Abstract
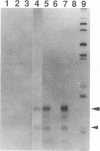
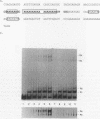
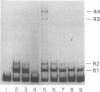
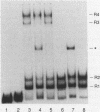
Lymphotoxin (LT; also known as tumor necrosis factor-beta) is a pleiotropic cytokine whose expression is tightly regulated in most cells and is repressed prior to activation signals. In some early B cells and Abelson murine leukemia virus-transformed pre-B-cell lines, LT mRNA is constitutively expressed. To examine the molecular regulation of the LT gene in a constitutively expressing cell line, we studied the Abelson murine leukemia virus-transformed lines PD and PD31. As demonstrated by primer extension analysis, constitutively expressed pre-B-cell-derived and inducibly expressed T-cell-derived LT mRNA were initiated at the same cap sites and predominant cap site utilization was conserved. Furthermore, we delineated an upstream activating sequence that was an important functional component of lymphotoxin transcriptional activation in PD and PD31 cells. The upstream activating sequence was localized to an essentially homopolymeric A + T-rich region (LT-612/-580), which was bound specifically by recombinant human high-mobility group I protein (HMG-I) and a PD/PD31 nuclear extract HMG-I (HMG-I-like) protein. The nuclear extract-derived HMG-I-like protein was recognized by anti-HMG-I antibody and bound to LT DNA to effect an electrophoretic mobility shift identical to that of bound recombinant human HMG-I. These findings implicate HMG-I in the regulation of constitutive lymphotoxin gene expression in PD and PD31 cells. HMG-I and HMG-I-like proteins could facilitate the formation of active initiation complexes by altering chromatin structure and/or by creating recognition sites for other activator DNA-binding proteins, some of which may be unique to or uniquely modified in these constitutive LT mRNA producers.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexeev D. G., Lipanov A. A., Skuratovskii IYa Poly(dA).poly(dT) is a B-type double helix with a distinctively narrow minor groove. 1987 Feb 26-Mar 4Nature. 325(6107):821–823. doi: 10.1038/325821a0. [DOI] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bussemakers M. J., van de Ven W. J., Debruyne F. M., Schalken J. A. Identification of high mobility group protein I(Y) as potential progression marker for prostate cancer by differential hybridization analysis. Cancer Res. 1991 Jan 15;51(2):606–611. [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Tabor S., Struhl K. Distinguishing between mechanisms of eukaryotic transcriptional activation with bacteriophage T7 RNA polymerase. Cell. 1987 Sep 25;50(7):1047–1055. doi: 10.1016/0092-8674(87)90171-1. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disney J. E., Johnson K. R., Magnuson N. S., Sylvester S. R., Reeves R. High-mobility group protein HMG-I localizes to G/Q- and C-bands of human and mouse chromosomes. J Cell Biol. 1989 Nov;109(5):1975–1982. doi: 10.1083/jcb.109.5.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckner R., Birnstiel M. L. Cloning of cDNAs coding for human HMG I and HMG Y proteins: both are capable of binding to the octamer sequence motif. Nucleic Acids Res. 1989 Aug 11;17(15):5947–5959. doi: 10.1093/nar/17.15.5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton T. S., Nissen M. S., Reeves R. Specific A . T DNA sequence binding of RP-HPLC purified HMG-I. Biochem Biophys Res Commun. 1987 Feb 27;143(1):260–265. doi: 10.1016/0006-291x(87)90659-0. [DOI] [PubMed] [Google Scholar]
- Elton T. S., Reeves R. Microheterogeneity of the mammalian high mobility group (HMG) proteins 1 and 2 investigated by reverse-phase high performance liquid chromatography. Anal Biochem. 1985 Feb 1;144(2):403–416. doi: 10.1016/0003-2697(85)90133-2. [DOI] [PubMed] [Google Scholar]
- Elton T. S., Reeves R. Microheterogeneity of the mammalian high-mobility group proteins 14 and 17 investigated by reverse-phase high-performance liquid chromatography. Anal Biochem. 1985 May 1;146(2):448–460. doi: 10.1016/0003-2697(85)90568-8. [DOI] [PubMed] [Google Scholar]
- Elton T. S., Reeves R. Purification and postsynthetic modifications of Friend erythroleukemic cell high mobility group protein HMG-I. Anal Biochem. 1986 Aug 15;157(1):53–62. doi: 10.1016/0003-2697(86)90195-8. [DOI] [PubMed] [Google Scholar]
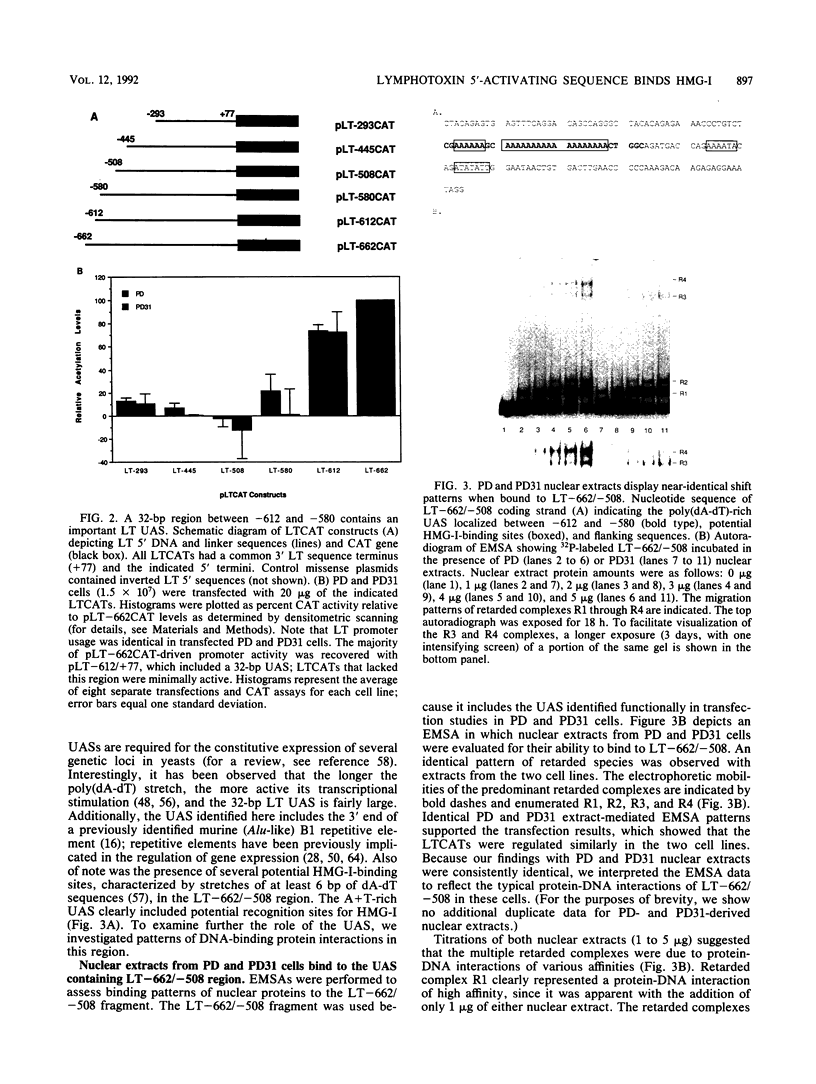
- Fashena S. J., Tang W. L., Sarr T., Ruddle N. H. The murine lymphotoxin gene promoter. Characterization and negative regulation. J Immunol. 1990 Jul 1;145(1):177–183. [PubMed] [Google Scholar]
- Giancotti V., Buratti E., Perissin L., Zorzet S., Balmain A., Portella G., Fusco A., Goodwin G. H. Analysis of the HMGI nuclear proteins in mouse neoplastic cells induced by different procedures. Exp Cell Res. 1989 Oct;184(2):538–545. doi: 10.1016/0014-4827(89)90352-2. [DOI] [PubMed] [Google Scholar]
- Giancotti V., Pani B., D'Andrea P., Berlingieri M. T., Di Fiore P. P., Fusco A., Vecchio G., Philp R., Crane-Robinson C., Nicolas R. H. Elevated levels of a specific class of nuclear phosphoproteins in cells transformed with v-ras and v-mos oncogenes and by cotransfection with c-myc and polyoma middle T genes. EMBO J. 1987 Jul;6(7):1981–1987. doi: 10.1002/j.1460-2075.1987.tb02461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Merlino G. T., Willingham M. C., Pastan I., Howard B. H. The Rous sarcoma virus long terminal repeat is a strong promoter when introduced into a variety of eukaryotic cells by DNA-mediated transfection. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6777–6781. doi: 10.1073/pnas.79.22.6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
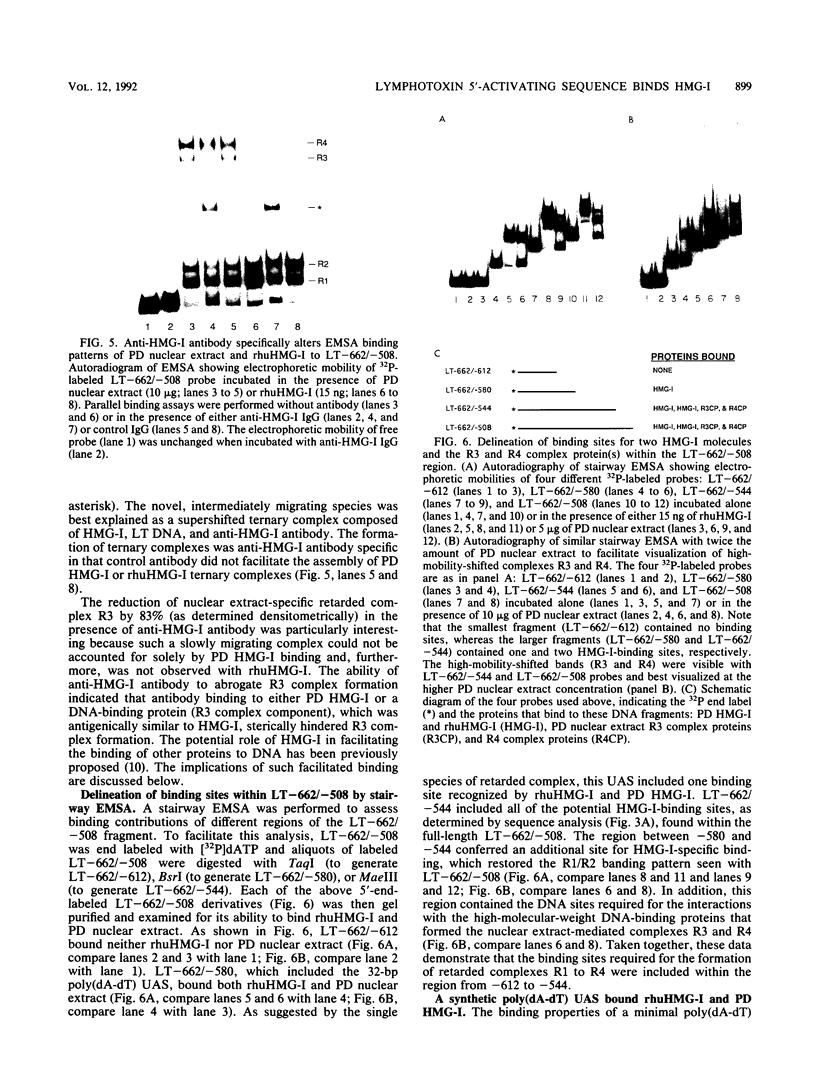
- Johnson K. R., Disney J. E., Wyatt C. R., Reeves R. Expression of mRNAs encoding mammalian chromosomal proteins HMG-I and HMG-Y during cellular proliferation. Exp Cell Res. 1990 Mar;187(1):69–76. doi: 10.1016/0014-4827(90)90118-t. [DOI] [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Elton T. S., Barr P. J., Reeves R. Complete murine cDNA sequence, genomic structure, and tissue expression of the high mobility group protein HMG-I(Y). J Biol Chem. 1988 Dec 5;263(34):18338–18342. [PubMed] [Google Scholar]
- Johnson K. R., Lehn D. A., Reeves R. Alternative processing of mRNAs encoding mammalian chromosomal high-mobility-group proteins HMG-I and HMG-Y. Mol Cell Biol. 1989 May;9(5):2114–2123. doi: 10.1128/mcb.9.5.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Karlson J. R., Mørk E., Holtlund J., Laland S. G., Lund T. The amino acid sequence of the chromosomal protein HMG-Y, its relation to HMG-I and possible domains for the preferential binding of the proteins to stretches of A-T base pairs. Biochem Biophys Res Commun. 1989 Feb 15;158(3):646–651. doi: 10.1016/0006-291x(89)92770-8. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- Kristie T. M., Roizman B. Alpha 4, the major regulatory protein of herpes simplex virus type 1, is stably and specifically associated with promoter-regulatory domains of alpha genes and of selected other viral genes. Proc Natl Acad Sci U S A. 1986 May;83(10):3218–3222. doi: 10.1073/pnas.83.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L., Holmgren-König M., Khoury G. Transcriptional "silencer" element in rat repetitive sequences associated with the rat insulin 1 gene locus. Proc Natl Acad Sci U S A. 1986 May;83(10):3151–3155. doi: 10.1073/pnas.83.10.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskov R., Lancz G., Ruddle N. H., McGrath K. M., Specter S., Klein T., Djeu J. Y., Friedman H. Production of tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) by murine pre-B and B cell lymphomas. J Immunol. 1990 May 1;144(9):3424–3430. [PubMed] [Google Scholar]
- Lenardo M. J., Fan C. M., Maniatis T., Baltimore D. The involvement of NF-kappa B in beta-interferon gene regulation reveals its role as widely inducible mediator of signal transduction. Cell. 1989 Apr 21;57(2):287–294. doi: 10.1016/0092-8674(89)90966-5. [DOI] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Lue N. F., Buchman A. R., Kornberg R. D. Activation of yeast RNA polymerase II transcription by a thymidine-rich upstream element in vitro. Proc Natl Acad Sci U S A. 1989 Jan;86(2):486–490. doi: 10.1073/pnas.86.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund T., Holtlund J., Fredriksen M., Laland S. G. On the presence of two new high mobility group-like proteins in HeLa S3 cells. FEBS Lett. 1983 Feb 21;152(2):163–167. doi: 10.1016/0014-5793(83)80370-6. [DOI] [PubMed] [Google Scholar]
- Müller U., Jongeneel C. V., Nedospasov S. A., Lindahl K. F., Steinmetz M. Tumour necrosis factor and lymphotoxin genes map close to H-2D in the mouse major histocompatibility complex. Nature. 1987 Jan 15;325(6101):265–267. doi: 10.1038/325265a0. [DOI] [PubMed] [Google Scholar]
- Nedospasov S. A., Hirt B., Shakhov A. N., Dobrynin V. N., Kawashima E., Accolla R. S., Jongeneel C. V. The genes for tumor necrosis factor (TNF-alpha) and lymphotoxin (TNF-beta) are tandemly arranged on chromosome 17 of the mouse. Nucleic Acids Res. 1986 Oct 10;14(19):7713–7725. doi: 10.1093/nar/14.19.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen M. S., Langan T. A., Reeves R. Phosphorylation by cdc2 kinase modulates DNA binding activity of high mobility group I nonhistone chromatin protein. J Biol Chem. 1991 Oct 25;266(30):19945–19952. [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Paul N. L., Lenardo M. J., Novak K. D., Sarr T., Tang W. L., Ruddle N. H. Lymphotoxin activation by human T-cell leukemia virus type I-infected cell lines: role for NF-kappa B. J Virol. 1990 Nov;64(11):5412–5419. doi: 10.1128/jvi.64.11.5412-5419.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul N. L., Ruddle N. H. Lymphotoxin. Annu Rev Immunol. 1988;6:407–438. doi: 10.1146/annurev.iy.06.040188.002203. [DOI] [PubMed] [Google Scholar]
- Peck L. J., Wang J. C. Sequence dependence of the helical repeat of DNA in solution. Nature. 1981 Jul 23;292(5821):375–378. doi: 10.1038/292375a0. [DOI] [PubMed] [Google Scholar]
- Portoles P., Rojo J., Golby A., Bonneville M., Gromkowski S., Greenbaum L., Janeway C. A., Jr, Murphy D. B., Bottomly K. Monoclonal antibodies to murine CD3 epsilon define distinct epitopes, one of which may interact with CD4 during T cell activation. J Immunol. 1989 Jun 15;142(12):4169–4175. [PubMed] [Google Scholar]
- Reeves R., Elton T. S., Nissen M. S., Lehn D., Johnson K. R. Posttranscriptional gene regulation and specific binding of the nonhistone protein HMG-I by the 3' untranslated region of bovine interleukin 2 cDNA. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6531–6535. doi: 10.1073/pnas.84.18.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Gorman C. M., Howard B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985 May 24;13(10):3599–3615. doi: 10.1093/nar/13.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Langan T. A., Nissen M. S. Phosphorylation of the DNA-binding domain of nonhistone high-mobility group I protein by cdc2 kinase: reduction of binding affinity. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1671–1675. doi: 10.1073/pnas.88.5.1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves R., Magnuson N. S. Mechanisms regulating transient expression of mammalian cytokine genes and cellular oncogenes. Prog Nucleic Acid Res Mol Biol. 1990;38:241–282. doi: 10.1016/s0079-6603(08)60713-8. [DOI] [PubMed] [Google Scholar]
- Rhodes D., Klug A. Sequence-dependent helical periodicity of DNA. Nature. 1981 Jul 23;292(5821):378–380. doi: 10.1038/292378a0. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. W., Smith M., Cox D., Williamson V. M., Young E. T. DNA sequences of two yeast promoter-up mutants. Nature. 1983 Aug 18;304(5927):652–654. doi: 10.1038/304652a0. [DOI] [PubMed] [Google Scholar]
- Russnak R. H., Candido E. P., Astell C. R. Interaction of the mouse chromosomal protein HMG-I with the 3' ends of genes in vitro. J Biol Chem. 1988 May 5;263(13):6392–6399. [PubMed] [Google Scholar]
- Saffer J. D., Thurston S. J. A negative regulatory element with properties similar to those of enhancers is contained within an Alu sequence. Mol Cell Biol. 1989 Feb;9(2):355–364. doi: 10.1128/mcb.9.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan K. C., Ruddle N. H., Schreiber R. D. Generation and characterization of hamster monoclonal antibodies that neutralize murine tumor necrosis factors. J Immunol. 1989 Jun 1;142(11):3884–3893. [PubMed] [Google Scholar]
- Solomon M. J., Strauss F., Varshavsky A. A mammalian high mobility group protein recognizes any stretch of six A.T base pairs in duplex DNA. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1276–1280. doi: 10.1073/pnas.83.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Strauss F., Varshavsky A. A protein binds to a satellite DNA repeat at three specific sites that would be brought into mutual proximity by DNA folding in the nucleosome. Cell. 1984 Jul;37(3):889–901. doi: 10.1016/0092-8674(84)90424-0. [DOI] [PubMed] [Google Scholar]
- Struhl K. Constitutive and inducible Saccharomyces cerevisiae promoters: evidence for two distinct molecular mechanisms. Mol Cell Biol. 1986 Nov;6(11):3847–3853. doi: 10.1128/mcb.6.11.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Molecular mechanisms of transcriptional regulation in yeast. Annu Rev Biochem. 1989;58:1051–1077. doi: 10.1146/annurev.bi.58.070189.005155. [DOI] [PubMed] [Google Scholar]
- Struhl K. Naturally occurring poly(dA-dT) sequences are upstream promoter elements for constitutive transcription in yeast. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8419–8423. doi: 10.1073/pnas.82.24.8419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen E., Palvimo J., Mahonen A., Linnala-Kankkunen A., Mäenpä P. H. Selective decrease in low-Mr HMG proteins HMG I and HMG Y during differentiation of mouse teratocarcinoma cells. FEBS Lett. 1988 Feb 8;228(1):45–48. doi: 10.1016/0014-5793(88)80581-7. [DOI] [PubMed] [Google Scholar]
- Wegner M., Zastrow G., Klavinius A., Schwender S., Müller F., Luksza H., Hoppe J., Wienberg J., Grummt F. Cis-acting sequences from mouse rDNA promote plasmid DNA amplification and persistence in mouse cells: implication of HMG-I in their function. Nucleic Acids Res. 1989 Dec 11;17(23):9909–9932. doi: 10.1093/nar/17.23.9909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter E., Varshavsky A. A DNA binding protein that recognizes oligo(dA).oligo(dT) tracts. EMBO J. 1989 Jun;8(6):1867–1877. doi: 10.1002/j.1460-2075.1989.tb03583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R. L., Ruddle N. H., Padula S. J., Lingenheld E. G., Bergman C. M., Rugen R. V., Epstein D. I., Clark R. B. Subtypes of helper cells. Non-inflammatory type 1 helper T cells. J Immunol. 1988 Nov 15;141(10):3329–3334. [PubMed] [Google Scholar]
- Wu J., Grindlay G. J., Bushel P., Mendelsohn L., Allan M. Negative regulation of the human epsilon-globin gene by transcriptional interference: role of an Alu repetitive element. Mol Cell Biol. 1990 Mar;10(3):1209–1216. doi: 10.1128/mcb.10.3.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Yen H. F., Rothblum L. I. Purification and characterization of a high-mobility-group-like DNA-binding protein that stimulates rRNA synthesis in vitro. Mol Cell Biol. 1988 Aug;8(8):3406–3414. doi: 10.1128/mcb.8.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]