Abstract
Background
Anaplastic thyroid cancer (ATC) is a rare but highly aggressive malignancy with a median survival of 3–5 months. The BRAF oncogene is mutated to its active form in up to 24% of ATC cases. Sorafenib is a tyrosine kinase inhibitor that acts on the RAF-1 serine/threonine kinase. In preclinical mouse models, sorafenib inhibits the growth of ATC xenografts and improves survival. No study of sorafenib in ATC has been conducted. We conducted a multi-institutional phase II trial of sorafenib in patients with ATC who had failed up to two previous therapies.
Methods
The primary endpoint of the trial was the Response Evaluation Criteria In Solid Tumors (RECIST)–defined imaging response rate. Twenty patients with ATC were treated with sorafenib 400 mg twice daily.
Results
Two of the 20 patients had a partial response (10%) and an additional 5 of 20 (25%) had stable disease. The duration of response in the two responders was 10 and 27 months, respectively. For the patients with stable disease, the median duration was 4 months (range 3–11 months). The overall median progression-free survival was 1.9 months with a median and a 1-year survival of 3.9 months and 20%, respectively. Toxicity was manageable and as previously described for sorafenib, including hypertension and skin rash.
Conclusion
Sorafenib has activity in ATC, but at a low frequency and similar to our previous experience with fosbretabulin. One patient with a response had previously progressed on fosbretabulin. Toxicities were both predictable and manageable.
Introduction
Anaplastic thyroid cancer (ATC) is a highly aggressive solid tumor that represents 2% of all thyroid cancer and occurs mainly in elderly patients. ATC is rare, with only 300–500 cases reported annually in the United States and an annual incidence of 1–2 cases per million population per year (1). It carries an ominous prognosis, with a median survival of 3–5 months from diagnosis, and the prognosis grows even worse upon failure of chemotherapy and radiation (2). All patients with ATC are classified as having stage IV disease by the TNM staging and American Joint Commission on Cancer staging systems (3). This reflects the highly metastatic and systemic nature of this disease. A multimodality approach consisting of surgical debulking, radiation therapy, and doxorubicin-based chemotherapy is an acceptable treatment (4). The common cause of death is the invasion of local structures with airway compromise; therefore, obtaining local control is an important palliative endpoint.
The molecular pathogenesis of ATC has been extensively studied over the past few years. Mutations in several oncogenes and tumor suppressor genes have been described, including the BRAF, RAS, CTNNB1 (catenin), PIK3CA, P53, PTEN, AXIN1, and APC genes (5). ATC carries many numerical and structural chromosomal changes (5). The BRAF oncogene, part of the RAS–RAF–MEK–ERK signal transduction pathway, is mutated to its active form in up to 24% of ATC cases; this is thought to be an important event in the evolution of ATC (6,7).
Sorafenib is a small-molecule tyrosine kinase inhibitor that acts on the RAF-1 serine/threonine kinase. Sorafenib also blocks vascular endothelial growth factor receptor 2 (VEGFR2) and platelet-derived growth factor receptor beta (PDGFR-β) and, thus, has antiangiogenic properties as well, all of which have been proven as potential therapeutic targets for thyroid cancer (7). In several preclinical studies, sorafenib has been shown to inhibit the growth of rat orthotopic ATC xenografts and the survival of test animals was improved; in two other nude mouse human tumor xenograft experiments, it was shown to inhibit tumor growth as well (8–10). Unfortunately, subsequent data have demonstrated that many of the presumed thyroid cell lines used in the literature are not unique or are not actually of thyroid origin (11). Nevertheless, some of the cell lines studied, such as FRO (9), C643, Hth-74 (8), and 8505C (10), were confirmed to be of thyroid origin.
There have been two phase II trials of sorafenib in patients with advanced or metastatic thyroid cancer. In the first study, among patients with advanced thyroid cancer regardless of histology, 2 of 30 patients had ATC, both of whom progressed (12). However, one patient had a 50% decrease in the size of a shoulder nodule at 4 weeks of therapy. In the second study, 4 of 56 patients had ATC, with a median duration of therapy of 2 months (ranging from 0.5 to 10 months and a total duration of therapy in all patients of 16 months) (13). On the basis of the aforementioned preclinical and clinical rationale, we embarked on a phase II study of patients with anaplastic thyroid carcinoma.
Materials and Methods
Patients
All participating Institutional Review Boards approved this study and written informed consent was obtained from all of the patients. Three institutions participated in this trial: University Hospitals Seidman Cancer Center/Case Western Reserve University, Henry Ford Hospital, and Mary Babb Randolph Cancer at West Virginia University. Patients with histologically confirmed ATC (diagnostic pathology material was required to be submitted to the pathology department of University Hospitals Seidman Cancer Center to confirm diagnosis of ATC by one pathologist [J.W.]) were eligible if their disease had progressed after treatment with cytotoxic chemotherapy (given alone or with radiation) and their disease was not considered amenable to radiation or surgery with curative intent. Patients may have received up to two prior systemic cytotoxic chemotherapy regimens. Any combined modality systemic cytotoxic chemotherapy was considered as one prior cytotoxic regimen. Other eligibility criteria included measurable disease, age >18 years, life expectancy of >8 weeks, and Eastern Cooperative Oncology Group (ECOG) performance status 0–2. Patients had normal organ and marrow function as defined as follows: absolute neutrophil count >1250 cells/μL, platelets >100,000 cells/μL, total bilirubin <1.5 times the upper limit of normal, aspartate aminotransferase/alanine aminotransferase (AST/ALT) <3.5 times the institutional upper limit of normal, and creatinine <1.5 times the upper limit of normal. Patients had to be able to swallow an oral medication. Patients with known brain metastases were excluded. Patients with hypertension must have had clinical documentation of controlled blood pressure/hypertension before study entry (i.e., no blood pressure determination exceeding 150 mmHg systolic or 100 mmHg diastolic). Patients could not be on therapeutic anticoagulation.
Treatment and study design
Sorafenib was administered on a fixed daily dose of 400 mg by mouth twice daily. A cycle of therapy was considered to be 28 days. Blood pressures were monitored weekly during the first cycle. The treatment was continued until one of the following criteria applied: disease progression; intercurrent illness preventing further treatment; unacceptable adverse events; or patient decision to withdraw. The primary objective of this study was to determine that the objective response rate of sorafenib given to patients with anaplastic carcinoma of the thyroid is 20% or greater. Secondary objectives were overall survival, progression-free survival (PFS), and toxicity.
Statistical considerations
The goal of this study was to examine the effectiveness of sorafenib against ATC. There is no standard therapy for ATC, and the median survival for relapse following radiation and chemotherapy (corresponding to previously treated, advanced regional disease) or metastatic disease is on the order of 3–5 months, and an objective response rate of 5% is typical, though a response rate of 20% has been reported for the most active agent (doxorubicin) and is thus feasible as our target response rate. Given the distinct natural history of this disease, two different efficacy endpoints were defined: objective response rate to sorafenib as well as survival endpoints. Accordingly, an increase in the overall objective response rate from 5% to 20% would be deemed clinically meaningful to embark on a larger confirmatory phase III study. Assuming an objective response rate of 5% for historic controls and a target response rate of 20%, we used Simon's MinMax two-stage design (13) with α=0.10 and β=0.10 (power=0.90) to determine that if none of the first 18 patients respond, the study is terminated; otherwise, accrual is continued to a total of 32 patients, at which point if ≤3 patients respond, the proposed treatment option is rejected. PFS was measured from the date of outset of treatment to the date of disease progression or death, whichever occurred first, and censored to the date of last follow-up for survivors. The partial response rate and the clinical benefit (partial response and stable disease) rate, along with their 95% confidence intervals [CIs], were estimated by Wilson's method (14), and the survivor functions were estimated by the Kaplan–Meier method.
Results
Patients
Twenty patients were enrolled between June 2005 and April 2011. Baseline patient characteristics are depicted in Table 1. The median age was 59, with the majority of patients being men. More than half the patients enrolled had an ECOG performance status of 1 or 2. A total of 84 cycles were administered with a median of 2 cycles (range 1–27). The median follow-up was 3.9 months (range 1.2–26.2 months). All of the patients enrolled had a diagnosis of ATC confirmed by one pathologist (J.W.). All patients had stage IVC (metastatic) disease.
Table 1.
Patient Characteristics
| n (%) | |
|---|---|
| Patients | 20 (100%) |
| Sex | |
| Male | 13 (65%) |
| Female | 7 (35%) |
| Median age (years) | 59 (range 28–79) |
| U.S. OMB race/ethnicity category | |
| Caucasian | 20 (100%) |
| African American | 0 (0%) |
| ECOG performance status | |
| 0 | 9 (45%) |
| 1 | 7 (35%) |
| 2 | 4 (20%) |
| Prior therapy | |
| Chemotherapy | 20 (100%) |
| Radiation | 18 (90%) |
| Surgery | 18 (90%) |
U.S. OMB, United States Office of Management and Budget; ECOG, Eastern Cooperative Oncology Group.
Efficacy
Among the 20 patients, 18 patients underwent serial computed tomography scanning for disease response evaluation. The two who did not were considered to have progressive disease. Two of the 20 patients (10%) experienced a partial response [CI 3%–30%]. In addition, 5 of the 18 patients (28%) had stable disease. The clinical benefit rate (partial response+stable disease) was 35% (7 of 20 patients [CI 18%–57%]). The first patient with partial response was a 67-year-old man with a long-standing history of papillary thyroid cancer since 1994, treated initially with a subtotal thyroidectomy and radioactive iodine ablation. He had many recurrences over the years, which were treated with additional surgery and radioactive iodine ablation. Ten years from his initial diagnosis, he developed progression for which pathology showed a clear anaplastic transformation. He was initially treated on a protocol utilizing fosbretabulin and chemotherapy with progression, and then treated on the current protocol and received a total of 10 cycles of sorafenib. The second patient was a 43-year-old woman who underwent an initial subtotal thyroidectomy in 2006. Pathology showed anaplastic undifferentiated thyroid cancer with focal areas of papillary features (<5% of tumor). She received 27 cycles of sorafenib.
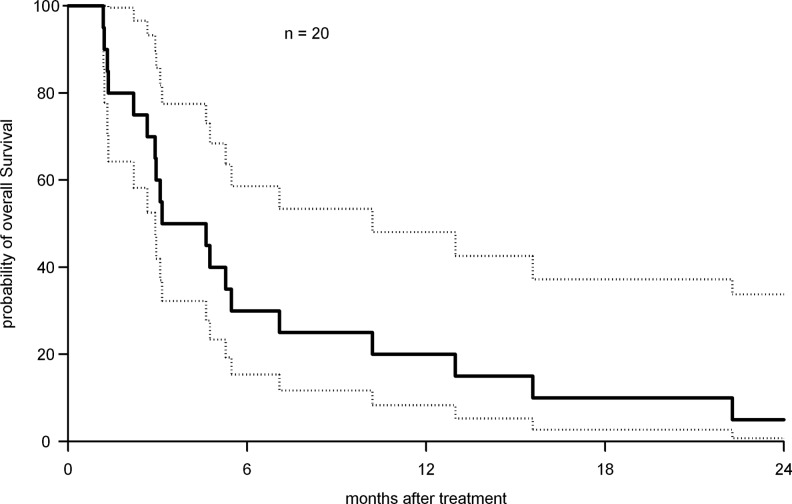
The median PFS was 1.9 months [CI 1.3–3.6 months] with a 6-month PFS of 15% and a 1-year PFS of 10%. The median overall survival time was 3.9 months [CI 2.2–7.1 months] Figure 1. The 6-month survival was 30%, and the 1-year survival was 20%.
FIG. 1.
Kaplan–Meier estimation of overall survival with 95% confidence interval [CI]. The median overall survival time was 3.9 months [CI 2.2–7.1 months].
Toxicity
The toxicities are shown in Table 2. In brief, hematological toxicities were uncommon and grade 1 or 2 in nature. In terms of nonhematological toxicities, the expected sorafenib toxicities were seen (e.g., hypertension and skin rash) with no safety concerns or signals. Electrolyte abnormalities were common but generally grade 1 or 2 in severity. The only grade 3 gastrointestinal toxicity seen was a single case of a gastrointestinal perforation. Constitutional symptoms of weight loss and fatigue occurred in the majority of cases. Grade 1 and 2 hypertension was seen in 20% of the patients. Rash and desquamation were very common, including 15% of patients with grade 3 events.
Table 2.
Drug-Related Adverse Events per NCI Version 3.0 in All Cycles
| |
Toxicity grade |
|||
|---|---|---|---|---|
| Adverse event | 1 | 2 | 3 | 4 |
| Hematological | ||||
| Neutropenia | 0 | 2 (10%) | 0 | 0 |
| Thrombocytopenia | 4 (20%) | 0 | 0 | 0 |
| Anemia | 7 (35%) | 4 (20%) | 0 | 0 |
| Nonhematological | ||||
| Hyponatremia | 7 (35%) | 0 | 2 (10%) | 0 |
| Hypokalemia | 3 (15%) | 1 (5%) | 0 | 0 |
| Hypomagnesemia | 4 (20%) | 0 | 0 | 0 |
| Hypocalcemia | 6 (30%) | 1 (5%) | 1 (5%) | 0 |
| Hypophosphatemia | 2 (10%) | 1 (5%) | 2 (10%) | 0 |
| Mucositis | 4 (20%) | 3 (15%) | 0 | 0 |
| Nausea | 3 (15%) | 2 (10%) | 0 | 0 |
| Vomiting | 1 (5%) | 1 (5%) | 0 | 0 |
| Diarrhea | 5 (25%) | 2 (10%) | 0 | 0 |
| Constipation | 1 (5%) | 1 (5%) | 0 | 0 |
| GI perforation | 0 | 0 | 1 (5%) | 0 |
| AST/ALT | 5 (25%) | 1 (5%) | 0 | 0 |
| Albumin | 1 (5%) | 2 (10%) | 0 | 0 |
| Weight loss | 6 (30%) | 5 (25%) | 1 (5%) | 0 |
| Anorexia | 1 (5%) | 0 | 1 (5%) | 0 |
| Hypoglycemia | 6 (30%) | 0 | 0 | 0 |
| Infection | 0 | 1 (5%) | 1 (5%) | 0 |
| Neuropathy | 5 (5%) | 0 | 0 | 0 |
| Fatigue | 8 (40%) | 3 (15%) | 1 (5%) | 0 |
| Cardiac ischemia | 0 | 1 (5%) | 0 | 0 |
| Thrombosis | 0 | 0 | 0 | 1 (5%) |
| Hemorrhage | 2 (10%) | 1 (5%) | 0 | 0 |
| Creatinine | 2 (10%) | 1 (5%) | 0 | 0 |
| Hypertension | 3 (15%) | 1 (5%) | 0 | 0 |
| Rash/desquamation | 6 (30%) | 4 (20%) | 3 (15%) | 0 |
Patients=20, total cycles=84. Data presented as the highest grade in each patient (percentage in parentheses). Only toxicities listed as at least possibly related to study drug are listed.
GI, gastrointestinal; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
Discussion
ATC represents a highly aggressive tumor with a median survival of 3–5 months and even worse survival after progression on chemotherapy and radiation. Clinical trials of novel agents in ATC are difficult, given the rarity of this disease and its decreasing incidence (16). Indeed, most published studies of novel agents for advanced ATC are small and have between 6 and 26 patients (7). Randomization at the phase II level would be impractical and difficult to complete. Thus, we conducted a single-arm phase II trial with the response rate as our primary endpoint. Our study met the prespecified criteria for enrollment of all planned 32 patients. However, due to poor accrual, the study was halted. The occurrence of two cases of prolonged partial response is highly significant in so far as it may indicate a subgroup of patients with ATC carrying a specific pathway activation that is blocked by sorafenib. Unfortunately, we do not have any molecular correlates in these patients, and the patients did not undergo a mutational analysis of BRAF. It is, however, now agreed that sorafenib is actually a poor BRAF inhibitor. From a pathological standpoint, it is noteworthy that the two cases of prolonged partial remission had an association with papillary thyroid cancer: one occurring after an anaplastic transformation of a longstanding papillary carcinoma, and the other with some areas of papillary findings on histology. Up to 20%–30% of ATCs are believed to occur in the setting of transformation from an underlying well-differentiated thyroid cancer (17). Sorafenib has previously been shown to have some activity in papillary thyroid cancer (18). An obvious hypothesis is that sorafenib may have had activity on the papillary component of these two patients and not on the anaplastic component. This is highly unlikely given the prolonged response seen in our two responding patients as well as the fact that, histologically, the papillary component represented a minor section of the tumor.
The median survival of 3.9 months seen on our trial was similar to our previous phase II trial of fosbretabulin, where a median survival was 4.7 months with 34% and 23% survival at 6 and 12 months, respectively (19). Therefore, sorafenib provides no superiority to fosbretabulin. The toxicities of sorafenib were overall manageable with only rare occurrences of grade 3 and 4 toxicities.
In conclusion, sorafenib has limited activity in ATC; however, the subgroup of ATC that occurs in the setting of anaplastic transformation of a more well-differentiated thyroid cancer or those ATC presenting with histological regions of papillary differentiation may respond to sorafenib. No clinical trials are currently evaluating the efficacy of adding sorafenib to cytotoxic chemotherapy in patients with advanced thyroid cancer, although this approach is currently under investigation in multiple other malignancies. Interestingly, there are three phase II clinical trials for patients with advanced thyroid cancer, including ATC, evaluating the combination of sorafenib with mTOR kinase inhibitors (everolimus or temsirolimus) and their results are expected with great interest (clinical trials.gov accessed on 02/01/2012).
Acknowledgments
This study was supported by National Institutes of Health Grant No. U01CA62502 and ClinicalTrials.gov Identifier No. NCT00126568.
Disclosure Statement
The authors declare no conflicts of interest.
References
- 1.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 2.Ain KB. Anaplastic thyroid carcinoma: a therapeutic challenge. Semin Surg Oncol. 1999;16:64–69. doi: 10.1002/(sici)1098-2388(199901/02)16:1<64::aid-ssu10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Edge SB. Byrd DR. Compton CC. Fritz AG. Greene FL. In: AJCC Cancer Staging Manual. 7th. Trotti A, editor. New York, NY: Springer Verlag; 2010. [Google Scholar]
- 4.Kim JH. Leeper RD. Treatment of locally advanced thyroid carcinoma with combination doxorubicin and radiation therapy. Cancer. 1987;60:2372–2375. doi: 10.1002/1097-0142(19871115)60:10<2372::aid-cncr2820601004>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Smallridge RC. Marlow LA. Copland JA. Anaplastic thyroid cancer: molecular pathogenesis and emerging therapies. Endocr Relat Cancer. 2009;16:17–44. doi: 10.1677/ERC-08-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagin JA. Molecular genetics of human thyroid neoplasms. Annu Rev Med. 1994;45:45–52. doi: 10.1146/annurev.med.45.1.45. [DOI] [PubMed] [Google Scholar]
- 7.Nagaiah G. Hossain A. Mooney CJ. Parmentier J. Remick SC. Anaplastic thyroid cancer: a review of epidemiology, pathogenesis, and treatment. J Oncol. 2011;2011:5423–5458. doi: 10.1155/2011/542358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S. Yazici YD. Calzada G. Wand ZY. Younes MN. Jasser SA. El-Naggar AK. Myers JN. Sorafenib inhibits the angiogenesis and growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer Ther. 2007;6:1785–1792. doi: 10.1158/1535-7163.MCT-06-0595. [DOI] [PubMed] [Google Scholar]
- 9.Ouyang B. Knauf JA. Smith EP. Zhang L. Ramsey T. Yussuff N. Batt D. Fagin JA. Inhibitors of Raf kinase activity block growth of thyroid cancer cells with RET/PTC or BRAF mutations in vitro and in vivo. Clin Cancer Res. 2006;12:1785–1793. doi: 10.1158/1078-0432.CCR-05-1729. [DOI] [PubMed] [Google Scholar]
- 10.Salvatore G. De Falco V. Salerno P. Jiang Y. Garbi C. Ugolini C. Miccoli P. Basolo F. Castellone MD. Cirafici AM. Melillo RM. Fusco A. Bittner ML. Santoro M. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res. 2006;12:1623–1629. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 11.Schweppe RE. Klopper JP. Korch C. Pugazhenthi U. Benezra M. Knauf JA. Fagin JA. Marlow LA. Copland JA. Smallridge RC. Haugen BR. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–4341. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta-Abramson V. Troxel AB. Nellore A. Puttaswamy K. Redlinger M. Ransone K. Mandel SJ. Flaherty KT. Loevner LA. O'Dwyer PJ. Brose MS. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–4719. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kloos RT. Ringel MD. Knopp MV. Hall NC. King M. Stevens R. Liang J. Wakely PE., Jr. Vasko VV. Saji M. Rittenberry J. Wei L. Arbogast D. Collamore M. Wright JJ. Grever M. Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–1684. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsai WY. Chi Y. Chen CM. Interval estimation of binomial proportion in clinical trials with a two-stage design. Stat Med. 2008;27:15–35. doi: 10.1002/sim.2930. [DOI] [PubMed] [Google Scholar]
- 16.Are C. Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 17.Wiseman SM. Loree TR. Rigual NR. Hicks WL., Jr Douglas WG. Anderson GR. Stoler DL. Anaplastic transformation of thyroid cancer: review of clinical, pathologic, and molecular evidence provides new insights into disease biology and future therapy. Head Neck. 2003;25:662–670. doi: 10.1002/hed.10277. [DOI] [PubMed] [Google Scholar]
- 18.Chen L. Shen Y. Luo Q. Yu Y. Lu H. Zhu R. Response to sorafenib at a low dose in patients with radioiodine-refractory pulmonary metastases from papillary thyroid carcinoma. Thyroid. 2011;21:119–124. doi: 10.1089/thy.2010.0199. [DOI] [PubMed] [Google Scholar]
- 19.Mooney CJ. Nagaiah G. Fu P. Wasman JK. Cooney MM. Savvides PS. Bokar JA. Dowlati A. Wang D. Agarwala SS. Flick SM. Hartman PH. Ortiz JD. Lavertu PN. Remick SC. A phase II trial of fosbretabulin in advanced anaplastic thyroid carcinoma and correlation of baseline serum-soluble intracellular adhesion molecule-1 with outcome. Thyroid. 2009;19:233–240. doi: 10.1089/thy.2008.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]