects of a moderate-to-high intensity, single modality aerobic training program over 14 weeks while Campbell et al. [25] investigated moderate-intensity training over 12 months. Allgayer et al. [27] on the other hand, only tested the effect of short-term (2 weeks) aerobic training. Given the heterogeneity in previously tested exercise prescriptions, definitive conclusions are not possible at this time. Methodological differences in study population may also explain the inconsistent findings. The study by Campbell et al. [25] consisted of sedentary, overweight/obese women without a history of breast cancer or other concomitant comorbid diseases. In this setting, aerobic training was associated with a non-significant reduction in markers of oxidative status. In stark contrast, our sample consisted of histologically-confirmed, postsurgical NSCLC, 39% were receiving adjuvant chemotherapy, and the majority presented with at least one comorbid disease. In this setting, moderate-to-high intensity aerobic training was associated with consistent increases in four isomers of F2-isoprostanes. Similarly, Allgayer et al. [27] reported that aerobic training was also associated with increased oxidative damage in subjects with a histologically-confirmed diagnosis of malignancy and prior treatment with cytotoxic adjuvant therapy. As such, it appears reasonable to suggest that the effects of exercise on markers of oxidative status may differ based on the physiological/disease status of an individual as well as the presence of ongoing or prior cytotoxic therapy.
The acute (short-term) effects of anticancer therapy on markers of oxidative status have been investigated by several groups. In general, anticancer therapy is associated with a statistical significant increase in markers of oxidative status which then subsequently decreases typically within ~48 hours following initial insult.[28,29] Similarly, in our prior work, we found statistical significant increases in F2-isoprostanes within one hour of administration of anthracycline-containing adjuvant chemotherapy in women with breast cancer that normalized to pre-treatment levels within 24 hours.[20] Fewer studies have assessed the chronic (repeated bouts) of therapy on markers of oxidative status. Urinary 8-oxo-dG levels, a marker of DNA damage, increased after pulmonary resection and the complete course of adjuvant chemotherapy and/or radiotherapy in lung cancer patients.[30,31] In the present study, there were no statistical significant differences in markers of oxidative status between patients receiving and not receiving adjuvant chemotherapy. In addition, aerobic training did not modify the chemotherapy–oxidative status relationship. The reasons for the contrasting findings are not known but our small sample size and methodological differences relating to timing and measurement of oxidative status may be partially responsible.
The prevailing dogma is that chronic (repeated), moderate-intensity aerobic training may reduce the primary risk of cancer as well as cancer progression through a reduction in the formation and release of ROS.[12,25] As such, F2-isoprostanes levels, which reflect the pro-oxidant–antioxidant balance or the constitutive level of ROS within tissues, and at the systemic level, would be expected to be lower following aerobic training. In contrast, our findings indicated that chronic aerobic training was associated with a statistical significant increase in F2-isoprostanes levels. Based on the current evidence the implications of our findings are not clear.
Moderate-to-high intensity aerobic training increases oxygen consumption and fat oxidation which, in turn, disturbs pro-oxidant–endogenous antioxidant homeostasis leading to generation of ROS in the skeletal muscle.[32] The concept of hormesis purports that repeated exposure to non-lethal damage causes enhanced protection against subsequent oxidative insults via increased expression of endogenous antioxidant machinery.[33] In one respect therefore, higher levels of F2-isoprostanes in our study may indicate that aerobic training-induced generation of ROS was not offset by sufficient antioxidant defense capacity. Such findings may be context specific. Specifically, aerobic training may augment other potent ROS-producing processes in lung cancer patients such as chemotherapy, smoking, surgery, other concomitant comorbidities that together overwhelm antioxidant defense capacity. An alternative explanation is that the aerobic training caused an increase in the constitutive oxidative status background (i.e., higher oxidant set-point) which may have decreased the amplitude in ROS with subsequent oxidative insults. Results of our exploratory analysis showing that the increase in F2-isoprostanes levels with aerobic training was lower in those patients with higher levels at baseline (pre-training) supports this notion (analyses not presented). However, high constitutive levels of ROS over an extended period of time may increase the potential for development of tissue injury.
Finally, it is important to consider the present findings in context of the effects of aerobic training on other outcomes in lung cancer patients. For example, we previously reported that aerobic training was associated with significant improvements in VO2peak, QOL, and fatigue in the parent study.[5] Both VO2peak and patient-reported outcomes have been shown to be independent predictors of prognosis in NSCLC.[10,34–36] Thus, it is important to interpret the effects or risk-to-benefit ratio of aerobic training in context of its pleiotropic properties. Second, we exploited urinary measures of F2-isoprostanes that reflect systemic levels of oxidative status; the precise mechanisms of how aerobic training-induced increases in systemic markers of oxidative status influence redox balance within the tumor microenvironment and subsequently, tumor progression and metastasis are not defined. Further studies exploiting appropriate pre-clinical models of NSCLC as well as other solid tumors are required to unravel the complex interaction between free radical production, tissue injury, tumor progression, and even response to cytotoxic therapy.
This study has important limitations. Obvious limitations are the relatively small sample size, lack of non-exercise control group, and relatively short exercise intervention period. To adequately investigative the effects of exercise training on biomarkers of oxidative stress, larger randomized controlled trials examining the effects of longer term exercise training (≥ 3 months) are required. In addition, studies evaluating the acute as well as chronic (constitutive) effects of exercise training on markers of oxidative status are required. In summary, supervised aerobic training was associated with statistical significant increases in urinary measures of oxidative stress in postsurgical NSCLC. Future, randomized trials are required to further investigate the effects of aerobic training on measures of oxidative stress and expression of antioxidant defense capacity in cancer patients both during and following adjuvant therapy. In conjunction, eloquently designed preclinical studies to understand how exercise-induced modulation of systemic oxidative stress modulates tumor progression and response to anticancer therapy will significantly facilitate translational research in this field.
Fig. 1.
Effects of aerobic training on: (a) iPF (2-alpha)-III, (b) 2,3-dinor-iPF (2-alpha)-III, (c) iPF (2-alpha)-VI, and (d) 8,12-Iso-iPF (2-alpha)-VI, and (e) Composite Index from baseline to postintervention (14 weeks).
Acknowledgments
Funding
This study was supported by a Pilot Grant from the Duke Older Americans Independence Center and Lance Armstrong Foundation Young Investigators Award awarded to LWJ. LWJ is also supported by NIH CA143254, CA142566, CA138634, CA133895, CA125458 and funds from George and Susan Beischer.
References
- 1.Jones LW, Eves ND, Waner E, et al. Exercise therapy across the lung cancer continuum. Curr Oncol Rep. 2009 Jul;11(4):255–262. doi: 10.1007/s11912-009-0036-0. [DOI] [PubMed] [Google Scholar]
- 2.Jones LW, Eves ND, Peddle CJ, et al. Effects of presurgical exercise training on systemic inflammatory markers among patients with malignant lung lesions. Appl Physiol Nutr Metab. 2009 Apr;34(2):197–202. doi: 10.1139/H08-104. [DOI] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, Goldberg S, et al. A structured exercise program for patients with advanced non-small cell lung cancer. J Thorac Oncol. 2009 May;4(5):595–601. doi: 10.1097/JTO.0b013e31819d18e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peddle CJ, Jones LW, Eves ND, et al. Effects of presurgical exercise training on quality of life in patients undergoing lung resection for suspected malignancy: a pilot study. Cancer Nurs. 2009 Mar-Apr;32(2):158–165. doi: 10.1097/NCC.0b013e3181982ca1. [DOI] [PubMed] [Google Scholar]
- 5.Jones LW, Eves ND, Peterson BL, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical non-small cell lung cancer patients: a pilot study. Cancer. 2008 Dec 15;113(12):3430–3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- 6.Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008 Jan;33(1):95–98. doi: 10.1016/j.ejcts.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Jones LW, Peddle CJ, Eves ND, et al. Effects of presurgical exercise training on cardiorespiratory fitness among patients undergoing thoracic surgery for malignant lung lesions. Cancer. 2007 Aug 1;110(3):590–598. doi: 10.1002/cncr.22830. [DOI] [PubMed] [Google Scholar]
- 8.Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007 Aug;57(2):175–180. doi: 10.1016/j.lungcan.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 9.Spruit MA, Janssen PP, Willemsen SC, et al. Exercise capacity before and after an 8-week multidisciplinary inpatient rehabilitation program in lung cancer patients: a pilot study. Lung Cancer. 2006 May;52(2):257–260. doi: 10.1016/j.lungcan.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Jones LW, Watson D, Herndon JE, II, et al. Peak oxygen consumption and long-term all-cause mortality in non-small cell lung cancer. Cancer. doi: 10.1002/cncr.25396. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones LW, Eves ND, Haykowsky M, et al. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009 Jun;10(6):598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 12.McTiernan A. Mechanisms linking physical activity with cancer. Nat Rev Cancer. 2008 Mar;8(3):205–211. doi: 10.1038/nrc2325. [DOI] [PubMed] [Google Scholar]
- 13.Ji LL, Leeuwenburgh C, Leichtweis S, et al. Oxidative stress and aging. Role of exercise and its influences on antioxidant systems. Ann N Y Acad Sci. 1998 Nov 20;854:102–117. doi: 10.1111/j.1749-6632.1998.tb09896.x. [DOI] [PubMed] [Google Scholar]
- 14.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999 Dec;222(3):283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 15.Shen J, Deininger P, Hunt JD, et al. 8-Hydroxy-2′-deoxyguanosine (8-OH-dG) as a potential survival biomarker in patients with nonsmall-cell lung cancer. Cancer. 2007 Feb 1;109(3):574–580. doi: 10.1002/cncr.22417. [DOI] [PubMed] [Google Scholar]
- 16.Gackowski D, Speina E, Zielinska M, et al. Products of oxidative DNA damage and repair as possible biomarkers of susceptibility to lung cancer. Cancer Res. 2003 Aug 15;63(16):4899–4902. [PubMed] [Google Scholar]
- 17.Ji LL, Gomez-Cabrera MC, Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006 May;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 18.Basu S. F2-isoprostanes in human health and diseases: from molecular mechanisms to clinical implications. Antioxid Redox Signal. 2008 Aug;10(8):1405–1434. doi: 10.1089/ars.2007.1956. [DOI] [PubMed] [Google Scholar]
- 19.Comporti M, Signorini C, Arezzini B, et al. F2-isoprostanes are not just markers of oxidative stress. Free Radic Biol Med. 2008 Feb 1;44(3):247–256. doi: 10.1016/j.freeradbiomed.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 20.II’yasova D. Urinary Biomarkers of Oxidative Status in a Clinical Model of Oxidative Assault. Cancer Epidemiology, Biomarkers, and Prevention. doi: 10.1158/1055-9965.EPI-10-0211. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor AW, Bruno RS, Frei B, et al. Benefits of prolonged gradient separation for high-performance liquid chromatography-tandem mass spectrometry quantitation of plasma total 15-series F-isoprostanes. Anal Biochem. 2006 Mar 1;350(1):41–51. doi: 10.1016/j.ab.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 22.Haschke M, Zhang YL, Kahle C, et al. HPLC-atmospheric pressure chemical ionization MS/MS for quantification of 15-F2t-isoprostane in human urine and plasma. Clin Chem. 2007 Mar;53(3):489–497. doi: 10.1373/clinchem.2006.078972. [DOI] [PubMed] [Google Scholar]
- 23.Jones LW, Eves ND, Haykowsky M, et al. Cardiorespiratory exercise testing in clinical oncology research: systematic review and practice recommendations. Lancet Oncol. 2008 Aug;9(8):757–765. doi: 10.1016/S1470-2045(08)70195-5. [DOI] [PubMed] [Google Scholar]
- 24.Brusasco V, Crapo R, Viegi G. Coming together: the ATS/ERS consensus on clinical pulmonary function testing. Eur Respir J. 2005 Jul;26(1):1–2. doi: 10.1183/09031936.05.00034205. [DOI] [PubMed] [Google Scholar]
- 25.Campbell PT, Gross MD, Potter JD, et al. Effect of Exercise on Oxidative Stress: A 12-Month Randomized, Controlled Trial. Med Sci Sports Exerc. Feb 4; doi: 10.1249/MSS.0b013e3181cfc908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts CK, Vaziri ND, Barnard RJ. Effect of diet and exercise intervention on blood pressure, insulin, oxidative stress, and nitric oxide availability. Circulation. 2002 Nov 12;106(20):2530–2532. doi: 10.1161/01.cir.0000040584.91836.0d. [DOI] [PubMed] [Google Scholar]
- 27.Allgayer H, Owen RW, Nair J, et al. Short-term moderate exercise programs reduce oxidative DNA damage as determined by high-performance liquid chromatography-electrospray ionization-mass spectrometry in patients with colorectal carcinoma following primary treatment. Scand J Gastroenterol. 2008 Aug;43(8):971–978. doi: 10.1080/00365520701766111. [DOI] [PubMed] [Google Scholar]
- 28.Siomek A, Tujakowski J, Gackowski D, et al. Severe oxidatively damaged DNA after cisplatin treatment of cancer patients. Int J Cancer. 2006 Nov 1;119(9):2228–2230. doi: 10.1002/ijc.22088. [DOI] [PubMed] [Google Scholar]
- 29.Olinski R, Jaruga P, Foksinski M, et al. Epirubicin-induced oxidative DNA damage and evidence for its repair in lymphocytes of cancer patients who are undergoing chemotherapy. Mol Pharmacol. 1997 Nov;52(5):882–885. doi: 10.1124/mol.52.5.882. [DOI] [PubMed] [Google Scholar]
- 30.Crohns M, Saarelainen S, Erhola M, et al. Impact of radiotherapy and chemotherapy on biomarkers of oxidative DNA damage in lung cancer patients. Clin Biochem. 2009 Jul;42(10–11):1082–1090. doi: 10.1016/j.clinbiochem.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 31.Lases EC, Duurkens VA, Gerritsen WB, et al. Oxidative stress after lung resection therapy: A pilot study. Chest. 2000 Apr;117(4):999–1003. doi: 10.1378/chest.117.4.999. [DOI] [PubMed] [Google Scholar]
- 32.Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: Role of redox signaling. Free Radic Biol Med. 2008 Jan 15;44(2):142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 33.Ristow M, Zarse K, Oberbach A, et al. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009 May 26;106(21):8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Movsas B, Moughan J, Sarna L, et al. Quality of life supersedes the classic prognosticators for long-term survival in locally advanced non-small-cell lung cancer: an analysis of RTOG 9801. J Clin Oncol. 2009 Dec 1;27(34):5816–5822. doi: 10.1200/JCO.2009.23.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Herndon JE, 2nd, Fleishman S, Kornblith AB, et al. Is quality of life predictive of the survival of patients with advanced nonsmall cell lung carcinoma? Cancer. 1999 Jan 15;85(2):333–340. doi: 10.1002/(sici)1097-0142(19990115)85:2<333::aid-cncr10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 36.Quinten C, Coens C, Mauer M, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009 Sep;10(9):865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]