Abstract
Background
Chronic sialadenitis is one of the most frequent chronic complications after radioactive iodine (RAI) therapy for thyroid cancer. To evaluate the long-term effects of RAI ablation on salivary gland function, we investigated scintigraphic changes in salivary glands by direct comparison of two salivary gland scintigraphies (SGSs) taken before and at 5 years after an RAI ablation.
Methods
SGS was performed just before RAI ablation (pre-SGS) and ∼5 years after RAI ablation (F/U SGS) in 213 subjects who underwent thyroidectomy for thyroid cancer. The uptake score (U score) was graded, and the ejection fraction (EF) was quantified for the parotid and submandibular glands at pre-SGS and F/U SGS. Changes in salivary gland function were graded as mild, moderate, or severe according to the differences in U score and EF between the two SGSs. Xerostomia was assessed and compared with the SGS findings.
Results
Worsening of the U score was observed in 182 of 852 salivary glands (total: 21.3%; mild: 4.2%, moderate: 7.4%, severe: 9.7%), and 47.4% of the patients had a worsening U score for at least one of four salivary glands. A decrease in EF was observed in 173 of 852 salivary glands (total: 20.3%; mild: 5.4%, moderate: 6.8%, severe: 8.1%), and 43.7% of the patients experienced a decrease in the EF of at least one of the four salivary glands. Bilateral parotid gland dysfunction was the most commonly observed condition. Thirty-five (16.4%) patients complained of xerostomia at 5 years after RAI ablation. Scintigraphic changes in salivary gland function and xerostomia were more common in patients receiving 5.55 GBq, compared with 3.7 GBq. Xerostomia was more common in patients with submandibular gland dysfunction than those with parotid gland dysfunction (68.8% vs. 33.3%, p<0.05). The number of dysfunctional salivary glands was correlated with xerostomia (p<0.01).
Conclusion
About 20% of the salivary glands were dysfunctional on SGS 5 years after a single RAI ablation, especially in patients who received higher doses of RAI. While parotid glands are more susceptible to 131I-related damage, xerostomia was more associated with submandibular gland dysfunction and the prevalence of dysfunctional salivary glands.
Introduction
The use of radioactive iodine (RAI) for the ablation of residual thyroid tissue after thyroidectomy is well-established as a part of the management of differentiated thyroid cancer (1). Serious, acute complications are extremely rare during treatment, but adverse reactions after RAI treatment have been reported (2–6). Chronic sialadenitis is one of the most frequent chronic complications after RAI therapy for thyroid cancer. The diagnosis of chronic sialadenitis can readily be accomplished when the patient's history of having received RAI is factored into his or her clinical symptomatology. Several studies of the associated symptomatology after a single RAI therapy have been published (7–10). However, many patients who have objective evidence of salivary gland dysfunction do not complain of symptoms related to salivary gland dysfunction, because the perception threshold for dry mouth or related symptoms is quite different between patients. Evaluation of objective salivary gland function is best accomplished with a salivary gland scintigraphy (SGS) using 99mTc-pertechnetate. SGS has been proposed as an effective alternative approach to functional evaluation of salivary gland involvement in patients with xerostomia (11–13).
Although a significant proportion of patients complain of symptoms related to salivary gland dysfunction after RAI therapy, long-term objective salivary gland dysfunction, including semiquantitative information, have not been well clarified. Only a few studies have objectively evaluated the long-term salivary gland dysfunction induced by a single RAI treatment; furthermore, those studies have included a relatively small number of patients within 3 years after RAI ablation (10,14,15). Therefore, we enrolled a relatively large number of patients and performed SGS both before RAI ablation (pre-SGS) and at a median follow-up of 60 months after RAI ablation (F/U SGS). Scintigraphic changes in each salivary gland pre-SGS and F/U SGS, subjective symptoms, and factors affecting development of scintigraphic salivary gland dysfunction were evaluated in patients who received a single dose of RAI.
Materials and Methods
Patients
We enrolled 213 patients who underwent RAI ablation between January 2003 and December 2006 to treat remnant thyroid tissue after thyroidectomy at a single institution. The inclusion criteria were the following: (i) histologically confirmed differentiated thyroid cancer, (ii) status after thyroidectomy, and (iii) a minimum of 52 months of follow-up after RAI ablation. Patients with additional neck surgery, external radiotherapy of the head and neck, and additional RAI therapy during the follow-up period were excluded. Patients were also excluded if they had a history of symptoms related to salivary dysfunction (dry mouth, salivary gland pain and swelling, altered taste, and difficulty in swallowing dry foods) before RAI ablation or if they were using corticosteroids at the time of remnant ablation. Patients who were receiving a drug that could affect salivary gland function were also excluded. The usual follow-up of the patients was performed in an outpatient clinic every 6 months for 5 years after RAI ablation. For the objective evaluation of salivary gland function, SGS was performed before RAI ablation and at a median follow-up of 60 months after RAI ablation. At the same visit, the patients were interviewed regarding their symptoms related to salivary gland dysfunction and underwent a subjective evaluation by an experienced physician.
All of the investigations were approved by the Research Ethics Committee of our university hospital, and informed consent was obtained from each patient.
Salivary gland scintigraphy
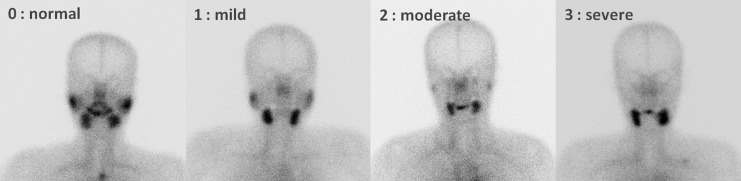
Salivary gland function was estimated by sequential SGS using 370 MBq (10 mCi) of 99mTc-pertechnetate. Scintigrams were taken by dynamic acquisition for the first 10 minutes (128×128, two frames per minute), and then images were acquired at 5-minute intervals for the next 15 minutes using a dual-head gamma camera (Infinia; GE Healthcare). The field of view included the head and cervical area. At 25 minutes after the injection, 10 mL of lemon juice was administered into the patient's mouth with a syringe for the stimulation of saliva. Images were taken for 5-minute intervals for the next 10 minutes after lemon juice intake. Grading of uptake was visually assessed, and ejection fraction (EF) of the each gland was calculated from pre- and postsialogogue images. Depending on the salivary gland uptake of both the parotid and submandibular glands, each salivary gland was visually graded and given a score from U0 to U3 (Fig. 1): U0, normal uptake and excretion; U1, mildly decreased uptake; U2, moderately decreased uptake and higher salivary gland activity than the background activity; U3, severely decreased uptake similar to the background (12). To calculate the EF of each salivary gland, regions of interest were placed over each observed salivary gland and the background region. The background-corrected activity of each salivary gland at pre- and postsialogogue was calculated, and EF was defined as the percentage of washout relative to the activity at presialogogue. Decay correction was not performed. The image analysis was independently reviewed by two experienced nuclear medicine physicians, who re-read all of the studies while blinded to the original clinical reports and clinical information and who reached a consensus.
FIG. 1.
Pattern of U0–U3 uptake scores in the parotid glands, as observed by salivary gland scintigraphy (SGS).
Pre-SGS and F/U SGS were obtained at 1–2 weeks before RAI ablation and at a median follow-up of 60 months after RAI ablation, respectively. The uptake scores (U scores) and the EFs of the bilateral parotid and submandibular glands at pre-SGS and F/U SGS were calculated, and the differences in the U scores and EFs of the bilateral parotid and submandibular glands between pre-SGS and F/U SGS were calculated. A mild worsening of the U score was defined as one grade difference between the pre-SGS and F/U SGS scores, and moderate worsening was defined as two grades of difference, and severe worsening of U score was defined as three grades of difference. A mild decrease in EF was defined as a decrease in EF of 11%–20%, a moderate decrease was 21%–30%, and a severe decrease was more than 30%, according to the literature (14,15).
RAI ablation
RAI ablation was performed using the thyroid hormone withdrawal method. In this hypothyroid protocol, levothyroxine treatment was withdrawn for 4 weeks, while triiodothyronine was given for 2 weeks and then withdrawn for 2 weeks. Serum thyrotropin (TSH) was then measured and exceeded 30 μU/mL in all of the patients. A low-iodine diet was recommended for 2 weeks before RAI ablation. RAI (131I) was orally administered in a single dose. The total doses of administered 131I ranged from 3.7 to 5.55 GBq (100–150 mCi), according to the clinical status. The patients fasted for 3 hours after therapy, both to ensure complete absorption and to reduce any risk of nausea and vomiting. After the administration of RAI, the patients were instructed to suck on sour candy at all times when awake, starting an hour after RAI and continuing for 2 days. In addition, all patients were awakened at least one time for the first two nights, first to urinate, and then to chew a piece of gum or suck candy. The patients were encouraged to drink plenty of water (at least 2400 mL) during the week after therapy, but otherwise no specific therapy was given unless they became symptomatic. Nonsteroidal anti-inflammatory medications were used in the presence of severe symptoms related to acute salivary gland swelling after RAI ablation.
Statistical analysis
The data are presented as means with standard deviations; median values are given when appropriate. For the statistical analysis, the means were compared between groups with an independent-sample t-test, and Pearson's chi-squared test and Fisher's exact test were used to compare the percentages of objective involvement of the salivary glands. Wilcoxon signed rank test was used to compare EF and U score between pre-SGS and F/U SGS. Additionally, Kendall's tau-b test for linear trends was used to study the relationship between the difference in the U score and in the EF of each salivary gland. Kappa statistic was used to study the interobserver agreement between two readers in the interpretation of the images. Statistical significance was set at p<0.05.
Results
Patient characteristics
Complete data were available from 852 salivary glands in 213 patients who underwent RAI ablation. Most of the patients (90.6%) were females and had papillary thyroid cancer (95.8%). Clinical follow-up was performed over 61.4±8.1 months after RAI ablation (median, 60 months; range, 52–83 months). The demographic data for the 213 patients are summarized in Table 1.
Table 1.
Patient Demographics
| Age (years) | |
| Mean±SD | 47±11 |
| Median/range | 47/20–75 |
| Sex | |
| Female/male | 91%/9% |
| RAI-administered activity (MBq [mCi]) | |
| Mean±SD | 5106±792 [138±21] |
| Range | 3700–5500 [100–150] |
| Follow-up period (months) | |
| Mean±SD | 61±8 |
| Median/range | 60/52–83 |
| Histology | |
| Papillary | 96% |
| Follicular | 4% |
| T stage | |
| T1 | 22% |
| T2 | 12% |
| T3 | 62% |
| T4 | 3% |
| Unknown | 1% |
| N stage | |
| N0 | 22% |
| N1 | 42% |
| Nx | 36% |
| M stage | |
| M0 | 100% |
| M1 | 0% |
| TNM stage | |
| I | 53% |
| II | 5% |
| III | 35% |
| IV | 7% |
| Risk group | |
| Low | 26% |
| Intermediate | 71% |
| High | 3% |
Symptom evaluation
Thirty-five of the 213 subjects (16.4%) complained of dry mouth symptoms possibly related to salivary gland dysfunction after RAI ablation. No significant differences were observed based on sex, age at ablation, duration of follow-up, stage of disease, primary tumor histology, risk group, or initial surgery between the patients with and without dry mouth symptoms after RAI ablation. The patients with dry mouth symptoms received significantly higher administered activities of RAI than those without dry mouth symptoms (5276±609 MBq vs. 5020±814 MBq, p=0.04). Dry mouth symptoms were more common in the patients given 5.55 GBq than in those given 3.7 GBq of RAI (17.9% vs. 7.8%, p=0.04).
Salivary gland scintigraphy
For the U score, the agreement ratio was 90.2% and the κ-value between the two independent readers was 0.78, indicating good agreement for each other's readings. In the pre-SGS, a normal salivary gland uptake (U0) was observed in 753 of 852 salivary glands (88.4%). Ninety-one of 852 salivary glands (10.7%) had scores of U1, and 8 (0.9%) scored U2. No U3 scores were observed before RAI ablation (Table 2). For the F/U SGS, normal salivary gland uptake (U0) was observed in 629 of 852 (73.8%); U1 was observed in 67 (7.9%); U2 was observed in 57 (6.7%); and U3 was observed in 99 (11.6%) salivary glands (Table 2 and Fig. 2). A single RAI ablation led to worsen the U score of both parotid glands (Wilcoxon signed rank test, right parotid gland: p=0.01; left parotid gland: p=0.02).
Table 2.
Comparison of Uptake Score in Salivary Gland Scintigraphies Between Preablation and 5 Years After Single Radioactive Iodine Ablation
| |
F/U U score |
|||
|---|---|---|---|---|
| Preablation U score | U0 | U1 | U2 | U3 |
| U0 | 589 (69.1%) | 31 (3.6%) | 50 (5.9%) | 83 (9.7%) |
| U1 | 40 (4.7%) | 36 (4.2%) | 2 (0.2%) | 13 (1.5%) |
| U2 | 0 (0%) | 0 (0%) | 5 (0.6%) | 3 (0.4%) |
| U3 | 0 | 0 | 0 | 0 |
U score, uptake score; F/U, follow-up.
FIG. 2.
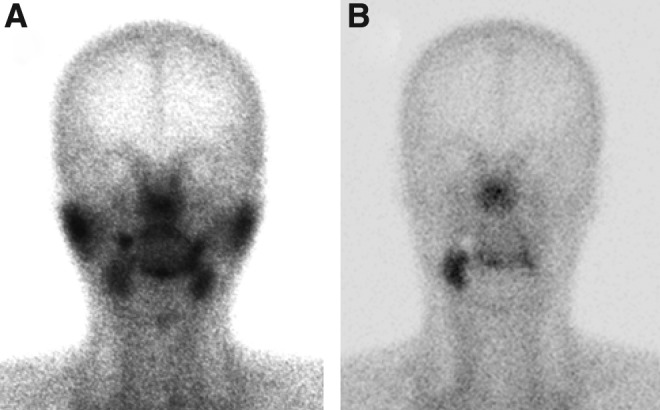
Salivary gland scintigraphies before radioactive iodine (RAI) ablation (A) and at 62 months after RAI ablation (B).
In the pre-SGS EF results, 811 of 852 salivary glands (95.2%; parotid glands: 96.8%; submandibular glands: 93.7%) had an EF of more than 40% with regard to sialogogue intake, whereas a slight decrease in EF was observed in 41 glands (4.8%). In the F/U SGS EF analysis, 585 salivary glands had a normal EF value of more than 40% (68.7%; parotid glands: 63.4%; submandibular glands: 74.2%), but decreases in salivary excretory function were observed in 267 glands (31.3%). The EF was 0%–9% in 7.9% of the salivary glands, 10%–19% in 6.6%, 20%–29% in 6.0%, and 30%–39% in 10.8%. A single RAI ablation did lead to a decrease in the EF of the left parotid and both submandibular glands (Wilcoxon signed rank test: left parotid gland, p=0.02; right submandibular gland, p=0.01; left submandibular gland, p=0.02).
Long-term changes in salivary gland function
With regard to the pre-SGS and F/U SGS, no change in the U score of each salivary gland was observed in 78.7% of 852 salivary glands after a single RAI ablation. However, worsening of uptake was observed in 190 of 852 salivary glands (21.3%), with worsening that was mild in 4.2%, moderate in 7.4%, and severe in 9.7% of the glands (Tables 2 and 3). Worsening of the U score was more frequently seen in parotid glands than in the submandibular glands (33.3% vs. 9.4%, p<0.01). Moderate-to-severe worsening of the U score was also more common in parotid glands (30.3%) than in submandibular glands (4.0%). In patient-based analyses, 101 of the 213 patients (47.4%) had worsening of the U score in at least one of the 4 salivary glands in F/U SGS after a single RAI ablation. Most of the patients had a worsening of the U score in one or two salivary glands (1 in 38.6%, 2 in 49.5%, 3 in 6.9%, and 4 glands in 5.0% of subjects with worsening of U scores). In patients with worsened U scores, 50.6% and 21.4% of subjects had bilateral involvement of the parotid glands and submandibular glands, respectively.
Table 3.
Worsening of the Uptake Score of Each Salivary Gland Between Preablation and Follow-Up Salivary Gland Scintigraphy
| |
Worsening of U score |
|||
|---|---|---|---|---|
| U0 | U1 | U2 | U3 | |
| Right parotid (3.7/5.55 GBq) | 67% (86%/61%) | 3% (0%/4%) | 13% (6%/15%) | 17% (8%/20%) |
| Left parotid (3.7/5.55 GBq) | 66% (94%/58%) | 4% (0%/4%) | 12% (2%/15%) | 18% (4%/23%) |
| Right submandibular (3.7/5.55 GBq) | 91% (88%/93%) | 6% (12%/4%) | 2% (0%/2%) | 1% (0%/1%) |
| Left submandibular (3.7/5.55 GBq) | 90% (86%/91%) | 5% (12%/3%) | 3% (2%/3%) | 2% (0%/3%) |
When comparing pre-SGS and F/U SGS, no change in EF was observed in 679 of 852 salivary glands (79.7%). A mild decrease in EF was observed in 5.4%, a moderate decrease in EF of 6.8%, and a severe decrease in EF of 8.1% of 852 salivary glands after a single RAI treatment (Table 4). A decrease in EF was more frequently detected in the parotid than in the submandibular glands (31.9% vs. 9.4%, respectively), and a moderate-to-severe decrease was also more common in the parotid than in the submandibular glands (30.3% vs. 8.7%). In patient-based analyses, a decrease in EF of at least one of the 4 salivary glands was observed in 93 of the 213 patients (43.7%) after a single RAI ablation. Most of the patients with a decrease in EF had involvement of one or two salivary glands (1 in 38.7%, 2 in 44.1%, 3 in 12.9%, and 4 in 4.3% of patients who had decreases in EF of more than 10%). In the patients with EF decreases, 49.2% and 22.2% of patients had bilateral involvement of the parotid and submandibular glands, respectively.
Table 4.
Decreases in the Ejection Fraction of Each Salivary Gland Between Preablation and Follow-Up Salivary Gland Scintigraphy
| |
Decrease in EF |
||||
|---|---|---|---|---|---|
| ≤0 | 0–10 | 11–20 | 21–30 | >30 | |
| Right parotid (3.7/5.55 GBq) | 57% (68%/54%) | 13% (10%/14%) | 3% (6%/2%) | 10% (4%/12%) | 17% (12%/18%) |
| Left parotid (3.7/5.55 GBq) | 56% (70%/52%) | 10% (10%/10%) | 8% (12%/7%) | 12% (6%/14%) | 14% (2%/17%) |
| Right submandibular (3.7/5.55 GBq) | 79% (78%/80%) | 13% (18%/11%) | 4% (2%/4%) | 3% (2%/3%) | 1% (0%/2%) |
| Left submandibular (3.7/5.55 GBq) | 79% (80%/79%) | 11% (14%/11%) | 7% (6%/7%) | 2% (0%/3%) | 1% (0%/1%) |
EF, ejection fraction.
The worsening of U scores was correlated with decreases in the EFs of both the parotid and submandibular glands (correlation coefficient: 0.739 for right, p<0.01 and 0.751 for left, p<0.01—parotid glands; and correlation coefficient: 0.199 for right, p=0.01 and 0.388 for left, p<0.01—submandibular glands).
Relationship between administered RAI dosage and change in salivary gland function
The demographic data were not different between the patients with changes in U scores or EFs and those without significant changes of salivary gland function, except for the administered dose of RAI. The administered RAI dosage was significantly higher in the patients with changes in U scores or EFs than in patients without changes of salivary gland function (5065±818 MBq vs. 5487±659 MBq, p=0.04). Moderate-to-severe worsening of U scores was more frequently observed in the patients given 5.55 GBq than in patients given 3.7 GBq of RAI (48.8% vs. 17.6%, p=0.02, Table 3). Moderate-to-severe worsening of the U scores of the parotid glands was observed in 36.7% of the patients given 5.55 GBq, but in 9.8% of the patients given 3.7 GBq of RAI. Mild worsening of the U score of the submandibular glands was seen in 3.4% of the patients given 5.55 GBq, compared with 11.8% of the patients given 3.7 GBq of RAI. In contrast, a moderate-to-severe decrease in EF was more common in the patients given 5.55 GBq than in those given 3.7 GBq of RAI (43.8% vs. 15.7%, p=0.03, Table 4). A moderate-to-severe decrease in the EFs of the parotid glands was observed in 31.2% of the patients given 5.55 GBq, but in 11.8% of the patients given 3.7 GBq of RAI.
Relationship between subjective and objective salivary gland dysfunction
When symptoms were compared with SGS findings, development of dry mouth was significantly more common in patients with moderate-to-severe worsening of the U scores of the submandibular glands than in those with worsening of the parotid glands (42.9% vs. 27.3%, p=0.01). Dry mouth symptoms were more frequently observed in patients with moderate-to-severe decreases in the EFs of the submandibular glands than in the parotid glands (44.4% vs. 29.9%, p=0.01). The number of salivary glands with moderate-to-severe worsening of the U score was positively correlated with the development of dry mouth (r=0.344, p<0.01, Table 5). The number of salivary glands with moderate-to-severe decreases in the EF was also correlated with the development of dry mouth (r=0.349, p<0.01, Table 5).
Table 5.
Dry Mouth Symptom Development According to the Number of Salivary Glands with Moderate-to-Severe Worsening of Uptake Score and Decreases in Ejection Fraction
| |
Number of salivary glands with moderate-to-severe worsening of U score |
Number of salivary glands with moderate-to-severe decrease in EF |
||||
|---|---|---|---|---|---|---|
| 0 | 1–2 | 3–4 | 0 | 1–2 | 3–4 | |
| Dry mouth (+) | 6% | 27% | 71% | 7% | 31% | 40% |
| Dry mouth (−) | 94% | 73% | 29% | 93% | 69% | 60% |
During follow-up, 17.8% of the patients who underwent RAI ablation experienced acute salivary gland swelling or pain after RAI ablation. The patients with a history of acute sialadenitis had a higher prevalence of a worsened U score and a decreased EF at the 5-year follow-up (worsening of U score: 76.5% vs. 34.8%, p<0.01 and decrease in EF: 61.8% vs. 32.3%, p<0.01).
Discussion
Acute sialadenitis is the most frequent complication after RAI therapy for thyroid cancer. Furthermore, acute sialadenitis may persist in as many as 15% of patients (9,10). The prevalence of objective salivary gland dysfunction is reported to be high in various reports (6,10,15,16). Thus, many patients who do not complain of symptoms related to salivary dysfunction have objective evidence of salivary dysfunction (9,15,16). Only 2 studies (10,16) that would be comparable to our series have examined long-term objective salivary gland dysfunction induced by RAI therapy (Table 6). These studies have reported objective salivary gland dysfunction rates in the range of 18.7%–64.7% in patients. In their studies, some patients had persistent, objective salivary dysfunction up to the end of follow-up, and newly developed objective salivary dysfunction was noted in some patients during the second or third year of follow-up. However, these studies dealt with relatively small numbers of patients within 3 years after RAI ablation and included patients with single and multiple RAI therapy.
Table 6.
Published Studies Examined Salivary Gland Dysfunction by Salivary Gland
| Study | No. of patients | Dose range of RAI (GBq) | Follow-up duration | Salivary gland dysfunctiona | Occurrence of xerostomia |
|---|---|---|---|---|---|
| Malpani et al. (6)b | 33 | 1.4–38.7 | 16 months | 73% | 36% |
| Solans et al. (10) | 48 | 0.9–3.7 | 3 years | 19% | 35% |
| 26 | 7.4–11.1 | 3 years | 35% | 31% | |
| Raza et al. (15)b | 50 | 1.4–≥11.1 | 6 months | 46% | 52% |
| Caglar et al. (16) | 34 | 3.7–5.5 | 20 months | 65% | 35% |
Patient-based analysis.
Include patients with multiple RAI therapies.
RAI, radioactive iodine.
The present study demonstrated a high prevalence of chronic salivary gland dysfunction at 5 years after a single RAI treatment by using serial SGS in 213 patients. Long-term objective salivary gland dysfunction was observed in ∼20% of parotid and submandibular glands, and roughly 40% of the patients had salivary gland dysfunction at a median follow-up of 60 months after RAI ablation. However, only 16.2% of the patients complained of dry mouth symptoms. Because the design of the study was different in terms of both the administered dose and the follow-up strategy, direct comparison between previous studies and our study is difficult. However, the strength of our study is demonstrated in the validation of previous results with objective, more long-term salivary gland dysfunction data that were determined in a larger series of patients. Salivary gland dysfunction was frequently observed regardless of symptomatology. In patients given 3.7 GBq of RAI, our results (moderate-to-severe worsening of the U score: 17.6%; moderate-to-severe decrease of EF: 11.8%) were not very different from those from a report by Solans et al., in which moderate-to-severe salivary dysfunction was found in 19.6% (10). The slightly lower prevalence of objective salivary gland dysfunction at 5 years in our study suggests that significant progression of dysfunction does not occur later than 3 years of an RAI ablation. Therefore, in theory, progressive damage in later years may not occur, but studies would be needed to determine if this is the case. Although sialogogue intake was encouraged for 2 days after administration of RAI, a high prevalence of chronic salivary gland dysfunction induced by RAI ablation was noted. Moreover, a higher prevalence of chronic salivary gland dysfunction was observed in patients with a history of acute sialadenitis. Therefore, prevention of acute sialadenitis is important to reduce the occurrence of chronic salivary gland dysfunction. Silberstein (17) reported that oral stimulation of the salivary gland using their own Cincinnati regimen was very efficient, as the prevalence of acute sialadenitis (4–10 days after RAI treatment) and chronic sialadenitis (6–8 months after RAI treatment) was very low (acute: 3%–7%, chronic: 0%–3%) with this regimen. Further randomized comparative studies are needed to confirm the universality of these findings and if they apply in longer follow-up.
Parotid gland dysfunction developed more frequently than submandibular dysfunction after RAI ablation. Furthermore, the parotid gland has a tendency to develop more severe dysfunction than the submandibular gland. In the parotid glands, moderate-to-severe dysfunction (moderate-to-severe worsening of the U score and a decrease in the EF) was more frequently observed than mild dysfunction (mild worsening of the U score and a decrease in the EF; Tables 3 and 4). Variations in parenchymal volumes, spontaneous secretion, and radiosensitivity may explain the increased damage to the parotid gland caused by RAI (2,4–6). The differences in radiosensitivity could be a result of higher concentrations of serous acinar cells in the parotid glands, which are selectively radiosensitive (18), whereas the mucinous tissue of the submandibular glands might have contributed to a radioprotective effect.
Salivary gland dysfunction and xerostomia after RAI therapy have been reported to be dependent on cumulative activity (2,10,16). Our data demonstrate that a higher administered dose results in a higher prevalence of salivary dysfunction based on the assessment of scintigraphic findings and subjective symptoms after a single RAI dose. It is interesting to note that moderate-to-severe salivary gland dysfunction (worsening of the U score or a decrease in EF) is more common than mild dysfunction in patients treated with 5.55 GBq of RAI. However, mild salivary gland dysfunction is rather common in patients treated with 3.7 GBq of RAI, and moderate-to-severe salivary gland dysfunction was observed in only a few patients. Thus, administered activities of 5.55 GBq were associated with more severe salivary gland (especially parotid gland) damage than administered activities of 3.7 GBq. Therefore, we believe that a minimal, but effective, RAI dose should be chosen for RAI ablation.
The present study is the first to demonstrate that dry mouth symptoms were more likely to be associated with worsening of the U score of the submandibular gland and with the number of dysfunctional salivary glands. The sensation of oral dryness can occur when a person's normal, unstimulated flow rate is reduced by ∼45%–50% (19,20). Whole saliva is the designation for mixed fluid in the mouth, which derives from the major salivary glands (the parotid, submandibular, and sublingual glands, which account for 90% of saliva production) and the minor salivary glands (which account for the remaining 10%). Under resting conditions, roughly two-thirds of saliva is produced by the submandibular glands (21). Therefore, submandibular gland dysfunction might be closely related to dry mouth symptoms, as seen in our study.
In the current study, we used a relatively large RAI dose for remnant ablation, and the development of objective salivary gland dysfunction after RAI ablation was more common in patients given 5.55 GBq than in those patients who received 3.7 GBq. The use of a lower dose of RAI for remnant ablation results in less-chronic salivary gland dysfunction. Future studies will be required in patients receiving lower RAI doses, such as 1.1 GBq, the dose commonly used on an outpatient basis. Although the development of chronic salivary gland dysfunction has been associated with increasing amounts of administered RAI activity, other factors, including remnant thyroid tissue, hydration status, uptake status of cold iodide, and the use of sialogogues or nonsteroidal inflammatory medications, can influence the development of chronic salivary gland dysfunction. Further studies of these factors are also required. Furthermore, salivary gland uptake was not quantitatively evaluated in the current study. We did not measure the absolute percentage uptake of the injected dose, because we believe that visual grading of uptake is quite acceptable for the clinical evaluation of salivary gland function. The absolute percentage of uptake is frequently unreliable due to uptake by other organs expressing the sodium iodide symporter, such as the stomach and remnant thyroid tissue, in addition to hydration status and urinary excretion.
Another unanswered question is whether the increased TSH level after short-term discontinuation of thyroid hormone, resulting in increased serum TSH levels, could stimulate the salivary glands via an activation of the sodium iodide symporter. Long-standing hypothyroidism might affect salivary gland function. However, the pre-SGS was likely obtained in a state of mild hypothyroidism, because the pre-SGS was performed within one week after triiodothyronine withdrawal. Of note, Dixit et al. reported that “stimulated parotid flow rates were not significantly different between healthy controls, subjects with hypothyroidism on thyroid replacement therapy, and subjects with hypothyroidism not on thyroid replacement therapy” (22). Therefore, we believe that changes in salivary gland uptake were not significantly influenced by differences in thyroid function at the time the patients underwent their preablation or their follow-up studies.
In conclusion, in the present study, we observed a worsening of the U score in 21.3% of the salivary glands, and there was a decrease in EF in 20.3% of the salivary glands at a median of 60 months after a single RAI ablation. Furthermore, 16.4% of the patients had symptoms related to salivary dysfunction at a median of 60 months after a single RAI dose. An apparent dose–response relationship was observed between objective and subjective parameters of salivary dysfunction. The development of dry mouth was associated with submandibular gland dysfunction and with the number of dysfunctional salivary glands. Therefore, minimal RAI activity should be used for RAI ablation. Further studies are required to determine the minimum RAI activity necessary to achieve successful remnant ablation and minimize side effects, as recently suggested by the American Thyroid Association guidelines (23).
Acknowledgments
This work was supported by Nuclear Research & Development Program of National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (MEST; grant code: 2010-0017515), and a grant of the Korea Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (Grant Number: A111345).
Disclosure Statement
The authors have nothing to disclose.
References
- 1.Samaan NA. Schultz PN. Hickey RC. Goepfert H. Haynie TP. Johnston DA. Ordonez NG. The results of various modalities of treatment of well differentiated thyroid carcinomas: a retrospective review of 1599 patients. J Clin Endocrinol Metab. 1992;75:714–720. doi: 10.1210/jcem.75.3.1517360. [DOI] [PubMed] [Google Scholar]
- 2.Alexander C. Bader JB. Schaefer A. Finke C. Kirsch CM. Intermediate and long-term side effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med. 1998;39:1551–1554. [PubMed] [Google Scholar]
- 3.Lin WY. Shen YY. Wang SJ. Short-term hazards of low-dose radioiodine ablation therapy in postsurgical thyroid cancer patients. Clin Nucl Med. 1996;21:780–782. doi: 10.1097/00003072-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Markitziu A. Lustmann J. Uzieli B. Krausz Y. Chisin R. Salivary and lacrimal gland involvement in a patient who had undergone a thyroidectomy and was treated with radioiodine for thyroid cancer. Oral Surg Oral Med Oral Pathol. 1993;75:318–322. doi: 10.1016/0030-4220(93)90144-s. [DOI] [PubMed] [Google Scholar]
- 5.Bohuslavizki KH. Brenner W. Lassmann S. Tinnemeyer S. Tönshoff G. Sippel C. Wolf H. Clausen M. Henze E. Quantitative salivary gland scintigraphy in the diagnosis of parenchymal damage after treatment with radioiodine. Nucl Med Commun. 1996;17:681–686. doi: 10.1097/00006231-199608000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Malpani BL. Samuel AM. Ray S. Quantification of salivary gland function in thyroid cancer patients treated with radioiodine. Int J Radiat Oncol Biol Phys. 1996;35:535–540. doi: 10.1016/s0360-3016(96)80016-2. [DOI] [PubMed] [Google Scholar]
- 7.Grewal RK. Larson SM. Pentlow CE. Pentlow KS. Gonen M. Qualey R. Natbony L. Tuttle RM. Salivary gland side effects commonly develop several weeks after initial radioactive iodine ablation. J Nucl Med. 2009;50:1605–1610. doi: 10.2967/jnumed.108.061382. [DOI] [PubMed] [Google Scholar]
- 8.Hoelzer S. Steiner D. Bauer R. Reiners C. Farahati J. Hundahl SA. Dudeck J. Current practice of radioiodine treatment in the management of differentiated thyroid cancer in Germany. Eur J Nucl Med. 2000;27:1465–1472. doi: 10.1007/s002590000333. [DOI] [PubMed] [Google Scholar]
- 9.Hyer S. Kong A. Pratt B. Harmer C. Salivary gland toxicity after radioiodine therapy for thyroid cancer. Clin Oncol (R Coll Radiol) 2007;19:83–86. doi: 10.1016/j.clon.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Solans R. Bosch JA. Galofre P. Porta F. Roselló J. Selva-O'Callagan A. Vilardell M. Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J Nucl Med. 2001;42:738–743. [PubMed] [Google Scholar]
- 11.Henriksen AM. Nossent HC. Quantitative salivary gland scintigraphy can distinguish patients with primary Sjögren's syndrome during the evaluation of sicca symptoms. Clin Rheumatol. 2007;26:1837–1841. doi: 10.1007/s10067-007-0586-1. [DOI] [PubMed] [Google Scholar]
- 12.Schall GL. Anderson LG. Wolf RO. Herdt JR. Tarpley TM., Jr Cummings NA. Zeiger LS. Talal N. Xerostomia in Sjogren's syndrome. Evaluation by sequential salivary scintigraphy. JAMA. 1971;216:2109–2116. [PubMed] [Google Scholar]
- 13.Vinagre F. Santos MJ. Prata A. da Silva JC. Santos AI. Assessment of salivary gland function in Sjogren's syndrome: the role of salivary gland scintigraphy. Autoimmun Rev. 2009;8:672–676. doi: 10.1016/j.autrev.2009.02.027. [DOI] [PubMed] [Google Scholar]
- 14.Umehara I. Yamada I. Murata Y. Takahashi Y. Okada N. Shibuya H. Quantitative evaluation of salivary gland scintigraphy in Sjörgen's syndrome. J Nucl Med. 1999;40:64–69. [PubMed] [Google Scholar]
- 15.Raza H. Khan AU. Hameed A. Khan A. Quantitative evaluation of salivary gland dysfunction after radioiodine therapy using salivary gland scintigraphy. Nucl Med Commun. 2006;27:495–499. doi: 10.1097/00006231-200606000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Caglar M. Tuncel M. Alpar R. Scintigraphic evaluation of salivary gland dysfunction in patients with thyroid cancer after radioiodine treatment. Clin Nucl Med. 2002;27:767–771. doi: 10.1097/00003072-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Silberstein EB. Reducing the incidence of I-131 induced sialadenitis: the role of pilocarpine. J Nucl Med. 2008;49:546–549. doi: 10.2967/jnumed.107.049411. [DOI] [PubMed] [Google Scholar]
- 18.Stephens LC. Schultheiss TE. Price RE. Ang KK. Peters LJ. Radiation apoptosis of serous acinar cells of salivary and lacrimal glands. Cancer. 1991;67:1539–1543. doi: 10.1002/1097-0142(19910315)67:6<1539::aid-cncr2820670613>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. J Dent Res. 1987;66:648–653. doi: 10.1177/00220345870660S107. [DOI] [PubMed] [Google Scholar]
- 20.Ghezzi EM. Lange LA. Ship JA. Determination of variation of stimulated salivary flow rates. J Dent Res. 2000;79:1874–1878. doi: 10.1177/00220345000790111001. [DOI] [PubMed] [Google Scholar]
- 21.Schneyer LH. Source of resting total mixed saliva of man. J Appl Physiol. 1956;9:79. doi: 10.1152/jappl.1956.9.1.79. [DOI] [PubMed] [Google Scholar]
- 22.Dixit PS. Ghezzi EM. Wagner-Lange LA. Ship JA. The influence of hypothyroidism and thyroid replacement therapy on stimulated parotid flow rates. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:55–60. doi: 10.1016/s1079-2104(99)70295-7. [DOI] [PubMed] [Google Scholar]
- 23.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]