Abstract
Background
This article describes our experience with inpatient hybrid closed-loop control (HCLC) initiated shortly after the diagnosis of type 1 diabetes in a randomized trial designed to assess the effectiveness of inpatient HCLC followed by outpatient sensor-augmented pump (SAP) therapy on the preservation of β-cell function.
Subjects and Methods
Forty-eight individuals with newly diagnosed type 1 diabetes and positive pancreatic autoantibodies (7.8–37.7 years old) received inpatient HCLC therapy for up to 93 h, initiated within 7 days of diagnosis.
Results
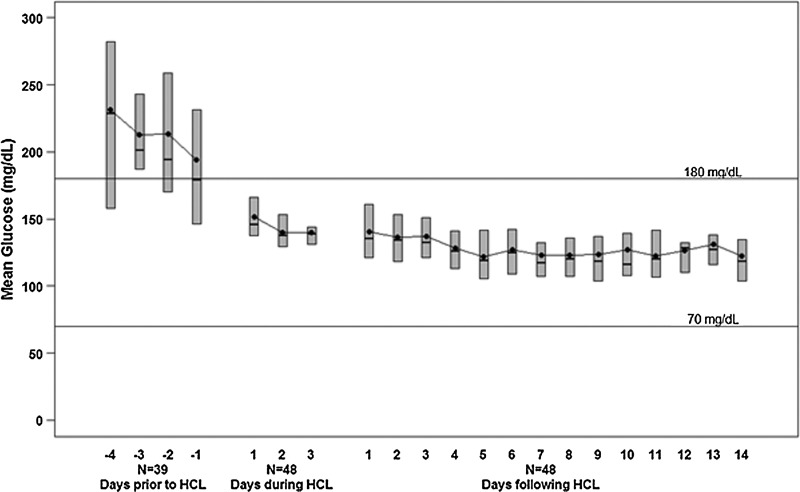
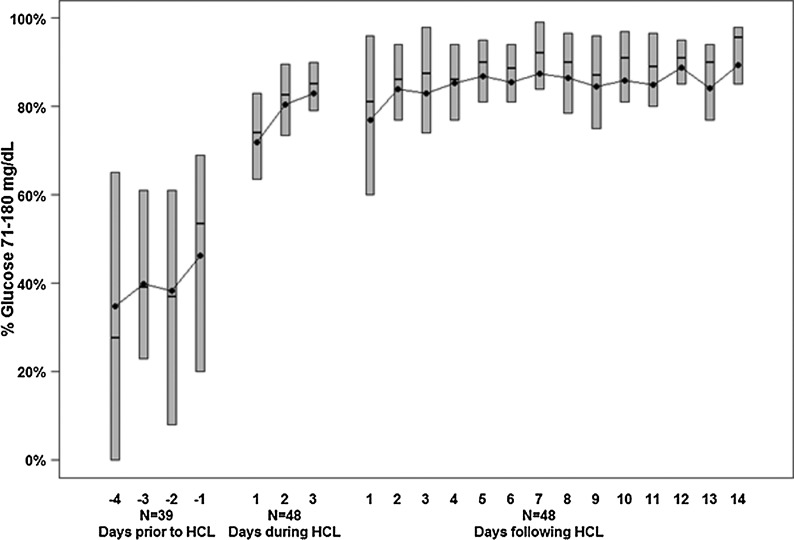
On initiation of HCLC, mean glucose concentration was 240±100 mg/dL. During the first day of HCLC, median of the participant's mean glucose concentrations fell rapidly to 146 mg/dL, a level of control that was sustained on Days 2 and 3 (138 mg/dL and 139 mg/dL, respectively). By Day 3, the median percentage of glucose values >250 and <60 mg/dL was <1%. During the first 2 weeks of SAP treatment at home, the median participant mean glucose level was 126 mg/dL (interquartile range, 117, 137 mg/dL), and the median percentage of values between 71 and 180 mg/dL was 85% (interquartile range, 80%, 90%).
Conclusions
Inpatient HCLC followed by outpatient SAP therapy can provide a safe and effective means to rapidly reverse glucose toxicity and establish near-normal glycemic control in patients with newly diagnosed type 1 diabetes.
Introduction
Optimizing glycemic control as soon as possible after the diagnosis of type 1 diabetes may serve to preserve residual β-cell function. A randomized trial involving 26 adolescents of a closed-loop system (BioStator™; Miles Laboratories, Elkhart, IN) using intravenous insulin and continuous venous blood glucose monitoring for 2 weeks after the clinical diagnosis of type 1 diabetes demonstrated significantly higher levels of stimulated C-peptide 1 year later.1 A more recent study did not find a benefit in preserving C-peptide levels with sensor-augmented pump (SAP) therapy initiated within 4 weeks of diagnosis compared with pump therapy alone.2 The Diabetes Control and Complications Trial showed that intensive therapy resulted in a longer retention of residual endogenous insulin secretion, lower hemoglobin A1c levels, and reduced risk of severe hypoglycemia and development of early retinopathy than conventional therapy.3,4
New technologies offer additional tools to improve glycemic control. In the inpatient research setting there are experimental closed-loop systems. In the outpatient setting there are commercially available insulin pumps and continuous glucose monitors (CGMs), which, when used together, have proven to be effective in lowering hemoglobin A1c levels in several randomized clinical trials.5,6 However, pumps and CGM devices generally are not prescribed at the time of diagnosis of type 1 diabetes, and there is little information on the effect of optimizing glycemic control as soon as possible after diagnosis.
To test the hypothesis that intensive glycemic control from the onset of type 1 diabetes will preserve endogenous insulin production, we conducted a randomized trial to evaluate inpatient hybrid closed-loop control (HCLC) followed by outpatient use of SAP therapy versus usual care in individuals enrolled within 7 days of diagnosis. The primary outcome is C-peptide levels measured with a mixed meal tolerance test (MMTT) at 12 months (these results will be reported in a separate article). Herein we describe our experience with the study participants in the intensive therapy group who were managed with inpatient HCLC and the subsequent first 2 weeks of outpatient SAP therapy.
Research Design and Methods
The study was conducted at five clinical centers. Participants were enrolled between May 2009 and October 2011. The protocol was approved by each local institutional review board. Written informed consent was obtained from participants ≥18 years of age and from parents/guardians of younger participants from whom written assent was obtained. Major eligibility criteria included ages from 6 to <46 years and diagnosis of type 1 diabetes with initiation of insulin therapy within the prior 7 days (with Day 0 considered the day that insulin was started). This report includes the results from the 48 participants who had at least one positive anti–islet cell autoantibody to insulin, glutamic acid decarboxylase, insulinoma antigen, zinc transporter-8, or an islet-cell antibody and were randomly assigned to the intervention group that received inpatient HCLC followed by outpatient SAP therapy. Two participants assigned to the intensive group did not have positive autoantibodies and as prespecified were not included in the analyses.
When possible, a blinded Guardian® CGM device (Medtronic MiniMed, Inc., Northridge, CA) was worn between enrollment and the hospital admission for initiation of HCLC. Prior to initiation of HCLC therapy, a 90-min MMTT was performed. At the start of the admission for HCLC therapy, an intravenous line was placed in the arm for blood draws to monitor glucose levels and for administration of glucose or insulin if needed for treatment of hypoglycemic or hyperglycemic events, respectively.
Inpatient HCLC used the Medtronic MiniMed external physiological insulin delivery (ePID) system,7,8 consisting of a Medtronic MiniMed subcutaneous glucose sensor and insulin infusion pump communicating wirelessly every minute with a bedside computer running Control Tool software (developed by Medtronic MiniMed) using the ePID algorithm. The goal was to complete a minimum of 72 h to a maximum of 96 h of HCLC. The ePID algorithm used every 1-min sensor readings to determine insulin administration. The protocol required that a physician or nurse practitioner trained in the care of persons with diabetes be available at all times during HCLC therapy. Study personnel calculated premeal boluses of insulin to assist in controlling postprandial hyperglycemia in association with the automated closed-loop control, thus the nomenclature “hybrid” closed-loop control. Sensor calibration was performed using the Control Tool software. Study personnel recalibrated the sensor whenever repeated sensor values deviated from the reference glucose concentrations by at least 15%.
The proportional-integral-derivative algorithm used in these studies has been previously described7–9 but was modified to incorporate insulin feedback (IFB).10–12 IFB uses a three-state subcutaneous insulin absorption model that captures the subcutaneous, plasma, and effective insulin concentrations and modifies the insulin dose to adjust for active insulin. The proportional-integral-derivative algorithm with IFB was initialized by entering the participant's weight and total daily insulin dose. The glucose set point was 110–120 mg/dL. Up to 20 min prior to each meal and snack, carbohydrates were counted, and a premeal bolus was given to provide insulin coverage for 75–80% of the meal based on the participant's current carbohydrate-to-insulin ratio, which was initially determined by dividing the total daily insulin dose into 45013 and then adjusted as needed based on postprandial glucose levels. There were no dietary restrictions, with participants choosing both the timing and the content of all meals and snacks. Participants were permitted short walks of up to 25 min between blood draws, during which time the closed-loop system and insulin delivery were suspended and then resumed upon return to the hospital room. During HCLC, blood glucose measurements were obtained every 30 min using a reference glucose analyzer (YSI [YSI Inc. Life Sciences, Yellow Springs, OH], GlucoScout [International Biomedical, Austin, TX], HemoCue® [HemoCue, Inc., Cypress, CA], or i-STAT® [Abbott Point of Care Inc., Princeton, NJ]) and every 15 min when glucose values were <70 mg/dL. Standard hypoglycemia treatment of approximately 15 g of carbohydrates was given for glucose values <70 mg/dL. For two consecutive glucose values >300 mg/dL, a serum ketone level was checked. Unless otherwise specified, reported glucose results refer to blood glucose measurements and not CGM measurements.
During the hospital admission, extensive teaching was provided for use of the insulin pump and CGM for diabetes management upon discharge. At discharge, participants were provided with all necessary supplies for the insulin pump and the CGM (including glucometers and testing strips) and were instructed to use the CGM daily.
Descriptive statistics are reported as mean±SD values or median with interquartile range (IQR) depending on the distribution of a variable.
Results
The 48 participants in the intensive treatment group had a mean (±SD) age of 12.7±4.7 years (range, 7.8–37.7 years; 46 of the 48 total patients were <18 years old), 35% were female, and 91% were non-Hispanic white, 2% were African-American, 4% were Hispanic, and 2% were more than one race. Three centers began recruitment from the onset of the study, enrolling 12, 14, and 11 subjects, respectively; two additional centers were added 2 years later, and they enrolled six and five subjects, respectively. At the time of diagnosis, mean initial glucose level was 501±193 mg/dL, mean hemoglobin A1c was 11.7±2.3% (105±25 mmol/mol), and mean body mass index percentile (excluding two participants >20 years of age) was 43±31%. Fourteen (29%) presented in diabetic ketoacidosis based on Diabetes Control and Complications Trial criteria. Intravenous insulin was received by 11 (79%) of the 14 with diabetic ketoacidosis and by none of the 34 without diabetic ketoacidosis; the mean initial glucose levels were 542±169 mg/dL and 483±202 mg/dL, respectively. Subcutaneous insulin was given on an outpatient basis prior to initiation of HCLC therapy.
Enrollment occurred 0–6 days after the initiation of insulin therapy (median, 3 days; IQR, 2, 4 days). For the 39 participants using a blinded CGM prior to the hospital admission, median participant mean glucose level by CGM was 179 mg/dL (IQR, 147, 231 mg/dL) during the day prior to initiating HCLC.
HCLC was initiated a mean of 5.7±1.2 days after diagnosis and was used for a median of 71.3 h (IQR, 70.3, 72.1 h; range, 29.9–93.2 h) by the 48 participants, with 45 of the 48 (94%) participants completing at least 68 h of HCLC. Three subjects stopped HCLC early (after 30, 42, and 56 h, respectively) because of problems with the intravenous line, which was required for use of HCLC.
On initiation of HCLC, mean glucose concentration was 240±100 mg/dL, which in some cases was measured shortly after the MMTT. Two participants had measurable blood ketones (0.3 and 0.5 mmol/L, respectively) within the first 12 h. During the first 6 h of HCLC, median participant mean glucose concentration was 170 mg/dL (IQR, 135, 208), with a median of 57% of values between 71 and 180 mg/dL (IQR, 35%, 81%). There was a progressive improvement in glycemic control over the rest of the first day, plateauing with a median among participants of about 80% of glucose values between 71 and 180 mg/dL (IQR, 75%, 90%) (Table 1 and Figs. 1 and 2). The median mean glucose level was lower, and the median percentage of values between 71 and 180 mg/dL was higher overnight (12 midnight to 7 a.m.) than during the daytime on all 3 days of HCLC (Table 2). The frequency of glucose levels ≤70 mg/dL was low (1.0% of glucose values) during HCLC with 13%, 17%, and 9% of participants having at least one value below 60 mg/dL on Days 1, 2, and 3, respectively. The percentage of readings ≤50 mg/dL was 0.1% on Day 1, 0% on Day 2, and 0% on Day 3, and for all days the Low Blood Glucose Index was 0.5. The percentages of glucose values 71–180 mg/dL according to clinical site are shown in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/dia).
Table 1.
Inpatient Reference Glucose Values
| Day 1 (n=48) | Day 2 (n=48) | Day 3 (n=46) | |
|---|---|---|---|
| Hours of glucose readings | 24 (24, 24) | 24 (24, 24) | 24 (24, 24) |
| Mean glucose (mg/dL) | 146 (135, 166) | 138 (129, 151) | 139 (131, 145) |
| Glucose values | |||
| 71–180 mg/dL | 78% (65%, 85%) | 81% (74%, 90%) | 84% (78%, 90%) |
| Glucose values >180 mg/dL | 20% (13%, 33%) | 15% (8%, 25%) | 16% (8%, 22%) |
| Glucose values >250 mg/dL | 4% (0%, 14%) | 0% (0%, 6%) | 0% (0%, 0%) |
| Percentage of glucose values ≤60 mg/dLa | 0.3% | 0.4% | 0.2% |
| Participants with [n (%)] | |||
| Glucose nadir ≤60 mg/dL | 6 (13%) | 8 (17%) | 4 (9%) |
| Glucose nadir 61–70 mg/dL | 16 (33%) | 8 (17%) | 10 (22%) |
| >5% values ≤60 mg/dL | 0 (0%) | 0 (0%) | 0 (0%) |
Data are median (25th, 75th percentiles) unless stated otherwise.
Pooled across participants.
FIG. 1.
Participant mean glucose values from a continuous glucose monitor worn prior to admission, during hybrid closed-loop (HCL) control, and 2 weeks following discharge (n=48). The bottom and top of each box denote the 25th and 75th percentiles, respectively, the line inside the box denotes the median, and the dot is the mean.
FIG. 2.
Distribution of percentage of continuous glucose monitor glucose values 71–180 mg/dL from a continuous glucose monitor worn prior to admission, during hybrid closed-loop (HCL) control, and 2 weeks following discharge (n=48). The bottom and top of each box denote the 25th and 75th percentiles, respectively, the line inside the box denotes the median, and the dot is the mean.
Table 2.
Inpatient Reference Glucose Values by Time of Day
| |
Daytime (7 a.m.–11 p.m.) |
Nighttime (11 p.m.–7 a.m.) |
||||
|---|---|---|---|---|---|---|
| Day 1 (n=48) | Day 2 (n=48) | Day 3 (n=46) | Day 1 (n=48) | Day 2 (n=47) | Day 3 (n=45) | |
| Hours of glucose readings | 16 (16, 16) | 16 (16, 16) | 16 (16, 16) | 8 (8, 8) | 8 (8, 8) | 8 (8, 8) |
| Mean glucose (mg/dL) | 159 (140, 176) | 146 (135, 160) | 143 (137, 157) | 123 (113, 134) | 123 (112, 138) | 122 (115, 137) |
| Glucose values | ||||||
| 71–180 mg/dL | 72% (59%, 81%) | 78% (68%, 88%) | 81% (75%, 88%) | 100% (82%, 100%) | 100% (88%, 100%) | 100% (94%, 100%) |
| >180 mg/dL | 28% (19%, 39%) | 22% (9%, 31%) | 19% (10%, 25%) | 0% (0%, 16%) | 0% (0%, 13%) | 0% (0%, 6%) |
| >250 mg/dL | 6% (0%, 13%) | 0% (0%, 8%) | 0% (0%, 0%) | 0% (0%, 0%) | 0% (0%, 0%) | 0% (0%, 0%) |
| Percentage of glucose values ≤60 mg/dLa | 0.4% | 0.4% | 0.1% | 0.0% | 0.3% | 0.3% |
| Participants with [n (%)] | ||||||
| Glucose nadir ≤60 mg/dL | 6 (13%) | 6 (13%) | 2 (4%) | 0 (0%) | 2 (4%) | 2 (4%) |
| Glucose nadir 61–70 mg/dL | 13 (27%) | 5 (10%) | 7 (15%) | 7 (15%) | 4 (9%) | 3 (7%) |
| >5% values ≤60 mg/dL | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (4%) | 1 (2%) |
Data are medians (25th, 75th percentiles) unless stated otherwise.
Pooled across participants.
Median (IQR) carbohydrate intake during the 3 days of HCLC was 72 (53, 88), 72 (59, 87), and 73 (59, 90) g for breakfast, lunch, and dinner, respectively, representing 1.7 (1.1, 2.3), 1.8 (1.3, 2.3), and 1.7 (1.3, 2.3) g/kg, respectively. There was an additional 51 (30, 88), 56 (33, 87), and 47 (27, 101) g for snacks on the 3 days. The median (IQR) total daily insulin dose was 1.2 (0.8, 1.5), 1.2 (0.8, 1.5), and 1.2 (0.9, 1.5) units/kg/day, and the percentage given by the ePID-IFB controller was 61%, 63%, and 58% on Days 1, 2, and 3, respectively, with the remainder given as manual premeal priming doses.
There were no episodes of diabetic ketoacidosis or severe hypoglycemia. One subject had an anaphylactic reaction (presumed peanut allergy) following his first dinner and received intravenous steroids, which resulted in increased insulin doses (1.8 units/kg/day) during the first 24 h.
In the 2 weeks following discharge from the hospital, during which time the participants were using SAP therapy at home, glycemic control improved further with a median participant mean sensor glucose level of 126 mg/dL (IQR, 117, 137) and a median percentage of values between 71 and 180 mg/dL of 85% (IQR, 80%, 90%) (Fig. 2). Median total daily insulin dose was 0.7 (IQR, 0.6, 1.0) units/kg/day.
Discussion
This study found that inpatient HCLC safely initiated soon after the diagnosis of type 1 diabetes resulted in a rapid decrease in blood glucose levels within 24 h of initiation. This was sustained during the subsequent 2 weeks of outpatient use of SAP therapy. While using HCLC, about 80% of glucose levels were in the target range of 71–180 mg/dL, with minimal hypoglycemia. The ePID controller in this setting was able to maintain excellent nocturnal glycemic control and good but not perfect daytime control, as has been demonstrated in prior inpatient studies of individuals with long-standing type 1 diabetes.7,8
In order to simulate normal food intake at home during inpatient HCLC, the participants freely selected the content of meals from the standard hospital menus. Because the majority of participants were adolescent boys, it is not surprising that total carbohydrate intake approached a mean of 2 g/kg/day. To reduce the anticipated postmeal hyperglycemia with HCLC, a physician-determined premeal bolus was given, which has been previously shown by Weinzimer et al.8 to significantly improve postprandial hyperglycemia during closed-loop control. It is of interest that despite the ePID-IFB controller not accounting for these premeal boluses, there was minimal postprandial hypoglycemia. All overnight insulin delivery was determined by the ePID controller.
It has previously been demonstrated that the metabolic decompensation at the time of diagnosis of type 1 diabetes is accompanied by a significant reduction in insulin sensitivity.14 Such insulin resistance was reflected in the median total daily insulin doses that exceed 1 unit/kg/day during initial inpatient HCLC. Conversely, the benefits of early intensive management using HCLC to acutely reverse glucotoxicity and improve insulin sensitivity are reflected in the rapid fall in daily insulin requirements to 0.7 unit/kg/day during the first 2 weeks of outpatient SAP therapy.
The use of closed-loop control in this study differs from the closed-loop control previously used by Shah et al.1 in that they began closed-loop control within 24–48 h from the onset of diabetes and continued inpatient closed-loop control for 14 days. They used the BioStator, which delivered intravenous insulin, whereas we delivered subcutaneous insulin, and their controller used blood glucose levels, whereas our controller was driven by subcutaneous glucose levels. Their target glucose levels were 63–80 mg/dL, and following meals glucose levels peaked between 110 to 150 mg/dL but returned within 1 h to the target glucose range.
In this study, we have shown that inpatient HCLC initiated shortly after the diagnosis of type 1 diabetes can be safe and effective in rapidly restoring near-normal glycemic control. It remains to be determined whether an “all-in” approach to diabetes technology that combines HCLC at the time of diagnosis with subsequent SAP therapy will result in a greater preservation of insulin production than the level that can be achieved with the current standards of care of newly diagnosed patients with type 1 diabetes.
Supplementary Material
Appendix
Writing Committee
Bruce A. Buckingham, MD, Pediatric Endocrinology, Stanford University, Stanford, CA; Roy W. Beck, MD, PhD, Jaeb Center for Health Research, Tampa, FL; Katrina J. Ruedy, MSPH, Jaeb Center for Health Research; Peiyao Cheng, MPH, Jaeb Center for Health Research; Craig Kollman, PhD, Jaeb Center for Health Research; Stuart A. Weinzimer, MD, Pediatric Endocrinology, Yale University, New Haven, CT; Linda A. DiMeglio, MD, Department of Pediatrics, Indiana University, Riley Hospital for Children, Indianapolis, IN; Andrew A. Bremer, MD, PhD, Division of Pediatric Endocrinology, Vanderbilt University Medical Center, Nashville, TN; Robert Slover, MD, Pediatrics, Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO; and Martin Cantwell, BSc, Medtronic Diabetes, Northridge, CA.
Participating Centers
Clinical Centers (listed in alphabetical order with clinical center name, city, and state, where PI indicates Principal Investigator, I indicates Co-Investigator, and C indicates Coordinator) (*indicates Clinical Centers that participate in both DirecNet and TrialNet; other clinical centers do not participate in DirecNet)
Pediatrics, Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, Aurora, CO: Robert Slover, MD (PI), H. Peter Chase, MD (I), David Maahs, MD (I), Carolyn Banion, CPNP (I), Cindy Cain, FNP (I), Christine (Maggie) Chan, MD (I), Christine L. Chan, MD (I), Rosanna Fiallo-Scharer, MD (I), David Howard, MD, PhD (I), Stephanie Kassels, FNP (I), Aaron Michels, MD (I), Kristen Nadeau, MD (I), Paul Wadwa, MD (I), Sandra Hoops, PA-C (I), Sarit Polsky, MD (I), Bahereh (Michelle) Schweiger, MD (I), Peter Gottlieb, MD (I), Lela Mansoori, MD (I), Laurel Messer, MSN (C), Sally Sullivan, (C), and Vicky Gage, RN, BSN (C).
Indiana University, Riley Hospital for Children, Indianapolis, IN: Linda A. DiMeglio, MD (PI), Linda Stroud, MD (I), Jill Meier, MD (I), Tamara Hannon, MD (I), Juan Sanchez, MD (I), Stephanie Woerner, FNP-C (I), Carmella Molina Evans, MD, PhD (I), Heather Jolivette, CPNP (I), Susanne Cabrera, MD (I), Emily Simms, MD (I), Tina Pottorff, CPNP (I), Andrea Goldyn, MD (I), John Fuqua, MD (I), Karen Stancombe, CPNP (I), Megan Behrmann, CPNP (I), Erica Eugster, MD (I), Nadine Haddad, MD (I), Mark Pescovitz, MD (I), Todd Nebesio, MD (I), Vidhya Viswanathan, MD (I), Katherine Lewis, MD (I), Leeann Ford (C), and Robin Hufferd (C).
*Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI), Darrell M. Wilson, MD (I), Kari Benassi, RN, NP (I), Tandy Aye, MD (I), Rajiv Kumar, MD (I), Parul Patel, MD (I), Avni Shah, MD (I), Sejal Shah, MD (I), Elizabeth Kunselman, NP, CDE (I), Kim Fuld, MD (I), Nikta Forghani, MD (I), Jennifer Block, ARNP, CDE (C), and Kimberly Caswell, APRN (C).
Division of Pediatric Endocrinology, Vanderbilt University Medical Center, Nashville, TN: Andrew A. Bremer, MD, PhD (PI), William Russell, MD (I), Anne Brown, APRN (I), Daniel Moore, MD, PhD (I), Margo Black, RN, BSN, CCRP (C), Faith Brendle
*Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI), William V. Tamborlane, MD (I), Jennifer Sherr, MD (I), Eda Cengiz, MD (I), Eileen Tichy, MMS (I), Miladys Palau-Collazo, MD (I), Grace Kim, MD (I), Robert Sherwin, MD (I), Amy Steffen, BS (C), Kate Weyman, MSN (C), and Melinda Zgorski, BSN (C).
Coordinating Center
Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD, Katrina J. Ruedy, MSPH, Craig Kollman, PhD, Peiyao Cheng, MPH, and Beth Stevens.
Protocol Development Committee
Bruce A. Buckingham, MD (Chair), Roy W. Beck, MD, PhD, Katrina J. Ruedy, MSPH, H. Peter Chase, MD, Darrell M. Wilson, MD, Jennifer Block, ARNP, CDE, Stuart A. Weinzimer, MD, William V. Tamborlane, MD, Francine Kaufman, Neil H. White, MD, Carla J. Greenbaum, MD, Karen Winer, MD, Ellen Leschek, MD, and Jay S. Skyler, MD, MACP.
Protocol Steering Committee
Bruce A. Buckingham, MD (Chair), Roy W. Beck, MD, PhD, Katrina J. Ruedy, MSPH, Robert Slover, MD, Stuart A. Weinzimer, MD, Linda A. DiMeglio, MD, Andrew A. Bremer, MD, PhD, H. Peter Chase, MD, Darrell M. Wilson, MD, William V. Tamborlane, MD, Jennifer Sherr, MD, William Russell, MD, Jennifer Block, ARNP, CDE, Laurel Messer, MSN, Amy Steffen, BS, Stephanie Woerner, FNP-C, Anne Brown, APRN Craig Kollman, PhD, Peiyao Cheng, MPH, Ellen Leschek, MD, Karen Winer, MD, Neil H. White, MD, Carla J. Greenbaum, MD, and Jay S. Skyler, MD, MACP.
Central Laboratories
Northwest Lipid Research Laboratories, Santica Marcovina, PhD, ScD, Jerry Palmer, MD, Jessica Chmielewski, Vineet Gaur, and Ann Wilson; TrialNet Core Screening Laboratory, University of Florida, William Winter, MD, David Pittman, and Melissa Sue Scott; Rutgers University Cell Repository, David Sokolowski and Michael Sheldon, MD; National Institute of Diabetes and Digestive and Kidney Diseases Biosample Repository, Heather Higgins; DNA Lab at the Barbara Davis Center for Childhood Diabetes, Sunanda Babu, PhD, George S. Eisenbarth, MD, and Liping Yu; and Jinfiniti Biosciences, John Nechtman, Richard McIndoe, PhD, and Haitao Liu.
The DirecNet Study Group
Clinical Centers (listed in alphabetical order with clinical center name, city, and state, where PI indicates Principal Investigator, I indicates Co-Investigator, and C indicates Coordinator)
Department of Pediatrics, University of Iowa Carver College of Medicine, Iowa City, IA: Eva Tsalikian, MD (PI), Michael J. Tansey, MD (I), Julie Coffey, MSN (C), Joanne Cabbage (C), and Sara Salamati (C).
Nemours Children's Clinic, Jacksonville, FL: Nelly Mauras, MD (PI), Larry A. Fox, MD (I), Kim Englert, RN (C), Joe Permuy, ARNP (C), and Kaitlin Sikes (C).
Division of Pediatric Endocrinology and Diabetes, Stanford University, Stanford, CA: Bruce A. Buckingham, MD (PI), Darrell M. Wilson, MD (I), Paula Clinton, RD, CDE (C), and Kimberly Caswell, APRN (C).
Department of Pediatrics, Yale University School of Medicine, New Haven, CT: Stuart A. Weinzimer, MD (PI), William V. Tamborlane, MD (I), Jennifer Sherr, MD (I), Amy Steffen, BS (C), Kate Weyman, MSN (C), Melinda Zgorski, BSN (C), and Eileen Tichy, MMS (C).
Washington University in St. Louis, St. Louis, MO: Neil H. White, MD (PI), Ana Maria Arbelaez, MD (I), Lucy Levandoski, PA-C (C), and Angie Starnes, RN, BSN, CDE (C).
Coordinating Center
Jaeb Center for Health Research, Tampa, FL: Roy W. Beck, MD, PhD, Katrina J. Ruedy, MSPH, Craig Kollman, PhD, Dongyuan Xing, MPH, Callyn Hall, and Beth Stevens.
National Institutes of Health
Gilman D. Grave, MD, PhD, Karen Winer, MD, and Ellen Leschek, MD.
The Type 1 Diabetes TrialNet Study Group
Chairman's Office
Jay S. Skyler, MD, MACP, Carla J. Greenbaum, MD, Norma S. Kenyon, PhD, Lisa Rafkin, MS, RD, CDE, Irene Santiago, and Jay M. Sosenko, MD.
National Institute of Diabetes and Digestive and Kidney Diseases staff
Judith Fradkin, MD, Ellen Leschek, MD, Peter Savage, MD, and Lisa Spain, MD.
Data Safety and Monitoring Board
Emily Blumberg, MD, Chair (University of Pennsylvania), Gerald Beck, PhD (Cleveland Clinic), Jonathan Braun, MD, PhD (University of California Los Angeles), David Brillon, MD, PhD (Cornell University), Rose Gubitosi-Klug MD, PhD (Case Western Reserve University), Lori Laffel, MD (Joslin Diabetes Center), Robert Veatch, PhD (Georgetown University), and Dennis Wallace, PhD (Research Triangle Institute).
Past members
Ake Lernmark (Lund University), Bernard Lo (University of California San Francisco), Herman Mitchell (Rho Inc.), Ali Naji (University of Pennsylvania), Jorn Nerup (University of Copenhagen), Trevor Orchard (University of Pittsburgh), Michael Steffes (University of Minnesota), Anastasios Tsiatis (North Carolina State University), and Bernard Zinman (University of Toronto).
Steering Committee
Jay S. Skyler, MD, Chairman (University of Miami Diabetes Research Institute), Mark Anderson, MD, PhD (University of California San Francisco), Mark Atkinson, PhD (University of Florida), Katarzyna Bourcier, PhD (National Institute of Allergy and Infectious Diseases), Dorothy Becker, MBBCh (University of Pittsburgh), Penelope Bingley, MD (University of Bristol), Janice Blum, PhD (Indiana University), Emanuele Bosi, MD (San Raffaele Hospital), Jane Buckner, MD (Benaroya Research Institute), H. Peter Chase, MD (University of Colorado Barbara Davis Center for Childhood Diabetes), Michael Clare-Salzler, MD (University of Florida), Peter Colman, PhD (Walter and Eliza Hall Institute of Medical Research), Linda A. DiMeglio, MD (Indiana University), George S. Eisenbarth, MD, PhD (University of Colorado Barbara Davis Center for Childhood Diabetes), C. Garrison Fathman, MD (Stanford University), Stephen Gitelman, MD (University of California San Francisco), Robin Goland, MD (Columbia University), Peter Gottlieb, MD (University of Colorado Barbara Davis Center for Childhood Diabetes), Gilman Grave, MD (Eunice Kennedy Shriver National Institute of Child Health and Human Development), Carla J. Greenbaum, MD (Benaroya Research Institute), Bernhard Hering, MD (University of Minnesota), Kevan Herold, MD (Yale University), Richard Insel, MD (Juvenile Diabetes Research Foundation), Jeffrey P. Krischer, PhD (University of South Florida), Jennifer Marks, MD (University of Miami Diabetes Research Institute), Antoinette Moran, MD (University of Minnesota), Jerry P. Palmer, MD (University of Washington), Mark Peakman, MD (Guy's, King's, and St. Thomas' School of Medicine), Alberto Pugliese, MD (University of Miami Diabetes Research Institute), Philip Raskin, MD (University of Texas Southwestern Medical School), Maria Grazia Roncarolo, MD (San Raffaele Scientific Institute), William Russell, MD (Vanderbilt University), Peter Savage, MD (National Institute of Diabetes and Digestive and Kidney Diseases), Desmond Schatz, MD (University of Florida), Robert Sherwin, MD (Yale University), Mark Siegelman, MD, PhD (University of Texas Southwestern Medical School), Olli Simell, MD (Hospital District of Southwest Finland), James Thomas, MD (Vanderbilt University), Massimo Trucco, MD (University of Pittsburgh), John Wentworth, MBBS, PhD, FRACP (Walter and Eliza Hall Institute of Medical Research), Diane Wherrett, MD (University of Toronto), Darrell M. Wilson, MD (Stanford University), William Winter, MD (University of Florida), Judith Fradkin, MD (National Institute of Diabetes and Digestive and Kidney Diseases, ex-officio), Ellen Leschek, MD (National Institute of Diabetes and Digestive and Kidney Diseases, ex-officio), and Lisa Spain, PhD (National Institute of Diabetes and Digestive and Kidney Diseases, ex-officio).
Past members
Christophe Benoist (Joslin Diabetes Center), Jeffrey Bluestone (University of California San Francisco), David Brown (University of Minnesota), Catherine Cowie (National Institute of Diabetes and Digestive and Kidney Diseases), Leonard Harrison (Walter and Eliza Hall Institute of Medical Research), Stanley Jordan (Cedars-Sinai Medical Center), Francine R. Kaufman (Childrens Hospital Los Angeles), John M. Lachin (George Washington University), Jeffrey Mahon (University of Western Ontario), Kirsti Nanto-Salonen (Hospital District of Southwest Finland), Gerald Nepom (Benaroya Research Institute), Tihamer Orban (Joslin Diabetes Center), Robertson Parkman (Childrens Hospital Los Angeles), Mark Pescovitz (Indiana University), John Peyman (National Institute of Allergy and Infectious Diseases), John Ridge (National Institute of Allergy and Infectious Diseases), Henry Rodriguez (Indiana University), John Wagner (University of Minnesota), and Anette Ziegler (Institut für Diabetesforschung).
Executive Committee
Jay S. Skyler, MD, MACP, Katarzyna Bourcier, PhD, Carla J. Greenbaum, MD, Jeffrey P. Krischer, PhD, Ellen Leschek, MD, Lisa Rafkin, MS, RD, CDE (University of Miami Diabetes Research Institute), Peter Savage, PhD, and Lisa Spain, MD.
Past members
Catherine Cowie, Mary Foulkes (George Washington University), Heidi Krause-Steinrauf (George Washington University), John M. Lachin, Saul Malozowski (National Institute of Diabetes and Digestive and Kidney Diseases), John Peyman, John Ridge, and Stephanie J. Zafonte (George Washington University).
Acknowledgments
We greatly appreciate the families who made a decision to participate in this study, a decision that was made within days of diagnosis, at a time of significant stress. This research was supported by National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development grants HD41890-10, HD41906-10, and HD41908-10 for the Diabetes Research in Children Network (DirecNet) Study Group and grants U01DK085509, U01DK06104211, 5U01DK085466-05, U01DK085505-02, and 5U01DK085465-04 for the Type 1 Diabetes TrialNet Study Group. Type 1 Diabetes TrialNet is a clinical trials network funded by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Center for Research Resources, the Juvenile Diabetes Research Foundation International, and the American Diabetes Association. This research was supported in part by the following: for the Barbara Davis Center for Childhood Diabetes, University of Colorado School of Medicine, CTRC grant 1UL1 RR025780; for the Division of Pediatric Endocrinology and Diabetes, Stanford University, Clinical and Translational Science Award SUL1 RR025744 for the Stanford Center for Clinical and Translational Education and Research (Spectrum) from the National Center for Research Resources, National Institutes of Health; for the Department of Pediatrics, Yale University School of Medicine, grant UL1 RR024139 from the National Center of Research Resources, a component of the National Institutes of Health, and the National Institutes of Health Roadmap for Medical Research (information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp); and for the Division of Pediatric Endocrinology, Vanderbilt University Medical Center, CTSA grant UL1TR000445 from the National Center for Advancing Translational Sciences. Medtronic MiniMed, Inc. loaned the insulin pumps and provided the reservoirs and infusion sets for the pumps at a discounted cost. The company also provided the MiniLink transmitters and UltraLink meters at no cost and provided the Sof-sensors at a discounted cost. LifeScan, Inc. provided the One Touch Ultra2 meters, test strips, and control solution at no cost, under the Investigator-Initiated Study Program of LifeScan, Inc.
Author Disclosure Statement
Among the members of the Writing Committee, B.A.B. received payment from Sanofi-Aventis, Glysense, and Roche for serving on Medical Advisory Boards, consulted for BD, received payment for a lecture (symposium at the European Association for the Study of Diabetes meeting) from DexCom, received payment from Medtronic for serving on the DSMB for STAR3 trial, and received institutional payment from Medtronic for other Principal Investigator–initiated studies and sponsored research. C.K. has consulted with Diabetes Technology Management (DTM). S.A.W. consulted for Johnson & Johnson and BD and received payment for lectures including service on speaker bureaus from Eli Lilly, stock/stock options from Insuline Medical, and in-kind support for research from Medtronic. L.D. reports payment to her institution from Medtronic for an investigator-initiated grant. A.A.B. consults for the American Humane Society on projects regarding animal-assisted therapy for pediatric patients and has received payment for lectures from Pfizer. M.C. is employed by Medtronic and has received payment for stock/stock options from Medtronic. R.S. serves on medical advisory board for Medtronic. No competing financial interests exist for R.W.B., K.J.R. and P.C.
References
- 1.Shah SC. Malone JI. Simpson NE. A randomized trial of intensive insulin therapy in newly diagnosed insulin-dependent diabetes mellitus. N Engl J Med. 1989;320:550–554. doi: 10.1056/NEJM198903023200902. [DOI] [PubMed] [Google Scholar]
- 2.Kordonouri O. Pankowska E. Rami B. Kapellen T. Coutant R. Hartmann R. Lange K. Knip M. Danne T. Sensor-augmented pump therapy from the diagnosis of childhood type 1 diabetes: results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53:2487–2495. doi: 10.1007/s00125-010-1878-6. [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW. Sibley S. Jackson M. Thomas W. β-Cell function and the development of diabetes-related complications in the Diabetes Control and Complications Trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- 4.The Diabetes Control and Complications Trial Research Group. Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the Diabetes Control and Complications Trial. A randomized, controlled trial. Ann Intern Med. 1998;128:517–523. doi: 10.7326/0003-4819-128-7-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 5.Bergenstal RM. Tamborlane WV. Ahmann A. Buse JB. Dailey G. Davis SN. Joyce C. Peoples T. Perkins BA. Welsh JB. Willi SM. Wood MA the STAR 3 Study Group. Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–320. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 6.The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464–1476. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 7.Steil GM. Rebrin K. Darwin C. Hariri F. Saad MF. Feasibility of automating insulin delivery for the treatment of type 1 diabetes. Diabetes. 2006;55:3344–3350. doi: 10.2337/db06-0419. [DOI] [PubMed] [Google Scholar]
- 8.Weinzimer SA. Steil GM. Swan KL. Dziura J. Kurtz N. Tamborlane WV. Fully automated closed-loop insulin delivery versus semi-automated hybrid control in pediatric patients with type 1 diabetes using an artificial pancreas. Diabetes Care. 2008;31:934–939. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 9.Steil G. Panteleon A. Rebrin K. Closed-loop insulin delivery—the path to physiological glucose control. Adv Drug Deliv Rev. 2004;56:125–144. doi: 10.1016/j.addr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Ruiz JL. Sherr JL. Cengiz E. Carria L. Roy A. Voskanyan G. Tamborlane WV. Weinzimer SA. Effect of insulin feedback on closed-loop glucose control: a crossover study. J Diabetes Sci Technol. 2012;6:1123–1130. doi: 10.1177/193229681200600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steil GM. Palerm CC. Kurtz N. Voskanyan G. Roy A. Paz S. Kandeel FR. The effect of insulin feedback on closed loop glucose control. J Clin Endocrinol Metab. 2011;96:1402–1408. doi: 10.1210/jc.2010-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loutseiko M. Voskanyan G. Keenan DB. Steil GM. Closed-loop insulin delivery utilizing pole placement to compensate for delays in subcutaneous insulin delivery. J Diabetes Sci Technol. 2011;5:1342–1351. doi: 10.1177/193229681100500605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davidson P. Hebblewhite H. Steed R. Bode B. Analysis of guidelines for basal-bolus insulin dosing: basal insulin, correction factor, and carbohydrate-to-insulin ratio. Endocr Pract. 2008;14:1095–1101. doi: 10.4158/EP.14.9.1095. [DOI] [PubMed] [Google Scholar]
- 14.Yki-Järvinen H. Koivisto VA. Natural course of insulin resistance in type I diabetes. N Engl J Med. 1986;315:224–230. doi: 10.1056/NEJM198607243150404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.